- 1Department of Haematology, University College London Hospitals NHS Foundation Trust, London, United Kingdom
- 2Department of Haematology, University College London Cancer Institute, London, United Kingdom
Cytopenia is a common side effect after chimeric antigen receptor T cell (CAR-T) therapy but is unusual at late timepoints when it is most commonly due to bone marrow (BM) failure, myelodysplasia (MDS) or other secondary malignancies. In this case report, we describe a patient who developed severe aplastic anaemia 18 months after CD19 CAR-T therapy, and shortly after COVID-19 vaccination. This is the first description of severe aplastic anaemia triggered by COVID-19 vaccination as a cause of post-CAR-T cytopenia. The patient described was treated with a combination of eltrombopag and ciclosporin due to frailty and had a rapid and complete clinical response to this therapy, providing an effective option for the treatment of immune-related cytopenia post-CAR-T in an increasingly elderly and frail population.
Introduction
COVID-19 vaccination has been linked to the development of a number of rare haematological conditions, but an association with immune-mediated bone marrow (BM) failure is less well established. Rare case reports describing this phenomenon are reported across vaccine formulations in patients without prior haematological history and in those who responded variably to immunosuppressive therapy, with some patients requiring allogeneic stem cell transplant (HSCT) rescue (10–16). To date, this phenomenon has not been described in the context of chimeric antigen receptor T cell (CAR-T) therapy. In this case report, we describe a patient who developed severe pancytopenia 18 months after CD19 CAR-T therapy and shortly after COVID-19 vaccination. This is the first description of a case of severe aplastic anaemia (AA) as a cause of late-onset post-CAR-T cytopenia, where a combination immunosuppressive treatment with eltrombopag and ciclosporin resulted in rapid clinical improvement without the need for an allogeneic stem cell transplant. The success of this strategy will be of interest to clinicians managing an increasingly frail and elderly population with improved survival after CAR-T therapy.
Case description
A 69-year-old woman with relapsed/refractory (r/r) Philadelphia chromosome-positive B-cell acute lymphoblastic leukaemia (B-ALL) successfully treated 18 months prior with CD19-directed CAR-T therapy presented with bruising and new-onset severe pancytopenia in June 2023.
Diagnosed in 2019, she was initially treated in the UKALL60+ trial with a very good MRD response. She was consolidated with reduced-intensity matched unrelated donor HSCT in the ALL-RIC trial in August 2019. Peri-transplant complications included pulmonary oedema and atrial flutter. Post-transplant, she developed grade II acute and then chronic skin graft-versus-host disease (GVHD) which was managed successfully with topical and oral steroids followed by ruxolitinib. She was off all immunosuppression by March 2021.
In September 2019 (1 month post-transplant), her chimerism analysis showed 100% donor chimerism in T cells and 95% donor chimerism in granulocytes. By November 2019 (3 months post-transplant), her T cell chimerism remained 100% but she had a slight drop in granulocyte donor chimerism (88%) and mixed chimerism was observed in B cells (81%). However, at 6 months post-transplant, her chimerism analysis was 100% in all three cell lineages and remained so until last checked in May 2021.
She received imatinib for a minimal residual disease (MRD) relapse in late 2019, but subsequently switched to nilotinib due to drug intolerance, and later to dasatinib in 2021 following a rise in MRD. A BM biopsy in mid-2021 confirmed morphological relapse with 37% blasts.
In July 2021, at 2 years post-transplant and prior to CAR-T therapy, her immunoglobulin G level remained low (3.86g/L) with normal immunoglobulin A (1.35g/L) and immunoglobulin M (0.40g/L) levels. Her CD19 levels were normal (0.419x10^9/L) and CD3 and CD4 levels were low (0.45x10^9/L, 0.33x10^9/L).
She received CAR-T therapy in a clinical trial in November 2021, achieving molecular remission at month 1, which was ongoing at month 18, at which point she did not require blood or platelet transfusion support. As expected, she became immunodeficient post-CAR-T. CAR-T persistence and B cell aplasia were ongoing at month 18 post-CAR-T. Her absolute CD19 count in June 2023 was 0.00x10^9/L, CD3 absolute count was low at 0.27x10^9/L, and CD4 absolute count was 0.13x10^9/L. Immunoglobulin G levels were low in the initial post-CAR T period but, by time of presentation in June 2023, had recovered to 5.70g/L.
