- 1Cátedra Rectoral de Medicina Basada en la Evidencia, Universidad de Carabobo, Madrid, Venezuela
- 2Faculty of Medicine, Universidad Francisco de Vitoria, Madrid, Spain
Background: Sickle cell disease (SCD) is a significant global health challenge, disproportionately affecting populations in low-resource regions, particularly sub-Saharan Africa, India, the Mediterranean, and the Caribbean. The Cochrane Collaboration has significantly contributed to evidence synthesis in SCD management, yet its impact has not been comprehensively assessed.
Research question: How has Cochrane’s evidence synthesis shaped research outputs and identified gaps in clinical evidence for SCD?
Objective: To systematically evaluate the scope, methodological rigour, and evidence gaps within Cochrane reviews on SCD interventions (1996–2024) and identify areas requiring further research.
Methods: We analysed 49 Cochrane systematic reviews using a mixed-methods approach, assessing both abstracted data and full-text methodology where available. Our quantitative analyses examined randomised clinical trials (RCTs), participant numbers, and meta-analytical techniques. We conducted qualitative analyses encompassing thematic categorisation and geographic distribution evaluation.
Results: Our analysis revealed significant methodological gaps: 34.7% (17/49) of reviews contained no RCTs (‘empty’ reviews), and notably, none of the 32 reviews incorporating RCTs conducted meta-analyses. Among the 32 reviews with RCTs, the median number of included trials was 3 (IQR: 1.75–5), with a median of 260 participants (IQR: 112–555). The research concentrated in three primary domains: Pain Management and Complications (22 reviews), Infection Prevention and Transfusion (15 reviews), and Genetic Therapies and Nutritional Support (12 reviews). The UK and Venezuela have produced the largest number of Cochrane reviews on SCD, positioning them as the primary contributors to evidence synthesis in this field. Additionally, 67.4% (33/49) of reviews involved international collaboration, reflecting a substantial degree of cross-border research engagement.
Conclusions: Cochrane reviews on SCD exhibit critical methodological limitations, particularly the absence of meta-analyses and the high prevalence of empty reviews. These gaps underscore the urgent need for enhanced primary research, especially RCTs, in underexplored therapeutic areas. Geographical analysis suggests opportunities for expanding international collaboration, particularly with researchers from high-burden, low-resource settings. To strengthen evidence-based SCD management, future research must prioritise: (1) standardising outcome measures, (2) applying innovative systematic review methodologies, and (3) closing identified evidence gaps. Addressing these issues will enhance the quality, reliability, and clinical applicability of systematic reviews in SCD research.
“Science is essentially a problem-solving activity” p. 111
“Science is accorded high value in our culture because, unlike many other intellectual endeavours, it appears capable of producing increasingly reliable knowledge” p 32
“Scientific knowledge differs from other kinds of knowledge, in particular from everyday knowledge, primarily by being more systematic.” p 143
1 Introduction
1.1 Sickle cell disease: a global health challenge
Sickle cell disease (SCD), encompassing a range of inherited erythrocyte disorders, affects millions globally, with the highest prevalence observed in sub-Saharan Africa, India, and the Mediterranean region (1). In the United States alone, approximately 100,000 individuals are afflicted by SCD, predominantly among those of African descent (2, 3). The disease is characterised by the synthesis of abnormal haemoglobin S, which causes erythrocytes to assume a crescent or “sickle” shape (4, 5). This genetic mutation triggers a cascade of severe health complications, including vaso-occlusive crises, which are episodes of intense pain emblematic of SCD; haemolytic anaemia resulting from the premature lysis of erythrocytes; an increased susceptibility to infections due to functional asplenia; and organ damage affecting the lungs, kidneys, liver, and brain. The intricate and chronic nature of SCD necessitates a comprehensive, evidence-based management strategy. Such strategies encompass the administration of analgesia to manage pain, prophylaxis against infections, and measures to mitigate both acute and long-term complications (6, 7). Effective management is thus contingent upon the integration of these multifaceted approaches to address the diverse and pervasive challenges posed by SCD.
1.2 The Cochrane Collaboration: championing evidence-based medicine
Founded in 1993, the Cochrane Collaboration has become a symbol of high-quality systematic reviews in the healthcare field (8). Named in honour of Archibald ―Archie― L. Cochrane, a distinguished British epidemiologist who passionately advocated for the use of randomised clinical trials in healthcare decision-making, this international non-profit organisation is committed to making up-to-date and accurate information about the effects of healthcare interventions widely accessible worldwide (9). Cochrane operates on fundamental principles that include cross-disciplinary and transnational collaboration, leveraging the passion and expertise of individuals, preventing redundant efforts, minimising bias through rigorous methodologies, keeping pace with emerging evidence, ensuring relevance to healthcare decision-making, facilitating access to systematic reviews, and continuously enhancing the quality of its outputs. These principles have established Cochrane as a leader in setting standards for systematic reviews, advancing methodologies for evidence synthesis, and shaping clinical practice guidelines and health policies on a global scale (10).
1.3 The intersection: a pathway to progress
The confluence of Cochrane’s methodological prowess with the urgent imperative for evidence-based management in SCD signifies a pivotal moment in healthcare research. Since the inaugural Cochrane review addressing SCD in 1996 (11), this collaboration has engendered a substantial corpus of high-quality evidence syntheses, thereby informing clinical practice, shaping research agendas, and guiding policy-making decisions.
As of August 2024, Cochrane has produced 62 reviews related to SCD, encompassing a diverse array of interventions ranging from pain management strategies to pioneering gene therapies. These systematic reviews have not only amalgamated extant knowledge but have also elucidated lacunae within current research paradigms, thereby catalysing subsequent studies and ensuring the judicious allocation of resources to the most exigent areas.
This manuscript endeavours to elucidate the profound ramifications of this intersection, critically analysing how Cochrane reviews have revolutionised SCD research and management over the past three decades. By scrutinising the breadth and profundity of Cochrane’s contributions, we identify key advancements, enduring challenges, and prospective trajectories in the ongoing battle against SCD.
Employing a data-driven methodology, augmented by illustrative graphics, we demonstrate how the rigorous standards of Cochrane reviews have enhanced the quality of evidence underpinning SCD research, ultimately translating into superior patient care and outcomes. This investigation not only underscores the efficacy of evidence-based medicine (12, 13) but also exemplifies how systematic reviews can propel progress across diverse healthcare domains.
As we navigate this exploration of Cochrane reviews within the context of SCD, we invite readers to recognise the synergistic interplay between these two titans of medical research and evidence-based practice. Furthermore, we encourage envisioning the future potential that their sustained collaboration holds for the millions afflicted by SCD globally.
In brief, the research question was: How has Cochrane’s evidence synthesis shaped research outputs and identified gaps in clinical evidence for SCD?
2 The intersection of Cochrane and sickle cell disease research: a pathway to progress
The convergence of the Cochrane Collaboration, now simply known as Cochrane, with SCD research represents a seminal moment in the progression of evidence-based medicine, particularly within the realm of chronic diseases. This timeline delineates the pivotal milestones of this journey, illustrating how Cochrane’s methodical approach to healthcare has profoundly influenced SCD research and clinical practice.
The narrative commences in 1972 with the visionary contributions of Archie Cochrane (9), a pioneering advocate for RCTs. Cochrane’s insistence on the necessity of rigorous clinical trials laid the foundational ethos for what would evolve into a global movement centred on systematic reviews—comprehensive evaluations of evidence that underpin informed medical decision-making. His enduring legacy established the groundwork for the eventual formation of Cochrane and its mission to ensure that healthcare decisions are anchored in the most reliable and current evidence available.
In 1993, the establishment of The Cochrane Collaboration marked a watershed in the field of medical research (8). This international consortium of researchers and healthcare professionals was conceived to systematically organise and appraise medical research, thereby ensuring that healthcare interventions are informed by dependable and exhaustive evidence. This initiative was transformative not only for healthcare at large but also had significant ramifications for diseases such as SCD, which had historically been under-researched and inadequately addressed.
The year 1996 emerges as a landmark in the context of SCD. It was during this year that Dr. Kassam Mahomed conducted the inaugural Cochrane review focused on SCD (11), encompassing one randomised clinical trial: Prophylactic versus selective blood transfusion for sickle cell anaemia during pregnancy (11). This review constituted a critical milestone in SCD research, providing a structured and evidence-based framework for understanding and enhancing the treatment of this debilitating condition. Dr. Mahomed’s work offered invaluable insights into the management of SCD in pregnant women, a demographic particularly susceptible to complications.
As of 17 August 2024, Cochrane’s unwavering commitment to advancing SCD research is epitomised by the completion of 62 Cochrane reviews pertaining to the disease (14). These reviews constitute a substantial body of work that has not only deepened the collective understanding of SCD but also informed clinical guidelines and treatment protocols, thereby directly influencing patient care and outcomes on a global scale (15–17).
Looking forward, the timeline envisages a future wherein sustained advancements in research and systematic reviews will continue to refine and enhance the management of SCD. The ongoing dedication to evidence-based research holds the promise of driving innovation, improving clinical practice, and ultimately transforming the lives of those afflicted by SCD worldwide (18).
The illustration demonstrates how these leaders have shaped SCD research within the Cochrane framework, offering a comprehensive summary of their lasting legacy. By outlining the chronological developments and key contributions, the figure highlights the profound impact of their work on Cochrane’s methodological standards and the advancements in SCD research. This visual not only honours the foundational efforts of these pioneers but also situates their influence within the broader context of evidence-based medicine (Figure 1).
