- 1Department of Internal Medicine, Sinai Hospital of Baltimore, Baltimore, MD, United States
- 2Department of Internal Medicine, Jacobi Medical Center, New York City, NY, United States
- 3Department of Internal Medicine and Infectious Diseases, University of South Florida, Tampa, FL, United States
- 4Department of Internal Medicine, George Washington University, Washington, DC, United States
Vitamin B12 deficiency is commonly known to cause hematological, neurological, and gastrointestinal disturbances including anemia, pancytopenia, sub-acute combined degeneration, and glossitis, among others. Hemolytic anemia is a rare but possible presentation of vitamin B12 deficiency. We present a case series of three elderly females with different past medical histories but with similar presentations of severe anemia. Initial investigation revealed pancytopenia and hemolytic anemia. Further evaluation indicated vitamin B12 deficiency and subsequently, pernicious anemia, as confirmed by low serum vitamin B12 levels and positive intrinsic cell antibody respectively. These findings directed the diagnosis toward vitamin B12 deficiency-induced hemolytic anemia, a rare clinical entity. All three patients responded to treatment with intramuscular vitamin B12 injections, showing significant improvement in their pancytopenia and hemolytic anemia. This case series highlight the importance of considering vitamin B12 deficiency as a potential cause of hemolytic anemia in patients presenting with pancytopenia and ineffective hematopoiesis. Further research is necessary to illuminate the therapeutic implications of this rare condition.
Introduction