Two weeks following her 18-month remission BM biopsy and 10 days following a booster dose of the Pfizer-BioNTech mRNA SARS-CoV-2 vaccine on 5th June 2023, she presented with spontaneous bruising, fatigue, and shortness of breath and was admitted to hospital. Her COVID-19 vaccination history included an initial two doses of the Pfizer BioNTech vaccine in January and April 2021. Her COVID-19 serology was negative following these initial doses, suggesting no antibody response, but became positive in September 2021 following a COVID-19 infection pre-CAR-T therapy. A third vaccine dose was administered immediately prior to CAR-T therapy in October 2021 without adverse events. In March, May, and December 2022 she had three further doses of the Pfizer BNT vaccine.
A full blood count (FBC) demonstrated severe pancytopenia (haemoglobin, 65g/L; mean corpuscular volume (MCV), 109.4; reticulocytes, 0.33%; total white cell count, 0.37 x109/L; neutrophils, 0.01x109/L; lymphocytes 0.29 x109/L; monocytes 0.02 x109/L; eosinophils 0.05 x109/L; basophils 0.00 x109/L; platelets, 2x109/L; immature platelet fraction, 2.7%) (Table 1) and a blood film showed pancytopenia with anisopoikilocytosis. Lactate dehydrogenase, Vitamin B12, and red cell folate levels were within normal range, and an autoimmune antibody panel was negative. Parvovirus IgG and IgM were positive