
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hematol., 09 April 2025
Sec. Red Cells, Iron and Erythropoiesis
Volume 4 - 2025 | https://doi.org/10.3389/frhem.2025.1550664
 Kemal Mohamed*
Kemal Mohamed* Wondwossen Tadesse
Wondwossen Tadesse Dagmawi Woldesenbet
Dagmawi Woldesenbet Milkias Abebe
Milkias Abebe Abdulhakim Mussema
Abdulhakim Mussema Elias Tamene
Elias Tamene Solomon Gebre
Solomon GebreBackground: Neonatal anemia occurs when the blood contains lower hemoglobin levels or erythrocytes than normal. Maternal obstetric and neonatal clinical characteristics and other medical conditions can contribute to this condition.
Methods: A facility-based cross-sectional study of 277 infants was conducted from October 14, 2023, to January 2, 2024. Consecutive sampling techniques were employed to enroll 277 mothers and their neonates, resulting in a response rate of 96.18%. Face-to-face interviews were conducted to gather information on neonates’ sex, sociodemographic characteristics, and nutritional status using a pre-tested structured questionnaire. A Mindray BC-3000Plus Hematology Analyzer was utilized to analyze the blood samples collected from the mothers and their neonates. All raw data were coded and entered into SPSS version 27 and analyzed using descriptive statistics, independent t-tests, and logistic regression. Statistical significance was established at a p-value of 0.05.
Results: The overall prevalence of neonatal anemia was 21.7% (60/277). Mean hemoglobin levels were significantly lower among neonates born to anemic mothers than among those born to non-anemic mothers [(12.26 ± 2.66) Vs (13.39 ± 1.59), p <0.001). The findings from this study also showed the protective effect of taking “iron and folic acid” supplementation during pregnancy on the development of neonatal anemia (Adjusted odds ratio [AOR] = 0.15, 0.07-0.34, p <0.001).
Conclusion: During pregnancy, taking “iron and folic acid” supplements can prevent the development of neonatal anemia. Maternal anemia is correlated with lower mean hemoglobin levels in neonates. Therefore, during antenatal care follow-up, policymakers should consider implementing maternal health education regarding infant anemia and relevant health interventions to reduce the incidence of infant anemia.
Neonatal anemia is when the blood contains less than 13.5 g/dl hemoglobin (1). The primary reasons for anemia in neonates include increased red blood cell breakdown, decreased erythropoiesis, and blood loss (2). In addition, other obstetric conditions include preterm birth, maternal iron deficiency during pregnancy, maternal anemia, blood loss after delivery, genetic disorders, low birth weight, and other neonatal conditions (3–6). The symptoms of neonatal anemia include pale skin, exhaustion, a fast heartbeat, and difficulty feeding (3).
In underdeveloped nations with inadequate interventional implementation programs, low iron intake, and inadequate infrastructure services for early detection and management of anemia at the level of health facilities, it becomes more prevalent (7, 8). Because breast milk contains little iron, infants under six months of age are particularly susceptible to anemia from their poor iron intake, and fast growth (9). They therefore mostly depend on iron from intrauterine life (10). When the mother, who supplies the fetal iron, is anemic, the situation is even more tragic. Anemia affects about 36.8% of pregnant women globally, 41.7% in Africa (11), and 22% of pregnant women in Ethiopia (11, 12).
Untreated anemia in neonates can lead to respiratory distress, sensorium depression, tissue hypoxia, stunted growth, chronic heart failure(CHF), viral infections, such as HIV/AIDS, and hepatitis due to repeated transfusions of blood products (13–15). In addition, the long-term implications of neonatal anemia include decreased neurocognitive development, growth failure, and increased vulnerability to infection at all ages (13, 14, 16). Given these factors, anemia is a contributing factor to infant morbidity and mortality (1, 17, 18).
There are insufficient data on neonatal anemia’s prevalence and risk factors in various regions of Ethiopia. Some studies conducted in Ethiopia reported variable rates of neonatal anemia of 9% in Addis Ababa (19), 25% in Gondar (1), and 29.1% in Nekemte western Ethiopia (13). Thus, the current study assessed the prevalence of anemia in neonates at Wachemo University Nigist Eleni Mohammed Memorial Comprehensive Specialized Hospital in Central Ethiopia.
The study was conducted at Wachemo University Nigist Eleni Mohammed Memorial Comprehensive Specialized Hospital (WUNEMMCSH), situated 232 km south of Addis Ababa, the capital city of Ethiopia, in Hossana, central Ethiopia. The hospital provides treatment, prevention, and rehabilitation services. These services are organized into case teams, including outpatient, inpatient, emergency, critical care, obstetrics, maternal-child health, orthopedics, oncology, and surgical departments. The hospital was selected due to its high patient and client attendance rate. This hospital serves residents of the Hadiya zone, as well as other zones and special woredas, including Gurage, Selte, Halab, and Kembata-Tembaro. The hospital provides 11580 delivery services annually (2023 annual facility reports). This institution is the sole referral hospital in the Hadiya region.
A facility-based cross-sectional study was conducted on neonates delivered at the obstetrics department of WUNEMMCSH from October 14, 2023, to January 2, 2024.
The source population for this study comprised all neonates delivered at the obstetrics department of WUNEMMCSH.
