
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Hematol., 04 March 2025
Sec. Blood Cancer
Volume 4 - 2025 | https://doi.org/10.3389/frhem.2025.1552200
 Andrea Serafin1
Andrea Serafin1 Alessandro Cellini1
Alessandro Cellini1 Francesco Angotzi1
Francesco Angotzi1 Valeria Ruocco1
Valeria Ruocco1 Arianna Bevilacqua1
Arianna Bevilacqua1 Marco Pizzi2
Marco Pizzi2 Livio Trentin1*
Livio Trentin1* Andrea Visentin1*
Andrea Visentin1*The management of accelerated chronic lymphocytic leukemia (A-CLL), an aggressive and rare variant of CLL characterized by increased proliferation and histologically defined features, remains a challenging area with limited evidence. A-CLL is distinguished by its intermediate behavior between indolent CLL and Richter Transformation (RT), often associated with high-risk genetic markers and rapid disease progression. Existing data from the era of targeted therapies are scarce, complicating the standardization of treatment approaches and prognostic assessments. While novel agents such as Bruton Tyrosine Kinase inhibitors (BTKi) and venetoclax have shown promise in individual cases, comprehensive evaluations in A-CLL are lacking. We present two cases of CLL that progressed through various phases, including the accelerated phase and suspected RT. These cases highlight the distinct clinical features of A-CLL, including elevated LDH levels, high SUV on PET-CT, and adverse genetic markers, alongside the limitations of traditional chemoimmunotherapy. Importantly, we detail the novel use of a triplet therapy combining a non-covalent BTKi, venetoclax, and rituximab, demonstrating promising outcomes that provide valuable insights into managing this aggressive CLL variant in the era of targeted therapies.
Chronic lymphocytic leukemia (CLL) is an indolent lymphoproliferative disorder. However, in 2% to 10% of cases, it can exhibit histological features of aggressiveness, a condition known as accelerated CLL (A-CLL). This entity is distinct from Richter’s transformation (RT).
A-CLL was first defined in 1988, but only in 2010 it has been recognized as a distinct histological subtype with aggressive clinical behavior (1, 2). The incidence of A-CLL remains unknown, since the diagnosis requires lymph node biopsy, which is not included routinely in the disease workup (3). Currently, there are no radiological or laboratory markers that can definitively identify A-CLL, making diagnosis a complex task that relies heavily on the expertise of specialized hematopathologist.