and negative respectively (indicating prior exposure), and viral PCR testing was negative for parvovirus, HHV6, EBV, CMV and HIV. Donor chimerism was 100%, flow cytometry for a paroxysmal nocturnal haemoglobinuria (PNH) clone was negative, and blood CAR-T marking indicated stable ongoing CAR-T persistence (5676 copies/ug genomic DNA). Repeat BM biopsies [including next-generation sequencing (NGS)] showed no evidence of leukaemia relapse or myelodysplasia but was severely hypocellular for her age (Figure 1A), consistent with very severe AA as per the British Committee for Standards in Haematology diagnostic criteria (6).
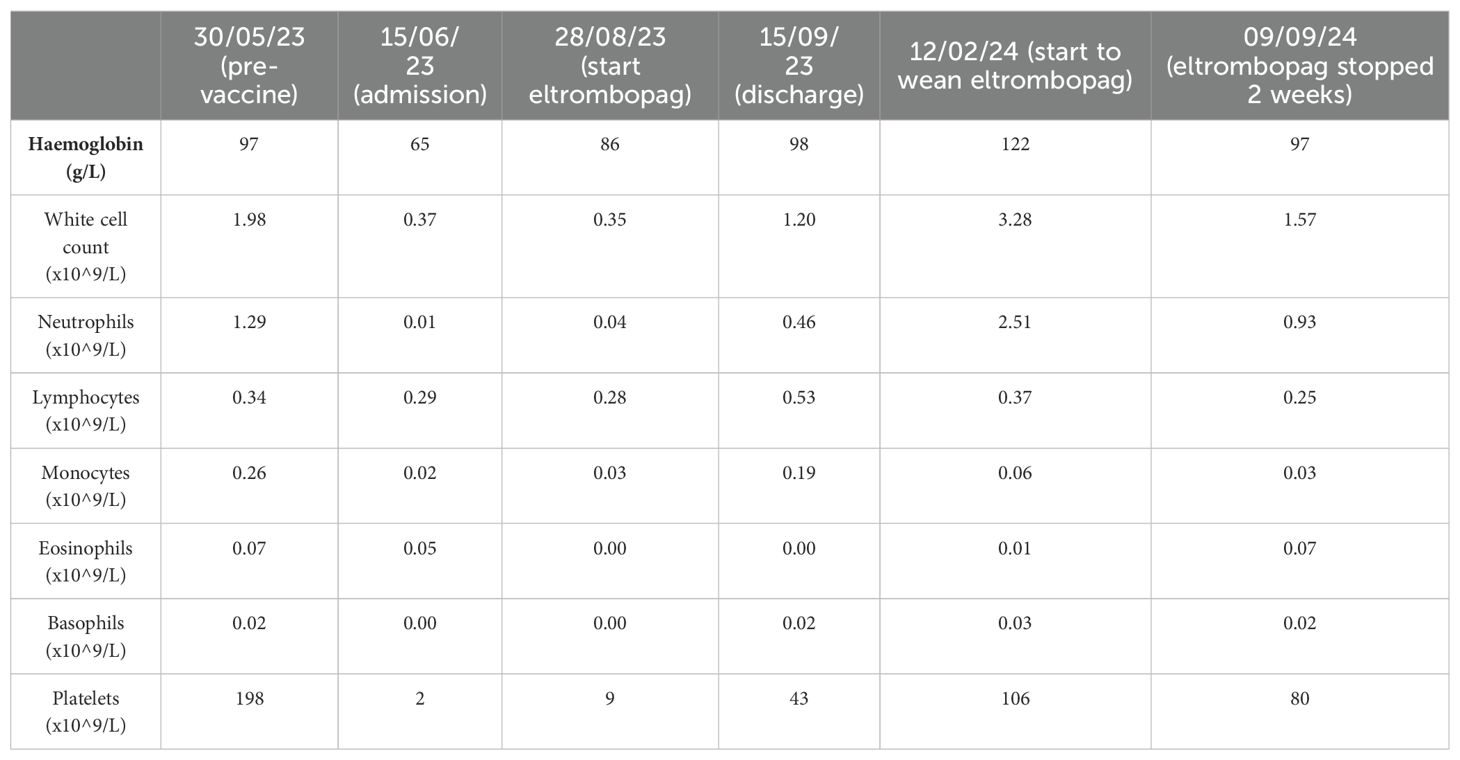
Table 1. Patient full blood count results at selected timepoints before and after presentation with pancytopenia and after initiation of treatment with eltrombopag.
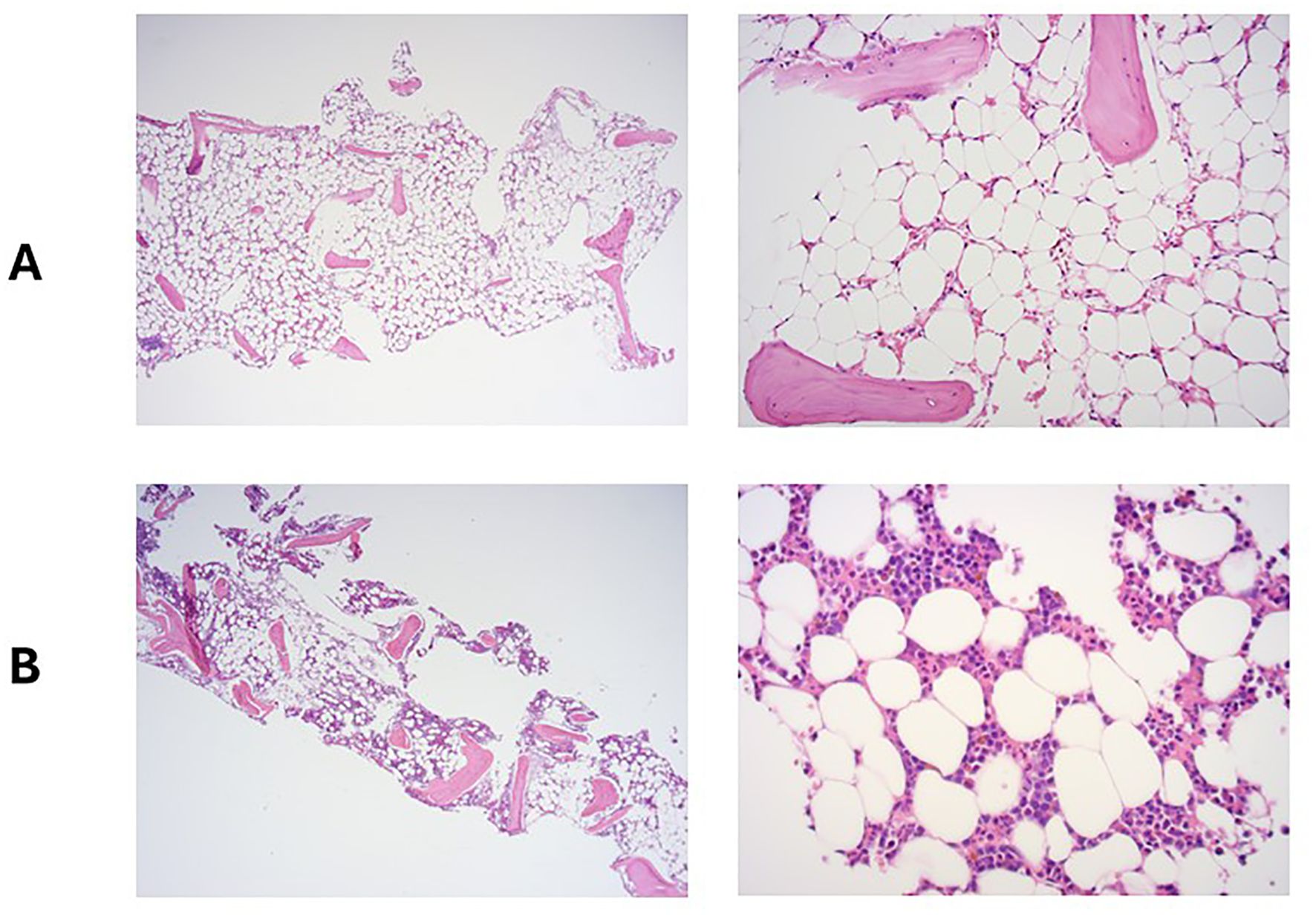
Figure 1. Bone marrow trephine at the time of admission with pancytopenia (A) and 5 months later, following treatment with eltrombopag and ciclosporin (B).
Despite supportive care with granulocyte colony-stimulating factor (GCSF), red cell, and platelet transfusions, her FBC did not spontaneously improve. An emergency CD34+ selected, unconditioned stem cell top-up from her HSCT donor was requested, but due to the donor’s ill health, this was repeatedly postponed and ultimately cancelled. A 2nd HSCT from an alternative donor was considered, but the risk of mortality (TRM) was considered too high given her age and frailty. Intensive aplastic anaemia management including anti-thymocyte globulin (ATG) therapy was considered high-risk given the patient’s frail state, and as an alternative, dual therapy with the thrombopoietin (TPO) agonist eltrombopag (150mg once daily) with ciclosporin (75mg twice daily with target levels of 100-300) was commenced (Figure 2) after funding approval was obtained for eltrombopag. Transiently deranged liver function tests precipitated an eltrombopag dose reduction to 100mg once daily. Following a 3-month hospital admission for refractory pancytopenia, combined eltrombopag and ciclosporin resulted in rapid count recovery, discharge from hospital on day 22 of treatment, GCSF independence on day 47, platelet independence on day 59, and red-cell transfusion independence on day 108. At 16 weeks of follow-up, she remained on eltrombopag 100mg once daily and a weaning dose of ciclosporin. Repeat bone marrow biopsies showed normal cellularity and trilineage haematopoiesis (Figure 1B). Eltrombopag was slowly weaned thereafter. An unsuccessful attempt was made to stop eltrombopag in August 2024 due to the recurrence of cytopenia and the patient continues on low dose eltrombopag at last follow up.
