
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hematol., 17 March 2025
Sec. Red Cells, Iron and Erythropoiesis
Volume 4 - 2025 | https://doi.org/10.3389/frhem.2025.1461498
This article is part of the Research TopicInvestigations and Management of Hereditary Red Blood Cells DiseasesView all 4 articles
 Sundar Periyavan1
Sundar Periyavan1 Sudha Kumar1
Sudha Kumar1 G. N. Mamatha1
G. N. Mamatha1 Santhosh Hegde1
Santhosh Hegde1 Suman Jain2
Suman Jain2 Rakesh Dhanya1
Rakesh Dhanya1 Rajat Kumar Agarwal1,3*†
Rajat Kumar Agarwal1,3*† Lawrence Faulkner1,4
Lawrence Faulkner1,4Introduction: This study aimed to study the effectiveness of high-performance liquid chromatography (HPLC) as the initial step for thalassemia/hemoglobinopathy carrier identification in contrast to the conventional complete blood count (CBC)-first approach.
Materials and methods: This multicenter study was conducted in four hospitals in South and Central India from July 2021 to December 2022, enrolling 6,549 antenatal women. Complete blood count and HPLC tests for the beta-thalassemia trait and common variants were performed. Cost-effectiveness and efficacy of three different approaches: CBC as the first step followed by HPLC for low mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) samples, HPLC as the first step, and HPLC and CBC performed together.
Results: The sensitivity and specificity of the first step with MCV and MCH cut-offs for all hemoglobinopathy carriers were 80.8% and 50% respectively. For beta-thalassemia carriers alone, the sensitivity and specificity were 96.1% and 50.6%. For sickle cell disease carriers, the sensitivity was 69% while the specificity was 51.4%. If using only CBC as the first step, we would have missed 14.1% of all hemoglobinopathy carriers. The cost of conducting HPLC first was 0.7% higher than conducting CBC as the first step and then following up with HPLC. The increase in cost was 47.8% if we performed HPLC and CBC together.
Conclusion: We conclude that conducting HPLC as the first step is cost-effective, saves time and labor, and seems to be the preferable approach for prevention programs. HPLC as the first step identified several types of hemoglobinopathies, including alpha and beta variants, that would have otherwise been missed by the conventional CBC-first screening method.
India faces a significant burden of ß-thalassemia major, with an estimated 100,000 to 150,000 patients currently living with the condition. The average prevalence of beta-thalassemia carriers is 3-4% (1, 2). The WHO estimates that only 12% of transfusion-dependent β-thalassemia (TDT) patients are properly transfused and only 39% have access to any chelation (3). As a result, most patients with TDT do not reach full adulthood. A study conducted in India found that the actuarial median survival for individuals with thalassemia was 26.9 years and under-5 mortality was 7 times higher than the general population (4). Ensuring systematic care for all current and new patients in India is going to impose a major financial burden on the healthcare system—a study in 2008 concluded that the cost of transfusing and chelating a child with a body weight of 30kg for 1 year was INR 200,000, i.e., approximately 2,500 USD (5).
Sickle cell disease (SCD) is the most common genetic blood disease recorded in various parts of India. With a variable carrier rate ranging from 0% to 40% depending on the geographical area, it has emerged as a major public health concern in the country, especially in tribal pockets. SCD was mainly believed to affect the tribal population of Central India, however, non-tribal persons are also affected in many states such as Odisha, Chhattisgarh, Gujarat, Maharashtra, and Madhya Pradesh, where the burden of disease is significant. SCD contributes significantly to a high infant mortality rate and maternal mortality and anemia, and inflicts a huge socio-economic burden on the people living with the disease and their families. Globally, some of the highest βS allele frequencies have been reported in the Indian population and India has been ranked as the country with the second heaviest burden in the world in terms of estimated numbers of babies born with the sickle β-globin gene variant, i.e., 42,016 births (inter-quartile range, IQR: 35,347–50,919) in 2010 according to the National guidelines on management and control of Sickle Cell Disease – 2022 (6). There is clearly a compelling need to have an effective prevention program in place.
