- 1Department of Radiology, Xuanwu Hospital, Capital Medical University, Beijing, China
- 2Beijing Key Laboratory of Magnetic Resonance Imaging and Brain Informatics, Beijing, China
- 3Department of Radiology, Dongfang Hospital Beijing University of Chinese Medicine, Beijing, China
- 4Department of Radiology, Tianjin Medical University General Hospital, Tianjin, China
The aim of this study was to explore possible changes in whole brain gray matter volume (GMV) after spinal cord injury (SCI) using voxel-based morphometry (VBM), and to study their associations with the injury duration, severity, and clinical variables. In total, 21 patients with SCI (10 with complete and 11 with incomplete SCI) and 21 age- and sex-matched healthy controls (HCs) were recruited. The 3D high-resolution T1-weighted structural images of all subjects were obtained using a 3.0 Tesla MRI system. Disease duration and American Spinal Injury Association (ASIA) Scale scores were also obtained from each patient. Voxel-based morphometry analysis was carried out to investigate the differences in GMV between patients with SCI and HCs, and between the SCI sub-groups. Associations between GMV and clinical variables were also analyzed. Compared with HCs, patients with SCI showed significant GMV decrease in the dorsal anterior cingulate cortex, bilateral anterior insular cortex, bilateral orbital frontal cortex (OFC), and right superior temporal gyrus. No significant difference in GMV in these areas was found either between the complete and incomplete SCI sub-groups, or between the sub-acute (duration <1 year) and chronic (duration >1 year) sub-groups. Finally, the GMV of the right OFC was correlated with the clinical motor scores of left extremities in not only all SCI patients, but especially the CSCI subgroup. In the sub-acute subgroup, we found a significant positive correlation between the dACC GMV and the total clinical motor scores, and a significant negative correlation between right OFC GMV and the injury duration. These findings indicate that SCI can cause remote atrophy of brain gray matter, especially in the salient network. In general, the duration and severity of SCI may be not associated with the degree of brain atrophy in total SCI patients, but there may be associations between them in subgroups.
Introduction
Previous studies on animals and humans have shown that brain cortex can be reshaped following spinal cord injury (SCI) (Pernet and Hepp-Reymond, 1975; Feringa and Vahlsing, 1985; Pons et al., 1991; Florence, 1998; Hains et al., 2003; Lee et al., 2004; Kim et al., 2006; Felix et al., 2012; Hou et al., 2014; Zheng et al., 2017). This cortical reorganization may contribute to the recovery of spared functions (Kaas et al., 2008; Ghosh et al., 2009; Kao et al., 2009), but it can also produce aberrant “sensations” without any external inputs, such as phantom sensations (Jain et al., 2000; Simoes et al., 2012) and neuropathic pain (Flor et al., 1995; Wrigley et al., 2009b; Henderson et al., 2011). To date, the mechanisms of cortical reorganization remain poorly understood, and most related research has been carried out on animals. Studies in humans require non-invasive approaches that were not available until relatively recently.
Currently, it remains uncertain whether these reorganizations result from new dendritic sprouting, or reflect an unmasking of already existing dormant synapses, or both. Endo et al. (2007) reported that in adult rats, the cortical representation in response to spare forelimb stimulation was enlarged and invaded into the adjacent sensory-deprived hind limb territory in S1 as early as 3 days after complete transection of the mid-thoracic spinal cord. The immediate cortical reorganization observed after spinal cord injury probably results from disinhibition or unmasking of existing dormant synapse after deafferentation (Aguilar et al., 2010). However, the extent of intracortical arborization of single thalamocortical axons is no >600 microns, and the shifts in cortical organization caused by latent synapses could be very small (<2–3 mm) (Rausell and Jones, 1995). Pons et al. (1991) reported dramatic expansion of S1 in monkeys following unilateral cervical dorsal rhizotomies, with the face representation expanding by up to 14 mm, so this cortical reorganization cannot be simply explained by the unmasking of latent connections and overlap of thalamocortical projection. The results from Pons and other researchers (Jain et al., 2000) support the idea that cortex reorganization may be driven dominantly by new dendritic sprouting rather than by the unmasking of already existing dormant synapses. Some researchers also indicated that the cortical changes caused by spinal cord injury were the sum of the two effects (Chen et al., 2002).
There have been a few non-invasive structural studies on brain gray matter (GM) change following SCI in humans, but these results were divergent (Crawley et al., 2004; Jurkiewicz et al., 2006; Wrigley et al., 2009a; Freund et al., 2011). For example, some researchers reported no changes in brain gray matter volume (GMV) in the primary motor cortex (Crawley et al., 2004; Jurkiewicz et al., 2006) after SCI, whereas others found a significant decrease in GMV in the same area in patients with SCI (Wrigley et al., 2009a; Freund et al., 2011). The wide range of disease duration, rehabilitation training, drug intervention, and different research methodology may have contributed to the inconsistencies between previous studies. Moreover, it is still unknown whether the anatomical changes occur only within the brain sensorimotor system, and furthermore, if there are any changes in brain GM following SCI, it is unclear whether these changes are correlated with injury severity, duration or clinical variables.
In this study, using a voxel-based morphometry (VBM) method (Ashburner and Friston, 2000), we aimed to explore the possible GMV changes voxel by voxel in whole brain regions after SCI and tried to elucidate the aforementioned issues. Based on previous studies (Hou et al., 2014), we hypothesized that brain GM impairment following SCI would be found in the primary sensorimotor cortex, and that longer and more severe injury would cause more significant changes in the GMV of the brain.
