
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Hematol., 10 January 2025
Sec. Blood Cancer
Volume 3 - 2024 | https://doi.org/10.3389/frhem.2024.1482891
Cyclic thrombocytopenia (CTP) is a rare hematological disorder characterized by significant oscillations in platelet counts, alternating between phases of severe thrombocytopenia and periods of normal or elevated platelet levels. Due to its clinical similarities with Immune Thrombocytopenia (ITP), CTP presents a diagnostic challenge. We report the case of a 54-year-old woman who first presented at age 45 with a petechial rash, heavy menstruation, and severe thrombocytopenia with a platelet count of less than 5,000 × 10^9/L. Her severe thrombocytopenia was followed by marked rebound thrombocytosis, ultimately leading to a clinical diagnosis of idiopathic cyclic thrombocytopenia (CTP). Unlike other cyclic hematological disorders, CTP exclusively affects platelet counts distinguishing it from other conditions. It is unresponsive to conventional ITP treatments, and thus accurate diagnosis through vigilant platelet monitoring in unexplained thrombocytopenia can help CTP patients avoid unnecessary therapies and their associated side effects.
Cyclic thrombocytopenia (CTP) is a rare hematological disorder, predominantly affecting female patients, characterized by periodic fluctuations in platelet counts. This often leads to misdiagnosis as immune thrombocytopenia (ITP) due to overlapping clinical manifestations (1, 2). With only about 70 cases reported to date, CT’Ps etiology remains largely elusive (3). The absence of standard treatment protocols and specific diagnostic criteria further complicates its management (4–6). This report discusses a case of CTP managed over nearly a decade, highlighting our approach to the challenges in the diagnosis and treatment of this rare hematological disorder.
A patient in her 50s, with a past medical history of inconsistent menorrhagia and benign positional vertigo, presented with intermittent petechiae and easy bruising. She has no other medical conditions or surgical history. Family history was remarkable for Multiple Myeloma in her brother. Her complete blood count (CBC) revealed thrombocytopenia with platelet count of 10.0x109L (normal range: 140,000-440,000) and microcytic anemia with hemoglobin (Hb) of 10.5 g/dL (12.0-16.0) and mean corpuscular volume (MCV) of 76.0 fL (80-99). Investigations including ANA, ESR, occult blood stool, coagulation panel, and bone marrow biopsy yielded unremarkable results. The peripheral smear did not show any schistocytes or other abnormal cells. Endocrinological associations with menstruation or thyroid disease were unfruitful. The infectious workup included viral screening for HIV and Hepatitis panels that were negative. Given the lack of any other symptoms, recent travels, or sick contacts, there was low suspicion for other infections etiologies. Flow cytometry of blood and bone marrow revealed no abnormalities.
Initial improvement with corticosteroids and intravenous immunoglobulin was followed by relapse, exhibiting platelet counts as low as 4.0x109L, and on other occasions, as high as 1.9x109L. The patient reported increased bruising/petechiae and weakness during nadirs and headaches during apices. Moreover, during the relapses, the patient’s thrombocytosis was accompanied by transaminitis with aspartate transaminase (AST) of 58 U/L (0 - 37), alanine transaminase (ALT) of 51 U/L (6 - 37) and alkaline phosphate of 135 U/L (35 - 104). She underwent an elective splenectomy with the discovery of the rebound thrombocytosis, though it provided no clear therapeutic benefit. She subsequently failed multiple treatment trials, including immunosuppressants and thrombopoietin (TPO) mimetics. TPO mimetics were administered in the form of eltrombopag (Promacta) at a dose of 75 mg daily for three months and romiplostim (Nplate), initially dosed at 0.5–1 mcg/kg and later increased to a maximum of 250 mcg weekly, for six months. While these therapies increased the peak (apex) platelet counts, they failed to improve the lowest (nadir) counts and were therefore discontinued. Ultimately, the patient was diagnosed with idiopathic cyclic thrombocytopenia (CTP), leading to the cessation of all immunosuppressive and TPO-directed therapies. Her course of platelet count fluctuations and interventions are further detailed in Figure 1.
