- 1Department of Molecular Biotechnology and Health Sciences, University of Turin, Turin, Italy
- 2Department of Clinical and Biological Sciences, University of Turin, Turin, Italy
- 3Department of Medical Sciences, University of Turin, Turin, Italy
- 4Department of Sciences of Public Health and Pediatrics, University of Turin, Turin, Italy
Chronic neutrophilic leukemia (CNL) is a rare myeloproliferative neoplasm characterized by CSF3R mutations. Here, we report the first case of a CNL patient positive for a de-novo germline CSF3R T618I variant effectively treated with ruxolitinib and pegylated interferon (peg-IFN), obtaining normalization of blood counts and maintaining good disease control for over 8 years. In addition, the RUNX1 L56S variant was detected at diagnosis and confirmed in subsequent next-generation sequencing (variant allele frequency of 50.4%). Although this variant has been debated, it is now widely recognized as a benign polymorphism and current evidence shows it does not modify prognosis. Our report adds to the growing body of evidence confirming the importance of a good evaluation of the mutational status of driver mutations in the setting of germline variants.
Introduction
Chronic neutrophilic leukemia (CNL) is a BCR-ABL1 negative myeloproliferative neoplasm (MPN) characterized by sustained mature neutrophil proliferation (white blood cell count ≥ 25 × 109/ L, with ≥80% segmented neutrophils plus bands), bone marrow (BM) granulocytic hyperplasia, and hepatosplenomegaly (1). It has a median age at diagnosis of 71 years and a median survival of 24 months (2). The discovery of oncogenic driver variants in the colony-stimulating factor 3 receptor (CSF3R), most frequently CSF3R T618I, in the majority of patients (3) led to the addition of this mutation to the diagnostic criteria for CNL in the 2016 World Health Organization (WHO) classification (4) and the International Consensus Classification (ICC) of Myeloid Neoplasms and Acute Leukemias (5). Though the presence of an activating CSF3R mutation supports the diagnosis of CNL, it is not definitive. In fact, not only have additional known pathogenic mutations, such as those in ASXL1 and SETBP1, been reported in CSF3R mutated patients (2), but this mutation has also been found in other myeloid neoplasms, with a major impact on prognosis (6–9).
Due to the rarity of the disease, no standard of care exists (2). An allogeneic stem cell transplant (allo-HSCT) is the only therapy that improves survival (10, 11). Other therapeutic approaches include splenectomy, sometimes complicated by postoperative worsening of neutrophilia (12, 13), and conventional chemotherapy with hydroxyurea (HU), often used as a first-line agent. Interferon-alpha (IFN-a) and pegylated interferon-a (peg-IFN-a) have been proven to be effective treatments and should be considered as a first-line therapy for young patients, or as a later option if previous therapies fail (2). Increasing evidence has shown the efficacy of the JAK1/JAK2 inhibitor ruxolitinib (3, 14–16).
While in most cases the clinical history of the disease indicates the presence of a CSF3R mutation, very few cases in literature have reported CNL resulting from germline mutations. We present the first case of a patient with CNL with a de novo germline CSF3R T618I variant who was effectively treated with ruxolitinib and peg-IFN.
Case description
Herein, we report a 29-year-old woman with CNL positive for a germline CSF3R T618I variant. At birth, her absolute neutrophil count was 80000/mmc [with neutrophil precursors on peripheral blood (PB)< 10%] and it was not associated with anemia or thrombocytopenia. BM studies performed at 1 and 5 months of age revealed myeloid hyperplasia with conserved maturation, <5% myeloblasts, and no dysplastic features; her karyotype was normal and the BCR::ABL1 fusion gene was absent. Her family history was negative for hematological diseases. At 6 months of age, her spleen size demonstrated by ultrasound was 8.8 cm. Her PB features (absence of anemia, thrombocytopenia, or monocytosis), the lack of monosomy 7 or other chromosomal abnormalities, normal levels of hemoglobin-F for age, and normal in vitro growth of granulocyte progenitors (dependent on and proportional to the addition of growth factors) permitted the exclusion of juvenile myelomonocytic leukemia (JMML) as a diagnosis (1, 4, 17). The absence of the Philadelphia chromosome and the absence of granulocytic dysplasia and dysgranulopoiesis in the BM allowed the exclusion of Ph+ Chronic Myeloid Leukemia (CML) and Myelodysplastic syndromes/myeloproliferative neoplasms (MDS/MPN) with neutrophilia (previously aCML) (1), respectively.