Materials and Methods
Participants
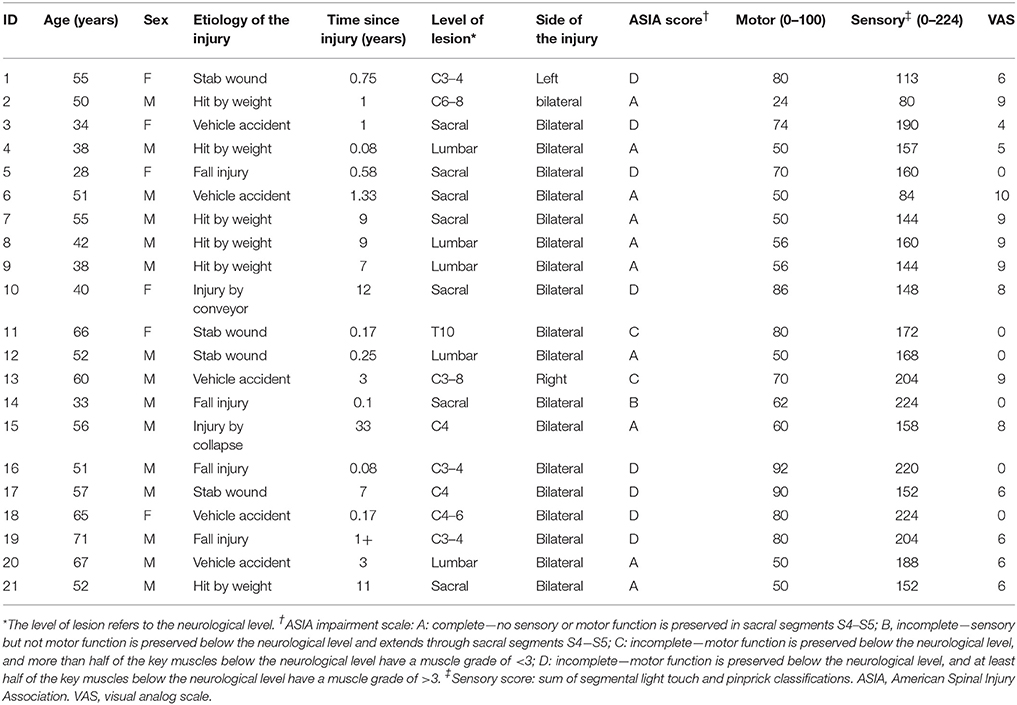
In this study, 21 right-handed patients with SCI (15 male and 6 female patients, with a mean age of 50.5 ± 12.1 years, and an age range from 28 to 71 years) were enrolled. Lesions included cervical (eight cases), thoracic (one case), lumbar (five cases), and sacral (seven cases) levels. Impairment was labeled as grade A in 10 patients, and grade B, C or D in the remaining 11 patients, according to the American Spinal Injury Association (ASIA) Impairment Scale 2012 (http://asia-spinalinjury.org). The duration of the disease was from 1 month to 33 years, with a mean of 5.2 ± 8.7 years. The SCI group was divided into complete SCI (CSCI) group (10 cases) and incomplete SCI (ISCI) group (11 cases) based on the injury severity, and into sub-acute (duration 1–12 months; 8 cases) and chronic (duration >12 months; 13 cases) group based on the injury duration (Macklin et al., 2016). All patients had various degrees of bilateral sensorimotor dysfunction, with the exception of two patients who exhibited only unilateral dysfunction (one on the left and the other on the right side). All the patients had imaging hyper-intensity at the injury level on conventional T2-weighted MRI. We excluded the following patients from this study: patients with brain diseases that were confirmed by conventional MRI and patients with any type of mental illness or cognitive disorder. All patients underwent a comprehensive clinical assessment before MRI, including light touch sensory score, pinprick sensory score, and motor score, which were assessed by two qualified clinicians using the ASIA classification scale (Marino et al., 2003, 2008). A visual analog scale (VAS) and the Beck Depression Inventory (BDI) were also administered to evaluate the severity of pain and depression at the time of MRI acquisition. Sensory levels were assessed by testing two aspects of sensation: light touch and pinprick (sharp/dull discrimination) of each dermatome (C4–S5, bilateral). Motor function assessment comprised key muscle functions testing of 10 paired myotomes (C5–T1 and L2–S1; Jutzeler et al., 2016). The healthy control (HC) group comprised 21 right-handed healthy volunteers (15 men and 6 women, with a mean age of 50.0 ± 12.4 years and an age range of 26–65 years), matched for age, sex, and years of education. Table 1 provides detailed information of the patients with SCI.
The study protocol was approved by the Ethics Committee of Xuanwu Hospital of Capital Medical University, Beijing, China. Written informed consent in accordance with the Declaration of Helsinki was obtained from each participant of this study.
Image Acquisition
Images were obtained using a 3.0 T MRI system (Trio Tim, Siemens, Erlangen, Germany) with a 12-channel phase-array head coil. Tight but comfortable foam padding was used to minimize head motion, and earplugs were used to reduce the imaging noise. The participants were asked to stay calm, close their eyes, breath evenly, and try to avoid specific thoughts. Conventional brain axial fluid-attenuated inverse recovery sequence was used to exclude brain visible abnormality. High-resolution three-dimensional (3D) structural T1-weighted images were acquired in sagittal orientation using a 3D magnetization-prepared rapid gradient-echo sequence (MP-RAGE) with the following parameters: repetition time (TR) = 1800 ms; echo time (TE) = 2.13 ms; inversion time (TI) = 1100 ms; flip angle (FA) = 9°; number of slices = 192; slice thickness = 1 mm; field of view (FOV) = 256 × 256 mm2; matrix = 256 × 256; resulting an isotropic voxel size of 1 × 1 × 1 mm3.
