
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hum. Neurosci. , 11 June 2012
Sec. Cognitive Neuroscience
Volume 6 - 2012 | https://doi.org/10.3389/fnhum.2012.00171
This article is part of the Research Topic Factors mediating performance monitoring in humans – from context to personality View all 14 articles
A commentary has been posted on this article:
An Updated Update to Personality and Error Monitoring
People differ considerably with respect to their ability to initiate and maintain cognitive control. A core control function is the processing and evaluation of errors from which we learn to prevent maladaptive behavior. People differ strongly in the degree of error processing, and how errors are interpreted and appraised. In the present study it was investigated whether a correlate of error monitoring, the error negativity (Ne or ERN), is related to personality factors. Therefore, the EEG was measured continuously during a task that provoked errors, and the Ne was tested with respect to its relation to personality traits. The results indicate a substantial trait-like relation of error processing and personality factors: the Ne was more pronounced for subjects scoring low on the “Openness” scale, the “Impulsiveness” scale and the “Emotionality” scale. Inversely, the Ne was less pronounced for subjects scoring low on the “Social Orientation” scale. The results implicate that personality traits related to emotional valences and rigidity are reflected in the way people monitor and adapt to erroneous actions.
From our everyday experience we know that the efficiency of monitoring behavior and coping with undesired outcomes of one's own actions differs considerably between subjects. Furthermore, subjects differ considerably with respect to how they interpret and appraise such outcomes. Especially the committing and processing of errors plays a crucial role with respect to the adequate adaptation of behavior. A correlate of response monitoring has caught considerable attention during the last two decades. Following erroneous responses in a typical choice reaction experiment a sharp negative deflection at fronto-central electrode position in a simultaneous EEG measurement can be observed: the error negativity (Ne, Falkenstein et al., 1990) or error-related negativity (ERN, Gehring et al., 1993). This finding supports the idea of a common response monitoring system, which is involved in monitoring and adapting behavior with respect to external or internal events. The sources of the Ne have been located repeatedly in the anterior cingulate cortex (ACC) (Dehaene et al., 1994; Debener et al., 2005; Hoffmann and Falkenstein, 2010), and it is assumed that the Ne is elicited by striatal dopamine projections (Holroyd and Coles, 2002).
Indeed, several neurophysiological studies indicate a relation of the Ne to the functioning of the dopamine system, for example studies which investigated schizophrenia (Mathalon et al., 2002), attention deficit disorder (Liotti et al., 2005), alcohol consumption (Holroyd and Yeung, 2003), or aging (Nieuwenhuis et al., 2002; Beste et al., 2009; Hoffmann and Falkenstein, 2011).
Most models about the functional role of the Ne assume that it reflects a detection mechanism of undesirable or unexpected events (Holroyd and Coles, 2002; Ridderinkhof et al., 2004; Alexander and Brown, 2011). The Ne is thought to reflect the degree by which an event (be it external or internal) is worse than expected Holroyd and Coles (2002), or unexpected (Alexander and Brown, 2011). A competing hypothesis, the conflict hypothesis suggests that the Ne is the result of a temporal overlap of competing responses. The higher the concordance between all activated possible responses, the higher the conflict and thus the larger is the Ne amplitude (Botvinick et al., 2001, 2004; van Veen et al., 2001). However, several more recent results contradict the conflict hypothesis (e.g., Carbonnell and Falkenstein, 2006; Masaki et al., 2007).
All hypotheses so far assume that the Ne is related to the detection of unexpected or undesirable or conflicting events. Such events need control to be avoided on-line or at least in the future (Hoffmann and Falkenstein, 2012). More recent studies suggest the Ne to reflect the activity of a common response monitoring system (Debener et al., 2005; Hoffmann and Falkenstein, 2010; Roger et al., 2010) evaluating state-goal discrepancies permanently and inducing cognitive control. This activity as reflected in the Ne appears to be integrated in pre-frontal cortex (PFC) functions dealing with the maintaining or execution of cognitive control. It is well known that the PFC, and more specifically the ACC, is central to inhibitory control (Braver et al., 2001). Also, ACC functioning has top down effects even on sensory processes (Sarter et al., 2006; Danielmeier et al., 2011). Thus, ACC and PFC appear to be central to the control of internal states (Devinsky et al., 1995).
Anyway, people differ considerably not only with respect to performance, but also with respect to how they are concerned with making errors. For example people suffering from obsessive compulsive disorder show an enhanced Ne, and its amplitude is related to the severity of symptoms (Gehring et al., 2000). Also, simply being more worried about committing errors is predictive for an enhanced Ne (Hajcak et al., 2003). Furthermore, the Ne appears to be related to negative affective experience (Hajcak et al., 2004). A reversed pattern can be observed in poorly socialized individuals, here the Ne is reduced (Dikman and Allen, 2000).
