- 1Hematology and Stem Cell Transplantation and Cellular Therapies Unit (CTMO), Department of Hemato-Oncology and Radiotherapy, Grande OspedaleMetropolitano “Bianchi-Melacrino-Morelli”, Reggio Calabria, Italy
- 2Stem Cell Transplant Program, Reggio Calabria, Italy
- 3Institute of Clinical Physiology - National Research Council (IFC-CNR), Rome, Italy
- 4Institute of Clinical Physiology (IFC-CNR), Reggio Calabria, Italy
- 5Pharmacy Unit, Grande Ospedale Metropolitano “Bianchi-Melacrino-Morelli”, Reggio Calabria, Italy
Objectives: To evaluate the efficacy of biosimilar (BIO) pegfilgrastim (PEG) in lymphoma patients after autologous stem cell transplantation (ASCT).
Methods: 86 consecutive lymphoma patients who received BIO/PEG after ASCT were assessed. The primary endpoints of this study were the incidence of febrile neutropenia (FN) and time to neutrophil engraftment.
Results: Most patients were males (67.4%) with a median age of 48 years. FN occurred in 66 patients (76.7%), and most of the fever was grade 1-2. The median time to neutrophil engraftment was 9 days. The incidence of FN differs based on lymphoma type (p-value <0.01) and was higher in non-Hodgkin lymphoma (NHL) than in Hodgkin Lymphoma (HL). No statistical difference was found between NHL and HL regarding the time to reach the neutrophil engraftment. Hospitalization lasted from a minimum of 9 to a maximum of 34 days. The restricted mean time to discharge was 15.9 days (95%CI 14-16), without differences based on lymphoma type.
Conclusion: Although the study has the significant limitation of not being randomized and not having a control arm, it highlights the efficacy and safety of a BIO-PEG formulation in patients with Lymphoma and undergoing ASCT.
1 Introduction
High-dose chemotherapy (HDC) and autologous stem cell transplantation (ASTC) remains a therapeutic option in patients with Hodgkin Lymphoma (HL) and Non-Hodgkin Lymphoma (NHL) who are refractory (R) or relapsed (R) after first-line therapy and who are responsive to salvage therapy (1–5). The BEAM (carmustine -BCNU-, etoposide, aracytin, and melphalan) has been widely used since 1990 as a standard conditioning regimen before ASCT in this setting (4).
Although advances in supportive care have dramatically improved the safety of ASCT, with expected rates of treatment-related mortality below 2%–3% (6), febrile neutropenia (FN) is a potentially fatal toxicity of many HDC regimens (7). The development of FN affects the cost and length of hospitalization (8), resulting in worse outcomes and high mortality (9, 10). Several studies show that the incidence of FN correlates with post-chemotherapy white blood cell recovery (11–13), so, early neutrophil engraftment post-transplantation is a goal to be pursued.
A strategy to reduce the risk of FN is the prophylactic use of granulocyte colony-stimulating factor (G-CSF) (14–17). The use of G-CSF has been associated with faster neutrophil engraftment, lower incidences of mortality rate due to infection, lower use of broad-spectrum antibiotics, a reduction of days of hospitalization, and a lower treatment cost (18, 19), and, in general, with an improvement in clinical outcomes (9, 20). Two types of G-CSF are available for reducing the duration of neutropenia: short-acting (SA) (e.g., lenograstim and filgrastim) (21–24) and long-acting (LA) (e.g., pegfilgrastim and lipegfilgrastim) (25–27). SA G-CSFs are administered as a daily subcutaneous injection (for a recommended ≥10 days per cycle), while LA G-CSFs are given as one shoot subcutaneous injection.
Biosimilars (BIO) are biological products that are highly like approved originator products with only minor differences in clinically inactive components and no clinically meaningful differences in efficacy and safety (28). BIO-PEG has been approved for prophylaxis of severe neutropenia duration and febrile neutropenia in cancer patients, including those affected by hematologic malignancies. However, poor data have been so far published among patients with Lymphoma undergoing ASCT.
This real-life study aimed to evaluate the efficacy and the safety of BIO-PEG when used in patients with Lymphoma undergoing ASCT.
2 Methods
2.1 Patients
This is a single-arm, longitudinal, real-life study investigating BIO-PEG’s effectiveness in a cohort of patients who received this drug.
The study included autologous transplantation-eligible lymphoma patients who were aged 18–65 years. The clinical criteria for ASCT eligibility were: 1) HL; 2) diffuse large B-cell Lymphoma (DLBCL) in first chemo-sensitive relapse or refractory to first-line therapy but sensitive to salvage therapy; 3) follicular lymphoma (FL) in first or subsequent relapse; 4) mantle-cell lymphoma (MCL) in first or second-line treatment; 5) peripheral T-cell lymphomas in first response and the relapse setting. Patients were excluded if they met any of the following criteria: a World Health Organization performance status >2; New York Heart Association class II–IV heart failure; abnormal pulmonary function findings; history of active malignancy during the past 5 years (excluding basal cell carcinoma or stage 0 cervical cancer); absolute neutrophil count (ANC) of ≤ 1.0 × 109/L; platelet count of ≤ 75 × 109/L; a creatinine clearance of ≤ 60 mL/min.
2.2 Treatment
Patients received a conditioning BEAM regimen consisting of BCNU (300 mg/m2 i.e., day − 7), etoposide (200 mg/m2 days − 6 to − 3), cytarabine (400 mg/m2 days − 6 to − 3), and melphalan (140 mg/m2 day −2). The minimum target dose of CD34+ cells required to support HDC safely was 2 × 106/kg. Twenty-four hours after stem cell infusion, patients received a single subcutaneous BIO/PEG (PEG-bmez) injection (6 mg). Antibiotic prophylaxis was not used. All patients received oral acyclovir 800 mg twice daily from day 3 until approximately day 90 post-ASCT. Pneumocystis jirovecii pneumonia prophylaxis was administered with trimethoprim/sulfamethoxazole (1 double-strength tablet; 2–3 times weekly) and initiated post-hematologic recovery for 3 months. Red blood cell (RBC) and platelet transfusions (PT) were administered to maintain hemoglobin levels of ≥ 8 mg/dL and platelet counts of ≥ 10 × 109/L or in patients with symptomatic anemia/minimal mucocutaneous hemorrhagic syndrome. Intravenous hydration and electrolyte support were also provided. Where FN occurred following a long period of neutropenia (ANC < 0.5 × 109/L or ANC of 1 × 109/L with a predicted decline to < 0.5 × 109/L over the subsequent 48 h) blood and catheter-drawn cultures were ordered, and intravenous Piperacillin/tazobactam was promptly started.