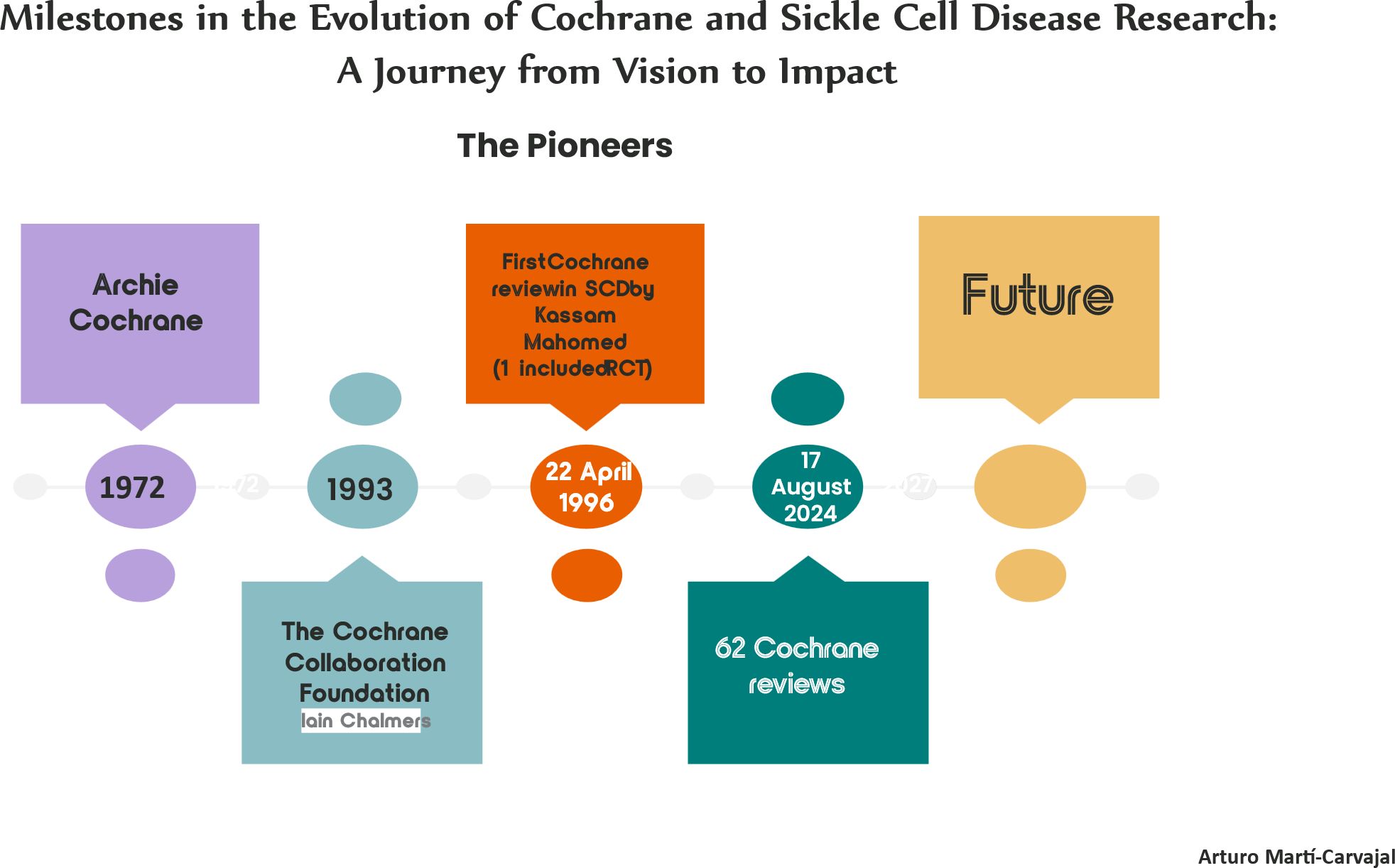
Figure 1. Key milestones in the development of cochrane and sickle cell disease research: a journey from vision to impact.. Milestones in the evolution of cochrane and sickle cell disease research: a journey from vision to impact. It provides a clear and concise visual representation of the key milestones in the development of Cochrane and SCD research, highlighting the main individuals who led these advancements. Source: references (8, 9, 11).
3 Materials and methods
We conducted a comprehensive analysis of Cochrane reviews focused on SCD. We mapped the thematic coverage and content domains of these reviews, rather than evaluating their methodological quality. We implemented a systematic framework with predetermined sequential steps to ensure rigorous examination of the review content.
3.1 Data collection
We undertook a thorough search of the Cochrane Library database through 17 August 2024. Our comprehensive search strategy identified 62 Cochrane reviews directly related to SCD.
3.2 Review selection
From our initial pool of 62 reviews, we meticulously selected 49 reviews that primarily addressed the management and treatment of SCD (11, 19–65). We judiciously excluded reviews that mentioned SCD peripherally or focused on related but distinct conditions, thereby ensuring the specificity and relevance of our analysis.
3.3 Data extraction
For each of the 49 selected reviews, we systematically extracted data from their respective abstracts. We collected the Cochrane ID (CD code), publication year, number of authors, countries of the corresponding author and co-authors, number of included randomised clinical trials (RCTs), total number of participants, presence or absence of meta-analyses, as well as key findings and recommendations. By gathering these specific pieces of information, we ensured a comprehensive and structured analysis of each review, facilitating a thorough understanding of the research landscape and the contributions of each included study.
3.4 Quantitative analysis
We conducted a quantitative examination of the extracted data, focusing on several key metrics. First, we assessed the average number of included RCTs per review to gauge the intensity of research within each review’s defined scope regarding RCT inclusion criteria. Next, we evaluated the average number of participants per review to determine the scale of participant involvement across the studies. We also analysed the temporal distribution of reviews to understand the number of reviews published over time. Additionally, we determined the proportion of reviews incorporating meta-analyses to assess the extent to which meta-analytical techniques were employed.
3.5 Thematic analysis
We performed a thematic analysis to categorise the reviews into principal domains of SCD management, including but not limited to pain control, infection prevention, and the management of complications. This qualitative assessment facilitated the identification of prevailing themes and gaps within the existing body of research, providing a clearer understanding of the focus areas and areas needing further investigation. We inserted mental map within the taxonomy, as we believe this approach offers a deeper and more epistemologically robust perspective.
3.6 Visualisation
To enhance data interpretation and clarify complex relationships, we developed several types of visual representations: bar graphs, scatter plots, and three-dimensional principal component analysis (PCA) graphs, and mental map. The assistance of ChatGPT 4.o in generating these graphical representations ensured clarity and precision in the visual dissemination of our findings.
Through this rigorous methodological approach, our study endeavoured to provide a comprehensive and nuanced understanding of the impact and evolution of Cochrane reviews in the realm of SCD research. The integration of both quantitative and qualitative analyses, complemented by sophisticated visualisations, ensures a robust and insightful examination of the subject matter, thereby contributing valuable knowledge to the field of evidence-based medicine.
4 Results
The analysis of 49 Cochrane’s abstracts on SCD revealed several key findings.
4.1 Review characteristics
We identified that 34.7% ≈ 35% (17/49) of Cochrane reviews did not include any RCTs. This phenomenon is renamed as “empty” systematic reviews.
After excluding systematic reviews that did not include RCTs, the median number of RCTs in the remaining reviews was three. For these reviews, the 25th percentile was 1.75, and the 75th percentile was five. Following this adjustment, among the systematic reviews that included at least one RCT, the participant data revealed the following insights: the median number of participants per review was 260, the 25th percentile was 112, and the 75th percentile was 555.
4.2 Meta-analyses characteristics
Remarkably, none of the 32 reviews incorporating RCTs conducted meta-analyses. Of 32 Cochrane reviews with RCTs, 5 (15.6%) included one RCT and 27 (84.4%) included multiple RCTs. A recent review illustrates this pattern: from 26 included studies, only eight reported important outcomes at 6 months, which were distributed across 17 different comparisons, precluding statistical pooling (61).
4.3 Temporal distribution
The first Cochrane review on SCD was published in 1996 (11), followed by a gradual increase in the number of reviews over the years. Figure 2 illustrates the annual trends in Cochrane reviews on SCD between 1994 and 2024, highlighting three key metrics: the total number of reviews published (blue solid line), the number of RCTs included in these reviews (orange dashed line), and the number of meta-analyses conducted (red dotted line). The period from 2015 to 2024 saw the highest concentration of reviews, reflecting a growing research focus and an accumulation of evidence on SCD management.
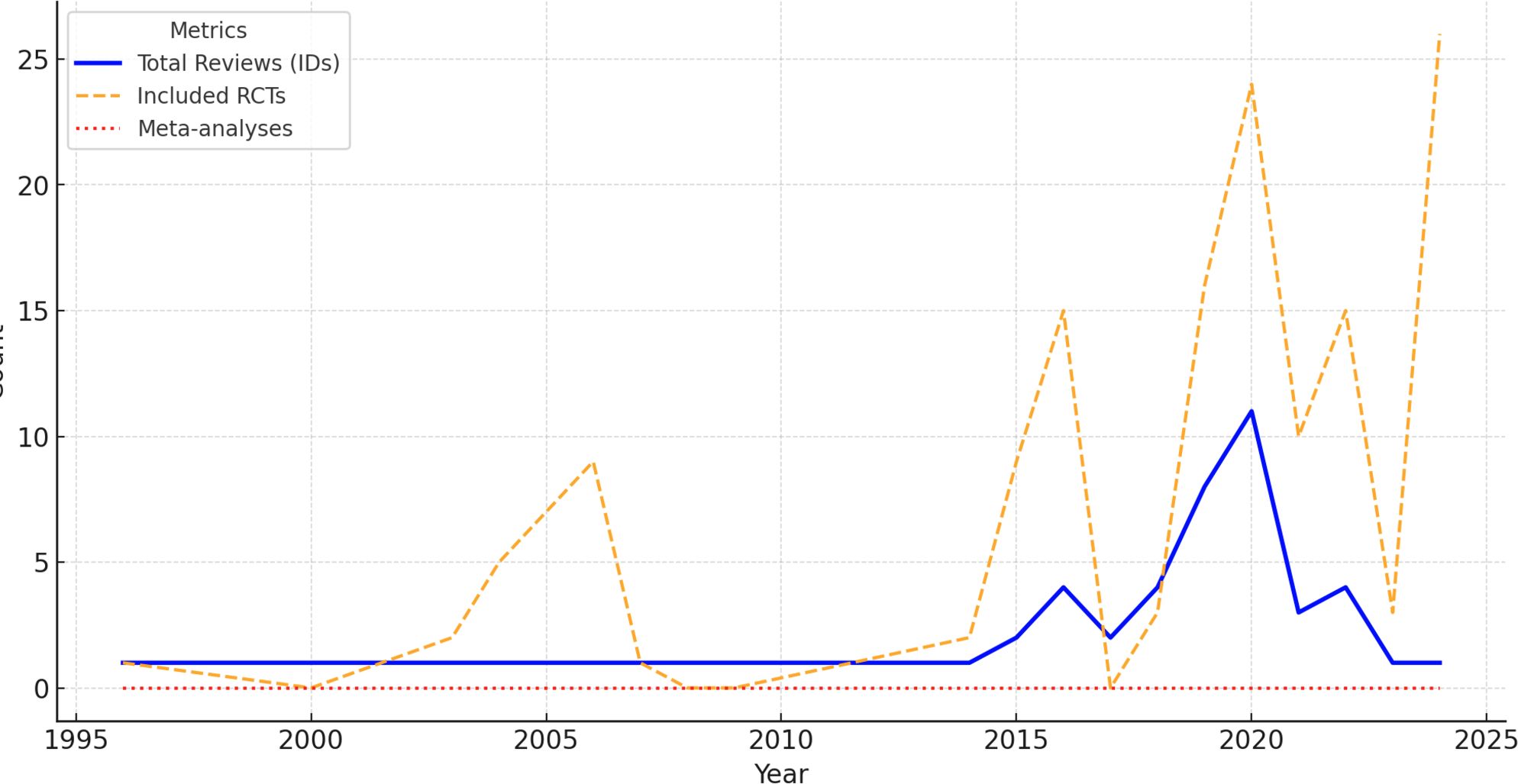
Figure 2. Yearly trends in cochrane reviews for sickle cell disease (1994–20224). The chart displays yearly trends of 49 Cochrane reviews on SCD (1994–2024), including RCTs and meta-analyses. While RCT inclusion increased, meta-analyses remain negligible. Source: Analysis of 49 Cochrane reviews on Sickle Cell Disease, 1996-2024 (Martí-Carvajal, 2024).
Over this 30-year period, a total of 49 reviews were published. While the inclusion of RCTs shows a notable rise, particularly after 2010, the near-complete absence of meta-analyses is striking, as indicated by the flat red line. Despite the increasing availability of RCTs, their synthesis through meta-analysis has not been undertaken, highlighting a significant gap in evidence synthesis within Cochrane’s research on SCD. See Figure 2.
4 Geographic distribution of Cochrane reviews
Our analysis of the 49 Cochrane reviews on SCD revealed a diverse but uneven global distribution of research leadership (Figure 3).