Data Processing and VBM Analyses
Post-processing was performed using Statistical Parametric Mapping (SPM8) software (http://www.fil.ion.ucl.ac.uk/spm/) implemented in MATLAB 2013a (Math Works, Natick, MA, USA), and included the following steps. All images were visually checked by two experienced radiologists to eliminate images with apparent artifacts caused by factors such as head motion, susceptibility artifacts, and instrument malfunction. All structural magnetic resonance images were then manually reoriented to place the anterior commissure at the origin and the anterior–posterior commissure in the horizontal plane. Next, the structural images were segmented into GM, white matter (WM), and cerebrospinal fluid (CSF) areas, using the unified standard segmentation option in SPM version 8. After segmentation, the individual WM and GM components were normalized into the standard Montreal Neurological Institute (MNI) space using the Diffeomorphic Anatomical Registration through Exponentiated Lie algebra (DARTEL) algorithm (Ashburner, 2007). The normalized GM component was modulated to generate the relative GMV by multiplied by the nonlinear part of the deformation field at the DARTEL step. The resulting GMV images were then smoothed with an 8-mm full-width at half-maximum Gaussian kernel.
Statistical Analysis
Both T-test and Partial correlation was used depend on the data with normal distribution, if not, rank sum test and spearman correlation test would be employed (Supplementary Material Tables S1, S2).
First, T-test was performed with age and sex between the SCI patients and HCs, and between the CSCI and ISCI sub-groups, and between the sub-acute and chronic sub-groups, no statistical significant differences were found (p > 0.05). Using the general linear model in SPM, a voxel-wise two-sample t-test was used to compare the GMV differences between the SCI group and the HC group (voxel-level uncorrected p < 0.0001, non-stationary cluster-level family-wise error correction with p < 0.05), with the age and sex as nuisance covariates. Next, the mean GMV values of clusters with statistical significance were extracted for subsequent post-hoc analyses. Then a region of interest (ROI)-based two-sample t-test was carried out to compare the GMV differences between the CSCI and ISCI sub-groups, and between the sub-acute and chronic sub-groups after removing age and sex effects (p < 0.05, uncorrected). Finally, partial correlation analysis was performed to explore any potential association between GMV and injury duration and clinical variables in patients with SCI, and between the GMV of each subgroup (CSCI and ISCI) and the duration of injury, and between the GMV and the clinical variables after removing age and sex effects (p < 0.05, uncorrected). The last several steps were also carried out in SPSS version 16 (IBM, Armonk, NY, USA).
Results
Brain GMV Changes in Patients with SCI
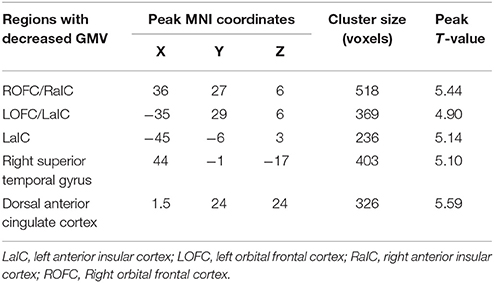
Voxel-wise comparisons demonstrated that compared with HCs, patients with SCI had lower GMV in the dorsal anterior cingulate cortex (dACC), bilateral orbital frontal cortex (OFC), bilateral anterior insular cortex (aIC), and right superior temporal gyrus (STG) (non-stationary cluster-level FWE correction with p < 0.05; Table 2; Figures 1, 2A). There were no brain regions demonstrating higher GMV in the patients with SCI relative to the HCs (Figure 1).
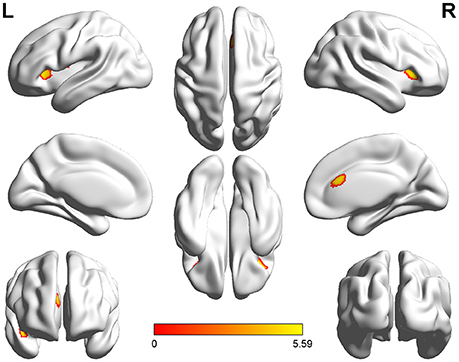
Figure 1. Three-dimensional images made by BrainNet Viewer based on the result of VBM. Regions showing significant higher GMV in healthy controls compared with the patients with SCI [cluster level, family-wise error (FWE) p ≤ 0.05]: dorsal anterior cingulate cortex (dACC), bilateral anterior insular cortex (aIC), bilateral orbital frontal cortex (OFC), and right superior temporal gyrus (STG).
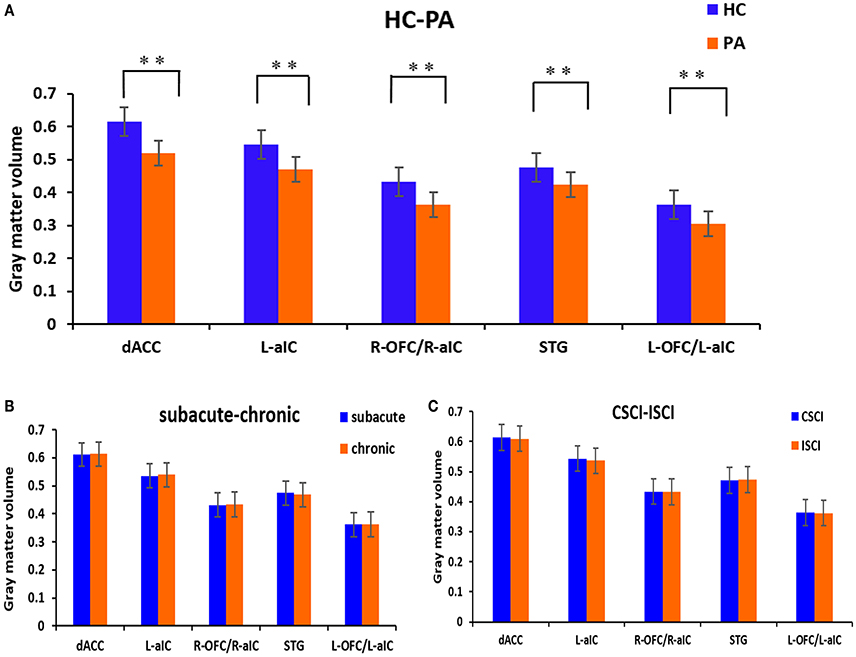
Figure 2. GM volumetric changes between different sub-groups based on region of interest (ROI) analysis. (A) Differences between the patients with SCI and healthy controls; (B) differences between the sub-acute and chronic sub-groups; (C) differences between the CSCI and ISCI sub-groups. ROIs were extracted based on the VBM findings. **Statistical significance with cluster wise FWE-corrected p ≤ 0.05. dACC, dorsal anterior cingulate cortex; L-aIC, left anterior insular cortex; R-aIC, right anterior insular cortex; L-OFC, left orbital frontal cortex; R-OFC, right orbital frontal cortex; STG, right superior temporal gyrus.