2.3 Endpoints
The primary endpoints of this study were the incidence of FN and time to neutrophil engraftment. FN was defined as a temperature of ≥ 38.2°C on at least two consecutive occasions or a persistent temperature of ≥ 38.0°C for at least 1 h, accompanied by an ANC of < 0.5 × 109/L in the absence of any documented infectious cause (e.g., transfusion reaction or administration of cytotoxic drugs). Time to neutrophil engraftment was defined as three consecutive days where the patient had an ANC of ≥ 0.5 × 109/L.
Secondary endpoints included platelet engraftment (platelet count ≥ 20 × 109/L, not requiring a platelets transfusion in the preceding 7 days), the incidence of diarrhea, and mucositis. An additional analysis evaluated the difference between HL and NLH subtypes. The safety endpoint of the study was the incidence of study drug-related adverse events.
Complete blood counts were collected using samples before chemotherapy and daily during the aplastic phase until hospital discharge.
2.4 Statistical analysis
Descriptive statistics presented data, including median, interquartile range (IQR), and percentage values. Univariate Kaplan- Meier analyses assessed the relationship between time to neutrophil engraftment and other patient variables. As the proportional hazard assumption was violated, the restricted mean survival time (RMST) was adopted to estimate the treatment effect. RMST, defined as the area under the survival function curve up to a specific time (t*), shows the mean survival time or, in our case, the mean time in which there was no neutrophil engraftment. Univariate logistic regression analysis evaluated the relationship between FN and other patient variables; identified covariates were used for multiple logistic regression analysis. For the logistic models, data were expressed as odds ratio (OR), 95% confidence intervals (CI), and p-values. All analyses were adjusted by patient sex and age, irrespective of the association with the outcome (significant/not significant).
3 Results
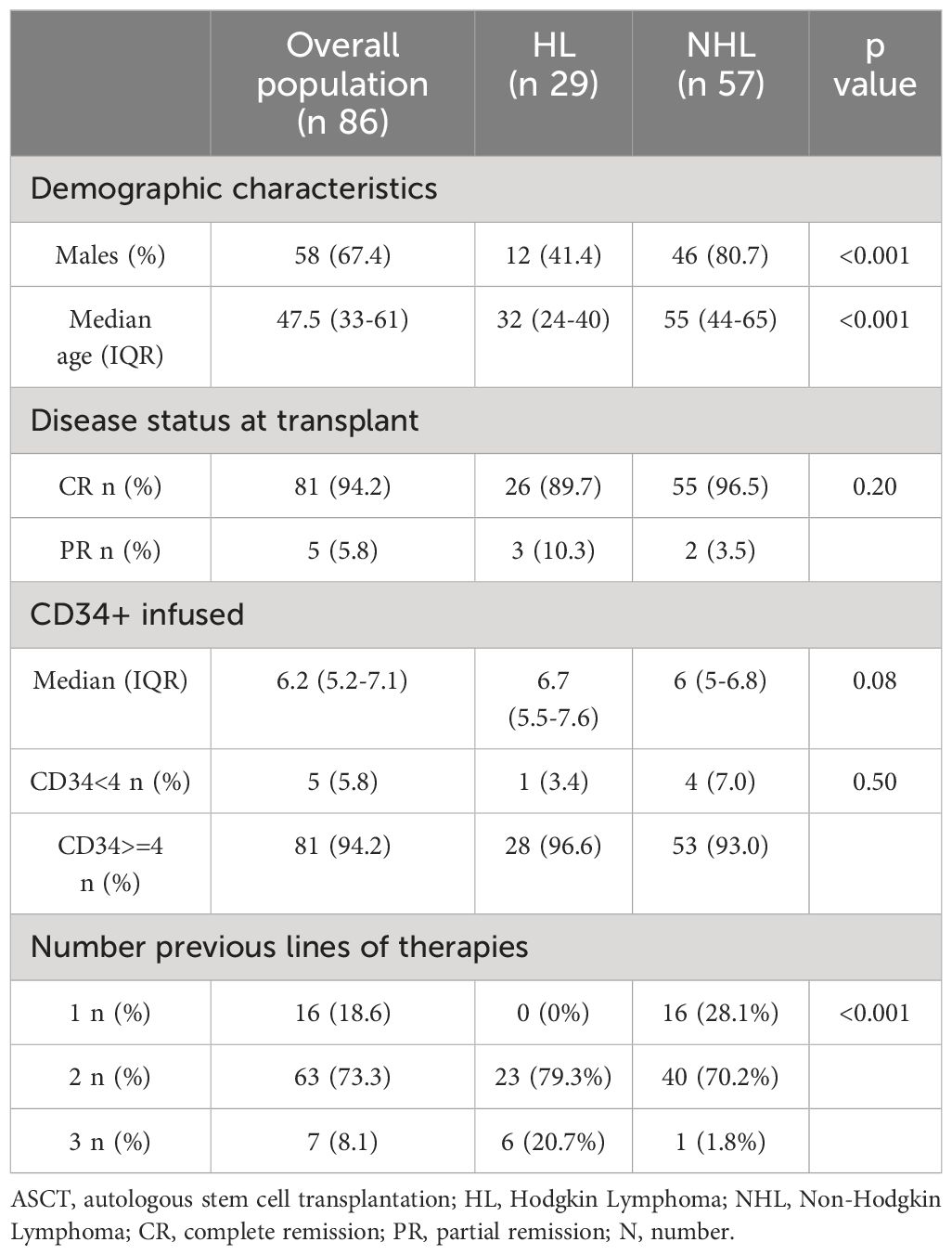
From January 2021 to June 2022, 86 consecutive lymphoma patients underwent ASCT and administration of PEG-bmez. Table 1 summarizes patient characteristics at the time of ASCT. The majority were males (n 44, 67.4%) with a median age of 48. Most patients (94.2%) had a complete response. The median basal CD34+ infusion was 6.2x106/kg (IQ 5.2-7.1), and for only five patients, the basal infusion was <4×106/kg. Mild bone pain was observed in approximately 20% of PEG patients (n = 24/86). Bone pain occurred primarily on days of neutrophil engraftment. In most patients, pain symptoms were controlled by the administration of paracetamol. No cardiac, neurological, renal, or pulmonary complications were reported, and no patients died in the first 100 days post-transplantation. Twenty-nine patients were affected by HL (33.7%) and 57 by NHL (66.3% - 32 DLBCL (56.1%), 16 MCL (28.1%), one FL (1.8%) and 8 PTCL-NOS). HL and NHL patients differed for gender, age at transplant.