The Jaivigyan project demonstrated that there are several different carriers for beta-globin gene defects including the beta-thalassemia trait (BTT), HbS trait (sickle cell trait), Hb E trait, Hb D trait, delta-beta thalassemia trait, Hb Lepore trait, and Hereditary persistence of fetal hemoglobin (HPFH) trait in India, often coexisting in the same population (2). Even though homozygous HbD and HbE are clinically silent or very mild, in combination with other traits, they may be severe. An effective prevention program strategy should cover all these syndromes (5). Although the guidelines recommend the use of red cell indices for screening, they are typically normal for the HPFH, Hb S, Hb D, and Hb E traits. Sometimes, the NESTROFT (Naked Eye Single Tube Red cell Osmotic Fragility Test) is recommended for beta-thalassemia carrier screening even though the Jaivigyan project observed that it missed an average of 13% beta-thalassemia carriers and had several limitations (5, 7). Similarly, solubility tests have an average specificity of 92% and a positive predictive value of 80% for Hb S. The option of using red cell indices in combination with the NESTROFT (for BTT), solubility test (for HbS), and dichlorophenolindopheno test (for HbE) to screen an individual for hemoglobinopathies prior to confirmation using high-performance liquid chromatography (HPLC)/Hb-electrophoresis, is both a labor-intensive, operator-dependent, and difficult to quality-control (7).
In the Guidelines for Antenatal Care and Skilled Attendance at Birth (8) published by the Government of India, screening for thalassemia does not appear in the list of essential care recommendations, nor does it appear in the Recommendations for Routine Antenatal Care for the Healthy Pregnant Women (8) by the Indian College of Obstetricians & Gynaecologists and The Federation of Obstetric and Gynaecological Societies of India.
Since hemoglobinopathy screening is not being routinely performed, in 2020, we started our program for the prevention of thalassemia by targeting pregnant women. With the known limitations and challenges of the two-step screening strategy which uses red cell indices and other tests as step one followed by confirmation with HPLC, we decided to start by conducting a red cell indices study in parallel with HPLC and use the generated data to identify the optimal approach. After 18 months, the data were analyzed retrospectively in order to assess the utility of red cell indices in the screening algorithm. Here, we share the efficacy of the strategy using red cell indices as a screening tool.
This multicenter study was conducted in busy maternity clinics in four hospitals in South and Central India between July 2021 and December 2022. Samples were transported by courier packed in UN3373-compliant packages to the Sankalp Labs in Bangalore. The samples reached the lab within 2 to 4 days from collection.
Women in their first pregnancy or who were not screened during prior pregnancies, before the 14th week of gestation, were counseled on the need to undergo an antenatal investigation and participation in this program. After counseling and obtaining informed consent, a complete blood count (CBC) and HPLC to test for the beta-thalassemia trait and common variants were performed. If the woman was found to be a thalassemia/hemoglobinopathy carrier, then her husband was tested for carrier status. Detection of carriers would be based mainly on the Hb pattern observed on HPLC.
For β-thalassemia carrier diagnosis, an HbA2 level cut-off of 3.5% was considered. This was correlated with the mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH). If MCV<80 fl and MCH<27 pg, along with HbA2>3.5 were observed, individuals were labeled as β-thalassemia carriers. Inconsistent MCV and MCH findings were confirmed using mutation analysis.
The key driver to using CBC first followed by HPLC when indicated is cost considerations. We compared the cost-effectiveness and efficacy of three different approaches: CBC as the first step where CBC was performed first and only the samples that had low MCV or low MCH were tested with HPLC; HPLC as the first step where HPLC was performed first and only for the samples that had borderline or abnormal HPLC values were tested with CBC; and the third approach, where both HPLC and CBC were performed together for each sample. We assessed how the difference between HPLC costs and CBC costs in the different setups contributes to the relative cost-effectiveness of the screening strategies using the prevalence data that we found. For this, we expressed the HPLC cost as a multiple of CBC cost, represented as m. The cost function was written for each approach in terms of how much it would cost to test each sample expressed in terms of the unit cost of CBC. The study was approved by the Institutional Ethics Committee at the Sankalp India Foundation.
Pregnant women with a median age of 26.7 years (IQR: 23.7 - 26.7, range: 18.1 - 48.2 years) were part of this study.
Table 1 summarizes the distribution of individuals who were categorized based on their thalassemia/hemoglobinopathy status. MCV and MCH cut-offs were taken from the guidelines for the screening, diagnosis, and management of hemoglobinopathies by Ghosh et al. (2014) (9).