The patient also demonstrated microcytic anemia that was determined to be an unrelated iron deficiency anemia and treated with iron supplements. Also, as can be seen in Figure 2, there was no correlation between the CTP and other blood lines. A parallel comparison of platelet count and mean platelet volume fluctuations are also demonstrated in Figure 3. In addition, bone marrow biopsy (Figure 4) and peripheral smear (Figure 5) were included from year 3 of presentation, showcasing normal WBC and Hb levels during an episode of thrombocytosis.
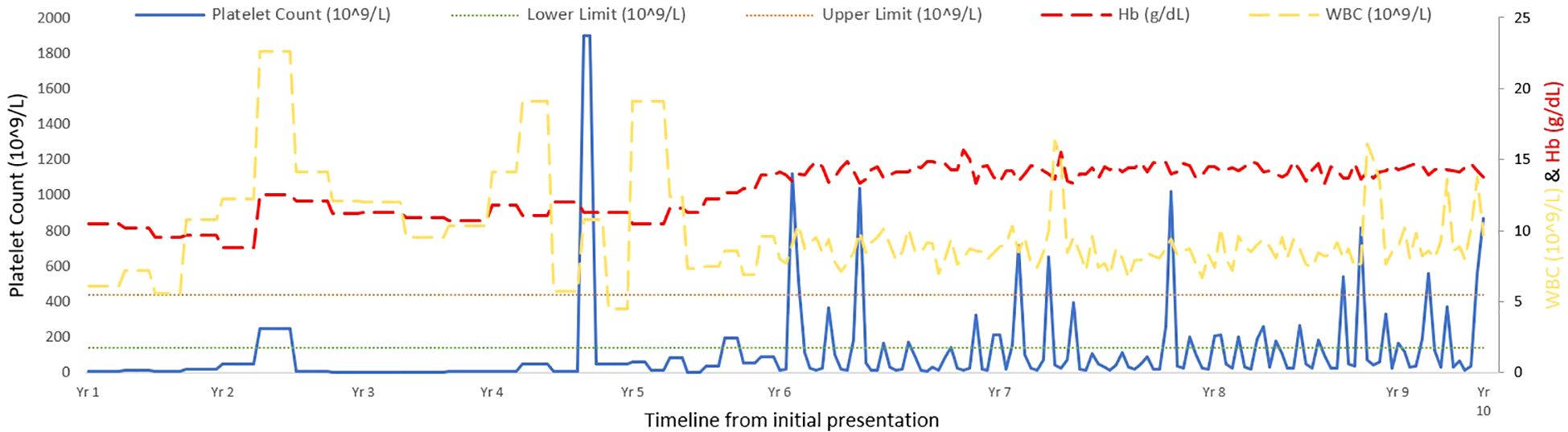
Figure 2. Longitudinal monitoring of platelet counts, white blood cell levels, and hemoglobin over 10 years in a cyclic thrombocytopenia patient. *Due to the weekly monitoring implemented from Year 6, the variance is more obviously seen from that point forward.