The study population comprised all neonates delivered at the obstetrics department of WUNEMMCSH during the data collection period, fulfilling the inclusion criteria.
The study included all neonates admitted to the WUNEMMCSH during the specified period who were born at or after 28 gestational weeks. However, the study excluded neonates and mothers with any medical conditions and neonates below 28 days of gestation.
The single-population proportion formula was used to determine the minimum sample size required. The sample size was calculated to be 288 after adding 5% non-response, using the 23.2% prevalence of anemia in neonates from a study conducted in Northwest Ethiopia (20), a 5% margin of error, and a 95% confidence interval (CI) of 1.96.
All women and their neonates who fulfilled the eligibility requirements of the study were consecutively added to the sample until the required number of participants was reached.
Face-to-face interviews were conducted to collect data on neonates’ sex, sociodemographic characteristics, and maternal nutritional status using a pre-tested structured questionnaire. The questionnaire was initially developed in English, translated into Amharic, and subsequently back-translated into English to ensure consistency. After receiving training on the study objectives and data collection instruments, the midwives and ward nurses administered the questionnaire. Maternal clinical data, including antenatal care (ANC) follow-up, iron levels, gravidity, iron and folic acid supplementation during pregnancy, and the occurrence of antepartum hemorrhage, were extracted from maternal medical records. We used Mid-Upper Arm Circumference (MUAC) simple non-invasive measurement of nutritional status, which involves measuring the circumference of the mid-upper arm using a flexible tape, particularly in children under five years of age (21).
After delivery, approximately 3 mL of umbilical cord blood was obtained from the clamped umbilical cord. Cord blood samples were collected from the clamped cord, excluding the placenta by two midwifery professionals. To stop blood clotting, the obtained sample was quickly placed in a test tube containing ethylenediaminetetraacetic acid (EDTA) and gently mixed. After birth, 3 mL of venous blood was drawn from the mother using a disposable syringe after 24 hours postpartum. The Mindray BC-3000Plus Hematology Analyzer was used to analyze the complete blood count (CBC). A skilled laboratory technician strictly follows the standard operating procedures (SOP) when performing CBC.
Data collectors received one day of instruction regarding ethical issues and data collection techniques before beginning data collection. A pre-test was conducted on 5% of the study sample, which comprised patients who were not included in the study. Consequently, the questions’ coherence, completeness, and flow, as well as the time required to complete them, were examined. The primary investigator and supervisor (nursing head) performed routine oversight to guarantee that all the required data were correctly gathered. Laboratory SOPs were followed throughout specimen collection and CBC analysis to ensure the quality of the laboratory results. Therefore, blood was transferred to the EDTA tube wall and mixed well by gently inverting the tube eight to ten times after collection to prevent hemolysis. Labeling was performed on the samples, and the request forms had the same unique number after collection to avoid confusion. Before the patient sample analysis, the expiration date of the reagent was determined. A routine background run was conducted to reduce the background error of the hematology analyzer.
After coding, the raw data were imported into SPSS version 27 for analysis. The data were analyzed, and the participants’ demographics were described using descriptive statistics. Independent tests were used to compare the hemoglobin levels of neonates according to maternal anemia status. Neonatal anemia was evaluated as a binary outcome of interest. Statistical relationships between the dependent and independent variables were ascertained using multivariate analysis and binary logistic regression. To account for potential confounders, a multivariate analysis was performed on the variables in the bivariate regression model linked to the dependent variable (p < 0.25). The degree of correlation between the occurrence of neonatal anemia and independent variables was assessed using the odds ratio (OR) with a 95% confidence interval and a p-value of 0.05, which was considered statistically significant.
Ethical approval was obtained from the Department of Medical Laboratory Sciences, School of Medicine and Health Sciences, Wachemo University: Ref No: MLS/3730/2023, Date: October 1, 2023. After ethical clearance was received, permission to conduct the research was obtained from the WUNEMMCSH. All participants were informed of the purpose of the study and their participation was voluntary. Written informed consent was obtained from the children’s parents or guardians; the confidentiality of the information provided was assured. All laboratory investigations were performed using an established standard operating procedure (SOP).
In total, 277 mothers and their newborns were included in this study, resulting in a response rate of 96.18%. The mean age of the mothers who gave birth to the neonates was 27.52 ± 1.29 years, with the majority of them (67.9%; 188/277) being between 25 and 29 years old. In addition, most of the mothers were from urban (183/277; 66.1%). Of the mothers, 88.8%(246/277) were married (Table 1).
