- 1Centre for Cognitive and Systems Neuroscience, School of Psychology, Social Work and Social Policy, University of South Australia, Adelaide, SA, Australia
- 2Sleep and Chronobiology Laboratory, School of Psychology, Social Work and Social Policy, University of South Australia, Adelaide, SA, Australia
- 3Department of Psychology, University of York, York, United Kingdom
We hypothesize a beneficial influence of sleep on the consolidation of the combinatorial mechanisms underlying incremental sentence comprehension. These predictions are grounded in recent work examining the effect of sleep on the consolidation of linguistic information, which demonstrate that sleep-dependent neurophysiological activity consolidates the meaning of novel words and simple grammatical rules. However, the sleep-dependent consolidation of sentence-level combinatorics has not been studied to date. Here, we propose that dissociable aspects of sleep neurophysiology consolidate two different types of combinatory mechanisms in human language: sequence-based (order-sensitive) and dependency-based (order-insensitive) combinatorics. The distinction between the two types of combinatorics is motivated both by cross-linguistic considerations and the neurobiological underpinnings of human language. Unifying this perspective with principles of sleep-dependent memory consolidation, we posit that a function of sleep is to optimize the consolidation of sequence-based knowledge (the when) and the establishment of semantic schemas of unordered items (the what) that underpin cross-linguistic variations in sentence comprehension. This hypothesis builds on the proposal that sleep is involved in the construction of predictive codes, a unified principle of brain function that supports incremental sentence comprehension. Finally, we discuss neurophysiological measures (EEG/MEG) that could be used to test these claims, such as the quantification of neuronal oscillations, which reflect basic mechanisms of information processing in the brain.
Introduction
The ability to form memory is essential for an organism to successfully adapt to changing environmental demands (Rasch and Born, 2013). While memory encoding and retrieval occur during periods of wake, sleep facilitates the consolidation of freshly encoded information through unique neuromodulatory activity (Staresina et al., 2015). Electrophysiological research demonstrates that sleep is composed of intensive variations in spatio-temporal oscillations across the brain. These oscillations, characterizing rapid- (REM) and non-rapid eye movement (NREM) sleep, originate from signals generated by specific cortical and subcortical networks, and play a key role in memory consolidation (Rauchs et al., 2005).
Evidence suggests the relation between sleep and memory extends to higher-order cognitive domains, such as language (Nieuwenhuis et al., 2013; Mirković and Gaskell, 2016). However, current research on sleep and language is largely limited to word learning and grammar generalization (for review, see Rasch, 2017), which does not account for the complex combinatorics of language at the sentence level. Here, we propose that sleep is a brain state necessary for the consolidation of the combinatorial mechanisms that underlie cross-linguistic variations in sentence comprehension, namely sequence-based (order-sensitive) and dependency-based (order-insensitive) combinatorics. In addition, we suggest that sleep’s effect on the consolidation of sentential combinatorics is reflected in various profiles of brain rhythmicity.
The spatiotemporal architecture of oscillatory rhythms is a fundamental principle of brain structure and function during both wake and sleep states (Buzsáki, 1996; Varela et al., 2001). Sleep-related oscillatory dynamics, such as the sleep-spindle, slow wave oscillation and REM theta activity, will be hypothesized to differentially consolidate sequence-dependent and sequence-independent combinatorics, manifesting in distinct oscillatory activity during sentence comprehension. To support this proposal, we briefly review evidence linking sleep to declarative and procedural memory consolidation, and recent research implicating sleep in language learning. We also review the proposed involvement of the declarative and procedural memory systems in language as posited by Ullman’s Declarative/Procedural Model (Ullman, 2001, 2004, 2016). We then outline a new perspective on the involvement of declarative and procedural memory in language by linking mechanisms of sleep-dependent memory consolidation to the neurobiological underpinnings of different types of sentence-level combinatorics (Bornkessel-Schlesewsky and Schlesewsky, 2013; Bornkessel-Schlesewsky et al., 2015). Finally, we will present testable hypotheses arising from this view, focusing on oscillatory brain activity.
Neurobiology of Sleep and Memory Consolidation
The notion that sleep facilitates memory consolidation and neural plasticity is long-standing (Graves, 1936; Klinzing et al., 2016b). Since its discovery (Aserinsky and Kleitman, 1953), REM sleep was thought to be the sleep stage that supported memory consolidation because of its wake-like EEG and oculomotor activity (Rasch and Born, 2015). However, mixed evidence from studies that selectively deprived subjects of REM sleep (for review, see Vertes and Eastman, 2000) prompted a shift in the sleep and memory field to focus on the role of NREM sleep in memory consolidation. Evidence implicating NREM sleep and associated SWS activity in memory consolidation has given rise to several theories, including the Active System Consolidation (ASC; Diekelmann and Born, 2010; Born and Wilhelm, 2012) and information overlap to abstract (iOtA; Lewis and Durrant, 2011) models, and the Synaptic Homeostasis hypothesis (SHY; Tononi and Cirelli, 2006, 2014). According to the ASC model, memory formation is supported by a hippocampal and neocortical system, such that mnemonic representations initially reliant on the hippocampal complex are integrated into the neocortex for long-term storage. From this perspective, sleep integrates hippocampally dependent memory traces with neocortical long-term memory (LTM) networks by facilitating cross-talk between the two systems (Born and Wilhelm, 2012; Mirković and Gaskell, 2016).
Slow oscillations (SOs; <1.0 Hz) and sleep spindles (10–16 Hz) – hallmarks of NREM sleep – are suggested to be involved in re-processing memory traces within the hippocampo-cortical network (Schabus et al., 2004; Lewis and Durrant, 2011). SOs reflect synchronized membrane potential fluctuations between hyperpolarised up-states and depolarised down-states of neocortical neurons (Lewis and Durrant, 2011; Klinzing et al., 2016a). During phases of depolarisation, sleep spindles are generated from thalamic reticular neurons and promote memory consolidation via cortico-thalamic loops, with individual differences in sleep spindle frequency and density associated with post-sleep memory for motor tasks (Peters et al., 2008), word-pair associations (Schabus et al., 2004), and emotional images (Kaestner et al., 2013). These findings are in line with a broader view (i.e., the ASC model; Born and Wilhelm, 2012) that SOs serve as a temporal gating mechanism for the flow of information between the hippocampus and neocortex, and that the nesting of sleep spindles in phases of depolarisation initiates synaptic change through LTP (Andrillon et al., 2011; Staresina et al., 2015). By contrast, SHY (Tononi and Cirelli, 2014) argues that the plastic processes occurring during wakefulness (e.g., memory encoding) result in a net increase in synaptic weight in networks subserving memory formation. Sleep is argued to facilitate the downscaling of synaptic weight to a baseline level that is homeostatically sustainable; a process posited to be performed by SOs during SWS (Mascetti et al., 2013). This process of synaptic renormalisation desaturates the capacity to encode new information during subsequent wake periods by decreasing neuronal excitability, which in turn, improves the signal-to-noise ratio in the reactivation of stored memory traces (Olcese et al., 2010; Tononi and Cirelli, 2012). The iOtA model (Lewis and Durrant, 2011) builds upon the ASC and SHY models, but makes predictions primarily about schema-conformant memory. According to iOtA, memory traces that are part of the same schemata are preferentially reactivated during sleep via nested spindle and SO activity, and thus develop stronger connections. After synaptic downscaling during SWS, the strongest connections between neurons that share encoded memory traces remain intact, supporting the formation of cognitive schemata (Lewis and Durrant, 2011).
The literature linking sleep and memory consolidation has focused to a large extent on the distinction between declarative and procedural memory, and the unique neurophysiology that contributes to their respective sleep-facilitated consolidation (Smith, 2001). Declarative and procedural memory differ in regard to their level of awareness and the neural networks subserving their computations (Barham et al., 2016). Declarative memory is primarily subserved by prefrontal and medial temporal lobe (MTL) structures, and supports the learning of general facts, namely semantic and episodic memory (Duff and Brown-Schmidt, 2012). In contrast, procedural memory is subserved by a basal ganglia cortico-striatal system, which facilitates the acquisition and execution of motor and sequence learning (Barnes et al., 2005; Albouy et al., 2013).
SWS is traditionally associated with the consolidation of declarative memory, assumedly via coordination of widespread neural synchrony that enable interactions between the hippocampus and neocortex (Rasch and Born, 2013). Conversely, REM is assumed to be associated with the facilitation of procedural memory consolidation, potentially through the activation of locally encoded memory traces in cortical-striatal networks (Barham et al., 2016). It is important to note, however, that the relationship between sleep and procedural memory consolidation is less clear than for declarative memory. In a recent meta-analysis, Pan and Rickard (2015; also see Rickard and Pan, 2017) argue that, for at least finger tapping tasks, sleep does not stabilize procedural memory, and that time of training (e.g., morning/evening), old age (i.e., >59 years), and a build-up of reactive inhibition over training, explain differences in procedural memory consolidation from training to delayed testing over and above that of sleep. There is, however, strong evidence implicating sleep in the consolidation of non-motor procedural tasks, such as auditory statistical learning paradigms (e.g., Durrant et al., 2013, 2016), suggesting a beneficial effect of sleep on procedural memory consolidation may be domain-specific.
Moreover, the claim that SWS is preferentially involved in declarative memory consolidation, and REM in procedural memory consolidation, is too simplistic and is not well supported by recent evidence (for a review, see Ackermann and Rasch, 2014). Alternatively, EEG phenomena associated with SWS (spindles, slow oscillations) and REM (theta oscillations, increases in acetylcholine; ACh) are posited to contribute sequentially to the consolidation of declarative and procedural memory (Fogel et al., 2007; Cairney et al., 2014; Llewellyn and Hobson, 2015; Rasch and Born, 2015). During SWS, low levels of ACh promote spontaneous reactivation of recently encoded memory traces within the hippocampal-cortical system, leading to a transfer of information from the hippocampus to the neocortex (Gais and Born, 2004; Hasselmo, 2006; Rasch et al., 2006). During REM, ACh is nine times greater than during wake (Hutchison and Rathore, 2015). This REM-related increase in ACh has been posited as one potential mechanism that promotes afferent input relative to feedback, an increase in theta oscillatory activity, and synaptic plasticity (McDevitt et al., 2015). These oscillatory and chemical changes within SWS and REM support proposals (e.g., the Sequential Hypothesis; Giuditta et al., 1995) that REM strengthens neocortical memory representations that have been selectively refined through the synaptic downscaling of SWS. It is important to note, however, that evidence implicating scalp-recorded REM theta activity in humans is scarce. Much of the evidence supporting this notion is based on invasive animal research (e.g., Boyce et al., 2016), which is difficult to generalize to higher-order mnemonic processes in humans, such as language learning. This is further complicated by REM sleep deprivation studies, which provide equal evidence for and against a role of REM in memory consolidation (Vertes and Eastman, 2000). From this perspective, the role of REM and associated theta activity in memory consolidation is less established than the role of SWS. See Figure 1 for a schematic of sleep architecture and associated oscillatory activity in humans.
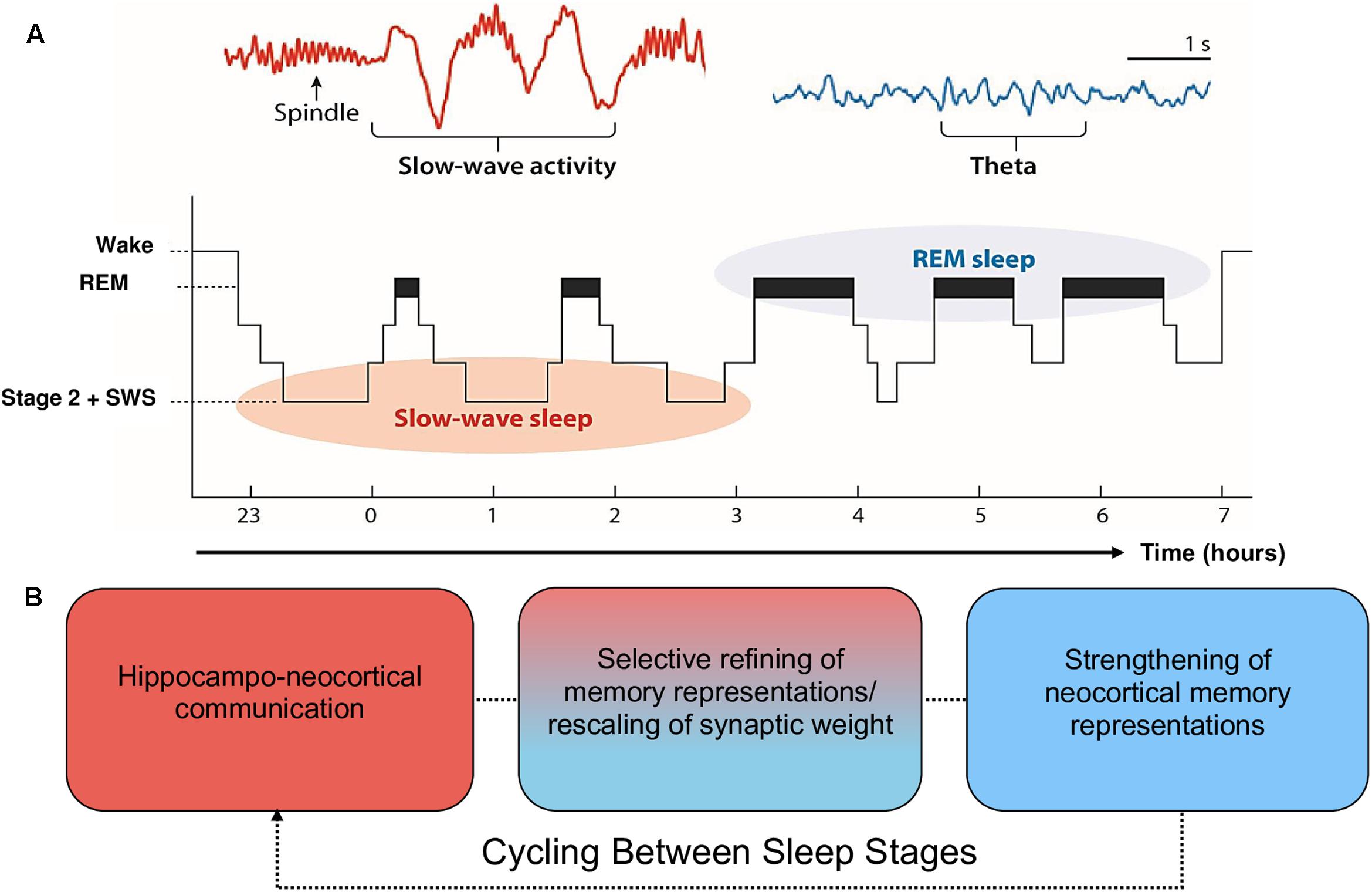
FIGURE 1. Schematic of sleep architecture in humans and associated oscillatory activity and stages of memory consolidation. (A) SWS is most prominent during the first half of the sleep period, and is dominated by neocortical slow oscillations (SOs) and thalamic spindles. By contrast, REM sleep is most prominent during the second half of the sleep period and is characterized by ponto-geniculo-occipital waves, increased acetylcholine (ACh) and cortical theta oscillations (reproduced from Vorster and Born, 2015; permission to reuse image is not required from the copyright holder for non-commercial use as determined by RightsLink®). (B) The cyclic occurrence of SWS and REM differentially facilitate memory consolidation. The hierarchical nesting of sharp-wave ripples and spindles within the up state of SOs during SWS facilitate the transfer of information from the hippocampal complex to the neocortex. These neocortically distributed memory representations are strengthened by REM theta oscillations and increases in ACh (Lewis and Durrant, 2011; Hutchison and Rathore, 2015). Each cycle of SWS induces large-scale rescaling of synaptic strength via widespread SO activity.
