
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hematol., 21 February 2025
Sec. Blood Cancer
Volume 4 - 2025 | https://doi.org/10.3389/frhem.2025.1521017
This article is part of the Research TopicElevating Supportive Care Measures for Multiple Myeloma in the Modern EraView all articles
 Amman Bhasin1
Amman Bhasin1 Adam Finn Binder2*
Adam Finn Binder2* Leland Damron3
Leland Damron3 Amry Majeed1
Amry Majeed1 Adam Barsouk3
Adam Barsouk3 Kelly Hughes1
Kelly Hughes1 Niketa Raj3
Niketa Raj3 Abdullateef O. Abdulkareem2
Abdullateef O. Abdulkareem2 Tingting Zhan4
Tingting Zhan4 Divya Polu3
Divya Polu3 Sai Gundepalli5
Sai Gundepalli5 Srinivas Devarakonda5
Srinivas Devarakonda5Purpose: Current treatment guidelines strongly support the use of antiresorptive therapy in patients with newly diagnosed multiple myeloma (NDMM) with the goal of preventing skeletal related events (SRE). Despite these concrete, data-driven recommendations, the impact of delays in antiresorptive initiation in NDMM patients is understudied. Through a multicenter retrospective study, we examined the impact of delays in antiresorptive initiation on the rates of SREs. We furthered our exploration of this topic in a separate retrospective analysis with a focus on reasons for delays in antiresorptive therapy initiation.
Methods: Electronic health records from two large academic institutions were used to identify patients with NDMM between July 1, 2016, and June 30, 2019. Exclusion criteria included patients with previous antiresorptive use and patients never prescribed antiresorptives. Time to antiresorptive initiation and its subsequent impact on the rate of SREs was analyzed using hazard ratios. A follow up, single-center retrospective study was conducted using EHR data with an emphasis on the identification of barriers to antiresorptive initiation. Here, descriptive, and inferential statistics were used to identify variables that have a statistically significant impact on antiresorptive initiation.
Results: A total of 759 patients with newly diagnosed MM met inclusion criteria for our multicenter study. Our study found that a delay in initiation of anti-resorptive therapy of greater than 31 days from diagnosis resulted in an increased risk for SRE with a hazard ratio of 1.654 (95% CI: 1.054-2.598; p-value = 0.029). In our follow up study, a total of 45.6% of patients with newly diagnosed MM were prescribed antiresorptive therapy, while 59% of patients with identified lytic lesions on screening imaging received anti-resorptive therapy. Statistically insignificant differences were observed in the time to initiation of anti-resorptive therapy based on health insurance. Variables such as race and gender were not found to have a statistically significant relationship with delays in antiresorptive initiation.
Conclusions: Patients with NDMM should be initiated on antiresorptive therapy without delay to minimize the rates of SREs, and clinicians should be diligent in anticipating delays in initiation such as need for dental clearance and renal disease.
Multiple myeloma (MM) is a hematologic malignancy characterized by an uncontrolled, monoclonal proliferation of B-lymphocyte derived plasma cells within the bone marrow (1). As many as 80-90% of patients with MM ultimately develop osteolytic lesions that often lead to further skeletal damage including diffuse osteopenia and skeletal-related events (SRE) in 22-34% of patients (2–4). SREs are defined as pathologic fractures, spinal cord compression, surgical or radiation intervention to stabilize bone lesions or other complications such as hypercalcemia, all of which can impact quality of life, morbidity, and mortality (4). Antiresorptive agents, such as bisphosphonates and denosumab, are commonly used in MM patients with skeletal disease as an adjunct to their myeloma therapy in order to prevent SREs (5, 6).
Bisphosphonates and denosumab inhibit bone resorption via different mechanisms. Bisphosphonates attach to hydroxyapatite binding sites on bony surfaces preventing reabsorption, while denosumab, a RANKL-directed monoclonal antibody, imitates osteoprotegerin and thus prevents bone turnover (4, 7). A meta-analysis of data from 24 randomized control trials demonstrated the benefit of bisphosphonates compared to placebo or no treatment in preventing SREs (6). Similarly, in a Phase 3 clinical trial, denosumab was found to have similar efficacy to bisphosphonates (Zoledronic Acid) in delaying SREs (8). Despite their benefits in reducing SREs, it is common for antiresorptive initiation to be delayed for a variety of reasons, including dental clearance, medical co-morbidities, or relative medication contraindications. Furthermore, several retrospective studies have found that antiresorptive therapy is underutilized in MM patients as a whole (9, 10).