Taking into account the core structures involved in response monitoring and learning, i.e., dopamine system, amygdala, insula, and PFC (de Bruijn et al., 2009; Gentsch et al., 2009; Jocham and Ullsperger, 2009; Ullsperger et al., 2010), reasonable predictions can be made about the expected morphology of the Ne with respect to the way errors are processed and interpreted by the individual (for a recent review see e.g., Hoffmann and Falkenstein, 2012). For example, in anxious subjects like obsessive subjects and worriers, negative affect arising from committing an error might activate the ACC more than in normal subjects which is reflected in a larger Ne (Gehring et al., 2000; Ernst et al., 2006; Segalowitz and Dywan, 2009). On the other hand, individuals with reduced amygdala activations might have reduced PFC activation to inhibit responses of the nucleus accumbens, which in turn might lead to a reduced Ne (Ernst et al., 2006). Typically, these subjects experience a heightened sensation-seeking tendency. Accordingly, risk-taking is correlated with the Ne amplitude (Santesso and Segalowitz, 2009). Also, subjects scoring high on neuroticism show a larger alteration of the Ne if motivation is being manipulated (Pailing and Segalowitz, 2004). In sum, it appears that the Ne is strongly affected by emotional aspects of personality. Boksem and his coworkers (Boksem et al., 2006) related the Ne to the Behavioral Inhibition System (BIS/BAS) scales, which are based on a biopsychological theory of personality (Gray, 1987, 1989). They found that subjects scoring high on the BIS scale, i.e., who are particularly sensitive to punishment, displayed larger Ne amplitudes. Also, it was already shown that the Ne was increased for subjects scoring high in negative affect and negative emotionality scales, at least at an early time point of the conducted experiment (Luu et al., 2000). Finally, Ruchsow et al. (2005) found the Ne to be related to impulsivity: highly impulsive subjects display a reduced Ne. However, Santesso and Segalowitz (2009) found no relation of the Ne to impulsivity and punishment, but rather to empathy and risk raking.
In sum there does not exist very much work on the relation of error processing and personality, and the results are partly contradictory. Thus, the purpose of the study was to test whether the Ne is related to a wider range of personality factors. Therefore, a classic personality inventory, the Freiburg Personality Inventory (FPI-R, Fahrenberg et al., 1989), was utilized in order to test relations of the Ne and personality factors which conceptually integrate the “normal” range of personality dimensions which are, if showing extreme expressions, indicators of pathologies typically mediating Ne effects (anxiety, violation of social norms, social orientation). Also, these personality dimensions should incorporate emotional reactivity (e.g., empathy, impulsivity). Therefore, the study focused on the personality traits “Openness”, “Social Orientation”, “Emotionality”, and “Impulsiveness” as measured by the FPI-R. Table 1 provides an overview of how these dimensions are conceptualized.
A student sample of 28 healthy young subjects participated (11 females). Subjects were aged between 19 and 30 years (mean = 22.7, SD = 2.5) and gave written informed consent prior to participation. Subjects received 10, –€/h payment for participation. The study was conducted according to the code of ethics of the World Medical Association (Declaration of Helsinki) and was proofed by the local ethics committee. The data of three participants had to be rejected due to too few errors (N = 1) and bad data channels in the EEG (N = 2).
Participants were seated in an ergonomic chair in front of a backprojection beamer display. The distance from display to participants was about 120 cm. The viewing angle was for all target stimuli 1.5°. Stimuli were presented in mild gray on a dark gray background. Subjects were asked to response by a button press of the left or right index finger. The experiment consisted of 720 trials, from which the first 80 trials (training trials) were discarded from further analysis. A short break was provided at 1/3 and 2/3 of the trials. Prior to participation of the EEG experiment all participants filled out the FPI-R and the STAI-T in a separate room. Instructions for the questionnaires were given according to the instruction of the manuals.
For inducing errors a modified global-local task was conducted. During the experiment a combination of two letters (F, H) of different size (small, large) was presented during each trial. The large letters had a viewing angle of 1.5°, and the small ones a viewing angle of 0.5°. The instruction of the participants was to respond to the smaller (local) or larger (global) letter according to a predefined instruction: “attend to the small letter” (local), “attend to the large letter”(global), “ignore the small letter”(global), and “ignore the large letter”(local). This instruction was presented to the subjects as a pre-cue 1000 ms prior to presentation of the target stimulus. Subject had to respond to the to-be-attended letter, or to the not-to-be ignored letter. The attended letter H required a button press of the right index finger, and the letter F a response with the left index finger. This stimulus-response mapping was fully balanced, i.e., half of the participants should respond the other way round.