A Role for Sleep in Language Learning
Interest in the role of sleep during language learning has increased dramatically in recent years, with evidence suggesting that sleep plays a critical role in the consolidation of lexico-semantic information and simple grammatical rules (for review: Rasch, 2017). These experiments consistently demonstrate that sleep consolidates novel word meanings and their respective phonological forms from early childhood by integrating them within the existing mental lexicon (Dumay and Gaskell, 2007; Simon et al., 2017). In particular, SWS promotes novel word production and recognition (Tamminen et al., 2010; Gaskell et al., 2014), and grammar generalization over and above that of time spent awake (Batterink and Paller, 2015). These findings fit within the ASC model of sleep and memory formation (Born and Wilhelm, 2012). From this perspective, the consolidation of linguistic information occurs during two stages (Davis and Gaskell, 2009; Schreiner and Rasch, 2016).
Initially, the hippocampal complex plays a crucial role in binding a distributed neural representation of the linguistic input, such as word form and meaning (Davis and Gaskell, 2009). During sleep, these newly encoded memory representations are spontaneously reactivated, resulting in localized synaptic downscaling and the distribution of lexical representations in neocortical LTM networks (Rasch and Born, 2013; Schreiner and Rasch, 2016). Thus, sleep is posited to facilitate the integration of newly encoded lexical representations with existing lexical schemata, such as phonological and word form-to-meaning mapping systems (Gaskell et al., 2014).
This idea has been further tested by investigating the effect of sleep on the consolidation of a hidden linguistic rule using event-related potentials (ERPs; Batterink et al., 2014), a derivative of EEG reflecting the synchronized firing of neuronal populations time-locked to specific cognitive or sensory events (Luck, 2014). In this nap study, participants were presented with novel two-word phrases, which included an (English) noun that was preceded by a novel word serving as an article. Unbeknownst to the participants, the novel articles predicted noun animacy, an important semantic feature that is relevant for sentence comprehension in many languages of the world (e.g., Bates et al., 2001). Relative to participants who only experienced SWS, participants who experienced both SWS and REM demonstrated a larger negative ERP occurring between 400 and 800 ms in response to animacy violations, suggesting greater sensitivity to the hidden linguistic rule. This ERP effect provides preliminary evidence for a modulatory role of SWS and REM in generating neural representations of linguistic information by generalizing novel linguistic rules in memory.
This claim is supported by language learning studies that find sleep-mediated effects on oscillatory brain dynamics, suggesting memory-related changes in the organization of local and distributed neuronal assemblies (Fellner et al., 2013; Hanslmayr and Staudigl, 2014; Hanslmayr et al., 2016). Oscillations within different frequency bands are posited to reflect a number of language-related computations, including the retrieval of newly learned word meanings (Bakker et al., 2015; Takashima et al., 2016) and the detection of violations in artificial languages (de Diego-Balaguer et al., 2011). For example, Bakker et al. (2015) reported that novel words encoded before a 12-h consolidation period elicited greater fronto-temporally distributed theta power at recall than novel words encoded immediately before recall, while de Diego-Balaguer et al. (2011) found that an increase in alpha and theta phase synchrony during encoding predicted the detection of violations in learned trisyllabic sequences. Research also reveals that greater theta power during encoding of word-pair associations predicts sleep spindle frequency, which in turn is associated with enhanced recall (Heib et al., 2015; Schreiner and Rasch, 2015; Schreiner et al., 2015). Theta activity is associated with memory encoding and retrieval, and is thought to facilitate the consolidation of memory representations in the neocortex via hippocampo-cortical loops (Schreiner and Rasch, 2016), while alpha activity coordinates the flow of information in thalamo-cortical connections that subserve attention and perception (Klimesch et al., 2007; Klimesch, 2012; Hanslmayr et al., 2016). Thus, modulations in theta and alpha activity may differentially modulate the encoding and consolidation of linguistic information, facilitating sleep-dependent memory consolidation, such as spindle-related memory reprocessing (Hanslmayr et al., 2016; Schreiner and Rasch, 2016).
Although this evidence suggests sleep may play a role in aspects of language learning, this does not mean that the consolidation process will be uniform regardless of the material to be learned. Two related factors that may be relevant to memory consolidation, particularly in the case of language, are prior knowledge and systematicity (Dingemanse et al., 2015; Mirković and Gaskell, 2016; Gilboa and Marlatte, 2017).
Prior knowledge has traditionally been viewed as crucial to successful encoding and retention of new knowledge, but research on memory consolidation has revived interest in the notion of schema integration in learning (Gilboa and Marlatte, 2017). A landmark study by Tse et al. (2007) demonstrated that rats’ ability to acquire new associations between flavors and locations depended on the rats’ prior knowledge. If new pairings were consistent with previously learned associations involving similar stimuli then the process of consolidation was swift, with new associations quickly becoming independent of the hippocampus. This result is consistent with the idea that a pre-existing mental schema can assist with the learning and integration of new memory traces, a claim supported by McClelland (2013), who demonstrated that this kind of schema-compatibility effect could be explained in the context of a Complementary Learning Systems (CLS) model.
A CLS account predicts that the relationship between the individual elements of a new set of associations can be influential in terms of their initial acquisition and subsequent consolidation. If a set of new associations (e.g., between form and meaning) are in some ways compatible or systematic then they should be acquired more easily by a cortical network with less reliance on the hippocampal complex (Mirković and Gaskell, 2016). If the hippocampus is involved to a lesser extent during initial acquisition, then hippocampo-cortical replay during sleep might also be less important for consolidation, meaning that sleep-facilitated consolidation effects of hippocampally dependent memory may be weaker. However, such predictions are quite difficult to make because of the potential interaction between prior knowledge and systematicity. That is, the same compatibility in a systematic mapping that leads to weak reliance on the hippocampus during initial acquisition might also lead to greater schema compatibility during consolidation.
Mirković and Gaskell (2016) examined the influence of systematicity empirically in the context of an artificial language learning experiment. They trained participants on a language in which some elements had an entirely arbitrary relationship between the form and the meaning (as is typical of monomorphemic content words), whereas other elements had a more consistent relationship (determiners were used that had a consistent relationship with the gender of the referent). They found that, in this case, only the arbitrary components showed an influence of sleep on performance, consistent with the argument that hippocampal reliance is affected by the level of systematicity.
Nevertheless, several open questions remain. Mirković and Gaskell’s (2016) study adopted an afternoon nap paradigm, which occurred at a different circadian phase than nocturnal sleep and was dominated by NREM sleep. In accordance with the sequential hypothesis (Giuditta, 2014; Giuditta et al., 1995), interactions between SWS and REM may mediate the influence of prior knowledge and systematicity on the retention of new (linguistic) knowledge. An interactive effect of SWS and REM was demonstrated by Batterink et al. (2014), who found that sensitivity to violations of systematic article–noun pairings was predicted by the combined time spent in SWS and REM. From this perspective, during SWS, spindles and SOs may support the consolidation of schema conformant memory (Lewis and Durrant, 2011; Tamminen et al., 2013), while cortical REM theta activity may strengthen systematic mappings between form-to-meaning associations, similar to the beneficial role of REM in facilitating the abstraction of stimuli in probabilistic classification learning paradigms (e.g., Barsky et al., 2015). However, as stated in section “Neurobiology of Sleep and Memory Consolidation,” the relationship between REM theta activity and memory is not as well established in humans as in animal models, making REM-related memory consolidation hypotheses tentative and open to further investigation. Thus, while recent research has produced important initial insights on sleep and the consolidation of novel words and simple grammatical rules, we still know relatively little about the neural basis of sleep-facilitated memory consolidation of sentence-level combinatorics, and how an effect of prior knowledge and systematicity may be differentially mediated by different sleep stage characteristics.
Beyond Single Words: Preliminary Evidence for a Role of Sleep in the Consolidation of Sentence-Level Combinatorics
A potential role for sleep in the consolidation of sentence-level combinatorics is identifiable based on studies using artificial and modified miniature languages (MML). Artificial and MMLs generally contain a limited number of words belonging to several syntactic categories that can be combined into meaningful sentences based on the grammatical regularities of a chosen language model (Mueller, 2006). These paradigms provide a useful framework not only to track the learning trajectory of single words, but also the extraction and generalization of the linguistic building blocks (e.g., sequencing and dependency formation) that underpin sentence comprehension.
Studies using these paradigms (Friederici et al., 2002; Mueller et al., 2007) have helped characterize the neural correlates of language learning by demonstrating that rule violations elicit a biphasic ERP pattern containing a negativity (e.g., N400) and a late positivity (e.g., P600), as observed in natural language studies. Additionally, in a recent functional magnetic resonance imaging (fMRI) study (Weber et al., 2016), speakers of Dutch were exposed to an artificial language made up of 36 transitive verbs, ten intransitive verbs and four nouns. Activation in the angular gyrus – a region associated with semantic representations and in unifying smaller concepts into larger representations (Seghier, 2013) – increased linearly across the learning phase (i.e., across 7–9 days), and predicted participants’ ability to detect illegal word-order variations. Further neuroanatomical research with shorter learning intervals (i.e., ∼1–2 days) corroborates Weber and colleagues’ findings, demonstrating that hippocampal activation systematically decreases, while activation of language-related neocortical regions (e.g., BA 45 of Broca’s area) systematically increase across (artificial) language exposure (Opitz and Friederici, 2003, 2004, 2007; Mueller et al., 2014). These findings are in line with two-stage models of memory consolidation (e.g., Davis and Gaskell, 2009; Kumaran et al., 2016), further substantiating the notion that newly encoded information is initially reliant on the hippocampal complex before becoming neocortically distributed. As described in section “Neurobiology of Sleep and Memory Consolidation,” neocortical LTM networks are strengthened during sleep, suggesting sleep may play a critical role in consolidating language at the sentence-level, but that such an effect may depend on factors related to schema integration and systematicity. Thus, although existing artificial and MML experiments have helped characterize (artificial) language learning, further research is required to expand our understanding of the neurobiological mechanisms underlying the consolidation of language at the sentence-level, such as mechanisms of sleep-dependent memory consolidation.
One model, namely the Declarative/Procedural Model (DP model; Ullman, 2001, 2004, 2016), attempts to ground language processing in the neurobiological systems subserving memory. The DP model argues for a one-to-one mapping between declarative/procedural and semantic/syntactic processing, respectively, and assumes that sleep plays a beneficial role in the consolidation of both memory systems (although it does not provide specific sleep-related predictions; see Ullman, 2016). Since, to the best of our knowledge, the DP model is the only model of language beyond the single word-level that assumes a beneficial role of sleep via the two memory systems as a shared basis, we will briefly review its theoretical underpinnings before introducing our perspective.
Contributions of the Declarative and Procedural Memory Systems to Language
For language, differential roles of the declarative and procedural memory systems have been posited and discussed extensively by Ullman (2001; 2004; 2016). It is assumed here that declarative memory underlies the associative memory system required for the mental lexicon and the processing of semantic relations, while procedural memory subserves all rule-based processes in language, including morphology and syntax. As such, the processing of lexico-semantic and syntactic information is argued to differentially engage the neurobiological substrates associated with the declarative and procedural memory systems, respectively.