Differences in GMV between SCI Sub-Groups
ROI-wise comparisons revealed that there was no statistical difference in GMV between the sub-acute and chronic sub-groups (Figure 2B) or between the ISCI and CSCI sub-groups (Figure 2C) for the brain areas mentioned above (p > 0.05, uncorrected).
Correlation between Clinical Variables and GMV in Patients with SCI and between Clinical Variables and GMV of SCI Sub-groups
In all patients with SCI, partial correlation analyses showed a trend toward negative correlation between right OFC GMV and the injury duration (r = −0.448, p = 0.055, uncorrected). A weak positive correlation was shown between the right OFC GMV and the clinical motor scores of left extremities (r = 0.492, p = 0.032, uncorrected; Figure 3A). In the CSCI subgroup, partial correlation analyses showed a significant positive correlation between the right OFC GMV and the clinical motor scores of left extremities(r = 0.712, p = 0.031, uncorrected; Figure 3B). In the sub-acute subgroup, a significant positive correlation was shown between the dACC GMV and the total clinical motor scores (r = 0.830, p = 0.011, uncorrected; Figure 3C), in addition, partial correlation analyses showed a significant negative correlation between right OFC GMV and the injury duration (r = 0.843, p = 0.035, uncorrected; Figure 3D). In the chronic subgroup, a trend toward positive correlation was found between the right OFC GMV and the clinical motor scores of left extremities (r = 0.599, p = 0.052, uncorrected).There were no statistical correlations between GMV and either VAS or BDI.
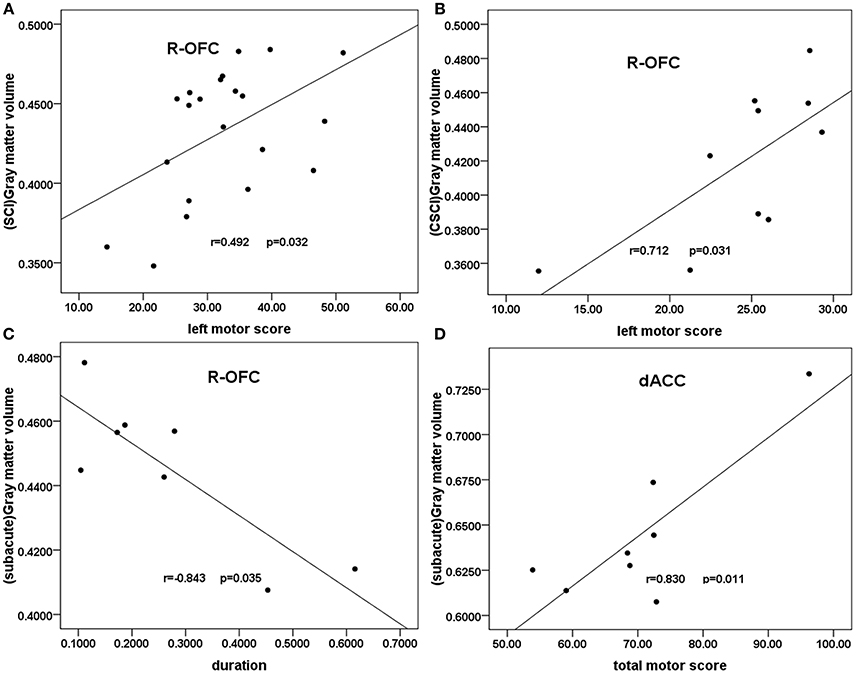
Figure 3. Correlation between GMV and clinical scores in patients with SCI and the SCI subgroups. Partial correlation showed (A) a moderate positive correlation between the GMV of the right OFC and the clinical left motor scores in patients with SCI (r = 0.492, p = 0.032); (B) a significant positive correlation between the right OFC GMV and the clinical left motor scores in the CSCI subgroup (r = 0.712, p = 0.031); (C) a significant positive correlation between the dACC GMV and the total clinical motor scores in the sub-acute sub-group (r = 0.830, p = 0.011); (D) a significant negative correlation between right OFC GMV and the injury duration in the sub-acute subgroup (r = −0.843, p = 0.035). R-OFG, right orbital frontal cortex; dACC, dorsal anterior cingulate cortex; GMV, gray matter volume; SCI, spinal cord injury.
Discussion
In the present study, we found significantly reduced GMV in many cognitive brain regions of patients with SCI compared with HCs. There were no statistical differences in GMV between the CSCI and ISCI sub-groups, or between the sub-acute and the chronic sub-groups. Finally, we found positive association between the GMV of patients with SCI and left motor scores, and between the GMV of CSCI patients and left motor scores. Additionally, in the sub-acute subgroup, a significant positive correlation was shown between the dACC GMV and the total motor scores, and a significant negative correlation was found between right OFC GMV and the injury duration. These findings may provide new insights into the structural plasticity of brain after SCI.
Brain GMV changes following SCI have been reported previously (Crawley et al., 2004; Jurkiewicz et al., 2006; Wrigley et al., 2009a; Freund et al., 2011, 2012, 2013; Felix et al., 2012), most of which were in the primary motor cortex (M1), primary somatosensory cortex (S1) and secondary somatosensory cortex (S2). Some researchers have suggested that these changes may be caused by direct or secondary Wallerian degeneration. As Freund et al. (2013) found that the corticospinal tracts and GM in the sensorimotor cortex of brain atrophied dramatically at 40 days after SCI, they proposed that volumetric changes in the brain GM and WM may be due to demyelination of axons and atrophy of neuronal cell bodies (Buss et al., 2004; Ramu et al., 2008; Cohen-Adad et al., 2011). In the present study, no volumetric changes were found in S1, M1, or S2, which is consistent with a few previous studies (Crawley et al., 2004; Yoon et al., 2013). An early study found that Wallerian degeneration of ascending fiber pathways following SCI can cause neuronal apoptosis, but the affected upward distance was only about 6–7 mm (Pallini et al., 1988). Thus, this short range of degeneration cannot involve the sensorimotor cortex, which could explain our findings.