Following their button press, participants received a feedback according to their performance (“good”, “error”, “too slow”). An adaptive deadline was utilized (Rinkenauer et al., 2004; Hoffmann and Falkenstein, 2010, 2011) in order to minimize speed-accuracy tradeoff. This means that the maximum time during which participants had to respond without receiving the feedback “too slow” was adapted online by estimating the percentage of errors during the last 40 trials. If the error rate was lower than 8% the deadline was decreased by 50 ms; if the error rate was above 12% the deadline was increased by 50 ms. This way the deadline was adapted to the error rate, and not to the mean RT, which prevents reliably from a strong speed-accuracy trade-off.
The EEG was recorded from 60 standard electrode sites using an active electrode system (actiCap©, BrainProducts). EOG was recorded vertically from above and below the right eye (vEOG) and from the outer canthi of both eyes (hEOG). EEG and EOG were digitized at 1000 Hz and the recording was conducted using an average reference amplifier system (QuickAmp©, BrainProducts).
The EEG was analyzed using EEGLAB (Delorme and Makeig, 2004) and Matlab©. Initially, data were filtered (0.1–30 Hz) using a phase-shift free IIR Butterworth filter. Subsequently, the data were segmented relative to the button press (–0.5 to 1.0 s) and pruned automatically from artifacts by a statistical thresholding procedure (Delorme et al., 2007). Then the EEG was re-referenced to linked mastoids. For removal of ocular artifacts independent component analysis was conducted by applying extended infomax (Lee et al., 1999) with default settings as implemented in EEGLAB. Ocular artifacts were removed by applying an objective automatic artifact removal algorithm which combines spatial and temporal features of independent components for artifact detection (Mognon et al., 2010).
Following this the automated thresholding protocol was conducted a second time in order to remove residual artifacts. Finally, event-related potentials were calculated for correct and erroneous responses, respectively. The Ne was measured as a difference wave between correct and erroneous response-related activity. From this difference wave the Ne was extracted as a peak-to-peak measure at FCz, i.e., as the difference between the local maximum in the time range from –50 to 0 ms and the local minimum in the time range from 0 to 100 ms. The rationale for this was to yield an estimate of the error-specific variation in the post-response ERP. In the following this difference is termed Ne. This measure was correlated with the personality questionnaire scores.
Additionally, sLORETA estimations of the source of variation between the response-related ERPs of correct and erroneous responses corresponding to the Ne peak were conducted. sLORETA can be used to estimate the sources generating the variance between two experimental conditions. sLORETA is an improved version of LORETA. The main difference is that sources are estimated based on standardized current density allowing a more precise source localization than the previous LORETA-method (Pascual-Marqui, 2002; Pascual-Marqui et al., 2002). sLORETA was performed with the scalp maps of the Ne and corresponding time window of the correct response to find the generators of these maps. This was done by comparison of the voxel-based sLORETA-images (6239 voxels at a spatial resolution of 5 mm; MNI template) of both response types. Statistical quantification was conducted by using the sLORETA-built-in voxelwise randomization tests (5000 permutations) based on statistical non-parametric mapping (SnPM), corrected for multiple comparisons (Nichols and Holmes, 2002). The voxels with significant differences (p < 0.05) were located in specific brain regions.
Reaction time data were analyzed by means of repeated measures ANOVAs with the factors accuracy (correct vs. erroneous responses) and cue (global vs. local). Only RTs in the time range from 100 to 1000 ms were included in the analysis. Error rates were analyzed by a T-test. Effect sizes are provided by means of partial eta squared (η2p) and Cohen's d.
For assessing personality dimensions the Freiburg Personality Inventory was utilized in its revised version (FPI-R, Fahrenberg et al., 1989). The FPI-R is a structured objective measure of personality traits. It consists of 128 items which are grouped on 10 primary scales and two secondary scales. The former consist of the dimensions “Life satisfaction”, “Social orientation”, “Achiement orientation”, “Inhibitedness”, “Impulsiveness”, “Aggressiveness”, “Strain”, “Somatic complaints”, “Health concern”, and “Frankness/Openness”. The two secondary scales consist of “Extraversion” and “Emotionality,” which shall measure Eysencks's Extraversion/Introversion and Neuroticism constructs. The FPI-R is one of the most used and best assessed personality inventories in the German language area and yields sufficient reliability (Cronbachs' alpha between 0.73 and 0.83). All analyses were restricted to personality factors from which it can be assumed that they are conceptually related to cognitive control, which are “Openness”, “Social Orientation”, “Emotionality”, and Impulsiveness”. Due to the sample size, which was not sufficient for multiple regression analysis, the sample was divided into high and low scoring subjects by a median split of the questionnaire data. Therefore, “high” and “low” groups did not consist exactly of the same number of subjects (which was due to a slight skewness in the data and the fact that the final sample consisted of 25 subjects). Subsequently, the relation of Ne and personality factors was assessed by means of Welch T-Tests that account for different N and unequal variances. Reported are always T-Values and corrected degrees of freedom. Additionally, effect sizes are provided by means of Cohen's d in order to ease power estimations.