For the declarative memory system, this is posited to include MTL regions, including the hippocampal complex and entorhinal and perihinal cortices; however, Ullman (2016) recently proposed that there should be a decrease in the involvement of the MTL and an increase in neocortical regions as a function of time and experience of language use. This proposal is in accordance with two-stage models of memory that were discussed in section “A Role for Sleep in Language Learning” (see Davis and Gaskell, 2009 for a discussion on novel word consolidation), such that during novel word learning the hippocampal complex binds relational aspects of the word (e.g., form and meaning), with these associations slowly becoming independent of the hippocampus and neocortically distributed over time. While the DP Model’s predictions of semantic and episodic memory in relation to language processing are in accordance with the well-established two-stage models of memory consolidation, evidence at the sentence-level is limited, and while sleep is assumed to play a beneficial role in the consolidation of both memory systems (see Ullman, 2016), specific sleep-related predictions are absent.
By contrast, the procedural memory system is thought to be comprised of parietal, cerebellar, basal ganglia and frontal structures, including premotor regions (Newman et al., 2001; Ullman, 2016). Moreover, Ullman (2001, 2016) argues that specific ERP components are rooted in the neuroanatomical structures of the two memory systems: the N400, which is often associated with lexico-semantic violations (but see, for example, Frisch and Schlesewsky, 2001, 2005; Choudhary et al., 2009; Dröge et al., 2016, for evidence against a narrow lexico-semantic function of the N400), is suggested to be tied to MTL and rhinal cortex activation, while left anterior negativities are tied to procedural memory activation (Ullman, 2001, 2016; Morgan-Short et al., 2012). Late positivities, such as the P600, are discussed as originating from ‘conscious syntactic integration’ processes (Ullman, 2016). In regard to (second) language learning, Ullman argues that the declarative system is engaged more strongly than procedural memory during the initial phases of learning, evidenced by greater MTL activation during early second language processing, and greater activation of ganglia cortico-striatal structures when processing becomes more “native-like.”
Ullman states that “procedural memory should underlie the learning and processing of sequences and rules in language” (Ullman, 2016, p. 960), but acknowledges that his predictions for procedural memory are less specific and more tentative than for declarative memory. However, the basic assumptions of the DP Model are closely tied to the affordances of processing English and languages of a similar type. Consideration of a broader range of languages calls for a somewhat more complex perspective on the combinatory mechanisms underlying sentence interpretation (e.g., MacWhinney et al., 1984; Bornkessel and Schlesewsky, 2006; Bornkessel-Schlesewsky and Schlesewsky, 2009). From this perspective, the dichotomy between the declarative and procedural memory systems does not appear to fully account for the inherent complexity of language. In the following section, we discuss the need to distinguish between different types of combinatory mechanisms depending on the language being processed.
A New Perspective on Higher-Level Language Combinatorics and the Potential Role of Sleep in Their Consolidation
Languages differ fundamentally with respect to the information sources that are relevant to sentence interpretation. The assignment of thematic roles to noun phrases (NPs) is a case in point. Thematic role assignment allows comprehenders to determine “who is doing what to whom” in the sentence currently being comprehended, and the way in which it occurs is thought to differ between languages (e.g., Bornkessel and Schlesewsky, 2006; Dominey et al., 2009). Specifically, following Bornkessel-Schlesewsky et al. (2015), we assume that languages differ as to whether they dominantly rely on order-sensitive (sequence-based) or order-insensitive (non-sequence-based) combinatorics.
Sequence-based sentence interpretation is the dominant mechanism in languages such as English or Dutch. Accordingly, native speakers of these languages typically interpret the first NP encountered as the actor (the active, controlling participant) and the second NP as the undergoer (the affected participant), irrespective of semantic cues (MacWhinney et al., 1984). This is apparent from example (1), which is an implausible sentence in English because “the javelin” must be interpreted as the actor. In fact, the reliance on word order for interpretation is so strong that the only plausible way of combining “javelin,” “athletes,” and “throw” – namely to mean that the athletes threw the javelin – is not a possible interpretation of this sentence for native speakers of English (Bornkessel and Schlesewsky, 2006; Bornkessel-Schlesewsky et al., 2011).
(1) The javelin has thrown the athletes. (Hoeks et al., 2004)
By contrast, in languages that rely primarily on non-sequence-based sentence interpretation thematic role assignment is based more strongly on cues other than word order, such as case marking and/or semantic information, including animacy (for a review, see Bates et al., 2001). A particularly striking example, from the Australian language Jiwarli, is given in (2).
(2) Jiwarli, Pama-Nyungan, Western Australia (Austin, 2001) Yinha nhurra parlura-rni-nma payipa nganaju. this.acc 2sg.erg full-caus-imper pipe.acc 1sg.dat.acc ‘You fill up this pipe of mine!’
As is apparent from (2), groups of words that must be interpreted together need to occur sequentially in Jiwarli. For example, “this” and “pipe” are separated from each other by two other words. In addition, the order in which the words occur is not important for the interpretation of the sentence. Rather, which words belong together and which role they play within the sentence is indicated by case marking, i.e., changes in the morphological form of NPs depending on their role in the current sentence (akin to the difference between subject and object personal pronouns in English, cf. “I saw her” versus “She saw me”). While (2) is a quite extreme example, the basic principle of sentence interpretation being based primarily on non-sequential (order-independent) cues is very common across the languages of the world, applying, for example, in German, Turkish and Japanese. For a review of the supporting behavioral evidence from a wide range of languages, see Bates et al. (2001).
These cross-linguistic dissociations in incremental sentence comprehension are captured in proposals that assume distinct combinatory mechanisms in the brain, namely sequence-based and dependency-based (sequence-independent) combinatorics (Bornkessel-Schlesewsky et al., 2015). From this perspective, speakers of sequence-dependent languages (e.g., English and Dutch) are posited to rely primarily on predictive sequence processing mechanisms for sentence comprehension (Bornkessel-Schlesewsky et al., 2011, 2015; Dröge et al., 2016). Conversely, speakers of languages such as German and Turkish rely more strongly on sequence-independent features such as case marking or animacy to combine linguistic input into successively more complex representations, thereby facilitating the establishment of relations between non-adjacent elements in a sentence. Note, however, that both types of combinatorics are thought to be operative in all languages: clearly, the processing of languages such as German and Turkish is not completely independent of the order in which the words in a sentence are encountered, and languages such as English allow for non-adjacent dependencies. Thus, rather than being a clear-cut dichotomy, the classification of languages as sequence-dependent or sequence-independent is a matter of degree.
This assumed distinction of dependency- and sequencing-based combinatorics as basic and dissociable components of the neurobiology of human language (Bornkessel-Schlesewsky et al., 2015) raises new questions about the relation between these combinatory mechanisms and different memory systems, and accordingly, about the role of sleep in their consolidation. While it appears reasonably straightforward to associate sequence-based combinatorics with the procedural memory system, the status of non-sequence-based combinatorics is less clear. This type of combinatorics is rule-based but sequence-independent (for a similar perspective, see Wilson et al., 2014). It thus shows characteristics of both memory systems (e.g., the requirement for relational binding as in declarative memory; rule-based combinatorics as assumed by Ullman for procedural memory). Consequently, the consolidation of non-sequence-based combinatorics may depend on an interaction between the two memory systems, or may work independently of both systems1.
This perspective is closely tied to theoretical advancements in cognitive neuroscience which view the brain as a predictive organ (Friston, 2010; Friston and Buzsáki, 2016), and which posit that the (lexico)semantic/syntax distinction can be better described as a segregation of what and when representations. This claim is supported by various neurobiological observations of sleep-dependent memory consolidation - as an optimisation of (Bayesian) model evidence (Hobson and Friston, 2012; Rauss and Born, 2017) – facilitating the generalization of ordinal sequences (the when) and the establishment of semantic schemas of unordered items (the what). These findings provide a promising basis for investigating the consolidation of sequence-dependent and non-sequence-dependent combinatorics from a neurobiological perspective. However, they also demonstrate a need to move beyond the current state of the art in the literature in order to fully capture the complexity of the two types of combinatorics. As described above, non-sequence-based combinatorics involve unordered schemas that are rule-based in their organization; that is, while these schemas are unordered from a sequence-based perspective, they do involve organizational principles of other types. Likewise, sequence-based combinatorics cannot be reduced to ordinal sequences. Rather, they require more richly structured sequence representations, involving asymmetric, hierarchical sequences of elements.
In the following, we derive novel hypotheses about the sleep-dependent consolidation of higher-order language combinatorics based on these assumptions. Specifically, we explore how such hypotheses can be linked to oscillatory brain dynamics, which have long been identified as a key feature of sleep neurophysiology, and which also play an essential role in information processing while awake.
Sleep-Dependent Consolidation of Higher-Order Language Combinatorics as Reflected in Oscillatory Brain Rhythms
Neural oscillations are ubiquitous in the central nervous system and play a key role in sensory, motor and cognitive computations during wake and sleep states (Buzsáki, 1996; Canolty and Knight, 2010). Wake oscillatory activity is typically divided into five bands: delta (δ; ∼0.5–3.5 Hz), theta (θ; ∼4–7.5 Hz), alpha (α; ∼8–12 Hz), beta (β; ∼13–30 Hz) and gamma (γ; >30 Hz; Mai et al., 2016; Cole and Voytek, 2017). Conversely, Stage 2 sleep is characterized by sigma (12–15 Hz) and θ oscillations, while SWS is predominantly characterized by sigma, δ and SO (0–1 Hz) activity. REM sleep is dominated by high-intensity, wake-like θ oscillations (Hutchison and Rathore, 2015).
Oscillatory cycles within each band can be conceptualized as temporal receptive windows, transmitting envelopes of information of varying size across or within neuronal pools (Buzsáki, 2010; Harmony, 2013; Buzsáki and Schomburg, 2015). It follows that slow oscillations, such as those within the δ and θ range, are involved in large-scale network activity, which in turn, modulate faster local events expressed as activity in higher frequencies (e.g., in β and γ activity; Buzsáki and Draguhn, 2004; Sirota et al., 2008). The coupling of activity between fast and slow frequencies allows regions that are part of the same functional network to bind together information that is differentially encoded in memory (Bastiaansen et al., 2012).