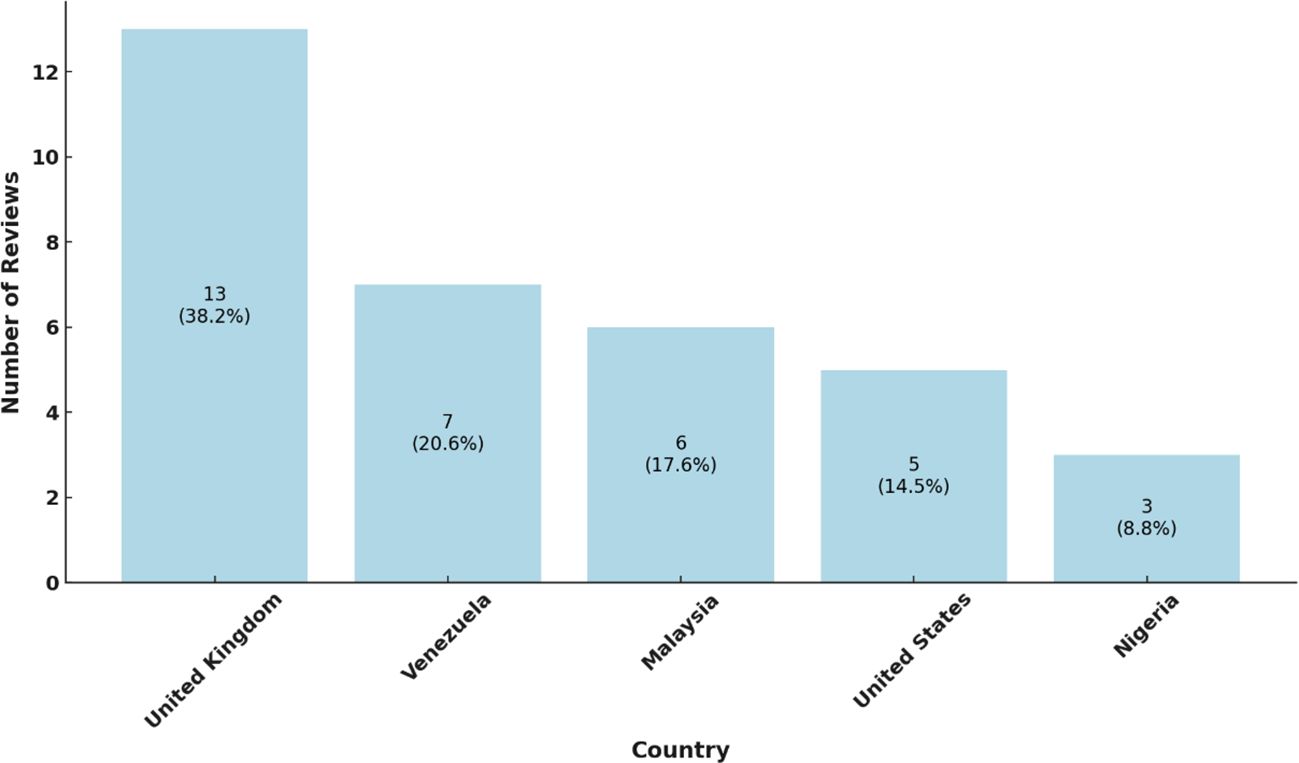
Figure 3. Review distribution by country. The chart illustrates the number of reviews per country, with each bar indicating the country’s share of the total reviews: United Kingdom, Venezuela, Malaysia, The United States and Nigeria. The percentages are showing each country’s proportion within the dataset. This distribution highlights regional contributions to the overall review count. Source: Analysis of 49 Cochrane reviews on SCD, 1996-2024 (Martí-Carvajal, 2024).
Meanwhile, Figure 4 illustrates the distribution of reviews by continent.
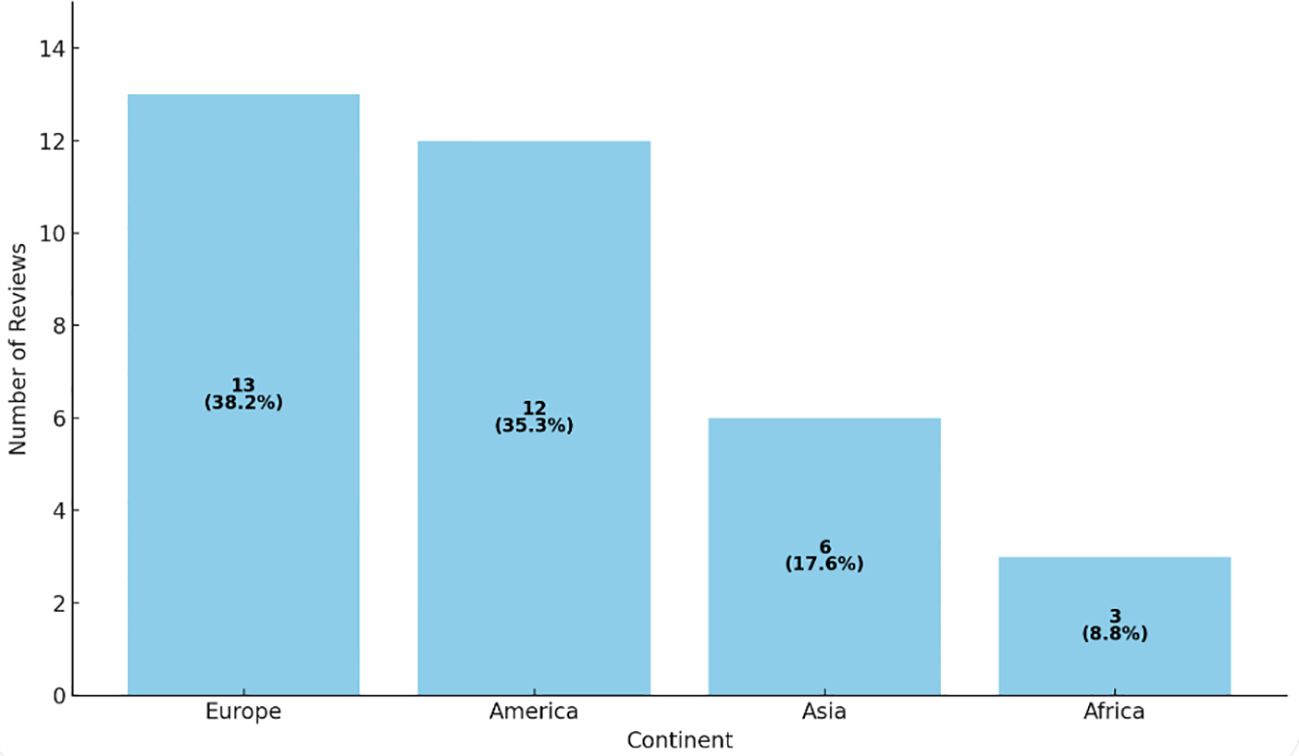
Figure 4. Distribution of reviews by continent. illustrates the distribution (number and percentage) of reviews by continent. It is indeed striking that most Cochrane reviews on SCD originate in Europe and the Americas, despite the condition’s high prevalence in Africa. This highlights a potential mismatch between research priorities and actual disease burden, suggesting gaps in funding, infrastructure, or production capacity in the regions most affected. Source: Analysis of 49 Cochrane reviews on Sickle Cell Disease, 1996-2024 (Martí-Carvajal, 2024).
5 International collaborative efforts
Our analysis revealed that 32 of the 49 reviews (67.43%) involved international collaborations. These efforts are detailed in references (11, 20–28, 30, 32–34, 38, 41, 43–47, 49–51, 54–56, and 58–63). Figure 5 illustrates the global network for sickle cell disease research within the Cochrane framework, with the United Kingdom at its core. The UK serves as a central hub, maintaining extensive collaborations across numerous countries and demonstrating its pivotal role and significant contributions to advancing this field. Venezuela maintains strong collaborative ties with Spain, Brazil and Jamaica, while its cooperation with the USA is more moderate. In contrast, the United States and Malaysia exhibit high connectivity within the network, reflecting their prominent roles in sickle cell research initiatives.
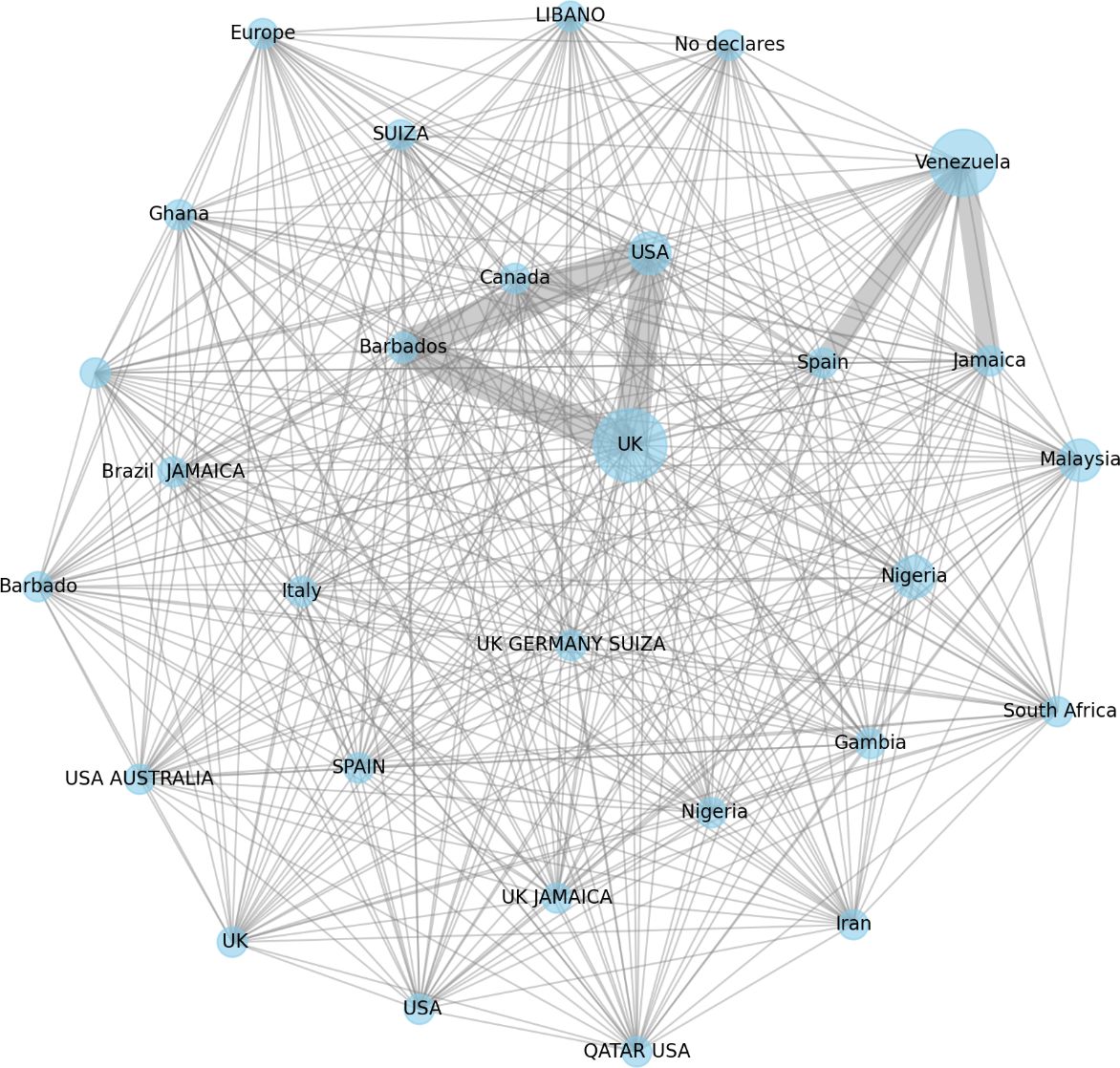
Figure 5. Global collaborative network on sickle cell disease research by cochrane. The graph depicts Cochrane-facilitated international collaborations in sickle cell disease research. Node size indicates each country’s central role, with the UK as the primary hub and Venezuela as a key collaborator with Brazil, Jamaica, and Spain. Edge thickness represents the strength of these partnerships, while missing connections, such as between Nigeria and other countries, highlight gaps in collaboration. In this figure includes a “no declares” label, which should be interpreted as indicating that the first Cochrane review on SCD was conducted by a single author. Therefore, there are no countries to list. Source: Analysis of 49 Cochrane reviews on Sickle Cell Disease, 1996-2024 (Martí-Carvajal, 2024).