Between 1% to 2% of anemia cases in the general population are attributable to vitamin B12 deficiency. This deficiency can stem from various causes, such as impaired gastric absorption due to factors like gastrectomy, bariatric surgery, and pernicious anemia (PA), impaired intestinal absorption from conditions like ileal resection and chronic pancreatitis, decreased intake, hemolysis, HIV, and drug-induced effects, such as from alcohol, proton pump inhibitors, and metformin. Notably, the prevalence of vitamin B12 insufficiency tends to be higher among the elderly (1, 2).
A deficiency of vitamin B12 can present as a mix of neurologic symptoms, including paresthesias, loss of proprioception, or autonomic nervous system dysfunction, and hematologic abnormalities, such as macrocytic anemia, thrombocytopenia, or pancytopenia (3). Although rare, there are a few reported cases in the literature of vitamin B12 deficiency presenting as hemolytic anemia. In this case series, we present three cases of hemolytic anemia from vitamin B12 deficiency.
Case description
Case 1
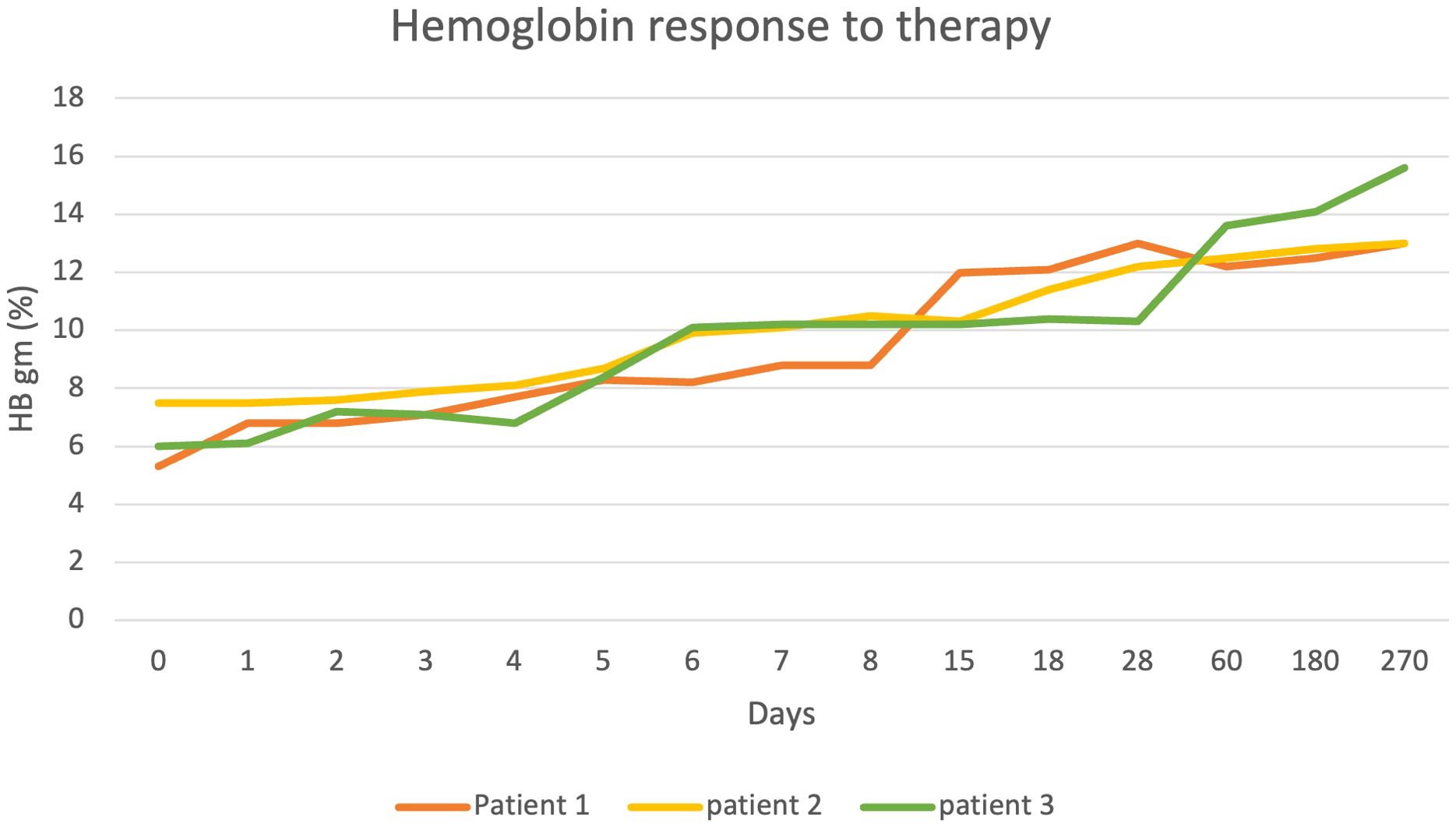
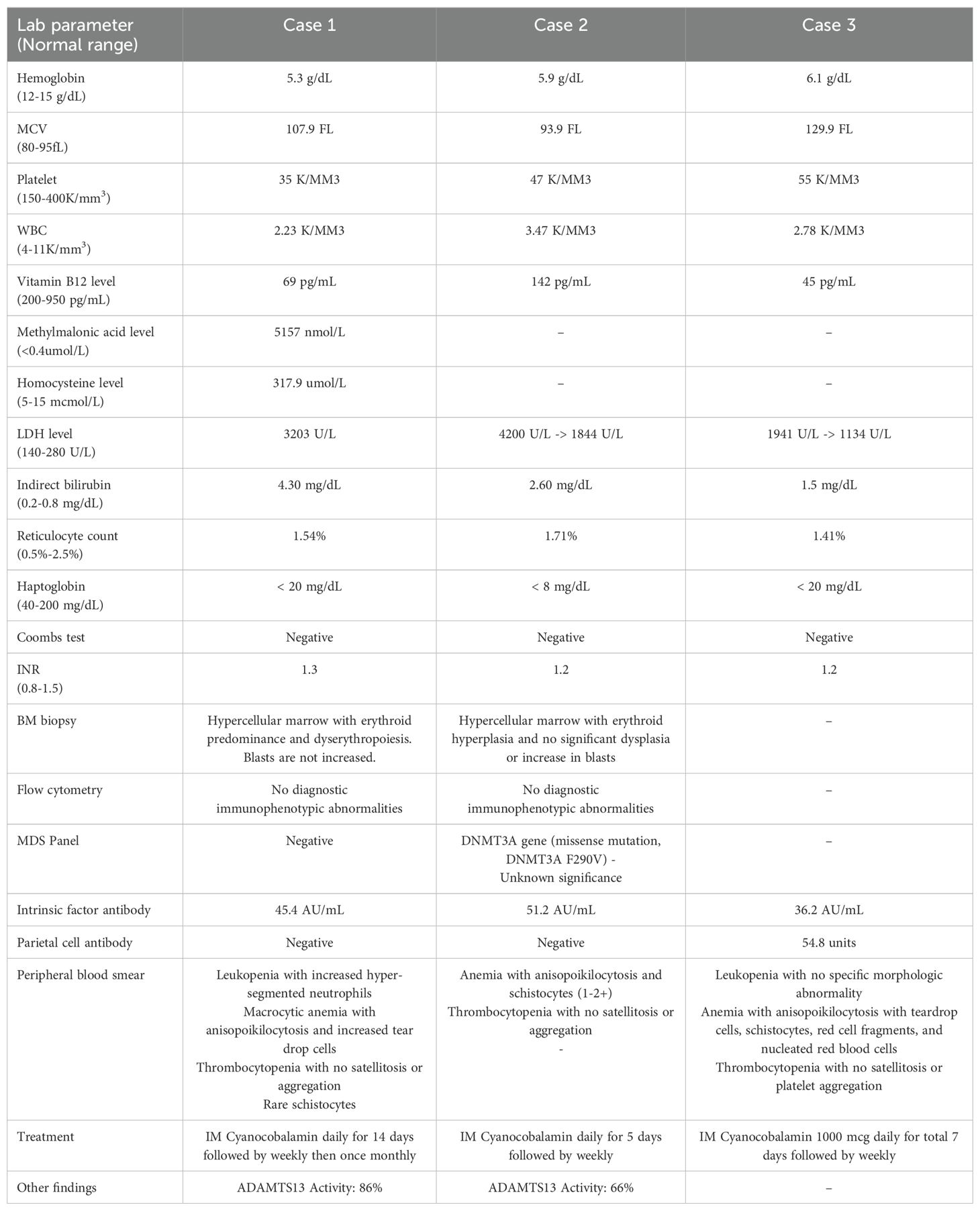
A 68-year-old female with a medical history of hypertension, diabetes mellitus, and asthma presented with left-sided chest pain. She also reported chronic fatigue for few months. Patient was initially found to be hypotensive with mean arterial pressure (MAP) in 50s but quickly improved with IV fluid administration. Her physical exam was largely unremarkable except for conjunctival pallor. To rule out cardiac and pulmonary causes, a comprehensive evaluation consisting of CT scans, EKGs, and cardiac biomarkers was performed, yielding negative results. Complete blood picture (CBC) revealed pancytopenia with a macrocytic anemia characterized by a hemoglobin level of 5.3 g/dL and mean corpuscular volume (MCV) of 107.9 fL. The patient’s white blood cell (WBC) count was 2.2 K/mm³, and the platelet count was 38 K/mm³. Comprehensive metabolic panel (CMP) was largely unremarkable renal and hepatic function except for elevated bilirubin of 5.3 mg/dL with indirect bilirubin of 4.3 mg/dl (Figure 1).
