- 1Hematology and Bone Marrow Transplantation Unit, Azienda Ospedaliero-Universitaria Policlinico S. Marco, Catania, Italy
- 2Postgraduate School of Hematology, University of Catania, Catania, Italy
- 3Hematology Unit, ARNAS Garibaldi, Catania, Italy
- 4Hematology Unit, Azienda Ospedaliera Papardo, Messina, Italy
- 5Hematology Unit, Ospedale San Vincenzo, Messina, Italy
Background: Waldenström macroglobulinemia (WM) is a rare and indolent B-cell lymphoproliferative disorder with greater incidence in elderly patients where a precise algorithm of initial therapy is still not clear. Immunochemotherapy regimen consisting of dexamethasone, rituximab, and oral cyclophosphamide (DRC) is considered a suitable first-line treatment because of its safety, efficacy, and manageability.
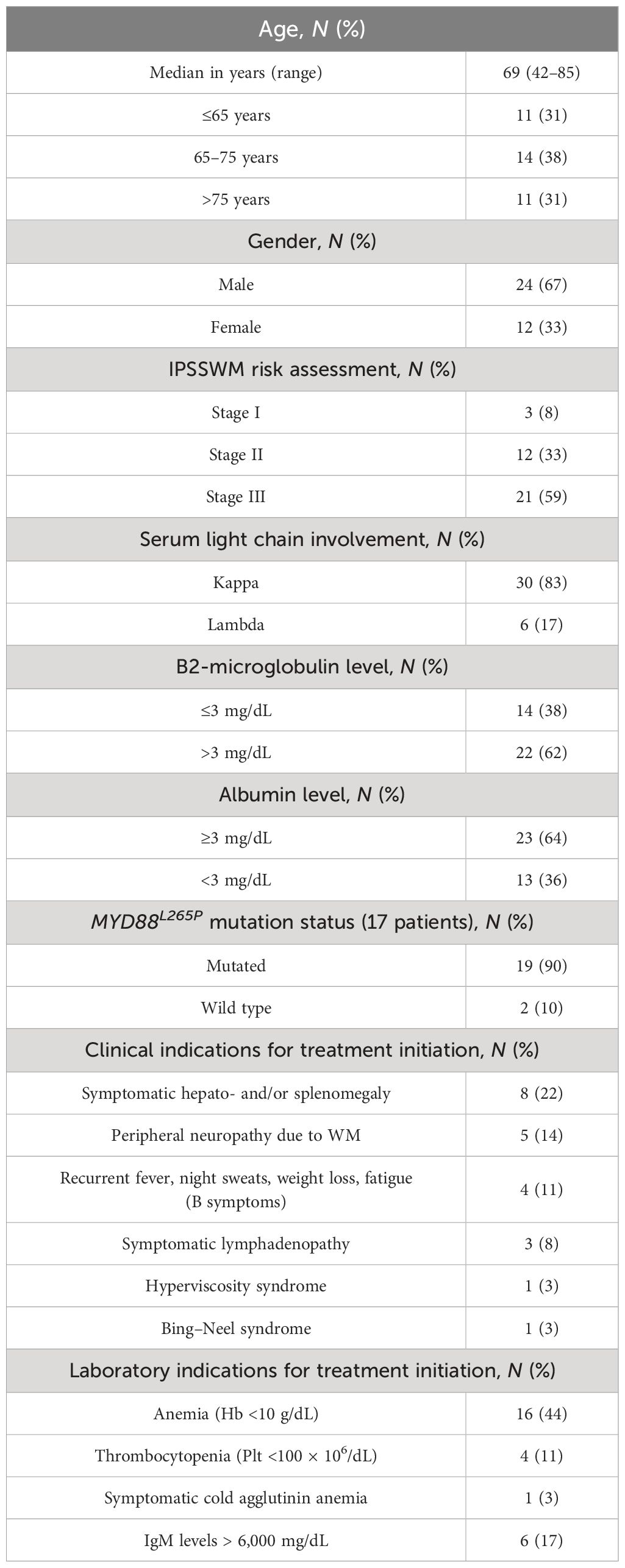
Patients and methods: We retrospectively describe the results of 36 consecutive treatment-naïve patients with WM who were treated from June 2013 until June 2021 with the DRC regimen every 4 weeks instead of 3 weeks, for six cycles. The median age was 69 years (range, 42–85 years), with one-third being older than 75 years. Most patients had features of advanced disease, with nearly 60% being high risk. Median IgM level prior to treatment initiation was 2.9 g/dL.
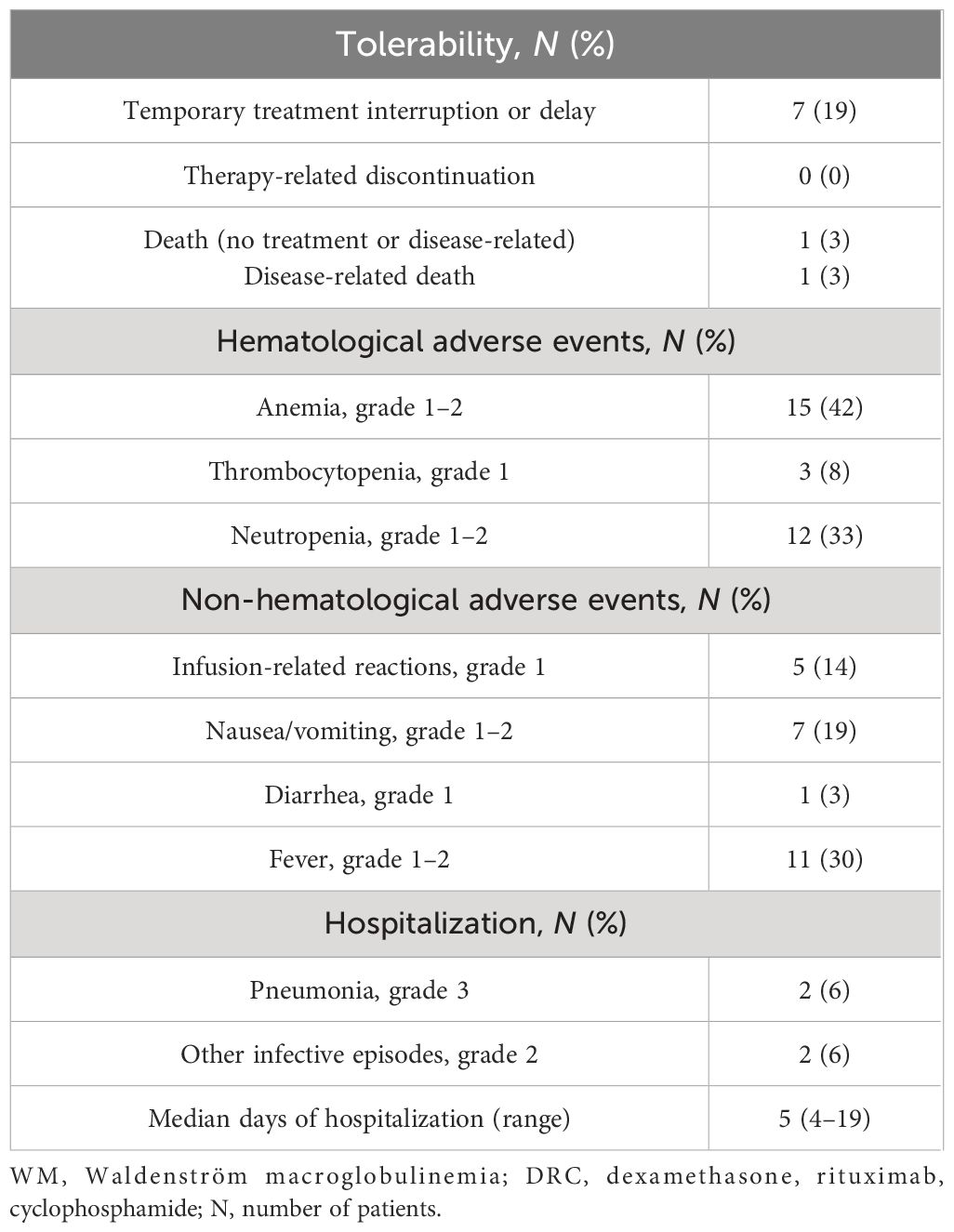
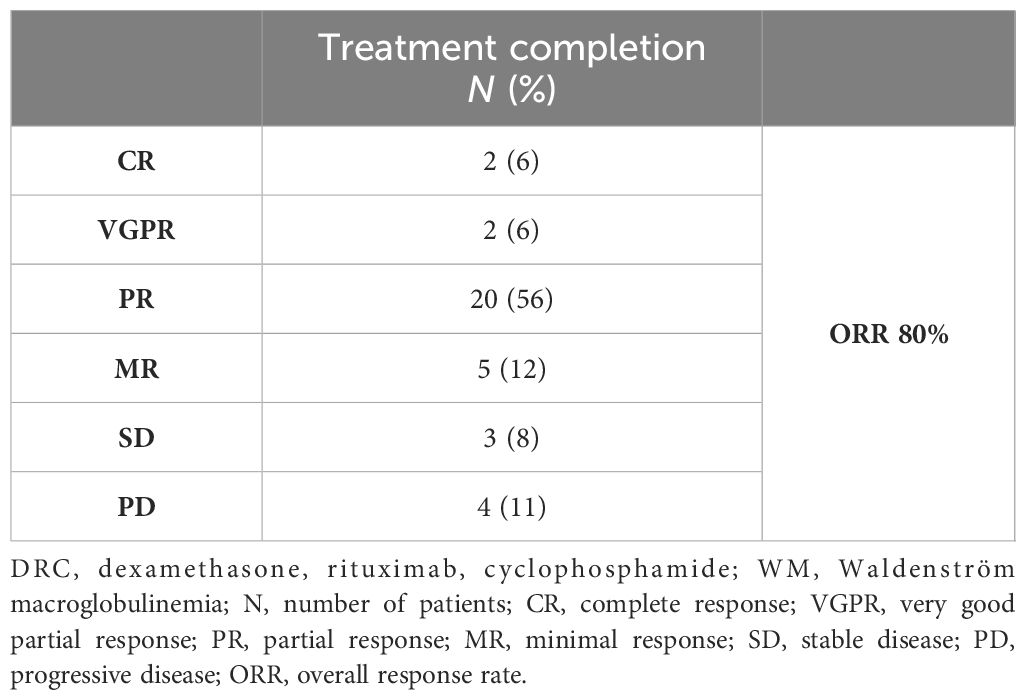
Results: Overall response rate was 80% after a median time of two cycles, with 67% of patients achieving at least partial response. After a median follow-up of 59 months, the median overall survival (OS) was not reached and the median time to next treatment (TTNT) was 48 months (95% CI 25–87 months). Approximately 70% of the evaluable study population had a 3-year survival without additional treatment, while 75% had a 3-year OS rate. The treatment was well-tolerated with only two patients (6%) recorded to have grade 3 pneumonia and no grade 3 hematological toxicity maybe due to the regular use of growth factors for red and white blood cells. Baseline albumin level and achievement of at least minimal or partial response had a significant impact on TTNT, while baseline hemoglobin and IgA level affected outcome in terms of OS (p < 0.05).
Conclusion: This is the first real-life experience describing the use of the DRC regimen in treatment-naive patients with WM with administration of therapy every 4 weeks instead of 3 weeks showing apparent comparable efficacy, along with good tolerability and safety, especially in terms of hematological toxicity, independently from comorbidity burden.
Introduction
Waldenström macroglobulinemia (WM) is a rare indolent B-cell lymphoproliferative disorder, accounting for 1%–2% of hematologic malignancies, that generally develops in elderly patients aged 65 years or above with concomitant comorbidities. The diagnosis is based on the identification of bone marrow infiltration by lymphoplasmacytic cells that produce any amount of monoclonal immunoglobulin M (IgM) protein (1, 2). Mutation of MYD88L265P in bone marrow is present in more than 90% of patients and represents a useful tool in differential diagnosis. Furthermore, its usefulness in disease prognosis, together with C-X-C chemokine receptor type 4 (CXCR4) mutation, present in 30% of patients with WM, has been demonstrated previously (3). Clinical manifestations of WM are heterogeneous and can be related to IgM gammopathy causing hyperviscosity syndrome, neuropathy, cold agglutinin or cryoglobulin hemolytic anemia, bone marrow infiltration by lymphoplasmacytic cells causing anemia, thrombocytopenia, and neutropenia. Furthermore, extramedullary tissue infiltration is presented with lymphadenopathy, hepatomegaly, and splenomegaly. Commonly known systemic B symptoms such as night sweats, fever, itching, and weight loss can also be present at diagnosis. Other extramedullary infiltrations, such as kidney, gastrointestinal tract, and skin, are present in less than 5% of patients, while central nervous system localization is positive in approximately 1% of WM (4). However, approximately 25% of patients are asymptomatic at the time of the diagnosis, and these patients should not be treated, but followed every 3–6 months, since treatment is recommended only in symptomatic disease (5).