The collaborative landscape spans North America, Europe, Africa, South America, and Asia, highlighting a unified global dedication to advancing sickle cell disease research. Historical and cultural relationships, such as those between Nigeria and the UK or Jamaica and the UK, further facilitate these medical research partnerships.
The varying thickness of the connecting lines in the network represents the frequency of collaborations, with thicker lines indicating more consistent partnerships. Notable collaborations, particularly those involving Venezuela with Spain, Brazil and Jamaica, suggest targeted joint research initiatives.
These gaps present opportunities for establishing new partnerships, which could enhance collaborative efforts and leverage mutual strengths and resources.
Overall, the global network visualisation underscores the extensive international commitment to Cochrane-led sickle cell disease research. The interconnectedness of diverse nations reflects a collective effort to advance medical knowledge and treatment through robust, cross-border collaborations, demonstrating a unified objective to combat and understand sickle cell disease worldwide.
6 Taxonomical distribution of three decades of Cochrane reviews in sickle cell disease
The systematic categorisation of 49 Cochrane reviews (1996-2024) (11, 19–66) reveals a sophisticated taxonomy of evidence across the spectrum of SCD management. This distribution reflects both the complexity of the condition and the evolving priorities in clinical research (Figure 6).
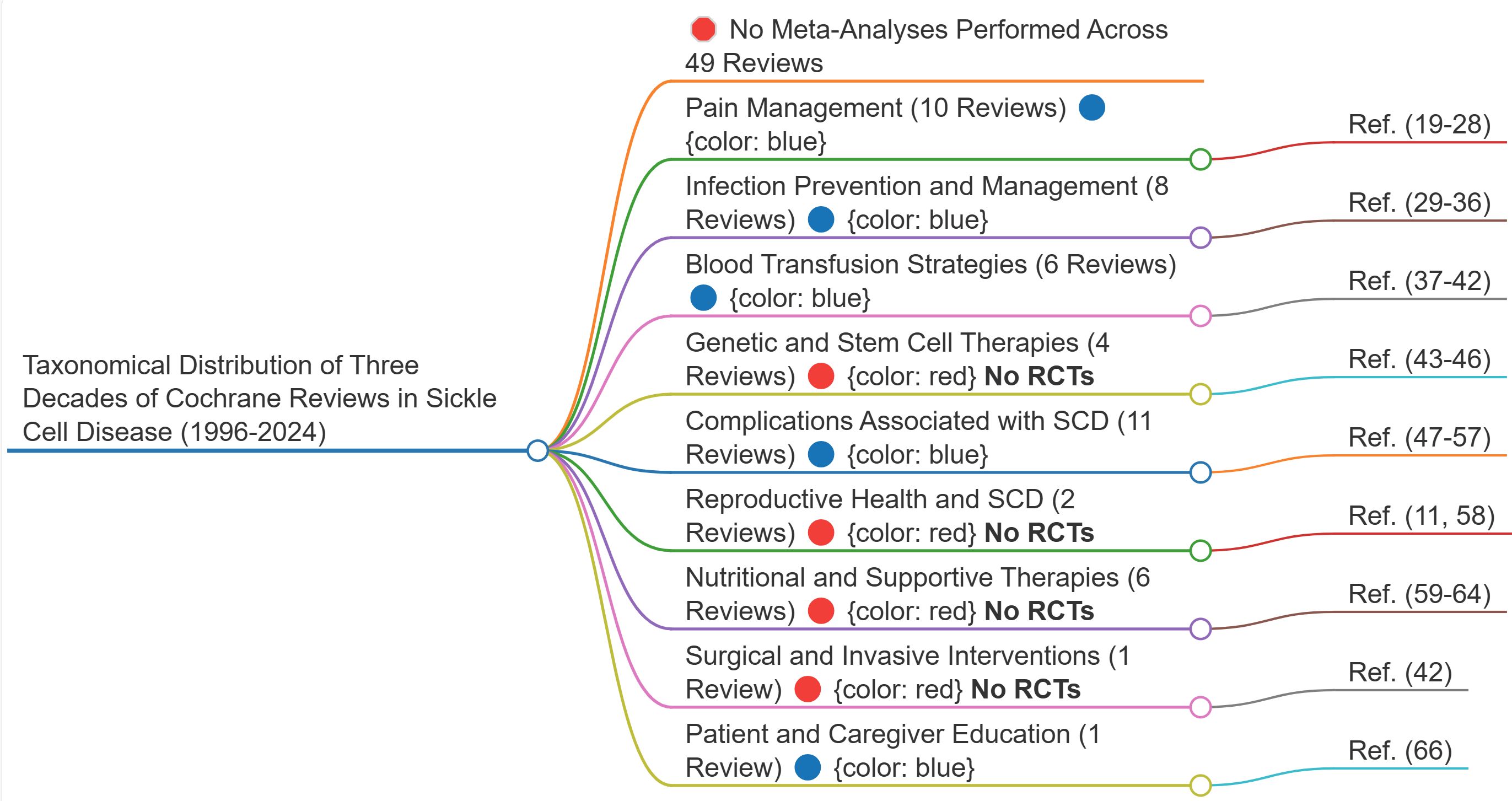
Figure 6. Mental map of the taxonomical distribution of three decades of cochrane reviews in sickle cell disease (1996-2024). This map does not merely visualise research gaps—it exposes a deeper epistemic structure. The fragmentation seen at every level of SCD research is not incidental; it follows a fractal pattern, where knowledge is produced but not synthesised, perpetuating a self-replicating cycle of disconnection. This structural failure prevents evidence from forming a coherent whole, reinforcing clinical uncertainty. The implications extend beyond research inefficiency: they reveal a form of epistemic injustice embedded in the very architecture of medical science. See Discussion for full analysis. Source: Analysis of 49 Cochrane reviews on Sickle Cell Disease, 1996-2024 (Martí-Carvajal, 2024).
This taxonomical distribution provides a strategic framework for understanding the current evidence landscape while highlighting areas requiring further investigation. The relative proportions of reviews across categories offer valuable insights into research priorities and the complexity of SCD management over three decades of systematic investigation.
The synthesis of three decades of Cochrane reviews (1996-2024) in SCD represents a landmark in evidence-based medicine, documenting the evolution of therapeutic approaches across nine interconnected management categories through 49 comprehensive reviews.
6.1 Pain management
Pain management stands as the most extensively researched domain in SCD, encompassing 10 comprehensive Cochrane reviews. The foundation begins with broad pain management strategies for both children and adults (19), recognising the lifelong impact of SCD-related pain. Innovative approaches include transcutaneous electrical nerve stimulation (20), which offers a non-pharmacological option for pain relief. The evidence extends to specific pharmacological interventions for adults experiencing vaso-occlusive crises (21), whilst piracetam has been evaluated for its potential in reducing crisis incidence (22).
Pregnancy presents unique challenges in pain management, warranting dedicated research into crisis management during this vulnerable period (23). The psychological dimension of pain receives attention through reviews of psychological therapies (24), acknowledging pain’s complex biopsychosocial nature. Fluid replacement therapy (25) and interventions for neuropathic pain (26) demonstrate the diverse therapeutic approaches available. Advanced interventions including low-molecular-weight heparins (27) and inhaled nitric oxide (28) represent more recent developments in crisis management.
6.2 Infection prevention and management
The infectious disease spectrum in SCD has garnered significant attention across eight reviews. Prophylactic antibiotics for pneumococcal infection in children (29) form a cornerstone of preventive care, complemented by research into conjugate Haemophilus influenzae type b vaccines (30). The management of community-acquired pneumonia (31) and prevention of invasive salmonella infections (32) reflect the breadth of infectious challenges faced by SCD patients.
Acute chest syndrome, a severe complication, has prompted specific antibiotic research (33), whilst pneumococcal vaccination strategies (34) remain crucial for prevention. In regions where SCD and malaria coexist, malaria chemoprophylaxis (35) proves vital. Osteomyelitis, a significant infectious complication, has warranted dedicated review of antibiotic approaches (36).
6.3 Blood transfusion strategies
Transfusion medicine in SCD encompasses six pivotal reviews, addressing various clinical scenarios. Pregnancy-related transfusion strategies compare prophylactic versus selective approaches (37), whilst stroke prevention through transfusion has received thorough evaluation (38). Acute chest syndrome management through transfusion (39) and long-term transfusion for chronic chest complications (40) demonstrate the versatility of transfusion therapy. Preoperative transfusion protocols (41) and management of acute sequestration crises through splenectomy versus conservative approaches (42) complete this comprehensive evidence base.
6.4 Genetic and stem cell therapies
The management of SCD has evolved significantly from early detection through neonatal screening programs, as established by Lees and colleagues’ landmark review, to current therapeutic interventions. Early identification through screening has been crucial in initiating timely interventions and improving patient outcomes (43). Building upon this foundation, the implementation of hydroxyurea therapy, thoroughly evaluated in Rankine-Mullings and Nevitt’s comprehensive review (44), has become a cornerstone in disease management, demonstrating significant clinical benefits in reducing pain crises and improving quality of life.
The frontier of curative approaches now extends beyond these conventional treatments, exploring groundbreaking therapeutic possibilities through gene therapy (45) and haematopoietic stem cell transplantation (46). These potentially transformative interventions represent a paradigm shift in SCD treatment, aiming to address the fundamental genetic defect underlying the disease. While neonatal screening enables early intervention and hydroxyurea provides symptomatic relief, these newer genetic and cellular approaches offer the promise of potential cure by targeting the disease at its genetic roots.
These reviews evaluate potentially transformative interventions that aim to address the fundamental genetic defect in SCD.
6.5 Complications associated with SCD
The multisystem nature of SCD complications is reflected in twelve detailed reviews. Priapism management (47) addresses a significant male health concern. Respiratory complications receive extensive coverage through reviews of acute chest syndrome treatments (39, 48) and bronchodilator therapy (49). Renal manifestations are addressed through reviews of chronic kidney disease interventions (50) and ACE inhibitors for proteinuria (51).
Musculoskeletal complications, particularly avascular necrosis (52), and dermatological issues such as leg ulcers (53) demonstrate the disease’s widespread impact. The spectrum extends to intrahepatic cholestasis (54), dental complications (55), silent cerebral infarcts (56), and retinopathy (57).
6.6 Reproductive health and SCD
Reproductive health considerations are captured in two essential reviews examining contraceptive options through steroid hormones (58) and transfusion strategies during pregnancy (11), addressing crucial aspects of women’s health in SCD.
6.7 Nutritional and supportive therapies
Nutritional support encompasses diverse approaches including folate supplementation (59), magnesium therapy (60), and antioxidant supplementation (61). Plant-derived medicines (62) offer traditional therapeutic perspectives, while prevention of red blood cell dehydration (63) addresses cellular pathophysiology. Vitamin D supplementation (64) and iron overload management through deferasirox (65) complete the nutritional support evidence base.
