
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci. , 20 February 2017
Sec. Brain Health and Clinical Neuroscience
Volume 11 - 2017 | https://doi.org/10.3389/fnhum.2017.00066
This article is part of the Research Topic Applied Neuroimaging, Neuromodulation and Neurofeedback Investigations in Autism Spectrum Disorder (ASD). View all 6 articles
 Dominique Endres1*†
Dominique Endres1*† Simon Maier1†
Simon Maier1† Bernd Feige1
Bernd Feige1 Nicole A. Posielski1
Nicole A. Posielski1 Kathrin Nickel1
Kathrin Nickel1 Dieter Ebert1
Dieter Ebert1 Andreas Riedel1
Andreas Riedel1 Alexandra Philipsen2
Alexandra Philipsen2 Evgeniy Perlov1†
Evgeniy Perlov1† Ludger Tebartz van Elst1†
Ludger Tebartz van Elst1†Background: Autism spectrum disorder (ASD) is often associated with epilepsy. Previous studies have also shown increased rates of electroencephalographic (EEG) alteration in ASD patients without epilepsy. The aim of this study was to compare the rate of intermittent rhythmic delta and theta activity (IRDA/IRTA) events between high-functioning adult patients with ASD and matched healthy controls.
Materials and Methods: Routine EEG records of 19 ASD patients and 19 matched controls were screened for IRDA/IRTA using a fully data driven analysis with fixed thresholds. IRDA/IRTA rates before and after hyperventilation (HV) as well as the HV-induced difference in IRDA/IRTA rates (HV difference) were analyzed. For inter-group measures, we used the Wilcoxon rank sum test.
Results: Significantly increased HV difference was detected in the ASD group (p = 0.0497). However, the groups showed no difference in IRDA/IRTA rates before HV (p = 0.564) and after HV (p = 0.163).
Conclusions: The lack of any group differences regarding IRDA/IRTA before HV might be related to the fact that we only studied non-secondary high-functioning autism in a small sample of epilepsy-free adult patients. A significantly increased HV difference might be regarded as a marker of subtle neuronal network instability possibly causing short-term disturbances via local area network inhibition and long-term effects via epileptic encephalopathy.
High-functioning autism spectrum disorder (ASD) is a common neurodevelopmental and basic psychiatric disorder (Tebartz van Elst, 2013; Tebartz van Elst et al., 2013). Its prevalence varies between 1% and 2.7%, with increased rates in men (Brugha et al., 2011; Kim et al., 2011). ASD is characterized by long-lasting deficits in social communication, repetitive/stereotypical behavior, and special interests (Tebartz van Elst, 2013; Tebartz van Elst et al., 2013; Lai et al., 2014). Patients with high-functioning forms of ASD have normal or above-average intelligence quotients (IQs) and are able to use language on a superficial level (Tebartz van Elst, 2013; Tebartz van Elst et al., 2013). ASD is associated with high rates of psychiatric comorbidity. Most frequently, patients suffer from comorbid depression, anxiety disorder, obsessive-compulsive disorder and psychotic disorders. It is also closely linked to other neurodevelopmental diseases, such as attention deficit hyperactivity disorder (ADHD) and tic disorders (Lai et al., 2014). Moreover, ASD is associated with epilepsy (see next section; Lai et al., 2014). The clinical assessment for ASD involves personal and developmental history (interviews of parents or caregivers), behavioral observations, a physical examination and psychometric testing as well as optional neuropsychological tests, structured interviews, or observational measures. In many clinical centers, instrument-based diagnostics, including laboratory measurements, electroencephalography (EEG) and cerebral magnetic resonance imaging (cMRI) are also performed during clinical assessments for ASD (Tebartz van Elst, 2013). From a pathophysiological perspective, primary forms of ASD without a recognizable cause and secondary forms with a recognizable cause can be distinguished. Patients with primary forms often display a familial liability to autism however without a well-recognized genetic disorder or syndrome. In such cases, hereditary ASD is interpreted as a polygenetic phenomenon. Secondary forms may be triggered by mono- or oligogenetic disorders (e.g., fragile X syndrome, tuberous sclerosis, etc.) or syndromes (e.g., isodicentric chromosome 15, DiGeorge syndrome, etc.), or they may be acquired via brain disorders (e.g., encephalitis with epilepsy, traumatic brain injury; Tebartz van Elst, 2013; Tebartz van Elst et al., 2013). Specific psychotherapy programs were developed to improve social interaction among ASD patients (e.g., The Freiburg Asperger Specific Therapy Manual for Adult Patients or the FASTER program; Ebert et al., 2013). So far no specific psychotropic drugs are approved for treatment of the autistic core symptoms, but pharmacological treatment of comorbidities or specific symptoms may be useful. For example, patients with comorbid depression can be treated with antidepressants, and in patients with EEG alterations, aggressiveness and impulsivity psychotropic anticonvulsants such as carbamazepine may be useful; however, further research regarding treatment of ASD symptoms is needed (Tebartz van Elst, 2013; Tebartz van Elst et al., 2013; Hirota et al., 2014).
The link between autism and epilepsy or EEG alterations is well known. In fact, several reviews explaining this connection were published in the last few years (Spence and Schneider, 2009; Berg and Plioplys, 2012; Ghacibeh and Fields, 2015; Lee et al., 2015). Epilepsy rates range from 5% to 46% in autistic patients (Spence and Schneider, 2009; Ghacibeh and Fields, 2015), and epilepsy was found to be associated with low IQ in many studies (e.g., Tuchman et al., 1991; Hara, 2007; Amiet et al., 2008; Spence and Schneider, 2009; Berg and Plioplys, 2012). Generalized tonic-clonic and complex partial seizures as well as absences were described (Spence and Schneider, 2009). In addition, one-third of childhood epilepsy cohorts fulfilled the psychometric criteria for ASD (Clarke et al., 2005). In ASD patients without epilepsy, increased rates of EEG pathologies ranging from 6.7% to 61% were reported (Ghacibeh and Fields, 2015). In groups of patients with ASD, epileptiform activity was detected in about 60% of children (Chez et al., 2006; Kim et al., 2006) and more rarely in adults (18% in Hara, 2007).