To date, immunochemotherapy is considered the standard of care for patients with WM in up-front treatment management, especially in specific clinical indications, such as cytopenias or hyperviscosity, based on long-term experience and disease presentation. The Eighth International Workshop on WM recommended using rituximab-based combinations as first-line treatment. One of the recommended first-line regimens is DRC, which consists of rituximab, cyclophosphamide, and dexamethasone every 3 weeks, for a total of six cycles (6), especially in patients with clinically relevant cytopenias from bone marrow infiltrations and/or IgM-related neuropathies (7). Another rituximab-based regimen consists of bendamustine (BR) (8), for a total of four to six cycles, indicated in patients with bulky disease and high tumor burden, according to ESMO (7). The proteasome inhibitor bortezomib in combination with rituximab (VR) (9) and dexamethasone (BDR) (10) is highly considered for patients with very high IgM levels and/or symptoms of hyperviscosity (7). Finally, Bruton’s tyrosine kinase (BTK) inhibitors represent a first-line treatment option for elderly unfit patients (7, 11). However, a precise algorithm of therapy is still missing due to the lack of comparative trials among regimens.
The DRC regimen has been investigated so far by only three prospective multicenter studies (6, 12, 13). This combination was designed on the evidence of rituximab and cyclophosphamide efficacy and minimal toxicity in patients with WM, along with the effect of dexamethasone, which increases the sensitivity of rituximab-mediated cytotoxicity. More than 80% of patients, with a median of 68 years, achieved at least minimal response and more than 50% of patients had 2-year progression-free survival between the two studies. Toxicity was mainly hematological with approximately 10%–20% of patients with grade 3 or worse neutropenia being the most frequent one, while infectious episodes grade 3 or worse were evidenced in 3%–10% of the study population.
Given the rarity of the disorder and the growing number of treatments, in the absence of high-quality recommendations, real-life studies are essential in improving the management of WM in everyday practice. Therefore, a multicenter data collection of all patients with WM in follow-up was performed, as part of Sicilian Myeloma Network (SMN) association. Here, we describe the results of a long-term follow-up in a cohort of 36 previously untreated symptomatic patients with WM, who received the DRC regimen every 4 weeks instead of 3 weeks as first-line therapy outside of clinical trials.
Patients and methods
Patient selection
Medical records of 79 patients diagnosed with WM and followed from January 1999 until September 2023 at four Sicilian centers, as part of the SMN, were screened for the purpose of retrospective study enrollment (Figure 1). In accordance with our clinical practice, the diagnosis of WM was based on the detection of monoclonal IgM in the serum by serum protein electrophoresis and immunofixation and histopathological confirmation of bone marrow infiltration by lymphoplasmacytic cells and monoclonal plasmacells. Among the evaluated patients, a total of 36 previously untreated patients received the DRC combination from June 2013 until June 2021.