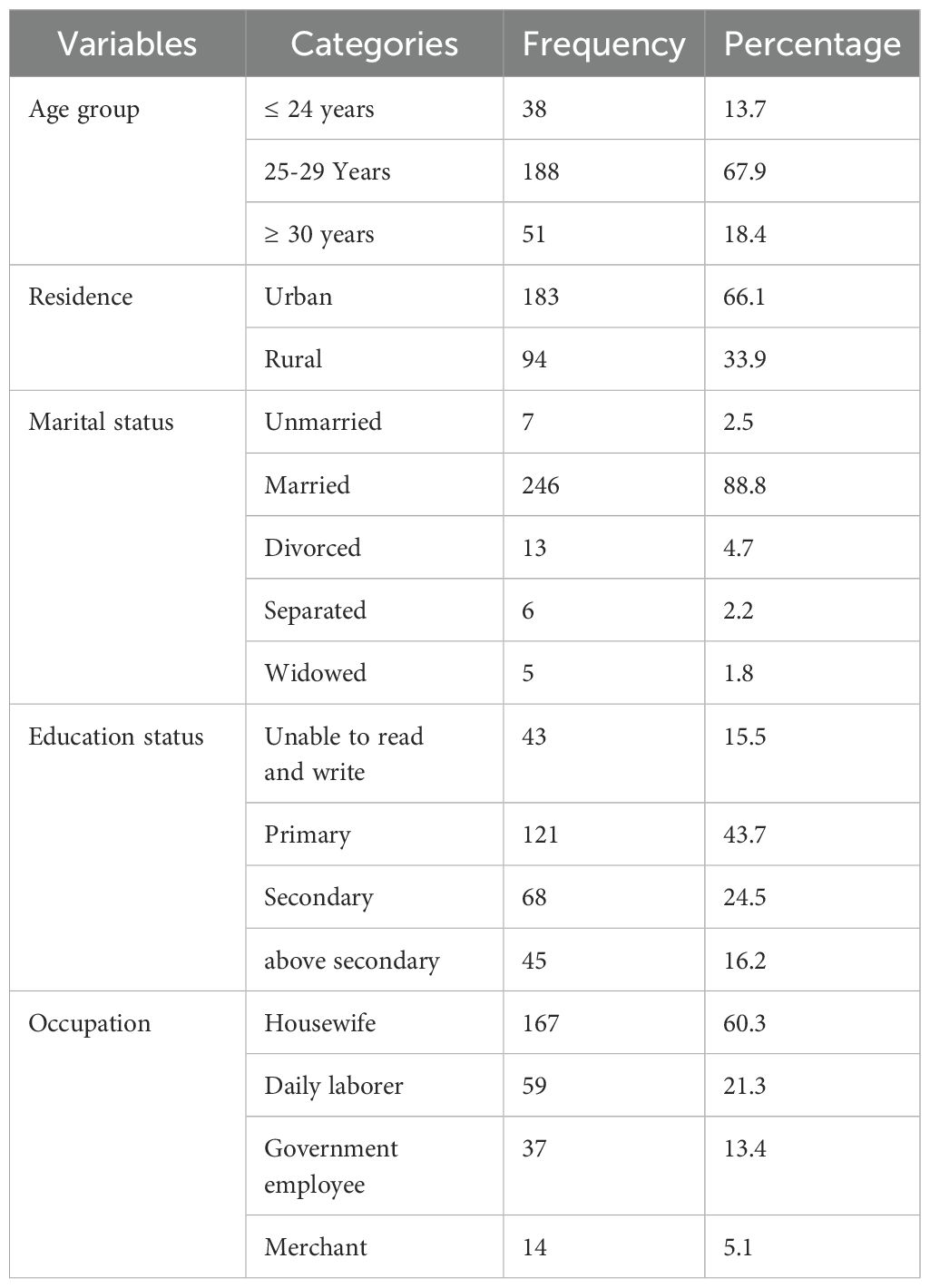
Table 1. Socio-demographic characteristics of neonates’ mothers at WUNEMMCSH, central Ethiopia, from October 14, 2023, to January 2, 2024.
In this study, 65.7% (182/277) of patients underwent a check-up during antenatal care(ANC). Of the mothers, 29.6% (82/277) were primiparous and 72.2% (200/277) reported taking “iron and folic acid” supplements during pregnancy. Regarding the mode of delivery, 88.4% (245/277) of the mothers delivered their children via the vagina. Regarding maternal MUAC, 89.5% (248/277) of the mothers had MUAC ≥ 23 cm. Of the mothers who participated in the study, 24.9% (69/277) had anemia, with an Hgb value of less than 11 g/dl. The female-to-male ratio of the neonates was 0.56 to 1 (100/177), with only 11.2% (31/277) weighing less than 2.5 kg (Table 2).