In this study, we investigated intermittent rhythmic delta and theta activity (IRDA/IRTA). IRDA was first described by Cobb (1945) and involves pathological EEG patterns with unclear significance (Brigo, 2011). Structural, metabolic, inflammatory, or epileptic reasons can cause theta activity (Cobb, 1945; Accolla et al., 2011; Brigo, 2011; van Vliet et al., 2012). Structurally, the delta rhythm might be generated in the thalamus or basal nuclei (Cobb, 1945). We earlier hypothesized that IRDA/IRTA can induce adaptive homeostatic processes, which could lead to functional alternations in neuronal networks (Tebartz van Elst et al., 2011, 2016a; Tebartz van Elst and Perlov, 2013; Endres et al., 2016).
Given this theoretical background, the aim of our study was to compare IRDA/IRTA rates between primary ASD patients without epilepsy and matched controls. To create a homogeneous study cohort, only high-functioning patients with ASD were included. We hypothesized that we would find: (1) increased IRDA/IRTA rates in the resting EEGs of ASD patients; and (2) a significantly more pronounced increase in IRDA/IRDA after hyperventilation (HV) as a correlate for unstable cerebral networks in ASD patients.
All patients and controls agreed to EEG examinations. EEG measurements were part of the routine clinical procedure for ASD patients. In the study, we included all available datasets from ASD patients of the Department of Psychiatry and Psychotherapy, University Medical Center Freiburg, between 2006 and 2012. The study was approved by the local ethics committee (Faculty of Medicine, Freiburg University, EK-Fr 233/14) and conformed to the principles of the Declaration of Helsinki. Control subjects were recruited from an earlier research project (Tebartz van Elst et al., 2014; van Elst et al., 2014; Endres et al., 2015; Maier et al., 2016).
Only patients fulfilling the ICD-10 criteria for Asperger syndrome were included1. The diagnostic procedure was conducted by experienced senior consultant psychiatrists. All clinical information about comorbidity and somatic diseases was received from medical reports. We excluded all secondary ASD forms to create a homogeneous study sample. Psychometric measurements included the autism spectrum quotient (AQ; Baron-Cohen et al., 2001) and the empathy quotient (EQ; Baron-Cohen and Wheelwright, 2004) for autistic symptoms. In uncertain cases, the Autism Diagnostic Interview-Revised (Lord et al., 1994), the autism diagnostic observation schedule-generic (Lord et al., 2000), or behavioral observations were performed. For retrospective detection of ADHD symptoms in childhood, the Wender Utah Rating Scale (WURS-k) was used (Retz-Junginger et al., 2002).
Control subjects were recruited from an earlier project (Tebartz van Elst et al., 2014; van Elst et al., 2014; Endres et al., 2015; Maier et al., 2016). Therefore, the control group was well investigated using cMRIs; interviews to obtain patients’ demographics, psychosocial characteristics and medical histories; psychometrics (i.e., multiple-choice vocabulary intelligence test (MWT-B; Lehrl et al., 1995); WURS-k (Retz-Junginger et al., 2002), AQ (Baron-Cohen et al., 2001), and EQ scores (Baron-Cohen and Wheelwright, 2004); and semi-structured interviews (i.e., Mini International Neuropsychiatric Interview; Sheehan et al., 1998). Only controls without psychotropic medication, without neurological disorders, normal intelligence, no current axis I or II disorders, WURS-k scores of <30, and AQ scores of <30 were included.
Between 2006 and 2012, we investigated 32 patients with ASD (20 males, 12 females). The control group included 34 healthy controls; one EEG was excluded due to technical reasons, one EEG was excluded due to incomplete psychometrics. To exclude age and gender effects, we automatically matched both variables. This resulted in 19 well-matched patient–control pairs (9 male pairs, 10 female pairs).
Topographical EEG was recorded using all 21 standard locations of the international 10–20 system (Klem et al., 1999). The recording reference was Fpz, ground Oz. Analog signals were recorded with a time constant of 0.3 s and a low pass of 70 Hz, sampled at 256 Hz and continuously stored for further processing. Off-line, the digital signals were filtered between 0.3 Hz and 45 Hz and downsampled to 100 Hz. EEG monitoring was performed for 11 min, and EEG recording was divided into three parts: first, a resting state was maintained for 6 min; second, a HV period was maintained for 3 min; and third, a post-HV period was maintained for 2 min. NEUROFILE software was used to register data2.
The data analysis was performed using in-house software, avg_q3. The main steps were: (1) marking artifacts, including signal jumps and “blocking”, i.e., sections without signal variation in any channel; (2) independent component analysis (ICA) on the artifact-free data sections avoiding 5 s before and after any artifact marker (extended ICA; Lee et al., 1999; Makeig et al., 1999); (3) detection and correction of electrooculographic (EOG) artifacts by exclusion of EOG-related ICA components; and (4) detection of IRDA/IRTA. Empirically determined thresholds were used for the detection of phase and jump artifacts, optimizing detection and minimizing false positive findings. IRDA/IRTA was detected in any non-excluded independent component irrespective of topography. IRDA/IRTA detection was achieved by filtering the activation time series of all non-excluded independent components between 2 Hz and 7 Hz and thresholding for maximal amplitude between 25 μV and 245 μV. Only IRDA/IRTA candidates within the artifact-free EEG sections were considered. IRDA/IRTA rates were computed as events per minute separately for the intervals before and after HV.