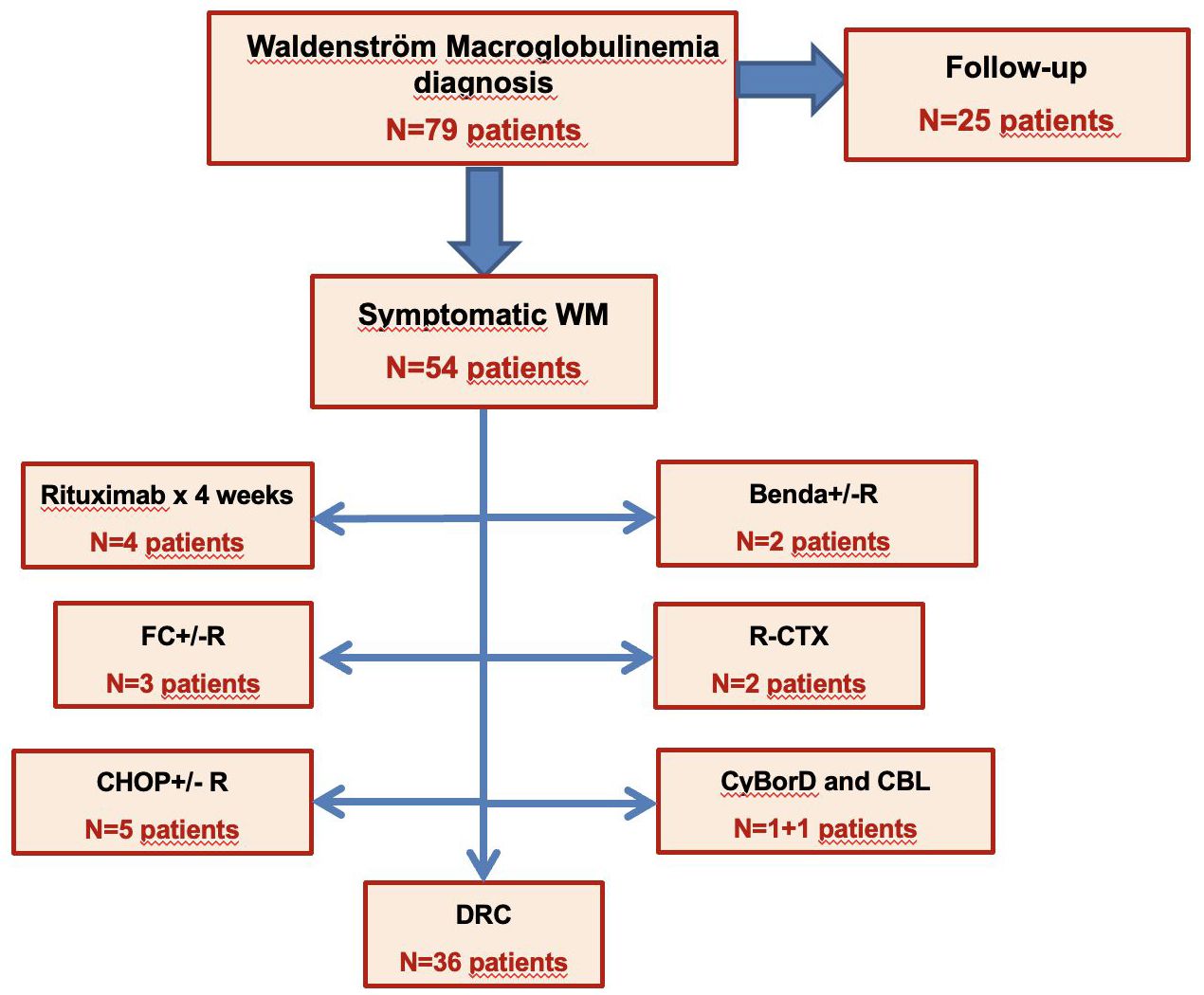
Figure 1 Screening of 79 treatment-naïve patients with WM based on the first line of treatment. WM, Waldenström macroglobulinemia; FC+/-R, fludarabine, cyclophosphamide +/- rituximab; CHOP+/-R, cyclophosphamide, doxorubicine, vincristine, prednisone +/- rituximab; Benda+/-R, bendamustine +/- rituximab; R-CTX, rituximab, cyclophosphamide; CyBorD, cyclophosphamide, bortezomib, dexamethasone; CBL, cholorambucile; DRC, dexamethasone, rituximab, cyclophosphamide.
The following factors were evaluated at the time of diagnosis: age, sex, cell blood count test, level of serum M-protein, type of light chain, concentration of uninvolved immunoglobulins, serum albumin, serum β2-microglobulin, International prognostic scoring system for Waldenström macroglobulinemia (IPSSWM) (14), onset symptoms, percentage of bone marrow involvement, and MYD88 status when possible. CXCR4 and TP53 mutations were not tested. Patient’s comorbidity status was calculated by using both Cumulative Illness Rating Scale-Geriatric (CIRS-G) (15) and Charlson Comorbidity Index (CCI) (16) excluding lymphoproliferative neoplasm diagnosis. Follow-up data included the review of the patients’ inpatient and outpatient medical records. At least one of the following criteria for initiation of treatment was required: presence of “B” symptoms (fever, night sweat, and weight loss), bulky disease (lymphadenopathy greater than 5 cm), and/or symptomatic lymphadenopathy, splenomegaly or hepatomegaly, hyperviscosity syndrome, peripheral neuropathy, cryoglobulinemia, cold agglutinin anemia, hemoglobin less than 10 g/dL, or platelet count less than 100 × 106/dL.
The study was approved by an independent ethics committee of the coordinating center (Policlinico Catania 1, n.34/2019/PO) and conducted in accordance with International Conference on Harmonization Guidelines on Good Clinical Practice and the principles of the Declaration of Helsinki. All patients provided written informed consent.
The objective of this study was to evaluate overall response rate (ORR), along with measurement of best response rate, time to next treatment (TTNT), overall survival (OS), and safety of the treatment regimen.
Procedures and drug administration
All patients received DRC regimen every 4 weeks, instead of 3 weeks in order to reduce drug-related toxicity and increase patient compliance, for six cycles at the following dosage: dexamethasone 20 mg intravenously (i.v.), followed by rituximab 375 mg/m2 i.v. on day 1 and cyclophosphamide 100 mg/m2 orally twice daily on days 1 to 5 for a total dose of 1,000 mg/m2.
Rituximab was administrated in sodium chloride 0.9% solution. The first infusion was initiated at 50 mg/h, and if tolerated, the rate was increased at 50 mg/h every 30 min to a maximum of 400 mg/h. Subsequent infusions were initiated at 100 mg/h and, if tolerated, increased at 100 mg/h increments every 30 min to a maximum of 400 mg/h. Premedication consisted of paracetamol 1 g i.v. 1 h prior to rituximab infusion, chlorphenamine 10 mg i.v. bolus 15 min prior to rituximab infusion, and dexamethasone 20 mg i.v. 30 min prior to rituximab (as part of the DRC regimen). Cyclophosphamide was available in 50-mg tablets; doses were rounded to the nearest 50 mg. Administration of rituximab was delayed to the second cycle of treatment in patients with high IgM levels (IgM > 5,000 mg/dL); therefore, no patient underwent plasmapheresis to prevent hyperviscosity symptoms due to IgM flare.
Concomitant medications
Antibiotic and antiviral prophylaxis was carried out with trimethoprim and sulfamethoxazole (800 mg twice a day, twice a week) and acyclovir 200 or 400 mg daily according to the policy of each center. Supportive therapy with erythropoietin (EPO) and granulocyte colony-stimulating factor (G-CSF) was administered accordingly to ASH/ASCO guidelines and the policy of each single center, involving the administration of G-CSF in case of moderate or severe neutropenia (17, 18), even in the absence of symptoms of infection. In addition, patients evaluated in this study were candidates for annual vaccination against influenza and pneumococcal pneumonia. From March 2021, both doses of anti-COVID-19 mRNA vaccination were given to all patients, mostly Pfizer vaccine (Comirnaty), and then also a third dose.
Efficacy and safety assessment
Each patient’s medical history was recorded on day 1 of each cycle. Physical examinations were conducted, and blood was collected for hematology, renal, and liver function tests at each cycle on day 1 and whenever it was considered necessary.
Efficacy assessment was recorded on day 1 of cycle 2 and every cycle thereafter. The response to the treatment was evaluated according to the criteria established by the VIth International WM Workshop. The response evaluation included complete remission [CR, 100% reduction of serum monoclonal IgM protein according to electrophoresis, with negative serum immunofixation and bone marrow biopsy, with complete resolution of extramedullary disease (lymphadenopathy and splenomegaly)], very good partial remission (VGPR, ≥90% reduction in serum M protein and complete resolution of extramedullary disease), partial remission (PR, 50%–90% reduction in serum M protein, and reduction of extramedullary disease), minor response (MR, 25%–50% reduction in serum M protein and reduction of extramedullary disease), stable disease (SD, <25% reduction and <25% increase in serum M protein), and progressive disease (PD, ≥25% increase in serum M protein) (1).
Adverse events during the DRC regimen were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCICTC) criteria (CTCAE) (19). IgM flare was defined as an increase in serum IgM level after immunochemotherapy initiation and prior to the second cycle of treatment administration, non-associated to disease progression.