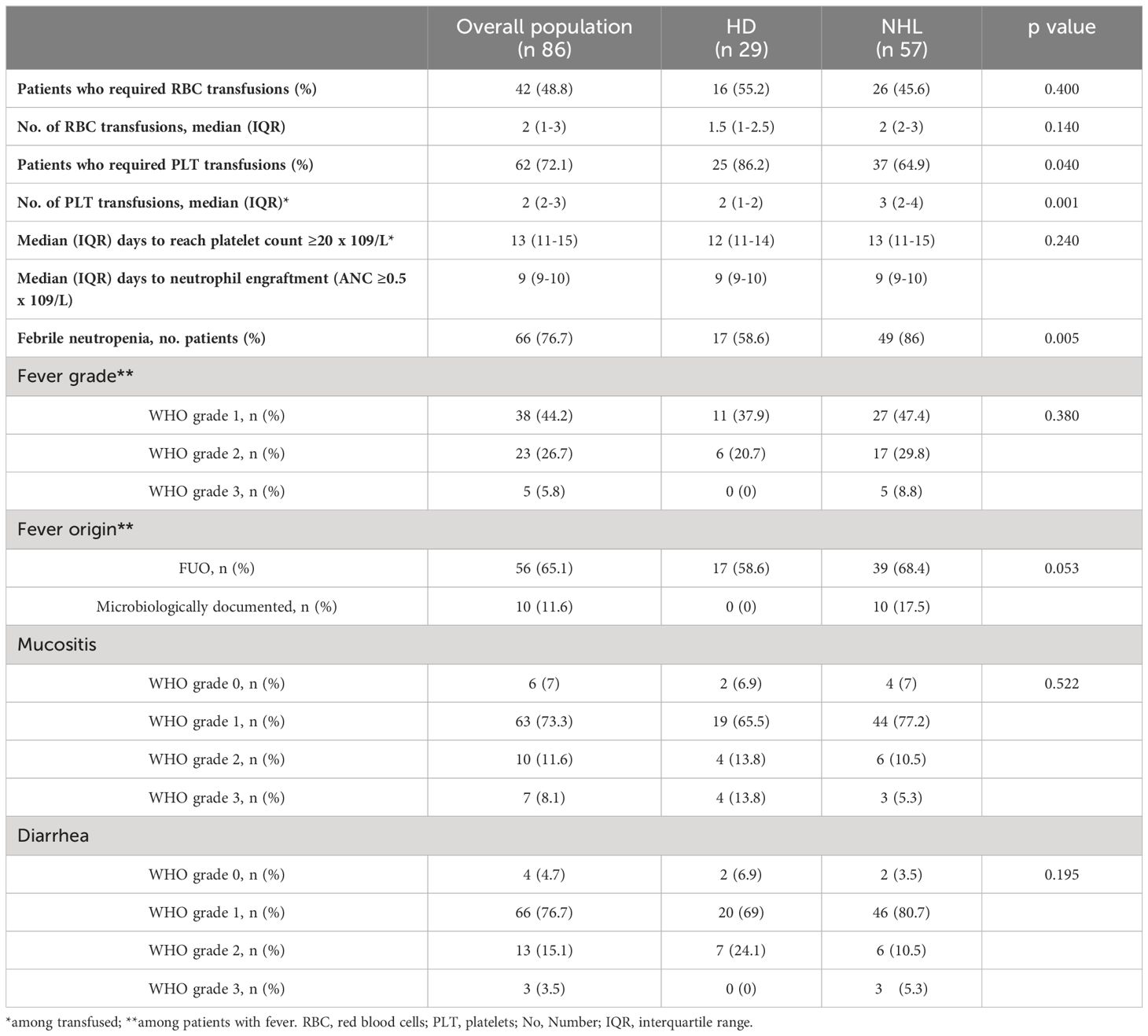
Outcome measurements are summarized in Table 2. FN occurred in 66 patients (76.7%). Grade 2-3 mucositis occurred in about 20% of patients, and grade 2-3 diarrhea in 19 cases. The median time to neutrophil and platelet engraftment was 9 days (range, 9-10) and 13 days (range, 11-15), respectively. The time to reach neutrophil engraftment was further investigated using a Kaplan–Meier analysis (Figure 1). Cumulative median and mean survival-free time of neutrophil engraftment was 9 days (95% CI 8.7-9.3) and 9.8 days (95% CI 9.4-10.1), respectively. HL and NHL patients differed for the incidence of febrile neutropenia, request for platelet support and number of platelet bags infused.
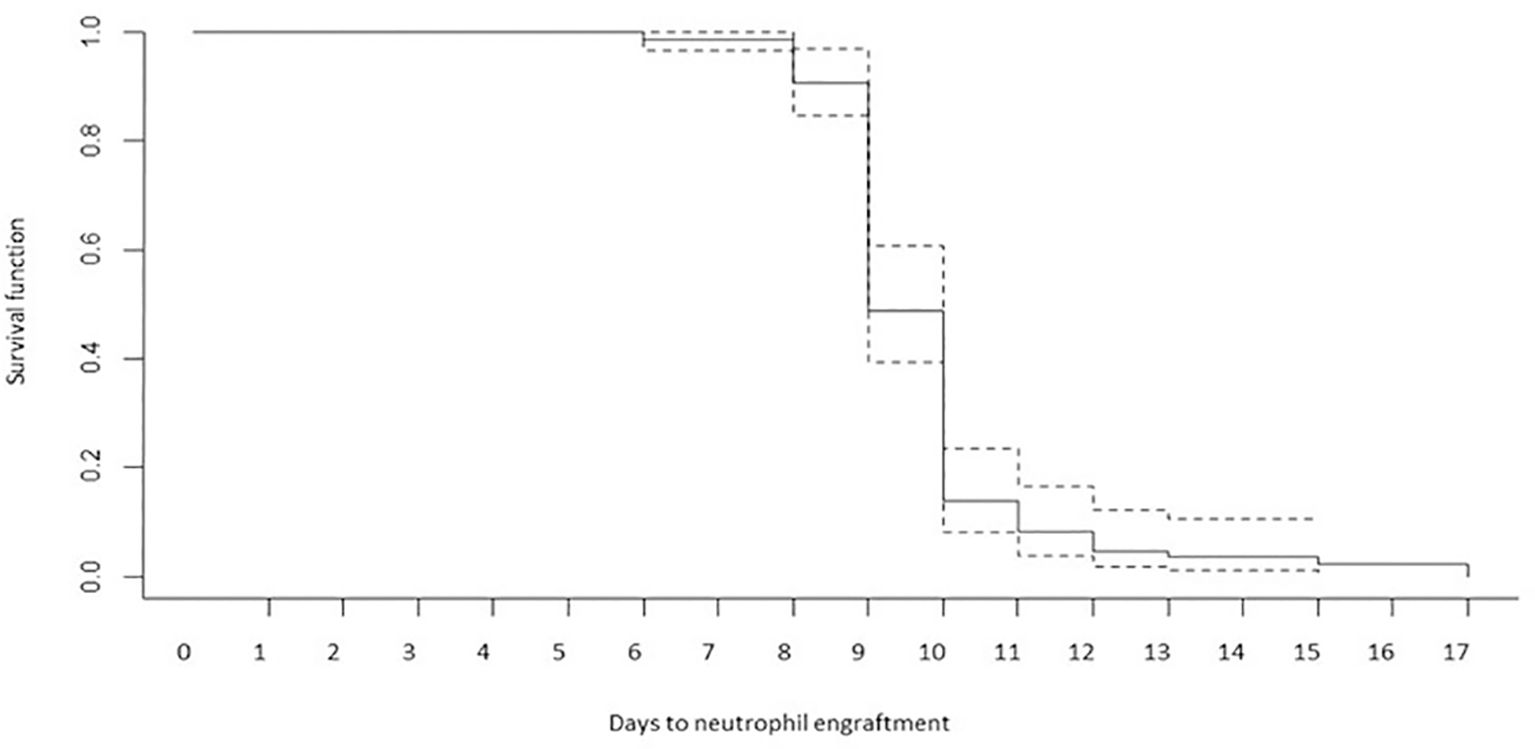
Figure 1 Time to reach neutrophil engraftment (Kaplan–Meier analysis). Cumulative median and mean survival-free time of neutrophil engraftment was 9 days (95% CI 8.7-9.3) and 9.8 days (95% CI 9.4-10.1).