Further evaluation of her pancytopenia revealed elevated lactate dehydrogenase (LDH) levels up to 3,211 U/L, haptoglobin levels less than 20 mg/dL, reticulocyte count 1.54%, iron level 150 mcg/dL, ferritin 470.5 ng/mL, total iron binding capacity (TIBC) 271 ug/dL, vitamin B12 69 pg/mL, and folate 9.50 ng/mL (Table 1). Peripheral blood smear revealed leukopenia with increased hyper segmented neutrophils (Figure 2C), macrocytic anemia with anisopoikilocytosis and increased tear drop cells, and thrombocytopenia. Methylmalonic acid and homocysteine levels were elevated at 5157 nmol/L and 317.9 μmol/L, respectively. Methylene-tetra-hydrofolate reductase (MTHFR) DNA analysis done for genetic mutation testing for hyperhomocystinemia was negative. Antibodies to intrinsic factor were positive at 45.4 AU/mL, while parietal cell antibodies were negative, concerning for PA. Low suspicion for disseminated intravascular coagulation (DIC) with largely unremarkable coagulation panel (PT/INR 15.9 seconds/1.3, aPTT 32.9 seconds, and fibrinogen 366 mg/dL), thrombotic thrombocytopenic purpura (TTP) with normal ADAMTS13 activity 86% and antibody levels 3, and other forms of thrombotic microangiopathy (TMA) with no significant schistocytes on peripheral blood smear, negative direct anti-globuin test (DAT), normal complement levels and kidney function. She was however treated with empiric steroids for few days due to severe thrombocytopenia and concern of TTP, while her investigations were pending. Infectious etiologies evaluated with testing for HIV, hepatitis C, and parvovirus was negative. We did not obtain zinc and copper levels as part of our evaluation.