Oscillatory neuronal activity is typically quantified using power spectrum analyses, which index local neuronal activity, and phase synchronization, which is a measure of functional connectivity between distant neuronal populations (Rubinov and Sporns, 2010; Bastiaansen et al., 2012). Possible separable functional roles of each band have been examined across a large body of research in a number of domains, including attention (Klimesch, 2012), memory (Duzel et al., 2010; Hanslmayr et al., 2016) and language (Lewis and Bastiaansen, 2015). Given that oscillatory activity is an inherent property of brain function, supporting both neural plasticity (Hanslmayr et al., 2016) and neural communication (Canolty and Knight, 2010), we posit that neuronal oscillations are a robust means of indexing any effect of sleep on the formation of the neural networks that subserve sentence comprehension. Throughout the remainder of the paper, we will attempt to provide neurobiologically grounded proposals for the role of oscillatory activity in the encoding, sleep-facilitated consolidation and retrieval of sentence-level combinatorics. While we consider proposals regarding the neurobiological underpinnings of the observed evidence from a variety of sources (e.g., scalp-recorded and intracranial EEG in humans), some hypotheses are more tentative than others. This includes REM θ activity in memory consolidation, given that the majority of supporting evidence stems from invasive animal studies (see Figure 2 for a schematic of the oscillatory mechanisms subserving the encoding, consolidation and retrieval of sentence-level combinatorics)
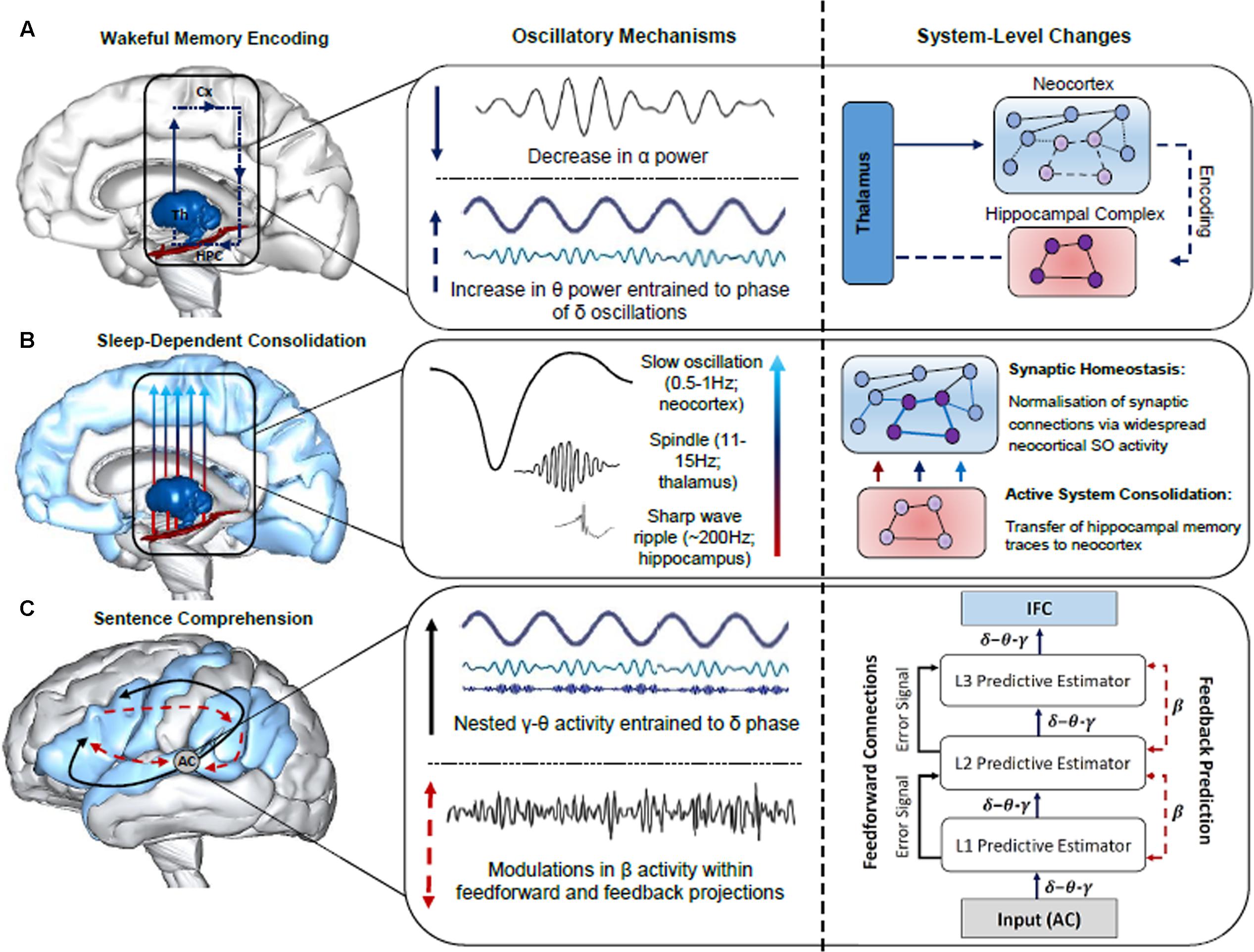
FIGURE 2. Summary of the oscillatory mechanisms subserving the encoding, consolidation and retrieval of information during language learning and sentence comprehension. (A) Decreases in α power facilitate enhanced information processing within the thalamo-neocortical-hippocampal system (TNHs), enabling freshly encoded memory traces to form in the hippocampal complex (HPC). An increase in θ power entrained to the phase of δ oscillations strengthens newly formed memory traces within the TNHs (thalamus, Th; neocortex, Cx). (B) Sleep-dependent neurophysiological activity, such as thalamic sleep spindles nested within the up-state of slow oscillations (SOs), enable hippocampal-cortical communication, and the transfer of information to the neocortex for long-term storage, as illustrated by the neural network grids in the right panel. SOs also induce a rescaling of synaptic weight, optimizing synaptic efficiency for post-sleep encoding and retrieval. (C) The construction of sentence-level meaning is facilitated by LTM networks recently established in the neocortex and a fine tuning of synaptic connections in the cortical hierarchy during sleep, as reflected by hierarchically nested δ–θ–γ and β activity, respectively (schematic of hierarchically nested δ–θ–γ oscillations modified from Calderone et al., 2014). Specifically, the schematic in the right panel illustrates the interplay between neuronal oscillations during sentence comprehension from a predictive-coding-based view of the brain following sleep-dependent consolidation. Once acoustic speech patterns are perceived by the auditory cortex (AC), hierarchically nested δ–θ–γ oscillations form successively more complex representations that are generated across the cortical hierarchy (i.e., L1, L2, L3 predictive estimators). Each level of the cortical hierarchy compares feedback (top-down) predictions to lower levels of the hierarchy, a process subserved by β oscillations. Error signals occur when there is a discrepancy between the predicted and actual sensory input, resulting in an update of the internal model. Brain models were generated using BodyParts3D/Anatomography service by DBCLS, Japan.
δ Oscillations Entrain the Activity of Higher Frequencies
Low frequency oscillations in the δ range have traditionally been associated with memory consolidation processes occurring during sleep (Harmony, 2013; Rasch and Born, 2013). While δ oscillations play a key role in hippocampo-cortical communication during sleep, they also play an active role in sensory processing during wakeful states (Schroeder and Lakatos, 2009; Basar and Duzgun, 2016). Research in the auditory domain indicates that δ–θ oscillations lock to various speech features (e.g., at the syllabic and phoneme level), facilitating the decoding and integration of complex sequences during speech perception (Doelling et al., 2014). Similarly, increases in the amplitude of θ and γ oscillations are entrained to the δ phase during attentionally demanding tasks (e.g., oddball tasks), such that increases in θ and γ cross-frequency coupling (CFC) in response to salient stimuli are predicted by the phase of δ oscillations (Schroeder and Lakatos, 2009; Calderone et al., 2014).
In general, the literature suggests that δ oscillations modulate the entrainment of higher frequencies (e.g., θ and γ) during the processing of higher-level information from a task-relevant input stream, such as language and general sequence processing (Canolty and Knight, 2010). From this perspective, δ oscillations may govern optimal excitability within and between neuronal assemblies, facilitating information transfer during wake, and memory consolidation during sleep. Thus, for language learning, we propose that the phase of δ oscillations will entrain higher frequency bands, facilitating the timing and spiking of synaptic activity, and in turn, facilitate memory encoding (Fell and Axmacher, 2011). For sentence comprehension, δ activity will depend on the predictability of the category sequence, which will further depend on which units have been successfully consolidated into LTM.
θ Oscillations and Hippocampo-Cortical Communication during Memory Encoding and Sentence Comprehension
θ oscillations are generated in the hippocampus and surrounding structures (Covington and Duff, 2016; Piai et al., 2016). They play a key role in the coordination of communication between the hippocampal complex and neocortical regions during wakeful states (Hanslmayr et al., 2016; Herweg et al., 2016). The hippocampus is implicated in relational binding and representational integration, which are important in language processing (Duff and Brown-Schmidt, 2012; Covington and Duff, 2016). Further, the hippocampus has been suggested to be involved in predictive processing by combining elements in memory, supporting the ability to predict future events (Bendor and Spiers, 2016; Friston and Buzsáki, 2016). The process of combining elements in memory to predict sensory input is critical during sentence comprehension, since as information unfolds, the brain generates predictions about upcoming information (Bornkessel-Schlesewsky et al., 2016; Dröge et al., 2016). The hippocampus might therefore support language processing by generating predictions for upcoming linguistic information, and θ activity may be modulated depending on whether the sensory input matches the internal model predictions, an idea also recently proposed by Covington and Duff (2016).
This proposal is supported by Friston and Buzsáki (2016, p. 508) who state that “Whether in space or time, ordinal sequences in the hippocampal system may ‘index’ the items (“what”) in the neocortex…[and] the organized access to neocortical representations (“what”) then becomes episodic [memory] information.” From this perspective, during incremental sentence comprehension, the hippocampus may encode the succession of words, accumulating evidence over the duration of the sentence. This evidence may then be used by the neocortex to test what predictions about prior beliefs (i.e., the probability distribution of the likelihood of upcoming words based on prior observations). Based on evidence indicating that θ oscillations bind neocortically distributed memory traces during memory encoding and retrieval (Herweg et al., 2016), θ activity might combine linguistic input into successively more complex representations, establishing relations between (non-adjacent) elements in a sentence. We posit that this may be a general mechanism for the processing of dependencies in linguistic input: dependencies necessarily require the relational binding of two elements; they may also lead to predictions of upcoming input items when the dependent element within a dependency precedes the independent element. From this perspective, dependency processing involves (neocortically computed) relational binding and (hippocampally driven) rule-based processing. Further, as what computations are posited to be performed by the neocortex, sleep should optimize the transmission of spatial sequences into more complex (unordered) representations by strengthening connections between the hippocampus and neocortex, and in turn, modulate θ activity during subsequent wakeful states.
Modulations in θ power may also index effects of systematicity and prior knowledge on the consolidation of sequence- and dependency-based combinatorics. As discussed in section “A Role for Sleep in Language Learning,” newly encoded associations that are compatible or systematic with existing schemata may be acquired more easily by a cortical network with less reliance on the hippocampal complex (Tse et al., 2007; Mirković and Gaskell, 2016; Gilboa and Marlatte, 2017). For example, the consolidation of a second language that shares combinatorial properties similar to a first language (e.g., morphosyntactic case marking in German and Hindi) may result in less hippocampal-dependence during initial learning, and thus weaker sleep-related consolidation effects during SWS (e.g., a reduction in the occurrence of spindles). Rather, effects of sleep may depend on interactions between SWS and REM – as demonstrated by Batterink et al. (2014) – resulting in an early emergence of cortical θ activity during incremental sentence comprehension.
In summary, we expect θ oscillations to reveal effects of sleep-facilitated memory consolidation of sentential combinatorics. Specifically, we posit that there will be an increase in θ power during incremental sentence comprehension after a language learning task followed by a period of sleep versus an equivalent wake period. We also hypothesize that θ oscillations index hippocampal when-based processing, and neocortically driven what-based relational binding, two mechanisms which may depend on hippocampo-cortical communication during SWS (reflected in an increase in spindles and SOs). We assume that this effect will accompany both sequence-dependent and sequence-independent combinatory processing, as both types of combinatorics are based on dependency relations. The two types of combinatorics differ in that, on top of basic dependency processing, sequence-based combinatorics include an additional restriction on the positioning of the elements in question as part of a structured sequence.
α Oscillations as a Thalamo-Cortical Gating Mechanism
According to the inhibition-timing hypothesis (Klimesch et al., 2007), oscillatory α activity modulates the activation of task-relevant cortical regions, facilitating the flow of information through thalamo-cortical networks, and enabling memory traces to form in the hippocampal complex (Bazanova and Vernon, 2014). The generation of α oscillations is posited to occur through GABAergic inter-neurons, an inhibitory neurotransmitter which receives input from excitatory output neurons, manifesting as oscillatory activity in cortico-thalamic and intra-cortical circuits (Mathewson et al., 2011). During NREM sleep, neocortical SOs, thalamic sleep spindles and hippocampal sharpwave ripples facilitate the reactivation of freshly encoded memory traces in the thalamo-neocortical-hippocampal system (TNHs; Bergmann and Staresina, 2017). Thus, α oscillations may serve as a thalamo-cortical gating mechanism, modulating wakeful memory encoding and subsequent reactivation of the TNHs during NREM sleep. From this perspective, α activity might modulate the timing and strength of language learning by facilitating the encoding of novel words and the regularities that govern the combination of words into sentences. Specifically, event-related changes in α power during encoding may index cortical processing in response to novel linguistic information, determining whether sensory input reaches the hippocampal complex via thalamo-cortical connections for long-term consolidation. To this end, we predict that changes in α activity during encoding will modulate language learning outcomes, manifesting behaviourally as greater accuracy of acceptability ratings, and neurophysiologically in distinct oscillatory profiles that reflect successful sentence comprehension. Finally, we expect that this effect will be more pronounced after sleep compared to wake through sleep-dependent reactivation of the TNHs during NREM sleep.
β Oscillations Reflect a Hierarchical Predictive Coding Architecture
β oscillations have recently been proposed to reflect the propagation of top-down predictions to lower levels of the cortical hierarchy during sentence comprehension (Lewis et al., 2016). During highly predictable sentence constructions, β activity is posited to increase, reflecting maintenance of the model predictions. Conversely, β activity is suggested to decrease when prediction errors occur in highly predictable sentences, possibly reflecting mismatches between internal predictions and the actual sensory input (Weiss and Mueller, 2012; Fontolan et al., 2014; Lewis and Bastiaansen, 2015; Lewis et al., 2015). From this perspective, we predict that β power will be modulated by sentences with unpredicted continuations, e.g., sentences deviating from the canonical word order, which are expected to elicit greater β desynchronization due to word-order-related prediction errors. We posit that this desynchronization reflects internal model updates based on mismatches with the actual sensory input, such as the abstract features (e.g., category) and sensory properties (e.g., word form) of the incoming linguistic item (Bornkessel-Schlesewsky et al., 2016). Finally, in addition to the TNHs facilitating offline reactivation of memory traces, homeostatic reductions in synaptic weight during sleep may accentuate prediction error-related β activity relative to an equivalent period of wake (for more on sleep and the formation of predictive codes, see Hobson and Friston, 2012; Rauss and Born, 2017).
γ Oscillations Reflect Local Network Activation during the Phase of Hippocampal θ Activity
Our hypotheses for γ oscillations are less specific and more tentative than for the slower frequency bands, since oscillations above ∼30 Hz are susceptible to artifact interference, making it difficult to interpret their functional role in information processing and cognition (Whitham et al., 2007; Kovach et al., 2011; Buzsáki and Schomburg, 2015). Specifically, electromyogram and oculomotor signals can contaminate scalp and cortically (i.e., electrocorticography; ECoG) recorded electrical activity >30 Hz, and cause widespread synchronized high frequency oscillations, leading to spurious inter- and intra-regional γ activity (Whitham et al., 2007). Scalp- and cortically recorded γ activity is also confounded by volume-conduction currents, which result from large fluctuations in subcortical γ rhythms that spread to and inflate γ activity in surrounding cortical layers (for a comprehensive discussion see Buzsáki and Schomburg, 2015). Recordings of cortical neuronal populations are particularly susceptible to volume-conduction currents, as cortical neurons share significant overlap in somatic and dendritic connections (Sirota et al., 2008; Buzsáki and Schomburg, 2015). For this reason, we suggest that the following predictions for γ oscillations be tested with depth electrodes, or at the very least, with magnetoencephalography (MEG), which can overcome spatially spread high frequency activity, since magnetic fields are less distorted by cortical tissue and the low conductivity of the skull (Cuffin and Cohen, 1979; Muthukumaraswamy and Singh, 2013). These approaches would be complemented by advanced analysis techniques, such as independent component analysis, in conjunction with appropriate filtering procedures (Buzsáki and Schomburg, 2015).