6.8 Surgical and invasive interventions
Surgical management, particularly regarding splenectomy versus conservative approaches for acute sequestration crises (42), represents a critical decision point in SCD care.
6.9 Patient and caregiver education
The vital role of education is addressed through comprehensive review of interventions to improve knowledge and complication recognition among patients and caregivers (66), underpinning the importance of informed self-management in SCD care.
This extensive body of Cochrane evidence, comprising 49 reviews spanning nearly three decades, demonstrates the evolution and sophistication of SCD management. The reviews reflect both the complexity of care required and the ongoing commitment to developing evidence-based approaches across all aspects of this challenging condition (Box 1).
Box 1. Key messages and future directions from three decades of Cochrane reviews in sickle cell disease (1996-2024).
1. Evidence Base
• 34.7% ‘empty’ reviews (no randomised clinical trials)
• Median of 3 trials per review (IQR: 1.75-5)
• 260 median participants (IQR: 112-555)
• Absence of meta-analyses across all reviews
2. Research Distribution
• Pain Management and Complications: 22 reviews
• Infection Prevention and Transfusion: 15 reviews
• Genetic Therapies and Nutritional Support: 12 reviews
3. International Collaboration
• 67.43% cross-border partnerships
• United Kingdom and Venezuela as primary collaborative hubs in Europe and South America, respectively.
• Limited representation from high-burden regions
4. Future Priorities
• Conduct randomised clinical trials in identified gap areas
• Implement meta-analyses where sufficient trials exist
• Expand collaboration with affected regions
• Standardise outcome measures across trials
IQR, Interquartile Range.
7 Fractal representation of evidence: Cochrane reviews on sickle cell disease — strengths and gaps in the systematic synthesis
This section builds upon the Taxonomical Distribution of Three Decades of Cochrane Reviews in Sickle Cell Disease, incorporating the number of participants in each included RCT to provide a more detailed representation of the evidence base. Figure 7 presents a fractal analogy between the tracheobronchial tree and the structure of evidence synthesis in systematic reviews, illustrating how systematic reviews develop and consolidate knowledge in a hierarchical, self-similar manner. This concept is rooted in fractality in biomedicine, where structures exhibit self-similarity across different scales, reinforcing the organisation of systematic evidence synthesis.
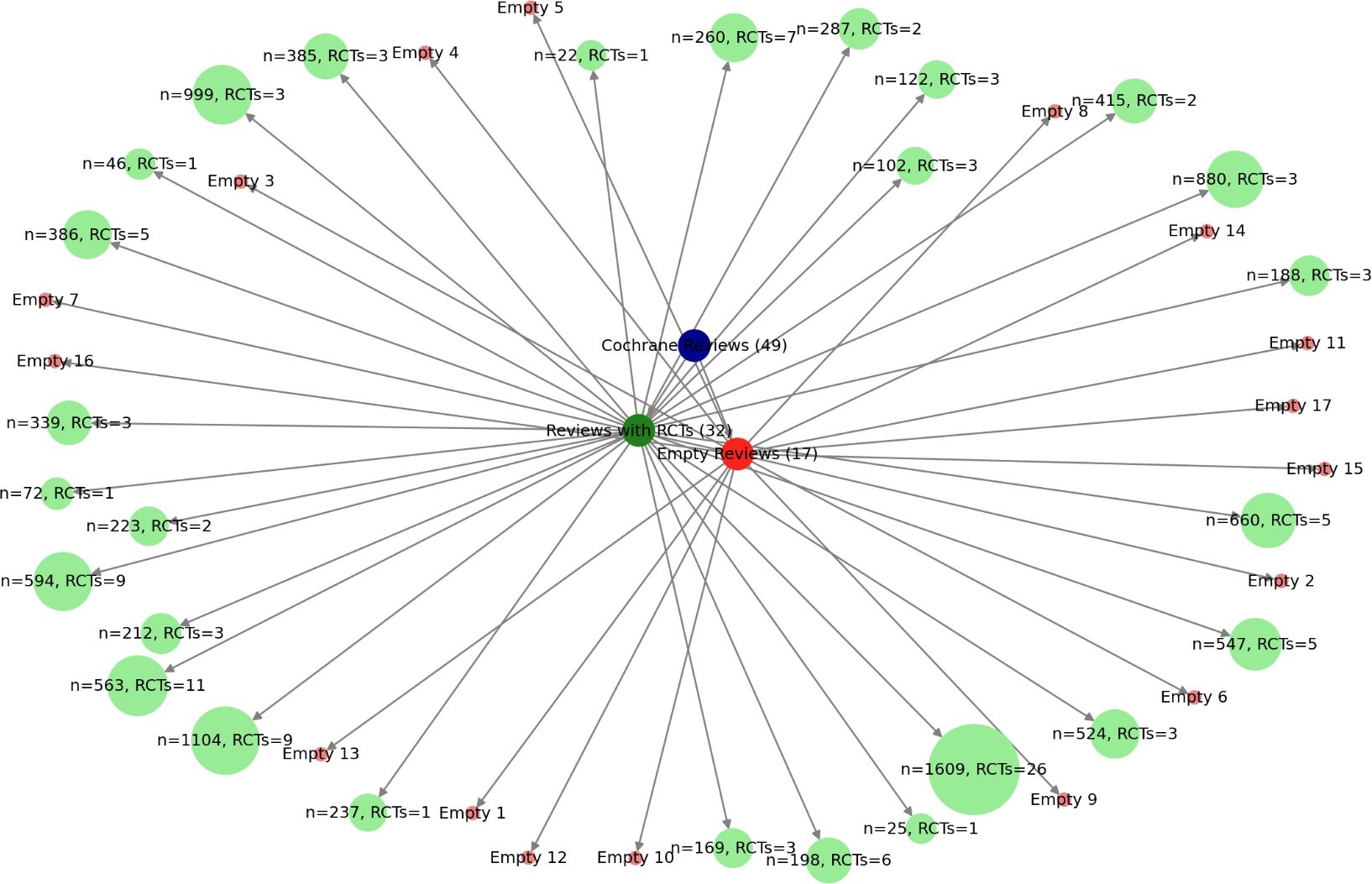
Figure 7. Fractal architecture of evidence: the tracheobronchial analogy in cochrane reviews on sickle cell disease (1996-2024). This fractal representation visualises the systematic structure of the 49 Cochrane reviews on SCD, highlighting the hierarchical organisation of evidence. Reviews with RCTs (green) form the backbone of evidence synthesis, while empty reviews (red) expose critical gaps where trials are lacking. The varying node sizes reflect the weight of each review based on participant numbers and included RCTs, illustrating the density and distribution of clinical research. This visualisation underscores the necessity of robust trial data for a truly consolidated meta-analytic synthesis. Source: Analysis of 49 Cochrane reviews on Sickle Cell Disease, 1996-2024 (Martí-Carvajal, 2024).
In this model:
● The root node with 49 reviews represents the trachea, the main conduit through which evidence enters the synthesis process.
● The green nodes (32 reviews with RCTs) represents a well-developed fractal bronchial tree, where hierarchical branching allows evidence to expand from individual trials to systematic aggregation, including potential meta-analyses.
● The red nodes (17 empty reviews) signifies an underdeveloped airway, where the absence of RCTs disrupts the fractal structure, preventing further branching and consolidation of evidence.
This analogy demonstrates that systematic reviews follow a fractal respiratory pattern, where the presence of RCTs enables the evidence base to expand and function effectively. Just as a healthy tracheobronchial tree ensures optimal gas exchange, a well-structured evidence synthesis process, rooted in RCTs, facilitates robust and meaningful conclusions. Conversely, when RCTs are absent, the evidence pathway is obstructed, mirroring the dysfunction of an atrophic airway incapable of efficient gas exchange.
Furthermore, the size of each node reflects the number of participants and RCTs included in each review, providing a visual representation of where evidence is concentrated and where gaps exist. Thus, just as bronchi are essential for respiration, RCTs are indispensable for building a coherent and reliable body of evidence in systematic reviews.
8 Research emphasis in sickle cell disease interventions: a comparative analysis of key categories
Figure 8, a 3D graph, provides a comparative visualisation of the focus areas in SCD interventions as documented in Cochrane reviews from 1996 to 2024. The plot aims to convey the relative research emphasis across three primary intervention categories: Pain Management and Complications, Infection Prevention and Transfusion, and Genetic Therapies and Nutritional Support. Each of these categories is represented as a distinct point in the 3D space, positioned according to the volume of references associated with it. The following breakdown shows the key aspects to consider:
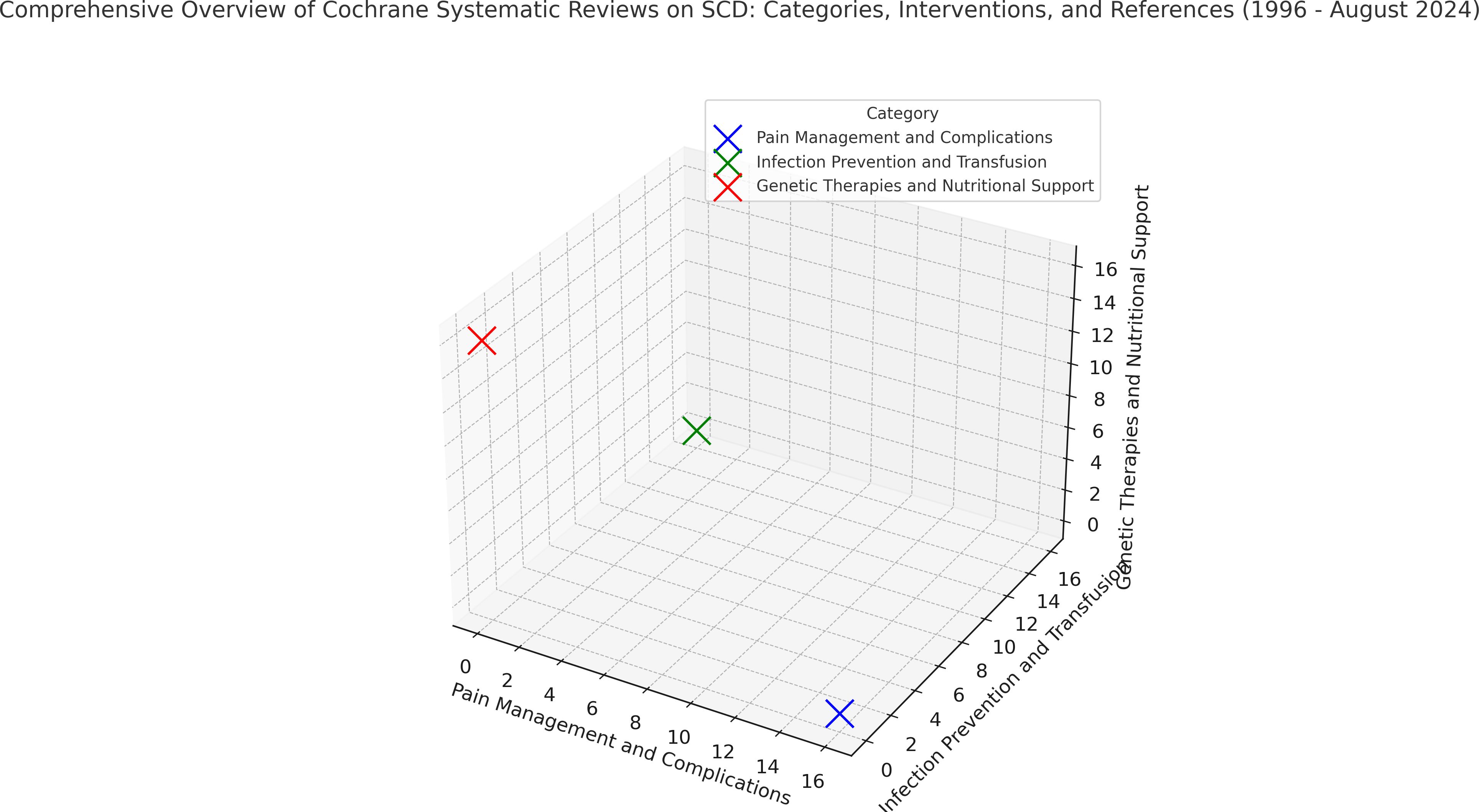
Figure 8. 3D representation of SCD intervention categories based on cochrane reviews (1996-2024). This 3D representation illustrates the comparative emphasis on primary intervention categories in Sickle Cell Disease based on Cochrane reviews from 1996 to 2024. Each point’s distance from the origin reflects the volume of systematic reviews within that category, with greater distances indicating higher research focus. This figure serves as an overview of research trends, highlighting areas of extensive study and potential gaps in intervention approaches. Source: Analysis of 49 Cochrane reviews on Sickle Cell Disease, 1996-2024 (Martí-Carvajal, 2024).