The established histological criteria for diagnosis focus on the presence of proliferation centers (PC) in lymph nodes, assessing their size and activity. Diagnosis requires at least one of the following three morphological criteria: 1) Proliferation centers that are larger than a ×20 microscopic field; 2) Increased mitotic activity, with more than 2.4 mitotic figures per PC; 3) a Ki-67 index exceeding 40% per PC (2, 4) (Figure 1).
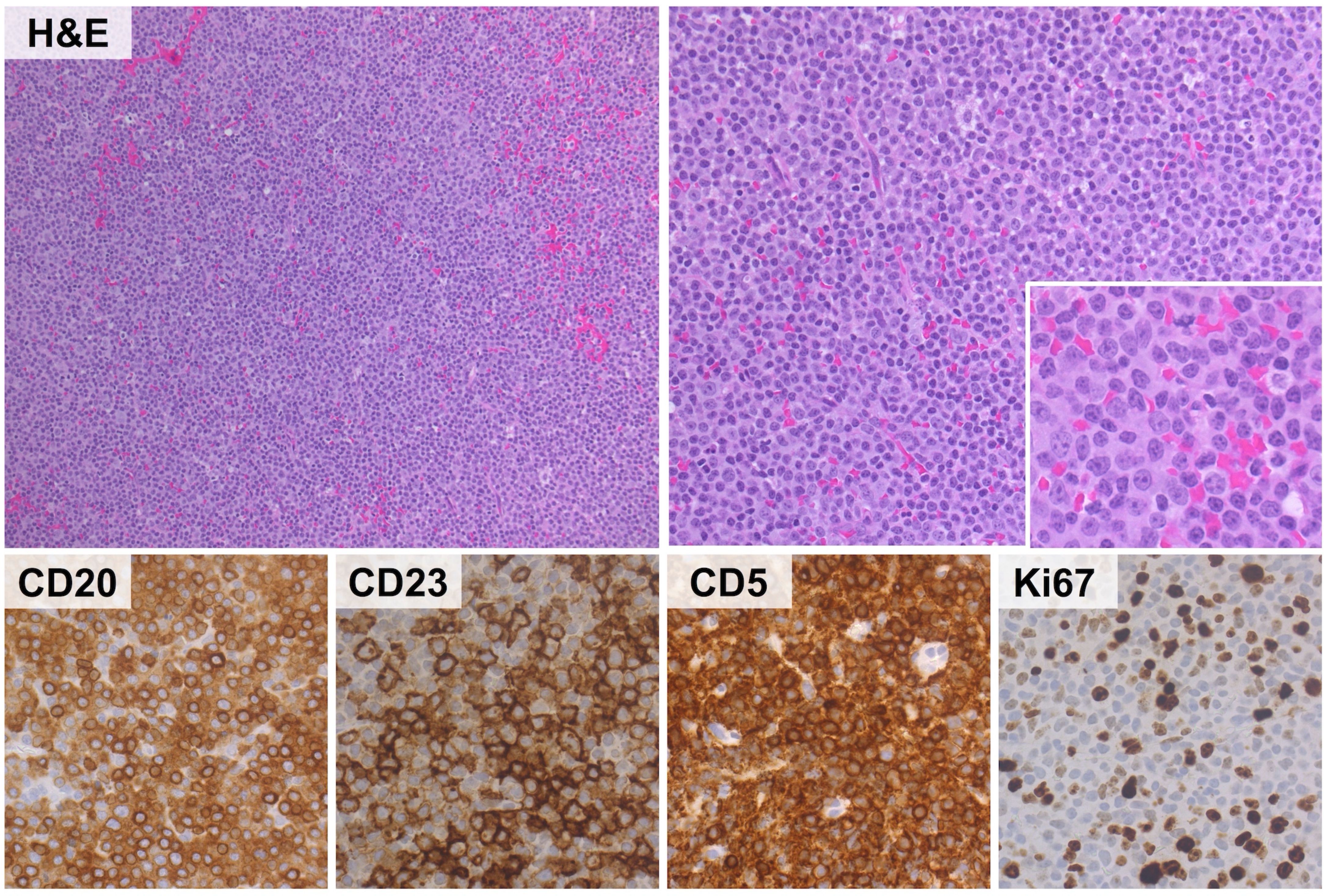
Figure 1. Representative histological features of aCLL. The lymphoid infiltrate discloses a vaguely nodular to diffuse growth pattern (upper left panel) and consists of small to medium-sized lymphocytes with round nuclei, open chromatin and small, centrally located nuclei. Mitoses are readily found (upper right panel and insert). The phenotype of the neoplastic population recapitulates that of conventional CLL (positivity for CD20, CD23, CD5) with moderate proliferation index (Ki67 stain: 20%). (H&E and immunoperoxidase stains; original magnification: 10x, 20x and 40x).
A-CLL is marked by rapidly progressing and widespread lymphadenopathy. While levels of lactate dehydrogenase (LDH) and Beta-2-microglobulin are higher compared to typical CLL cases, they do not reach the values seen in Richter Transformation (5). Additionally, the utility of 18FDG PET-CT scans in this context remains unclear, although elevated standardized uptake values (SUV) have been observed.
A-CLL often arises in the relapsed setting, with disease progression frequently associated with the acquisition of high-risk genetic alterations such as TP53 mutations, del(17p), and complex karyotypes. These genomic lesions may develop over the course of the disease rather than being present at initial diagnosis (5). Patients with A-CLL experience a worse prognosis and shorter overall survival compared to those with CLL treated in the era of chemo-immunotherapy (CIT); however, data coming from the era of targeted therapies are limited and based only on few cases reported. Successful treatment of A-CLL has been reported with Bruton Tyrosine Kinase inhibitors (BTKi) such as ibrutinib and acalabrutinib as well as venetoclax-rituximab (VenR) combination (Table 1) (6–9).