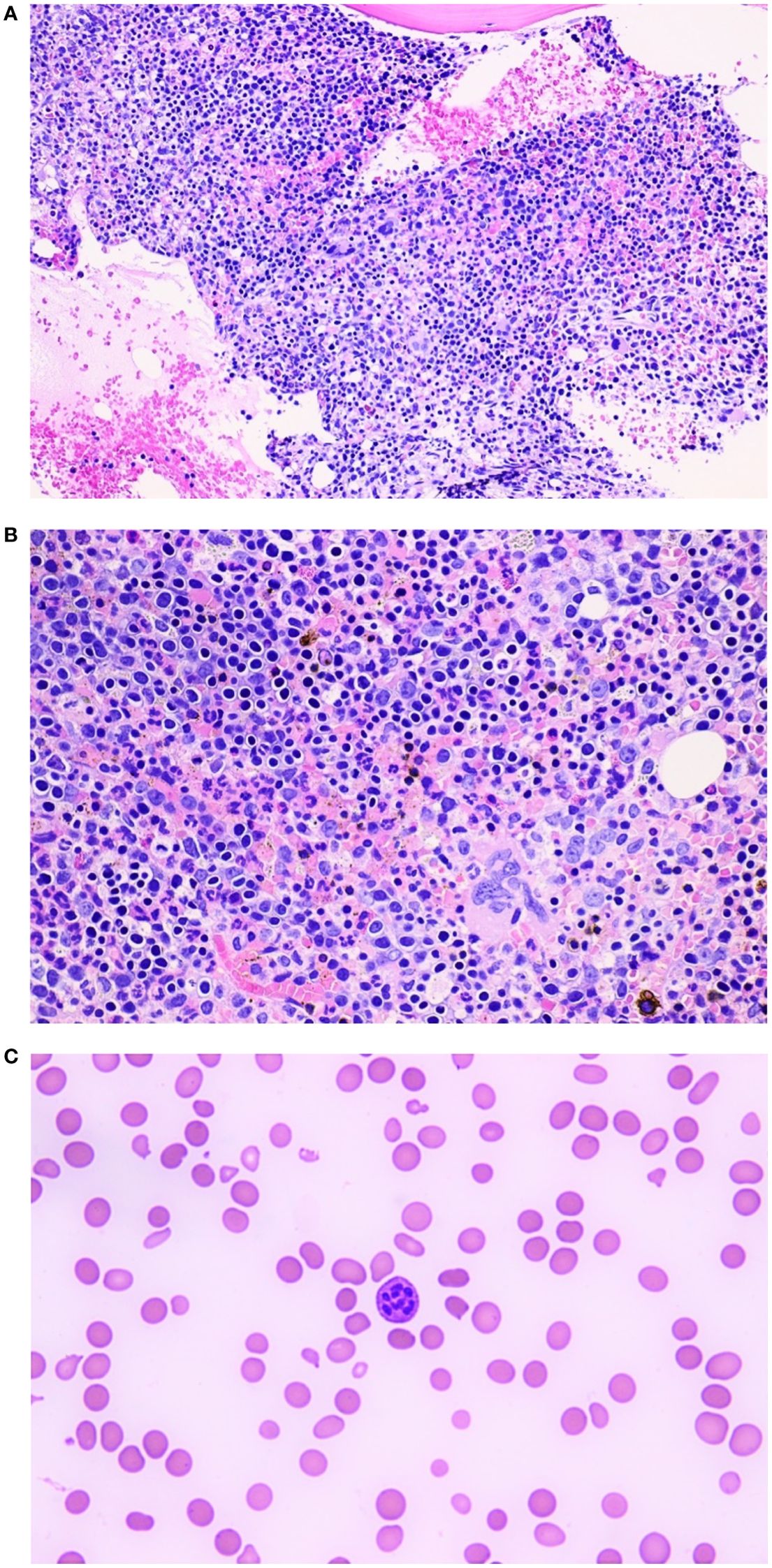
Figure 2. (A) Bone marrow aspirate, 20X maginificaton: Hypercellular bone marrow for age (68 years, case one) with an erythroid hyperplasia. (B) Bone marrow aspirate, 40X magnification: Hypercellular bone marrow for age (68 years, case one) with an erythroid hyperplasia. (C) Peripheral smear 60X magnification (case one): Hyper segmented neutrophil. The background red blood cells show anisopoikylocytosis with occasional red cell fragments and tear drop cells.
