
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Hematol. , 06 March 2024
Sec. Blood Cancer
Volume 3 - 2024 | https://doi.org/10.3389/frhem.2024.1334577
Introduction: Anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma (LBCL) is a rare subtype of diffuse large B-cell lymphoma (DLBCL). Patients with ALK+ LBCLs have poor response and survival outcomes when treated with traditional chemotherapy regiments. The efficacy of second- and third-generation ALK inhibitors has been reported in treating ALK+ LBCLs. Additionally, owing to the plasmablastic morphology and immune features observed in ALK+ LBCLs, plasma cell tumor therapies may be effective for this patient population. In this case report, we utilized a myeloma-like therapy combined with a third-generation ALK inhibitor for a newly diagnosed ALK+ LBCL patient.
Case presentation: We reported a 32-year-old male patient diagnosed with ALK+ LBCL. Immunohistochemistry (IHC) analysis revealed a plasma cell immunophenotype characterized by CD138 positivity but negativity for mature B lymphocyte markers. The patient received six cycles of VRD (bortezomib 1.3 mg/m2 d1, 4, 8, and 11; lenalidomide 25 mg qd d1–14; dexamethasone 20 mg d1–2, d4–5, d8–9, and d11–12) and cyclophosphamide (1.0 g q3w) treatment. Lorlatinib (100 mg once daily) was added starting from the second cycle of treatment onwards. After four cycles of treatment, the patient achieved complete remission, which was maintained for more than 6 months after completing chemotherapy, without any significant safety concerns.
Conclusion: VRD and cyclophosphamide combined with a third-generation ALK inhibitor resulted in durable complete remission for an individual with ALK+ LBCL, suggesting it as a therapeutic option for patients with this subtype.
Anaplastic lymphoma kinase (ALK)-positive large B-cell lymphoma (LBCL) is a rare subtype of diffuse large B-cell lymphoma (DLBCL), accounting for less than 1% of cases. The median age at diagnosis ranges from 35 to 43 years old, with a male predominance (1, 2). The most common symptom is painless lymph node enlargement, but half of patients have B symptoms (1). Approximately 75% of ALK+ LBCLs harbor t (2;17) (p23; q23) chromosomal translocation, resulting in the fusion gene CLTC-ALK by fusing the clathrin heavy-chain gene (CLTC) on chromosome 17q23 with the ALK gene on chromosome 2p23 (1). NPM-ALK [t (2;5) (p23; q35)] is the most frequent fusion gene observed in anaplastic large cell lymphomas (ALCLs), occurring in approximately 70%–80% of cases, but only in approximately 17% of ALK+ LBCLs (1, 3). ALK+ LBCL displays immunoblastic or plasmablastic features in histomorphology. Similar to ALCL, tumor cells in ALK+ LBCL express ALK and epithelial membrane antigen (EMA), but negative for CD30 and mature B lymphocyte markers such as CD20, CD79a, and PAX-5. Tumor cells usually contain monoclonal light-chain (κ or λ) of immunoglobulin (Ig) and are positive for plasma cell antigens like CD138. A previous study reported 26 cases of ALK+ LBCLs (1). All cases expressed ALK protein. Half of these cases exhibited a plasmablastic morphology and expressions of CD138 were detected in 96% of cases. The histomorphology and immune features of ALK+ LBCL indicate the potential efficacy of agents used for plasmacytoma.
ALK+ LBCLs have poor clinical outcome when treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-derived regimens (2). Therefore, novel therapeutic approaches should be evaluated specifically for this patient group. ALK inhibitors are reported effective against ALK+ lymphomas, including ALCL, ALK-positive non-small-cell lung cancer (NSCLC) and ALK+ LBCL (4–6). Here, we reported that a patient with ALK+ LBCL achieved durable complete remission using myeloma-like treatment combined with the third-generation ALK inhibitor, lorlatinib.