Repeated measures ANOVAs revealed a significant effect for the factors cue [F(1, 24) = 24.76, p = 4.42−05, η2p = 0.51] and accuracy [F(1, 24) = 27.48, p = 2.25e−05, η2p = 0.53]. However, a significant interaction of cue and accuracy [F(1, 24) = 36.23, p = 3.25e−06, η2p = 0.6] revealed that RTs did not differ between the global and local conditions if the responses were erroneous, but they were considerably longer for the local condition of correct responses compared to correct responses in the global conditions (compare Figure 1). Error rates were lower for the global compared to the local condition [4.6 vs. 6%; t(24) = −2.36, p = 0.013, d = 0.47].
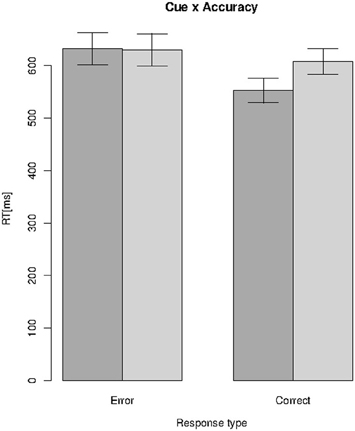
Figure 1. Reaction times (RT) as a function of Cue (global = dark gray, local = gray) and response type (errors vs. correct), bars = standard error of the mean.
There was a clear error negativity observable in the data [Figure 2; t(24) = 8.81, p = 2.76e−09, d = 1.32]. sLORETA localized the Ne complex in the ACC [Tal(x,y,z) = Tal(x,y,z) = −10, 35, 25, t < −4.3, p = 0.01]. In this task, the Ne amplitude was negatively correlated with the error rate [r = −0.53, t(23) = −2.99, p = 0.006].
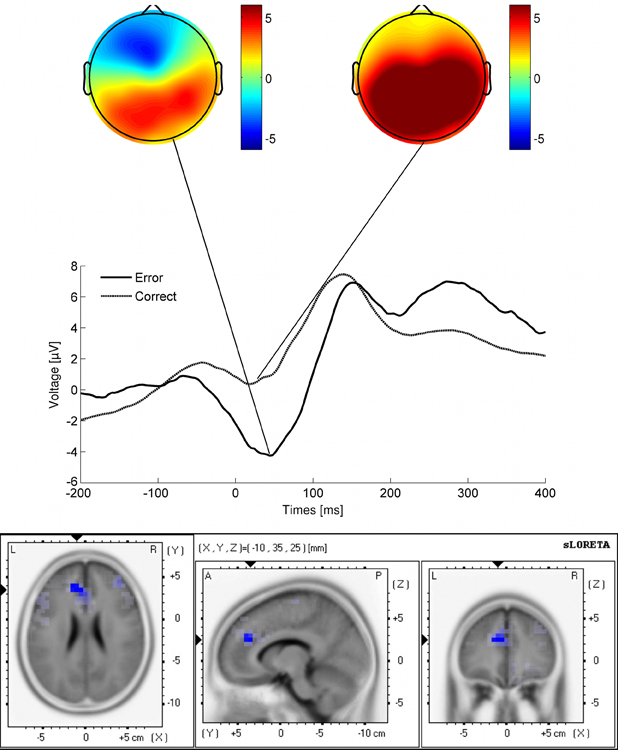
Figure 2. Response-related potentials for correct and erroneous responses (response: time point zero) as well as corresponding topographies at the time point of maximum deflection in the Ne. Lower panel: sLORETA estimate of the Ne (Tal(x,y,z) = −10, 35, 25). The sLORETA estimations of all participants' topographies were projected on an averaged normalized brain (MNI template).
The Ne was larger for subjects scoring high vs. low on the “Social Orientation” scale [t(21.71) = 2.29, p = 0.01, d = 0.91] and smaller for subjects scoring high vs. low on the scales “Openness” [t(18.6) = 3.01, p = 0.003, d = 1.22], “Impulsiveness” [t(17.889) = 2.19, p = 0.02, d = 0.88] and “Emotionality” [t(17.75) = 1.92, p = 0.03, d = 0.78]. Figure 3 summarizes these results.