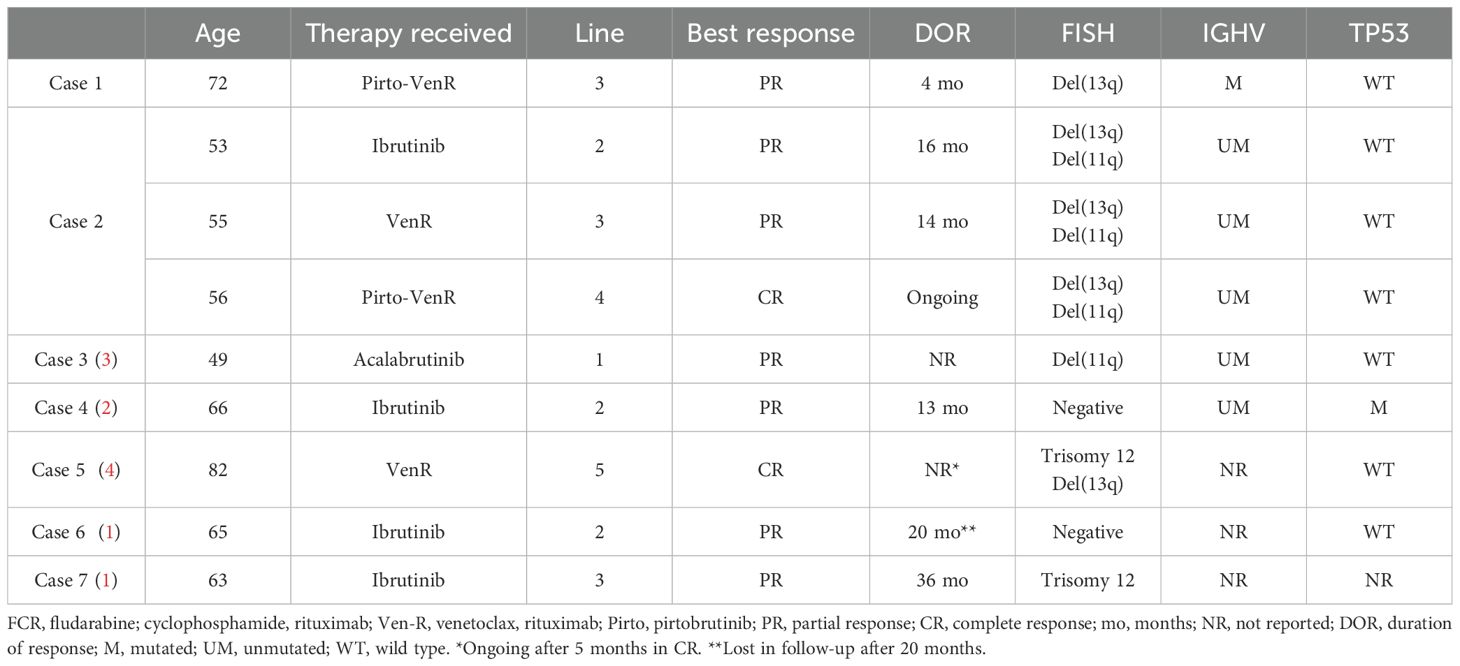
Table 1. Clinical and biological characteristics of the cases presented and review of the existing literature on A-CLL treated with novel therapies (1–4).
We examine the cases of two patients with CLL who experienced disease progression through various phases, including the accelerated phase and suspected RT. By detailing the clinical presentation, diagnostic evaluations, therapeutic interventions, and disease progression, we try to underscore the complexities and advances in managing CLL, particularly in high-risk scenarios. The insights gained from this case can inform future therapeutic strategies aimed at improving patient outcomes in A-CLL. Cases reported are graphically summarized in Figure 2.
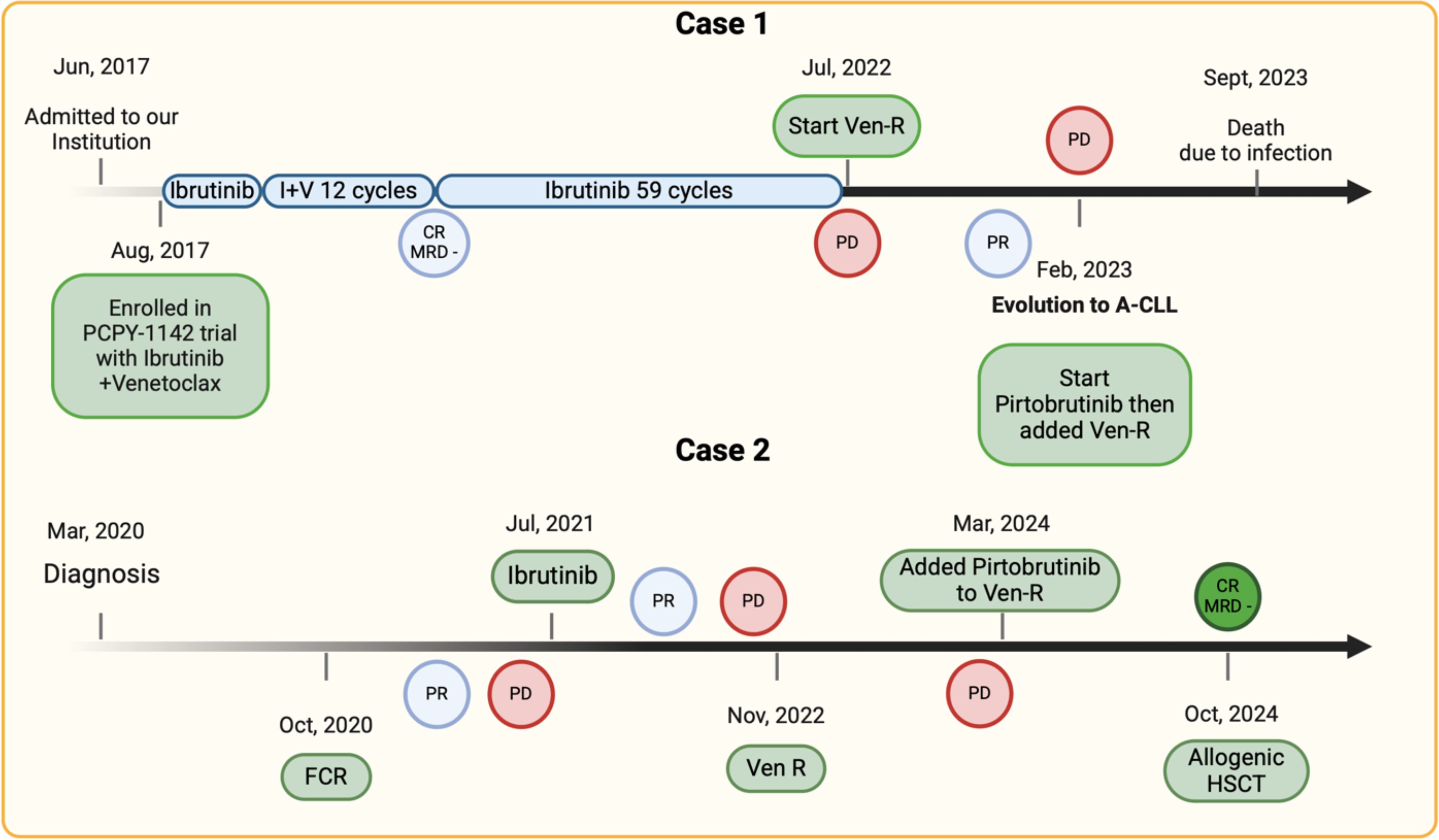
Figure 2. Treatment timeline of the cases presented. PR, partial response; CR, complete response; MRD, minimal residual disease; PD, progressive disease; Ven-R, venetoclax-rituximab; FCR, fludarabine-cyclophosphamide-rituximab; A-CLL, accelerated chronic lymphocytic leukemia.
In June 2017, a 69-year-old male patient with chronic lymphocytic leukemia (CLL) was admitted to our institution, presenting with lymphocytosis (96.20 × 10^9/L), while other hematological parameters were within normal limits. The patient exhibited numerous lymphadenopathies both above and below the diaphragm, with the largest measuring