Based on the findings of ineffective blood cell production and hemolysis in the setting of vitamin B12 deficiency, intramuscular cyanocobalamin supplementation was initiated at a dose of 1000 mcg daily for 14 days. She also received three units of packed red blood cells (PRBC) on admission. Despite the treatment with vitamin B12 injections, the patient showed an initial poor response with worsening thrombocytopenia with platelet counts as low as 18K/mm3. Consequently, a bone marrow (BM) aspirate and biopsy were performed, revealing a hypercellular marrow with dyserythropoiesis and erythroid predominance, without evidence of blasts (Figures 2A, B). As morphological changes associated with vitamin B12 deficiency in the marrow significantly overlaps with those seen in myelodysplastic syndrome, we obtained myelodysplastic syndrome (MDS) panel using next-generation sequencing (NGS) and it was negative for MDS.
With the continuation of intramuscular cyanocobalamin, the patient’s cell counts gradually improved. The treatment regimen was modified to one injection weekly for 12 weeks, followed by monthly injections as patient has poor absorption of vitamin B12 in view of PA.
Case 2
A 74-year-old female with a past medical history of hypertension, hypothyroidism, hyperlipidemia, diabetes, obstructive sleep apnea, and allergic rhinitis was referred to the emergency department by her primary care physician due to anemia, with a hemoglobin level of 5.8 g/dL and MCV 93.9 fL. The patient reported transient gum bleeding, along with generalized weakness, poor appetite, and weight loss (Figure 1).
In addition to severe anemia, thrombocytopenia with a platelet counts of 47/mm³ and leukopenia at 3.47 K/mm3 were noted. Laboratory tests showed an elevated LDH level (>4200 U/L), haptoglobin levels < 8 mg/dL, increased indirect bilirubin levels (2.6 mg/dL), reticulocyte count 1.71%, iron level 100 mcg/dL, ferritin 93 ng/mL, TIBC 216 ug/dL, vitamin B12 level 142 pg/mL, folate 6.62 ng/mL, INR 1.2, aPTT 33.8 seconds, and negative direct Coombs test (Table 1). Peripheral blood smear revealed anemia with anisopoikilocytosis and 1-2+ schistocytes, as well as thrombocytopenia without satellitosis or aggregation. Infectious etiologies evaluated with testing for HIV, hepatitis C, and parvovirus was negative. We did not obtain zinc and copper levels as part of our evaluation.
Elevated D-dimer levels prompted further investigation, revealing a pulmonary embolism in the right lower lobe. Fibrinogen levels and ADAMTS13 activity were within normal ranges. The patient was put initially on a heparin drip and then was transitioned to apixaban. For vitamin B12 deficiency, patient was initiated on intramuscular cyanocobalamin 1000 mcg once daily. However, given the high suspicion of 2+ schistocytes in the peripheral blood smear, worsening thrombocytopenia, and pulmonary embolism (PE); patient was initially started on methylprednisolone at a dose of 1mg/kg daily. BM biopsy was obtained as patient’s cell lines continued to drop despite giving two units of PRBC, vitamin B12 injections, and steroids. BM biopsy revealed hypercellular marrow with erythroid hyperplasia and no significant dysplasia or increase in blasts along with a DNMT3A missense mutation of unknown significance on myelodysplastic syndrome panel testing. Methylprednisolone was subsequently tapered to prednisone (1 mg/kg daily), followed by a further taper to 60 mg prednisone daily.
Continuation of cyanocobalamin supplementation led to improvement in the patient’s anemia. The presence of positive intrinsic factor antibodies (51.2 AU/mL) confirmed the diagnosis of PA. The patient is currently awaiting a gastroenterology referral for an upper endoscopy and is undergoing a prednisone taper.
Case 3
An 82-year-old female patient with a past medical history of coronary artery disease, cervical stenosis, and well-controlled diabetes, presented to the emergency department after recurrent falls. In the weeks leading to her hospitalization, the patient noted peripheral neuropathy, and significant weight loss from 140 lbs to 121 lbs, which she attributed to loss of taste. She also reported experiencing night sweats and chills. The patient’s daughter reported a recent change in the patient’s gait, and both reported a perceived slowing in the patient’s cognition. The patient had been on metformin for more than 20 years, recently discontinued due to well-controlled HbA1c levels (Figure 1).