For statistical analyses, we used Statistical Package for the Social Sciences, version 22 (SPSS 224) and R, version 3.2.25. The continuous variables of age, and psychometric scores were compared using two-sided independent sample t-tests. The gender ratio was calculated using Pearson’s two-sided chi-square test. IRDA/IRTA rates were corrected for age, using a linear model to estimate the predicted influence of subjects’ age on IRDA/IRTA rates. These estimates were then subtracted from the individual IRDA/IRTA rates. Group comparisons for the target variables (IRDA/IRTA before HV, IRDA/IRTA after HV, and difference in IRDA/IRTA from before HV to after HV [abbreviated as HV difference]) were performed using a Wilcoxon rank sum test (for inter-group comparisons). Moreover a parametric robust (non-parametric) repeated measures ANOVA (regular ranks) was performed using the npIntFactRep package in R to analyze the interaction between group and HV. We chose the robust repeated measure ANOVA since the assumption of homogeneity of variance was violated. In addition, medicated and unmedicated patients were compared using a Wilcoxon rank sum test. For outlier detection we performed a Tukey’s test. For correlation analyses of age corrected IRDA/IRTA rates with AQ und EQ scores, we calculated Spearman’s rank correlation. P < 0.05 served as the criterion of significance for all statistical analyses.
ASD patients and controls were matched in age and gender. In the patient group, 42% were medicated, mostly with antidepressants (37%). As expected psychometric autistic and ADHD scores were significantly increased in ASD patients (Table 1).
No significant difference was found between ASD patients and control subjects regarding the rate of IRDA/IRTA before (W = 201, r = −0.175, p = 0.564) and after HV (W = 229, r = −0.283, p = 0.163). However, significantly increased HV difference was detected in the ASD group (W = 248, r = −0.364, p = 0.0497; Table 2). The comparison between medicated and unmedicated patients showed no differences in the frequency of IRDA/IRTA before (W = 42, r = −0.0277, p = 0.9039), after HV (W = 53, r = −0.1576, p = 0.492), and for HV difference (W = 52, r = −0.1389, p = 0.5448). In the robust ANOVA the interaction group by HV was significant (F(1,36) = 6.50, p = 0.015). Outlier detection led to the exclusion of one ASD value for before, after and HV difference. The Wilcoxon test remained significant for the HV difference (W = 229, r = −0.3372, p = 0.0403) and not significant for IRDA/IRTA rates before (W = 182, r = −0.1455 p = 0.3763) and after HV (W = 210, r = −0.2538, p = 0.1226).
The correlation analysis in the ASD group showed no significant correlations between IRDA/IRTA and autism scores (AQ, EQ).
The main finding of our study is the significantly increased HV difference in primary non-syndromal ASD patients. Contradictory to our hypothesis, we found no significant difference between the ASD and control groups in terms of the IRDA/IRTA rate in resting EEGs. Still the IRDA/IRTA-HV- difference might turn out to be a marker of discrete but possible relevant neuronal network instability.
To our knowledge, this is the first study analyzing IRDA/IRTA in ASD patients. Our findings (significant increase in HV difference) are in line with earlier reports of increased rates of EEG alterations for children and adults with autism. In most previous studies, there was no differentiation between EEG alterations before and after HV. However, the absence of EEG alterations before HV is contrary to most findings (Spence and Schneider, 2009; Ghacibeh and Fields, 2015). The most likely explanation for this is the fact that we concentrated on high-functioning non-secondary autism in adults while most other previous studies did not differentiate between secondary and primary autism. Therefore other study groups contained also secondary and syndromal autism which is known to be more closely linked to epilepsy.
Furthermore most studies have focused on children, mostly with mental retardation (see overviews from Kawasaki et al., 1997; Spence and Schneider, 2009; Ghacibeh and Fields, 2015). Although some studies included adult patients with autism (e.g., Tuchman et al., 1991; Kawasaki et al., 1997; Giovanardi Rossi et al., 2000; Hughes and Melyn, 2005; Chez et al., 2006; Hartley-McAndrew and Weinstock, 2010), studies focusing only on adult cohorts are rare. Hara observed 130 patients with autistic disorder or atypical autism. In adulthood (18–35 years), 25% exhibited epileptic seizures; 68% of the epileptic group revealed epileptiform EEG findings before onset of epilepsy, 18% of the non-epileptic group exhibited epileptic discharges, and lower IQs were observed in the epileptic group (Hara, 2007). To our knowledge, no studies have analyzed high-functioning cohorts, as we did. EEG alterations in autism research might be influenced by different evaluation methods and the inclusion of different ASD subgroups (see “Discussion” Section about the nosology problem). For evaluation methods, we used routine EEGs. Earlier studies analyzing short routine EEGs found lower rates of EEG alterations (Hara, 2007). In contrast, high rates of EEG alterations were found with video EEG monitoring (epileptiform abnormalities in 59%; Kim et al., 2006) or 24 h digital overnight EEGs (epileptiform abnormalities in 61%; Chez et al., 2006).