Since the patient was asymptomatic, no treatment was started. She received regular checks of her BM, including cytogenetics, to exclude dysplastic evolution. Her neutrophil levels during childhood stabilized at 30-40000/mmc without the need for treatment and without evidence of major infectious episodes. The persistence of a significant splenomegaly (21 cm at the age of 14) determined a mild thrombocytopenia, with values just above 100x10^9/L. At 20 years of age, because of her spleen size (24 cm) and consequent symptoms, she underwent a splenectomy; after the procedure, an increase in neutrophil and platelet counts occurred, forcing attending physicians to start therapy with HU 1500 mg/die.
At this point, the patient was referred to our adult hematological center. BM evaluation revealed expansion of granulocyte lineage, <1% myeloblasts, and no dysplastic features. A biological study using next-generation sequencing (NGS) on a panel of 30 myeloid genes1 identified NM_000760.4(CSF3R):c.1853C>T, p.Thr618Ile (T618I), and NM_001754.5(RUNX1):c.167T>C, p.Leu56Ser (L56S) variants on PB. No other variants were detected. In addition, no rearrangements involving PDGFRA, PDGFRB, FGFR1, or PCM1-JAK2 were found in this patient. Of note, at that time, variant allele frequency (VAF) was not reported. The CSF3R T618I is a pathogenic variant associated with CNL both in somatic and germline states (Clinvar Variation ID:208339). The patient’s clinical history and the results obtained supported the diagnosis of CNL (4). The first treatment option proposed was allo-HSCT, which she has consistently declined.
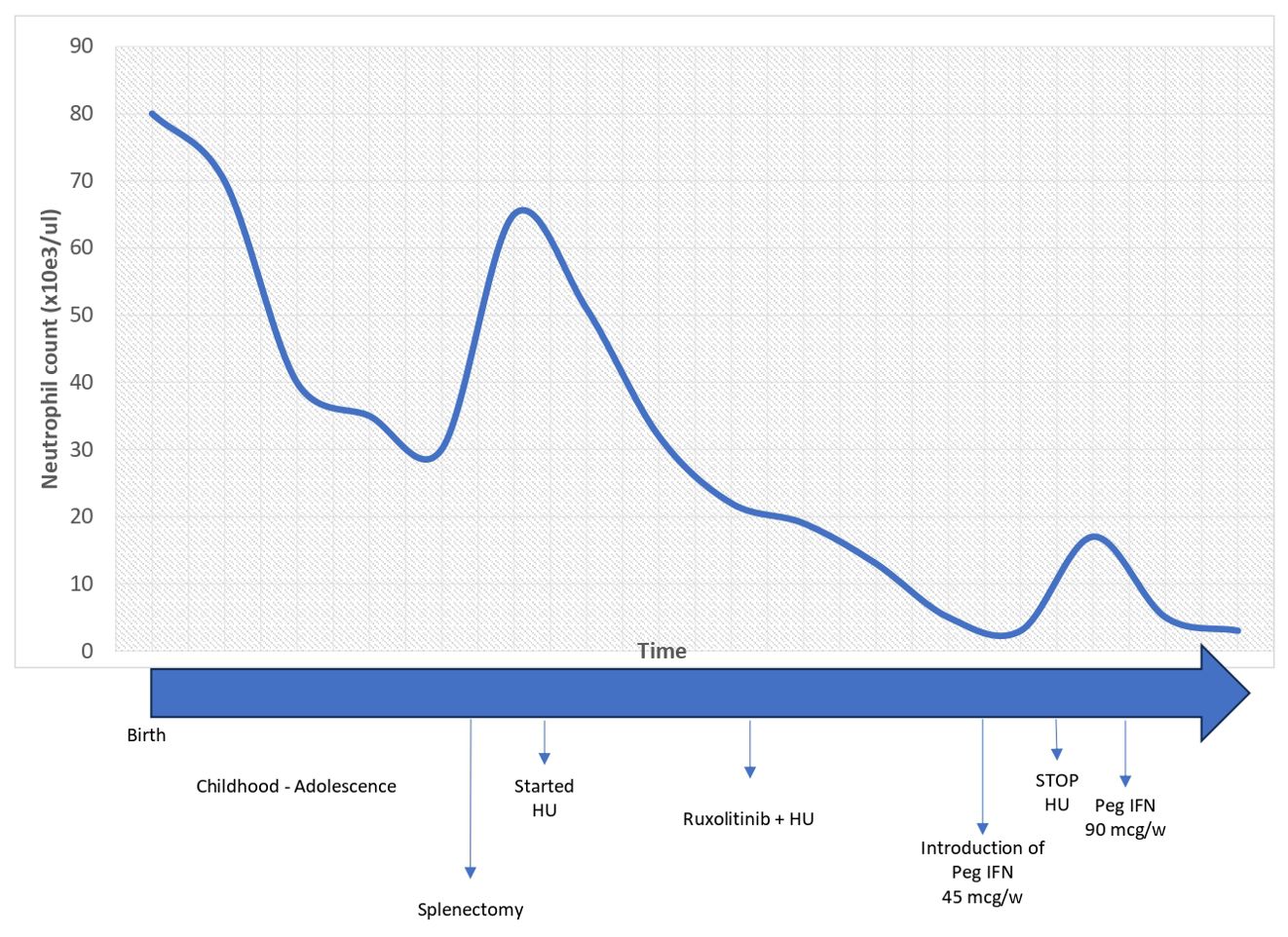