Laboratory tests obtained on admission revealed pancytopenia with macrocytic anemia (hemoglobin of 6.1 g/dL with MCV 129.9 fL), WBC count of 2.78 K/mm3, and platelet count of 55 K/mm3. Further investigation, including elevated lactate dehydrogenase up to 1941 U/L, low haptoglobin <20 mg/dL, iron level 90 mcg/dL, ferritin 93 ng/mL, TIBC 187 ug/dL, vitamin B12 level 45 pg/mL, folate 29.90 ng/mL, INR 1.2, and a negative DAT, pointed to non-immune hemolytic anemia (Table 1). A CT scan of the chest, abdomen, and pelvis did not reveal lymphadenopathy or hepatomegaly.
The patient was promptly started on intramuscular cyanocobalamin therapy 1000 mcg daily for seven days for her vitamin B12 deficiency. Evaluation for PA was positive with intrinsic factor antibody positive at 36.2 AU/mL and parietal cell antibody elevated at 54.8 units deeming PA as the potential cause of her vitamin B12 deficiency. Her LDH continued to trend down with the cyanocobalamin treatment. She is currently undergoing once-weekly injections. She is pending further gastroenterology evaluation.
Discussion
Vitamin B12 is a critical nutrient necessary for several pivotal biochemical processes. These include the conversion of homocysteine to methionine, 5-methyltetrahydrofolate to tetrahydrofolate, and methylmalonic acid to succinyl coenzyme A. These metabolic processes are instrumental for maintaining optimal hematopoiesis, DNA synthesis, and neurological function (4).
The prevalence of vitamin B12 deficiency increases in the elderly population across all causal mechanisms, with approximately 20% of individuals aged over 60 years affected (1, 2). Also, vitamin B12, primarily found in animal-derived food sources, may be deficient in strict vegan populations (3). The primary etiological factors of vitamin B12 deficiency encompass autoimmune causes, malabsorption, and dietary insufficiency.
In all of our patients, the underlying etiology was PA affecting absorption of vitamin B12. PA is an autoimmune disease that leads to the gradual destruction of the stomach lining, particularly affecting the body and fundus, where parietal cells reside. This cell loss reduces production of intrinsic factor, a protein essential for vitamin B12 absorption. Without sufficient vitamin B12, the body cannot produce healthy red blood cells (erythropoiesis) or maintain nerve function (myelin synthesis). The autoimmune basis of PA is indicated by the presence of antibodies against intrinsic factor and parietal cells, and it often coexists with other autoimmune conditions (5).
Due to impaired DNA synthesis, vitamin B12 deficiency presents with megaloblastic anemia, demyelinating neurological disease or both. Megaloblastic anemia is a type of macrocytic anemia characterized by large, abnormal red blood cells (megaloblasts) due to impaired DNA synthesis. It is also seen with folate deficiency, both of which are essential for DNA replication in red blood cell precursors. Macrocytic anemia, on the other hand, is a broader term that includes all forms of anemia with larger than normal red blood cells (high MCV) and result from various causes, not limited to vitamin B12 or folate deficiency (6). Hemolytic anemia and pancytopenia from vitamin B12 deficiency are rare. In a study of 201 patients with vitamin B12 deficiency, the observed prevalence of pancytopenia was 5% and hemolytic anemia was 1.5% (7). Vitamin B12 deficiency that presents with hemolytic picture is characterized by elevated LDH, low haptoglobin, and increased indirect bilirubin. This pattern is mainly attributed to ineffective erythropoiesis and intramedullary destruction of red blood cells (8).
Vitamin B12 deficiency-related hemolysis may be a result of the accumulation of homocysteine, given its conversion to methionine is impeded (7). Homocysteine exhibits distinct properties that make it a potential “hemolytic toxin”. These include the pro-oxidant effect that stems from the generation of free radicals during the oxidation of varied mixed disulfide compounds or the autoxidation of homocysteine itself. Additionally, it has a stronger propensity to form disulfide bonds when compared to other thiols (9, 10). Correlation between higher levels of homocysteine and increased lipid peroxidation, LDH, and hemolysis have been identified in in vitro studies.
The clinical manifestation of hemolytic anemia associated with vitamin B12 deficiency can differ significantly based on the severity of the deficiency. Symptoms may encompass fatigue, weakness, dyspnea, pallor, and jaundice (11).