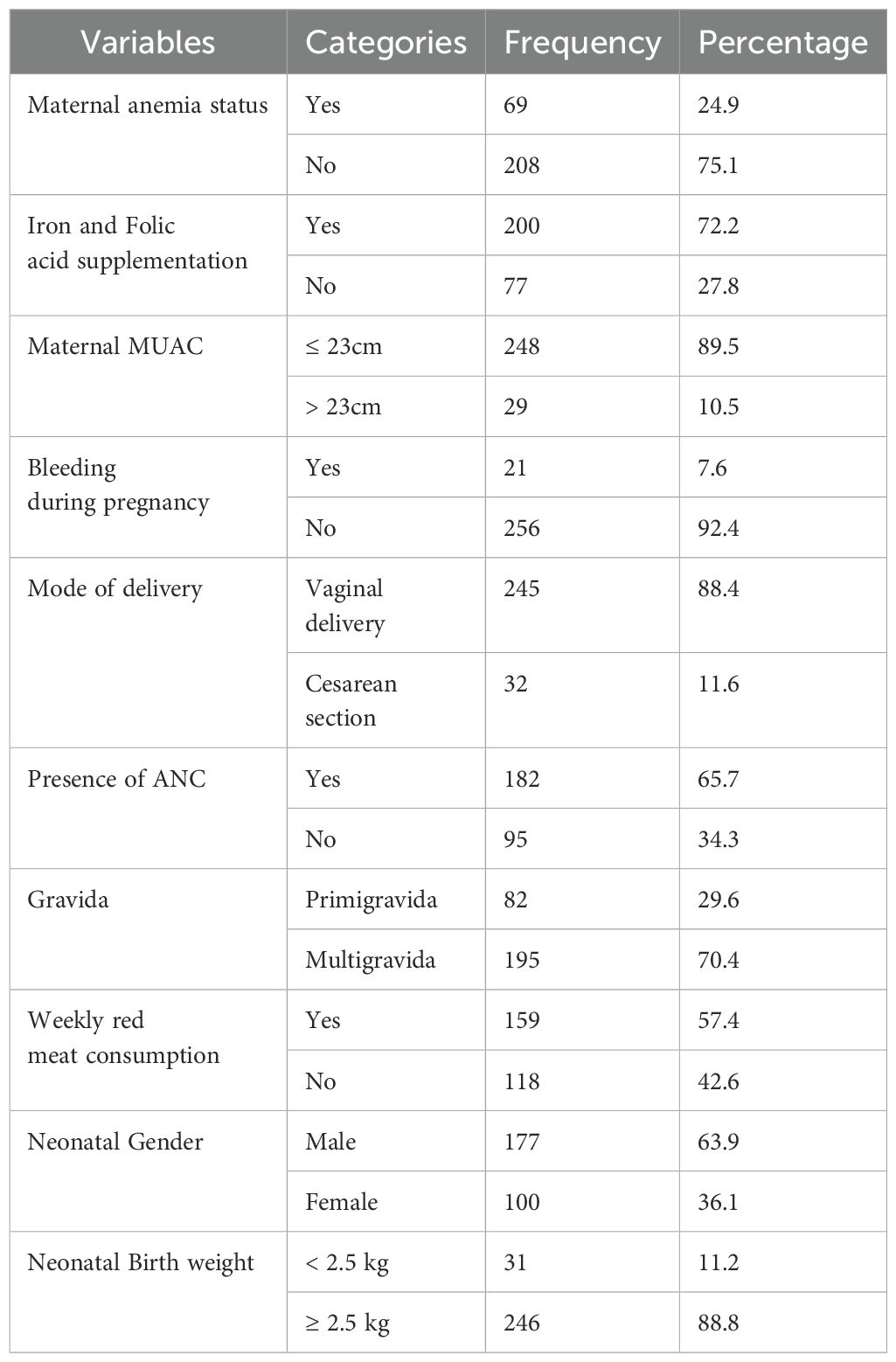
Table 2. Socio-demographic characteristics and clinical data of the study participants at WUNEMMCSH, central Ethiopia, from October 14, 2023, to January 2, 2024.
The neonate hemoglobin levels ranged from 4.8 g/dl to 17.9 g/dl, with a mean hemoglobin level of 13.1 g/dl ± 1.97. In this study, the overall prevalence of neonatal anemia was 21.7%(60/277). Mean hemoglobin levels were significantly lower among neonates born from anemic mothers than among those born from [(12.26 ± 2.66) Vs (13.39 ± 1.59), p <0.001)] (Figure 1).
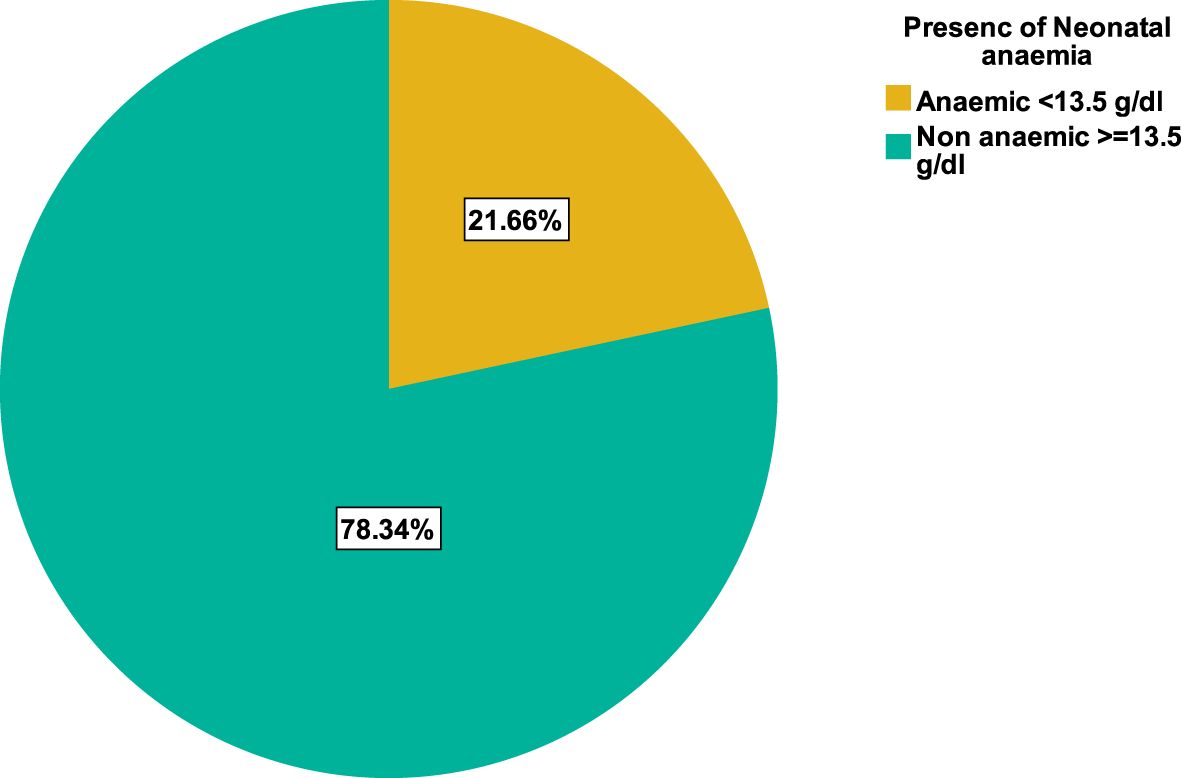
Figure 1. Prevalence of anemia among neonates delivered at the obstetrics department of WUNEMMCSH, Central Ethiopia.