In the language comprehension literature, γ synchronization is argued to reflect accurate model predictions. That is, the matching between top-down (e.g., memory representations of word meaning, contextual information derived from prior discourse) and bottom-up (i.e., the incoming word) information is hypothesized to be reflected in γ synchronization (Lewis et al., 2015; Lam et al., 2016). However, this research is largely based on cortical (EEG) recordings, which may be confounded by volume conduction currents. Given the possible artifactual nature of scalp-recorded γ oscillations, we will focus on research that has utilized more reliable measures of neurophysiological activity, such as depth electrode recordings.
Research using depth electrodes reveal that γ oscillations occur within the hippocampal complex as well as throughout the cortex (Sirota et al., 2008; Buzsáki and Schomburg, 2015). Further, the selective coupling between regions CA1/CA3 and the medial entorhinal cortex appears to be mediated by γ oscillations that are phase-locked to θ activity (Colgin, 2015). Hippocampally generated θ oscillations entrain isolated bursts of γ activity through widespread, reciprocal connections between the hippocampal complex and neocortex (Lisman and Jensen, 2013). For example, in a study on waking rats, a large proportion of neocortically generated γ oscillations were dependent on the phase of hippocampally generated θ oscillations (Sirota et al., 2008). Thus, the temporal organization between CFC neocortical γ and hippocampal θ oscillations may facilitate information transfer between regionally distant neocortical neural ensembles, which in turn, may support information processing within the hippocampo-cortical system.
This interpretation is in accordance with a θ–γ neural code proposed by Lisman and Jensen (2013), who posit that θ–γ CFC facilitates the generation of ordered multi-item representations within the hippocampo-cortical network, providing information to down-stream regions about the sequence of upcoming sensory input. This interpretation aligns with our proposed role of θ oscillations in dependency-based combinatorial computations, and with Friston and Buzsáki’s (2016) perspective on hippocampal when-based processing. Within this framework, the hippocampal complex encodes the succession of sensory input, which is then used by the neocortex to perform what-based predictions. While θ oscillations may support hippocampo-cortical communication, self-organized γ oscillations may help to bind memory representations by (1) allowing neural ensembles that have coded individual memory traces to spike, and (2) generating gaps between temporally encoded items that prevent errors in decoding hippocampally driven sequences, since up to four γ cycles can occur within one θ cycle (Lisman and Jensen, 2013). This proposal is in line with evidence implicating hierarchically nested δ–θ–γ activity in sensory and memory computations, including the perception of speech (see Giraud and Poeppel, 2012; Arnal et al., 2016; Ding et al., 2016). It is also in accordance with the observation that slow cortical oscillations (e.g., δ and θ) reflect large network activation, which in turn modulates the activity of more regionally isolated, faster oscillations (e.g., γ; Sirota et al., 2008).
To this end, bursts of regionally isolated γ activity may reflect the activation of locally encoded memory traces during incremental sentence comprehension, such as the meaning of single words and morphological case marking cues. The entrainment of γ activity to the phase of θ oscillations may then facilitate the binding of these individual memory traces within the hippocampo-cortical network, providing information to down-stream regions about the meaning of the sentence, a process which may be supported by inter-regional δ oscillations (for a similar perspective, see Murphy, 2016). Finally, in line with the notion that SWS and REM play complementary roles in memory consolidation (Giuditta et al., 1995), we posit that REM will strengthen regionally isolated neocortical memory representations that have been selectively refined through the synaptic downscaling of SWS, which will manifest in increased γ activity during incremental sentence comprehension.
Summary of Hypotheses
To summarize, we will restate the above as concrete predictions that follow our proposed functional role of neuronal oscillations in reflecting effects of sleep on the consolidation of sequence-based (order-sensitive) and dependency-based (order-insensitive) combinatorics during language learning and sentence comprehension.
Hypothesis 1: The phase of δ oscillations entrain the activity of higher frequencies, modifying learning and large-scale neuronal network communication in an attention-dependent manner.
Evidence for this prediction stems from research with rodents and monkeys, which demonstrate that δ and θ cross-frequency phase synchronization coordinates interactions between deep and superficial cortical layers, modifying sensory perception and learning processes, particularly for task-relevant stimuli (Carracedo et al., 2013; Harmony, 2013). Thus, we hypothesize that the phase of δ oscillations will entrain higher frequency bands, such as θ and γ oscillations, facilitating the timing and spiking of synaptic activity and regulating large-scale network communication during language learning and sentence comprehension. Specifically, δ and θ cross-frequency phase synchronization will predict enhanced memory encoding and retrieval, which will predict greater accuracy of acceptability ratings during sentence comprehension tasks requiring grammaticality judgements.
Hypothesis 2: θ oscillations bind relational elements from LTM during sentence comprehension that have been consolidated during sleep-dependent reactivation of the thalamo-neocortical-hippocampal system.
This hypothesis is supported by intracranial EEG evidence reported by Piai et al. (2016), who found that θ power increased in the hippocampal complex during ongoing relational processing during sentence comprehension. In accordance with the general memory literature, θ activity is posited to reflect the synchronization between neocortical regions and the hippocampal complex, binding neocortically distributed memory representations during encoding and retrieval (Osipova et al., 2006; Herweg et al., 2016). This interpretation is supported by sleep and memory research (Bakker et al., 2015; Schreiner et al., 2015), which reports increased neocortical θ power during memory retrieval after a period of sleep, possibly reflecting stronger connectivity between the hippocampal complex and neocortex. These findings are in line with the ASC model (Born and Wilhelm, 2012), which predicts that SOs, spindles and sharp-wave ripples facilitate memory consolidation by modulating hippocampo-cortical communication. Thus, our prediction is two-fold: (1) θ power during incremental sentence comprehension of a newly learned language will be increased following a period of sleep versus an equivalent period of wake, with this increase in power predicted by the occurrence of SOs, spindles and ripples; and, (2) an increase in θ power will occur for both sequence-independent and sequence-dependent interpretation, as both rely on basic dependency formation, which involves the binding of multiple memory traces to form coherent representations.
Hypothesis 3: Decreases in α power facilitate enhanced information processing within the thalamo-neocortical-hippocampal system, promoting the encoding of novel words and the regularities that govern the combination of words into sentences.
α oscillations facilitate cortical processing, acting as a gating mechanism for information flow within thalamocortical loops (Klimesch, 2012; Sadaghiani and Kleinschmidt, 2016). In terms of power, α desynchronization reflects the activation of cortical areas with increased neuronal excitability (a decrease in amplitude), whereas α synchronization reflects the inhibition of brain regions (Klimesch, 2012). From this perspective, we hypothesize that α desynchronization will enhance language learning by enabling novel linguistic information to be processed by the thalamus, promoting the formation of memory traces in the hippocampal complex via the entorhinal cortex. This effect will manifest behaviourally as greater accuracy of acceptability ratings, and neurophysiologically in distinct oscillatory rhythms engaged during sentence comprehension, such as increases in θ-band power. Finally, we expect that this effect will be more pronounced after sleep compared to wake through sleep-dependent neurophysiology, such that a decrease in α power at encoding and an increase in SOs and thalamic spindles during sleep will predict (1) enhanced behavioral performance on grammaticality judgment tasks and (2) increases in θ- and β-band power during incremental sentence comprehension.
Hypothesis 4: During incremental sentence comprehension, β synchronization reflects maintenance of model predictions, while β desynchronization reflects prediction error signals.
From a predictive-coding-based view of the brain, internal generative models, which predict unfolding linguistic input, update when there is a mismatch between predicted sensory input and the actual sensory input (Pickering and Garrod, 2013; Bornkessel-Schlesewsky et al., 2015). In principle, because of the time-dependent nature of sensory-related predictions, β oscillations may reflect the maintenance of model predictions of sensory input. From this perspective, during sentence comprehension, feedback projections (reflecting model predictions) that conflict with prediction error signals projected by feedforward connections may increase β-band desynchronization. This prediction is in line with in vivo recordings demonstrating that β oscillations are generated in deep cortical layers, which propagate prediction-related error signals backward on the cortical hierarchy to more superficial layers (Arnal, 2012). It is also in accordance with the proposal that β desynchronization is elicited by bottom-up information that conflicts with top-down predictions during sensory processing (Arnal et al., 2011), or conversely, that β synchronization occurs when “the cognitive set has to be maintained; (Engel and Fries, 2010, p. 160). Thus, we hypothesize that β power will be modulated by whether incoming linguistic items match internal model predictions. We further posit that SOs will fine tune synaptic connections in the cortical hierarchy, optimizing information flow between feedforward and feedback projections, and in turn, optimize accurate model predictions and minimize prediction errors.
Hypothesis 5: γ oscillations are temporally entrained to the phase of θ and δ oscillations, which subserves the binding of spatially distant neocortical memory traces that have been strengthened during REM sleep.
As stated above, our hypotheses for γ oscillations are more tentative than for the slower frequency bands. Based on depth electrode recordings (e.g., Sirota et al., 2008) and MEG research on speech perception (e.g., Ding et al., 2016), we hypothesize that locally generated cortical γ oscillations are temporally entrained to the phase of θ and δ oscillations during incremental sentence comprehension. We further posit that such a hierarchical nesting reflects the following: (1) bursts of regionally isolated γ activity allow neuronal ensembles that code specific memory traces – such as for the meaning of single words – to optimally spike; (2) during sentence comprehension, hippocampally generated θ activity binds together single memory traces activated by γ activity, and; (3) large-scale δ oscillations facilitate the transfer of γ–θ bound memory representations to regions further downstream. Finally, we predict that increases in REM and associated θ activity after language learning will predict increases in γ synchronization during subsequent sentence comprehension by potentially reorganizing inter- and intracortical memory representations that have been selectively refined during SWS (see Durrant et al., 2015, for a discussion on REM θ oscillations and schema-conformant memory consolidation).
Concluding Remarks and Future Directions
We have proposed that sleep is an optimal brain-state for consolidating sequence-based (order-sensitive) and dependency-based (order-insensitive) combinatorics. To this end, we argued that sleep-dependent memory consolidation optimizes synaptic efficacy, which maximizes the ability of the brain to generate predictions of upcoming sensory input during incremental sentence comprehension. We have provided testable predictions for this proposal, focussing on sleep-mediated effects on oscillatory brain activity during language learning and sentence comprehension. During encoding and sentence comprehension, δ oscillations entrain the activity of higher frequencies that serve as windows of various size for processing information within and between neuronal pools. α oscillations coordinate the flow of information in a thalamo-neocortical-hippocampal system that subserves memory encoding, and subsequent sleep-dependent memory consolidation. In turn, we have proposed that θ oscillations index a sleep-dependent transfer of information from MTL to neocortex, a process which supports both dependency- and sequence-based combinatorial computations. β oscillations reflect the propagation of predictions and prediction errors via a hierarchically organized predictive coding architecture that is instantiated by sleep-dependent synaptic downscaling. Finally, γ oscillations are entrained to the phase of hippocampally generated θ oscillations, a temporally coordinated process which subserves the binding of spatially distinct, neocortically stored information during sentence comprehension.
Although not within the scope of this paper, it would be worthwhile to consider how mechanisms of sleep-dependent memory consolidation influence the ontogenesis of the functional neuroanatomy of sentence comprehension, such as the dorsal-ventral stream architecture (Brauer et al., 2013; Bornkessel-Schlesewsky et al., 2015). In the visual domain, sleep drives plastic changes in early (V1) and late (i.e., parietal lobe) visual areas, facilitating top-down attentional modulations of primary visual cortex, enhancing visual object recognition (Walker et al., 2005). Similar effects may hold in the auditory domain, such that sleep may trigger large-scale, system-level changes, modifying acoustic memory representations beyond primary auditory cortex, facilitating the recognition of successfully more complex auditory objects (e.g., from syllables to words), a process subserved by the ventral stream (Rauschecker and Scott, 2009; Bornkessel-Schlesewsky et al., 2015).
Clinically, understanding the relationship between sleep neurophysiology and language learning could inform treatments for individuals with language-related disorders, including those with Autism Spectrum Disorder, Specific Language Impairment, and Aphasia, who experience greater sleep disturbances than healthy controls (McGregor and Alper, 2015). Specifically, SOs may serve as a sensitive biomarker of local cortical reorganization during aphasia therapy post-stroke (Sarasso et al., 2010, 2014). Research on both animals and humans indicates that SOs play a homeostatic role in synaptic plasticity by facilitating synaptic depression to obtain a general rescaling of synaptic strength (Sarasso et al., 2014; Tononi and Cirelli, 2014). In this view, if the hypotheses proposed in this paper hold, such that SOs – at least partially – underlie the consolidation of sentential combinatorics, SOs could be selectively increased via stimulation methods (e.g., transcranial magnetic or closed looped stimulation methods; Ngo et al., 2013) to accelerate aphasia-based speech and language therapy. Finally, this paper provides a theoretical framework for understanding how sleep may affect foreign language learning in adults beyond the single word level (e.g., Schreiner and Rasch, 2016), influencing approaches to foreign language learning, which is critical in an increasingly multilingual world.
Author Contributions
ZC is responsible for the construction and presentation of Figures 1, 2. All authors contributed to the preparation, writing and proof-reading of the manuscript.