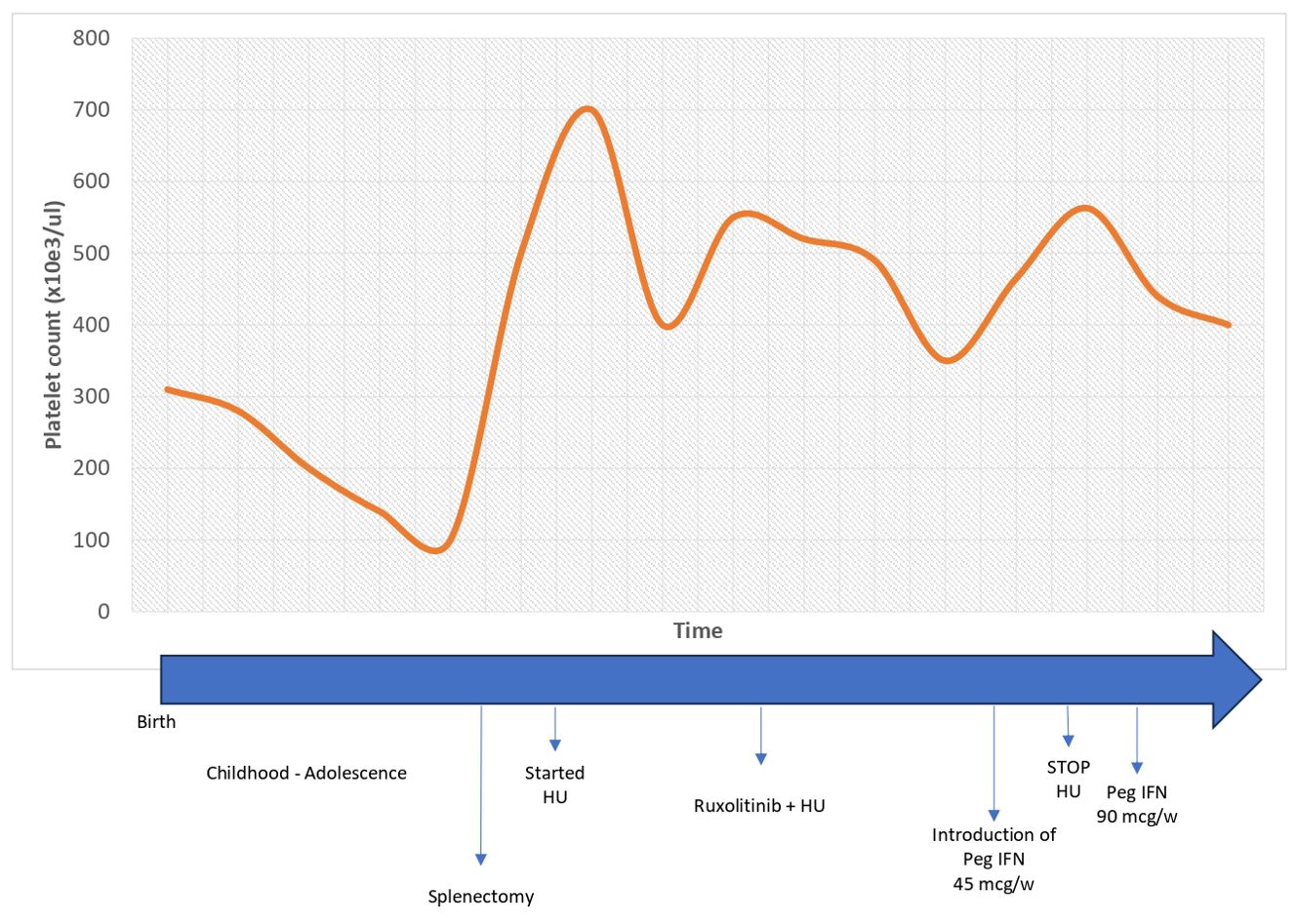
Since HU did not contain neutrophil counts (21 years, 98,100/mmc), therapy with ruxolitinib was started at a dose of 20 mg with good tolerance. However, it was not sufficient to control the neutrophilia (25 years, 12,750/mmc), therefore, based on expert opinion, literature on combination therapy in MPN (18), and in vitro studies of the role of IFNs in neutrophil apoptosis (19, 20), peg-IFN was introduced at a weekly dose of 45 mcg. After 3 months, it was increased to 90 mcg weekly in association with ruxolitinib 40 mg, while HU was discontinued. With ruxolitinib and peg-IFN, good control of the disease was obtained, with normalization of both neutrophil and platelet counts after 6 weeks of treatment, which is still being maintained after 8 years with good tolerance and no significant side effects (the lifelong trend of neutrophil and platelet counts are described in Figures 1, 2, respectively). The risk of progression based on the score by Szuber et al. (15) remained low, supporting the maintenance of the cytoreductive therapy. BM studies, performed at 26 and 29 years (Figure 3), remained unchanged, as did molecular and cytogenetic studies. NGS confirmed the presence of the T618I variant in the CSF3R gene (VAF 49,8%) and RUNX1 L56S (VAF 50,4%). Sanger sequencing of exon 14 of the CSF3R gene in DNA extracted from BM, PB, and saliva confirmed the presence of the variant in a heterozygous state in all three tissues, consistent with the germline origin. In addition, Sanger sequencing in the proband’s parents established its de novo nature (absent in parents).