The independent variables were analyzed using bivariate and multivariate logistic regression analyses. Multivariate logistic regression analysis showed that after adjusting for possible confounders, taking “iron and folic acid” supplements during pregnancy can decrease the chance of developing neonatal anemia (AOR = 0.15, 0.07-0.34, p <0.001). Maternal anemia, residence, follow-up, maternal occupation, bleeding during pregnancy, gravidity, maternal educational status, maternal MUAC, maternal age group, neonatal age, neonatal birth weight, and meat consumption were not significantly associated with neonatal anemia (Table 3).
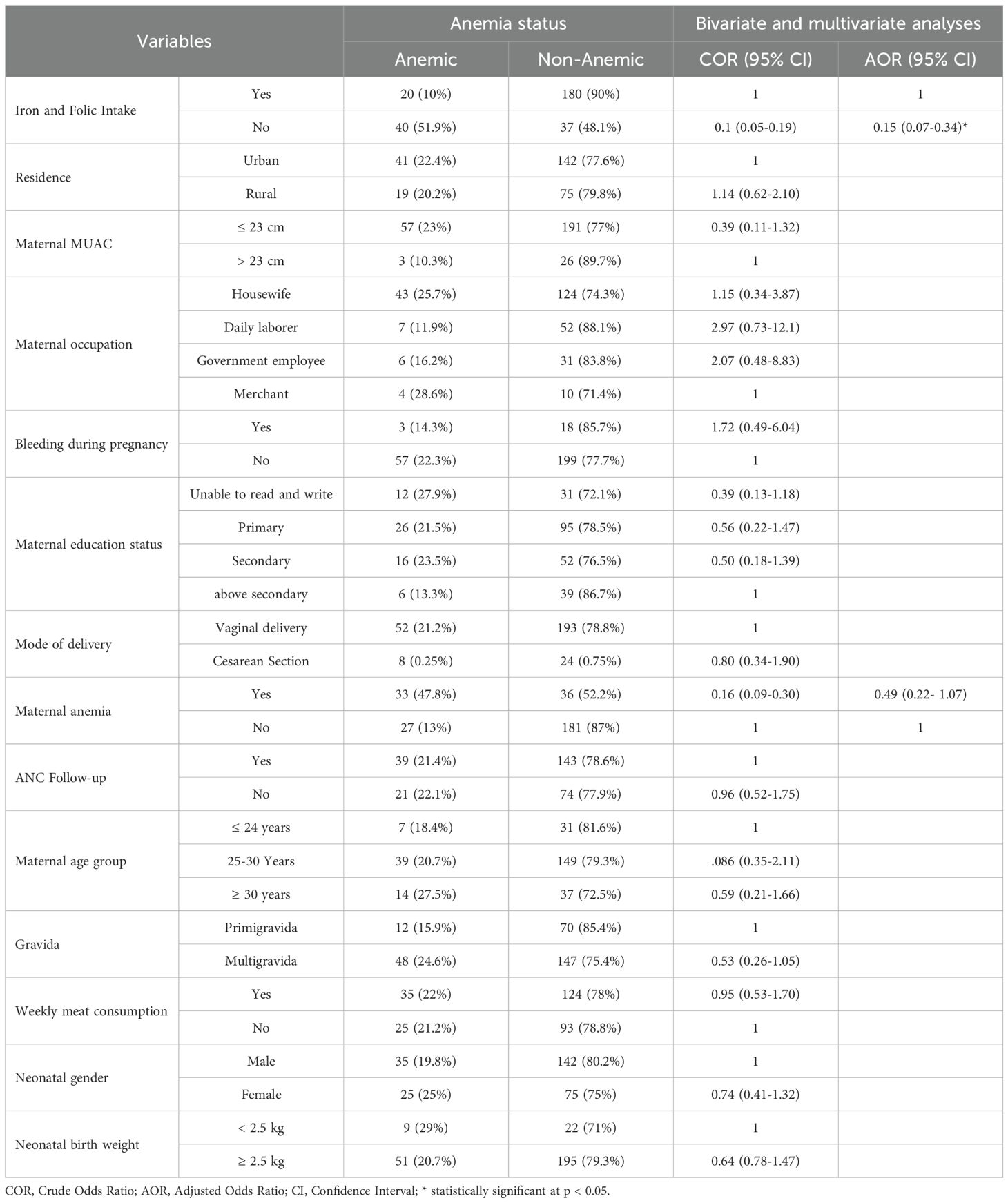
Table 3. Bivariable and Multivariate Binary Logistic Regression Analysis for Factors of Anemia among Neonate at WUNEMMCSH, Central Ethiopia, from October 14, 2023, to January 2, 2024.