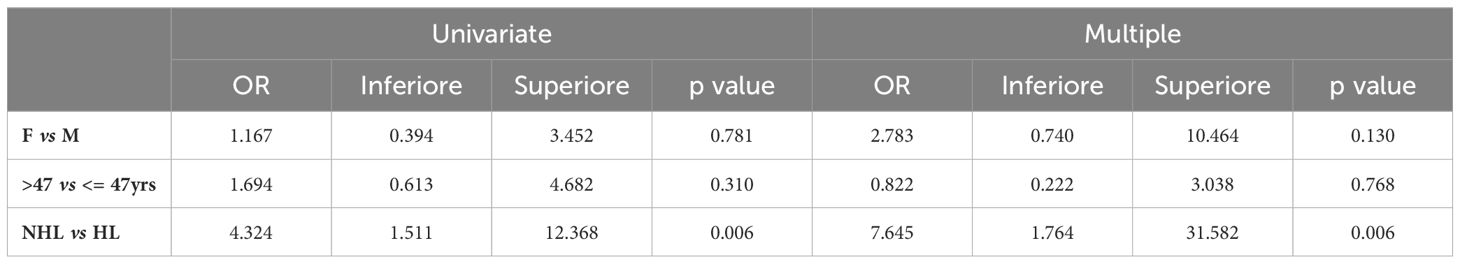
Univariate logistic analyses show a significant association between FN and NHL in univariable and multivariable logistic analyses, although with large confidence intervals due to a relatively low sample size (Table 3).
No statistical difference was found between HL and NHL regarding the time to reach the neutrophil engraftment. Figure 2 shows the same pattern of time to engraftment in HL and NHL patients. The restricted mean survival time analysis (RMST) confirms that over 17 days of follow-up, neutrophil engraftment occurred, on average, 9.9 days after the transplant (95% CI 9.2-10.5) in HL and 9.7 days (95% CI 9.3-10.1) in NHL (ΔRMST HD-NHL, -0.16 days; 95% CI -0.91–0.59, p 0.67) without any significant difference. Age and sex-adjusted analyses also confirmed these results.
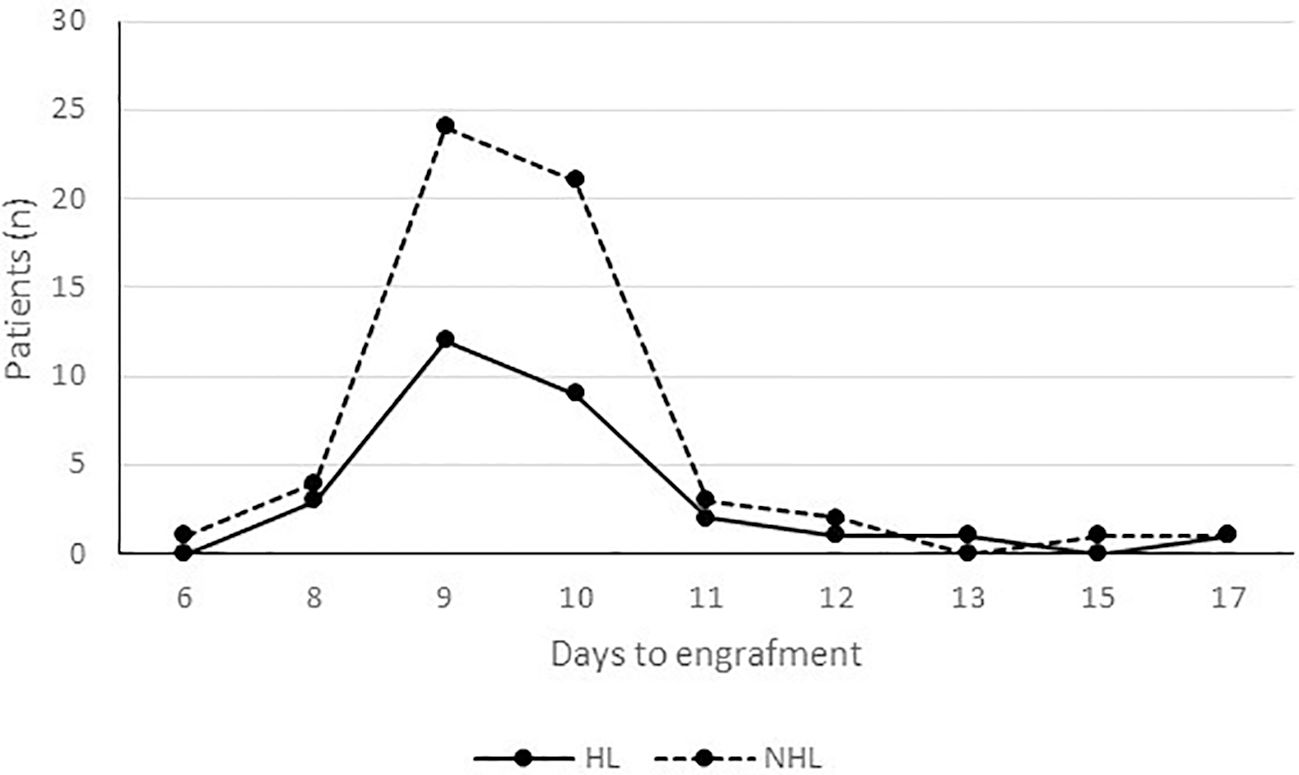
Figure 2 Time to engraftment by lymphoma type. Neutrophil engraftment occurred, on average, 9.9 days after the transplant (95% CI 9.2-10.5) in HL and 9.7 days (95% CI 9.3-10.1) in NHL (ΔRMST HD-NHL, -0.16 days; 95% CI -0.91–0.59, p 0.67).