If we look at previous EEG studies on autism, the nosology problem i.e., the fact that ASD comprises patient subgroups with diverse pathophysiologies, becomes apparent (Tebartz van Elst et al., 2006, 2013, 2014; Endres et al., 2015). Different subgroups of ASD can be distinguished by age, gender, course of disease, level of functioning, etiological variables and comorbidity. Regarding age, children, adolescent and adult patients must be distinguished; the dynamic changes in the brain during childhood and adolescence might result in different electrophysiological properties. In the current study, we investigated only adult patients and compared them with age-matched control subjects; therefore, developmental changes should be of no consequence. Regarding gender, earlier research reported increased prevalence of EEG alterations in young females on a meta-analytic-level (Amiet et al., 2008). In this study, we compared gender-matched ASD and control groups to exclude gender effects. Regarding the course of the disease, patients with early infantile autism, atypical autism, or Asperger syndrome must be distinguished. Developmental regression, which is observed in one-third of children with autism (Kawasaki et al., 1997), has been found to be associated with EEG alterations (Ghacibeh and Fields, 2015). EEG alterations were frequent in patients with regressive autism, and epileptiform activity was found more often in patients with regression than in patients without regression (Tuchman and Rapin, 1997; Lewine et al., 1999; Shinnar et al., 2001). However, other studies found no such differences in patients with developmental regression (Rossi et al., 1995; Chez et al., 2006; Giannotti et al., 2008). Regarding the level of functioning, high-functioning patients with normal or above-average IQs and effective use of language should be distinguished from low-functioning patients with low IQs and no communicative language. In support of this distinction a meta-analysis reported a higher prevalence of epilepsy in patients with intellectual disability (21.5%) compared to patients without intellectual disability (8%; Amiet et al., 2008). Regarding etiological variants, primary idiopathic forms of ASD must be distinguished from secondary non-idiopathic forms caused by acquired brain disorders, genetic disorders, or syndromal autism (Tebartz van Elst, 2013; Tebartz van Elst et al., 2013). Patients with secondary non-idiopathic forms were affected by epilepsy more often than patients suffering from primary idiopathic forms (Pavone et al., 2004; Miles et al., 2005). Finally, patients must be distinguished by comorbidity. Frequent developmental comorbidities are ADHD and tic disorders, and epilepsy itself is also an important neurological comorbid disorder (Lai et al., 2014). It can be assumed that EEG alterations are more prevalent in ASD patients with comorbid epilepsy (Hara, 2007). Until now, most studies have analyzed low-functioning children and adolescents (i.e., the typical patients of child and adolescent psychiatrists). To create a homogeneous study group and exclude developmental influences, we focused on high-functioning adults with primary variants and without relevant somatic comorbidities (particularly epilepsy but also a history of relevant birth complications or inflammatory brain disease). Such patients might suffer from a less severe form of ASD. In line with that idea, epileptiform activity was less common in patients with Asperger syndrome than in patients with more severe forms of autism (Mulligan and Trauner, 2014).
In summary, it can be noted that ASD is a heterogeneous disorder comprising many etiological subgroups and underlying pathophysiologies. The fact that we found no difference in the IRDA/IRTA rate before HV between groups might be explained by our inclusion of less severe forms of ASD without intellectual disability or developmental regression.
In our study, the HV difference was significantly different. Therefore, the question of the role of HV arises. HV is an effective provocation method leading to decreased blood carbon dioxide levels, and therefore to cerebral vasoconstriction (Barker et al., 2012). This might result in EEG changes, such as physiological slowing, IRDA/IRTA, or epileptiform activity (Siddiqui et al., 2011; Barker et al., 2012). Interictal epileptiform activity was found particularly in patients with generalized epilepsy, but also in patients with focal epilepsy (Ahdab and Riachi, 2014; Kane et al., 2014). The role of EEG changes in patients without epileptic seizures is still unresolved. The phenomenon of HV-induced high-amplitude rhythmic slow activity with altered awareness in children without epilepsy was described as an epileptiform occurrence and physiological process (Barker et al., 2012). In this study, we focused on automatic detection of IRDA/IRTA, which is clearly pathological excitatory neuronal network activity (Tebartz van Elst et al., 2016a).
The most important clinical question is whether there is a causal relationship between EEG alterations and ASD symptoms. We hypothesize that local area network inhibition could cause short-term effects and epileptic encephalopathy could cause long-term effects. Alternatively, EEG alterations might simply be an epiphenomenon of a pathophysiological process responsible for ASD (Spence and Schneider, 2009).
As described above, IRDA/IRTA is a pathological excitatory neuronal network activity. If such primary excitatory activity is focal and frequent enough, it could trigger a secondary homoeostatic reaction to which we have referred in previous publication using the term local area neuronal network inhibition (LANI). These putative inhibitory processes are conceptualized as a cybernetic homoeostatic mechanism of the brain to keep the functional equilibrium of the local neuronal network. While discrete and distributed LANI might not present with any clinical symptoms, ongoing suprathreshold LANI could in fact cause such symptoms. The nature of such phenomena then depends on the location of inhibited brain regions or anatomical loops (Tebartz van Elst et al., 2011; Tebartz van Elst and Perlov, 2013). Ongoing LANI in the frontal lobe might lead to stimulus satiation, and LANI in the left temporal lobe might trigger hallucinations.
Neuronal network instability as represented by IRDA/IRTA activity may lead to short term LANI effects illustrated above. But it may also cause long term effects stimulating the brain to restructure itself into a connectivistic pattern that minimizes the spread of epileptic activity (Tebartz van Elst et al., 2016b). Frequent epileptiform patterns can also interfere with normal neuronal physiology and brain development, disrupting various neurocognitive processes (i.e., plasticity, memory encoding and language processing) and causing developmental delays, regression or disruption. While this notion is controversial, respective results have been observed for other established syndromes (Holmes and Lenck-Santini, 2006; Ghacibeh and Fields, 2015). For instance, the Landau–Kleffner syndrome is characterized by language regression in normally developing children and is associated with EEG alterations mainly during sleep (Tebartz van Elst and Perlov, 2013). Cognitive functioning in patients with epileptic encephalopathy improves following normalization of their EEGs (Holmes and Lenck-Santini, 2006). Studies on children showed that antiepileptic treatment of interictal epileptiform activity could improve psychosocial function (Binnie, 2003; García-Peñas, 2011). On the background of such observations the question arises if the IRDA/IRTA-HV difference might serve as a biomarker indicating pathophysiological relevant neuronal network instability in a given patient.
The study was retrospective; we included only patients from our clinic with ASD that were diagnosed between 2006 and 2012. To create a homogeneous study group, we included only adult patients with primary high-functioning forms of ASD. Patients with comorbid epilepsy and atypical autism were excluded from the study. In our previous studies on ASD, we established study cohorts with normal or above-average IQs as a filter for primary forms of ASD (Riedel et al., 2014; Tebartz van Elst et al., 2014). In the current study, IQ values were not available from all patients. To exclude secondary forms of ASD, the available clinical information was checked precisely. Additionally, we included medicated and unmedicated patients, which might have influenced our results. However, the IRDA/IRTA rates in medicated and unmedicated patients did not differ in statistical comparisons. Moreover, the total sample size could have been larger than 38, and further studies with increased numbers of subjects should be performed to confirm our findings. To subtract the possible variance of age and gender, we performed pairwise matching for these variables, which created highly comparable patient and control groups.