Anemia poses a significant public health concern in Africa, particularly in children aged 5 years, and is associated with substantial morbidity and mortality. The determinants of anemia in children under 5 years were sex, residence, maternal education level, and family size (22). Not only under five children anemia also affects neonates with significant health impacts. This health impact is particularly pronounced in Sub-Saharan Africa, where health infrastructure is inadequate (23). In Ethiopia, the prevalence of neonatal anemia is influenced by geographical factors, obstetric variables, and maternal characteristics (24). Neonatal anemia can be managed by red blood cell transfusion (25), but red blood cell transfusion is linked to a higher risk of necrotizing enterocolitis, the spread of infectious diseases, and adverse neurodevelopmental outcomes (25).
This study found that the prevalence of neonatal anemia was 21.6%. This finding agrees with the studies done in New York, which found 21% (6), and the Netherlands, which found 21% (26). Possible explanations for these similarities include the operationalization of the hemoglobin level cut-off point for neonates. Similar to our research, the study described above classified neonate anemia as having the 13.5 g/dl hemoglobin threshold. In contrast, this finding is greater than that of studies conducted in the USA 14% (6), 5.7% in Nepal (27), 11.7% in Iran (28), and in Addis Ababa Ethiopia 9% (19). The discrepancies in clinical characteristics, variations in sample size, and socioeconomic status among the study subjects could be the cause of these variations.
In contrast to our findings, a study done in Brazil 32.6% (29), Pakistan 79.3% (30), Iran 53% (31), Ghana 57.3% (18), Benin 61.1% (17), Ethiopia 29.1% (32) reported a higher prevalence of neonatal anemia. One potential explanation for the observed discrepancy between the present study and the Benin study could be attributed to the latter’s exclusive inclusion of infants born to mothers infected with malaria, whereas the former also included neonates from uninfected mothers. The fetus acquires malaria parasites congenitally, which subsequently infiltrate the fetus’s erythrocytes intracellularly. Consequently, this condition leads to an increased prevalence of infant anemia and decreased hemoglobin levels.
Depending on the differences in the modes of delivery of the research participants, this could account for the divergence from the Iranian study. Of the study participants, 88.4% (245/277) were born through the vagina, whereas all the study participants in Iran were born via cesarean section. In contrast to vaginal delivery, accidental incision of the placenta during cesarean delivery may result in hemorrhage, leading to anemia. A potential explanation for the discrepancy between the findings of the Addis Ababa study, Ethiopia, and the present study could be attributed to the former’s exclusive inclusion of neonates with low birth weight. In contrast, the latter encompassed both low birth weight and normal weight neonates.
In this study, neonates born to mothers with anemia had significantly lower mean hemoglobin levels than those born to non-anemic mothers. One explanation for this finding could be that maternal anemia can increase the risk of developing severe neonatal anemia (33), and it is a risk factor for anemia among neonates (20). An increased chance of premature birth has been linked to maternal anemia, which can increase the risk of neonatal anemia as well. Given their shortened gestation time and immature erythrocyte production, premature neonates are more likely to develop anemia. Iron, folate, or vitamin B12 deficiencies are critical elements that can lead to maternal anemia (34). Anemia in infants can result from the fetus not receiving enough of these nutrients during development if the mother suffers from deficiency. Overall, maternal anemia can affect fetal health and increase the risk of neonatal anemia, including insufficient oxygen transfer, hereditary factors, nutritional deficiency, and preterm birth.
In the present study, pregnant women who take “iron and folic acid” supplements can decrease the chance of developing neonatal anemia. This finding is supported by findings from studies conducted in Italy, Iraq, and Peru (35–37). Mothers lacking iron supplementation during pregnancy have decreased maternal iron reserves; consequently, the quantity of placental iron transferred to the fetus is reduced, thereby increasing the risk of anemia among neonates (38).
Taking “iron and folic acid” supplements during pregnancy can reduce the risk of neonatal anemia. Maternal anemia significantly lowers hemoglobin levels in neonates. Therefore, during antenatal care follow-up, policymakers should consider maternal health education regarding infant anemia and relevant health care interventions to lessen the burden.