In addition to direct degeneration of the sensorimotor cortex, SCI can also lead to atrophy of the non-sensorimotor cortex, such as the anterior cingulate cortex, insular cortex, and middle frontal gyrus (Wrigley et al., 2009a; Yoon et al., 2013; Jutzeler et al., 2016). For example, Wrigley et al. (2009a) have reported that patients with SCI had significant GM atrophy in the medial prefrontal cortex (MPFC) and anterior cingulate cortex. Consistent with their findings, we also found significantly decreased GMV in the dACC and bilateral anterior insular cortex, which are the core hubs of the salient network (Seeley et al., 2007). The anterior insula is one of the core hubs of the salient network that serves to identify the most relevant salient stimuli and forward them to higher cognitive regions to guide behavior (Menon and Uddin, 2010), while the dACC mainly plays roles in conflict monitoring between competing stimuli and provides a continuously updated prediction of expected cognitive demand to optimize future behavioral responses (Sheth et al., 2012). For patients with SCI, their sensory inputs were partially or fully lost. Thus, the reduced GMV in the core hubs of the salient network may reflect a disuse-induced transneuronal degeneration (Eidelberg et al., 1989; Bose et al., 2005; Jurkiewicz et al., 2006). We also found decreased GMV in the bilateral OFC. The OFC is an important relay of limbic system, and it is not only involved in regulating feeling and the visceral activities, but also in generating reward activity and some other complex psychological activity (Rolls, 2004; Cauda et al., 2011). Therefore, the decrease of GMV in OFC in patients with SCI may be associated with depression and with emotional and cognitive impairment after injury.
Although some earlier studies reported brain GM changes in patients with CSCI or patients with ISCI (Henderson et al., 2011; Villiger et al., 2015) they did not compare the potential differences directly between the two groups in one study. Thus, it is still uncertain whether injury severity can affect the level of GMV change. In this study, we directly compared brain GMV changes between patients with CSCI and patients with ISCI, but we found no statistical differences between them. Possible interpretations for this result are that: (1) the degeneration caused by SCI may be very slow and non-specific to both ISCI and CSCI; and (2) differences of the degree of brain atrophy between the CSCI and ISCI may be slight; and (3) the sample size of each SCI sub-groups was too small to identify tiny differences in GMV between the CSCI and ISCI.
The effect of SCI duration on cortical reorganization is not very clear. To date, only a few studies have explored the correlation between GMV and disease duration. Using an animal model, Rao et al. (2015) found positive correlations between regional homogeneity (ReHo) changes in the left ACC and the postoperative time. Several studies have shown that brain structure or function can be influenced by intervention during the duration of extensive disease following SCI (Corbetta et al., 2002; Cramer et al., 2007; Jurkiewicz et al., 2007; Jung et al., 2011; Lundell et al., 2011). However, there few studies have directly explored the correlation between injury duration and brain GMV changes. In the present study, we found a negative trend for the correlation between right OFC GMV of patients with SCI and injury duration (p = 0.055) and a significant negative correlation between right OFC GMV of sub-acute subgroup and injury duration (p = 0.035), indicating that longer disease course may lead to severer GM atrophy, especially in the sub-acute stage (duration 1–12 months), which is consistent with the findings of some previous studies (Freund et al., 2011, 2013). Of course, the reliability of this finding may benefit from larger sample sizes in future studies.
To date, there have been few studies exploring the correlation between GM changes and clinical variables. Hou et al. (2014) found a significant correlation between M1 GMV and total ASIA motor scores in patients with SCI. Villiger et al. (2015) reported a positive correlation between volumetric changes in the left middle temporal gyrus and the improvements in performance of patients with ISCI in rehabilitative training. In the present study, we found that right OFC GMV was positively related to left motor score not only in all the patients with SCI but also in the CSCI subgroup, moreover, a trend toward positive correlation was found between the right OFC GMV and the left motor scores in the chronic subgroup. Currently, a direct correlation between the OFC and motor function is lacking. It may contribute to the motor function indirectly, such as regulating reward activity (Rolls, 2004). Because the correlation is somewhat weak, we cannot make a clear inference. Additionally, we found a significant positive correlation between the dACC GMV and the total motor scores in the sub-acute subgroup(p = 0.011), indicating that the greater GMV in the dACC, the better clinical performance, which is consistent with the findings of some previous researches that: dACC play an important role in the brain work underlying motor control through its undirected and directed interactions with the supplementary motor area (SMA; Asemi et al., 2015; Diwadkar et al., 2017).
Several limitations of the present study should be addressed. First, we investigated GMV changes in patients with SCI with a very broad range of disease durations (range 1 month to 33 years). Second, the injured spinal segments were heterogeneous. Third, the severity of pain made the explanation more complex; however, we did not find a significant correlation between the GMV and the severity of pain. Finally, the relatively small sample size diminished the statistical power, especially when we tried to divide the patients with SCI into sub-groups.
In conclusion, our study provides evidence that SCI can cause significant atrophy of brain GM. Furthermore, brain atrophy was not evident in the sensorimotor system that directly innervates the paralyzed limbs, but in brain areas without direct connections, especially in the salient network. There were no statistical differences in GMV between the CSCI and ISCI sub-groups or between the sub-acute and the chronic sub-groups. But the GMV of the right OFC was correlated with the clinical motor scores of left extremities in not only all SCI patients, but especially the CSCI subgroup. In the sub-acute subgroup, a significant positive correlation was shown between the dACC GMV and the total clinical motor scores, and a significant negative correlation between right OFC GMV and the injury duration, indicating that the duration and severity of SCI may not be associated with the degree of brain atrophy in total SCI patients, but there may be associations between them in subgroups, which is need further study in future.