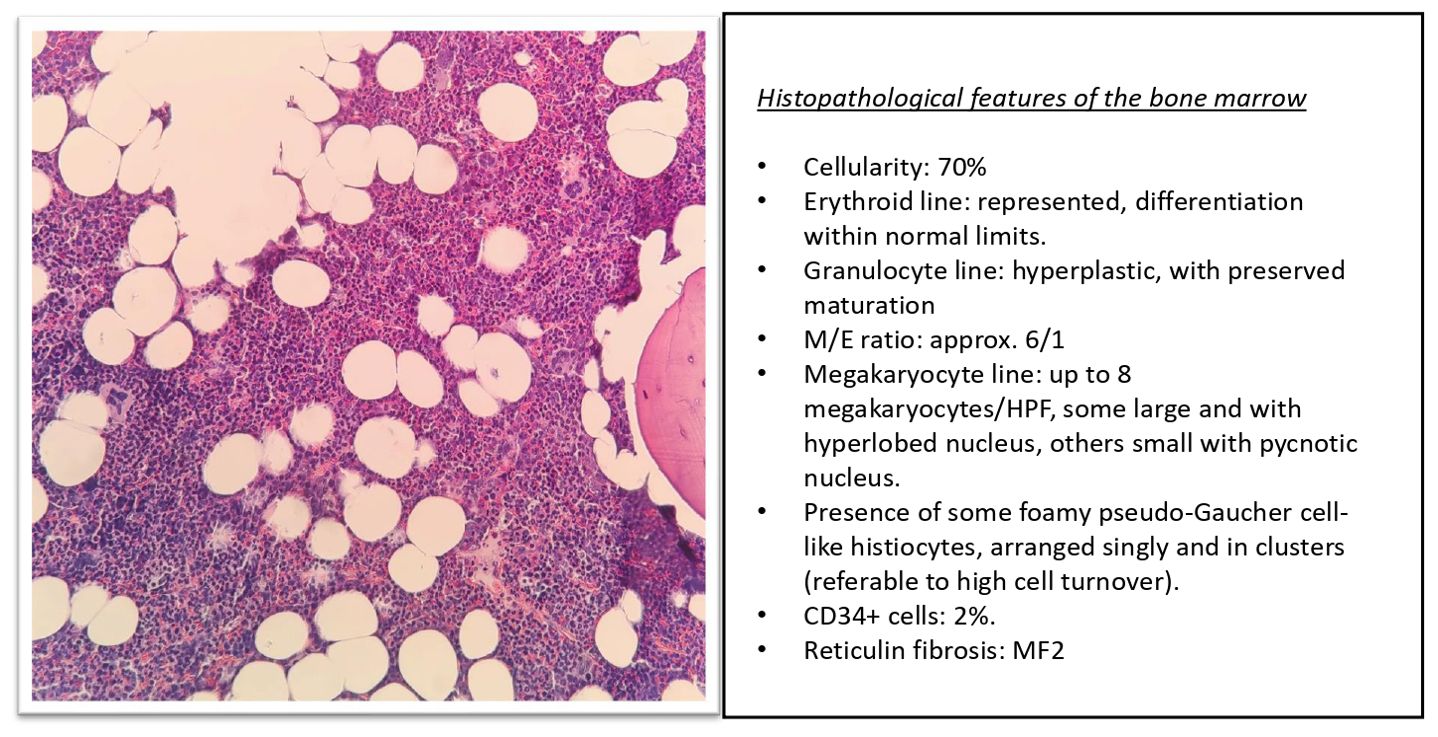
Figure 3. Histopathological panel with the morphological details of the patient’s bone marrow biopsy.