Hospitalization lasted from a minimum of 9 to a maximum of 34 days (Figure 3). The restricted mean time to discharge was 15.9 days (95%CI 14-16), without differences by lymphoma type (HL 16.2, 95%CI 14.4-18.0; NHL 15.8, 95%CI 14.6-17.0).
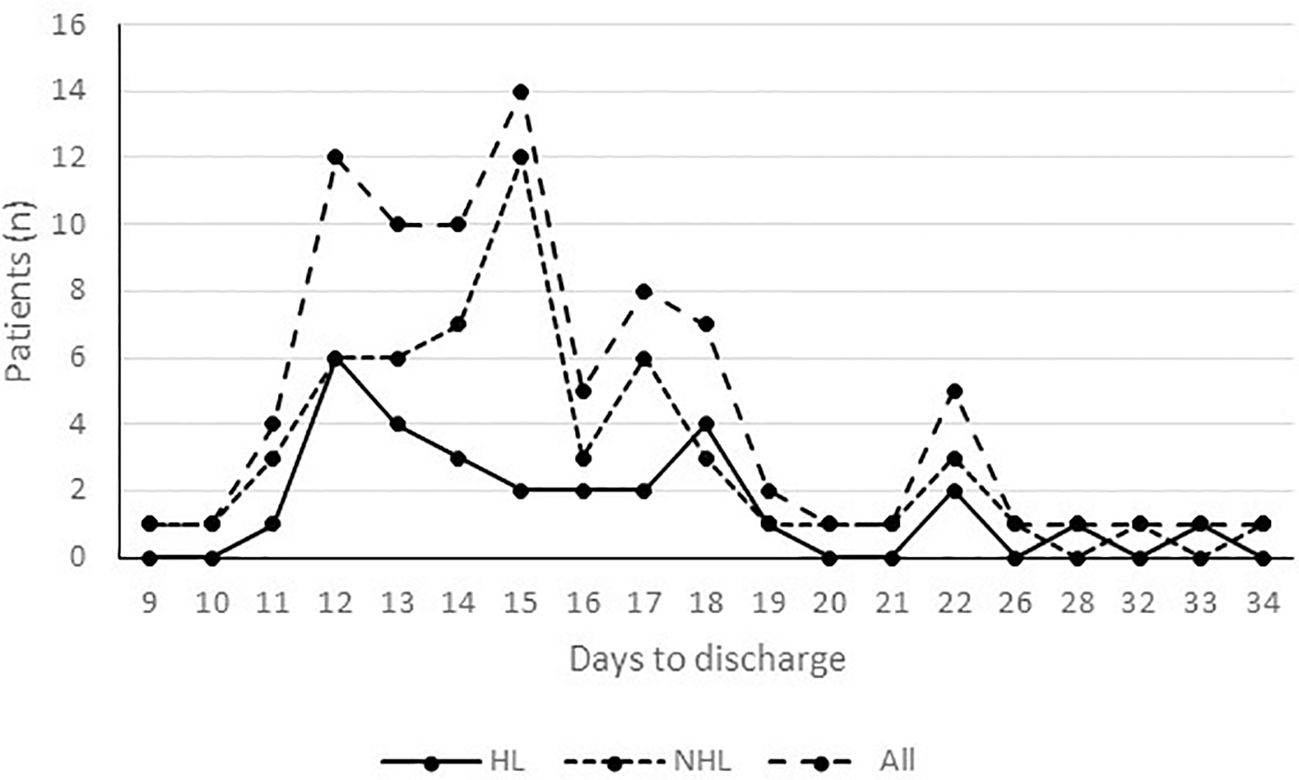
Figure 3 Time to discharge by lymphoma type. Hospitalization lasted from a minimum of 9 to a maximum of 34 days. The restricted mean time to discharge was 15.9 days (95%CI 14-16), without differences by lymphoma type (HL 16.2, 95%CI 14.4-18.0; NHL 15.8, 95%CI 14.6-17.0).
4 Discussion
BEAM regimen followed by ASCT causes myelosuppression, which can result in FN and potentially lead to severe infections. The risk of neutropenia and its complications can be reduced with ST G-CSF administration. ST G-CSF is safe and effective, cleared rapidly from the body, with a half-life of approximately 3.5 hours, and requires daily administration for up to 14 days. The alternative to ST G-CSF is an LT G-CSF, such as PEG. PEG is a pegylated form of G-CSF with similar indications and adverse events, although because of its longer half-life, it requires a one-shoot of 6 mg for a single HDC (29, 30). This dosage is sufficient in adult patients, regardless of body weight, making PEG a simple, effective, and well-tolerated option for managing HDC-induced neutropenia.
Although few studies compare the two drugs after HDC and ASCT, clinical trials have shown that a single, subcutaneous dose of PEG is as safe and effective as daily ST G-CSF.
Wannesson et al. (31) showed that neutrophil engraftment was reduced with PEG in multiple myeloma (MM) and lymphoma patients. Some authors reported that PEG had similar efficacy and safety profiles compared with SA G-CSF after ASCT (32), with a lower incidence of FN in the PEG group (33). Wang et al. showed in a retrospective analysis that PEG prophylaxis was more effective than SA G-CSF for FN prophylaxis in patients post-ASCT, especially for MM patients (34). Other studies characterized by heterogeneity in trial design, conditioning regimen and initiation of post-transplant drug administration, compared PEG versus G-CSF, demonstrated substantial equivalence in terms of days to engraftment, incidence of febrile neutropenia, antibiotic use and length of hospitalization (35–37). However, the efficacy of neutropenia prophylaxis may differ for G-CSF derivatives and different diseases (38, 39).