8.1 Axes and categories
● The X-axis represents Pain Management and Complications, which includes studies focusing on managing acute and chronic pain in SCD, as well as addressing related complications such as organ damage due to vaso-occlusive episodes (Total reviews: 22) (19–28, 42, 47–57).
● The Y-axis represents Infection Prevention and Transfusion. This category encompasses interventions aimed at preventing infections, which are common in SCD due to a compromised immune system, and various strategies surrounding blood transfusions (Total reviews: 15) (11, 29–41, 65).
● The Z-axis represents Genetic Therapies and Nutritional Support, an area that covers advancements in genetic treatments, nutritional recommendations, and supplementary care designed to support overall health in SCD patients (Total reviews: 12) (43–46, 58–64, 66).
8.2 Position and distance from the origin
● The origin is the point at which all three axes intersect at zero. It serves as the starting point in 3D space, representing a zero reference count for each category. In this graph, each category’s point is placed at a certain distance from the origin based on the volume of references associated with that category.
● A greater distance from the origin means a higher volume of references, indicating that this category has received more research attention. Conversely, a point closer to the origin reflects a smaller number of references, suggesting less emphasis on that category in the analysed Cochrane reviews.
8.3 Interpretation of each category’s placement
● In this specific plot:
➢ Pain Management and Complications (in blue on the X-axis) appears furthest from the origin, signalling that this category has received substantial research attention and has a larger body of references (N=22). This aligns with the recognised importance of managing pain, one of the most distressing and common symptoms for SCD patients (19–28, 42, 47–57).
➢ Infection Prevention and Transfusion (in green on the Y-axis) holds an intermediate position, reflecting a moderate number of reviews in this area (N =15). This suggests a considerable focus on managing infections and transfusions, crucial due to the high infection risk in SCD and the role of transfusions in managing anaemia (11, 29–41, 65).
➢ Genetic Therapies and Nutritional Support (in red on the Z-axis) is closest to the origin, indicating that it has the smallest number of references among the three (N = 12). While it represents emerging and supplementary approaches, this position suggests relatively less research focus on genetic and nutritional support in the Cochrane reviews of the specified years (43–46, 58–64, 66).
8.4 Overall insights and research trends
● This graph allows researchers, clinicians, and stakeholders to quickly assess which intervention areas have been more extensively studied and which may benefit from further research.
● The emphasis on Pain Management and Complications likely reflects a prioritised response to one of the most immediate and distressing symptoms of SCD. Meanwhile, the moderate focus on Infection Prevention and Transfusion is consistent with the critical role of these strategies in managing SCD’s systemic impacts.
● The positioning of Genetic Therapies and Nutritional Support may indicate either a relatively new interest in these interventions or a complementary role, which could change as the field progresses towards more personalised and holistic approaches to SCD management.
This graph effectively summarises 30 years of Cochrane research focused on SCD, illustrating the areas of highest emphasis and shedding light on potential gaps where further investigation could support comprehensive patient care.
9 Discussion
This pioneering meta-research epidemiological study of 49 Cochrane reviews on SCD from 1996 to 2024 underscores the profound impact of Cochrane on SCD research and clinical practice over nearly three decades.
9.1 Main results
The breadth and depth of evidence synthesised across three wide categories embody seven domains, highlighting significant advancements in evidence-based medicine, clinical practice, patient-centred care, and public health policy. However, the study also reveals critical limitations that illuminate substantial gaps in SCD research, emphasising the need for targeted future investigations.
9.1.1 Systematic review characteristics
o 35% of the reviews lacked RCTs, termed “empty” reviews, revealing limited evidence in certain areas.
o Reviews with RCTs had a median of three trials (25th percentile: 1.75; 75th percentile: 5) and a median participant count of 260 (25th percentile: 112; 75th percentile: 555).
9.1.2 Meta-analyses
o None of the 32 reviews with RCTs conducted a meta-analysis.
9.1.3 Temporal trends
o The number of reviews grew steadily, peaking between 2015 and 2024. However, the absence of meta-analyses persisted, as indicated by a flat trendline.
9.1.4 Geographic and collaborative contributions
o The UK emerged as a central collaboration hub, maintaining partnerships across Europe, North America, Africa, and Asia. Venezuela demonstrated strong regional ties with Spain, Brazil and Jamaica, reflecting targeted collaborative initiatives.
9.1.5 Research taxonomy
o Nine domains were identified. Pain management dominated with 22 reviews, followed by infection prevention and transfusion strategies (15 reviews) and genetic therapies and nutritional support (12 reviews).
9.1.6 Research gaps
o Figure 7 reveal contrasting trends: some reviews included substantial data, while others lacked participants or RCTs, highlighting under-researched areas.
9.1.7 Collaborative synergies
o International collaborations spanned five continents, showcasing a collective effort to advance SCD research. The UK’s leading role underscored its significant contributions, while Venezuela’s active partnerships in Latin America highlighted regional strengths.
9.2 Overall breadth and relevance of evidence
This study provides a comprehensive assessment of the completeness and applicability of evidence within Cochrane reviews on SCD management. As the first meta-research investigation systematically evaluating these reviews over three decades, it offers a novel perspective on the evolution and current state of evidence synthesis in SCD. The findings highlight critical gaps that hinder the development of comprehensive and equitable treatment protocols.
The absence of RCTs in 35% of Cochrane reviews represents a substantial gap in primary research. While domains such as pain management and infection prevention have received considerable attention, others, including genetic therapies and nutritional interventions, remain insufficiently explored. This disparity limits the establishment of reliable, evidence-informed clinical practices across the complex spectrum of SCD care. Furthermore, the absence of meta-analyses in reviews incorporating RCTs underscores a missed opportunity to synthesise findings into actionable, generalisable insights. This lack of synthesis restricts the translational impact of existing research, delaying the adoption of evidence-based approaches. As highlighted in broader discussions of scientific advancement (67), limitations in evidence synthesis—such as incomplete data or uneven research capacity—impede progress in critical areas like SCD management.
The findings also highlight significant challenges in applying existing evidence to clinical practice. The predominance of studies conducted in high-resource environments limits the relevance of findings to low-income regions, where the burden of SCD is most severe. Additionally, the absence of context-specific interventions for key issues, such as infection control and transfusion strategies, underscores the need for research tailored to diverse healthcare settings. The uneven distribution of global research capacity further exacerbates these challenges, leaving certain regions underrepresented in the evidence base.
Systemic barriers to generating complete and applicable evidence persist. Conducting large-scale RCTs in emerging areas, such as genetic therapies, is often constrained by rare target populations and ethical considerations. Similarly, logistical and infrastructural limitations disproportionately affect research efforts in resource-limited settings. These systemic inequities sustain disparities in both the evidence base and its application, creating variability in clinical practice and outcomes.
By systematically evaluating SCD research across three decades, this study highlights the urgent need to prioritise under-researched domains, adopt meta-analytical approaches, and enhance inclusivity in research design. The findings advocate for a globally coordinated effort to bridge capacity gaps and align research priorities with the needs of diverse populations. Strengthening the evidence base in SCD management will facilitate more equitable, evidence-driven advances in care.
9.3 The epistemic value of taxonomy in sickle cell disease research: a comprehensive analysis of Cochrane reviews
The taxonomy used in this study extends beyond categorising the body of evidence in SCD research within Cochrane reviews. It serves as a structural tool that reveals the limitations in how knowledge has been accumulated and processed. By systematically organising the Cochrane reviews, the taxonomy not only highlights evidence gaps but also uncovers deep fractures in the way this evidence is synthesised. The division of evidence into isolated reviews, without the unifying force of meta-analyses, exposes a broader issue—the fragmentation of knowledge in medical science.
This fragmentation becomes particularly evident when examining specific research areas—such as hydroxyurea, transfusions, or gene therapy. At every level of analysis, a recurrent pattern emerges: isolated findings, contradictory results, and an absence of convergence. This issue is not purely methodological—it represents a form of epistemic injustice, where knowledge is generated but not effectively integrated. The absence of meta-analyses at multiple levels of research leads to clinical uncertainty, undermining the potential for evidence-based decision-making (68–70). The true value of this taxonomical framework, therefore, lies in its capacity to expose failures in the architecture of medical knowledge.
What this taxonomy further reveals is not just the absence of meta-analyses, but a deeper, structural epistemic injustice4 that hinders the integration of knowledge across Cochrane reviews on SCD published between 1996 and 2024. Research fragmentation at global, thematic, and individual study levels reflects the same systemic failure. This failure does not stem solely from a lack of trials or data but from an epistemic system that prioritises accumulation over synthesis.
While each review contributes valuable insights, the absence of systematic integration of findings results in fragmented knowledge that fails to provide clinicians with stable, evidence-based guidance. This leads to medical uncertainty5—for clinicians, patients, and researchers alike. Anjum and Rocca define uncertainty as knowing the possible negative outcomes of an action but not knowing the odds, whereas risk involves knowing both the possible outcomes and their relative likelihoods (71).
This is not merely inefficiency; it represents an epistemic crisis that reveals the failure of medical science to construct knowledge as a coherent whole. The implications extend beyond methodological critiques. It is not just about study quality or the number of trials conducted, but about the failure to organise, synthesise, and consolidate research findings into actionable medical knowledge. Unless this epistemic system is restructured, medical practice will continue to be guided by fragmented evidence, leading to inconsistent clinical outcomes and suboptimal patient care.
9.4 Fractal epistemic injustice and the organisation of evidence in systematic reviews
Systematic reviews are the foundation of evidence-based medicine, guiding clinical decisions and health policies (72). However, the structure and completeness of the evidence base within systematic reviews are often overlooked when interpreting their impact (73). This study introduces a fractal analogy between the tracheobronchial tree and the distribution of evidence in systematic reviews, providing a novel perspective on how evidence synthesis is organised and where gaps exist.
Anjum and Rocca state that when scientists evaluate the risks of an intervention, conflicting predictions often arise—even when substantial evidence is gathered (71). This inconsistency stems not only from study heterogeneity but also from structural gaps in the evidence base, conceptualised here as fractal epistemic injustice.