Discussion
CNL is a rare neoplasm and its true incidence is unknown (2). It mainly affects older adults, and CSF3R T618I or other activating CSF3R mutations in these patients are present. However, recent evidence suggests that CNL may also develop from germline mutations (described patients with a germline CSF3R T618I mutation are summarised in Table 1). The first case of a CNL patient with a germline CSF3R T618I variant was reported in 2016 (21). The patient in question had a stable leukocytosis for 11 years, splenomegaly, and mildly decreased platelets; no additional variants were identified and she was never treated. A different variant in the CSF3R gene (T617N), transmitted with an autosomal dominant pattern of inheritance with complete penetrance and which is responsible for hereditary chronic neutrophilia, was previously identified by Plo et al. (22). The first case of a family with CNL resulting from a constitutional CSF3R T618I variant was reported by Duployez et al. (23). The proband, an 18-year-old man with splenomegaly and leukocytosis, was diagnosed with CNL. The finding of an overlapping asymptomatic clinical picture in his sister (age: 9 years) led physicians to study the whole family. A heterozygous germline CSF3R T618I variant was identified in both children and the mother (with stable leukocytosis and splenomegaly that have never been investigated). In addition, a heterozygous variant of uncertain significance (VUS) in NRAS (c.54_55delAC:p.L19Dfs*12; VAF 49%) was identified in the proband’s sister and mother. The proband, due to symptomatic splenomegaly, started ruxolitinib treatment up to 30 mg. Unfortunately, the course of the disease is not known since regular follow-ups were not conducted. Recently, a four-generation family with germline transmission of the T618I mutation has been reported (20). The proband, who was diagnosed with CSF3R T618I-mutated CNL at 48 years of age, had several family members showing neutrophilia in their blood tests; in these relatives (from 4 to 51 years of age), analyses using whole-exome sequencing revealed the presence of a germline CSF3R T618I variant. Interestingly, none of them progressed to acute leukemia.
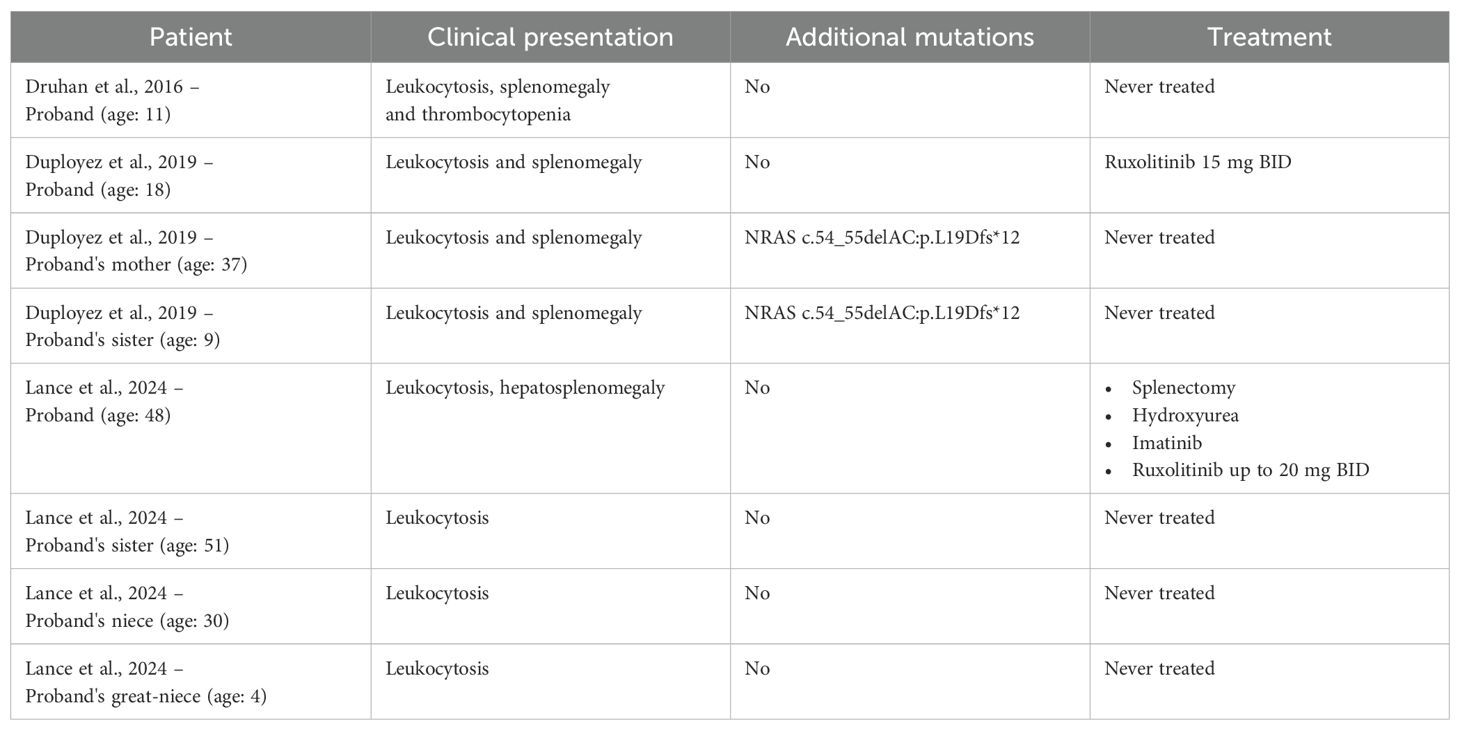
Table 1. Clinical presentation, molecular data, and therapy of the described patients with a germline CSF3R T618I mutation.