Funding
Preparation of this work was supported by Australian Commonwealth Government funding awarded to ZC under the Research Training Program (number 212190). IB-S is supported by an Australian Research Council Future Fellowship (FT160100437). MG is supported by a UK Economic and Social Research Council grant (ES/N009924/1).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Louise Kyriaki, Andrew Corcoran, and Alex Chatburn for their valuable feedback on an earlier version of this paper. We would also like to thank Albrecht Vorster, who very kindly provided us with a high-resolution image of part A of Figure 1, and to the two reviewers for their insightful and constructive comments.
Footnotes
- ^Note also that it is not straightforwardly apparent whether existing findings on the sleep-facilitated consolidation of word learning (see section “A Role for Sleep in Language Learning”) might generalize to sentence-level combinatorics. The learning of novel words entails the learning of sound sequences, i.e., sequences of phonemes. This raises the question of whether phoneme sequence consolidation operates via similar mechanisms to word sequence consolidation at the sentence level. However, a crucial difference between the two types of sequences is that sequences of words involve the combination of meaningful units into larger meaningful units. Phonemes, by contrast, do not themselves bear meaning, but are rather the smallest units in language that differentiate meaning. Consider, for example, the difference between the English words “map” and “nap”: here, “m” and “n” lead to a difference in meaning without being meaningful in and of themselves (See Collier et al., 2014, for an accessible summary of how phonological and syntactic combinatorics differ). It is an open question whether the sequencing property common to both phonological and sequence-based sentence-level combinatorics constitutes a common denominator for mechanisms of consolidation, or whether the two are subject to differential consolidation processes due to the difference in the types of units being combined.
References
Ackermann, S., and Rasch, B. (2014). Differential effects of non-REM and REM sleep on memory consolidation?. Curr. Neurol. Neurosci. Rep. 14:430. doi: 10.1007/s11910-013-0430-8
Albouy, G., King, B. R., Maquet, P., and Doyon, J. (2013). Hippocampus and striatum: dynamics and interaction during acquisition and sleep-related motor sequence memory consolidation. Hippocampus, 23, 985–1004. doi: 10.1002/hipo.22183
Andrillon, T., Nir, Y., Staba, R. J., Ferrarelli, F., Cirelli, C., Tononi, G., et al. (2011). Sleep spindles in humans: insights from intracranial EEG and unit recordings. J. Neurosci. 31, 17821–17834. doi: 10.1523/JNEUROSCI.2604-11.2011
Arnal, L. H. (2012). Predicting “When” using the motor system’s Beta-band oscillations. Front. Hum. Neurosci. 6:225. doi: 10.3389/fnhum.2012.00225
Arnal, L. H., Poeppel, D., and Giraud, A.-L. (2016). “A neurophysiological perspective on speech processing,” in The Neurobiology of Language, eds G. Hickok, and S. L. Small (Amsterdam: Elsevier), 463–478. doi: 10.1016/b978-0-12-407794-2.00038-9
Arnal, L. H., Wyart, V., and Giraud, A.-L. (2011). Transitions in neural oscillations reflect prediction errors generatred in audivisual speech. Nat. Neurosci. 14, 797–803. doi: 10.1038/nn.2810
Aserinsky, E., and Kleitman, N. (1953). Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science 118, 273–274. doi: 10.1176/jnp.15.4.454
Austin, P. (2001). “Word order in a free word order language: the case of Jiwarli,” in Forty Years on: Ken Hale and Australian Languages, eds J. Simpson, D. Nash, M. Laughren, P. Austin, and B. Alpher (Canberra ACT: Australian National University), 205–323.
Bakker, I., Takashima, A., van Hell, J. G., Janzen, G., and McQueen, J. M. (2015). Changes in theta and beta oscillations as signatures of novel word consolidation. J. Cogn. Neurosci. 27, 1286-1297. doi: 10.1162/jocn_a_00801
Barham, M. P., Enticott, P. G., Conduit, R., and Lum, J. A. (2016). Transcranial electrical stimulation during sleep enhances declarative (but not procedural) memory consolidation: evidence from a meta-analysis. Neurosci. Biobehav. Rev. 63, 65–77. doi: 10.1016/j.neubiorev.2016.01.009
Barnes, T. D., Kubota, Y., Hu, D., Jin, D. Z., and Graybiel, A. M. (2005). Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature 437, 1158–1161. doi: 10.1038/nature04053
Barsky, M. M., Tucker, M. A., and Stickgold, R. (2015). REM sleep enhancement of probabilistic classification learning is sensitive to subsequent interference. Neurobiol. Learn. Mem. 122, 63–68. doi: 10.1016/j.nlm.2015.02.015
Basar, E., and Duzgun, A. (2016). Links of consciousness, perception, and memory by means of delta oscillations of brain. Front. Psychol. 7:275. doi: 10.3389/fpsyg.2016.00275
Bastiaansen, M., Mazaheri, A., and Jensen, O. (2012). “Beyond ERPs: oscillatory neuronal dynamic,” in The Oxford handbook of event-related potential components, eds S. J. Luck, and E. S. Kappenman (New York, NY: Oxford University Press), 31–50.
Bates, E., Devescovi, A., and Wulfeck, B. (2001). Psycholinguistics: a cross-language perspective. Annu. Rev. Psychol. 52, 369–396. doi: 10.1146/annurev.psych.52.1.369
Batterink, L. J., Oudiette, D., Reber, P. J., and Paller, K. A. (2014). Sleep facilitates learning a new linguistic rule. Neuropsychologia 65, 169-179. doi: 10.1016/j.neuropsychologia.2014.10.024
Batterink, L. J., and Paller, K. A. (2015). Sleep-based memory processing facilitates grammatical generalization: evidence from targeted memory reactivation. Brain Lang. 167 83–93. doi: 10.1016/j.bandl.2015.09.003
Bazanova, O. M., and Vernon, D. (2014). Interpreting EEG alpha activity. Neurosci. Biobehav. Rev. 44, 94–110. doi: 10.1016/j.neubiorev.2013.05.007
Bendor, D., and Spiers, H. J. (2016). Does the hippocampus map out the future? Trends Cogn. Sci. 20, 167–169. doi: 10.1016/j.tics.2016.01.003
Bergmann, T. O., and Staresina, B. P. (2017). Neuronal Oscillations and Reactivation Subserving Memory Consolidation Cognitive Neuroscience of Memory Consolidation. Berlin: Springer, 185–207. doi: 10.1007/978-3-319-45066-7_12
Born, J., and Wilhelm, I. (2012). System consolidation of memory during sleep. Psychol. Res. 76, 192–203. doi: 10.1007/s00426-011-0335-6
Bornkessel, I., and Schlesewsky, M. (2006). The extended argument dependency model: a neurocognitive approach to sentence comprehension across languages. Psychol. Rev. 113, 787–821. doi: 10.1037/0033-295X.113.4.787
Bornkessel-Schlesewsky, I., Kretzschmar, F., Tune, S., Wang, L., Genc, S., Philipp, M., et al. (2011). Think globally: cross-linguistic variation in electrophysiological activity during sentence comprehension. Brain Lang. 117, 133–152. doi: 10.1016/j.bandl.2010.09.010
Bornkessel-Schlesewsky, I., and Schlesewsky, M. (2013). Reconciling time, space and function: a new dorsal-ventral stream model of sentence comprehension. Brain Lang. 125, 60–76. doi: 10.1016/j.bandl.2013.01.010
Bornkessel-Schlesewsky, I., Schlesewsky, M., Small, S. L., and Rauschecker, J. P. (2015). Neurobiological roots of language in primate audition: common computational properties. Trends Cogn. Sci. 19, 142–150. doi: 10.1016/j.tics.2014.12.008
Bornkessel-Schlesewsky, I., Staub, A., and Schlesewsky, M. (2016). “The timecourse of sentence processing in the brain,” in Neurobiology of Language, eds G. Hickok, and S. L. Small (Amsterdam: Elsevier), 607–620. doi: 10.1016/b978-0-12-407794-2.00049-3
Bornkessel-Schlesewsky, I., and Schlesewsky, M. (2009). The role of prominence information in the real-time comprehension of transitive constructions: a cross-linguistic approach. Lang. Linguist. Compass 3, 19–58. doi: 10.1111/j.1749-818X.2008.00099.x
Boyce, R., Glasgow, S. D., Williams, S., and Adamantidis, A. (2016). Causal evidence for the role of REM sleep theta rhythm in contextual memory consolidation. Science 352, 812–816. doi: 10.1126/science.aad5252
Brauer, J., Anwander, A., Perani, D., and Friederici, A. D. (2013). Dorsal and ventral pathways in language development. Brain Lang. 127, 289–295. doi: 10.1016/j.bandl.2013.03.001
Buzsáki, G. (1996). The hippocampo-neocortical dialogue. Cereb. Cortex 6, 81–92. doi: 10.1093/cercor/6.2.81
Buzsáki, G. (2010). Neural syntax: cell assemblies, synapsembles, and readers. Neuron 68, 362–385. doi: 10.1016/j.neuron.2010.09.023
Buzsáki, G., and Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science 304, 1926–1929. doi: 10.1126/science.1099745
Buzsáki, G., and Schomburg, E. W. (2015). What does gamma coherence tell us about inter-regional neural communication? Nat. Neurosci. 18, 484–489. doi: 10.1038/nn.3952
Cairney, S. A., Durrant, S. J., Power, R., and Lewis, P. A. (2014). Complementary roles of slow-wave sleep and rapid eye movement sleep in emotional memory consolidation. Cereb. Cortex 25, 1565–1575. doi: 10.1093/cercor/bht349
Calderone, D. J., Lakatos, P., Butler, P. D., and Castellanos, F. X. (2014). Entrainment of neural oscillations as a modifiable substrate of attention. Trends Cogn. Sci. 18, 300–309. doi: 10.1016/j.tics.2014.02.005
Canolty, R. T., and Knight, R. T. (2010). The functional role of cross-frequency coupling. Trends Cogn. Sci. 14, 506–515. doi: 10.1016/j.tics.2010.09.001
Carracedo, L. M., Kjeldsen, H., Cunnington, L., Jenkins, A., Schofield, I., Cunningham, M. O., et al. (2013). A neocortical delta rhythm facilitates reciprocal interlaminar interactions via nested theta rhythms. J. Neurosci. 33, 10750–10761. doi: 10.1523/JNEUROSCI.0735-13.2013
Choudhary, K. K., Schlesewsky, M., Roehm, D., and Bornkessel-Schlesewsky, I. (2009). The N400 as a correlate of interpretively relevant linguistic rules: evidence from Hindi. Neuropsychologia 47, 3012–3022. doi: 10.1016/j.neuropsychologia.2009.05.009
Cole, S. R., and Voytek, B. (2017). Brain oscillations and the importance of waveform shape. Trends Cogn. Sci. 21, 137–149. doi: 10.1016/j.tics.2016.12.008
Colgin, L. L. (2015). Theta-gamma coupling in the entorhinal-hippocampal system. Curr. Opin. Neurobiol. 31, 45–50. doi: 10.1016/j.conb.2014.08.001
Collier, K., Bickel, B., van Schaik, C. P., Manser, M. B., and Townsend, S. W. (2014). Language evolution: syntax before phonology? Proc. R. Soc. B Biol. Sci. 281:20140263. doi: 10.1098/rspb.2014.0263
Covington, N. V., and Duff, M. C. (2016). Expanding the language network: direct contributions from the hippocampus. Trends Cogn. Sci. 20, 869–870. doi: 10.1016/j.tics.2016.10.006
Cuffin, B. N., and Cohen, D. (1979). Comparison of the magnetoencephalogram and electroencephalogram. Electroencephalogr. Clin. Neurophysiol. 47, 132–146. doi: 10.1016/0013-4694(79)90215-3
Davis, M. H., and Gaskell, M. G. (2009). A complementary systems account of word learning: neural and behavioural evidence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 3773–3800. doi: 10.1098/rstb.2009.0111
de Diego-Balaguer, R., Fuentemilla, L., and Rodriguez-Fornells, A. (2011). Brain dynamics sustaining rapid rule extraction from speech. J. Cogn. Neurosci. 23, 3105–3120. doi: 10.1162/jocn.2011.21636
Diekelmann, S., and Born, J. (2010). The memory function of sleep. Nat. Rev. Neurosci. 11, 114–126. doi: 10.1038/nrn2762
Ding, N., Melloni, L., Zhang, H., Tian, X., and Poeppel, D. (2016). Cortical tracking of hierarchical linguistic structures in connected speech. Nat. Neurosci. 19, 158–164. doi: 10.1038/nn.4186
Dingemanse, M., Blasi, D. E., Lupyan, G., Christiansen, M. H., and Monaghan, P. (2015). Arbitrariness, iconicity, and systematicity in language. Trends Cogn. Sci. 19, 603–615. doi: 10.1016/j.tics.2015.07.013
Doelling, K. B., Arnal, L. H., Ghitza, O., and Poeppel, D. (2014). Acoustic landmarks drive delta-theta oscillations to enable speech comprehension by facilitating perceptual parsing. Neuroimage 85, 761–768. doi: 10.1016/j.neuroimage.2013.06.035