Our analysis shows that systematic reviews with a well-developed fractal structure—i.e., a sufficient number of RCTs with large sample sizes—allow for a stable, hierarchical synthesis of evidence. These reviews resemble a fully developed tracheobronchial tree, where branching ensures a coherent flow of evidence that supports robust risk assessments.
9.5 Limitations and gaps in global research
The lack of significant collaboration from regions such as the Middle East and India, despite the high burden of SCD in these areas, highlights a critical gap in the global research landscape. This shortfall is clearly outlined in our review, emphasising the need for greater research attention and collaboration in these regions. It relates to Defining Global Strategies to Improve Outcomes in Sickle Cell Disease: A Lancet Haematology Commission (1).
This observation aligns with the principle of the economy of thought6, which advocates for the efficient and strategic use of information to guide decision-making and resource allocation. By identifying these underrepresented regions, our review serves as a foundational tool for redirecting research focus and collaborative efforts where they are most needed.
Identifying these deficiencies is not just a critique of existing research practices but a success of the review process itself—bringing attention to areas that require urgent engagement. These findings should not be seen merely as gaps but as opportunities for new collaborations and initiatives that could significantly impact global health outcomes in SCD management.
9.6 Implications for future research and clinical practice
The findings underscore the imperative for future studies to target underexplored domains within SCD management. Addressing these gaps is vital for developing comprehensive clinical guidelines and improving patient care. Furthermore, the incorporation of meta-analyses in future Cochrane reviews, where feasible, will strengthen the evidence base and support more informed clinical decision-making.
The study also highlights the necessity for continuous updates to systematic reviews to reflect advancements in personalised medicine and context-specific interventions. Regular updates ensure alignment between evidence synthesis and the latest innovations in SCD management, thereby promoting practices that reflect current scientific knowledge and clinical needs.
9.7 Recommendations
To overcome the identified challenges and enhance the evidence base for SCD management, several strategic measures are proposed:
1. Standardisation of Clinical Trial Design
Harmonise outcome measures and study designs across SCD trials to facilitate data pooling, improve meta-analytical feasibility, and ensure the comparability of findings. Researchers should prioritise areas identified by “empty” reviews, including non-pharmacological interventions and long-term outcomes, to build a comprehensive evidence base.
2. Enhancement of International Collaborations
Foster global research networks, particularly with researchers in high-burden, low-resource settings. Strengthening these partnerships will increase the volume of high-quality studies, ensure global relevance of findings, and promote equitable healthcare outcomes.
3. Patient-Centred Research Approaches
Integrate patient-reported outcomes and quality-of-life measures into SCD studies. This focus on patient perspectives will guide the development of treatments that prioritise both longevity and quality of life.
4. Focus on Long-Term Outcomes
Prioritise systematic reviews and primary research that address long-term outcomes and quality of life.
By implementing these recommendations, the SCD research community can address existing gaps, enhance the quality and applicability of evidence, and improve patient outcomes globally. Cochrane remains central to this effort, guiding research priorities and advancing evidence-based clinical practice.
10 Conclusion
This meta-research epidemiological study underscores the pivotal role of Cochrane reviews in shaping evidence-based care for SCD while identifying critical gaps requiring urgent attention. Over nearly three decades, Cochrane reviews have advanced clinical practice and informed public health policy. However, the large number of reviews lacking RCTs, along with the prevalence of empty reviews, reflects a fragmented and inconsistent evidence base, hindering progress.
The findings highlight the urgent need to prioritise rigorous RCTs in underexplored areas such as pain management, infection prevention, and genetic therapies. Addressing these gaps is essential for developing comprehensive clinical guidelines, improving patient outcomes, and reducing global inequities in SCD care. The absence of meta-analyses in reviews containing RCTs highlights the pressing need for data synthesis to enhance the impact of existing studies and provide clinicians with actionable insights.
This study serves as both a call to action and a roadmap for future research. By fostering international collaboration, embracing innovative trial methodologies, and addressing systemic barriers to evidence generation, the research community can transform the SCD evidence landscape. Achieving the ultimate goal—delivering equitable, patient-centred care—requires these efforts, firmly grounded in scientific rigour and guided by the highest standards of evidence.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AM: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. Generative AI was used for the creation of this Manuscript. Authors used GPT 4.o to generate a new version of Figure 8 in PDF and EPS.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ Laudan L. Progress and its Problems: Towards a Theory of Scientific Growth. Berkeley and Los Angeles (CA): University of California Press; 1977.
- ^ Laudan R, Laudan L, Donovan A, editors. Scrutinizing Science: Empirical Studies of Scientific Change. Dordrecht: Springer Netherlands; 1988. (Synthese Library; vol. 193).
- ^ Hoyningen-Huene P. Systematicity: The Nature of Science. New York: Oxford University Press; 2013. (Oxford Studies in Philosophy of Science).
- ^ Fricker M. Epistemic Injustice: Power and the Ethics of Knowing. Oxford University Press; 2007.
- ^ “Medicine is a science of uncertainty and an art of probability” Sir William Osler
- ^ Banks EC. The philosophical roots of Ernst Mach's economy of thought. Synthese. 2004;139(1):23-53. Available from: https://doi.org/10.1023/b:synt.0000021306.66850.a3.
References
1. Piel FB, Rees DC, DeBaun MR, Nnodu O, Ranque B, Thompson AA, et al. Defining global strategies to improve outcomes in sickle cell disease: a Lancet Haematology Commission. Lancet Haematol. (2023) 10:e633–86. doi: 10.1016/S2352-3026(23)00096-0
2. Edelstein SJ. The Sickled Cell: From Myths to Molecules. Cambridge (MA: Harvard University Press (1986).
3. Wailoo K. Dying in the City of the Blues: Sickle Cell Anemia and the Politics of Race and Health. Chapel Hill (NC: The University of North Carolina Press (2014).
4. Pauling L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science. (1949) 110:543–8. doi: 10.1126/science.110.2865.543
5. Ingram VM. Sickle-cell anemia hemoglobin: the molecular biology of the first “molecular disease”—the crucial importance of serendipity. Genetics. (2004) 167:1–7. doi: 10.1534/genetics.167.1.1
6. World Health Organization Regional Office for Africa. Sickle cell disease . Available online at: https://www.afro.who.int/health-topics/sickle-cell-disease (Accessed March 6, 2025).
7. Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. (2014) 312:1033–48. doi: 10.1001/jama.2014.10517
8. Chalmers I. The Cochrane collaboration: preparing, maintaining, and disseminating systematic reviews of the effects of health care. Ann N Y Acad Sci. (1993) 703:156–63; discussion 163-5. doi: 10.1111/j.1749-6632.1993.tb26345.x
9. Cochrane AL. Effectiveness and Efficiency: Random Reflections on Health Services. London: The Nuffield Provincial Hospitals Trust (1972).
10. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.5 (updated August 2024). Cochrane (2024). Available online at: www.training.cochrane.org/handbook (Accessed March 6, 2025).
11. Mahomed K. Prophylactic versus selective blood transfusion for sickle cell anemia during pregnancy. Cochrane Database Syst Rev. (1996), CD000040. doi: 10.1002/14651858.CD000040
12. Evidence-Based Medicine Working Group. Evidence-based medicine: a new approach to teaching the practice of medicine. JAMA. (1992) 268:2420–5. doi: 10.1001/jama.1992.03490170092032
13. Sackett DL, Rosenberg W, Gray J, Haynes RB, Richardson WS. Evidence-based medicine: what it is and what it isn’t. BMJ. (1996) 312:71–2. doi: 10.1136/bmj.312.7023.71
14. The Cochrane library. Available online at: https://www.cochranelibrary.com/search (Accessed March 6, 2025).
15. Liem RI, Lanzkron S, Coates TD, DeCastro L, Desai AA, Ataga KI, et al. American Society of Hematology 2019 guidelines for sickle cell disease: cardiopulmonary and kidney disease. Blood Adv. (2019) 3:3867–97. doi: 10.1182/bloodadvances.2019000916
16. Kanter J, Liem RI, Bernaudin F, Bolaños-Meade J, Fitzhugh CD, Hankins JS, et al. American Society of Hematology 2021 guidelines for sickle cell disease: stem cell transplantation. Blood Adv. (2021) 5:3668–89. doi: 10.1182/bloodadvances.2021004394C
17. Murad MH, Liem RI, Lang ES, Akl EA, Meerpohl JJ, DeBaun MR, et al. 2019 sickle cell disease guidelines by the American Society of Hematology: methodology, challenges, and innovations. Blood Adv. (2019) 3:3945–50. doi: 10.1182/bloodadvances.2019000931
18. Cochrane evidence resources for the treatment of Sickle cell disorders. Available online at: https://cam.cochrane.org/news/cochrane-evidence-resources-treatment-sickle-cell-disorders (Accessed March 6, 2025).