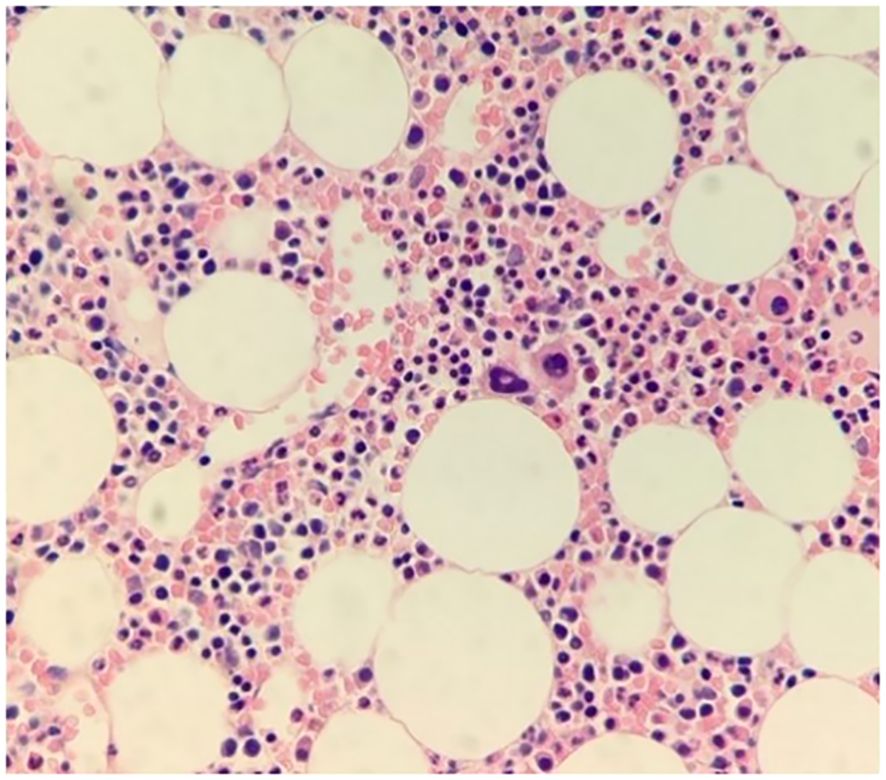
Figure 4. Bone marrow biopsy. Hematoxylin and eosin (H&E)-stained bone marrow biopsy slides at 400X magnification show normocellular bone marrow with trilineage hematopoiesis, showcasing thrombocytosis yet adequate megakaryocytes with normal morphology.
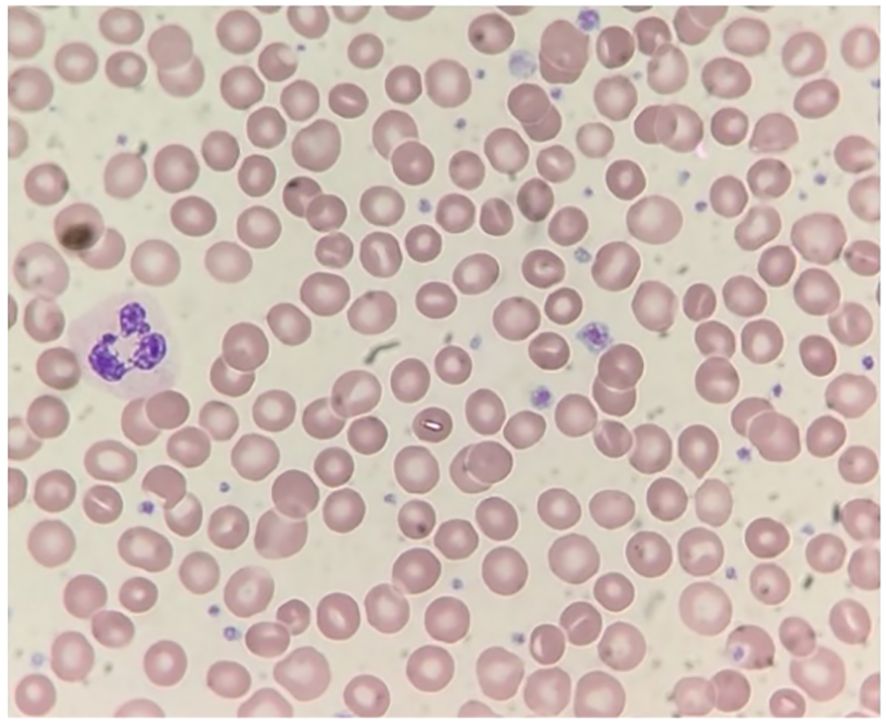
Figure 5. Peripheral blood smear. Wright-stained slide at 1000X magnification) showing normal morphology including variably sized, well granulated platelets (including some larger forms), and no abnormal cell types.
Over a 10-year follow-up, her thrombocytopenia and thrombocytosis cycles seemed to last approximately 5-6 weeks. After inefficacy of multiple therapy lines, all medications were ceased. Currently, she is managed with monthly CBC monitoring and intermittent platelet transfusion at a threshold of platelet count under 10.0x109L.