The role of antiresorptive therapy in the prevention of SREs has been well-established by multiple studies and has been shown to reduce SREs by an estimated 44% (5, 6, 11). However, the effect of delayed antiresorptive initiation on the development of SRE has not been explored. To elucidate the impact of antiresorptive timing on SRE development, we conducted a multi-center, retrospective analysis of patients undergoing MM treatment at two large academic institutions. Additionally, a follow up study was conducted at a large, single-center academic institution with the goal of identifying potential barriers to initiation of antiresorptive therapy and investigation of screening modalities utilized to detect lytic lesions at the time of diagnosis. The goal of this study is to investigate time to initiation of antiresorptive therapy in real world practice and barriers to its initiation, and their subsequent impact on clinical outcomes to optimize prevention of SREs in MM patients.
This report is comprised of two distinct studies. The primary study was a retrospective analysis of patients with newly diagnosed MM (NDMM) from July 1, 2016, to June 30, 2019. Data was collected using the Electronic Health Records (EHR) from two large academic institutions, Sidney Kimmel Cancer Center (SKCC) at Thomas Jefferson University Hospitals (TJUH) and The Ohio State University Comprehensive Cancer Center (OSUCCC). Information was collected on patient demographics, including a past medical history of CKD, osteopenia, osteoporosis, vitamin D deficiency, obesity, initial MM therapy, status of myeloma bone disease, ISS stage at diagnosis, use of antiresorptives, and incidence of SRE during follow-up. Patients previously treated with antiresorptive therapy and patients not treated with antiresorptive therapy were excluded. The primary endpoint was hazard ratio for developing a SRE based on time to antiresorptive therapy.
The follow up study was a retrospective analysis of NDMM patients between January 1, 2022, and November 1, 2022 at a single academic center, SKCC. All patients with NDMM were included in the analysis. Patient demographics and medical information were collected from the EHR. Specific variables analyzed in this study included age, race, and status and type of health insurance. IRB approval was obtained for all research activities in accordance with the declaration of Helsinki.
In the primary study, baseline patient characteristics, including a past medical history of CKD, osteopenia, osteoporosis, vitamin D deficiency, obesity, underwent univariable analysis using chi-square and fisher’s exact tests. A p-value level of < 0.05 was considered significant. The relationship between incidence of SREs and time to antiresorptive therapy, baseline patient characteristics, gender of patients, age, International Staging System (ISS) stage at diagnosis, and prior SRE present at diagnosis was analyzed by using a multivariable Cox proportional hazards model. The cutoff point of antiresorptive therapy delay was based on the recursive partitioning of univariable Cox model. A p-value level of < 0.05 was considered significant.
In the follow up study, data was analyzed using descriptive statistics such as mean, standard deviation, median, frequency, and interquartile range (IQR). Inferential statistics such as t-tests, chi-square, ANOVA, and Kruskal Wallis analyses were performed where appropriate. A p-value level of < 0.05 was considered significant.
From July 2016 to June 2019, patients with NDMM were identified at two large academic institutions, SKCC and OSUCCC. Of these patients, 759 subjects met eligibility criteria for study inclusion. Among these patients, the median age at diagnosis was 60.2 years, 57% were male, 76.7% of patients were Caucasian, and 20% were African American. At the time of diagnosis, 210 patients (27.7%) had osteopenia, 45 (5.9%) had osteoporosis, 229 (30.2%) were vitamin D deficient, 121 (15.9%) had CKD, and 319 (42%) were obese. ISS stage at diagnosis was known in 86.4% and unknown in 13.6% of patients with known staging noted to be 34.5% stage I, 27% stage II, and 24.9% stage III. At diagnosis, a SRE was present in 338 (45.1%) of patients.
The subsequent follow-up study was conducted at a single, large academic center (SKCC) to identify barriers to antiresorptive therapy initiation. From January 2022 to November 2022, 68 patients with NDMM met eligibility criteria for study inclusion. The median age was 66.5 years, 51.5% were male, 51.4% were Caucasian, and 39.7% were African American. Of these patients, health insurance analysis found that 28 patients (41%) had traditional Medicare, 21 (30.9%) had private insurance, 10 (14.7%) had Medicare Advantage, 6 (8.8%) had state insurance, and 3 (3.4%) had Medicaid Expansion. Complete baseline characteristics are outlined in Table 1.