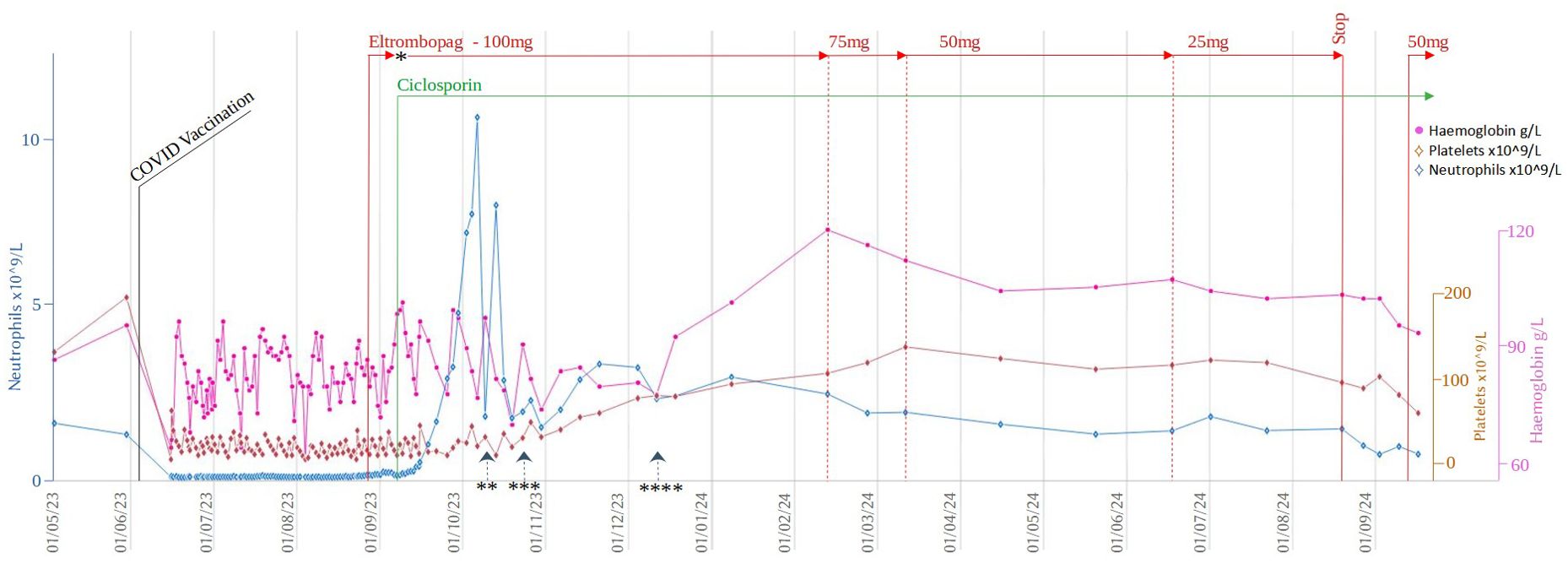
Figure 2. Timeline of cell counts and treatment during the initial 5 months after her presentation with pancytopenia. *Eltrombopag treatment was paused for 4 days due to deranged LFTs. Restarted at 50mg OD and up-titrated to 100mg OD after a further 4 days ** Last dose of GCSF *** Last platelet transfusion **** Last red cell transfusion.
Patient perspective
I woke up in June 2023 and noticed several small purple bruises on my neck, chest and arms. I contacted my haematology support team for advice. On review, and following blood tests, I was advised I would need to be admitted to hospital, the start of my three months stay as an inpatient! I knew the team were doing all they could to find out what was causing my low blood counts, but it was a stressful and worrying time although I tried to stay positive. As I eventually began to stabilise with eltrombopag I started to look forward to being discharged. I continue to take both ciclosporin and eltrombopag daily and make regular maintenance visits to haematology outpatients which is wonderfully reassuring. I have found that my mobility has suffered along with experiencing joint discomfort, but I do not appear to have any adverse reaction to the medication.
Discussion
Immune effector cell-associated haematotoxicity (ICAHT) is the most commonly reported CAR-T therapy side effect in both a clinical trial and real-world setting. Such cytopenia following CAR-T therapy is commonly biphasic; with early and intermittent neutrophil recovery often followed by a further decline in neutrophil count. Some patients, however, have more severe and long-lasting haematotoxicity (1). In conjunction with the expected B cell aplasia and hypogammaglobulinemia after CD19 CAR-T treatment, such haematotoxicity increases the risk of severe infectious complications in these patients. Infections are responsible for the majority of non-relapse mortality after CAR-T therapy (2).
Early cytopenia (day 0-30), often lasting until around day 10, is associated with lymphodepleting chemotherapy and delays in count recovery here are often seen in patients with high-grade CRS. Prolonged or late onset cytopenia is those occurring after day 30 post-CAR-T therapy (3). The pathophysiology of such late onset and/or prolonged cytopenia after CAR-T therapy remains incompletely understood but is likely multi-factorial. A CAR-T therapy patient’s bone marrow HSC reserve will be influenced by the extent of previous cytotoxic chemotherapies, the ageing process, and the presence of age-related clonal haematopoiesis of indeterminate potential (CHIP). Both systemic and local inflammation in the bone marrow microenvironment from CAR-T targeting tumour cells and the release of cytokines and growth factors are thought to contribute to cytopenia (4).