19. Dunlop R, Bennett KCLB. Pain management for sickle cell disease in children and adults. Cochrane Database Systematic Rev. (2014), CD003350. doi: 10.1002/14651858.CD003350.pub3
20. Pal S, Dixit R, Moe S, Godinho MA, Abas ABL, Ballas SK, et al. Transcutaneous electrical nerve stimulation (TENS) for pain management in sickle cell disease. Cochrane Database Systematic Rev. (2020), CD012762. doi: 10.1002/14651858.CD012762.pub2
21. Cooper TE, Hambleton IR, Ballas SK, Cashmore BA, Wiffen PJ. Pharmacological interventions for painful sickle cell vaso-occlusive crises in adults. Cochrane Database Systematic Rev. (2019), CD012187. doi: 10.1002/14651858.CD012187.pub2
22. Al Hajeri A, Fedorowicz Z. Piracetam for reducing the incidence of painful sickle cell disease crises. Cochrane Database Systematic Rev. (2016), CD006111. doi: 10.1002/14651858.CD006111.pub3
23. Martí-Carvajal AJ, Peña-Martí GE, Comunián-Carrasco G, Martí-Peña AJ. Interventions for treating painful sickle cell crisis during pregnancy. Cochrane Database Systematic Rev. (2009), CD006786. doi: 10.1002/14651858.CD006786.pub2
24. Anie KA, Green J. Psychological therapies for sickle cell disease and pain. Cochrane Database Systematic Rev. (2015), CD001916. doi: 10.1002/14651858.CD001916.pub3
25. Okomo U, Meremikwu MM. Fluid replacement therapy for acute episodes of pain in people with sickle cell disease. Cochrane Database Systematic Rev. (2017), CD005406. doi: 10.1002/14651858.CD005406.pub5
26. Asnani MR, Francis DK, Brandow AM, Hammond Gabbadon CEO, Ali A. Interventions for treating neuropathic pain in people with sickle cell disease. Cochrane Database Systematic Rev. (2019), CD012943. doi: 10.1002/14651858.CD012943.pub2
27. van Zuuren EJ, Fedorowicz Z. Low-molecular-weight heparins for managing vaso-occlusive crises in people with sickle cell disease. Cochrane Database Systematic Rev. (2015), CD010155. doi: 10.1002/14651858.CD010155.pub3
28. Aboursheid T, Albaroudi O, Alahdab F. Inhaled nitric oxide for treating pain crises in people with sickle cell disease. Cochrane Database Systematic Rev. (2022), CD011808. doi: 10.1002/14651858.CD011808.pub3
29. Rankine-Mullings AE, Owusu-Ofori S. Prophylactic antibiotics for preventing pneumococcal infection in children with sickle cell disease. Cochrane Database Systematic Rev. (2021). CD003427. doi: 10.1002/14651858.CD003427.pub5
30. Allali S, Chalumeau M, Launay O, Ballas SK, de Montalembert M. Conjugate Haemophilus influenzae type b vaccines for sickle cell disease. Cochrane Database Systematic Rev. (2018), CD011199. doi: 10.1002/14651858.CD011199.pub3
31. Martí-Carvajal AJ, Conterno LO. Antibiotics for treating community-acquired pneumonia in people with sickle cell disease. Cochrane Database Systematic Rev. (2016), CD005598. doi: 10.1002/14651858.CD005598.pub4
32. Odey F, Okomo U, Oyo-Ita A. Vaccines for preventing invasive salmonella infections in people with sickle cell disease. Cochrane Database Systematic Rev. (2018), CD006975. doi: 10.1002/14651858.CD006975.pub4
33. Martí-Carvajal AJ, Conterno LO, Knight-Madden JM. Antibiotics for treating acute chest syndrome in people with sickle cell disease. Cochrane Database Systematic Rev. (2019), CD006110. doi: 10.1002/14651858.CD006110.pub5
34. Davies EG, Hirst C, Lottenberg R, Dower N. Pneumococcal vaccines for sickle cell disease. Cochrane Database Systematic Rev. (2004), CD003885. doi: 10.1002/14651858.CD003885.pub2
35. Oniyangi O, Omari AAA. Malaria chemoprophylaxis in sickle cell disease. Cochrane Database Systematic Rev. (2006), CD003489. doi: 10.1002/14651858.CD003489.pub2
36. Martí-Carvajal AJ, Agreda-Pérez LH. Antibiotics for treating osteomyelitis in people with sickle cell disease. Cochrane Database Systematic Rev. (2019), CD007175. doi: 10.1002/14651858.CD007175.pub5
37. Okusanya BO, Oladapo OT. Prophylactic versus selective blood transfusion for sickle cell disease in pregnancy. Cochrane Database Systematic Rev. (2016), CD010378. doi: 10.1002/14651858.CD010378.pub3
38. Estcourt LJ, Kohli R, Hopewell S, Trivella M, Wang WC. Blood transfusion for preventing primary and secondary stroke in people with sickle cell disease. Cochrane Database Systematic Rev. (2020), CD003146. doi: 10.1002/14651858.CD003146.pub4
39. Dolatkhah R, Dastgiri S. Blood transfusions for treating acute chest syndrome in people with sickle cell disease. Cochrane Database Systematic Rev. (2020), CD007843. doi: 10.1002/14651858.CD007843.pub4
40. Estcourt LJ, Hopewell S, Trivella M, Hambleton IR, Cho G. Regular long-term red blood cell transfusions for managing chronic chest complications in sickle cell disease. Cochrane Database Systematic Rev. (2019), CD008360. doi: 10.1002/14651858.CD008360.pub5
41. Estcourt LJ, Kimber C, Trivella M, Doree C, Hopewell S. Preoperative blood transfusions for sickle cell disease. Cochrane Database Systematic Rev. (2020), CD003149. doi: 10.1002/14651858.CD003149.pub4
42. Owusu-Ofori S, Remmington T. Splenectomy versus conservative management for acute sequestration crises in people with sickle cell disease. Cochrane Database Systematic Rev. (2017), CD003425. doi: 10.1002/14651858.CD003425.pub4
43. Lees C, Davies SC, Dezateux C. Neonatal screening for sickle cell disease. Cochrane Database Systematic Rev. (2000), CD001913. doi: 10.1002/14651858.CD001913
44. Rankine-Mullings AE, Nevitt SJ. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Systematic Rev. (2022), CD002202. doi: 10.1002/14651858.CD002202.pub3
45. Olowoyeye A, Okwundu CI. Gene therapy for sickle cell disease. Cochrane Database Systematic Rev. (2020), CD007652. doi: 10.1002/14651858.CD007652.pub7
46. Oringanje C, Nemecek E, Oniyangi O. Hematopoietic stem cell transplantation for people with sickle cell disease. Cochrane Database Systematic Rev. (2020), CD007001. doi: 10.1002/14651858.CD007001.pub5
47. Chinegwundoh FI, Smith S, Anie KA. Treatments for priapism in boys and men with sickle cell disease. Cochrane Database Systematic Rev. (2020), CD004198. doi: 10.1002/14651858.CD004198.pub4
48. Al Hajeri A, Serjeant GR, Fedorowicz Z. Inhaled nitric oxide for acute chest syndrome in people with sickle cell disease. Cochrane Database Systematic Rev. (2008), CD006957. doi: 10.1002/14651858.CD006957
49. Knight-Madden JM, Hambleton IR. Inhaled bronchodilators for acute chest syndrome in people with sickle cell disease. Cochrane Database Systematic Rev. (2022), CD003733. doi: 10.1002/14651858.CD003733.pub5
50. Roy NBA, Carpenter A, Dale-Harris I, Dorée C, Estcourt LJ. Interventions for chronic kidney disease in people with sickle cell disease. Cochrane Database Systematic Rev. (2023), CD012380. doi: 10.1002/14651858.CD012380.pub3
51. Sasongko TH, Nagalla S. Angiotensin-converting enzyme (ACE) inhibitors for proteinuria and microalbuminuria in people with sickle cell disease. Cochrane Database Systematic Rev. (2021), CD009191. doi: 10.1002/14651858.CD009191.pub4
52. Martí-Carvajal AJ, Solà I, Agreda-Pérez LH. Treatment for avascular necrosis of bone in people with sickle cell disease. Cochrane Database Systematic Rev. (2019), CD004344. doi: 10.1002/14651858.CD004344.pub7
53. Martí-Carvajal AJ, Knight-Madden JM, Martinez-Zapata MJ. Interventions for treating leg ulcers in people with sickle cell disease. Cochrane Database Systematic Rev. (2021), CD008394. doi: 10.1002/14651858.CD008394.pub4
54. Martí-Carvajal AJ, Martí-Amarista CE. Interventions for treating intrahepatic cholestasis in people with sickle cell disease. Cochrane Database Systematic Rev. (2020), CD010985. doi: 10.1002/14651858.CD010985.pub4
55. Mulimani P, Ballas SK, Abas ABL, Karanth L. Treatment of dental complications in sickle cell disease. Cochrane Database Systematic Rev. (2019), CD011633. doi: 10.1002/14651858.CD011633.pub3
56. Estcourt LJ, Kimber C, Hopewell S, Trivella M, Doree C, Abboud MR. Interventions for preventing silent cerebral infarcts in people with sickle cell disease. Cochrane Database Systematic Rev. (2020), CD012389. doi: 10.1002/14651858.CD012389.pub3
57. Myint KT, Sahoo S, Thein AW, Moe S, Ni H. Laser therapy for retinopathy in sickle cell disease. Cochrane Database Systematic Rev. (2022), CD010790. doi: 10.1002/14651858.CD010790.pub3
58. Manchikanti Gomez A, Grimes DA, Lopez LM, Schulz KF. Steroid hormones for contraception in women with sickle cell disease. Cochrane Database Systematic Rev. (2007), CD006261. doi: 10.1002/14651858.CD006261.pub2
59. Dixit R, Nettem S, Madan SS, Soe HH, Abas ABL, Vance LD, et al. Folate supplementation in people with sickle cell disease. Cochrane Database Systematic Rev. (2018), CD011130. doi: 10.1002/14651858.CD011130.pub3
60. Than NN, Soe HHK, Palaniappan SK, Abas ABL, De Franceschi L. Magnesium for treating sickle cell disease. Cochrane Database Systematic Rev. (2019), CD011358. doi: 10.1002/14651858.CD011358.pub3
61. Bolarinwa AB, Oduwole O, Okebe J, Ogbenna AA, Otokiti OE, Olatinwo AT. Antioxidant supplementation for sickle cell disease. Cochrane Database Systematic Rev. (2024), CD013590. doi: 10.1002/14651858.CD013590.pub2
62. Oniyangi O, Cohall DH. Phytomedicines (medicines derived from plants) for sickle cell disease. Cochrane Database Systematic Rev. (2020), CD004448. doi: 10.1002/14651858.CD004448.pub7
63. Nagalla S, Ballas SK. Drugs for preventing red blood cell dehydration in people with sickle cell disease. Cochrane Database Systematic Rev. (2018), CD003426. doi: 10.1002/14651858.CD003426.pub6
64. Soe HH, Abas ABL, Than NN, Ni H, Singh J, Said AR, et al. Vitamin D supplementation for sickle cell disease. Cochrane Database Systematic Rev. (2020), CD010858. doi: 10.1002/14651858.CD010858.pub3
65. Meerpohl JJ, Schell LK, Rücker G, Motschall E, Fleeman N, Niemeyer CM, et al. Deferasirox for managing transfusional iron overload in people with sickle cell disease. Cochrane Database Systematic Rev. (2014), CD007477. doi: 10.1002/14651858.CD007477.pub3
66. Asnani MR, Quimby KR, Bennett NR, Francis DK. Interventions for patients and caregivers to improve knowledge of sickle cell disease and recognition of its related complications. Cochrane Database Systematic Rev. (2016), CD011175. doi: 10.1002/14651858.CD011175.pub2
67. Stegenga J. Justifying scientific progress. Philos Sci. (2024) 91:543–60. doi: 10.1017/psa.2023.118
68. Gheihman G, Johnson M, Simpkin AL. Twelve tips for thriving in the face of clinical uncertainty. Med Teach. (2020) 42:493–99. doi: 10.1080/0142159X.2019.1579308
69. Patel B, Gheihman G, Katz JT, Simpkin Begin A, Solomon SR. Navigating uncertainty in clinical practice: a structured approach. J Gen Intern Med. (2024) 39:829–36. doi: 10.1007/s11606-023-08596-4
70. Scott IA, Doust JA, Keijzers GB, Wallis KA. Coping with uncertainty in clinical practice: a narrative review. Med J Aust. (2023) 218:418–25. doi: 10.5694/mja2.v218.9
71. Anjum RL, Rocca E. Philosophy of Science. Cham: Springer Nature Switzerland AG (2024). doi: 10.1007/978-3-031-56049-1
72. Egger M, Higgins JPT, Davey Smith G eds. Systematic reviews in health research: meta-analysis in context. 3rd ed. Hoboken, NJ: John Wiley & Sons (2022).
Keywords: sickle cell disease, Cochrane reviews, evidence-based medicine, meta research, systematic reviews, research methodology, global health, health equity
Citation: Martí-Carvajal A (2025) Cochrane’s contribution to global health: three decades of progress in sickle cell disease research. Front. Hematol. 4:1535100. doi: 10.3389/frhem.2025.1535100
Received: 27 November 2024; Accepted: 24 February 2025;
Published: 21 March 2025.
Edited by:
John Strouboulis, King’s College London, United KingdomReviewed by:
Steven Okoli, Imperial College London, United KingdomRachel Kesse-Adu, Guy’s and St Thomas’ NHS Foundation Trust, United Kingdom
Copyright © 2025 Martí-Carvajal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arturo Martí-Carvajal, YXJ0dXJvLm1hcnRpLmNhcnZhamFsQGdtYWlsLmNvbQ==; YW1hcnRpQHVjLmVkdS52ZQ==
†ORCID: Arturo Martí-Carvajal, orcid.org/0000-0001-8677-3351
 Arturo Martí-Carvajal
Arturo Martí-Carvajal