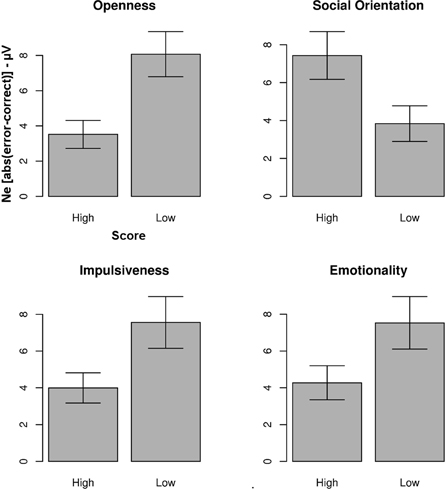
Figure 3. Average absolute values of the error-correct difference for four personality dimensions according to the FPI-R (bars = standard errors of the mean). The Ne was more pronounced for subjection scoring low on the “Openness” scale, the “Impulsiveness” scale and the “Emotionality” scale. Inversely, the Ne was less pronounced for subjects scoring low on the “Social Orientation” scale.
However, since the error rate was correlated significantly with the Ne, and the error rate itself might well be correlated with personality traits further analyses were necessary. Therefore, partial correlations between Ne and personality dimensions, controlled for error rates, were calculated. This was done by calculating a linear regression between Ne and error rate (Ne = b*error rate) and correlating the residuals of this linear fit with the personality scores. Again, the Ne was less pronounced for subjects scoring high on the scales “Openness” [t(18.1) = 1.833, p = 0.04, d = 0.74], “Impulsiveness” [t(19.028) = 2.304, p = 0.01, d = 0.93], and “Emotionality” [t(17.97) = 1.89, p = 0.037, d = 0.77]. However, the relation of Ned and “Social Orientation” did only show a trend toward significance [t(21.57) = 1.34, p = 0.096, d = 0.53].
The core result of the present study is that the Ne was clearly related to personality traits closely associated with social and emotional dimensions as well as behavioral flexibility. As such the Ne was correlated with the factors “Openness”, “Impulsiveness”, “Emotionality”, and “Social Orientation”. After cancelling out the variance due to error rate, the first three dimensions still correlated with the Ne. The large effect sizes (Cohen's d > 0.7 on average) indicate a strong relation, which cannot be due to spurious correlations.
This is, at least to our knowledge, the first time that such strong relations are shown. Although a moderate relation of the Ne and personality factors has already been shown (e.g., Pailing and Segalowitz, 2004; Santesso and Segalowitz, 2009), this pattern is a bit heterogeneous. This might be due to different tasks, measurement systems and more importantly, different personality constructs. For example the study of Pailing and Segalowitz (2004) utilized the NEO-PI-R that measures five more global personality dimensions (with comparable reliability like the FPI-R), whereas the FPI-R measures 12 dimensions. Also, the number of participants (N = 18) was relatively low for regression analyses. Though the present study tested at least 25 subjects this number is still not sufficient for an adequate multivariate testing, i.e., multiple regression analyses. Therefore, we focused on “dimensions of interest” in one specific test that has been tested extensively in the German area. For more general analyses with higher statistical power more participants are necessary (Tabachnick and Fidell, 2001). Also, it has to be kept in mind that personality tests are sensitive to cultural influences. Furthermore, personality questionnaires do not have the same reliability as performance measures. This difference in the measurement error might lead to false insignificant correlations: if one of two samples is less reliable than the other one, the correlation of both might be underestimated. From the Ne it is known that it is quite reliable across measurements and within subjects and thus it can be interpreted as a trait-like correlate of ACC integrity (Segalowitz et al., 2010; Weinberg and Hajcak, 2011). Moreover, the reported Ne reliability was between 0.56 and 0.67, which is compared to modern psychological tests relatively low. For example the reliability of the test utilized in the study reported here lies between 0.73 and 0.81 (according to the test manual). These coefficients are typical for good scales in this research area. Also, it has to be kept in mind that in contrast to psychometric tests the measurement of the Ne is not standardized, nor exist norm values. Furthermore, the acquisition and analysis protocol differs from laboratory to laboratory. Thus, in the case of the Ne it is not clear where the measurement error comes from: is the Ne itself less reliable or is this reliability due to a measurement error? This question cannot be answered from concepts of classical test theory (like reliability). In sum this might be one reason why there exist only few studies reporting relations of the Ne and personality factors. Nevertheless, it can be expected that the Ne, as a correlate of response monitoring per se, should be correlated with personality factors which incorporate the evaluation of one's own behavior.