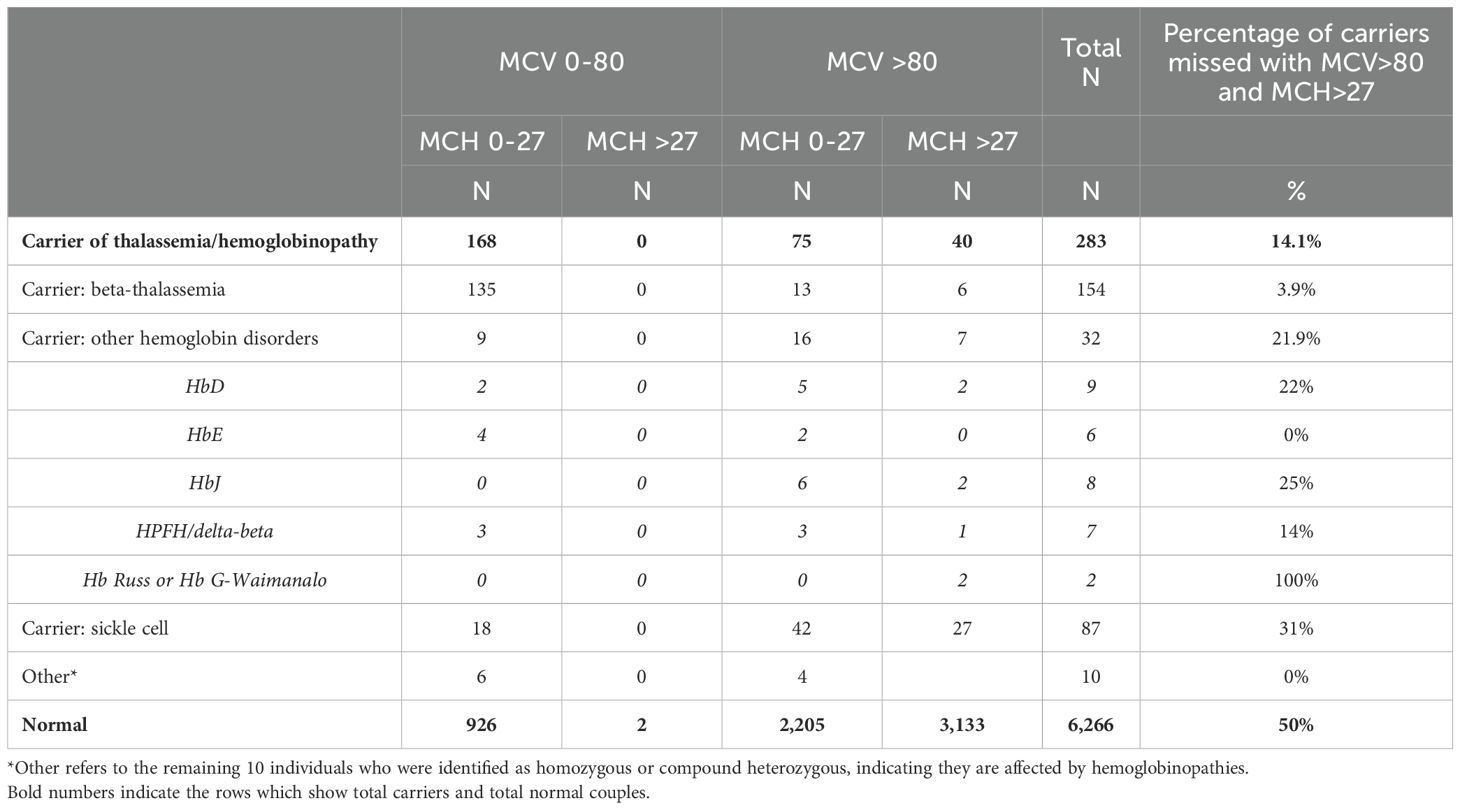
Table 1. Distribution of individuals who were categorized based on their thalassemia/hemoglobinopathy status.
The sensitivity and specificity of the first step with MCV and MCH cut-offs for all hemoglobinopathy carriers were 80.8% and 50%, respectively. For carriers of beta-thalassemia alone, the sensitivity was 96.1% but the specificity was 50.6%. For sickle cell disease, the sensitivity was 69% while the specificity was 51.4%. Regarding the other hemoglobinopathies (HbD, HbJ, HPFH/delta-beta, and Hb Russ/Hb G-Waimanalo), the sensitivity and specificity were 78.1% and 51.4%, respectively.
If using only CBC as the first step and with the MCV and MCH cutoffs, we would have missed 14.1% of all the hemoglobinopathy carriers. This would have included missing 3.9% of the beta-thalassemia carriers, 21.9% of the sickle cell carriers, and 31% of the carriers of other hemoglobinopathies. Of the other hemoglobinopathies, 22% of HbD, 25% of HbJ, 14% of HPFH/delta-beta, and all Hb Russ/Hb G-Waimanalo would have been missed. The findings also show that of all the women who were finally categorized as normal, if we first screened using red cell indices, 50% would have required further testing to confirm their status.