New severe trilineage bone marrow failure at very late timepoints post-CAR-T infusion is less common and should prompt a bone marrow examination. BM failure syndromes and secondary malignancies, particularly treatment-related myeloid neoplasms, should be considered (5).
In this case, our patient was diagnosed with very severe aplastic anaemia. AA is an immune-mediated BM failure syndrome characterised by predominantly T cell-dependent destruction of haematopoietic stem and progenitor cells. In the majority of cases (70-80%), the initiating event is unknown but triggers can include toxins, viral infections, and, rarely, vaccinations (6).
In our case, there was a strong temporal link between the onset of BM failure and the recent administration of COVID vaccination. Since the development of multiple COVID-19 vaccines, there have been reports of haematological complications across all vaccine formulations. A rare vaccine-induced immunothrombotic thrombocytopenia (VITT) syndrome has been described (7), as well as autoimmune haemolytic anaemia and immune thrombocytopenic purpura (8, 9).
In addition, there have been case reports and a small case series describing a link between COVID-19 vaccination and de novo aplastic anaemia. The pathogenic mechanisms by which SARS-CoV-2 vaccine administration may cause severe bone marrow failure are not known although researchers speculate that it may be due to vaccine-induced immune reactions. These case reports have all been in patients without significant prior medical/haematological history across varying vaccine formulations. Patients were treated with varying success with different regimens of immunosuppression (ATG/ciclosporin) (10–12). De novo aplastic anaemia has also been described in relation to the Pfizer-BioNTech mRNA vaccine used in our patient. Tabata et al. reported a case of a previously well male diagnosed with severe AA 4 days after a second dose of the Pfizer-BioNTech mRNA vaccine which was treated to remission with haematopoietic stem cell transplantation (13). Cecchi et al. presented a healthy 76-year-old man who developed AA 1 month after the Pfizer-BioNTech mRNA vaccine and was treated with ATG and ciclosporin (14). Chen et al. described a series of four cases in Taiwan that were treated with ATG/CSA, eltrombopag, and HSCT (15). Babakhanlou et al. also described four patients in a small case series who had received both the Pfizer and Moderna vaccines. AA developed on average 3 weeks after a second dose of vaccine and was treated with a combination of ATG/CSA and eltrombopag (16).
High-intensity AA management comprises immunosuppression with ATG/ciclosporin and allogeneic HSCT, but this is only suitable in fit patients with an available donor. Older patients experience more adverse events and higher mortality when undergoing treatment with both ATG and HSCT (6). Here, we would have preferred (but were unable) to administer a CD34+ donor-derived stem cell boost, as this has been shown to be effective in the allo-SCT and post-CAR-T space, leading to good neutrophil engraftment (17). Our experience of difficulty in obtaining an HSC boost for this patient is backed up by a recent 2023 survey from EHA and EBMT showing that HSC boosts are often unavailable (18).
Eltrombopag is an oral TPO mimetic. TPO regulates mature platelet production by binding the c-MPL receptor on megakaryocytes. Eltrombopag has therefore been approved for the treatment of immune thrombocytopenic purpura (ITP). C-MPL is also present on the cell surface of stem and progenitor cells and so recombinant thrombopoietin has been shown to additionally promote the expansion of haematopoietic stem cells (19). Eltrombopag has subsequently been found to be effective in both front-line AA in combination with ciclosporin as an ATG-sparing agent and in immunosuppression-refractory AA (20, 21).
Similarly to BM failure, retrospective studies and case reports suggest that eltrombopag for post-CAR-T haematotoxicity can lead to improvements in all blood lineages with minimal toxicity and transfusion independence. In one retrospective review of 71 cases at a single institution, TPO-mimetics allowed 10/11 treated patients to achieve platelet transfusion independence at a median of 17 days from treatment initiation (22). In a second larger analysis of 42 patients treated with eltrombopag for severe leukopenia and thrombocytopenia, recovery in trilineage haematopoiesis occurred by day 180 in 94% (23).
In this case, combinatorial eltrombopag and ciclosporin was a well-tolerated and highly effective alternative to ATG/allo-HSCT, resulting in a swift clinical response, discharge from hospital, and resolution of growth factor and transfusion dependency. This approach is particularly attractive in frail patients who may not tolerate more aggressive interventions.