Due to constraints in research facilities and budget, this study did not evaluate micronutrient deficiency, thalassemia, or other hemoglobinopathies in study participants. These considerations may strengthen the findings of this study.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
The studies involving humans were approved by Department of Medical Laboratory Sciences, School of Medicine and Health Sciences, Wachemo University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
KM: Conceptualization, Data curation, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing, Software, Supervision, Validation. WT: Data curation, Investigation, Methodology, Resources, Visualization, Writing – review & editing. DW: Methodology, Writing – original draft, Writing – review & editing. MA: Conceptualization, Methodology, Writing – review & editing. AM: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. ET: Conceptualization, Investigation, Methodology, Resources, Supervision, Writing – original draft. SG: Conceptualization, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Tiruneh T, Shiferaw E, Enawgaw B. Prevalence and associated factors of anemia among full-term newborn babies at University of Gondar comprehensive specialized hospital, Northwest Ethiopia: a cross-sectional study. Ital J Pediatr. (2020) 46:1–7. doi: 10.1186/s13052-019-0764-1
2. Kollamparambil TG, Carroll W, Rayaroth DK. Neonatal anaemia. Paediatrics Child Health. (2024) 34(5):154–9. doi: 10.1016/j.paed.2024.02.007
3. Kilpatrick SJ. Guidelines for perinatal care. United States: American Academy of Pediatrics (2017).
4. Arakhita Swain S, Trilochan M, Satpathy S, Saiprasanna B. Aetiopathological and clinical study of anemia in newborns admitted to a tertiary care centre. Shock. (2017) 4:3–77.
5. Sandhya V, Patil S, Rau A. Iron store status in newborns born to anemic and non-anemic mothers. J Pediatr Sci. (2012) 4:1–4. doi: 10.17334/jps.97621
6. Lee S, Guillet R, Cooper EM, Westerman M, Orlando M, Kent T, et al. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr Res. (2016) 79:42–8. doi: 10.1038/pr.2015.183
7. Zainel AJAL, Osman SRO, Al-Kohji SMS, Selim NA. Iron deficiency, its epidemiological features and feeding practices among infants aged 12 months in Qatar: a cross-sectional study. BMJ Open. (2018) 8:e020271. doi: 10.1136/bmjopen-2017-020271
8. Carter RC, Jacobson JL, Burden MJ, Armony-Sivan R, Dodge NC, Angelilli ML, et al. Iron deficiency anemia and cognitive function in infancy. Pediatrics. (2010) 126:e427–e34. doi: 10.1542/peds.2009-2097
9. Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. (2006) 64:S34–43. doi: 10.1111/j.1753-4887.2006.tb00243.x
10. Dereje I, Etefa T, Gebremariam T, Getaye A, Tunta A, Gerbi A. Prevalence of anemia and associated factors among term newborns in nekemte specialized hospital, western Ethiopia. J Multidiscip Healthcare. (2021) 14:2607–15. doi: 10.2147/JMDH.S326962
11. Karami M, Chaleshgar M, Salari N, Akbari H, Mohammadi M. Global prevalence of anemia in pregnant women: a comprehensive systematic review and meta-analysis. Maternal Child Health J. (2022) 26:1473–87. doi: 10.1007/s10995-022-03450-1
12. Bekele A, Tilahun M, Mekuria A. Prevalence of anemia and its associated factors among pregnant women attending antenatal care in health institutions of Arba Minch town, Gamo Gofa Zone, Ethiopia: A cross-sectional study. Anemia. (2016) 2016:1073192. doi: 10.1155/2016/1073192
13. Christensen RD, Ohls RK. Anemia in the neonatal period. Neonatology: A Pract Approach to Neonatal Diseases. (2012), 784–98. doi: 10.1007/978-88-470-1405-3_103
14. Hegade S. Etiological profile, clinical course, immediate outcome and short team follow up of anemia in new born. India: Rajiv Gandhi University of Health Sciences (2009).
15. Aher S, Malwatkar K, Kadam S. Neonatal anemia. Semin fetal neonatal Med. (2008) 13(4):239–47. doi: 10.1016/j.siny.2008.02.009
16. Molla A, Egata G, Mesfin F, Arega M, Getacher L. Prevalence of anemia and associated factors among infants and young children aged 6–23 months in Debre Berhan Town, North Shewa, Ethiopia. J Nutr Metab. (2020) 2020:2956129. doi: 10.1155/2020/2956129
17. Koura GK, Ouedraogo S, Le Port A, Watier L, Cottrell G, Guerra J, et al. Anaemia during pregnancy: impact on birth outcome and infant haemoglobin level during the first 18 months of life. Trop Med Int Health. (2012) 17:283–91. doi: 10.1111/j.1365-3156.2011.02932.x
18. Laar AK, Grant FE, Addo Y, Soyiri I, Nkansah B, Abugri J, et al. Predictors of fetal anemia and cord blood malaria parasitemia among newborns of HIV-positive mothers. BMC Res notes. (2013) 6:1–9. doi: 10.1186/1756-0500-6-350
19. Terefe B, Birhanu A, Nigussie P, Tsegaye A. Effect of maternal iron deficiency anemia on the iron store of newborns in Ethiopia. Anemia. (2015) 2015:808204. doi: 10.1155/2015/808204
20. Alamneh TT, Tilahun SF, Beyne MB, Fekadu SA, Assem AS, Kassa SF. Prevalence and associated factors of anemia among newborns at tibebe ghion specialized hospital, northwest Ethiopia. Int J Gen Med. (2022) 15:6465. doi: 10.2147/IJGM.S365817
21. WHO. Child growth standards and the identification of severe acute malnutrition in infants and children: a joint statement by the World Health Organization and the United Nations Children’s Fund. Carroll W, editor. Geneva: World Health Organization Press (2009).