Table 2 shows the cost of different screening approaches as a function of multiples of CBC cost. In our setup, the cost of CBC is Rs 100/-, while the cost of HPLC is Rs 200/-, and thus m=2. With this, the cost of conducting HPLC first was 0.7% higher than conducting CBC as the first step and then following up with HPLC. The increase in cost was 47.8% if we performed HPLC and CBC together.
The guidelines for the screening, diagnosis, and management of hemoglobinopathies identify conditions that need to be identified during antenatal screening in the Indian context. The guidelines also indicate that β-thalassemia carriers may have a normal MCV and/or MCH sometimes, and these individuals may be missed during screening and that the carriers of variant Hbs such as Hb E and Hb S may also have normal indices (9). Our findings confirm this and suggest that MCV and MCH cut-offs of 80 fl and 27 pg respectively may not be appropriate, at least when screening Indian pregnant women. Our findings suggest the following:
a) We would have missed identifying 14.1% carriers, 3.9% for beta-thalassemia, 21.9% for sickle cell disease, and 31% for other hemoglobinopathies;
b) The specificity of MCV and MCH cut-offs is low. We would also have had to conduct HPLC for 50% of the normal individuals since their MCV or MCH was below our cut-offs due to an iron deficiency;
c) Our findings show that an HPLC-first approach allows for 14% more carriers to be identified with no increase in cost.
This approach is faster and less labor-intensive, thus it seems to be more suited for screening antenatal families.
Recently, the lab took the decision to run red cell indices only for borderline cases where HPLC alone seems insufficient to draw conclusions. These are cases with Hb A2 between 3.5% and 4%. Our position of using HPLC as an upfront screening test seems to be validated, at least for the population that we serve.
In order to minimize missing couples at risk, a quantitative evaluation of HbA2 was recommended by Cao and Kan to be added in the first step of screening (10). The latest practice advisory developed by the American College of Obstetricians and Gynecologists recommends universal hemoglobinopathy testing using hemoglobin electrophoresis or molecular genetic testing in the initial prenatal visit even as the prevalence of hemoglobinopathies is lower in the United States (11). The “Significant haemoglobinopathies: guidelines for screening and diagnosis” by the British Society for Haematology also indicates that in areas of high prevalence (fetal prevalence >1.5 per 10,000 pregnancies), as is the case in India, simultaneous CBC and HPLC are recommended (12). The clinical practice guidelines from the Genetics Committee of the Society of Obstetricians and Gynaecologists of Canada (SOGC) and the Prenatal Diagnosis Committee of the Canadian College of Medical Geneticists (CCMG) also call for simultaneous CBC and HPLC (13). The advantages of directly testing by HPLC, capillary electrophoresis, or other comparable technologies as opposed to screening using red cell indices, NESTROFF, solubility tests, etc., are well acknowledged. Another study on the screening of antenatal women for beta-thalassemia also acknowledges that a high prevalence of anemia interferes with the use of red cell indices (14). The main driver for adopting multi-step screening approaches has been cost control (7, 15–17). Our experience shows that antenatal screening of pregnant women can be accomplished with improved carrier detection as compared to the two-step screening process without an increase in costs by directly screening using the gold standard test.
We conclude that conducting HPLC as the first step is cost-effective. With this approach, we have identified several types of hemoglobinopathies, including alpha and beta variants, that would have otherwise been missed by the CBC-first conventional screening method. Additionally, this approach saves time and labor by minimizing the number of tests that need to be performed. Conducting HPLC as the first method seems to be the preferable approach for prevention programs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Institutional Ethics Committee Sankalp India Foundation. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SP: Conceptualization, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing. SK: Formal analysis, Investigation, Validation, Writing – original draft, Writing – review & editing. MN: Data curation, Investigation, Writing – original draft, Writing – review & editing. SH: Data curation, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing. SJ: Conceptualization, Resources, Writing – original draft, Writing – review & editing. RD: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. RA: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Software, Validation, Writing – original draft, Writing – review & editing. LF: Conceptualization, Formal analysis, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Thalassemia and Sickle Cell Society, Hyderabad - India and Sankalp India Foundation, Bangalore, India.