up to 6 cm. Additionally, there was hepatosplenomegaly. Prognostic factor analysis revealed a borderline mutated IGHV3-21 rearrangement (97.92%), del(13q) by FISH, and no TP53 mutations via Sanger sequencing. Cytogenetic examination identified five chromosomal alterations, indicating a high complex karyotype. The patient was diagnosed with CLL Rai II/Binet B and initially began observation. However, less than a year later, the patient developed progressive lymph node enlargement and was treated with a combination of ibrutinib and venetoclax as part of a clinical trial. He received ibrutinib monotherapy for three cycles, followed by the combination of ibrutinib and venetoclax for 12 cycles, achieving a partial response (PR) with some residual lymph nodes measuring 2 cm and detectable minimal residual disease (MRD4, cutoff 10^-4) in peripheral blood, as assessed by flow cytometry. The patient then continued ibrutinib therapy, ultimately achieving a complete response (CR) with undetectable MRD4.
In March 2022, after 59 cycles of ibrutinib, the patient experienced his first relapse, characterized by the recurrence of lymphocytosis and mild thrombocytopenia. A new total body CT scan did not reveal any lymphadenopathy or hepatosplenomegaly. However, cytogenetic examination confirmed the selection of a clone from the previously described population, with del(13q) and no TP53 disruptions.