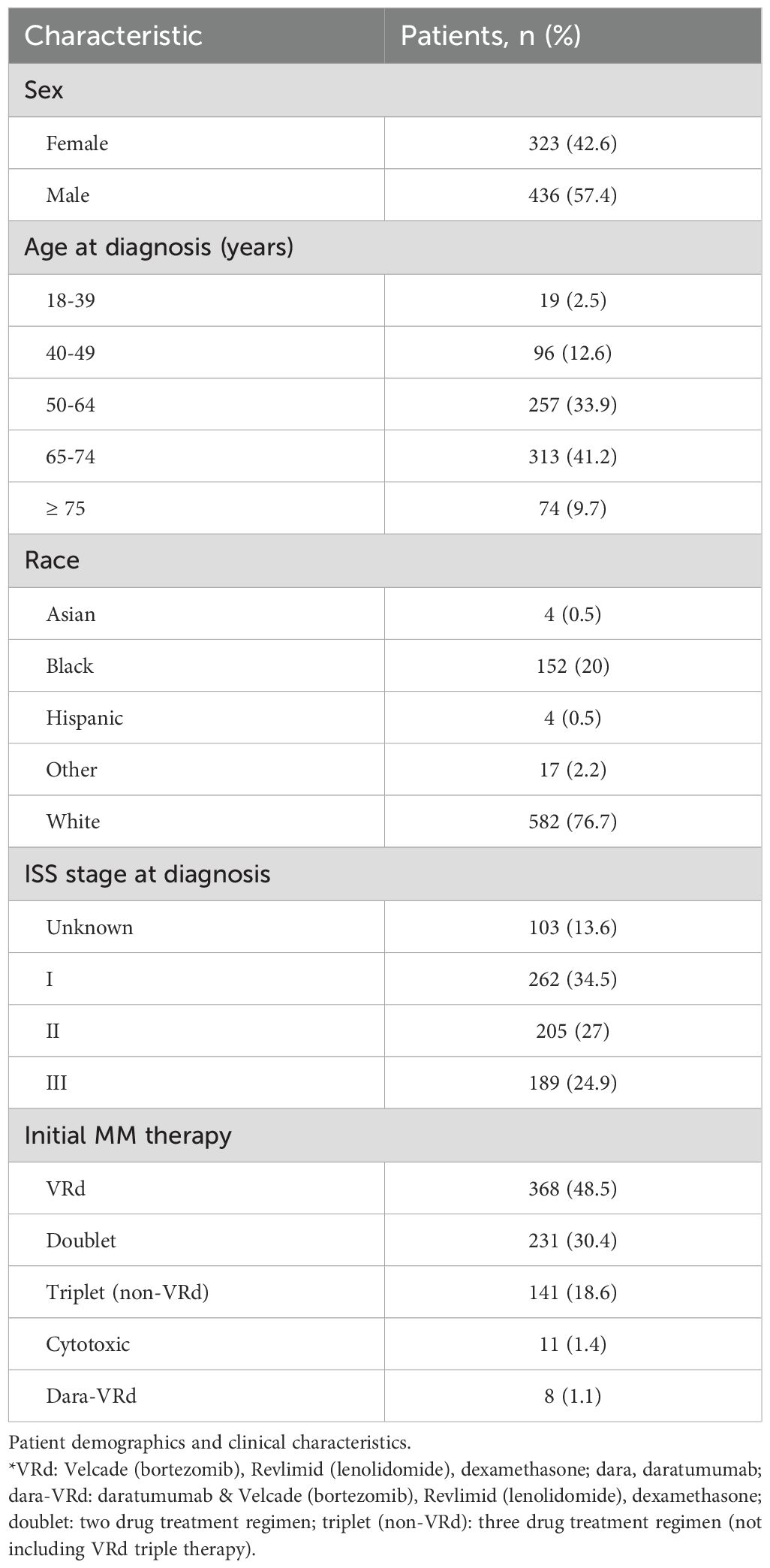
Table 1. Baseline characteristics and treatment information of patients with multiple myeloma at the time of the diagnosis.
Results of the multi-center retrospective study of NDMM patients indicated that a total of 368 (48.5%) of patients received Velcade, Lenalidomide (Revlimid), dexamethasone (VRd) therapy, 231 (30.4%) patients received doublet therapy, 141 (18.6%) received non-VRd triplet therapy, 11 (1.4%) patients received cytotoxic therapy, and 8 (1.1%) patients received daratumumab with VRd as induction therapy. Denosumab was initiated in 43.2% of patients with NDMM, corresponding to a total of 328 patients receiving bone-preserving agents. Median time to anti-resorptive therapy was 54.0 days with an interquartile range (IQR) of 146.0 days. Further analysis found that a delay in initiation of antiresorptive therapy of greater than 31 days from diagnosis resulted in an increased risk for SRE with a calculated hazard ratio of 1.654 (95% confidence interval = 1.054-2.598; p-value = 0.029) (Figure 1). A total of 180 (34%) patients received anti-resorptive agents within 31 days. No statistically significant associations were noted between an increased risk for SRE and baseline patient characteristics, gender of patients, age, International Staging System (ISS) stage at diagnosis, and prior SRE present at diagnosis.