Conclusion
The use of CAR-T therapies continues to grow exponentially for not only haematological malignancies but now potentially also in solid tumour and autoimmune conditions. We are faced with an expanding immunosuppressed (and often elderly and comorbid) population for whom the use of vaccination against COVID-19 to prevent serious infection is critically important. Whilst the incidence of AA following COVID-19 vaccination is very low overall, and a limitation of this report is that it is not possible to prove causality in our patient, for those patients affected by this complication, the consequences are life-threatening, and conventional therapies such as allo-HSCT and ATG may not be safe or feasible. Here we show that the combination of eltrombopag and ciclosporin potentially represents a valid alternative. Further research is needed to investigate the potential pathogenesis of COVID-19 vaccine-induced aplastic anaemia.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NM: Writing – original draft, Writing – review & editing. DS: Data curation, Writing – original draft, Writing – review & editing. PM: Supervision, Writing – original draft, Writing – review & editing. AC: Data curation, Writing – original draft, Writing – review & editing. MO: Supervision, Writing – original draft, Writing – review & editing. EP: Supervision, Writing – original draft, Writing – review & editing. CR: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rejeski K, Subklewe M, Aljurf M, Bachy E, Balduzzi A, Barba P, et al. Immune effector cell-associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood. (2023) 142:865–77. doi: 10.1182/blood.2023020578
2. Cordas dos Santos DM, Tix T, Rejeski K. Infections Drive Non-Relapse Mortality Following CAR-T Therapy across Disease Entities and CAR Products–a Meta-Analysis of Clinical Trials and Real-World Studies. Blood. (2023) 142:1064. doi: 10.1182/blood-2023-187516
3. Brudno JN, Natrakul D, Lam N, Dulau-Florea A, Yuan CM, Kochenderfer JN, et al. Acute and delayed cytopenias following CAR T-cell therapy: an investigation of risk factors and mechanisms. Leuk Lymphoma. (2022) 63:1849–60. doi: 10.1080/10428194.2022.2056172
4. Rejeski K, Jain MD, Shah NN, Perales MA, Subklewe M. Immune effector cell-associated haematotoxicity after CAR T-cell therapy: from mechanism to management. Lancet Haematol. (2024) 11:e459–470. doi: 10.1016/S2352-3026(24)00077-2
5. Tix T, Alhomoud M, Shouval R, Cliff ERS, Perales MA, Cordas Dos Santos DM, et al. Second primary malignancies after CAR T-cell therapy: A systematic review and meta-analysis of 5,517 lymphoma and myeloma patients. Clin Cancer Res. (2024) (20):4690–700. doi: 10.1158/1078-0432.CCR-24-1798
6. Killick SB, Bown N, Cavenagh J, Dokal I, Foukaneli T, Hill A, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. (2016) 172:187–207. doi: 10.1111/bjh.2016.172.issue-2
7. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S, et al. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. (2021) 384:2092–101. doi: 10.1056/NEJMoa2104840
8. Murdych TM. A case of severe autoimmune hemolytic anemia after a receipt of a first dose of SARS-CoV-2 vaccine. Int J Lab Hematol. (2022) 44:e10–2. doi: 10.1111/ijlh.13653
9. Paulsen FO, Schaefers C, Langer F, Frenzel C, Wenzel U, Hengel FE, et al. Immune thrombocytopenic purpura after vaccination with COVID-19 vaccine (ChAdOx1 nCov-19). Blood. (2021) 138:996–9. doi: 10.1182/blood.2021012790
10. Röth A, Bertram S, Schroeder T, Haverkamp T, Voigt S, Holtkamp C, et al. Acquired aplastic anemia following SARS-CoV-2 vaccination. Eur J Haematol. (2022) 109:186–94. doi: 10.1111/ejh.13788