Statistical analysis
Descriptive statistics were generated for analysis of results, and two-sided p-values under 0.05 were considered significant. Qualitative results were summarized in counts and percentages. ORR was defined as MR or better (CR + VGPR + PR + MR). The correlation of clinical variables with various parameters was assessed with Pearson’s chi-square test (or Fisher’s exact test) for categorical parameters and with Mann–Whitney or Wilcoxon signed rank test for continuous parameters. Receiver operating characteristic (ROC) curve was used for CCI and CIRS-G in order to evidence cutoff values for both comorbidity scores. Association between treatment interruption or hospitalization and comorbidity scores was evaluated with odds ratio (OR).
TTNT was calculated from the start of the first-line treatment to the start of the second-line treatment or the date the patient was last known to be alive. OS was calculated from treatment initiation until the date of death for any reason or the date the patient was last known to be alive. TTNT and OS were analyzed by Kaplan–Meier test. Standard errors were calculated by the method of Greenwood; the 95% confidence intervals (CIs) are computed as 1.96 times the standard error in each direction. The Cox proportional hazards model was used to evaluate prognostic markers for TTNT and OS. All calculations were performed using IBM SPSS Statistics v.29.0.2.0, IBM Corp, and MedCalc version 12.30.0.0 (Producer: MedCalc Software bvba, Ostend, Belgium.
Results
Patient characteristics
This survey included 36 patients treated with the DRC regimen outside of the clinical trial every 4 weeks instead of 3 weeks, from June 2013 until June 2021, and evaluated according to an intention-to-treat analysis. The baseline demographics of treatment-naive patients who received DRC are reported in Table 1. The median follow-up was 59 months (range, 3–177 months) and the median age of the patients prior to treatment start was 69 years (range, 42–85 years), with 67% of patients with WM being male. The median CIRS-G comorbidity score in our study population was 5 (range, 0–15), while the median CCI was 3 (range, 0–7).
The light chain type was kappa in most of the patients (83%) and the median percentage of bone marrow involvement was 63% (range, 10%–100%). Patients had features of advanced disease such as anemia with hemoglobin level below 11.5 g/dL in nearly 3/4 of the study population and platelet count below 150 × 109/dL in 9 patients and leukopenia in 3 patients, respectively. According to the IPSSWM, 21 patients (59%) were classified as stage III. Clinical and laboratory indications to treatment are described in Table 1. MYD88L265P mutation status was evaluated in 58% of DRC-treated patients with 90% resulting positive. Median IgM level prior to treatment initiation was 2.9 g/dL (range, 0.34–17.16 g/dL), while a total of 18 (50%) and 16 patients (49%) had IgG and IgA levels below the lower limit of normal, lower than 700 mg/dL and 70 mg/dL, respectively. The combined deficit of both IgG and IgA values was found in 12 patients (34%).
Safety
The immunochemotherapy DRC regimen was relatively well tolerated (Table 2). The most common hematological adverse events were anemia grade 1–2 in nearly half of the study population and neutropenia in one-third, while thrombocytopenia grade 1 was present in only three patients. There were no grade 3 or 4 hematological adverse events, while all patients with low-grade toxicity were managed with EPO and G-CSF supportive treatment: EPO was prescribed for values <10 g/dL with the intention of maintaining Hb values between 11 and 12 g/dL, while G-CSF was prescribed for neutrophil counts lower than 1,000/mmc even in the absence of signs of infection. Among non-hematological adverse events, nausea, vomiting, and fever were more frequently present. Rituximab infusion-related reactions were of grade 1, such as chills, fever, hypotension, and headache in a limited number of patients.
The treatment was temporarily interrupted and postponed in seven patients. Infusion-related reactions and subsequent patient choice were the reason in three cases, whereas in four patients, other complications independent from treatment determined treatment delay, mainly due to infection-related hospitalizations. In fact, two patients experienced dental infections, but they had both a personal history of dental disease, while two other patients were hospitalized for grade 3 pneumonia and chronic obstructive pulmonary disease (COPD) flare, with a median hospitalization time of 5 days (range, 4–6). Two patients passed away in the course of treatment, one due to disease progression, while the second patient died 1 month after starting due to critical conditions related to pre-existing chronic cardiac failure.
Efficacy
Patients were evaluated after every cycle until completion of six DRC cycles. Apart from the abovementioned patient who died from cardiac failure, and three patients who had disease progression in the course of treatment, all other patients completed the scheduled therapy. On an-intent-to-treat basis, ORR was 80%, with 4 patients achieving deep response (VGPR or better), 20 patients having PR, and 5 patients achieving MR. On the other hand, SD was present in three patients, while four patients suffered PD, one of them having extramedullary disease relapse (Table 3).
Approximately half of the 29 responding patients obtained at least MR after two cycles and PR after four cycles, respectively. Five patients had improved response after treatment completion; three patients achieved PR subsequently, from MR and SD in two and one patient, respectively. Furthermore, in two patients, CR was obtained from PR and VGPR in one patient each. Approximately 36% of patients experienced IgM flare not associated to disease progression after one cycle of treatment with a median duration of 2 months. However, IgM flare did not have any clinical consequences.
With a median follow-up of 59 months, the median TTNT was 48 months (95% CI 25–87 months) and median OS was not reached, as described in Figure 2. Approximately 70% of the evaluable study population, 26 patients in total, had 3-year survival without additional treatment. As for the OS rate at 36 months out of 22 evaluable patients, approximately 75% remained alive, respectively. Five patients passed away after the initiation of the DCR regimen, the abovementioned patient due to cardiac failure and another patient due to disease progression in the course of treatment, while three patients died in response to causes unrelated to both disease and treatment.
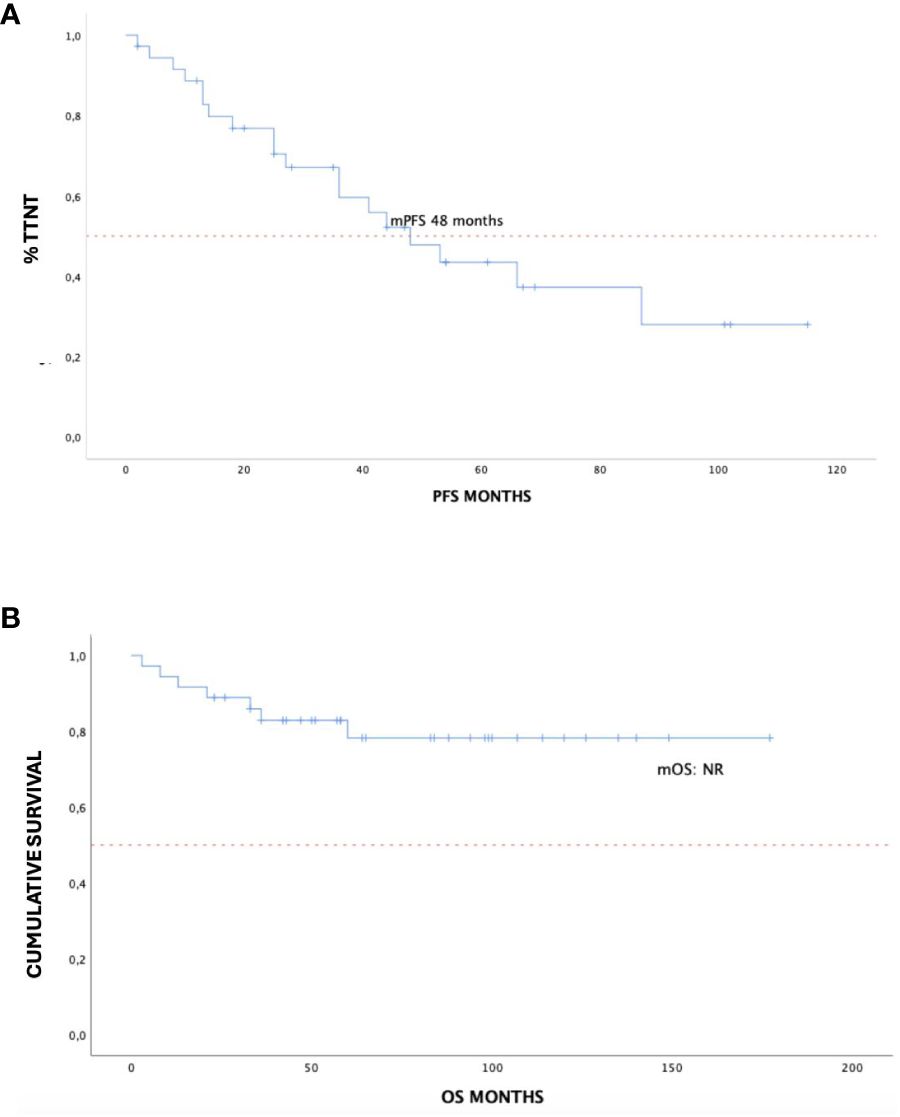
Figure 2 Time to next treatment (A) and overall survival (B) in 36 treatment-naïve patients with WM treated with DRC outside of clinical trials. WM, Waldenström macroglobulinemia; DRC, dexamethasone, rituximab, cyclophosphamide.