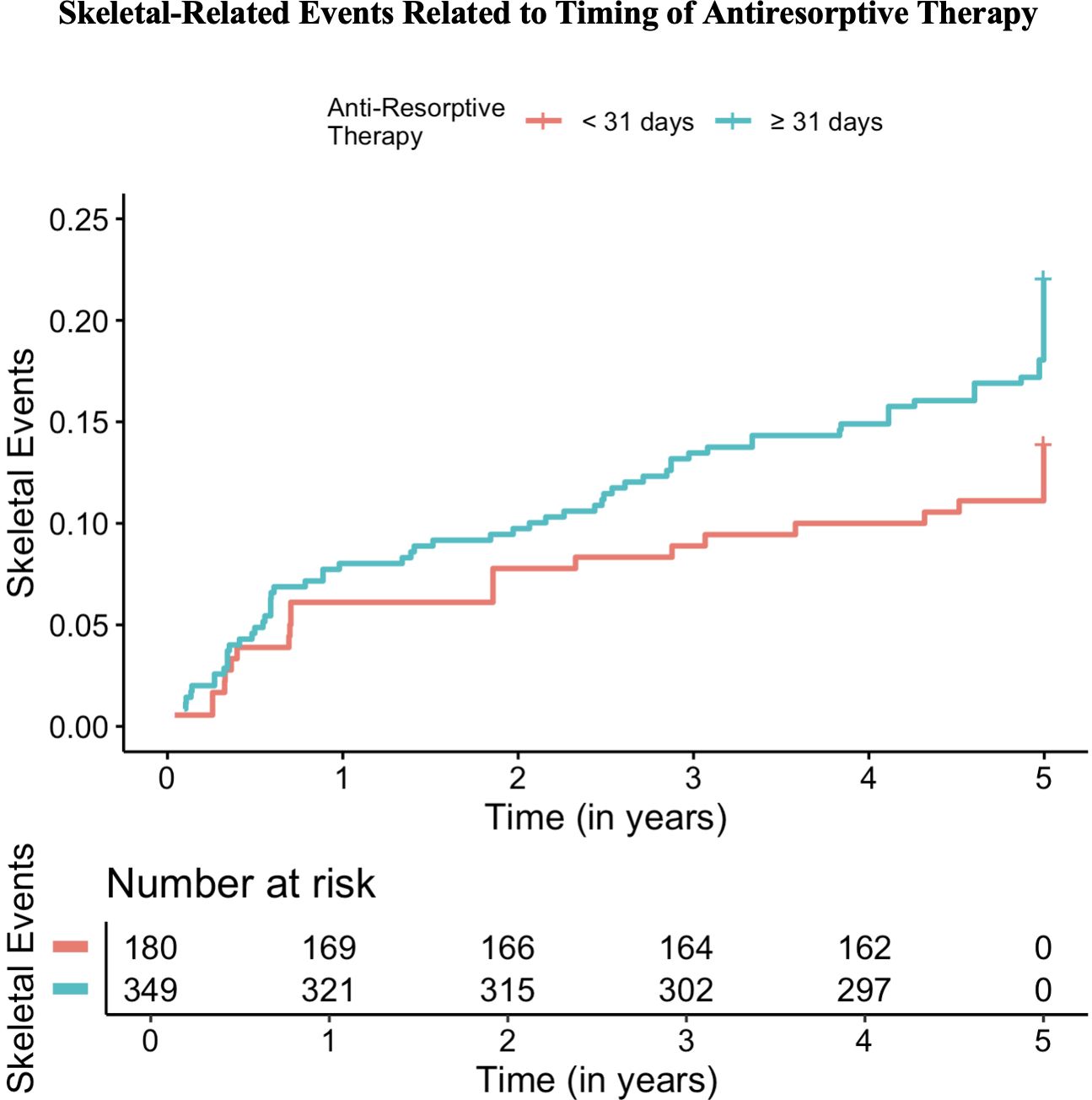
Figure 1. Rates of skeletal-related events (SREs) based on timing of initiation of anti-resorptive therapy in newly diagnosed multiple myeloma patients.
Follow up study results at a single, large academic center showed that skeletal survey (x-ray) was used to screen for lytic disease in 66.2% of patients, PET-CT scan in 75.6% of patients, axial MRI in 45.6% of patients, and PET-CT scan or axial MRI in 86.8% of patients (Table 2). Out of 68 patients with NDMM included in this study, 39 (57.4%) patients demonstrated lytic lesions on imaging. A total of 45.6% of patients with NDMM were prescribed antiresorptive therapy, while 59% of patients with NDMM and lytic lesions at the time of diagnosis received antiresorptive therapy. In NDMM patients, frequency of prescription was 17.6% for a bisphosphonate only, 17.6% for denosumab only, and 10.3% of patients received both therapies (Table 3). The median time from MM diagnosis to initiation of either antiresorptive agent was 39.5 days (IQR=79.8), 14.5 days (IQR=55.5) for a bisphosphonate, and 57.5 days (IQR=97.8) for denosumab.