Several authors (2) have argued that a natural history of CNL, like other MPNs, predicts a chronic phase, which may evolve into accelerated and blastic phases, and that further genetic variations are required for disease evolution (24, 25). Furthermore, the presence of a germline heterozygous CSF3R variant in patients with hematologic malignancies was identified as a risk factor for the development of the disease (26). A case report suggested that de novo mutations in GATA2 and KIT and the increased allelic burden of RUNX1 (c.1009_1022del; p.337_341del) were possible contributors to ruxolitinib resistance in a CNL patient (27). Stoner et al. proved that RUNX1 and STAG2 mutant subclones were present at disease progression and that cooperating mutations of CSF3R and RUNX1 could be an early marker of acute myeloid leukemia (AML) transformation (28). Zhang et al. demonstrated that CSF3R mutations are rare in AML, occasionally associated with core-binding factor gene abnormalities, such as RUNX1-RUNX1T1 (29). The presence of a concurrent RUNX1 germline variant (quite likely given the VAF of 50%), could have resulted in doubts regarding the diagnosis, since in the current WHO classification, the patient should be formally diagnosed with “Myeloid neoplasms associated with germline predisposition” (1). However, her clinical presentation (neutrophilia vs. thrombocytopenia, which is most commonly associated with a RUNX1 germline mutation) supported the diagnosis of CNL (30). In addition, even though at the time of diagnosis a RUNX1 L56S variant was controversially classified, it is now largely considered a benign polymorphism (31, Clinvar Variation ID:239045). Therefore, it is not currently possible to correlate a RUNX1 L56S variant with a prognosis.
Conclusion
In this report, we described the first case of CNL with a germline CSF3R mutation that was effectively treated with a combination therapy of ruxolitinib and peg-IFN. The treatment allowed good control of the disease and we did not record clonal evolution or changes in the mutational status. Although the therapy is effectively controlling neutrophil levels, the germline nature of the CSF3R mutation implies that the disease cannot be considered curable. The considerable risk of disease progression to acute leukemia emphasizes the importance of strict surveillance.
In conclusion, this report confirms that CNL patients may have different clinical courses; besides an analysis of mutational status, identification of germline origin may lay the foundations for studies on genetic predisposition and the need for screening in at-risk families. For the study of germline variants, it will be useful for clinicians to consider fibroblasts as the preferred alternative for confirming germline mutations. The disease can be stabilized and controlled with novel agents such as ruxolitinib; the new therapeutic combination of ruxolitinib and peg-IFN suggests that effective personalized strategies can improve clinical results.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Approval by an ethics committee is not required for single case reports that involve a non-vulnerable patient. The patient signed their written informed consent. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SG: Writing – original draft, Writing – review & editing. VB: Writing – review & editing. DCa: Writing – review & editing. VP: Writing – review & editing. LF: Writing – review & editing. UR: Writing – review & editing. DCi: Writing – review & editing. CF: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ NGS technology was used to examine a panel of 30 myeloid genes: ABL1, ASXL1, BRAF, CALR, CBL, CEBPA, CSF3R, DNMT3A, ETV6, EZH2, FLT3, HRAS, IDH1, IDH2, JAK2, KIT, KRAS, MPL, NPM1, NRAS, PTPN11, RUNX1, SETBP1, SF3B1, SRSF2, TET2, TP53, U2AF1, WT1, and ZRSR2.