The FDA has approved 6 biosimilars to PEG: PEG-apgf, PEG-bmez, PEG-cbqv, PEG-fpgk, PEG-jmdb, and PEG-pbbk (40–43). There were no significant differences between the BIO-PEGs and reference PEG in the rate of FN (44). To date, there are no studies on the use of BIO-PEG in patients with Lymphoma and undergoing ASCT. We conducted the first single-center real-life analysis of lymphoma patients undergoing PEG-bmez post-ASCT. Our study demonstrated that PEG-bez prophylaxis was safe and effective for neutrophil engraftment and FN prophylaxis. Single-arm, real-life studies complement traditional randomized controlled trials (RCTs) by providing valuable insights into how treatments work in real-world settings (45). While RCTs are considered the reference standard for evaluating interventions, they often have strict inclusion criteria and controlled environments that may not fully reflect the complexities of everyday clinical practice. Single-arm studies allow clinicians to assess interventions’ effectiveness, safety, and tolerability in diverse patient populations under real-world conditions. They provide valuable data on how treatments work outside controlled clinical trials, capturing nuances such as comorbidities, concomitant medications, and patient adherence that can influence outcomes. These studies are particularly important for evaluating interventions in rare diseases, where recruiting sufficient participants for traditional RCTs may be challenging. Additionally, they offer insights into the long-term effects of treatments as they follow patients over extended periods, providing valuable information on real-world outcomes and helping clinicians make informed decisions about treatment strategies. Overall, single-arm, real-life studies complement RCTs by providing essential data on the effectiveness and safety of interventions in diverse patient populations, ultimately contributing to improved patient care and clinical decision-making. This type of study is of relevance when the evolution of the disease is well known and when there is no evidence of the placebo effect.
Our real-life study was not aimed at a cost analysis. It is crucial, however, to emphasize that the recent licensing of BIO-PEG-containing products offers the opportunity to deliver the additional advantages of long-term SA G-CSF at a reduced cost. For countries using reference PEG, evident cost savings are reported by switching to BIO-PEG (46–48). Recently, the introduction of SA G-CSF biosimilars favored their adoption due to their cost-efficiency in reducing the incidence of FN in chemotherapy-treated patients than SA G-CSF originator and LA G-CSFs (48); hence, a similar pattern is expected for the LA GCSFs category. These results are consistent with a study’s findings, which showed that the introduction of BIO-PEGs in place of SA G-CSF treatments has a substantial cost-saving potential for the Italian National Healthcare Service (49). The analysis highlighted the economic advantage of using BIO-PEGs in place of SA G-CSF treatments in the FN treatment setting, providing a substantial cumulative cost saving of € 59,650 and € 41,539, respectively, for a 1000 patients population with solid tumors and lymphomas over a 3-years’ timeframe.
In conclusion, our study highlights the efficacy and safety of a BIO-PEG formulation in patients with Lymphoma and undergoing ASCT. Even though the study has the significant limitation of not being randomized and not having a control arm, it can be considered a preliminary assessment of effectiveness. Thus, these results can be helpful for the design of randomized Phase III studies. Compared with SA G-CSF, the development of randomized trials that can confirm the advantage of BIO-PEGs is desirable. Advantages may be related to overall cost (drug, reduced complications, and hospitalization), single and not daily administration, standardized dosing, and not related to patient weight.
Data availability statement
Raw data were generated at the Institute of Clinical Physiology (IFC-CNR), Reggio Calabria, Italy. Derived data supporting the findings of this study are available from the corresponding author upon request.
Ethics statement
The studies involving humans were approved by Calabria region ethics committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. All procedures performed in this study were in accordance with the ethical standards of the International Conference on Harmonization Guidelines for Good Clinical Practice and with the 1964 Helsinki Declaration and its later amendments.
Author contributions
BL: Conceptualization, Investigation, Writing – original draft. AP: Data curation, Formal Analysis, Writing – original draft. MP: Project administration, Writing – original draft, Writing – review & editing. CA: Investigation, Resources, Writing – original draft. GT: Data curation, Writing – original draft. MMi: Formal Analysis, Investigation, Writing – original draft. MP: Formal Analysis, Methodology, Writing – original draft. FC: Supervision, Writing – original draft. GaP: Formal Analysis, Methodology, Writing – original draft. GiP: Data curation, Writing – original draft. GU: Data curation, Writing – original draft. ID: Data curation, Writing – original draft. ASg: Data curation, Formal Analysis, Writing – original draft. ASc: Data curation, Writing – original draft. AI: Data curation, Writing – original draft. GL: Data curation, Writing – original draft. MA: Supervision, Writing – original draft. GD: Supervision, Validation, Writing – review & editing. MG: Resources, Supervision, Validation, Writing – original draft. MMa: Project administration, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin's disease: a randomised trial. Lancet. (2002) 359:2065–71. doi: 10.1016/S0140-6736(02)08938-9
2. Perales MA, Ceberio I, Armand P, Burns LJ, Chen R, Cole PD, et al. Role of cytotoxic therapy with hematopoietic cell transplantation in the treatment of Hodgkin lymphoma: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. (2015) 21:971–83. doi: 10.1016/j.bbmt.2015.02.022
3. Passweg JR, Baldomero H, Ciceri F, Corbacioglu S, de la Cámara R, Dolstra H, et al. Hematopoieticcelltransplantation and cellular therapies in Europe 2021. The second year of the SARS-CoV-2 pandemic. A Report from the EBMT Activity Survey. Bone MarrowTransplant. (2023) 58:647–58. doi: 10.1038/s41409-023-01943-3
4. Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin's lymphoma. N Engl J Med. (1995) 333:1540–5. doi: 10.1056/NEJM199512073332305
5. Oliansky DM, Czuczman M, Fisher RI, Dillon H, Ratko TA, Wall D, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of diffuse large B cell lymphoma: update of the 2001 evidence-based review. Biol Blood Marrow Transplant. (2011) 17:20–47 e30. doi: 10.1016/j.bbmt.2010.07.008
6. Bolon YT, Atshan R, Allbee-Johnson M, Estrada-Merly N, et al. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides. Transplant Cell Ther. (2022).