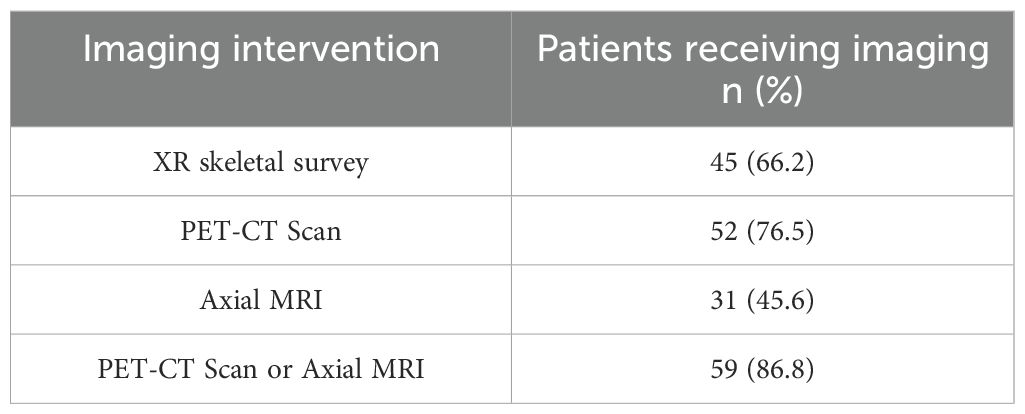
Table 2. Imaging interventions used to assess for bone involvement on newly diagnosed multiple myeloma patients in the study group.
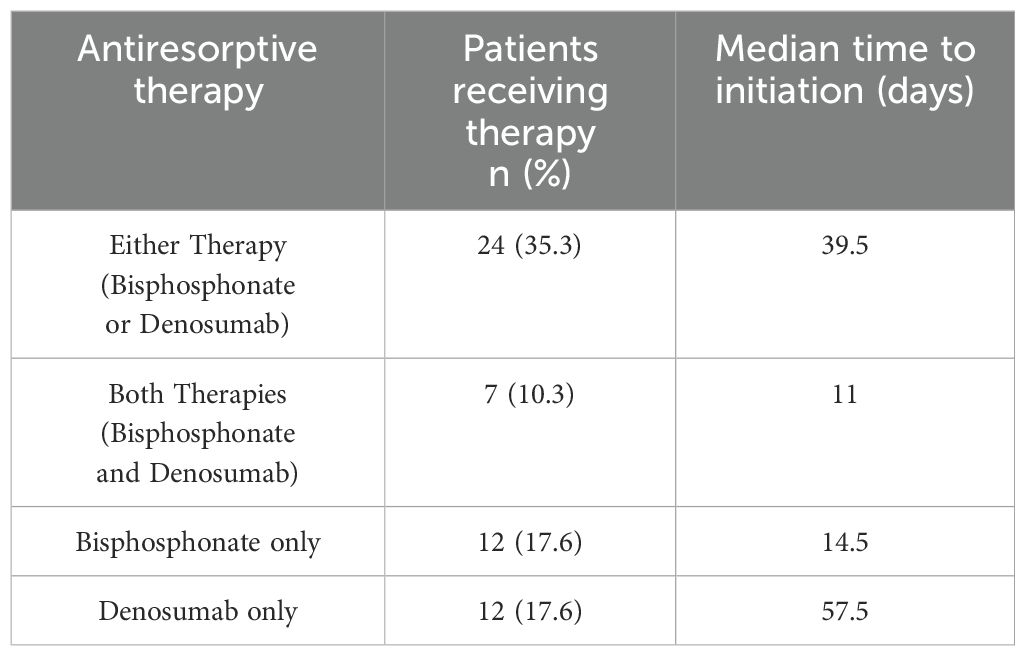
Table 3. Antiresorptive therapies used on patients with newly diagnosed multiple myeloma in the study group and median days to initiation of antiresorptive therapy.
Additionally, the follow up study analyzed several variables for possible associations related to delays in the initiation of antiresorptive. These variables included patient insurance type, race, sex, and dental clearance. In patients who received antiresorptive therapy, median time to prescription by insurance type, in days, was 23.5 for state, 36 for private, 48 for traditional Medicare, 144.5 for Medicaid expansion, and 175 for Medicare advantage (p = 0.21) (Figure 2). In NDMM patients, dental clearance referrals were provided to 58.8% of all patients and 71.8% of patients with lytic lesions prior to initiation of anti-resorptive therapy due to the risk of osteonecrosis of the jaw (ONJ). Due to the external nature of dental referrals outside of our institution, no further data could be collected and analyzed regarding the association of delays in antiresorptive therapy related to delays in dental clearance. Analysis of patient race (p=0.53), and sex (p=0.41) related to antiresorptive prescription were not statistically significant and were found to be unrelated to the lack of or delay in the administration of antiresorptive therapy.