References
1. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: myeloid and Histiocytic/Dendritic neoplasms. Leukemia. (2022) 36(7):1703–19. doi: 10.1038/s41375-022-01613-1
2. Szuber N, Orazi A, Tefferi A. Chronic neutrophilic leukemia and atypical chronic myeloid leukemia: 2024 update on diagnosis, genetics, risk stratification, and management. Am J Hematol. (2024) 99(7):1360–87. doi: 10.1002/ajh.27321
3. Maxson JE, Gotlib J, Pollyea DA, Fleischman AG, Agarwal A, Eide CA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. (2013) 368:1781–90. doi: 10.1056/NEJMoa1214514
4. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. (2016) 127:2391–405. doi: 10.1182/blood-2016-03-643544
5. Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. International Consensus Clas-sification of Myeloid Neoplasms and Acute Leukemias: integrating morphologic, clinical, and genomic data. Blood. (2022) 140(11):1200–28. doi: 10.1182/blood.2022015850
6. Loghavi S, Kanagal-Shamanna R, Khoury JD, Medeiros LJ, Naresh KN, Nejati R, et al. Fifth edition of the world health classification of tumors of the hematopoietic and lymphoid tissue: myeloid neoplasms. Mod Pathol. (2024) 37:100397. doi: 10.1016/j.modpat.2023.100397
7. Mohamed A, Gao J, Chen YH, Abaza Y, Altman J, Jennings L, et al. CSF3R mutated myeloid neoplasms: Beyond chronic neutrophilic leukemia. Hum Pathol. (2024) 149:66–74. doi: 10.1016/j.humpath.2024.06.008
8. Guastafierro V, Ubezio M, Manes N, Milanesi C, Della Porta M, Bonometti A. CSF3R-mutant chronic myelomonocytic leukemia is a distinct clinically subset with abysmal prognosis: a case report and systematic review of the literature. Leuk Lymphoma. (2023) 64:1566–73. doi: 10.1080/10428194.2023.2227750
9. Palomo L, Meggendorfer M, Hutter S, Twardziok S, Ademà V, Fuhrmann I, et al. Molecular landscape and clonal architecture of adult myelodysplastic/myeloproliferative neoplasms. Blood. (2020) 136:1851–62. doi: 10.1182/blood.2019004229
10. Ruan GJ, Smith CJ, Day C, Harmsen WS, Zblewski DL, Alkhateeb H, et al. A population-based study of chronic neutrophilic leukemia in the United States. Blood Cancer J. (2020) 10:68. doi: 10.1038/s41408-020-0334-1
11. Menezes J, Cigudosa JC. Chronic neutrophilic leukemia: a clinical perspective. Onco Targets Ther. (2015) 8:2383–90. doi: 10.2147/OTT.S49688
12. Tuohy E. A case of splenomegaly with polymorphonuclear neutrophil hyperleukocytosis. Am J Med Sci. (1920) 160:18–25. doi: 10.1097/00000441-192007000-00003
13. You W, Weisbrot IM. Chronic neutrophilic leukemia: report of two cases and review of the literature. Am J Clin Pathol. (1979) 72:233–42. doi: 10.1093/ajcp/72.2.233
14. Fleischman AG, Maxson JE, Luty SB, Agarwal A, Royer LR, Abel ML, et al. The CSF3R T618I mutation causes a lethal neutrophilic neoplasia in mice that is responsive to therapeutic JAK inhibition. Blood. (2013) 122:3628–31. doi: 10.1182/blood-2013-06-509976
15. Szuber N, Finke CM, Lasho TL, Elliott MA, Hanson CA, Pardanani A, et al. CSF3R-mutated chronic neutrophilic leukemia: long-term outcome in 19 consecutive patients and risk model for survival. Blood Cancer J. (2018) 8:21. doi: 10.1038/s41408-018-0058-7
16. Dao KT, Gotlib J, Deininger MMN, Oh ST, Cortes JE, Collins RH Jr, et al. Efficacy of ruxolitinib in patients with chronic neutrophilic leukemia and atypical chronic myeloid leukemia. J Clin Oncol. (2020) 38:1006–18. doi: 10.1200/JCO.19.00895
17. Gupta AK, Meena JP, Chopra A, Tanwar P, Seth R. Juvenile myelomonocytic leukemia-A comprehensive review and recent advances in management. Am J Blood Res. (2021) 11(1):1–21.