What is surprising in the present study is the relation between the Ne and several personality dimensions. However, this becomes clearer if having a look at the inter-correlation of the personality factors: of course they are, at least to some degree, conceptually related to each other. Thus, with respect to the relation of the measured personality dimensions these results are not so unexpected. Subjects scoring low in the “Openness”, “Impulsivity”, and “Emotionality” scale showed a larger Ne, which is in line with results from clinical samples and previous studies dealing with the relation of personality and error monitoring.
For recapitulation: “Openness/frankness” refers to a personality ranging from oriented toward social norms (following norms, introverted, making a good impression) to admitting violations of social norms or being unconventional. In the present study, subjects that tended to admit social norms or to be unconventional showed decreased error negativities compared to subjects who reported of being oriented toward social norms. According to the authors of the FPI-R, high scoring subjects just don't care very much about conventions (Fahrenberg et al., 1989, p. 77). The analogy to the results from studies dealing with psychopathy lies at hand: in subjects with a psychopathy diagnosis, the Ne is reduced (Munro et al., 2007; von Borries et al., 2010). Typically, psychopaths show a lack of interest in social norms.
With respect to the trait “Impulsiveness,” which ranges from calm, self-controlled to aggressive and spontaneous the results indicate that subjects that reported being self-controlled showed enhanced error negativities, which appears also to be predictable: maybe these subjects are able to gain more “self-control,” which is reflected in their capability of monitoring errors.
With respect to “Emotionality,” which ranges from emotionally stable, controlled to being a bit anxiously and being more pessimistic the results indicate that subjects which reported of being more emotionally stable showed increased Ne amplitudes. This appears to be not in line with studies showing enhanced Ne amplitudes for subjects suffering from anxiety disorders or depression (Olvet and Hajcak, 2008; Weinberg et al., 2010). However, in sum it may be that low scoring subjects (in all three scales) do not engage to a comparable degree like high scoring subjects in the task. Here further research is necessary, for example with studies experimentally varying task engagement. Also, it has to be kept in mind, that the personality traits have to be distinguished from pathologies and thus only cautious conclusion might be made.
The final scale, “social orientation” ranges from being self-concerned, being uncooperative to feeling social responsible and being helpful. Here the data pattern is inversed to the one that could be observed in the other scales: subjects that scored low, i.e., who reported being more helpful than being self-concerned showed enhanced error negativities. If this scale is interpreted as a scale that might capture a concept like empathy, this data pattern reminds of the results of Santesso and Segalowitz (2009). They showed that the Ne was enhanced in subjects scoring high on an empathy scale.
In a nutshell, it could be concluded that since the personality variables measured herein are trait measures, the found effects might reflect the degree to which individuals are concerned with the outcome of their behaviors. Thus, the Ne might also reflect trait-like emotional reactivity.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Alexander, W. H., and Brown, J. W. (2011). Medial prefrontal cortex as an action-outcome predictor. Nat. Neurosci. 14, 1338–1344.
Beste, C., Willemssen, R., Saft, C., and Falkenstein, M. (2009). Error processing in normal aging and in basal ganglia disorders. Neuroscience 159, 143–149.
Boksem, M. A., Tops, M., Wester, A. E., Meijman, T. F., and Lorist, M. M. (2006). Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Res. 1101, 92–101.
Botvinick, M. M., Braver, T. S., Barch, D. M., Carter, C. S., and Cohen, J. D. (2001). Conflict monitoring and cognitive control. Psychol. Rev. 108, 624–652.
Botvinick, M. M., Cohen, J. D., and Carter, C. S. (2004). Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. 8, 539–546.
Braver, T. S., Barch, D. M., Gray, J. R., Molfese, D. L., and Snyder, A. (2001). Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cereb. Cortex 11, 825–836.
Carbonnell, L., and Falkenstein, M. (2006). Does the error negativity reflect the degree of response conflict? Brain Res. 1095, 124–130.
Danielmeier, C., Eichele, T., Forstmann, B. U., Tittgemeyer, M., and Ullsperger, M. (2011). Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. J. Neurosci. 31, 1780–1789.
de Bruijn, E. R. A., de Lange, F. P., Von Cramon, D. Y., and Ullsperger, M. (2009). When errors are rewarding. J. Neurosci. 29, 12183–12186.
Debener, S., Ullsperger, M., Siegel, M., Fiehler, K., Von Cramon, D. Y., and Engel, A. K. (2005). Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J. Neurosci. 25, 11730–11737.
Dehaene, S., Posner, M., and Tucker, D. M. (1994). Localization of an neural system for error detection and compensation. Psychol. Sci. 5, 303–305.
Delorme, A., and Makeig, S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 134, 9–21.