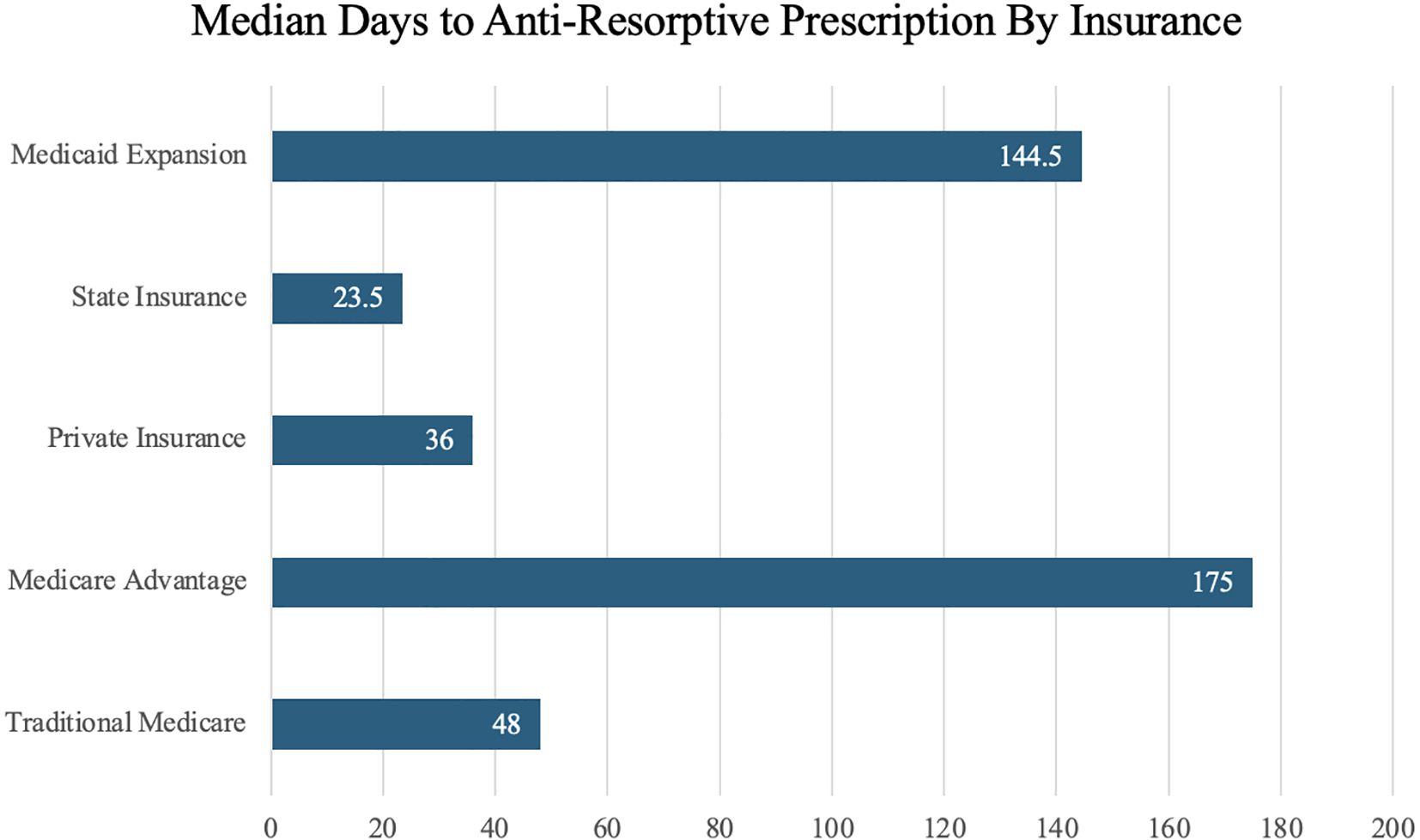
Figure 2. Median days to initiation of antiresorptive therapy by patient insurance type at the time of multiple myeloma diagnosis.
Current guidelines strongly support the initiation of antiresorptive agents in NDMM patients; however, there are no data-driven recommendations regarding specific timing of medication initiation. Previous retrospective studies have demonstrated that 64% of patients with NDMM received IV bisphosphonate administration within 30 days of diagnosis, while those not initiated on therapy within this timeframe were far less likely to ever receive antiresorptive therapy (10). Furthermore, an additional retrospective study of over 11,000 patients found that less than 66% of patients received bisphosphonate therapy with a median time to administration of 29 days (9). In our study, 43.2% of patient received anti-resorptive therapy and median time to anti-resorptive therapy was 54 days. Rates of anti-resorptive prescription were noted to be lower but comparable to the study conducted by Kim et al. (9). Notably, further analysis demonstrated that patients initiated on antiresorptive therapy more than 31 days after their MM diagnosis were 1.65 times more likely to experience a SRE. These findings demonstrate the clear utility of early initiation of antiresorptive therapy in reducing rates of SREs. Based on these significant findings, it is our recommendation to initiate antiresorptive therapy for NDMM patients within the first 31 days of diagnosis to mitigate the risk of SREs.