Dominey, P. F., Inui, T., and Hoen, M. (2009). Neural network processing of natural language: II. Towards a unified model of corticostriatal function in learning sentence comprehension and non-linguistic sequencing. Brain Lang. 109, 80–92. doi: 10.1016/j.bandl.2008.08.002
Dröge, A., Fleischer, J., Schlesewsky, M., and Bornkessel-Schlesewsky, I. (2016). Neural mechanisms of sentence comprehension based on predictive processes and decision certainty: electrophysiological evidence from non-canonical linearizations in a flexible word order language. Brain Res. 1633, 149–166. doi: 10.1016/j.brainres.2015.12.045
Duff, M. C., and Brown-Schmidt, S. (2012). The hippocampus and the flexible use and processing of language. Front. Hum. Neurosci. 6:69. doi: 10.3389/fnhum.2012.00069
Dumay, N., and Gaskell, M. G. (2007). Sleep-associated changes in the mental representation of spoken words. Psychol. Sci. 18, 35–39. doi: 10.1111/j.1467-9280.2007.01845.x
Durrant, S. J., Cairney, S. A., and Lewis, P. A. (2013). Overnight consolidation aids the transfer of statistical knowledge from the medial temporal lobe to the striatum. Cereb. Cortex 23, 2467–2478. doi: 10.1093/cercor/bhs244
Durrant, S. J., Cairney, S. A., and Lewis, P. A. (2016). Cross-modal transfer of statistical information benefits from sleep. Cortex 78, 85–99. doi: 10.1016/j.cortex.2016.02.011
Durrant, S. J., Cairney, S. A., McDermott, C., and Lewis, P. A. (2015). Schema-conformant memories are preferentially consolidated during REM sleep. Neurobiol. Learn. Mem. 122, 41–50. doi: 10.1016/j.nlm.2015.02.011
Duzel, E., Penny, W. D., and Burgess, N. (2010). Brain oscillations and memory. Curr. Opin. Neurobiol. 20, 143–149. doi: 10.1016/j.conb.2010.01.004
Engel, A. K., and Fries, P. (2010). Beta-band oscillations – signalling the status quo? Curr. Opin. Neurobiol. 20, 155–165. doi: 10.1016/j.conb.2010.02.015
Fell, J., and Axmacher, N. (2011). The role of phase synchronization in memory processes. Nat. Rev. Neurosci. 12, 105–118. doi: 10.1038/nrn2979
Fellner, M. C., Bauml, K. H., and Hanslmayr, S. (2013). Brain oscillatory subsequent memory effects differ in power and long-range synchronization between semantic and survival processing. Neuroimage 79, 361–370. doi: 10.1016/j.neuroimage.2013.04.121
Fogel, S. M., Smith, C. T., and Cote, K. A. (2007). Dissociable learning-dependent changes in REM and non-REM sleep in declarative and procedural memory systems. Behav. Brain Res. 180, 48–61. doi: 10.1016/j.bbr.2007.02.037
Fontolan, L., Morillon, B., Liegeois-Chauvel, C., and Giraud, A. L. (2014). The contribution of frequency-specific activity to hierarchical information processing in the human auditory cortex. Nat. Commun. 5:4694. doi: 10.1038/ncomms5694
Friederici, A. D., Steinhauer, K., and Pfeifer, E. (2002). Brain signatures of artificial language processing: evidence challenging the critical period hypothesis. Proc. Natl. Acad. Sci. U.S.A. 99, 529–534. doi: 10.1073/pnas.012611199
Frisch, S., and Schlesewsky, M. (2001). The N400 reflects problems of thematic hierarchizing. Neuroreport 12, 3391–3394. doi: 10.1097/00001756-200110290-00048
Frisch, S., and Schlesewsky, M. (2005). The resolution of case conflicts from a neurophysiological perspective. Cogn. Brain Res. 25, 484–498. doi: 10.1016/j.cogbrainres.2005.07.010
Friston, K. (2010). The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 11, 127–138. doi: 10.1038/nrn2787
Friston, K., and Buzsáki, G. (2016). The functional anatomy of time: what and when in the brain. Trends Cogn. Sci. 20, 500–511. doi: 10.1016/j.tics.2016.05.001
Gais, S., and Born, J. (2004). Declarative memory consolidation: mechanisms acting during human sleep. Learn. Mem. 11, 679–685. doi: 10.1101/lm.80504
Gaskell, M. G., Warker, J., Lindsay, S., Frost, R., Guest, J., Snowdon, R., et al. (2014). Sleep underpins the plasticity of language production. Psychol. Sci. 25, 1457–1465. doi: 10.1177/0956797614535937
Gilboa, A., and Marlatte, H. (2017). Neurobiology of schemas and schema-mediated memory. Trends Cogn. Sci. 21, 618–631. doi: 10.1016/j.tics.2017.04.013
Giraud, A. L., and Poeppel, D. (2012). Cortical oscillations and speech processing: emerging computational principles and operations. Nat. Neurosci. 15, 511–517. doi: 10.1038/nn.3063
Giuditta, A. (2014). Sleep memory processing: the sequential hypothesis. Front. Syst. Neurosci. 8:219. doi: 10.3389/fnsys.2014.00219
Giuditta, A., Ambrosini, M. V., Montagnese, P., Mandile, P., Cotugno, M., Zucconi, G. G., et al. (1995). The sequential hypothesis of the function of sleep. Behav. Brain Res. 69, 157–166. doi: 10.1016/0166-4328(95)00012-I
Graves, E. A. (1936). The effect of sleep upon retention. J. Exp. Psychol. 19, 316–322. doi: 10.1037/h0057827
Hanslmayr, S., Staresina, B. P., and Bowman, H. (2016). Oscillations and episodic memory: addressing the synchronization/desynchronization conundrum. Trends Neurosci. 39, 16–25. doi: 10.1016/j.tins.2015.11.004
Hanslmayr, S., and Staudigl, T. (2014). How brain oscillations form memories–a processing based perspective on oscillatory subsequent memory effects. Neuroimage 85, 648–655. doi: 10.1016/j.neuroimage.2013.05.121
Harmony, T. (2013). The functional significance of delta oscillations in cognitive processing. Front. Integr. Neurosci. 7:83. doi: 10.3389/fnint.2013.00083
Hasselmo, M. E. (2006). The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 16, 710–715. doi: 10.1016/j.conb.2006.09.002
Heib, D. P., Hoedlmoser, K., Anderer, P., Gruber, G., Zeitlhofer, J., and Schabus, M. (2015). Oscillatory theta activity during memory formation and its impact on overnight consolidation: a missing link? J. Cogn. Neurosci. 27, 1648–1658. doi: 10.1162/jocn_a_00804
Herweg, N. A., Apitz, T., Leicht, G., Mulert, C., Fuentemilla, L., and Bunzeck, N. (2016). Theta-Alpha oscillations bind the hippocampus, prefrontal cortex, and striatum during recollection: evidence from simultaneous EEG-fMRI. J. Neurosci. 36, 3579–3587. doi: 10.1523/JNEUROSCI.3629-15.2016
Hobson, J. A., and Friston, K. J. (2012). Waking and dreaming consciousness: neurobiological and functional considerations. Prog. Neurobiol. 98, 82–98. doi: 10.1016/j.pneurobio.2012.05.003
Hoeks, J. C., Stowe, L. A., and Doedens, G. (2004). Seeing words in context: the interaction of lexical and sentence level information during reading. Cogn. Brain Res. 19, 59–73. doi: 10.1016/j.cogbrainres.2003.10.022
Hutchison, I. C., and Rathore, S. (2015). The role of REM sleep theta activity in emotional memory. Front. Psychol. 6:1439. doi: 10.3389/fpsyg.2015.01439
Kaestner, E. J., Wixted, J. T., and Mednick, S. C. (2013). Pharmacologically increasing sleep spindles enhances recognition for negative and high-arousal memories. J. Cogn. Neurosci. 25, 1597–1610. doi: 10.1162/jocn_a_00433
Klimesch, W. (2012). Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 16, 606–617. doi: 10.1016/j.tics.2012.10.007
Klimesch, W., Sauseng, P., and Hanslmayr, S. (2007). EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 53, 63–88. doi: 10.1016/j.brainresrev.2006.06.003
Klinzing, J. G., Molle, M., Weber, F., Supp, G., Hipp, J. F., Engel, A. K., et al. (2016a). Spindle activity phase-locked to sleep slow oscillations. Neuroimage 134, 607–616. doi: 10.1016/j.neuroimage.2016.04.031
Klinzing, J. G., Rasch, B., Born, J., and Diekelmann, S. (2016b). Sleep’s role in the reconsolidation of declarative memories. Neurobiol. Learn. Mem. 136, 166–173. doi: 10.1016/j.nlm.2016.10.004
Kovach, C. K., Tsuchiya, N., Kawasaki, H., Oya, H., Howard, M. A. III, and Adolphs, R. (2011). Manifestation of ocular-muscle EMG contamination in human intracranial recordings. Neuroimage 54, 213–233. doi: 10.1016/j.neuroimage.2010.08.002
Kumaran, D., Hassabis, D., and McClelland, J. L. (2016). What learning systems do intelligent agents need? complementary learning systems theory updated. Trends Cogn. Sci. 20, 512–534. doi: 10.1016/j.tics.2016.05.004
Lam, N. H., Schoffelen, J. M., Udden, J., Hulten, A., and Hagoort, P. (2016). Neural activity during sentence processing as reflected in theta, alpha, beta, and gamma oscillations. Neuroimage 142, 43–54. doi: 10.1016/j.neuroimage.2016.03.007
Lewis, A. G., and Bastiaansen, M. (2015). A predictive coding framework for rapid neural dynamics during sentence-level language comprehension. Cortex 68, 155–168. doi: 10.1016/j.cortex.2015.02.014
Lewis, A. G., Schoffelen, J. M., Schriefers, H., and Bastiaansen, M. (2016). A predictive coding perspective on beta oscillations during sentence-level language comprehension. Front. Hum. Neurosci. 10:85. doi: 10.3389/fnhum.2016.00085
Lewis, A. G., Wang, L., and Bastiaansen, M. (2015). Fast oscillatory dynamics during language comprehension: unification versus maintenance and prediction? Brain. Lang. 148, 51–63. doi: 10.1016/j.bandl.2015.01.003
Lewis, P. A., and Durrant, S. J. (2011). Overlapping memory replay during sleep builds cognitive schemata. Trends Cogn. Sci. 15, 343–351. doi: 10.1016/j.tics.2011.06.004
Lisman, J. E., and Jensen, O. (2013). The theta-gamma neural code. Neuron 77, 1002–1016. doi: 10.1016/j.neuron.2013.03.007
Llewellyn, S., and Hobson, J. A. (2015). Not only… but also: REM sleep creates and NREM Stage 2 instantiates landmark junctions in cortical memory networks. Neurobiol. Learn. Mem. 122, 69–87. doi: 10.1016/j.nlm.2015.04.005
Luck, S. J. (2014). An Introduction to the Event-Related Potential Technique. Cambridge, MA: MIT Press.
MacWhinney, B., Bates, E., and Kliegl, R. (1984). Cue validity and sentence interpretation in English, German, and Italian. J. Verbal Learning Verbal Behav. 23, 127–150. doi: 10.1016/S0022-5371(84)90093-8
Mai, G., Minett, J. W., and Wang, W. S. (2016). Delta, theta, beta, and gamma brain oscillations index levels of auditory sentence processing. Neuroimage, 133, 516–528. doi: 10.1016/j.neuroimage.2016.02.064
Mascetti, L., Foret, A., Schrouff, J., Muto, V., Dideberg, V., Balteau, E., et al. (2013). Concurrent synaptic and systems memory consolidation during sleep. J. Neurosci. 33, 10182-10190. doi: 10.1523/JNEUROSCI.0284-13.2013
Mathewson, K. E., Lleras, A., Beck, D. M., Fabiani, M., Ro, T., and Gratton, G. (2011). Pulsed Out of Awareness: EEG Alpha oscillations represent a pulsed inhibition of ongoing cortical processing. Front. Psychol. 2:99. doi: 10.3389/fpsyg.2011.00099
McClelland, J. L. (2013). Incorporating rapid neocortical learning of new schema-consistent information into complementary learning systems theory. J. Exp. Psychol. 142, 1190–1210. doi: 10.1037/a0033812
McDevitt, E. A., Duggan, K. A., and Mednick, S. C. (2015). REM sleep rescues learning from interference. Neurobiol. Learn. Mem. 122, 51–62. doi: 10.1016/j.nlm.2014.11.015
McGregor, K. K., and Alper, R. M. (2015). Sleep disorders as a risk to language learning and use. EBP Briefs 10, 1–21.
Mirković, J., and Gaskell, M. G. (2016). Does sleep improve your grammar? preferential consolidation of arbitrary components of new linguistic knowledge. PLOS ONE 11:e0152489. doi: 10.1371/journal.pone.0152489
Morgan-Short, K., Steinhauer, K., Sanz, C., and Ullman, M. T. (2012). Explicit and implicit second language training differentially affect the achievement of native-like brain activation patterns. J. Cogn. Neurosci. 24, 933–947. doi: 10.1162/jocn_a_00119
Mueller, J. L. (2006). L2 in a nutshell: the investigation of second language processing in the miniature language model. Lang. Learn. 56, 235–270. doi: 10.1111/j.1467-9922.2006.00363.x
Mueller, J. L., Hirotani, M., and Friederici, A. D. (2007). ERP evidence for different strategies in the processing of case markers in native speakers and non-native learners. BMC Neurosci. 8:18. doi: 10.1186/1471-2202-8-18
Mueller, J. L., Rueschemeyer, S. A., Ono, K., Sugiura, M., Sadato, N., and Nakamura, A. (2014). Neural networks involved in learning lexical-semantic and syntactic information in a second language. Front. Psychol. 5:1209. doi: 10.3389/fpsyg.2014.01209
Murphy, E. (2016). A Theta-gamma neural code for feature set composition with phase-entrained delta nestings. UCL Work. Pap. Linguist. 28, 1–23.