After four months, due to symptomatic lymph node enlargement, the patient began second-line therapy with Venetoclax-Rituximab in July 2022. Initially, he responded clinically, but by February 2023, elevated LDH levels and new lymph node enlargement raised suspicion of RT. A PET/CT scan revealed numerous hypermetabolic adenopathies (SUV max 20) involving both major lymphatic regions above and below the diaphragm. An excisional biopsy of a laterocervical lymph node in February 2023 showed nodal involvement of a peripheral B-cell lymphoproliferative disorder, predominantly consisting of paraimmunoblasts with intermediate proliferative kinetics (Ki-67 40%). The growth pattern in confluent nodules, alongside the cytology of the lymphoid population, confirmed the diagnosis of A-CLL. After a multidisciplinary discussion, therapy with pirtobrutinib was initiated in March 2023, leading to rapid PR. Venetoclax was reintroduced in June 2023, followed by rituximab. The triplet regimen was well tolerated, with neutropenia G2 as the main observed hematological complication. However, the patient had pre-existing moderate hypogammaglobulinemia. After four months of treatment, while maintaining a partial response, he developed pneumonia requiring hospitalization, complicated by severe hyponatremia, and subsequently passed away.
A 52 year-old woman attended our institution in March 2020 after progressive enlargement of laterocervical and submandibular lymphadenopathies over the previous two years. A PET-CT scan revealed SUVmax of 8 in several supra- and sub-diaphragmatic lymphadenopathies. Biopsy of a laterocervical lymph node performed in another institute reported B cell characterized by medium to large blasts with a diffuse growth pattern and confluent nodular aggregates, positive for CD5 and CD23, indicating RT, despite blood tests, clinical presentation, and PET-CT findings were not suggestive for aggressive disease (Figure 1). The revision of the histological section performed at our Institute and the bone marrow (BM) biopsy showed a pattern consistent with A-CLL. FISH analysis showed del(11q) and del(13q), while TP53 and NOTCH1 were not mutated. Peripheral blood testing for IGHV gene status showed an unmutated state (100% homology). The patients commenced observation.
By July 2020, there was a progressive increase in lymphocytosis and adenopathies and in October 2020 FCR (fludarabine, cyclophosphamide and rituximab) therapy was started since at that time the national drug agency reimbursed ibrutinib only for patients with more than 65 years old. Despite initial response, the end of treatment evaluation showed a progressive disease. Another excisional biopsy confirming A-CLL was performed in March 2021, the patient underwent treatment with ibrutinib in July 2021, reaching partial response (PR) after 1 year of treatment. However, in November 2022, increased lymphocytosis was observed, along with severe anemia and thrombocytopenia, leading to treatment discontinuation and switch to Ven-R, achieving PR due to persistent iliac lymph nodes while MRD testing on peripheral blood (PB) and BM were undetectable. Given the good response to the ongoing therapy, the search for a MUD donor was opened in June 2023.
Notwithstanding, in January 2024, a 5cm swelling appeared in the right iliac area. PET-CT showed increased uptake and size of supra- and subdiaphragmatic lymphadenopathies with a SUV max of 7.8 at the inguinal level, where a biopsy of the left inguinal lymphadenopathy confirmed A-CLL.
Given the progressive disease, the patient began Pirtobrutinib in March 2024, that was added to the ongoing therapy (Ven-R). By August 2024, at the 6-month mark from the start of therapy, a complete response (CR) with undetectable MRD4 on PB and detectable MRD4 on BM was documented, and the patient was admitted for allogeneic stem cell transplantation (Allo-SCT) in early October 2024.
In this article we present 2 different cases of A-CLL in order to explore the diagnostic and therapeutic challenges of this limited group of patients, of whose we have few data, especially in the new era of targeted agents.
Regarding diagnostic issues, as illustrated in the second case, initial histological assessment suggested RT rather than A-CLL. This highlights the difficulty in distinguishing between aggressive transformations of CLL, which can often mimic one another in presentation, underlining the need of expert hematopathological evaluation (10). Additionally, the use of PET-CT and LDH values in differentiating A-CLL from other aggressive forms of CLL remains limited and can be misleading (11, 12), as suggested in the first clinical case, in which despite a SUV max of 20 and elevated LDH, the final diagnosis was not RT.