In addition, this study sought to explore the reasons for delays in antiresorptive initiation. Many studies have been published that validate the importance of antiresorptive therapy and stress early administration of these medications. However, few studies have investigated factors related to delayed administration. Thus, to identify barriers that delay antiresorptive administration, a follow up, single center study was conducted and identified several possible contributing variables. In both studies, many patients were noted to have delays in antiresorptive initiation due to obstacles in obtaining dental clearance in order to minimize risk for ONJ, a documented adverse effect of antiresorptive therapy (12). In our study group, a majority (58.8%) of patients were referred for dental clearance. However, given provider knowledge of delays in dental clearance, most patients at high risk for SREs (e.g. patients with existing lytic lesions, osteoporosis or osteopenia, and/or symptomatic bone pain) were initiated on antiresorptive medications prior to dental clearance and monitored closely for symptoms concerning for ONJ.
Patient insurance type was another variable that delayed initiation of antiresorptive therapy. Differences in insurance type demonstrated observable differences in median time to initiation of antiresorptive therapy. Despite lacking statistically significant differences in time to therapy, notable trends were observed. Patients with state and private traditional Medicare insurance received therapy at quicker median times of 23.5 and 36 days compared to patients with traditional Medicare, Medicaid expansion, Medicare advantage insurances with times of 48, 144.5, & 175 days, respectively. These trends illustrate that patients with certain insurance types may be at higher risk of experiencing SREs due to delays in initiation of antiresorptive therapies. Further studies and a larger sample size are required to further investigate the relationship between insurance type and delays to initiation of antiresorptive therapy. Other variables such as race and sex were not found to have a statistically significant relationship with delays in therapy initiation.
Finally, our study sought to investigate the efficacy of our current screening imaging modalities to detect lytic lesions at the time of MM diagnosis. The latest International Myeloma Working Group (IMWG) imaging guidelines published in 2019 recommend screening imaging in patients with suspected or NDMM to undergo low-dose whole-body PT/PET-CT scans or axial MRI imaging for identification and characterization of lytic lesions diagnostic of MM. These recommendations have been validated and shown to improve detection of lytic osseous lesions in patients with MM (13–16). Despite updated recommendations, many patients receive skeletal survey only to detect bone lesions, which are often not detected on plain radiographs alone (13, 15, 16). Results of our study (Table 2) demonstrate increased utilization of PET-CT or axial MRI imaging over skeletal survey x-ray screening. These results indicate that guideline-directed screening modalities are being utilized at higher rates for the purpose of screening for myeloma bone disease.
When evaluating the results of our multicenter analysis, there are several limitations to consider. First, it has been well documented that proteasome inhibitor (PI) treatment, namely Bortezomib, can demonstrate positive effects on bone metabolism. However, only approximately 10% of patients in this study received a treatment regimen without a PI based drug. Thus, the rates of SREs between PI and non-PI MM treatment regiments were unable to be analyzed to determine the potential protective effects of PI regimens. Furthermore, patient data was gathered from the EHRs of two, large academic institutions and therefore may not be generalizable to all populations. Further, given the retrospective nature of this study, our results may be affected by selection bias and incomplete data. We sought to minimize bias and increase sample size by including multiple centers in this study; including more patients from more diverse areas would make future results more robust and generalizable.
Our follow up, single center study was also a retrospective analysis and therefore is subjected to the same biases inherent to this type of study. In addition, the sample size of this study was low with a total of 68 patients meeting inclusion criteria. In order to accurately identify factors leading to delayed initiation of antiresorptive therapy, particularly regarding the impact of insurance type on antiresorptive initiation delays, a larger sample size is required. We sought to minimize bias by maximizing sample size within the limits of our inclusion criteria and blinding reviewers to the results of the study.
The results of our study have created the opportunity for additional research on this topic. As previously mentioned, a larger sample size would likely yield more robust recommendations regarding the specific factors resulting in delayed antiresorptive initiation. While insurance type was not found to have a statistically significant impact on antiresorptive initiation, our data certainly showed an observable effect. This could mean that there are unstudied variables impacting antiresorptive initiation that should be explored in future studies, preferably with larger sample sizes. Finally, to determine the optimal timing of antiresorptive therapy, prospective data may assist in aiding clinical decision-making.