11. Sridhara S, Nair R, Stanek M. Severe Aplastic Anemia After Receiving SARS-CoV-2 Moderna mRNA Vaccination. J Hematol. (2022) 11:34–9. doi: 10.14740/jh954
12. Pvs S, Koka H, Suvvari TK, Mounish Reddy R, Godavari ST, Thomas V, et al. An unusual case of acquired aplastic anemia following SARS-CoV-2 vaccination: A case report. ID cases. (2023) 33:e01826. doi: 10.1016/j.idcr.2023.e01826
13. Tabata S, Hosoi H, Murata S, Takeda S, Mushino T, Sonoki T, et al. Severe aplastic anemia after COVID-19 mRNA vaccination: Causality or coincidence? J Autoimmun. (2022) 126:102782. doi: 10.1016/j.jaut.2021.102782
14. Cecchi N, Giannotta JA, Barcellini W, Fattizzo B. A case of severe aplastic anaemia after SARS-CoV-2 vaccination. Br J Haematol. (2022) 196:1334–6. doi: 10.1111/bjh.v196.6
15. Chen CY, Chen TT, Hsieh CY, Lien MY, Yeh SP, Chen CC, et al. Case reports of management of aplastic anemia after COVID-19 vaccination: a single institute experience in Taiwan. Int J Hematol. (2023) 117:149–52. doi: 10.1007/s12185-022-03445-2
16. Babakhanlou R, Kadia T, Chien K, Thompson P. Aplastic anemia following the sars-cov-2 vaccine. HemaSphere. (2022) 6:701–2. doi: 10.1097/01.HS9.0000846112.16677.44
17. Mullanfiroze K, Lazareva A, Chu J, Williams L, Burridge S, Silva J, et al. CD34+-selected stem cell boost can safely improve cytopenias following CAR T-cell therapy. Blood Adv. (2022) 6:4715–18. doi: 10.1182/bloodadvances.2022007572
18. Rejeski K, Greco R, Onida F, Sánchez-Ortega I, Bonini C, Sureda A, et al. An international survey on grading, diagnosis, and management of immune effector cell-associated hematotoxicity (ICAHT) following CAR T-cell therapy on behalf of the EBMT and EHA. HemaSphere. (2023) 7:e889. doi: 10.1097/HS9.0000000000000889
19. Zeigler FC, de Sauvage F, Widmer HR, Keller GA, Donahue C, Schreiber RD, et al. In vitro megakaryocytopoietic and thrombopoietic activity of c-mpl ligand (TPO) on purified murine hematopoietic stem cells. Blood. (1994) 84:4045–52. doi: 10.1182/blood.V84.12.4045.bloodjournal84124045
20. Scheinberg P, Finelli C, Montaňo-Figueroa EH, Vallejo C, Norasetthada L, Calado RT, et al. Activity and safety of eltrombopag in combination with cyclosporin A as first-line treatment of adults with severe aplastic anaemia (SOAR): a phase 2, single-arm study. Lancet Haematology. (2024) 11:e206 –e215. doi: 10.1016/S2352-3026(23)00395-2
21. Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, et al. Eltrombopag Added to Standard Immunosuppression for Aplastic Anemia. N Engl J Med. (2017) 376:1540–50. doi: 10.1056/NEJMoa1613878
22. Drillet G, Lhomme F, De Guibert S, Manson G, Houot R. Prolonged thrombocytopenia after CAR T-cell therapy: the role of thrombopoietin receptor agonists. Blood Adv. (2023) 7:537–40. doi: 10.1182/bloodadvances.2022008066
Keywords: case report, COVID-19, CAR-T cell, aplastic anaemia, eltrombopag
Citation: Maciocia N, Spencer D, Maciocia P, Childerhouse A, O’Reilly M, Payne E and Roddie C (2024) Late onset pancytopenia post chimeric antigen receptor T cell therapy triggered by a COVID-19 vaccination: a case report. Front. Hematol. 3:1435584. doi: 10.3389/frhem.2024.1435584
Received: 20 May 2024; Accepted: 17 October 2024;
Published: 15 November 2024.
Edited by:
Andrew J. Innes, Imperial College London, United KingdomReviewed by:
Sarita Jaiswal, Narayana Health, IndiaKristina Hölig, University Hospital Dresden, Germany
Copyright © 2024 Maciocia, Spencer, Maciocia, Childerhouse, O’Reilly, Payne and Roddie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Maciocia, bi5tYWNpb2NpYUB1Y2wuYWMudWs=
†These authors share first authorship
 Nicola Maciocia
Nicola Maciocia Dean Spencer1†
Dean Spencer1† Paul Maciocia
Paul Maciocia Elspeth Payne
Elspeth Payne Claire Roddie
Claire Roddie