Cyclic thrombocytopenia, with its characteristic wave-like rhythm in platelet count, presents a diagnostic challenge due to its similarities with Immune Thrombocytopenia (ITP) in clinical presentation (7). The disorder is defined by oscillating platelet counts, leading to phases of thrombocytopenia that can cause symptoms such as easy bruising and prolonged bleeding, interspersed with periods of normal or increased platelet levels, which carry the risk of excessive clotting (8). Notably, the condition is distinguished by its exclusive impact on the platelet line, unlike other cyclic hematological disorders that affect multiple blood cell lines (9).
The pathophysiology and genetic underpinnings of CTP are complex and not fully understood, yet recent studies have identified a correlation with Thrombopoietin (TPO) expression levels (10). Moreover, other research suggests that cyclic thrombocytopenia could arise either from a destabilization in a peripheral control mechanism specific to platelets, from megakaryocyte dysfunction that impairs production of platelets, or from an autoantibody-mediated immune destruction targeting specific platelet antigens (9, 11, 12). Other reports have described hormonal and infectious etiologies; where CTP was observed to correspond with symptoms of menstrual cycles or development of CTP after an elevation of cytokines secondary to an infection with Ehrlichia or Hepatitis B (12,–13). Furthermore, treatment approaches for CTP show variable efficacy. While ITP is often treated with corticosteroids and rituximab, these treatments often fail in CTP. Some patients have shown improvement with cyclosporin A or danazol, others have not responded to these or other medications (5). In the context of multiple myeloma, treatments involving the proteasome inhibitor bortezomib have improved platelet counts, but the applicability of this finding to CTP remains under-explored (4).
Similar to Şumnu and Reyhan’s case, our patient was initially diagnosed with ITP until she exhibited a rebound thrombocytosis (12). Like their findings, our patient also did not respond to steroids or other immunosuppressive agents, suggesting that the underlying mechanism in her condition is unlikely to be autoimmune. Additionally, we ruled out a hormonal etiology given lack of correlation with menstruation, normal labs, and her extensive monitoring over a 10-year period, which included experiencing menopause without any observed changes in her disease symptoms or prognosis.
Our patient did not exhibit any infections mentioned in the literature, thus excluding an infectious cause for her chronic thrombocytopenia (CTP). A splenectomy was also performed, which failed to alleviate the symptoms of the disease. At one point in her treatment course, she received supplementary thrombopoietin (TPO), which was unable to resolve the thrombocytopenia, suggesting that her condition might be more related to TPO receptor issues or a dysfunction in megakaryocyte functionality rather than a deficiency in TPO production. Interestingly, our patient experiences mild transaminitis during episodes of thrombocytosis, potentially implicating liver involvement in the pathogenesis of her disease. Considering that TPO is primarily produced by the liver and that the liver also plays a role in the breakdown of platelets, further research into the liver’s and TPO receptors’ roles in CTP could be instrumental in understanding the etiology of this rare disease.
In light of these findings, we concluded that the best treatment approach is conservative, involving platelet transfusions when her platelet count drops to 10.0x109L or lower. To date, the patient is managing well and has reported no significant issues in dealing with this rare diagnosis.
In summary, clinical evidence has pointed to multiple potential origins for CTP, such as autoimmune responses, megakaryocyte production deficiencies, and disruptions in thrombopoietin receptor function. The rarity and elusive nature of CTP contributes to frequent misdiagnoses, complicating efforts to accurately determine its incidence and prevalence (14). It is important to make the distinction between CTP and ITP given the former’s poor response to corticosteroids and/or splenectomy. Thus, an accurate CTP diagnosis can prevent unwarranted immunosuppression therapy and its associated side effects, or an unnecessary splenectomy, in patients with CTP. The case presented here illustrates the diagnostic and management challenges posed by CTP, emphasizing the urgency for enhanced awareness and dedicated research. Lastly, we advise providers to be on the lookout for recurrent, immunosuppressant-resistant, episodes of thrombocytosis, followed by rebound thrombocytosis, especially in the female patient population.