We assessed routine 11-min EEG studies. Repeated EEG recordings, sleep and sleep deprivation measurements, or video EEG telemetry probably would have led to higher rates of EEG alteration. Therefore, our findings should be regarded as representing the minimum detection rate in this sample. The data analysis was performed completely automatically and independent of an investigator. Thus we can exclude elements of rating bias. The method is published on the Internet and is therefore accessible to the reader. Regarding statistical analyses, there is potential to criticize our inclusion of outliers (Figure 1). However, we believe that these outliers could present the subgroup of subjects with network instability. In these patients (and in all the other patients), no secondary explanation (e.g., epilepsy or brain disease) of the IRDA/IRTA rates was found. The inclusion of these subjects in the statistical calculation resulted in a non-normal distribution of the IRDA/IRTA rates, which was considered in the statistics through the application of a Wilcoxon rank sum test. However, even with using outlier detection, the results remained significant for the HV difference.
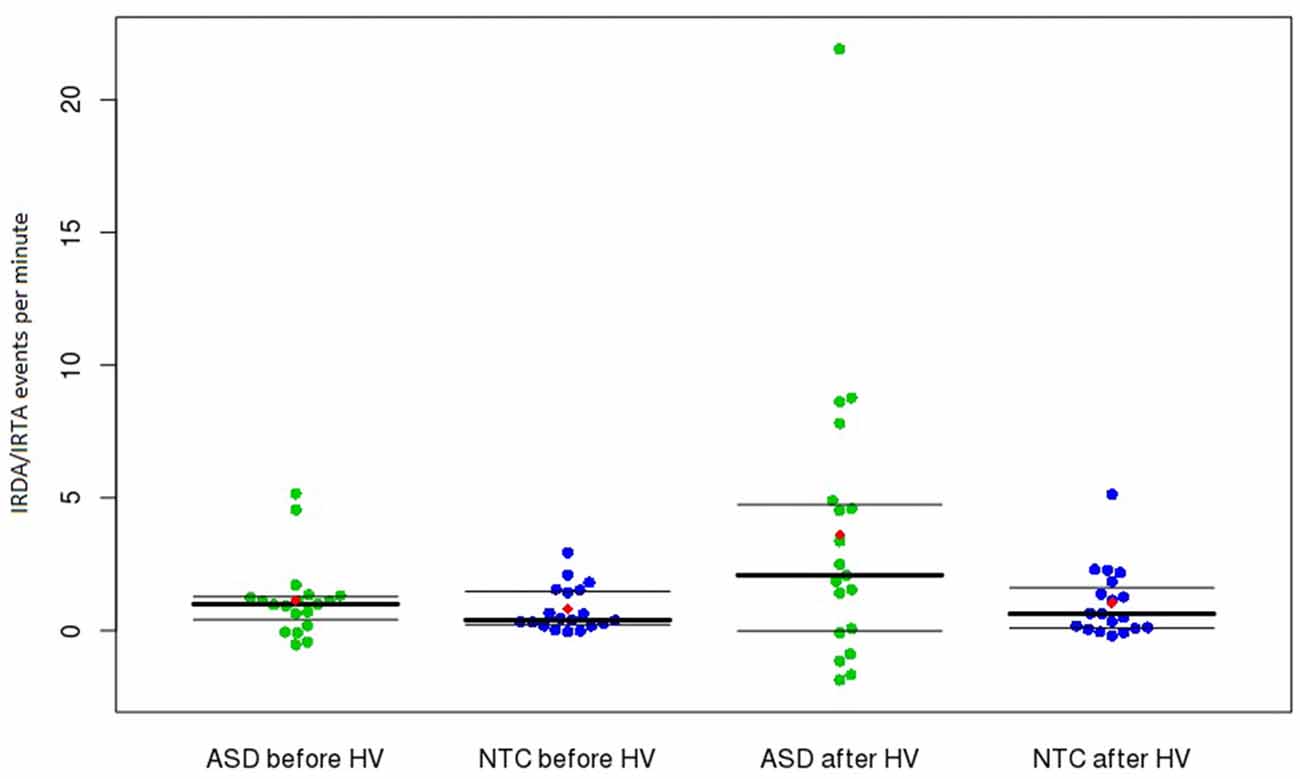
Figure 1. Frequency of IRDA/IRTA events before and after HV in the ASD and control groups. Presented are medians with upper and lower quantiles of corrected IRDA/IRDA rates. The use of age-corrected IRDA/IRTA-rates could result in negative corrected densities (i.e., IRDA/IRTA rates < 0). The red dots show the means. Abbreviations: ASD, autism spectrum disorder; HV, hyperventilation; IRDA, intermittent rhythmic delta activity; IRTA, intermittent rhythmic theta activity; NTC, neurotypical controls.
The primary finding of our study is a significantly increased HV difference in non-syndromal, high-functioning, epilepsy-free adult ASD patients. Further studies analyzing EEG alterations in larger cohorts of high-functioning adults with ASD are needed. An increased rate of IRDA/IRTA might be a neurophysiological marker of neuronal network instability which could have etiological and predictive relevance. For example it might modify cerebral information processing via short-term effects in terms of LANI or long-term effects via epileptic encephalopathy. The therapeutic implication of our findings is still unclear. Treatment with antiepileptic medication might be effective in such constellations. However, to prove this assumption, further clinical research with blinded, placebo-controlled intervention trials of ASD patients with EEG alterations are necessary.
LTvE, EP, DEn and SM initiated the study. BF developed the software for the EEG analysis. DEn, SM, NAP and BF conducted the data analysis. DEn and SM wrote the article. All authors were crucially involved in the theoretical discussion and performing of the manuscript. All authors read and approved the final version of the manuscript.
Parts of the study were funded by the German Federal Ministry of Science and Education (ADHD-NET: 01GV0605, 01GV0606). The rest of the study was financed in-house by the Department of Psychiatry and Psychotherapy at the Medical Center of the University of Freiburg.