Author Contributions
QC and WZ: conception/design of the work; the acquisition, analysis, and the interpretation of data for the work; drafting of the work; final approval of the version to be published; and agreement to be accountable for all aspects of the work. XC/LW/WQ/ZQ: responsible for: the analysis data for the work; drafting of the work; final approval of the version to be published; and agreement to be accountable for all aspects of the work. NC: design of the work; revision of the work; final approval of the version to be published; and agreement to be accountable for all aspects of the work. KL: revising the work; final approval of the version to be published; and agreement to be accountable for all aspects of the work.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81271556), National Natural Science Foundation of China (No. 81401394), Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201609), Beijing Municipal Natural Science Foundation (No. 7113155), and Science Foundation of Beijing Municipal Commission of Education (No. KM201210025013). We thank Xiuqin Jia and Peipeng Liang for their assistance in post-processing of images.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fnhum.2017.00211/full#supplementary-material
Figure S1. Brain white matter atrophy after spinal cord injury.
Abbreviations
GM, gray matter; GMV, gray matter volume; WM, white matter; CSF, cerebrospinal fluid; HC, healthy control; SCI, spinal cord injury; CSCI, complete spinal cord injury; ISCI, incomplete spinal cord injury; VBM, voxel-based morphometry; OFC, orbital frontal cortex; aIC, anterior insular cortex; STG, superior temporal gyrus; dACC, dorsal anterior cingulate cortex; SMA, supplementary motor area; TR, repetition time; TE, echo time TI, inversion time; FA, flip angle; FOV, field of view; ReHo, regional homogeneity; MNI, Montreal Neurological Institute.
References
Aguilar, J., Humanes-Valera, D., Alonso-Calvino, E., Yague, J. G., Moxon, K. A., Oliviero, A., et al. (2010). Spinal cord injury immediately changes the state of the brain. J. Neurosci. 30, 7528–7537. doi: 10.1523/JNEUROSCI.0379-10.2010
Asemi, A., Ramaseshan, K., Burgess, A., Diwadkar, V. A., and Bressler, S. L. (2015). Dorsal anterior cingulate cortex modulates supplementary motor area in coordinated unimanual motor behavior. Front. Hum. Neurosci. 9:309. doi: 10.3389/fnhum.2015.00309
Ashburner, J. (2007). A fast diffeomorphic image registration algorithm. Neuroimage 38, 95–113. doi: 10.1016/j.neuroimage.2007.07.007
Ashburner, J., and Friston, K. J. (2000). Voxel-based morphometry—the methods. Neuroimage 11, 805–821. doi: 10.1006/nimg.2000.0582
Bose, P., Parmer, R., Reier, P. J., and Thompson, F. J. (2005). Morphological changes of the soleus motoneuron pool in chronic midthoracic contused rats. Exp. Neurol. 191, 13–23. doi: 10.1016/j.expneurol.2004.08.028
Buss, A., Brook, G. A., Kakulas, B., Martin, D., Franzen, R., Schoenen, J., et al. (2004). Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain 127, 34–44. doi: 10.1093/brain/awh001
Cauda, F., D'Agata, F., Sacco, K., Duca, S., Geminiani, G., and Vercelli, A. (2011). Functional connectivity of the insula in the resting brain. Neuroimage 55, 8–23. doi: 10.1016/j.neuroimage.2010.11.049
Chen, R., Cohen, L. G., and Hallett, M. (2002). Nervous system reorganization following injury. Neuroscience 111, 761–773. doi: 10.1016/S0306-4522(02)00025-8
Cohen-Adad, J., El, M. M., Lehericy, S., Pradat, P. F., Blancho, S., Rossignol, S., et al. (2011). Demyelination and degeneration in the injured human spinal cord detected with diffusion and magnetization transfer MRI. Neuroimage 55, 1024–1033. doi: 10.1016/j.neuroimage.2010.11.089
Corbetta, M., Burton, H., Sinclair, R. J., Conturo, T. E., Akbudak, E., and McDonald, J. W. (2002). Functional reorganization and stability of somatosensory-motor cortical topography in a tetraplegic subject with late recovery. Proc. Natl. Acad. Sci. U.S.A. 99, 17066–17071. doi: 10.1073/pnas.262669099
Cramer, S. C., Orr, E. L., Cohen, M. J., and Lacourse, M. G. (2007). Effects of motor imagery training after chronic, complete spinal cord injury. Exp. Brain Res. 177, 233–242. doi: 10.1007/s00221-006-0662-9
Crawley, A. P., Jurkiewicz, M. T., Yim, A., Heyn, S., Verrier, M. C., Fehlings, M. G., et al. (2004). Absence of localized grey matter volume changes in the motor cortex following spinal cord injury. Brain Res. 1028, 19–25. doi: 10.1016/j.brainres.2004.08.060
Diwadkar, V. A., Asemi, A., Burgess, A., Chowdury, A., and Bressler, S. L. (2017). Potentiation of motor sub-networks for motor control but not working memory: interaction of dACC and SMA revealed by resting-state directed functional connectivity. PLoS ONE 12:e0172531. doi: 10.1371/journal.pone.0172531
Eidelberg, E., Nguyen, L. H., Polich, R., and Walden, J. G. (1989). Transsynaptic degeneration of motoneurones caudal to spinal cord lesions. Brain Res. Bull. 22, 39–45. doi: 10.1016/0361-9230(89)90125-1
Endo, T., Spenger, C., Tominaga, T., Brene, S., and Olson, L. (2007). Cortical sensory map rearrangement after spinal cord injury: fMRI responses linked to Nogo signalling. Brain 130, 2951–2961. doi: 10.1093/brain/awm237