The standard methodology for evaluating vitamin B12 deficiency typically starts with a CBC, peripheral smear, and vitamin B12 levels. Macrocytic anemia characterized by a mean corpuscular volume (MCV) typically exceeding 95 fL, hyper segmented neutrophils, and reduced serum vitamin B12 levels are common findings. In all our cases, the patients presented with severe anemia. A review indicated vitamin B12 levels in the range of 46 – 89 pg/mL, well below the normal reference range of 233 – 914 pg/mL (11). In the rare set of patients that presents with hemolytic picture, further hemolysis workup including LDH, haptoglobin, reticulocyte count, elevated indirect bilirubin should be pursued (7). As seen in our patients, severe vitamin B12 deficiency can mimic TTP and other MAHAs. Prompt recognition of this rare presentation can prevent the misdiagnosis of TTP and the associated use of high steroids and plasmapheresis, preventing potential adverse effects and high cost (12). Other causes of hemolytic anemia including TMA, DIC, and auto-immune hemolytic anemia should be evaluated as well.
BM biopsy and aspiration are generally not required to diagnose megaloblastic anemia and may create diagnostic confusion in cases of severe pancytopenia. In such cases, findings like hypercellularity, increased erythroblasts, or cytogenic abnormalities might misleadingly suggest leukemia, leading to extensive workup and aggressive treatment measures (3, 13). However, it can assist in ruling out other hemolytic anemia causes, such as myelodysplastic syndromes, paroxysmal nocturnal hemoglobinuria, and aplastic anemia. A review highlighted 11 cases in which a BM biopsy proved a valuable diagnostic instrument in evaluating hemolytic anemia associated with vitamin B12 deficiency. In our first patient, platelet count continued to drop to as low as 18 K/mm3 along with no meaningful improvement in hemoglobin levels despite receiving three units of PRBC on admission. In our second patient, we had a strong suspicion for a concomitant auto-immune hemolytic anemia along with additional concern of continued drop in cell lines including hemoglobin despite blood transfusion. This warranted to pursue BM biopsy in both these patients and helped to complete full work of a combined presentation of pancytopenia with hemolytic anemia. The biopsy findings typically show megaloblastic erythropoiesis with hyperplastic and dysplastic changes. These alterations are characterized by an increase in the number of immature erythroid cells, including megaloblasts, along with cytoplasmic vacuolation, internuclear bridging, and multinucleated cells. The BM biopsy images as shown in Figures 2A, B were consistent with similar cases in the literature.
The therapeutic strategy for vitamin B12-associated hemolytic anemia centers on the rectification of vitamin B12 deficiency. The ideal dosage and frequency of intramuscular vitamin B12 injections to treat hemolytic anemia linked with vitamin B12 deficiency are yet to be definitively established. Nevertheless, regimens comprise of one to multiple times weekly intramuscular injections of 1,000 mcg of cyanocobalamin in the first week then weekly for a duration of four to six weeks, subsequently followed by monthly maintenance injections of the same dosage. One case report used a regimen of 1,000 μg intramuscular vitamin B12 daily for 14 days followed by monthly injections. The resolution was achieved after 3 months (14). In our case, the duration of the cyanocobalamin injections varied, but all helped to improve the cell counts. Further studies are needed to determine the optimal duration for this condition.
Elhiday et al. cites evidence derived from limited studies suggesting high oral doses (1000 and 2000 µg) can have the same efficacy as intramuscular administrations (15). One case report on this condition used oral cyanocobalamin 1000 mcg with improvement of blood counts after one week (16).
Hematological disturbances such as anemia, leukopenia, changes in mean corpuscular volume (MCV), and thrombocytopenia are projected to show improvement within 8 weeks of initiating the treatment (17).
Conclusion
In conclusion, this case series highlights the importance of considering vitamin B12 deficiency as a reversible cause of hemolytic anemia. Prompt diagnosis and treatment are essential, as vitamin B12 replacement can lead to rapid hematologic improvement and prevent potential neurological complications. Recognizing this association is crucial, particularly in patients presenting with unexplained anemia and hemolysis. It is also important to recognize that BM biopsy in patients with vitamin B12 deficiency could be misleading and can lead to an extensive unwarranted evaluation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CS: Conceptualization, Writing – original draft, Writing – review & editing, Supervision, Visualization. PD: Writing – original draft. SK: Writing – original draft. JT: Writing – original draft. VP: Writing – original draft. VJ: Supervision, Writing – review & editing. SP: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Devi A, Rush E, Harper M, Venn B. Vitamin B12 status of various ethnic groups living in New Zealand: an analysis of the adult nutrition survey 2008/2009. Nutrients. (2018) 10. doi: 10.3390/nu10020181
2. Röhrig G, Gütgemann I, Kolb G, Leischker A. Klinisch-hämatologisches Bild des Vitamin-B12-Mangels im Alter. Z für Gerontologie und Geriatrie. (2018) 51:446–52.