Delorme, A., Sejnowski, T., and Makeig, S. (2007). Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. Neuroimage 34, 1443–1449.
Devinsky, O., Morrell, M. J., and Vogt, B. A. (1995). Contributions of anterior cingulate cortex to behaviour. Brain 118(Pt 1), 279–306.
Dikman, Z. V., and Allen, J. J. (2000). Error monitoring during reward and avoidance learning in high- and low-socialized individuals. Psychophysiology 37, 43–54.
Ernst, M., Pine, D. S., and Hardin, M. (2006). Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 36, 299–312.
Fahrenberg, J., Hampel, R., and Selg, H. (1989). Das Freiburger Persönlichkeitsinventar FPI. Revidierte Fassung FPI-R. Göttingen, Toronto, Zürich: Verlag für Psychologie, Hogrefe.
Falkenstein, M., Hohnsbein, J., Hoomann, J., and Blanke, L. (1990). “Effects of errors in choice reaction tasks on the {ERP} under focused and divided attention,” in Psychophysiological Brain Research, eds C. H. M. Brunia, A. W. K. Gaillard, and A. Kok (Tilburg: Tilburg University Press), 192–195.
Gehring, W. J., Goss, B., Coles, M. G. H., Meyer, D. E., and Donchin, E. (1993). A neural system for error-dedection and compensation. Psychol. Sci. 4, 385–390.
Gehring, W. J., Himle, J., and Nisenson, L. G. (2000). Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol. Sci. 11, 1–6.
Gentsch, A., Ullsperger, P., and Ullsperger, M. (2009). Dissociable medial frontal negativities from a common monitoring system for self- and externally caused failure of goal achievement. Neuroimage 47, 2023–2030.
Gray, J. A. (1987). “The neuropsychology of emotion and personality,” in Cognitive Neurochemistry, eds S. M. Stahl, S. D. Iverson, and E. C. Goodman (Oxford: Oxford University Press), 171–190.
Gray, J. A. (1989). “Fundamental systems of emotion in the mammalian brain,” in Coping with Uncertainty: Behavioral and Developmental Perspectives, ed D. S. Palermo (Hillsdale, NJ: Lawrence Erlbaum), 173–195.
Hajcak, G., McDonald, N., and Simons, R. F. (2003). Anxiety and error-related brain activity. Biol. Psychol. 64, 77–90.
Hajcak, G., McDonald, N., and Simons, R. F. (2004). Error-related psychophysiology and negative affect. Brain Cogn. 56, 189–197.
Hoffmann, S., and Falkenstein, M. (2010). Independent component analysis of erroneous and correct responses suggests online response control. Hum. Brain Mapp. 31, 1305–1315.
Hoffmann, S., and Falkenstein, M. (2011). Aging and error processing: age related increase in the variability of the error-negativity is not accompanied by increase in response variability. PLoS ONE 6:e17482. doi: 10.1371/journal.pone.0017482
Hoffmann, S., and Falkenstein, M. (2012). Predictive information processing in the brain: errors and response monitoring. Int. J. Psychophysiol. 83, 208–212.
Holroyd, C. B., and Coles, M. G. (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol. Rev. 109, 679–709.
Jocham, G., and Ullsperger, M. (2009). Neuropharmacology of performance monitoring. Neurosci. Biobehav. Rev. 33, 48–60.
Lee, T. W., Girolami, M., and Sejnowski, T. J. (1999). Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Comput. 11, 417–441.
Liotti, M., Pliszka, S. R., Perez, R., Kothmann, D., and Woldorff, M. G. (2005). Abnormal brain activity related to performance monitoring and error detection in children with ADHD. Cortex 41, 377–388.
Luu, P., Collins, P., and Tucker, D. M. (2000). Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. J. Exp. Psychol. Gen. 129, 43–60.
Masaki, H., Falkenstein, M., Sturmer, B., Pinkpank, T., and Sommer, W. (2007). Does the error negativity reflect response conflict strength? Evidence from a simon task. Psychophysiology 44, 579–585.
Mathalon, D. H., Fedor, M., Faustman, W. O., Gray, M., Askari, N., and Ford, J. M. (2002). Response-monitoring dysfunction in schizophrenia: an event-related brain potential study. J. Abnorm. Psychol. 111, 22–41.
Mognon, A., Jovicich, J., Bruzzone, L., and Buiatti, M. (2010). ADJUST: an automatic EEG artifact detector based on the joint use of spatial and temporal features. Psychophysiology 48, 229–240.
Munro, G. E., Dywan, J., Harris, G. T., McKee, S., Unsal, A., and Segalowitz, S. J. (2007). ERN varies with degree of psychopathy in an emotion discrimination task. Biol. Psychol. 76, 31–42.