Felix, M., Popa, N., Djelloul, M., Boucraut, J., Gauthier, P., Bauer, S., et al. (2012). Alteration of forebrain neurogenesis after cervical spinal cord injury in the adult rat. Front. Neurosci. 6:45. doi: 10.3389/fnins.2012.00045
Feringa, E. R., and Vahlsing, H. L. (1985). Labeled corticospinal neurons one year after spinal cord transection. Neurosci. Lett. 58, 283–286. doi: 10.1016/0304-3940(85)90067-9
Flor, H., Elbert, T., Knecht, S., Wienbruch, C., Pantev, C., Birbaumer, N., et al. (1995). Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 375, 482–484. doi: 10.1038/375482a0
Florence, S. L. (1998). Large-scale sprouting of cortical connections after peripheral injury in adult macaque monkeys. Science 282, 1117–1121. doi: 10.1126/science.282.5391.1117
Freund, P., Schneider, T., Nagy, Z., Hutton, C., Weiskopf, N., Friston, K., et al. (2012). Degeneration of the injured cervical cord is associated with remote changes in corticospinal tract integrity and upper limb impairment. PLoS ONE 7:e51729. doi: 10.1371/journal.pone.0051729
Freund, P., Weiskopf, N., Ashburner, J., Wolf, K., Sutter, R., Altmann, D. R., et al. (2013). MRI investigation of the sensorimotor cortex and the corticospinal tract after acute spinal cord injury: a prospective longitudinal study. Lancet Neurol. 12, 873–881. doi: 10.1016/S1474-4422(13)70146-7
Freund, P., Weiskopf, N., Ward, N. S., Hutton, C., Gall, A., Ciccarelli, O., et al. (2011). Disability, atrophy and cortical reorganization following spinal cord injury. Brain 134, 1610–1622. doi: 10.1093/brain/awr093
Ghosh, A., Sydekum, E., Haiss, F., Peduzzi, S., Zorner, B., Schneider, R., et al. (2009). Functional and anatomical reorganization of the sensory-motor cortex after incomplete spinal cord injury in adult rats. J. Neurosci. 29, 12210–12219. doi: 10.1523/JNEUROSCI.1828-09.2009
Hains, B. C., Black, J. A., and Waxman, S. G. (2003). Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J. Comp. Neurol. 462, 328–341. doi: 10.1002/cne.10733
Henderson, L. A., Gustin, S. M., Macey, P. M., Wrigley, P. J., and Siddall, P. J. (2011). Functional reorganization of the brain in humans following spinal cord injury: evidence for underlying changes in cortical anatomy. J. Neurosci. 31, 2630–2637. doi: 10.1523/JNEUROSCI.2717-10.2011
Hou, J. M., Yan, R. B., Xiang, Z. M., Zhang, H., Liu, J., Wu, Y. T., et al. (2014). Brain sensorimotor system atrophy during the early stage of spinal cord injury in humans. Neuroscience 266, 208–215. doi: 10.1016/j.neuroscience.2014.02.013
Jain, N., Florence, S. L., Qi, H. X., and Kaas, J. H. (2000). Growth of new brainstem connections in adult monkeys with massive sensory loss. Proc. Natl. Acad. Sci. U.S.A. 97, 5546–5550. doi: 10.1073/pnas.090572597
Jung, J. K., Oh, C. H., Yoon, S. H., Ha, Y., Park, S., and Choi, B. (2011). Outcome evaluation with signal activation of functional MRI in spinal cord injury. J. Korean Neurosurg. Soc. 50, 209–215. doi: 10.3340/jkns.2011.50.3.209
Jurkiewicz, M. T., Crawley, A. P., Verrier, M. C., Fehlings, M. G., and Mikulis, D. J. (2006). Somatosensory cortical atrophy after spinal cord injury: a voxel-based morphometry study. Neurology 66, 762–764. doi: 10.1212/01.wnl.0000201276.28141.40
Jurkiewicz, M. T., Mikulis, D. J., McIlroy, W. E., Fehlings, M. G., and Verrier, M. C. (2007). Sensorimotor cortical plasticity during recovery following spinal cord injury: a longitudinal fMRI study. Neurorehabil. Neural Repair 21, 527–538. doi: 10.1177/1545968307301872
Jutzeler, C. R., Huber, E., Callaghan, M. F., Luechinger, R., Curt, A., Kramer, J. L., et al. (2016). Association of pain and CNS structural changes after spinal cord injury. Sci. Rep. 6:18534. doi: 10.1038/srep18534
Kaas, J. H., Qi, H. X., Burish, M. J., Gharbawie, O. A., Onifer, S. M., and Massey, J. M. (2008). Cortical and subcortical plasticity in the brains of humans, primates, and rats after damage to sensory afferents in the dorsal columns of the spinal cord. Exp. Neurol. 209, 407–416. doi: 10.1016/j.expneurol.2007.06.014
Kao, T., Shumsky, J. S., Murray, M., and Moxon, K. A. (2009). Exercise induces cortical plasticity after neonatal spinal cord injury in the rat. J. Neurosci. 29, 7549–7557. doi: 10.1523/JNEUROSCI.2474-08.2009
Kim, B. G., Dai, H. N., McAtee, M., Vicini, S., and Bregman, B. S. (2006). Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp. Neurol. 198, 401–415. doi: 10.1016/j.expneurol.2005.12.010
Lee, B. H., Lee, K. H., Kim, U. J., Yoon, D. H., Sohn, J., Choi, S. S., et al. (2004). Injury in the spinal cord may produce cell death in the brain. Brain Res. 1020, 37–44. doi: 10.1016/j.brainres.2004.05.113
Lundell, H., Christensen, M. S., Barthelemy, D., Willerslev-Olsen, M., Biering-Sorensen, F., and Nielsen, J. B. (2011). Cerebral activation is correlated to regional atrophy of the spinal cord and functional motor disability in spinal cord injured individuals. Neuroimage 54, 1254–1261. doi: 10.1016/j.neuroimage.2010.09.009
Macklin, R. A., Bae, J., Orell, M., Anderson, K. D., Ellaway, P. H., and Perez, M. A. (2016). Time-dependent discrepancies between assessments of sensory function after incomplete cervical spinal cord injury. J. Neurotrauma. doi: 10.1089/neu.2016.4433. [Epub ahead of print].