4. Langan RC, Goodbred AJ. Vitamin B12 deficiency: recognition and management. Am Fam Physician. (2017) 96:384–9.
5. Bizzaro N, Antico A. Diagnosis and classification of pernicious anemia. Autoimmun Rev. (2014) 13:565–8. doi: 10.1016/j.autrev.2014.01.042
6. Green R. Vitamin B12 deficiency from the perspective of a practicing hematologist. Blood. (2017) 129:2603–11. doi: 10.1182/blood-2016-10-569186
7. Andrès E, Affenberger S, Zimmer J, Vinzio S, Grosu D, Pistol G, et al. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clin Lab Haematol. (2006) 28:50–6. doi: 10.1111/j.1365-2257.2006.00755.x
8. Yeruva SLH, Manchandani RP, Oneal P. Pernicious anemia with autoimmune hemolytic anemia: A case report and literature review. Case Rep Hematology. (2016) 2016:1–4. doi: 10.1155/2016/7231503
9. Stamler JS, Slivka A. Biological chemistry of thiols in the vasculature and in vascular-related disease. Nutr Rev. (2009) 54:1–30. doi: 10.1111/j.1753-4887.1996.tb03770.x
10. Loscalzo J. The oxidant stress of hyperhomocyst(e)inemia. J Clin Invest. (1996) 98:5–7. doi: 10.1172/JCI118776
11. Yamanishi M, Koba S, Jo T, Kotera T, Imashuku S. Thrombotic microangiopathy-like hemolysis in vitamin B12 deficiency-related macrocytic anemia. Clin Lab. (2018) 64. doi: 10.7754/Clin.Lab.2017.171138
12. Yadav SK, Liu B, Hussein G, Xu Q, Byrd D, Le-Kumar V, et al. Vitamin B12 deficiency and hemolytic anemia presenting as pseudo thrombotic microangiopathy: A systematic review. Blood. (2023) 142:5220. doi: 10.1182/blood-2023-190932
13. Konda M, Godbole A, Pandey S, Sasapu A. Vitamin B12 deficiency mimicking acute leukemia. Baylor Univ Med Center Proc. (2019) 32:589–92. doi: 10.1080/08998280.2019.1641045
14. Hassouneh R, Shen S, Lee O, Hart RA, Rhea LP, Fadden P. Severe vitamin B12 deficiency mimicking microangiopathic hemolytic anemia. J Hematology. (2021) 10:202–5. doi: 10.14740/jh889
15. Butler CC, Vidal-Alaball J, Cannings-John R, McCaddon A, Hood K, Papaioannou A, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials. Fam Pract. (2006) 23:279–85. doi: 10.1093/fampra/cml008
16. Elhiday H, Musa M, Hussaini SA, Al-Warqi A, Alfitori G. An unusual presentation of vitamin B12 deficiency associated with massive splenomegaly, hemolytic anemia, and pancytopenia: A case report. Cureus. (2022) 14:e26058. doi: 10.7759/cureus.26058
Keywords: vitamin B12 deficiency, hemolysis, anemia, pancytopenia, hemolytic anemia, intramedullary hemolysis
Citation: Sainatham C, Dy PS, Kaushik S, Tallapalli JR, Patel V, Jindal V and Paul S (2024) Case report: Vitamin B12 deficiency-associated hemolytic anemia. Front. Hematol. 3:1446241. doi: 10.3389/frhem.2024.1446241
Received: 09 June 2024; Accepted: 18 November 2024;
Published: 09 December 2024.
Edited by:
Pascal Amireault, INSERM U1134 Biologie Intégrée du Globule Rouge, FranceReviewed by:
Imo J. Akpan, Columbia University, United StatesTayler Van Denakker, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2024 Sainatham, Dy, Kaushik, Tallapalli, Patel, Jindal and Paul. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chiranjeevi Sainatham, c2FpbmF0aGFtY2hpcmFuamVldmlAZ21haWwuY29t
 Chiranjeevi Sainatham
Chiranjeevi Sainatham Paul Stendahl Dy
Paul Stendahl Dy Sharanya Kaushik
Sharanya Kaushik Jayanth Reddy Tallapalli
Jayanth Reddy Tallapalli Viral Patel1
Viral Patel1 Sonal Paul
Sonal Paul