AP: Received advisory board fees from Lilly, advisory board, and lecture fees from Medice, Novartis, and Shire, congress support from Servier, and a travel grant from Lundbeck. She has also authored books and articles on adult ADHD published by Elsevier, Hogrefe, Schattauer, MWV, Kohlhammer, and Karger. LTvE: Lectures, work shops or travel grants within the last 3 years: Eli Lilly, Medice, Shire, UCB, Servier, and Cyberonics.
The other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ADHD, attention deficit hyperactivity disorder; AQ, autism spectrum quotient; ASD, autism spectrum disorder; cMRI, cerebral magnetic resonance imaging; EEG, electroencephalography; EOG, electrooculography; EQ, empathy quotient; ICA, independent component analysis; IQ, intelligence quotient; IRDA, intermittent rhythmic delta activity; IRTA, intermittent rhythmic theta activity; HV, hyperventilation; MWT-B, multiple-choice vocabulary intelligence test; WURS, Wender Utah Rating Scale.
Accolla, E., Kaplan, P., Maeder-Ingvar, M., Jukopila, S., and Rossetti, A. (2011). Clinical correlates of frontal intermittent rhythmic delta activity (FIRDA). Clin. Neurophysiol. 122, 27–31. doi: 10.1016/j.clinph.2010.06.005
Ahdab, R., and Riachi, N. (2014). Reexamining the added value of intermittent photic stimulation and hyperventilation in routine EEG practice. Eur. Neurol. 71, 93–98. doi: 10.1159/000353650
Amiet, C., Gourfinkel-An, I., Bouzamondo, A., Tordjman, S., Baulac, M., Lechat, P., et al. (2008). Epilepsy in autism is associated with intellectual disability and gender: evidence from a meta-analysis. Biol. Psychiatry 64, 577–582. doi: 10.1016/j.biopsych.2008.04.030
Barker, A., Ng, J., Rittey, C. D., Kandler, R. H., and Mordekar, S. R. (2012). Outcome of children with hyperventilation-induced high-amplitude rhythmic slow activity with altered awareness. Dev. Med. Child Neurol. 54, 1001–1005. doi: 10.1111/j.1469-8749.2012.04337.x
Baron-Cohen, S., and Wheelwright, S. (2004). The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism and normal sex differences. J. Autism Dev. Disord. 34, 163–175. doi: 10.1023/b:jadd.0000022607.19833.00
Baron-Cohen, S., Wheelwright, S., Skinner, R., Martin, J., and Clubley, E. (2001). The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 31, 5–17. doi: 10.1023/A:1005653411471
Berg, A., and Plioplys, S. (2012). Epilepsy and autism: is there a special relationship? Epilepsy Behav. 23, 193–198. doi: 10.1016/j.yebeh.2012.01.015
Binnie, C. D. (2003). Cognitive impairment during epileptiform discharges: is it ever justifiable to treat the EEG? Lancet Neurol. 2, 725–730. doi: 10.1016/s1474-4422(03)00584-2
Brigo, F. (2011). Intermittent rhythmic delta activity patterns. Epilepsy Behav. 20, 254–256. doi: 10.1016/j.yebeh.2010.11.009
Brugha, T. S., McManus, S., Bankart, J., Scott, F., Purdon, S., Smith, J., et al. (2011). Epidemiology of autism spectrum disorders in adults in the community in England. Arch. Gen. Psychiatry 68, 459–465. doi: 10.1001/archgenpsychiatry.2011.38
Chez, M., Chang, M., Krasne, V., Coughlan, C., Kominsky, M., and Schwartz, A. (2006). Frequency of epileptiform EEG abnormalities in a sequential screening of autistic patients with no known clinical epilepsy from 1996 to 2005. Epilepsy Behav. 8, 267–271. doi: 10.1016/j.yebeh.2005.11.001
Clarke, D., Roberts, W., Daraksan, M., Dupuis, A., McCabe, J., Wood, H., et al. (2005). The prevalence of autistic spectrum disorder in children surveyed in a tertiary care epilepsy clinic. Epilepsia 46, 1970–1977. doi: 10.1111/j.1528-1167.2005.00343.x
Cobb, W. A. (1945). Rhythmic slow discharges in the electroencephalogram. J. Neurol. Neurosurg. Psychiatry 8, 65–78. doi: 10.1136/jnnp.8.3-4.65
Ebert, D., Fangmeier, T., Lichtblau, A., Peters, J., Biscaldi-Schäfer, M., and van Tebartz Elst, L. (2013). Asperger-Autismus und hochfunktionaler Autismus bei Erwachsenen: Ein Therapiemanual der Freiburger Autismus-Studiengruppe. 1st Edn. Göttingen: Hogrefe Verlag.
Endres, D., Perlov, E., Feige, B., Fleck, M., Bartels, S., Altenmüller, D., et al. (2016). Electroencephalographic findings in schizophreniform and affective disorders. Int. J. Psychiatry Clin. Pract. 20, 157–164. doi: 10.1080/13651501.2016.1181184
Endres, D., Perlov, E., Maier, S., Feige, B., Nickel, K., Goll, P., et al. (2015). Normal neurochemistry in the prefrontal and cerebellar brain of adults with attention-deficit-hyperactivity disorder. Front. Behav. Neurosci. 9:242. doi: 10.3389/fnbeh.2015.00242
García-Peñas, J. J. (2011). Interictal epileptiform discharges and cognitive impairment in children. Rev. Neurol. 1, S43–S52.