7. Aapro M, Boccia R, Leonard R, Camps C, Campone M, Choquet S, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. (2017) 25:3295–304. doi: 10.1007/s00520-017-3842-1
8. Lyman GH, Kuderer NM. Epidemiology of febrile neutropenia. Support Cancer Ther. (2003) 1:23–35. doi: 10.3816/SCT.2003.n.002
9. Lalami Y, Klastersky J. Impact of chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) on cancer treatment outcomes: an overview about well-established and recently emerging clinical data. Crit Rev Oncol Hematol. (2017) 120:163–79. doi: 10.1016/j.critrevonc.2017.11.005
10. Wang L, Baser O, Kutikova L, Page JH, Barron R. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and Meta-analysis of randomized controlled trials. Support Care Cancer. (2015) 23:3131–40. doi: 10.1007/s00520-015-2686-9
11. Wey E, Kibbler C. Infections associated with neutropenia and transplantation. Antibiot Chemother. (2012) 2010:502–23. doi: 10.1016/B978-0-7020-4064-1.00040-3
12. Baluch A, Shewayish S. Neutropenic Fever. In: Velez A, Lamarche J, Greene J, editors. Infections in Neutropenic Cancer Patients. Springer, Cham (2019). doi: 10.1007/978-3-030-21859-1_8
13. Castagnola E, Fontana V, Caviglia I, Caruso S, Faraci M, Fioredda F, et al. A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation. Clin Infect Dis. (2007) 45:1296–304. doi: 10.1086/522533
14. Bennett CL, Djulbegovic B, Norris LB, Armitage JO. Colony-stimulating factors for febrile neutropenia during cancer therapy. New Engl J Med. (2013) 368:1131–39. doi: 10.1056/NEJMct1210890
15. Aapro MS, Bohlius J, Cameron DA, Dal Lago L, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. (2011) 47:8–32. doi: 10.1016/j.ejca.2010.10.013
16. Crawford J, Armitage J, Balducci L, Becker PS, Blayney DW, Cataland SR, et al. Myeloid growth factors. J Natl Compr Canc Netw. (2013) 11:1266–90. doi: 10.6004/jnccn.2013.0148
17. Becker PS, Griffiths EA, Alwan LM, Bachiashvili K, Brown A, Cool R, et al. NCCN guidelines insights: hematopoietic growth factors, version 1.2020. J Natl Compr Canc Netw. (2020) 18:12–22. doi: 10.6004/jnccn.2020.0002
18. Lee SM, Radford JA, Dobson L, Huq T, Ryder WD, Pettengell R, et al. Recombinant human granulocyte colony-stimulating factor (filgrastim) following high-dose chemotherapy and peripheral blood progenitor cell rescue in high-grade non-Hodgkin's lymphoma: clinical benefits at no extra cost. Br J Cancer. (1998) 77:1294–9. doi: 10.1038/bjc.1998.216
19. Linch DC, Milligan DW, Winfield DA, Kelsey SM, Johnson SA, Littlewood TJ, et al. G-CSF after peripheral blood stem cell transplantation in lymphoma patients significantly accelerated neutrophil recovery and shortened time in hospital: results of a randomized BNLI trial. Br J Haematol. (1997) 99:933–8. doi: 10.1046/j.1365-2141.1997.4703274.x
20. Singh AD, Parmar S, Patel K, Shah S, Shore T, Gergis U, et al. Granulocyte colony-stimulating factor use after autologous peripheral blood stem cell transplantation: comparison of two practices. Biol Blood Marrow Transplant. (2018) 24:288–93. doi: 10.1016/j.bbmt.2017.10.026
21. Bolwell B, Goormastic M, Dannley R, Andresen S, Overmoyer B, Mendez Z, et al. G-CSF post-autologous progenitor cell transplantation: a randomized study of 5, 10, and 16 micrograms/kg/day. Bone Marrow Transplant. (1997) 19:215–9. doi: 10.1038/sj.bmt.1700645
22. Grosso D, Leiby B, Wilde L, Carabasi M, Filicko-O'Hara J, O'Hara W, et al. A prospective, randomized trial examining the use of G-CSF versus no G-CSF in patients post-autologous transplantation. Transplant Cell Ther. (2022) 28:831.e1–7. doi: 10.1016/j.jtct.2022.09.012
23. Klein EM, Sauer S, Klein S, Tichy D, Benner A, Bertsch U, et al. Antibiotic prophylaxis or granulocyte-colony stimulating factor support in multiple myeloma patients undergoing autologous stem cell transplantation. Cancers (Basel). (2021) 13:3439. doi: 10.3390/cancers13143439
24. U.S. Food and Drug Administration. ZARXIO®(filgrastim-sndz) injection, for subcutaneous or intravenous use: highlights of prescribing information (2015). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125553s007lbl.pdf. (Accessed 14 February 2023).
25. Martino M, Gori M, Porto G, Pellicano M, Santoro L, Verduci C, et al. Effectiveness of biosimilar pegfilgrastim in patients with multiple myeloma after high-dose melphalan and autologous stem cell transplantation. Ann Hematol. (2023) 102:1915–25. doi: 10.1007/s00277-023-05228-z
26. U.S. Food and Drug Administration. ZIEXTENZO™ (pegfilgrastim-bmez) injection, for subcutaneous use: highlights of prescribing information (2019). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761045lbl.pdf. (Accessed 14 February 2023).
27. Martino M, Gori M, Tripepi G, Recchia AG, Cimminiello M, Provenzano PF, et al. A comparative effectiveness study of lipegfilgrastim in multiple myeloma patients after high dose melphalan and autologous stem cell transplant. Ann Hematol. (2020) 99:331–41. doi: 10.1007/s00277-019-03901-w
28. U.S. Food and Drug Administration. Biosimilar and interchangeable products (2017). Available online at: https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products (Accessed 16 December 2019).
29. Bedell C. Pegfilgrastim for chemotherapy-induced neutropenia. Clin J Oncol Nurs. (2003) 7:55–6, 63-4. doi: 10.1188/03.CJON.55-56
30. Neulasta (pegfilgrastim) injection, for subcutaneous use [prescribing information] (2021). Amgen. Available online at: https://www.pi.amgen.com/-/media/Project/Amgen/Repository/pi-amgen-com/neulasta/neulasta_pi_hcp_english.pdf (Accessed July 12, 2023).