A 32-year-old male patient visited our hospital in November 2022. He complained a painless left neck mass for 1 year that had gradually increased in size over the past month. Since the onset of the disease, the patient had no discomfort including fever, weight loss, or night sweating. Physical examination revealed enlarged lymph nodes that had fused together to form a mass in the left neck and supraclavicular region. A biopsy of the neck mass was performed, which revealed ALK+ LBCL through histopathologic examination and immunohistochemistry (IHC). IHC showed CD45+, CD138+, MUM1+, CD20−, CD19−, PAX-5−, CD79a−, CD30−, CD3−, CK−, CD38−, S100−, and EBER−, and Ki-67 index was approximately 40%–50% (Figures 1A–H). Fluorescence in situ hybridization (FISH) revealed that 85% of tumor cells exhibited ALK rearrangement (Figure 1I). Blood routine examination results were mostly within the normal range. Lactic dehydrogenase (LDH) level was measured at 227 U/L (range: 109–245 U/L). An 18F-FDG-PET scan revealed multiple lymph nodes fused into a cluster in the left neck and supraclavicular region with a maximum standardized uptake value (SUVmax) of 24.96. Another lymph node with an SUVmax of 30.96 and maximum diameter of 4.5 cm was observed in the hepatic hilar region (Figure 2). The patient strongly refused bone marrow aspiration and biopsy; hence, bone marrow involvement was only excluded by normal PETCT scanning and normal blood routine examination. The detection of peripheral blood flow was not conducted; thus, the presence of tumor cells in peripheral blood was unknown. The diagnosis of ALK+ LBCL (stage III) was confirmed by a multidisciplinary team consisting of a pathologist, a radiologist, and an oncologist. In addition, the patient had no hereditary family history of any disease, and no one else in the family had the same disease.