Ghacibeh, G. A., and Fields, C. (2015). Interictal epileptiform activity and autism. Epilepsy Behav. 47, 158–162. doi: 10.1016/j.yebeh.2015.02.025
Giannotti, F., Cortesi, F., Cerquiglini, A., Miraglia, D., Vagnoni, C., Sebastiani, T., et al. (2008). An investigation of sleep characteristics, EEG abnormalities and epilepsy in developmentally regressed and non-regressed children with autism. J. Autism Dev. Disord. 38, 1888–1897. doi: 10.1007/s10803-008-0584-4
Giovanardi Rossi, P., Posar, A., and Parmeggiani, A. (2000). Epilepsy in adolescents and young adults with autistic disorder. Brain Dev. 22, 102–106. doi: 10.1016/s0387-7604(99)00124-2
Hara, H. (2007). Autism and epilepsy: a retrospective follow-up study. Brain Dev. 29, 486–490. doi: 10.1016/j.braindev.2006.12.012
Hartley-McAndrew, M., and Weinstock, A. (2010). Autism spectrum disorder: correlation between aberrant behaviors, EEG abnormalities and seizures. Neurol. Int. 2:e10. doi: 10.4081/ni.2010.e10
Hirota, T., Veenstra-Vanderweele, J., Hollander, E., and Kishi, T. (2014). Antiepileptic medications in autism spectrum disorder: a systematic review and meta-analysis. J. Autism Dev. Disord. 44, 948–957. doi: 10.1007/s10803-013-1952-2
Holmes, G., and Lenck-Santini, P. P. (2006). Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav. 8, 504–515. doi: 10.1016/j.yebeh.2005.11.014
Hughes, J., and Melyn, M. (2005). EEG and seizures in autistic children and adolescents: further findings with therapeutic implications. Clin. EEG Neurosci. 36, 15–20. doi: 10.1177/155005940503600105
Kane, N., Grocott, L., Kandler, R., Lawrence, S., and Pang, C. (2014). Hyperventilation during electroencephalography: safety and efficacy. Seizure 23, 129–134. doi: 10.1016/j.seizure.2013.10.010
Kawasaki, Y., Yokota, K., Shinomiya, M., Shimizu, Y., and Niwa, S. (1997). Brief report: electroencephalographic paroxysmal activities in the frontal area emerged in middle childhood and during adolescence in a follow-up study of autism. J. Autism Dev. Disord. 27, 605–620.
Kim, H., Donnelly, J., Tournay, A., Book, T., and Filipek, P. (2006). Absence of seizures despite high prevalence of epileptiform EEG abnormalities in children with autism monitored in a tertiary care center. Epilepsia 47, 394–398. doi: 10.1111/j.1528-1167.2006.00434.x
Kim, Y. S., Leventhal, B. L., Koh, Y.-J., Fombonne, E., Laska, E., Lim, E.-C., et al. (2011). Prevalence of autism spectrum disorders in a total population sample. Am. J. Psychiatry 168, 904–912. doi: 10.1176/appi.ajp.2011.10101532
Klem, G., Lüders, H., Jasper, H., and Elger, C. (1999). The ten-twenty electrode system of the international federation. The international federation of clinical neurophysiology. Electroencephalogr. Clin. Neurophysiol. 52, 3–6.
Lai, M.-C., Lombardo, M. V., and Baron-Cohen, S. (2014). Autism. Lancet 383, 896–910. doi: 10.1016/S0140-6736(13)61539-1
Lee, W., Girolami, M., and Sejnowski, T. (1999). Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Comput. 11, 417–441. doi: 10.1162/089976699300016719
Lee, B., Smith, T., and Paciorkowski, A. (2015). Autism spectrum disorder and epilepsy: disorders with a shared biology. Epilepsy Behav. 47, 191–201. doi: 10.1016/j.yebeh.2015.03.017
Lehrl, S., Triebig, G., and Fischer, B. (1995). Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol. Scand. 91, 335–345. doi: 10.1111/j.1600-0404.1995.tb07018.x
Lewine, J., Andrews, R., Chez, M., Patil, A., Devinsky, O., Smith, M., et al. (1999). Magnetoencephalographic patterns of epileptiform activity in children with regressive autism spectrum disorders. Pediatrics 104, 405–418. doi: 10.1542/peds.104.3.405
Lord, C., Risi, S., Lambrecht, L., Cook, E. H. Jr., Leventhal, B. L., DiLavore, P. C., et al. (2000). The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J. Autism Dev. Disord. 30, 205–223. doi: 10.1023/A:1005592401947
Lord, C., Rutter, M., and Le Couteur, A. (1994). Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J. Autism Dev. Disord. 24, 659–685. doi: 10.1007/bf02172145
Maier, S., Perlov, E., Graf, E., Dieter, E., Sobanski, E., Rump, M., et al. (2016). Discrete global but no focal gray matter volume reductions in unmedicated adult patients with attention-deficit/hyperactivity disorder. Biol. Psychiatry 80, 905–915. doi: 10.1016/j.biopsych.2015.05.012
Makeig, S., Westerfield, M., Jung, T., Covington, J., Townsend, J., Sejnowski, T., et al. (1999). Functionally independent components of the late positive event-related potential during visual spatial attention. J. Neurosci. 19, 2665–2680.
Miles, J. H., Takahashi, T. N., Bagby, S., Sahota, P. K., Vaslow, D. F., Wang, C. H., et al. (2005). Essential versus complex autism: definition of fundamental prognostic subtypes. Am. J. Med. Genet. A. 135, 171–180. doi: 10.1002/ajmg.a.30590
Mulligan, C. K., and Trauner, D. A. (2014). Incidence and behavioral correlates of epileptiform abnormalities in autism spectrum disorders. J. Autism Dev. Disord. 44, 452–458. doi: 10.1007/s10803-013-1888-6
Pavone, P., Incorpora, G., Fiumara, A., Parano, E., Trifiletti, R. R., and Ruggieri, M. (2004). Epilepsy is not a prominent feature of primary autism. Neuropediatrics 35, 207–210. doi: 10.1055/s-2004-821079
Retz-Junginger, P., Retz, W., Blocher, D., Weijers, H. G., Trott, G. E., Wender, P. H., et al. (2002). Wender utah rating scale (WURS-k) die deutsche kurzform zur retrospektiven erfassung des hyperkinetischen syndroms bei erwachsenen [Wender utah rating scale. The short-version for the assessment of the attention-deficit hyperactivity disorder in adults]. Nervenarzt 73, 830–838. doi: 10.1007/s00115-001-1215-x
Riedel, A., Maier, S., Ulbrich, M., Biscaldi, M., Ebert, D., Fangmeier, T., et al. (2014). No significant brain volume decreases or increases in adults with high-functioning autism spectrum disorder and above average intelligence: a voxel-based morphometric study. Psychiatry Res. 223, 67–74. doi: 10.1016/j.pscychresns.2014.05.013
Rossi, P. G., Parmeggiani, A., Bach, V., Santucci, M., and Visconti, P. (1995). EEG features and epilepsy in patients with autism. Brain Dev. 17, 169–174. doi: 10.1016/0387-7604(95)00019-8
Sheehan, D. V., Lecrubier, Y., Sheehan, K. H., Amorim, P., Janavs, J., Weiller, E., et al. (1998). The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 22–33; quiz 34–57.