Regarding therapeutic approaches, both patients experienced early relapse during treatment with VenR. However, when pirtobrutinib was combined with VenR, a new clinical response was observed. It remains unclear whether this reflects a true synergistic effect or whether the predominant role was played by the introduction of pirtobrutinib. In the first case, VenR was added after the patient had already achieved a partial response with pirtobrutinib, making it difficult to determine the specific contribution of each agent. In the second case, pirtobrutinib was introduced when the patient was beginning to progress on VenR, leading to an excellent response with CR and uMRD4 in the PB. These results allowed her to undergo for an Allo-SCT, after a careful evaluation of both the risk of transplant-related mortality and the risk that, in the event of a relapse or progression to RT, there would be few remaining viable strategies to guarantee disease remission, i.e. bispecific antibodies or CAR T-cell.
Pirtobrutinib, a non-covalent BTKi (ncBTKi), was investigated in the phase I/II BRUIN trial, including 317 patients with relapsed/refractory (R/R) CLL/SLL (13). Among these heavily pre-treated patients (median prior lines of therapy: 3, range 1-11), the overall response rate (ORR) was 82.2%. Median progression-free survival (PFS) was 19.6 months at a median follow-up of 19.4 months, with a reduced PFS of 13.8 months among patients treated with all five classes of CLL therapy (BTKi, anti-BCL2, PI3K inhibitors, chemotherapy and an anti-CD20 antibody). Patients with high-risk genetic features had a median PFS of 16.9 months for TP53 mutations or del(17p) and 18.7 months for unmutated IGHV. Giving these data, pirtobrutinib should be considered a valuable option in selected cases to achieve remission in heavily pretreated patients eligible for allogenic stem cell transplantation. In addition, in the phase 1b portion of the BRUIN study patients were eligible for the combination of pirtobrutinib with venetoclax ± rituximab (14). In this case prior cBTKi therapy was allowed but not prior venetoclax. Promising efficacy results were shown with 100% ORR and 70% uMRD rate at cycle 13. In contrast, the presented cases showed a favorable clinical response even if they had already received the VenR combination.
The role of CIT (in our case FCR) as a first-line treatment for A-CLL, as supported by existing literature, warrants reevaluation (2). The inherent genetic heterogeneity and the high-risk profile associated with A-CLL, often characterized by TP53 mutations and complex karyotype, suggest that chemoimmunotherapy may not provide adequate control or may worse the genome instability of the disease (2, 5). On the other hand, novel therapies, such as BTK and BCL2 targeted agents, remain unexplored in A-CLL and only limited to few case reports (6–9) (Table 1).
In contrast, many new drugs for RT have been studied, from the addition of new molecules such as venetoclax or polatuzumab to the backbone therapy based on R-CHOP or R-DaEPOCH, to multi-agent chemo-free therapies, check-point inhibitors, bispecific antibodies and CAR T-cell therapy, which have proven efficacy (15–18). However, no clinical trials specifically include A-CLL, so it remains unclear whether this form of CLL benefits more from CLL traditional regimens or from CHOP-based chemoimmunotherapy regimens as in RT.
The choice to add pirtobrutinib to the ongoing Ven-R regimen in case 2 derives from the in vitro synergistic effect and mitochondrial priming of BTKi combined with venetoclax (19, 20) and the preliminary data of the ongoing phase II trials on triplet regimens (BTKi + anti-BCL2 + anti-CD20 monoclonal antibodies) in CLL, showing high rates of undetectable MRD remissions both in first line and relapsed/refractory (R/R) setting (21–23). Obinutuzumab, ibrutinib and venetoclax was investigated in a phase II trial in 25 treatment naïve (TN) and 25 R/R patients with CLL, showing 67% and 50% uMRD4, respectively (22). Similarly, a phase II study of obinutuzumab, acalabrutinib and venetoclax in 45 R/R patients achieved uMRD4 in PB in 93.3% of cases, including 94% of patients previously exposed to venetoclax/BTKi and 93% with TP53 mutations (23). Another phase II trial of zanubrutinib, obinutuzumab, and venetoclax in 39 TN patients resulted in uMRD4 in 95% of PB and 89% of BM samples (21). Finally, the triplet combination of ncBTKi pirtobrutinib with venetoclax and obinutuzumab in TN patients is currently under investigation, and impressive preliminary data were presented at ASH2024 by N. Jain, with 100% uMRD4 in PB and 96% uMRD4 in BM after 1 year of therapy, showing promising results for the nearly CLL future especially in high risk (24).