Muthukumaraswamy, S. D., and Singh, K. D. (2013). Visual gamma oscillations: the effects of stimulus type, visual field coverage and stimulus motion on MEG and EEG recordings. Neuroimage 69, 223–230. doi: 10.1016/j.neuroimage.2012.12.038
Newman, A. J., Pancheva, R., Ozawa, K., Neville, H. J., and Ullman, M. T. (2001). An event-related fMRI study of syntactic and semantic violations. J. Psycholinguist. Res. 30, 339–364. doi: 10.1023/A:1010499119393
Ngo, H. V. V., Martinetz, T., Born, J., and Mölle, M. (2013). Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78, 545–553. doi: 10.1016/j.neuron.2013.03.006
Nieuwenhuis, I. L., Folia, V., Forkstam, C., Jensen, O., and Petersson, K. (2013). Sleep promotes the extraction of grammatical rules. PLOS ONE 8:e65046. doi: 10.1371/journal.pone.0065046.g001
Olcese U., Esser, S. K., and Tononi, G. (2010). Sleep and synaptic renormalization: a computational study. J. Neurophysiol. 104, 3476–3493. doi: 10.1152/jn.00593.2010
Opitz, B., and Friederici, A. D. (2003). Interactions of the hippocampal system and the prefrontal cortex in learning language-like rules. Neuroimage 19, 1730–1737. doi: 10.1016/s1053-8119(03)00170-8
Opitz, B., and Friederici, A. D. (2004). Brain correlates of language learning: the neuronal dissociation of rule-based versus similarity-based learning. J. Neurosci. 24, 8436–8440. doi: 10.1523/JNEUROSCI.2220-04.2004
Opitz, B., and Friederici, A. D. (2007). Neural basis of processing sequential and hierarchical syntactic structures. Hum. Brain Mapp. 28, 585–592. doi: 10.1002/hbm.20287
Osipova, D., Takashima, A., Oostenveld, R., Fernandez, G., Maris, E., and Jensen, O. (2006). Theta and gamma oscillations predict encoding and retrieval of declarative memory. J. Neurosci. 26, 7523–7531. doi: 10.1523/JNEUROSCI.1948-06.2006
Pan, S. C., and Rickard, T. C. (2015). Sleep and motor learning: is there room for consolidation? Psychol. Bull. 141, 812–843. doi: 10.1037/bul0000009
Peters, K. R., Ray, L., Smith, V., and Smith, C. (2008). Changes in the density of stage 2 sleep spindles following motor learning in young and older adults. J. Sleep Res. 17, 23–33. doi: 10.1111/j.1365-2869.2008.00634.x
Piai, V., Anderson, K. L., Lin, J. J., Dewar, C., Parvizi, J., Dronkers, N. F., et al. (2016). Direct brain recordings reveal hippocampal rhythm underpinnings of language processing. Proc. Natl. Acad. Sci. U.S.A. 113 11366–11371. doi: 10.1073/pnas.1603312113
Pickering, M. J., and Garrod, S. (2013). An integrated theory of language production and comprehension. Behav. Brain Sci. 36, 329–347. doi: 10.1017/S0140525X12001495
Rasch, B., and Born, J. (2015). In search of a role of REM sleep in memory formation. Neurobiol. Learn. Mem. 122, 1–3. doi: 10.1016/j.nlm.2015.04.012
Rasch, B. (2017). Sleep and language learning. Brain Lang. 167, 1–2. doi: 10.1016/j.bandl.2017.02.002
Rasch, B., and Born, J. (2013). About sleep’s role in memory. Physiol. Rev. 93, 681–766. doi: 10.1152/physrev.00032.2012
Rasch, B. H., Born, J., and Gais, S. (2006). Combined blockade of cholinergic receptors shifts the brain from stimulus encoding to memory consolidation. J. Cogn. Neurosci. 18, 793–802. doi: 10.1162/jocn.2006.18.5.793
Rauchs, G., Desgranges, B., Foret, J., and Eustache, F. (2005). The relationships between memory systems and sleep stages. J. Sleep Res. 14, 123–140. doi: 10.1111/j.1365-2869.2005.00450.x
Rauschecker, J. P., and Scott, S. K. (2009). Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat. Neurosci. 12, 718–724. doi: 10.1038/nn.2331
Rauss, K., and Born, J. (2017). A Role of Sleep in Forming Predictive Codes Cognitive Neuroscience of Memory Consolidation. Berlin: Springer, 117–132. doi: 10.1007/978-3-319-45066-7_8
Rickard, T. C., and Pan, S. C. (2017). Time for considering the possibility that sleep plays no unique role in motor memory consolidation: reply to Adi-Japha and Karni (2016). Psychol. Bull. 143, 454–458. doi: 10.1037/bul0000094
Rubinov, M., and Sporns, O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52, 1059–1069. doi: 10.1016/j.neuroimage.2009.10.003
Sadaghiani, S., and Kleinschmidt, A. (2016). Brain networks and alpha-oscillations: structural and functional foundations of cognitive control. Trends Cogn. Sci. 20, 805–817. doi: 10.1016/j.tics.2016.09.004
Sarasso, S., Maatta, S., Ferrarelli, F., Poryazova, R., Tononi, G., and Small, S. L. (2014). Plastic changes following imitation-based speech and language therapy for aphasia: a high-density sleep EEG study. Neurorehabil. Neural Repair 28, 129–138. doi: 10.1177/1545968313498651
Sarasso, S., Santhanam, P., Määtta, S., Poryiazova, R., Ferrarelli, F., Tononi, G., et al. (2010). Non-fluent aphasia and neural reorganization after speech therapy: insights from human sleep electrophysiology and functional magnetic resonance imaging. Arch. Ital. Biol. 148, 271–278.
Schabus, M., Gruber, G., Parapatics, S., Sauter, C., Klosch, G., Anderer, P., et al. (2004). Sleep spindles and their significance for declarative memory consolidation. Sleep 27, 1479–1485. doi: 10.1093/sleep/27.7.1479
Schreiner, T., Goldi, M., and Rasch, B. (2015). Cueing vocabulary during sleep increases theta activity during later recognition testing. Psychophysiology 52, 1538–1543. doi: 10.1111/psyp.12505
Schreiner, T., and Rasch, B. (2015). Boosting vocabulary learning by verbal cueing during sleep. Cereb. Cortex 25, 4169–4179. doi: 10.1093/bcercor/bhu139
Schreiner, T., and Rasch, B. (2016). The beneficial role of memory reactivation for language learning during sleep: a review. Brain Lang. 167 94–105. doi: 10.1016/j.bandl.2016.02.005
Schroeder, C. E., and Lakatos, P. (2009). Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci 32, 9–18. doi: 10.1016/j.tins.2008.09.012
Seghier, M. L. (2013). The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist 19, 43–61. doi: 10.1177/1073858412440596
Simon, K. N., Werchan, D., Goldstein, M. R., Sweeney, L., Bootzin, R. R., Nadel, L., et al. (2017). Sleep confers a benefit for retention of statistical language learning in 6.5 month old infants. Brain Lang. 167, 3–12. doi: 10.1016/j.bandl.2016.05.002
Sirota, A., Montgomery, S., Fujisawa, S., Isomura, Y., Zugaro, M., and Buzsaki, G. (2008). Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60, 683–697. doi: 10.1016/j.neuron.2008.09.014
Smith, C. (2001). Sleep states and memory processes in humans: procedural versus declarative memory systems. Sleep Med. Rev. 5, 491–506. doi: 10.1053/smrv.2001.0164
Staresina, B. P., Bergmann, T. O., Bonnefond, M., van der Meij, R., Jensen, O., Deuker, L., et al. (2015). Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 18, 1679–1686. doi: 10.1038/nn.4119
Takashima, A., Bakker, I., van Hell, J. G., Janzen, G., and McQueen, J. M. (2016). Interaction between episodic and semantic memory networks in the acquisition and consolidation of novel spoken words. Brain Lang. 167, 44–60 doi: 10.1016/j.bandl.2016.05.009
Tamminen, J., Lambon Ralph, M. A., and Lewis, P. A. (2013). The role of sleep spindles and slow-wave activity in integrating new information in semantic memory. J. Neurosci. 33, 15376–15381. doi: 10.1523/JNEUROSCI.5093-12.2013
Tamminen, J., Payne, J. D., Stickgold, R., Wamsley, E. J., and Gaskell, M. G. (2010). Sleep spindle activity is associated with the integration of new memories and existing knowledge. J. Neurosci. 30, 14356–14360. doi: 10.1523/JNEUROSCI.3028-10.2010
Tononi, G., and Cirelli, C. (2006). Sleep function and synaptic homeostasis. Sleep Med. Rev. 10, 49–62. doi: 10.1016/j.smrv.2005.05.002
Tononi, G., and Cirelli, C. (2012). Time to be SHY? Some comments on sleep and synaptic homeostasis. Neural Plast. 2012:415250. doi: 10.1155/2012/415250
Tononi, G., and Cirelli, C. (2014). Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34. doi: 10.1016/j.neuron.2013.12.025
Tse, D., Langston, R. F., Kakeyama, M., Bethus, I., Spooner, P. A., Wood, E. R., et al. (2007). Schemas and memory consolidation. Science 316, 76–82. doi: 10.1126/science.1135935
Ullman, M. T. (2001). A neurocognitive perspective on language: the declarative/procedural model. Nat. Rev. Neurosci. 2, 717–726. doi: 10.1038/35094573
Ullman, M. T. (2004). Contributions of memory circuits to language: the declarative/procedural model. Cognition 92, 231–270. doi: 10.1016/j.cognition.2003.10.008
Ullman, M. T. (2016). “The declarative/procedural model: a neurobiological model of language learning, knowledge, and use,” in Neurobiology of Language, eds G. Hickok and S. L. Small (Cambridge, MA: Academic Press), 953–968. doi: 10.1016/b978-0-12-407794-2.000076-6
Varela, F., Lachaux, J. P., Rodriguez, E., and Martinerie, J. (2001). The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci. 2, 229–239. doi: 10.1038/35067550
Vertes, R. P., and Eastman, K. E. (2000). The case against memory consolidation in REM sleep. Behav. Brain Sci. 23, 867–876; discussion 904–1121. doi: 10.1017/S0140525X00004003
Vorster, A. P., and Born, J. (2015). Sleep and memory in mammals, birds and invertebrates. Neurosci. Biobehav. Rev. 50, 103–119. doi: 10.1016/j.neubiorev.2014.09.020
Walker, M. P., Stickgold, R., Jolesz, F. A., and Yoo, S. S. (2005). The functional anatomy of sleep-dependent visual skill learning. Cereb. Cortex 15, 1666–1675. doi: 10.1093/cercor/bhi043
Weber, K., Christiansen, M. H., Petersson, K. M., Indefrey, P., and Hagoort, P. (2016). fMRI syntactic and lexical repetition effects reveal the initial stages of learning a new language. J. Neurosci. 36, 6872–6880. doi: 10.1523/JNEUROSCI.3180-15.2016
Weiss, S., and Mueller, H. M. (2012). “Too many betas do not spoil the broth”: the role of beta brain oscillations in language processing. Front. Psychol. 3:201. doi: 10.3389/fpsyg.2012.00201
Whitham, E. M., Pope, K. J., Fitzgibbon, S. P., Lewis, T., Clark, C. R., Loveless, S., et al. (2007). Scalp electrical recording during paralysis: quantitative evidence that EEG frequencies above 20 Hz are contaminated by EMG. Clin. Neurophysiol. 118, 1877–1888. doi: 10.1016/j.clinph.2007.04.027
Wilson, S. M., DeMarco, A. T., Henry, M. L., Gesierich, B., Babiak, M., Mandelli, M. L., et al. (2014). What role does the anterior temporal lobe play in sentence-level processing? Neural correlates of syntactic processing in semantic variant primary progressive aphasia. J. Cogn. Neurosci. 26, 970–985. doi: 10.1162/jocn_a_00550
Keywords: language learning, sentence comprehension, sleep and memory, neuronal oscillations, predictive coding
Citation: Cross ZR, Kohler MJ, Schlesewsky M, Gaskell MG and Bornkessel-Schlesewsky I (2018) Sleep-Dependent Memory Consolidation and Incremental Sentence Comprehension: Computational Dependencies during Language Learning as Revealed by Neuronal Oscillations. Front. Hum. Neurosci. 12:18. doi: 10.3389/fnhum.2018.00018
Received: 30 July 2017; Accepted: 15 January 2018;
Published: 31 January 2018.
Edited by:
Juliana Yordanova, Institute of Neurobiology (BAS), BulgariaReviewed by:
Erin J. Wamsley, Furman University, United StatesCaroline L. Horton, Bishop Grosseteste University, United Kingdom
Copyright © 2018 Cross, Kohler, Schlesewsky, Gaskell and Bornkessel-Schlesewsky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zachariah R. Cross, emFjaGFyaWFoLmNyb3NzQG15bWFpbC51bmlzYS5lZHUuYXU=
 Zachariah R. Cross
Zachariah R. Cross Mark J. Kohler
Mark J. Kohler Matthias Schlesewsky
Matthias Schlesewsky M. G. Gaskell
M. G. Gaskell Ina Bornkessel-Schlesewsky
Ina Bornkessel-Schlesewsky