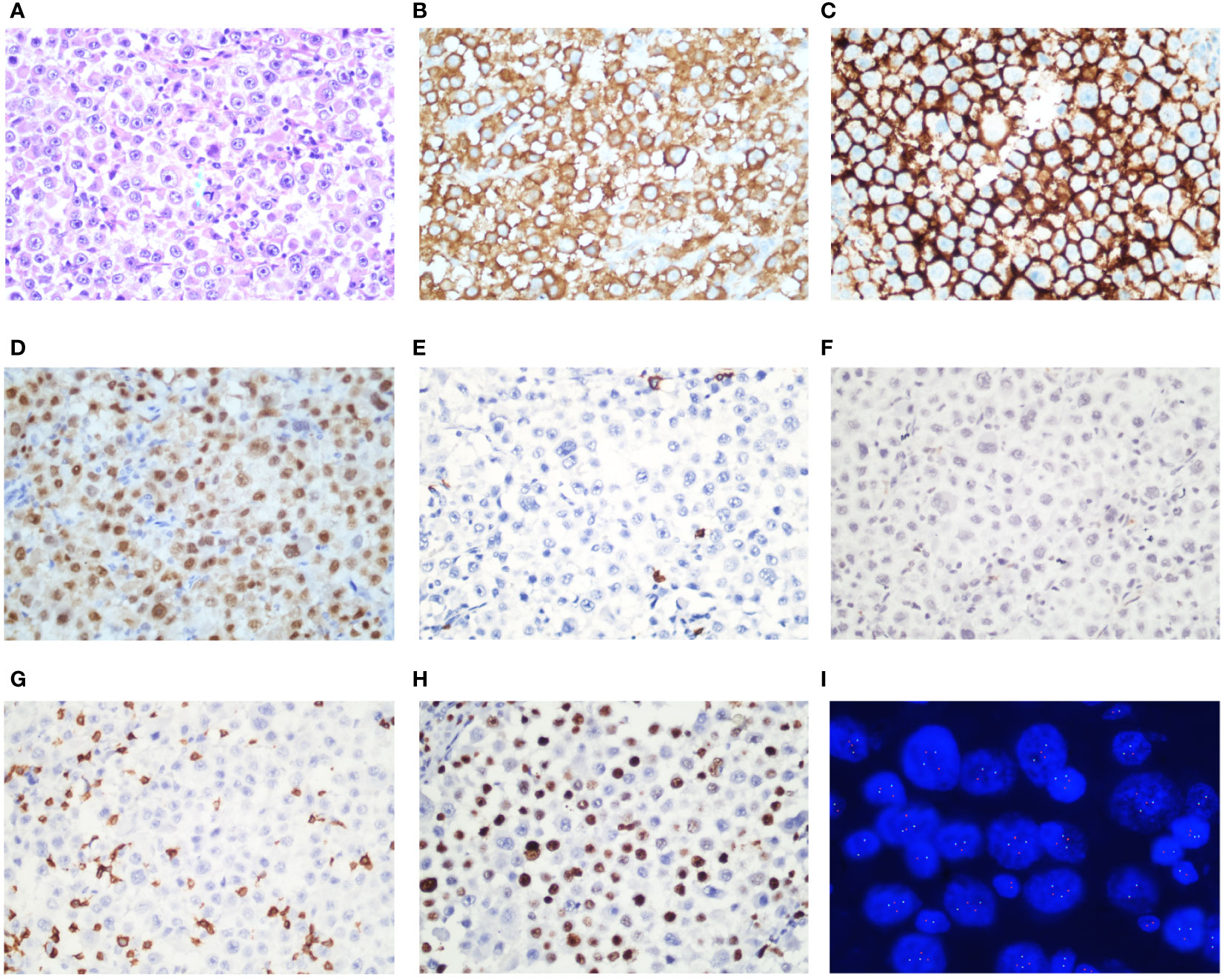
Figure 1 Immunohistochemical stains of ALK-positive large B-cell lymphoma. ALK-positive large B-cell lymphoma morphology shows sheets of large neoplastic lymphoid cells with plasmablastic morphologies (A, Wright-Giemsa). Tumor cells are positive for ALK in the cytoplasma rather than nucleus (B). Tumor cells are positive for CD138 (C) and MUM-1 (D), but negative for CD20 (E), CD30 (F), and CD3 (G). Ki-67 index was approximately 40%–50% (H). Fluorescence in situ hybridization (FISH) revealed that tumor cells had ALK rearrangement (I).
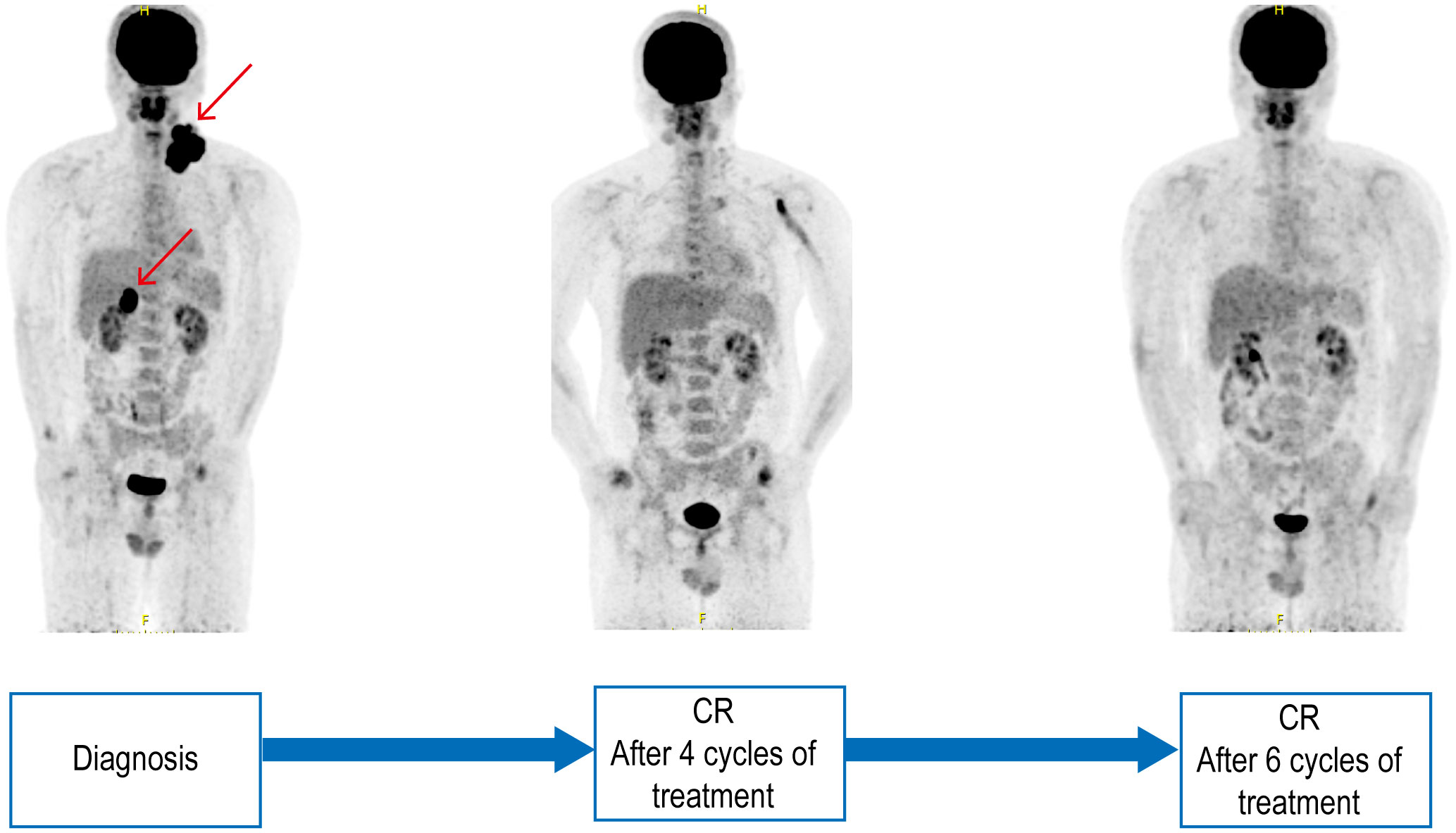
Figure 2 Pre- and post-treatment PETCT scanning. PETCT scanning of pre-treatment, after four and six cycles of treatment.
As CD20 was tested negative in this patient, rituximab was not administered. The efficacy of CHOP regimen in ALK+ LBCLs was discouraged (1, 7). IDH analysis revealed plasma cell differentiation in this patient. Following a comprehensive discussion and obtaining informed consent from the patient, we initiated myeloma-like therapy comprising bortezomib, lenalidomide, dexamethasone (VRD), and cyclophosphamide. The patient received the first cycle of VRD and cyclophosphamide treatment from 20 November 2022 (bortezomib 1.3 mg/m2 d1, 4, 8, and 11; lenalidomide 25 mg qd d1–14; dexamethasone 20 mg d1–2, d4–5, d8–9, and d11-12; cyclophosphamide 1.0 g d1). The neck mass reduced during the first cycle of treatment. The second cycle of treatment was postponed due to a COVID-19 infection. The neck mass had regrown larger upon his return to the hospital on 9 January 2023. This occurred 7 weeks after the initial VRD regimen. Encouraging results have been reported