Predictors of response
Patient and disease characteristics were evaluated with Kaplan–Meier univariate analysis in terms of both OS and TTNT and are reported in Table 4. ROC analysis evidenced CIRS-G score greater than four and CCI score greater than two as cutoff values, although without statistical significance. In terms of response evaluation, both the achievement of at least PR and MR determined improved outcome in terms of TTNT with p < 0.0001, and hazard ratio (HR) of 0.06 [95% CI 0.016–0.23] and 0.03 [95% CI 0.0005–0.14], respectively (Figures 3A, B). Baseline albumin level lower than 3 g/dL also had a statistically significant impact on TTNT, p = 0.02 [HR 0.16, 95% CI 0.03–0.76] (Figure 3C).
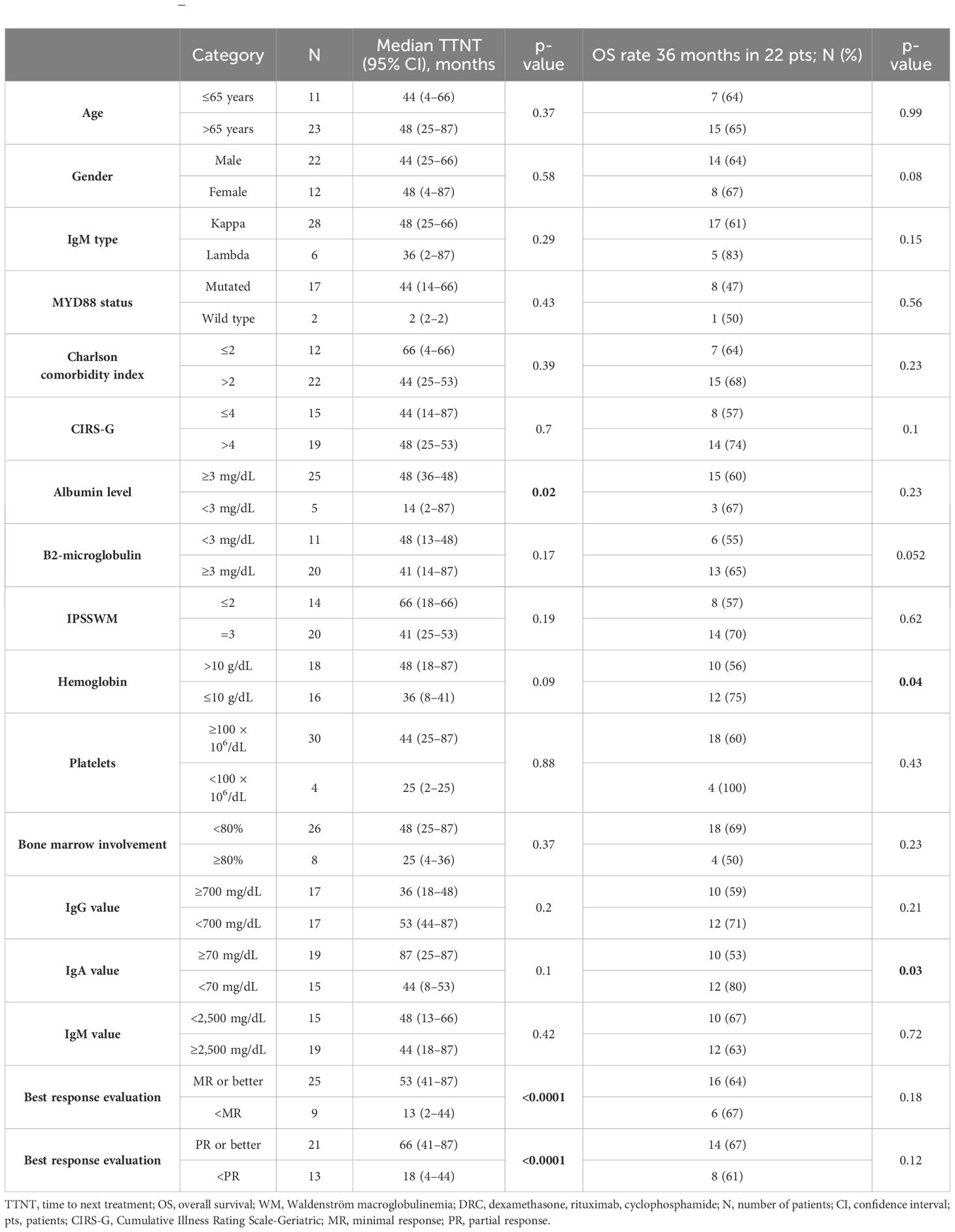
Table 4 Univariate Kaplan–Meier analysis of TTNT and OS in 36 patients with WM treated with DRC regimen.
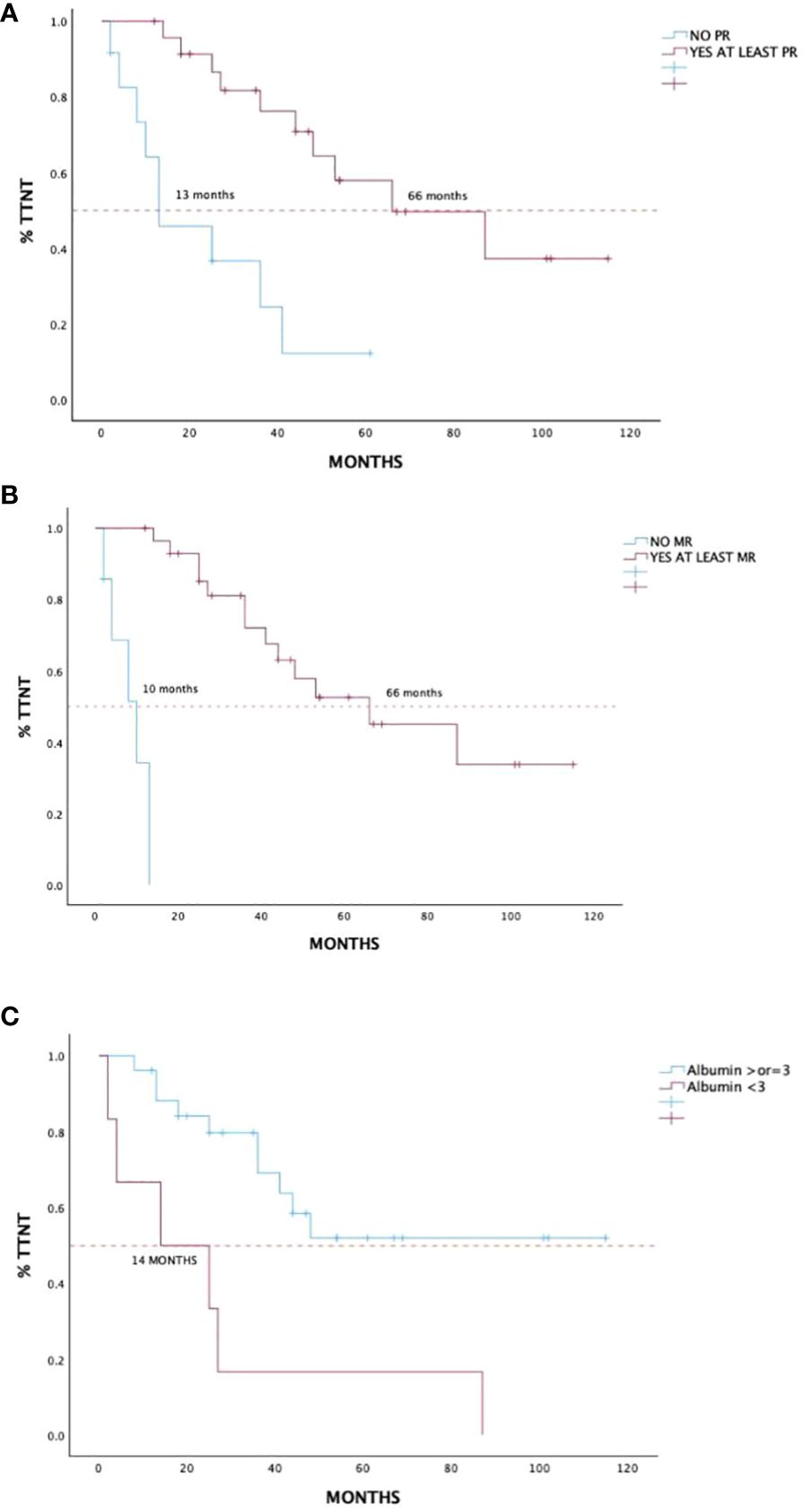
Figure 3 Time to next treatment in 36 treatment-naïve patients with WM treated with DRC outside of clinical trials according to achievement of partial response or better (A), minimal response or better (B), and baseline albumin level (C). WM, Waldenström macroglobulinemia; DRC, dexamethasone, rituximab, cyclophosphamide.