18. Sørensen AL, Mikkelsen SU, Knudsen TA, Bjørn ME, Andersen CL, Bjerrum OW, et al. Ruxolitinib and interferon-α2 combination therapy for patients with polycythemia vera or myelofibrosis: a phase II study. Haematologica. (2020) 105:2262–72. doi: 10.3324/haematol.2019.235648
19. Glennon-Alty L, Moots RJ, Edwards SW, Wright HL. Type I interferon regulates cytokine-delayed neutrophil apoptosis, reactive oxygen species production and chemokine expression. Clin Exp Immunol. (2021) 203:151–9. doi: 10.1111/cei.13525
20. Lance A, Chiad Z, Seegers SL, Paschall SC, Drummond K, Steuerwald NM, et al. Hereditary chronic neutrophilic leukemia in a four-generation family without transformation to acute leukemia. Am J Hematol. (2024) 99:1877–86. doi: 10.1002/ajh.v99.10
21. Druhan LJ, McMahon DP, Steuerwald N, Price AE, Lance A, Gerber JM, et al. Chronic neutrophilic leukemia in a child with a CSF3R T618I germ line mutation. Blood. (2016) 128:2097–9. doi: 10.1182/blood-2016-07-730606
22. Plo I, Zhang Y, Le Couédic JP, Nakatake M, Boulet JM, Itaya M, et al. An activating mutation in the CSF3R gene induces a hereditary chronic neutrophilia. J Exp Med. (2009) 206:1701–7. doi: 10.1084/jem.20090693
23. Duployez N, Willekens C, Plo I, Marceau-Renaut A, de Botton S, Fenwarth L, et al. Inherited transmission of the CSF3R T618I mutational hotspot in familial chronic neutrophilic leukemia. Blood. (2019) 134:2414–6. doi: 10.1182/blood.2019003206
24. Langabeer SE, Haslam K, Kelly J, Quinn J, Morrell R, Conneally E. Targeted next-generation sequencing identifies clinically relevant mutations in patients with chronic neutrophilic leukemia at diagnosis and blast crisis. Clin Transl Oncol. (2018) 20:420–3. doi: 10.1007/s12094-017-1722-2
25. Zhang H, Wilmot B, Bottomly D, Dao KT, Stevens E, Eide CA, et al. Genomic landscape of neutrophilic leukemias of ambiguous diagnosis. Blood. (2019) 134:867–79. doi: 10.1182/blood.2019000611
26. Trottier AM, Druhan LJ, Kraft IL, Lance A, Feurstein S, Helgeson M, et al. Heterozygous germ line CSF3R variants as risk alleles for development of hematologic Malignancies. Blood Adv. (2020) 4:5269–84. doi: 10.1182/bloodadvances.2020002013
27. Nooruddin Z, Miltgen N, Wei Q, Schowinsky J, Pan Z, Tobin J, et al. Changes in allele frequencies of CSF3R and SETBP1 mutations and evidence of clonal evolution in a chronic neutrophilic leukemia patient treated with ruxolitinib. Haematologica. (2017) 102:e207–9. doi: 10.3324/haematol.2016.163089
28. Stoner RC, Press RD, Maxson JE, Tyner JW, Dao KT. Insights on mechanisms of clonal evolution in chronic neutrophilic leukemia on ruxolitinib therapy. Leukemia. (2020) 34:1684–8. doi: 10.1038/s41375-019-0688-1
29. Zhang Y, Wang F, Chen X, Zhang Y, Wang M, Liu H, et al. CSF3R Mutations are frequently associated with abnormalities of RUNX1, CBFB, CEBPA, and NPM1 genes in acute myeloid leukemia. Cancer. (2018) 124:3329–38. doi: 10.1002/cncr.31586
30. Brown AL, Arts P, Carmichael CL, Babic M, Dobbins J, Chong CE, et al. RUNX1-mutated families show phenotype heterogeneity and a somatic mutation profile unique to germline predisposed AML. Blood Adv. (2020) 4(6):1131–44. doi: 10.1182/bloodadvances.2019000901
Keywords: case report, chronic neutrophilic leukemia, myeloproliferative neoplasm, CSF3R mutation, NGS
Citation: Grano S, Bonuomo V, Carli D, Peri V, Farinasso L, Ramenghi U, Cilloni D and Fava C (2024) Ruxolitinib and pegylated interferon in Chronic Neutrophilic Leukemia due to a pathogenic CSF3R germline variant: a case report. Front. Hematol. 3:1508463. doi: 10.3389/frhem.2024.1508463
Received: 09 October 2024; Accepted: 19 November 2024;
Published: 12 December 2024.
Edited by:
Valentina Giudice, University of Salerno, ItalyReviewed by:
Lawrence Druhan, Levine Cancer Institute, United StatesArturo Bonometti, Humanitas University, Italy
Yuta Baba, Showa University Fujigaoka Hospital, Japan
Copyright © 2024 Grano, Bonuomo, Carli, Peri, Farinasso, Ramenghi, Cilloni and Fava. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Selene Grano, c2VsZW5lLmdyYW5vQHVuaXRvLml0
 Selene Grano
Selene Grano Valentina Bonuomo
Valentina Bonuomo Diana Carli
Diana Carli Veronica Peri1
Veronica Peri1 Loredana Farinasso
Loredana Farinasso Ugo Ramenghi
Ugo Ramenghi Daniela Cilloni
Daniela Cilloni Carmen Fava
Carmen Fava