Current guidelines strongly support the administration of antiresorptive agents to prevent SREs in patients with NDMM. Based on the results from our multicenter retrospective analysis, we recommend early initiation of antiresorptive therapy within the first 31 days following a new diagnosis of MM to reduce the rates of SREs. Clinicians must be expedient in their administration of antiresorptive agents with special attention given to common barriers to initiation, such as dental clearance and renal disease. Though lacking statistical significance, our data shows a pronounced disparity in antiresorptive prescription based on insurance type. Variables such as race and sex were not found to be statistically significant. Finally, our study found close adherence to newly updated guidelines that indicate the use of PET-CT and axial MRI for the detection of bone involvement in NDMM.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Jefferson Office of Human Research Protection. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
ABh: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. ABi: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LD: Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing, Data curation. AM: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology. ABa: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. KH: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. NR: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. AA: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. TZ: Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. DP: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. SG: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. SD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor MB declared a past co-authorship with the author ABi.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. (2009) 23:3–9. doi: 10.1038/leu.2008.291
2. Hameed A, Brady JJ, Dowling P, Clynes M, O’Gorman P. Bone disease in multiple myeloma: pathophysiology and management. Cancer Growth Metastasis. (2014) 7:33–42. doi: 10.4137/CGM.S16817
3. Silbermann R, Roodman GD. Myeloma bone disease: Pathophysiology and management. J Bone Oncol. (2013) 2:59–69. doi: 10.1016/j.jbo.2013.04.001
4. Terpos E, Ntanasis-Stathopoulos I, Dimopoulos MA. Myeloma bone disease: from biology findings to treatment approaches. Blood. (2019) 133:1534–9. doi: 10.1182/blood-2018-11-852459
5. Anderson K, Ismaila N, Flynn PJ, Halabi S, Jagannath S, Ogaily MS, et al. Role of bone-modifying agents in multiple myeloma: american society of clinical oncology clinical practice guideline update. J Clin Oncol. (2018) 36:812–8. doi: 10.1200/JCO.2017.76.6402
6. Terpos E, Zamagni E, Lentzsch S, Drake MT, García-Sanz R, Abildgaard N, et al. Treatment of multiple myeloma-related bone disease: recommendations from the Bone Working Group of the International Myeloma Working Group. Lancet Oncol. (2021) 22:e119–30. doi: 10.1016/S1470-2045(20)30559-3
7. van Beek E, Pieterman E, Cohen L, Löwik C, Papapoulos S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun. (1999) 264:108–11. doi: 10.1006/bbrc.1999.1499
8. Raje N, Terpos E, Willenbacher W, Shimizu K, García-Sanz R, Durie B, et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: an international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. (2018) 19:370–81. doi: 10.1016/S1470-2045(18)30072-X
9. Kim C, Hernandez RK, Cyprien L, Liede A, Cheng PC. Patterns of bisphosphonate treatment among patients with multiple myeloma treated at oncology clinics across the USA: observations from real-world data. Support Care Cancer. (2018) 26:2833–41. doi: 10.1007/s00520-018-4133-1
10. McGrath LJ, Hernandez RK, Overman R, Reams D, Liede A, Brookhart MA, et al. Initiation and interruption in intravenous bisphosphonate therapy among patients with multiple myeloma in the United States. Cancer Med. (2018) 8:374–82. doi: 10.1002/cam4.2019.8.issue-1
11. Morgan GJ, Davies FE, Gregory WM, Cocks K, Bell SE, Szubert AJ, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet. (2010) 376:1989–99. doi: 10.1016/S0140-6736(10)62051-X
12. Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw–2014 update. J Oral Maxillofac Surg. (2014) 72:1938–56. doi: 10.1016/j.joms.2014.04.031
13. Hillengass J, Usmani S, Rajkumar SV, Durie BGM, Mateos MV, Lonial S, et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. (2019) 20:e302–12. doi: 10.1016/S1470-2045(19)30309-2
14. Lee K, Kim KW, Ko Y, Park HY, Chae EJ, Lee JH, et al. Comprehensive updates in the role of imaging for multiple myeloma management based on recent international guidelines. Korean J Radiol. (2021) 22:1497–513. doi: 10.3348/kjr.2020.0886
15. Mosebach J, Thierjung H, Schlemmer HP, Delorme S. Multiple myeloma guidelines and their recent updates: implications for imaging. Rofo. (2019) 191:998–1009. doi: 10.1055/a-0897-3966
Keywords: multiple myeloma, skeletal related events, SRE, antiresorptive, bisphosphonate, denosumab, lytic lesions, myeloma therapy
Citation: Bhasin A, Binder AF, Damron L, Majeed A, Barsouk A, Hughes K, Raj N, Abdulkareem AO, Zhan T, Polu D, Gundepalli S and Devarakonda S (2025) Time to initiation of antiresorptive agents in multiple myeloma to reduce skeletal related events. Front. Hematol. 4:1521017. doi: 10.3389/frhem.2025.1521017
Received: 01 November 2024; Accepted: 04 February 2025;
Published: 21 February 2025.
Edited by:
Muhamed Baljevic, Vanderbilt University Medical Center, United StatesReviewed by:
Adisak Tantiworawit, Chiang Mai University, ThailandCopyright © 2025 Bhasin, Binder, Damron, Majeed, Barsouk, Hughes, Raj, Abdulkareem, Zhan, Polu, Gundepalli and Devarakonda. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam Finn Binder, YWRhbS5iaW5kZXJAamVmZmVyc29uLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.