On the other hand, hemoglobin below 10 g/dL and IgA lower than 70 mg/dL prior to treatment initiation had a negative impact on OS, p = 0.04 [HR 0.2, 95% CI 0.05–0.91] and p = 0.03 [HR 0.18, 95% CI 0.04–0.81], respectively (Figures 4A, B). Comorbidity score in terms of CCI and CIRS-G had no impact on outcome in both TTNT and OS. Furthermore, OR analysis found no association between treatment interruption or hospitalization and CCI and CIRS-G (p > 0.05).
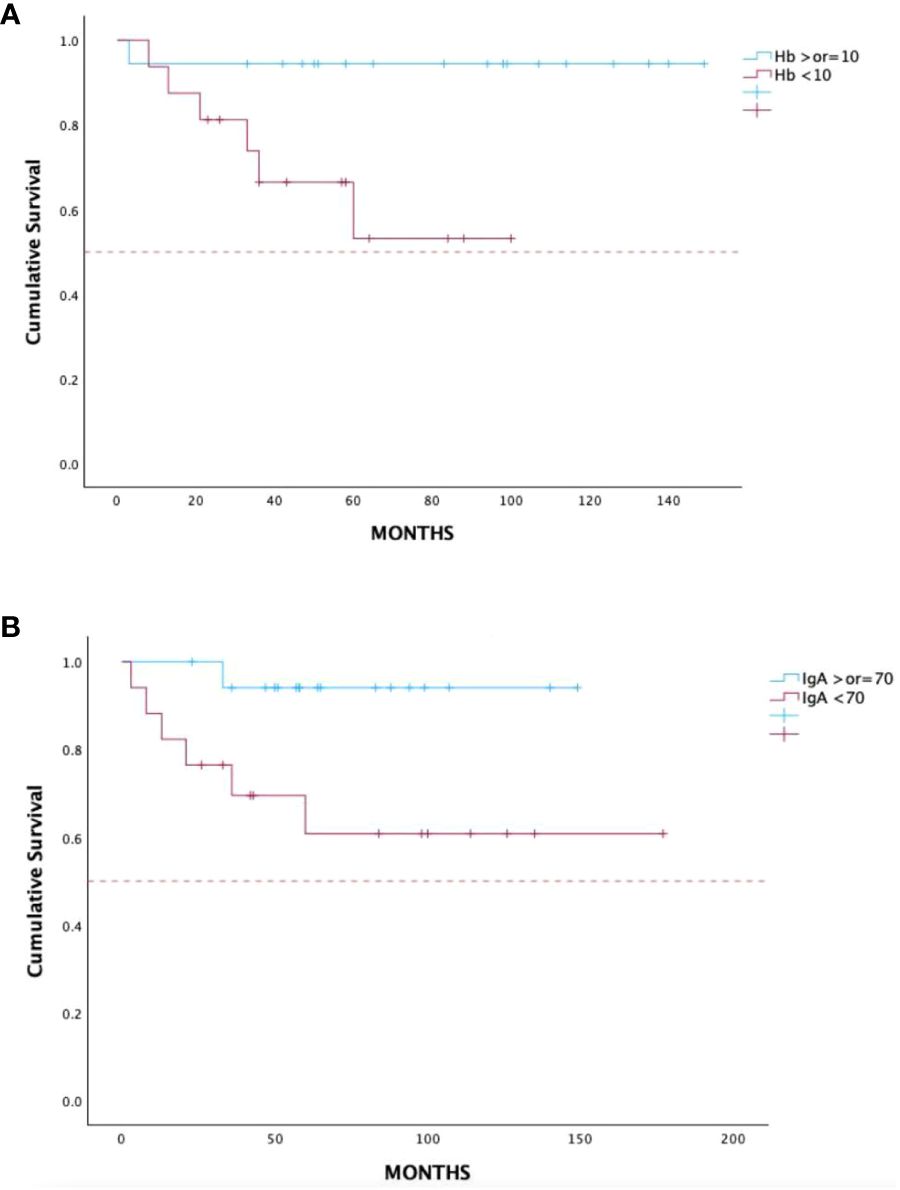
Figure 4 Overall survival in 36 treatment-naïve patients with WM treated with DRC outside of clinical trials according to baseline hemoglobin (A) and IgA levels (B). WM, Waldenström macroglobulinemia; DRC, dexamethasone, rituximab, cyclophosphamide.
Cox proportional hazards model was used to evaluate statistically significant parameters from univariate analysis. Baseline albumin level and PR or better confirmed their significance in terms of TTNT, with p = 0.01 [HR 7.5, 95% CI 1.62–34.8] and p = 0.02 [HR 24.1, 95% CI 1.67–344.7], while MR or better response failed to do so as well (p > 0.05). On the other hand, neither hemoglobin value nor IgA level remained statistically significant in terms of OS (p > 0.05).
Further treatment lines and patient outcome
Out of 19 patients with disease progression following the DRC regimen with indications to therapy, 16 underwent second-line treatment. Three patients suffered from lymphoma transformation, diffuse large B-cell lymphoma, hairy cell lymphoma, and cutaneous T-cell lymphoma. Twelve patients were treated with ibrutinib, including the patient with CNS involvement; in two patients, a bendamustine–rituximab regimen was used, while rituximab once a week for 4 weeks and prednisone–cyclophosphamide oral therapy were used in one patient each. We recorded no secondary neoplasms. ORR was 86%, with deep response achieved in three patients (CR in two patients and VGPR in one patient), while half of the population had PR. Minimal response was present in two patients, while in one patient, stable disease remained in the course of treatment.
Median TTNT after second line (TTNT 2) was 46 months (95% CI 41–46 months); five out of six patients remained in response after 36 months of treatment, while out of three patients who suffered from PD, one passed away due to disease progression. As for the rest, one patient developed multiple myeloma with amyloidosis IgM lambda and was subsequently treated with daratumumab-bortezomib-dexamethasone, achieving PR and still remains in response after 18 months of treatment. The third patient was treated with ibrutinib, passing away after 4 months in the absence of response.
Discussion
Because of its rarity and limited number of randomized clinical trials, a precise algorithm for the treatment of WM is still missing. To date, immunochemotherapy is still considered the standard of care for patients with WM. In particular, rituximab-based combinations are the most widely used in the management of both newly diagnosed and relapsed/refractory WM disease. The DRC regimen was designed on the basis of well-known combined efficacy of rituximab and alkylating agents, particularly cyclophosphamide (20, 21), along with a dexamethasone synergistic effect that increases the sensitivity of the malignant WM cells to the rituximab-mediated cytotoxicity (22). Furthermore, both myelosuppression level and therapy-related toxicity were tolerable, given the advanced patient’s age and concomitant comorbidities. Even though the DRC regimen has been considered a suitable choice for first-line treatment (7), to date, there are only three studies that describe its use in previously untreated patients (6, 12, 13).
The first study was a multicenter trial, conducted in Greece by Dimopoulos and colleagues between 2002 and 2007, in which 72 patients were enrolled. The ORR was 83%, and the reported toxicity was low, with 9% of grade 3 or 4 adverse reactions (6). Final study analysis after a median follow-up of 8 years revealed a median TTNT of 51 months and a median OS of 95 months (23).
The second one was a multicenter retrospective study, conducted in USA by Paludo and colleagues between 2007 and 2014. The aim of this study was to prove the efficacy of the DRC combination in both untreated and relapsed/refractory (R/R) patients. Therefore, a total of 100 patients were enrolled, 50 were treatment-naive and 50 were R/R. In previously untreated patients the ORR was 96%, while the median TTNT was not reached after a median follow-up of 51 months with a 4-year TTNT of 67%. Adverse reactions of grade 3 or 4 were reported in 30% of the patients, in particular neutropenia (20%), thrombocytopenia (7%), and infections (3%) (12).