Marino, R. J., Barros, T., Biering-Sorensen, F., Burns, S. P., Donovan, W. H., Graves, D. E., et al. (2003). International standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 26(Suppl. 1), S50–S56. doi: 10.1080/10790268.2003.11754575
Marino, R. J., Jones, L., Kirshblum, S., Tal, J., and Dasgupta, A. (2008). Reliability and repeatability of the motor and sensory examination of the international standards for neurological classification of spinal cord injury. J. Spinal Cord Med. 31, 166–170. doi: 10.1080/10790268.2008.11760707
Menon, V., and Uddin, L. Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 214, 655–667. doi: 10.1007/s00429-010-0262-0
Pallini, R., Fernandez, E., and Sbriccoli, A. (1988). Retrograde degeneration of corticospinal axons following transection of the spinal cord in rats. A quantitative study with anterogradely transported horseradish peroxidase. J. Neurosurg. 68, 124–128. doi: 10.3171/jns.1988.68.1.0124
Pernet, U., and Hepp-Reymond, M. C. (1975). [Retrograde degeneration of the pyramidal cells in the motor cortex of apes (Macaca fascicularis)]. Acta Anat. 91, 552–561. doi: 10.1159/000144414
Pons, T. P., Garraghty, P. E., Ommaya, A. K., Kaas, J. H., Taub, E., and Mishkin, M. (1991). Massive cortical reorganization after sensory deafferentation in adult macaques. Science 252, 1857–1860. doi: 10.1126/science.1843843
Ramu, J., Herrera, J., Grill, R., Bockhorst, T., and Narayana, P. (2008). Brain fiber tract plasticity in experimental spinal cord injury: diffusion tensor imaging. Exp. Neurol. 212, 100–107. doi: 10.1016/j.expneurol.2008.03.018
Rao, J. S., Ma, M., Zhao, C., Liu, Z., Yang, Z. Y., and Li, X. G. (2015). Alteration of brain regional homogeneity of monkeys with spinal cord injury: a longitudinal resting-state functional magnetic resonance imaging study. Magn. Reson. Imaging 33, 1156–1162. doi: 10.1016/j.mri.2015.06.011
Rausell, E., and Jones, E. G. (1995). Extent of intracortical arborization of thalamocortical axons as a determinant of representational plasticity in monkey somatic sensory cortex. J. Neurosci. 15, 4270–4288.
Rolls, E. T. (2004). The functions of the orbitofrontal cortex. Brain Cogn. 55, 11–29. doi: 10.1016/S0278-2626(03)00277-X
Seeley, W. W., Menon, V., Schatzberg, A. F., Keller, J., Glover, G. H., Kenna, H., et al. (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007
Sheth, S. A., Mian, M. K., Patel, S. R., Asaad, W. F., Williams, Z. M., Dougherty, D. D., et al. (2012). Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 488, 218–221. doi: 10.1038/nature11239
Simoes, E. L., Bramati, I., Rodrigues, E., Franzoi, A., Moll, J., Lent, R., et al. (2012). Functional expansion of sensorimotor representation and structural reorganization of callosal connections in lower limb amputees. J. Neurosci. 32, 3211–3220. doi: 10.1523/JNEUROSCI.4592-11.2012
Villiger, M., Grabher, P., Hepp-Reymond, M. C., Kiper, D., Curt, A., Bolliger, M., et al. (2015). Relationship between structural brainstem and brain plasticity and lower-limb training in spinal cord injury: a longitudinal pilot study. Front. Hum. Neurosci. 9:254. doi: 10.3389/fnhum.2015.00254
Wrigley, P. J., Gustin, S. M., Macey, P. M., Nash, P. G., Gandevia, S. C., Macefield, V. G., et al. (2009a). Anatomical changes in human motor cortex and motor pathways following complete thoracic spinal cord injury. Cereb. Cortex 19, 224–232. doi: 10.1093/cercor/bhn072
Wrigley, P. J., Press, S. R., Gustin, S. M., Macefield, V. G., Gandevia, S. C., Cousins, M. J., et al. (2009b). Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain 141, 52–59. doi: 10.1016/j.pain.2008.10.007
Yoon, E. J., Kim, Y. K., Shin, H. I., Lee, Y., and Kim, S. E. (2013). Cortical and white matter alterations in patients with neuropathic pain after spinal cord injury. Brain Res. 1540, 64–73. doi: 10.1016/j.brainres.2013.10.007
Keywords: spinal cord injury, gray matter volume, voxel-based morphometry, brain atrophy, clinical variables
Citation: Chen Q, Zheng W, Chen X, Wan L, Qin W, Qi Z, Chen N and Li K (2017) Brain Gray Matter Atrophy after Spinal Cord Injury: A Voxel-Based Morphometry Study. Front. Hum. Neurosci. 11:211. doi: 10.3389/fnhum.2017.00211
Received: 14 January 2017; Accepted: 11 April 2017;
Published: 28 April 2017.
Edited by:
Mikhail Lebedev, Duke University, USAReviewed by:
Igor Jakovcevski, German Center for Neurodegenerative Diseases (HZ), GermanyFlorence Evelyne Perrin, Université de Montpellier, France
Copyright © 2017 Chen, Zheng, Chen, Wan, Qin, Qi, Chen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Chen, Y2hlbnplbjgwNTdAc2luYS5jb20=
†These authors have contributed equally to this work.
 Qian Chen
Qian Chen Weimin Zheng
Weimin Zheng Xin Chen
Xin Chen Lu Wan1
Lu Wan1 Wen Qin
Wen Qin Nan Chen
Nan Chen Kuncheng Li
Kuncheng Li