In conclusion, A-CLL still remains one of the most relevant unmet clinical need in the CLL field due to its low incidence and difficulties with diagnosis and treatment. Given the findings of our case reports, the few cases reported in the existing literature (Table 1), and the results of the ongoing trials, prospective evaluation of novel therapies including triplet combinations for A-CLL may be warranted.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by study 4430/AO/18, which was approved by the local ethics board on 27th Jun 2019. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
AS: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AC: Data curation, Supervision, Writing – review & editing. FA: Writing – review & editing. VR: Data curation, Writing – review & editing. AB: Writing – review & editing. MP: Supervision, Validation, Writing – review & editing. LT: Data curation, Funding acquisition, Supervision, Validation, Writing – review & editing. AV: Data curation, Methodology, Supervision, Validation, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by Associazione Italiana per la Ricerca sul Cancro (A.I.R.C.) IG 25024 to LT, Progetti di Rilevanza Nazionale PRIN PNRR (P2022PSMX4) to AV, the ODV Ricerca per Credere nella Vita (RCV), Padua, Italy.
AV and LT received research funding and participated to advisory boards organized by Abbvie, Johnson&Johnson, AstraZeneca, BeiGene and Lilly.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pugh WC, Manning JT, Butler JJ. Paraimmunoblastic variant of small lymphocytic lymphoma/leukemia. Am J Surg Pathol. (1988) 12:907–17. doi: 10.1097/00000478-198812000-00002
2. Gine E, Martinez A, Villamor N, Lopez-Guillermo A, Camos M, Martinez D, et al. Expanded and highly active proliferation centers identify a histological subtype of chronic lymphocytic leukemia (“accelerated” chronic lymphocytic leukemia) with aggressive clinical behavior. Haematologica. (2010) 95:1526–33. doi: 10.3324/haematol.2010.022277
3. Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. (2018) 131:2745–60. doi: 10.1182/blood-2017-09-806398
4. Cui B, Ghia EM, Chen L, Rassenti LZ, DeBoever C, Widhopf GF, et al. High-level ROR1 associates with accelerated disease progression in chronic lymphocytic leukemia. Blood. (2016) 128:2931–40. doi: 10.1182/blood-2016-04-712562
5. Vadasz B, Zak T, Aldinger J, Sukhanova M, Gao J, Wolniak KL, et al. Accelerated” chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL): unraveling the biological gray zone of CLL/SLL in the era of novel therapies. Virchows Archiv. (2024). doi: 10.1007/s00428-024-03920-7
6. Xie J, Jang A, Vegel A, Hajja Y, Mouawad Y, Baghian A, et al. Successful treatment of “accelerated” chronic lymphocytic leukemia with single agent ibrutinib: A report of two cases. Leuk Res Rep. (2021) 15:100247. doi: 10.1016/j.lrr.2021.100247
7. Yavorkovsky LL. Atypical “accelerated” chronic lymphocytic leukemia with abnormal lymphocyte chromatin clumping, bone involvement, and exceptional response to Imbruvica. Cancer Rep (Hoboken). (2022) 5:e1601. doi: 10.1002/cnr2.1601
8. Catania G, Tavarozzi R, Pini GM, Borra T, Gandolfo C, Zacchi G, et al. The role of Bruton’s kinase inhibitors (BTKi) in accelerated Chronic Lymphocytic Leukemia (a-CLL): a case of successful response to acalabrutinib. J Basic Clin Physiol Pharmacol. (2023) 34:401–4. doi: 10.1515/jbcpp-2023-0051
9. Robak E, Jesionek-Kupnicka D, Stelmach P, Kupnicki P, Szataniak M, Robak T. Leukemia cutis in accelerated chronic lymphocytic leukemia: successful treatment with venetoclax and rituximab. Ann Hematol. (2022) 101:1387–92. doi: 10.1007/s00277-022-04753-7
10. Moore ME, Aguilera NS, Obiorah I, Williams E, Courville E. Assessment for acceleration and transformation of chronic lymphocytic leukemia/small lymphocytic lymphoma using histologic and immunohistochemical features: a case series. J Hematop. (2024) 17:139–47. doi: 10.1007/s12308-024-00598-3
11. Sigmund AM, Kittai AS. Richter’s transformation. Curr Oncol Rep. (2022) 24:1081–90. doi: 10.1007/s11912-022-01274-4