31. Wannesson L, Luthi F, Zucca E, Rosselet-christ A, Baglioni M, Marelli L, et al. Pegfilgrastim to accelerate neutrophil engraftment following peripheral blood stem cell transplant and reduce the duration of neutropenia, hospitalization, and use of intravenous antibiotics: a phase II study in multiple myeloma and lymphoma and comparison with filgrastim-treated matched controls. Leuk Lymph. (2011) 52:436–43. doi: 10.3109/10428194.2010.545462
32. Vanstraelen G, Frere P, Ngirabacu MC, Willems E, Fillet G, Beguin Y. Pegfilgrastim compared with filgrastim after autologous hematopoietic peripheral blood stem cell transplantation. Exp Hematol. (2006) 34:382–8. doi: 10.1016/j.exphem.2005.11.013
33. Jagasia MH, Greer JP, Morgan DS, Mineishi S, Kassim AA, Ruffner KL, et al. Pegfilgrastim after high-dose chemotherapy and autologous peripheral blood stem cell transplant: phase II study. Bone Marrow Transplant. (2005) 35:1165–9. doi: 10.1038/sj.bmt.1704994
34. Wang X, Ren J, Liang X, He P. Efficacy and cost of G-CSF derivatives for prophylaxis of febrile neutropenia in lymphoma and multiple myeloma patients underwent autologous hematopoietic stem cell transplantation. Hematology. (2021) 26:950–5. doi: 10.1080/16078454.2021.2003071
35. Samaras P, Buset EM, Siciliano RD, Haile SR, Petrausch U, Mischo A, et al. Equivalence of pegfilgrastim and filgrastim in lymphoma patients treated with BEAM followed by autologous stem cell transplantation. Oncology. (2010) 79:93–7. doi: 10.1159/000320604
36. Ballestrero A, Boy D, Gonella R, Miglino M, Clavio M, Barbero V, et al. Pegfilgrastim compared with filgrastim after autologous peripheral blood stem cell transplantation in patients with solid tumours and lymphomas. Ann Hematol. (2008) 87:49–55. doi: 10.1007/s00277-007-0366-7
37. Gerds A, Fox-Geiman M, Dawravoo K, Rodriguez T, Toor A, Smith S, et al. Randomized phase III trial of pegfilgrastim versus filgrastim after autologus peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. (2010) 16:678–85. doi: 10.1016/j.bbmt.2009.12.531
38. Martino M, Pratico G, Messina G, Irrera G, Massara E, Messina G, et al. Pegfilgrastim compared with filgrastim after high-dose melphalan and autologous hematopoietic peripheral blood stem cell transplantation in multiple myeloma patients. Eur J Haematol. (2006) 77:410–5. doi: 10.1111/j.1600-0609.2006.00736.x
39. Nyvepria (pegfilgrastim-apgf) injection, for subcutaneous use [prescribing information] (2023). Pfizer. Available online at: https://labeling.pfizer.com/ShowLabeling.aspx?format=PDF&id=13622. (Accessed July 12, 2023).
40. Udenyca (pegfilgrastim-cbqv) injection, for subcutaneous use [prescribing information] (2023). Available online at: https://udenyca.com/hcp/wp-content/pdfs/udenyca-pi.pdf. (Accessed July 12, 2023).
41. Stimufend (pegfilgrastim-fpgk) injection, for subcutaneous use [prescribing information] (2022). Fresenius Kabi USA. Available online at: www.accessdata.fda.gov/drugsatfda_docs/label/2022/761173Orig1s000correctedlbl.pdf. (Accessed July 12, 2023).
42. Fulphila (pegfilgrastim-jmdb) injection, for subcutaneous use [prescribing information] (2021). Mylan Institutional. Available online at: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=3ea915d7-2feb-4e75-91f7-913c965b7d8a&type=display. (Accessed July 12, 2023).
43. Waller CF, Ranganna GM, Pennella EJ, Blakeley C, Bronchud MH, Mattano LA Jr, et al. Randomized phase 3 efficacy and safety trial of proposed pegfilgrastim biosimilar MYL-1401H in the prophylactic treatment of chemotherapy-induced neutropenia. Ann Hematol. (2019) 98:1217–24. doi: 10.1007/s00277-019-03639-5
44. Saturni S, Bellini F, Braido F, Paggiaro P, Sanduzzi A, Scichilone N, et al. Randomized Controlled Trials and real life studies. Approaches and methodologies: a clinical point of view. Pulm Pharmacol Ther. (2014) 27:129–38. doi: 10.1016/j.pupt.2014.01.005
45. Cornes P, Gascon P, Vulto AG, Aapro M. Biosimilar pegflgrastim: improving access and optimising practice to supportive care that enables cure. BioDrugs. (2020) 34:255–63. doi: 10.1007/s40259-020-00411-4
46. Ravasio R, Antonuzzo L, Danova M, Pronzato P. Budget impact analysis of pegfilgrastim biosimilar in the treatment of febrile neutropenia in Italy. AboutOpen. (2020) 7:04–8. doi: 10.33393/abtpn.2020.2030
47. Aapro M, Cornes P, Abraham I. Comparative cost-efficiency across the European countries of various regimens of filgrastim, biosimilar filgrastim, and pegfilgrastim to reduce the incidence of chemotherapy-induced febrile neutropenia. J Oncol Pharm Pract. (2012) 18:171–9. doi: 10.1177/1078155211407367
48. Sun D, Andayani TM, Altyar A, MacDonald K, Abraham I. Potential cost savings from chemotherapy-induced febrile neutropenia with biosimilar filgrastim and expanded access to targeted antineoplastic treatment across the European Union G5 countries: a simulation study. Clin Ther. (2015) 37:842–57. doi: 10.1016/j.clinthera.2015.01.011
Keywords: lymphoma, biosimilar pegfilgrastim, autologous stem cell transplantation, chemotherapy, BIO-PEG
Citation: Loteta B, Pitino A, Pitea M, Alati C, Tripepi G, Mico' MC, Pellicano' M, Cogliandro F, Porto G, Policastro G, Utano G, Delfino IM, Sgarlata A, Scopelliti A, Idato A, Laenza G, Altomonte M, D'Arrigo G, Gori M and Martino M (2024) Effectiveness of biosimilar pegfilgrastim in patients with lymphoma after high-dose chemotherapy and autologous stem cell transplantation: a real-life study. Front. Hematol. 3:1441070. doi: 10.3389/frhem.2024.1441070
Received: 04 June 2024; Accepted: 10 July 2024;
Published: 25 July 2024.
Edited by:
Guido Gini, Azienda Ospedaliero Universitaria Ospedali Riuniti, ItalyReviewed by:
Raffaele Palmieri, University of Rome Tor Vergata, ItalyCostantino Riemma, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), Italy
Copyright © 2024 Loteta, Pitino, Pitea, Alati, Tripepi, Mico', Pellicano', Cogliandro, Porto, Policastro, Utano, Delfino, Sgarlata, Scopelliti, Idato, Laenza, Altomonte, D'Arrigo, Gori and Martino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martina Pitea, bWFydGluYS5waXRlYUBvc3BlZGFsZXJjLml0
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Barbara Loteta
Barbara Loteta Annalisa Pitino3†
Annalisa Pitino3† Martina Pitea
Martina Pitea Ilaria Maria Delfino
Ilaria Maria Delfino Annalisa Sgarlata
Annalisa Sgarlata Massimo Martino
Massimo Martino