22. Tadesse SE, Zerga AA, Mekonnen TC, Tadesse AW, Hussien FM, Feleke YW, et al. Burden and determinants of anemia among Under-Five children in Africa: systematic review and meta-analysis. Anemia. (2022) 2022:1382940. doi: 10.1155/2022/1382940
23. Tekelab T, Chojenta C, Smith R, Loxton D. The impact of antenatal care on neonatal mortality in sub-Saharan Africa: A systematic review and meta-analysis. PloS One. (2019) 14:e0222566. doi: 10.1371/journal.pone.0222566
24. Gudayu TW. Epidemiology of neonatal mortality: a spatial and multilevel analysis of the 2019 mini-Ethiopian demographic and health survey data. BMC pediatrics. (2023) 23:26. doi: 10.1186/s12887-023-03838-0
25. Von Lindern JS, Lopriore E. Management and prevention of neonatal anemia: current evidence and guidelines. Expert Rev hematology. (2014) 7:195–202. doi: 10.1586/17474086.2014.878225
26. Kalteren WS, Ter Horst HJ, den Heijer AE, de Vetten L, Kooi EM, Bos AF. Perinatal anemia is associated with neonatal and neurodevelopmental outcomes in infants with moderate to severe perinatal asphyxia. Neonatology. (2018) 114:315–22. doi: 10.1159/000490369
27. Timilsina S, Karki S, Gautam A, Bhusal P, Paudel G, Sharma D. Correlation between maternal and umbilical cord blood in pregnant women of Pokhara Valley: a cross sectional study. BMC Pregnancy Childbirth. (2018) 18:1–5. doi: 10.1186/s12884-018-1697-1
28. Mamoury G, Hamedy A, Akhlaghi F. Cord hemoglobin in newborns in correlation with maternal hemoglobin in northeastern Iran. Iranian J Med Sci. (2015) 28:166–8.
29. de Sá SA, Willner E, Pereira TAD, de Souza VR, Boaventura GT, de Azeredo VB. Anemia in pregnancy: impact on weight and in the development of anemia in newborn. Nutricion hospitalaria. (2015) 32:2071–9. doi: 10.3305/nh.2015.32.5.9186
30. Lone F, Qureshi R, Emmanuel F. Maternal anaemia and its impact on perinatal outcome in a tertiary care hospital in Pakistan. EMHJ-Eastern Mediterr Health J. (2004) 10:801–7. doi: 10.26719/2004.10.6.801
31. Baharvand P, Fathi M, Eliyasy H, Abdolkarimi B, Kiani AA. The effect of delivery type on neonatal blood indices in an Iranian population. Biomed Res Ther. (2018) 5:2768–75. doi: 10.15419/bmrat.v5i10.492
32. Alemu T, Umeta M. Prevalence and predictors of” small size” babies in Ethiopia: in-depth analysis of the Ethiopian demographic and health survey, 2011. Ethiopian J Health Sci. (2016) 26:243–50. doi: 10.4314/ejhs.v26i3.7
33. Mudher-Al-Hilli N. The effect of maternal anaemia on cord blood haemoglobin and newborn birth weight. Kerbala J Med. (2009) 2:589–93.
34. Benson CS, Shah A, Frise MC, Frise CJ. Iron deficiency anaemia in pregnancy: a contemporary review. Obstetric Med. (2021) 14:67–76. doi: 10.1177/1753495x20932426
35. Al-bakka AA, Umran RM. Effect of maternal supplementation with iron on neonatal iron status and birth weight. Med J Babylon. (2013) 10.
36. Parisi F, Berti C, Mandò C, Martinelli A, Mazzali C, Cetin I. Effects of different regimens of iron prophylaxis on maternal iron status and pregnancy outcome: a randomized control trial. J Maternal-Fetal Neonatal Med. (2017) 30:1787–92. doi: 10.1080/14767058.2016.1224841
37. O’Brien KO, Zavaleta N, Abrams SA, Caulfield LE. Maternal iron status influences iron transfer to the fetus during the third trimester of pregnancy. Am J Clin Nutr. (2003) 77:924–30. doi: 10.1093/ajcn/77.4.924
Keywords: prevalence, neonatal anemia, iron and folic acid supplementation, risk factors, Ethiopia
Citation: Mohamed K, Tadesse W, Woldesenbet D, Abebe M, Mussema A, Tamene E and Gebre S (2025) Anemia and its predictors among neonates at Wachemo University teaching hospital, Central Ethiopia: a facility-based cross-sectional study. Front. Hematol. 4:1550664. doi: 10.3389/frhem.2025.1550664
Received: 23 December 2024; Accepted: 17 March 2025;
Published: 09 April 2025.
Edited by:
Josef Prchal, The University of Utah, United StatesReviewed by:
Suzie Noronha, University of Rochester, United StatesCopyright © 2025 Mohamed, Tadesse, Woldesenbet, Abebe, Mussema, Tamene and Gebre. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kemal Mohamed, YWRvdGUxMjBAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.