12. Falchi L, Keating MJ, Marom EM, Truong MT, Schlette EJ, Sargent RL, et al. Correlation between FDG/PET, histology, characteristics, and survival in 332 patients with chronic lymphoid leukemia. Blood. (2014) 123:2783–90. doi: 10.1182/blood-2013-11-536169
13. Mato AR, Woyach JA, Brown JR, Ghia P, Patel K, Eyre TA, et al. Pirtobrutinib after a covalent BTK inhibitor in chronic lymphocytic leukemia. New Engl J Med. (2023) 389:33–44. doi: 10.1056/NEJMoa2300696
14. Roeker LE, Woyach JA, Cheah CY, Coombs CC, Shah NN, Wierda WG, et al. Fixed-duration pirtobrutinib combined with venetoclax ± Rituximab in relapsed/refractory chronic lymphocytic leukemia: updated results, including MRD data, from the BRUIN phase 1b study. Blood. (2023) 142:3269–9. doi: 10.1182/blood-2023-181090
15. Bajwa A, Habib A, Kittai AS. Treatment of richter’s transformation with novel therapies. Curr Hematol Malig Rep. (2024) 19:45–55. doi: 10.1007/s11899-023-00721-8
16. Kittai AS, Bond D, Huang Y, Bhat SA, Blyth E, Byrd JC, et al. Anti-CD19 chimeric antigen receptor T-cell therapy for richter transformation: an international, multicenter, retrospective study. J Clin Oncol. (2024) 42:2071–9. doi: 10.1200/JCO.24.00033
17. Tedeschi A, Frustaci AM, Condoluci A, Coscia M, Chiarle R, Zinzani PL, et al. Atezolizumab, venetoclax, and obinutuzumab combination in Richter transformation diffuse large B-cell lymphoma (MOLTO): a multicentre, single-arm, phase 2 trial. Lancet Oncol. (2024) 25:1298–309. doi: 10.1016/S1470-2045(24)00396-6
18. Jain N, Senapati J, Thakral B, Ferrajoli A, Thompson P, Burger J, et al. A phase 2 study of nivolumab combined with ibrutinib in patients with diffuse large B-cell Richter transformation of CLL. Blood Adv. (2023) 7:1958–66. doi: 10.1182/bloodadvances.2022008790
19. Haselager MV, Kielbassa K, ter Burg J, Bax DJC, Fernandes SM, Borst J, et al. Changes in Bcl-2 members after ibrutinib or venetoclax uncover functional hierarchy in determining resistance to venetoclax in CLL. Blood. (2020) 136:2918–26. doi: 10.1182/blood.2019004326
20. Liu Y, Yan F, Jiang VC, Li Y, Che Y, McIntosh J, et al. Pirtobrutinib and venetoclax combination overcomes resistance to targeted and chimeric antigen receptor T-cell therapy in aggressive mantle cell lymphoma. Haematologica. (2022) 108:1412–6. doi: 10.3324/haematol.2022.282031
21. Soumerai JD, Mato AR, Dogan A, Seshan VE, Joffe E, Flaherty K, et al. Zanubrutinib, obinutuzumab, and venetoclax with minimal residual disease-driven discontinuation in previously untreated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Haematol. (2021) 8:e879–90. doi: 10.1016/S2352-3026(21)00307-0
22. Rogers KA, Huang Y, Ruppert AS, Abruzzo LV, Andersen BL, Awan FT, et al. Phase II study of combination obinutuzumab, ibrutinib, and venetoclax in treatment-naïve and relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. (2020) 38:3626–37. doi: 10.1200/JCO.20.00491
23. Fürstenau M, Giza A, Weiss J, Kleinert F, Robrecht S, Franzen F, et al. Acalabrutinib, venetoclax, and obinutuzumab in relapsed/refractory CLL: final efficacy and ctDNA analysis of the CLL2-BAAG trial. Blood. (2024) 144:272–82. doi: 10.1182/blood.2023022730
Keywords: chronic lymphocytic leukemia, A-CLL, BTKi, ncBTKi, triplet regimens, novel therapies, venetoclax
Citation: Serafin A, Cellini A, Angotzi F, Ruocco V, Bevilacqua A, Pizzi M, Trentin L and Visentin A (2025) Case Report: Triplet combination with pirtobrutinib/venetoclax/rituximab in accelerated phase of chronic lymphocytic leukemia. Front. Hematol. 4:1552200. doi: 10.3389/frhem.2025.1552200
Received: 27 December 2024; Accepted: 12 February 2025;
Published: 04 March 2025.
Edited by:
Francesco Di Raimondo, University of Catania, ItalyReviewed by:
Matilde Scaldaferri, AOU Città della Salute e della Scienza di Torino, ItalyCopyright © 2025 Serafin, Cellini, Angotzi, Ruocco, Bevilacqua, Pizzi, Trentin and Visentin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Visentin, YW5kcmVhLnZpc2VudGluQHVuaXBkLml0; Livio Trentin, bGl2aW8udHJlbnRpbkB1bmlwZC5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.