Finally, data from prospectively randomized multicenter European phase II trial, presented as abstract, described outcome in 204 treatment-naïve patients with WM treated with either the DRC regimen or bortezomib in combination with DRC (B-DRC). ORR in the DRC group was 87%, and after a median follow-up of 27.5 months, median progression-free survival was 50.1 months, while OS was not reached (13).
In this multicenter study, we retrospectively examined a cohort of 79 patients with WM, followed at four different Sicilian Hematology Centers, members of SMN. After preliminary cohort evaluation, a total of 36 consecutive patients who underwent first-line DRC treatment regimen was examined (Figure 1). Treatment selection was based on case-to-case evaluation in each center, choosing the DRC regimen for patients without bulky disease. Our initial experience revealed frequent treatment delay in a significant proportion of patients, given the advanced age and slow hematological recovery, together with significant comorbidity burden measured in terms of both CIRS-G and CCI scale. Therefore, the regimen was administered every 4 weeks, instead of 3 weeks, in order to reduce drug-related toxicity and increase patient compliance, hopefully without reducing the efficacy.
In accordance with the results from the abovementioned prospective trials, our data showed that the DRC combination is an active treatment for symptomatic treatment-naïve patients with WM, even with a 4-week schedule treatment. In our cohort, the ORR was 80%, with four patients in deep response, while PR and MR were present in 56% and 12%, respectively, and in three patients, SD was maintained (Table 3). Furthermore, after a median follow-up of 59 months, the median OS was not reached and the median TTNT for the entire cohort was 48 months, comparable to the prospective studies. The results of the studies including the DRC regimen in a treatment-naïve setting is summarized in Table 5.
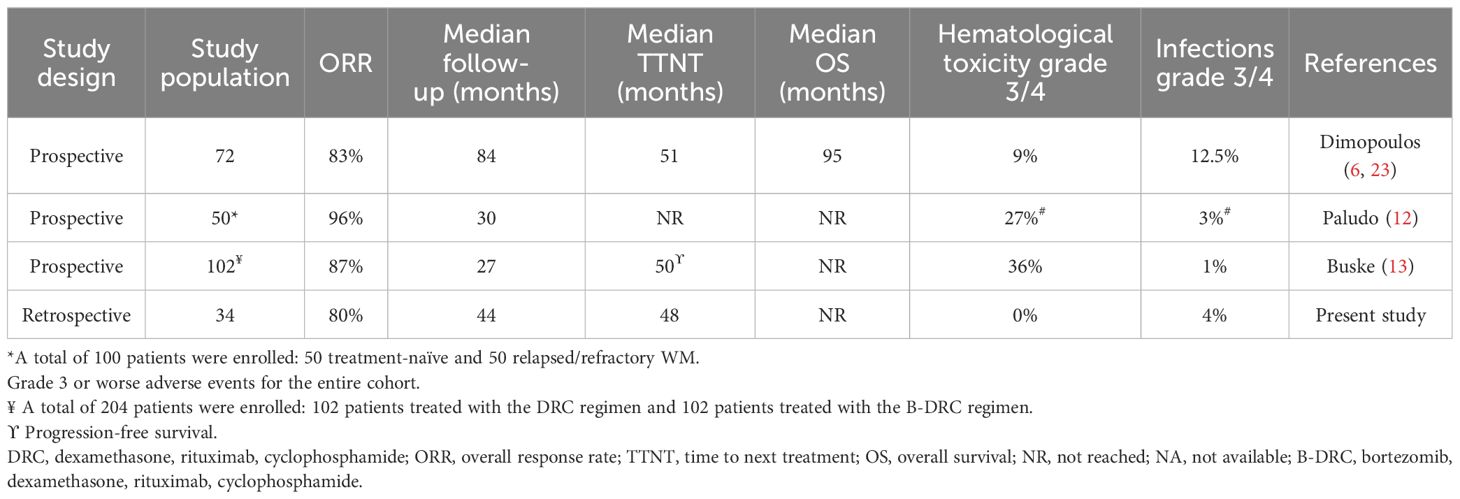
Table 5 Evaluation of the DRC regimen in treatment-naïve patients with WM in clinical trials and real-world practice.
Even if a comparison between clinical trials and our study is not applicable, interestingly our data showed an apparent non-inferior tolerability and safety of the DRC regimen, especially in terms of hematological adverse events grade 3 or higher (Table 5). This could be related to the different timing between cycles (6, 12, 13). Furthermore, in our cohort of patients, G-CSF was administered as prophylactic therapy in patients who experienced grade 1–2 neutropenia to prevent infections, as described by our previous experience (24–27), and EPO was prescribed for Hb values < 10 g/dL, thus reducing fatigue and asthenia. Finally, all patients were advised to receive annual vaccination against influenza and pneumococcal pneumonia based on each center’s policy. Two patients alone had grade 3 pneumonia, needing i.v. antibiotics and in-patient hospitalization, resolved without sequela and with prompt treatment resumption, comparable to other studies. There were no patients that discontinued treatment permanently due to adverse events; two patients passed away in the course of treatment, one due to disease progression, while the second patient died 1 month after starting due to critical conditions related to cardiac failure. Lastly, four patients suffered from disease transformation following DRC. Two patients switched to indolent types: hairy cell leukemia and cutaneous CD30+ T-cell lymphoma; one developed high-grade diffuse large B-cell lymphoma, while the last one suffered from IgM lambda multiple myeloma together with amyloidosis. Currently, all four patients are alive and in course of treatment.
Finally, univariate statistical analysis suggested that baseline albumin levels together with achievement of at least MR and PR in the course of therapy had a positive impact in terms of TTNT (Figure 3). Cox proportional hazard analysis confirmed a favorable outcome in patients based on baseline albumin levels (p = 0.01) and achievement of at least PR (p = 0.02), independently from the timing of treatment response (data not shown). On the other hand, baseline hemoglobin below 10 g/dL and IgA level below 70 mg/dL had a negative impact in terms of OS (Figure 4), although Cox analysis failed to confirm their statistical significance. In any case, these findings are in accordance with the literature, given that some of these factors are well-known indicators of aggressive phenotype and poorer outcome and are considered for the risk stratification in the ISSWM (14). Interestingly, neither comorbidity index had an impact on outcome, in terms of both TTNT and OS. Furthermore, there was no association between treatment interruption or hospitalization and both CCI and CIRS-G scale.
The limitations of the study include retrospective observational study design, together with a small study population compared to other studies. Furthermore, molecular analysis was available in a relatively small proportion of patients and those with an exclusively MYD88L265P status. However, considering the rarity of the disease, our study represents a relevant real-life experience that retrospectively describes the use of the DRC regimen in treatment-naïve patients with WM using a 28-day cycle schedule, with apparent non-inferiority in terms of safety and efficacy and with a good compliance. We can conclude that this work confirms the efficacy of the DRC combination as first-line treatment in patients with WM, even with 28-day instead of 21-day cycles resulting in good tolerability and safety and non-inferior efficacy.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The study was approved by an independent ethics committee of the coordinating center (Policlinico Catania 1, n.34/2019/PO) and was conducted in accordance with International Conference on Harmonization Guidelines on Good Clinical Practice and the principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects involved in the study.
Author contributions
VF: Writing – original draft. UM: Writing – original draft. SF: Writing – original draft. RS: Writing – original draft. CB: Writing – original draft. MG: Writing – original draft. VL: Writing – original draft. AB: Writing – original draft. AC: Writing – original draft. DM: Writing – original draft. UC: Writing – original draft. GM: Writing – original draft. FE: Writing – original draft. CG: Writing – original draft. AR: Writing – review & editing. FR: Writing – review & editing. CC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.This work was supported by Università degli Studi di Catania, “Fondi di Ateneo 2020–2022, Università di Catania, linea Open Access”.
Acknowledgments
The authors are thankful to all patients, families, and nurses who participated.