Nichols, T. E., and Holmes, A. P. (2002). Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 15, 1–25.
Nieuwenhuis, S., Ridderinkhof, K. R., Talsma, D., Coles, M. G., Holroyd, C. B., Kok, A., and van der Molen, M. W. (2002). A computational account of altered error processing in older age: dopamine and the error-related negativity. Cogn. Affect. Behav. Neurosci. 2, 19–36.
Olvet, D. M., and Hajcak, G. (2008). The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clin. Psychol. Rev. 28, 1343–1354.
Pailing, P. E., and Segalowitz, S. J. (2004). The error-related negativity as a state and trait measure: motivation, personality, and ERPs in response to errors. Psychophysiology 41, 84–95.
Pascual-Marqui, R. D. (2002). Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 24(Suppl. D), 5–12.
Pascual-Marqui, R. D., Esslen, M., Kochi, K., and Lehmann, D. (2002). Functional imaging with low-resolution brain electromagnetic tomography (LORETA): a review. Methods Find. Exp. Clin. Pharmacol. 24(Suppl. C), 91–95.
Ridderinkhof, K. R., Ullsperger, M., Crone, E. A., and Nieuwenhuis, S. (2004). The role of the medial frontal cortex in cognitive control. Science 306, 443–447.
Rinkenauer, G., Osman, A., Ulrich, R., Muller-Gethmann, H., and Mattes, S. (2004). On the locus of speed-accuracy trade-off in reaction time: inferences from the lateralized readiness potential. J. Exp. Psychol. Gen. 133, 261–282.
Roger, C., Benar, C. G., Vidal, F., Hasbroucq, T., and Burle, B. (2010). Rostral cingulate zone and correct response monitoring: ICA and source localization evidences for the unicity of correct- and error-negativities. Neuroimage 51, 391–403.
Ruchsow, M., Spitzer, M., Gron, G., Grothe, J., and Kiefer, M. (2005). Error processing and impulsiveness in normals: evidence from event-related potentials. Brain Res. Cogn. Brain Res. 24, 317–325.
Santesso, D. L., and Segalowitz, S. J. (2009). The error-related negativity is related to risk taking and empathy in young men. Psychophysiology 46, 143–152.
Sarter, M., Gehring, W. J., and Kozak, R. (2006). More attention must be paid: the neurobiology of attentional effort. Brain Res. Rev. 51, 145–160.
Segalowitz, S. J., and Dywan, J. (2009). Individual differences and developmental change in the ERN response: implications for models of ACC function. Psychol. Res. 73, 857–870.
Segalowitz, S. J., Santesso, D. L., Murphy, T. I., Homan, D., Chantziantoniou, D. K., and Khan, S. (2010). Retest reliability of medial frontal negativities during performance monitoring. Psychophysiology 47, 260–270.
Tabachnick, B. G., and Fidell, L. S. (2001). Using Multivariate Statistics. Boston, MA: Allyn and Bacon.
Ullsperger, M., Harsay, H. A., Wessel, J. R., and Ridderinkhof, K. R. (2010). Conscious perception of errors and its relation to the anterior insula. Brain Struct. Funct. 214, 629–643.
van Veen, V., Cohen, J. D., Botvinick, M. M., Stenger, V. A., and Carter, C. S. (2001). Anterior cingulate cortex, conflict monitoring, and levels of processing. Neuroimage 14, 1302–1308.
von Borries, A. K., Brazil, I. A., Bulten, B. H., Buitelaar, J. K., Verkes, R. J., and de Bruijn, E. R. (2010). Neural correlates of error-related learning deficits in individuals with psychopathy. Psychol. Med. 40, 1559–1568.
Weinberg, A., and Hajcak, G. (2011). Longer term test-retest reliability of error-related brain activity. Psychophysiology 48, 1420–1425.
Keywords: personality, response monitoring, error monitoring, error negativity, EEG
Citation: Hoffmann S, Wascher E and Falkenstein M (2012) Personality and error monitoring: an update. Front. Hum. Neurosci. 6:171. doi: 10.3389/fnhum.2012.00171
Received: 28 February 2012; Accepted: 25 May 2012;
Published online: 11 June 2012.
Edited by:
Christian Bellebaum, Ruhr University Bochum, GermanyReviewed by:
Marco Steinhauser, University of Konstanz, GermanyCopyright: © 2012 Hoffmann, Wascher and Falkenstein. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Sven Hoffmann, Leibniz Research Centre for Working Environment and Human Factors, Ardeystr. 67, 44139 Dortmund, Germany. e-mail:c2hvZmZtYW5uQGlmYWRvLmRl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.