Shinnar, S., Rapin, I., Arnold, S., Tuchman, R. F., Shulman, L., Ballaban-Gil, K., et al. (2001). Language regression in childhood. Pediatr. Neurol. 24, 183–189. doi: 10.1016/S0887-8994(00)00266-6
Siddiqui, S. R., Zafar, A., Khan, F. S., and Shaheen, M. (2011). Effect of hyperventilation on electroencephalographic activity. J. Pak. Med. Assoc. 61, 850–852.
Spence, S., and Schneider, M. (2009). The role of epilepsy and epileptiform eegs in autism spectrum disorders. Pediatr. Res. 65, 599–606. doi: 10.1203/PDR.0b013e31819e7168
Tebartz van Elst, L. (2013). Das Asperger-Syndrom im Erwachsenenalter: Und Andere Hochfunktionale Autismus-Spektrum-Störungen. Berlin: Medizinisch Wissenschaftliche Verlagsgesellschaft.
Tebartz van Elst, L., Ebert, D., and Hesslinger, B. (2006). Depression–augmentation or switch after initial SSRI treatment. N. Engl. J. Med. 354, 2611–2613; author reply 2611–2613. doi: 10.1056/nejmc066200
Tebartz van Elst, L., Fleck, M., Bartels, S., Altenmüller, D., Riedel, A., Bubl, E., et al. (2016a). Increased prevalence of intermittent rhythmic delta or theta activity (IRDA/IRTA) in the electroencephalogram (EEG) of patients with borderline personality disorder. Front. Behav. Neurosci. 10:12. doi: 10.3389/fnbeh.2016.00012
Tebartz van Elst, L., Riedel, A., and Maier, S. (2016b). Autism as a disorder of altered global functional and structural connectivity. Biol. Psychiatry 79, 626–627. doi: 10.1016/j.biopsych.2016.02.003
Tebartz van Elst, L., Krishnamoorthy, E. S., Schulze-Bonhage, A., Altenmüller, D.-M., Richter, H., Ebert, D., et al. (2011). Local area network inhibition: a model of a potentially important paraepileptic pathomechanism in neuropsychiatric disorders. Epilepsy Behav. 22, 231–239. doi: 10.1016/j.yebeh.2011.06.016
Tebartz van Elst, L., Maier, S., Fangmeier, T., Endres, D., Mueller, G. T., Nickel, K., et al. (2014). Disturbed cingulate glutamate metabolism in adults with high-functioning autism spectrum disorder: evidence in support of the excitatory/inhibitory imbalance hypothesis. Mol. Psychiatry 19, 1314–1325. doi: 10.1038/mp.2014.62
Tebartz van Elst, L., and Perlov, E. (2013). Epilepsie und Psyche: Psychische Störungen bei Epilepsie–Epileptische Phänomene in der Psychiatrie. 1st Edn. Stuttgart: Kohlhammer.
Tebartz van Elst, L., Pick, M., Biscaldi, M., Fangmeier, T., and Riedel, A. (2013). High-functioning autism spectrum disorder as a basic disorder in adult psychiatry and psychotherapy: psychopathological presentation, clinical relevance and therapeutic concepts. Eur. Arch. Psychiatry Clin. Neurosci. 263, S189–S196. doi: 10.1007/s00406-013-0459-3
Tuchman, R., and Rapin, I. (1997). Regression in pervasive developmental disorders: seizures and epileptiform electroencephalogram correlates. Pediatrics 99, 560–566. doi: 10.1542/peds.99.4.560
Tuchman, R., Rapin, I., and Shinnar, S. (1991). Autistic and dysphasic children. II: Epilepsy. Pediatrics 88, 1219–1225.
van Elst, L. T., Maier, S., Fangmeier, T., Endres, D., Mueller, G. T., Nickel, K., et al. (2014). Magnetic resonance spectroscopy comparing adults with high functioning autism and above average IQ. Mol. Psychiatry 19:1251. doi: 10.1038/mp.2014.160
Keywords: Asperger syndrome, autism spectrum disorder, EEG, adults, IRDA
Citation: Endres D, Maier S, Feige B, Posielski NA, Nickel K, Ebert D, Riedel A, Philipsen A, Perlov E and Tebartz van Elst L (2017) Altered Intermittent Rhythmic Delta and Theta Activity in the Electroencephalographies of High Functioning Adult Patients with Autism Spectrum Disorder. Front. Hum. Neurosci. 11:66. doi: 10.3389/fnhum.2017.00066
Received: 11 August 2016; Accepted: 31 January 2017;
Published: 20 February 2017.
Edited by:
Robert Coben, Integrated Neuroscience Services, USAReviewed by:
Jordi Costa-Faidella, University of Barcelona, SpainCopyright © 2017 Endres, Maier, Feige, Posielski, Nickel, Ebert, Riedel, Philipsen, Perlov and Tebartz van Elst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution and reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dominique Endres, ZG9taW5pcXVlLmVuZHJlc0B1bmlrbGluaWstZnJlaWJ1cmcuZGU=
† These authors have contributed equally to this work.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.