Conflict of interest
CC, FR, AR, and UM received honoraria from Amgen. CC, FR, and AR received honoraria from Celgene.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Owen RG, Kyle RA, Stone MJ, Rawstron AC, Leblond V, Merlini G, et al. Response assessment in Waldenström macroglobulinaemia: update from the VIth International Workshop. Br J Haematol. (2013) 160:171–6. doi: 10.1111/bjh.12102
2. Stone MJ, Pascual V. Pathophysiology of Waldenstrom’s macroglobulinemia. Haematologica. (2010) 95:359–64. doi: 10.3324/haematol.2009.017251
3. Schmidt J, Federmann B, Schindler N, Steinhilber J, Bonzheim I, Fend F, et al. MYD88 L265P and CXCR4 mutations in lymphoplasmacytic lymphoma identify cases with high disease activity. Br J Haematol. (2015) 169:795–803. doi: 10.1111/bjh.13361
4. Kapoor P, Paludo J, Vallumsetla N, Greipp PR. Waldenström macroglobulinemia: What a hematologist needs to know. Blood Rev. (2015) 29:301–19. doi: 10.1016/j.blre.2015.03.001
5. Oza A, Rajkumar SV. Waldenstrom macroglobulinemia: Prognosis and management. Blood Cancer J. (2015) 5(3):e394. doi: 10.1038/bcj.2015.28
6. Dimopoulos MA, Anagnostopoulos A, Kyrtsonis M-C, Zervas K, Tsatalas C, Kokkinis G, et al. Primary treatment of waldenström macroglobulinemia with dexamethasone, rituximab, and cyclophosphamide. J Clin Oncol. (2007) 25:3344–9. doi: 10.1200/JCO.2007.10.9926
7. Kastritis E, Leblond V, Dimopoulos MA, Kimby E, Staber P, Kersten MJ, et al. Correction to: “Waldenström’s macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2019) 30:860–2. doi: 10.1093/annonc/mdy466
8. Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grünhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. (2013) 381:1203–10. doi: 10.1016/S0140-6736(12)61763-2
9. Ghobrial IM, Xie W, Padmanabhan S, Badros A, Rourke M, Leduc R, et al. Phase II trial of weekly bortezomib in combination with rituximab in untreated patients with Waldenström Macroglobulinemia. Am J Hematol. (2010) 85:670–4. doi: 10.1002/ajh.21788
10. Gavriatopoulou M, García-Sanz R, Kastritis E, Morel P, Kyrtsonis M-C, Michalis E, et al. BDR in newly diagnosed patients with WM: final analysis of a phase 2 study after a minimum follow-up of 6 years. Blood. (2017) 129:456–9. doi: 10.1182/blood-2016-09-742411
11. Dimopoulos MA, Trotman J, Tedeschi A, Matous JV, Macdonald D, Tam C, et al. Ibrutinib for patients with rituximab-refractory Waldenström’s macroglobulinaemia (iNNOVATE): an open-label substudy of an international, multicentre, phase 3 trial. Lancet Oncol. (2017) 18:241–50. doi: 10.1016/S1470-2045(16)30632-5
12. Paludo J, Abeykoon JP, Kumar S, Shreders A, Ailawadhi S, Gertz MA, et al. Dexamethasone, rituximab and cyclophosphamide for relapsed and/or refractory and treatment-naïve patients with Waldenstrom macroglobulinemia. Br J Haematol. (2017) 179:98–105. doi: 10.1111/bjh.14826
13. Buske C, Dimopoulos MA, Grunenberg A, Kastritis E, Tomowiak C, Mahé B, et al. Bortezomib in combination with dexamethasone, rituximab and cyclophosphamide (B-DRC) as first - line treatment of Waldenstrom’s macroglobulinemia: results of a prospectively randomized multicenter European phase II trial. Blood. (2020) 136:26–6. doi: 10.1182/blood-2020-140933
14. Morel P, Duhamel A, Gobbi P, Dimopoulos MA, Dhodapkar MV, McCoy J, et al. International prognostic scoring system for Waldenström macroglobulinemia. Blood. (2009) 113:4163–70. doi: 10.1182/blood-2008-08-174961
15. Parmelee PA, Thuras PD, Katz IR, Lawton MP. Validation of the cumulative illness rating scale in a geriatric residential population. J Am Geriatr Soc. (1995) 43:130–7. doi: 10.1111/j.1532-5415.1995.tb06377.x
16. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8
17. Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Somerfield MR, Temin S. American society of clinical oncology/american society of hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Oncol Pract. (2010) 6:317–20. doi: 10.1200/JOP.2010.000132
18. Aapro MS, Bohlius J, Cameron DA, Lago LD, Donnelly JP, Kearney N, et al. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. (2011) 47:8–32. doi: 10.1016/j.ejca.2010.10.013
19. Thanarajasingam G, Minasian LM, Baron F, Cavalli F, De Claro RA, Dueck AC, et al. Beyond maximum grade: modernizing adverse event assessment and reporting in haematologic Malignancies. Lancet Haematol. (2018) 5(11):e563–98. doi: 10.1016/S2352-3026(18)30051-6
20. Dimopoulos MA, Zervas C, Zomas A, Kiamouris C, Viniou NA, Grigoraki V, et al. Treatment of waldenström’s macroglobulinemia with rituximab. J Clin Oncol. (2002) 20:2327–33. doi: 10.1200/JCO.2002.09.039
21. Hensel M, Villalobos M, Kornacker M, Krasniqi F, Ho AD. Pentostatin/cyclophosphamide with or without rituximab: an effective regimen for patients with Waldenström’s macroglobulinemia/lymphoplasmacytic lymphoma. Clin Lymphoma Myeloma. (2005) 6:131–5. doi: 10.3816/CLM.2005.n.039
22. Rose AL, Smith BE, Maloney DG. Glucocorticoids and rituximab in vitro: synergistic direct antiproliferative and apoptotic effects. Blood. (2002) 100:1765–73. doi: 10.1182/blood.V100.5.1765.h81702001765_1765_1773
23. Kastritis E, Gavriatopoulou M, Kyrtsonis M-C. Dexamethasone, rituximab, and cyclophosphamide as primary treatment of Waldenstrom macroglobulinemia: final analysis of a phase 2 study. Blood. (2015) 126:1392–4. doi: 10.1182/blood-2015-05-647420
24. Parisi MS, Calafiore V, Martino E, Leotta S, Pirosa MC, Sapienza G, et al. Long term disease control with Pomalidomide and Dexamethasone in Relapsed/Refractory Multiple Myeloma patients: a real life experience. J Clin Med. (2018) 8(10):1695. doi: 10.3390/jcm8101695
25. Conticello C, Romano A, Del Fabro V, Martino EA, Calafiore V, Sapienza G, et al. Feasibility, tolerability and efficacy of carfilzomib in combination with lenalidomide and dexamethasone in relapsed refractory myeloma patients: A retrospective real-life survey of the sicilian myeloma network. J Clin Med. (2019) 8(6):877. doi: 10.3390/jcm8060877
26. Markovic U, Romano A, Del Fabro V, Bellofiore C, Bulla A, Parisi MS, et al. Daratumumab as single agent in relapsed/refractory myeloma patients: A retrospective real-life survey. Front Oncol. (2021) 11:624405. doi: 10.3389/fonc.2021.624405
Keywords: Waldenström macroglobulinemia, first line treatment, DRC regimen, four-week cycle, safety
Citation: Del Fabro V, Markovic U, Frazzetto S, Sciortino R, Bellofiore C, Di Giorgio MA, Leotta V, Bulla A, Pelle AC, Elia F, Mannina D, Consoli U, Mineo G, Giallongo C, Romano A, Di Raimondo F and Conticello C (2024) Effectiveness, safety, and tolerability of delayed dexamethasone, rituximab, and cyclophosphamide as first-line treatment in patients with Waldenström macroglobulinemia: data from the Sicilian Myeloma Network. Front. Hematol. 3:1425677. doi: 10.3389/frhem.2024.1425677
Received: 30 April 2024; Accepted: 24 May 2024;
Published: 20 June 2024.
Edited by:
Shirley D’Sa, University College London Hospitals NHS Foundation Trust, United KingdomReviewed by:
Jindriska Lindsay, University College London, United KingdomJosephine Vos, Amsterdam University Medical Center, Netherlands
Copyright © 2024 Del Fabro, Markovic, Frazzetto, Sciortino, Bellofiore, Di Giorgio, Leotta, Bulla, Pelle, Elia, Mannina, Consoli, Mineo, Giallongo, Romano, Di Raimondo and Conticello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Frazzetto, ZnJhenpldHRvc2FyYUBnbWFpbC5jb20=
†These authors have contributed equally to this work
 Vittorio Del Fabro
Vittorio Del Fabro Uros Markovic
Uros Markovic Sara Frazzetto
Sara Frazzetto Roberta Sciortino3
Roberta Sciortino3 Angelo Curto Pelle
Angelo Curto Pelle Donato Mannina
Donato Mannina Ugo Consoli
Ugo Consoli Giuseppe Mineo
Giuseppe Mineo Cesarina Giallongo
Cesarina Giallongo Francesco Di Raimondo
Francesco Di Raimondo Concetta Conticello
Concetta Conticello