Author RA was employed by the company Jagriti InnoHealth Platforms Pvt. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Colah R, Italia K, Gorakshakar A. Burden of thalassemia in India: The road map for control. Pediatr Hematol Oncol J. (2017) 2:79–84. doi: 10.1016/j.phoj.2017.10.002
2. Mohanty D, Colah RB, Gorakshakar AC, Patel RZ, Master DC, Mahanta J, et al. Prevalence of β-thalassemia and other haemoglobinopathies in six cities in India: a multicentre study. J Community Genet. (2013) 4:33–42. doi: 10.1007/s12687-012-0114-0
3. Modell B, Darlison M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ. (2008) 86:480–7. doi: 10.2471/BLT.06.036673
4. Life expectancy and risk factors for early death in patients with severe thalassemia syndromes in South India. Blood Advances (2020) 4(7):1448–57. doi: 10.1182/bloodadvances.2019000760
5. Prevention and control of hemoglobinopathies in India - Thalassemias, sickle cell disease and other variant hemoglobins. (2016). Available online at: https://nhm.gov.in/images/pdf/programmes/RBSK/Resource_Documents/Guidelines_on_Hemoglobinopathies_in%20India.pdf (Accessed August 26, 2017).
6. Indian College of Hematology on behalf of Indian Society of Hematology and Blood Transfusion, Indian Council of Medical Research (ICMR). National guidelines on management and control of Sickle Cell Disease – 2022. (2022). Available online at: https://sickle.nhm.gov.in/uploads/english/OperationalGuidelines.pdf.
7. Colah RB. The use of NESTROFT for screening pregnant women for detection of β-thalassemia carriers. J Fetal Med. (2015) 02:9–10. doi: 10.1007/s40556-015-0040-4
8. Guidelines for antenatal care and skilled attendance at birth. (2010). Available online at: https://nhm.gov.in/images/pdf/programmes/maternal-health/guidelines/sba_guidelines_for_skilled_attendance_at_birth.pdf (Accessed February 26, 2017).
9. Ghosh K, Colah R, Choudhry V, Das R, Manglani M, Madan N, et al. Guidelines for screening, diagnosis and management of hemoglobinopathies. Indian J Hum Genet. (2014) 20:101. doi: 10.4103/0971-6866.142841
10. Cao A, Kan YW. The prevention of thalassemia. Cold Spring Harb Perspect Med. (2013) 3:a011775. doi: 10.1101/cshperspect.a011775
11. Elk AC, Gandhi M, Kaimal AJ, Moniz M, Shields A. Hemoglobinopathies in Pregnancy. Practice Advisory by the American College of Obstetricians and Gynecologists (2023). Available online at: https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2022/08/hemoglobinopathies-in-pregnancy.
12. Ryan K, Bain BJ, Worthington D, James J, Plews D, Mason A, et al. Significant haemoglobinopathies: guidelines for screening and diagnosis. Br J Haematol. (2010) 149:35–49. doi: 10.1111/j.1365-2141.2009.08054.x
13. Langlois S, Ford JC, Chitayat D, Chitayat D, Désilets VA, Farrell SA, et al. Carrier screening for thalassemia and hemoglobinopathies in Canada. J Obstet Gynaecol Can. (2008) 30:950–9. doi: 10.1016/S1701-2163(16)32975-9
14. Sharma A, Uppal N, Kukreja S, Kaur M, Kaur S. Screening of Thalassemia in pregnant females visitng tertiary hospital in Amritsar. Int J Clin Biochem Res. (2020) 7:226–31. doi: 10.18231/j.ijcbr.2020.049
15. Jahan A, Singh G, Gupta R, Sarin N, Singh S. Role of red cell indices in screening for beta thalassemia trait: an assessment of the individual indices and application of machine learning algorithm. Indian J Hematol Blood Transfus. (2021) 37:453–7. doi: 10.1007/s12288-020-01373-x
16. Mendiratta SL, Bajaj S, Popli S, Singh S. Screening of women in the antenatal period for thalassemia carrier status: comparison of NESTROFT, red cell indices, and HPLC analysis. J Fetal Med. (2015) 02:21–5. doi: 10.1007/s40556-015-0036-0
Keywords: thalassemia, hemoglobinopathy, CBC, HPLC, cost, detection, prevention
Citation: Periyavan S, Kumar S, Mamatha GN, Hegde S, Jain S, Dhanya R, Agarwal RK and Faulkner L (2025) HPLC first approach in detecting thalassemia and other common hemoglobinopathies is more cost and time effective. Front. Hematol. 4:1461498. doi: 10.3389/frhem.2025.1461498
Received: 08 July 2024; Accepted: 14 February 2025;
Published: 17 March 2025.
Edited by:
Imen Moumni, Pasteur Institute of Tunis, TunisiaReviewed by:
Prabhakar S. Kedar, Indian Council of Medical Research, IndiaCopyright © 2025 Periyavan, Kumar, Mamatha, Hegde, Jain, Dhanya, Agarwal and Faulkner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rajat Kumar Agarwal, cmFqYXRAc2Fua2FscGluZGlhLm5ldA==
†ORCID: Rajat Kumar Agarwal, orcid.org/0000-0003-4864-6479
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.