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The requirement of ethical approval was waived by Western Michigan University Homer Stryker M.D. School of Medicine Institutional Review Board for the studies involving humans because Western Michigan University Homer Stryker M.D. School of Medicine Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
TA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. SE: Data curation, Investigation, Validation, Visualization, Writing – original draft, Writing – review & editing. OI: Investigation, Writing – original draft, Writing – review & editing. MO: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. We would like to acknowledge and thank Western Michigan University Homer Stryker M.D. School of Medicine for funding the article publication cost via their contract with Frontiers.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Langlois GP, Arnold DM, Potts J, Leber B, Dale DC, Mackey MC. Cyclic thrombocytopenia with statistically significant neutrophil oscillations. Clin Case Rep. (2018) 6:1347–52. doi: 10.1002/ccr3.1611
2. Engström K, Lundquist A, Söderström N. Periodic thrombocytopenia or tidal platelet dysgenesis in a man. Scand J Haematol. (1966) 3:290–4. doi: 10.1111/j.1600-0609.1966.tb02373.x
3. Fogarty PF, Stetler-Stevenson M, Pereira A, Dunbar CE. Large granular lymphocytic proliferation-associated cyclic thrombocytopenia. Am J Hematol. (2005) 79:334–6. doi: 10.1002/ajh.20375
4. Lonial S, Waller EK, Richardson PG, Jagannath S, Orłowski RZ, Giver CR, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood. (2005) 106:3777–84. doi: 10.1182/blood-2005-03-1173
5. Kyrle PA, Eichinger S. How i manage cyclic thrombocytopenia. Blood. (2021) 137:178–84. doi: 10.1182/blood.2020008218
6. George JN. The thrombotic thrombocytopenic purpura and hemolytic uremic syndromes: evaluation, management, and long-term outcomes experience of the Oklahoma TTP-HUS Registry, 1989-2007. Kidney Int Suppl. (2009) 112):S52–4. doi: 10.1038/ki.2008.622
7. Apostu R, Mackey MC. Understanding cyclical thrombocytopenia: a mathematical modeling approach. J Theor Biol. (2008) 251:297–316. doi: 10.1016/j.jtbi.2007.11.029
8. Swinburne J, Mackey MC. Cyclical thrombocytopenia: characterization by spectral analysis and a review. J Theor Med. (2000) 2:81–91. doi: 10.1080/10273660008833039
9. Colijn C, Mackey MC. A mathematical model of hematopoiesis-I. Periodic chronic myelogenous leukemia. J Theor Biol. (2005) 237:117–32. doi: 10.1016/j.jtbi.2005.03.033
10. Zhang H, Chien M, Hou Y, Shomali W, Brar R, Ho C, et al. Longitudinal study of 2 patients with cyclic thrombocytopenia, stat3 and mpl mutations. Blood Adv. (2023) 7:190–4. doi: 10.1182/bloodadvances.2021006701
11. Langlois GP, Craig M, Humphries AR, Mackey MC, Mahaffy JM, Bélair J, et al. Normal and pathological dynamics of platelets in humans. J Math Biol. (2017) 75:1411–62. doi: 10.1007/s00285-017-1125-6
12. Go RS. Idiopathic cyclic thrombocytopenia. Blood Rev. (2005) 19:53–5. doi: 10.1016/j.blre.2004.05.001
13. Şumnu R, Diz-Küçükkaya R. Cyclic thrombocytopenia: A case report. Siklik trombositopeni: Olgu sunumu. Turkish J Haematol: Official J Turkish Soc Haematol. (2008) 27(3):196–9. doi: 10.5152/tjh.2010.28
Keywords: thrombocytopenia, thrombocytosis, cyclic thrombocytopenia, ITP (idiopathic thrombocytopenic purpura), splenectomy, TPO (thermoplastic polyolefin)
Citation: Al-Assil T, Ellythy S, Idris OA and Omaira M (2025) Case report: A decade-long journey with cyclic thrombocytopenia. Front. Hematol. 3:1482891. doi: 10.3389/frhem.2024.1482891
Received: 19 August 2024; Accepted: 16 December 2024;
Published: 10 January 2025.
Edited by:
Bhavana Bhatnagar, West Virginia University, United StatesReviewed by:
Suzie Noronha, University of Rochester, United StatesCopyright © 2025 Al-Assil, Ellythy, Idris and Omaira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Talal Al-Assil, dGFsYWwuYWxhc3NpbEB3bWVkLmVkdQ==
†Present address: Omer A. Idris, Malate Institute for Medical Research, Malate Inc., Grandville, MI, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.