for ALK inhibitors in ALK+ DLBLCs; thus, we recommended combining an ALK inhibitor with VRD for a more favorable response, which was accepted by the patient. Lorlatinib (100 mg once daily) was added since the second cycle of treatment. The neck mass rapidly reduced after the second cycle of treatment.
After four cycles of treatment, the patient achieved complete repression by PET-CT (Figure 2). Only small lymph nodes were still present, exhibiting slightly increased radiation uptake with an SUVmax of 2.7. The Deauville score was determined to be three points. After six cycles of treatment, Deauville score was one point (Figure 2). ALK+ LBCLs might benefit from hematopoietic stem cell transplantation (HSCT) (6). However, owing to the unavailability of a sibling donor for allogeneic HSCT and concerns regarding fertility preservation, alternative donor or autogenetic HSCT options were declined by the patient. Lorlatinib is being administered as maintenance therapy and has resulted in sustained complete remission for over 6 months since completion of chemotherapy, with a progression-free survival exceeding 10 months since initiation of ALK inhibitor therapy. No hematologic toxicity or other adverse effects have been observed during treatment.
The prognosis of ALK+ LBCLs is unfavorable. A systematic review included 184 cases of ALK+ LBCLs, and reported a median overall survival (OS) of 1.8 years and a 5-year OS rate of 28% (8). There is currently no standard treatment regimen for ALK+ LBCLs. Because of CD20 negativity in this cohort of patients, the efficiency of rituximab is discouraged. Previous studies revealed poor survival outcomes with CHOP or CHOP-like regimens in ALK+ LBCL patients. Laurent et al. (2) retrospectively analyzed 38 patients with ALK+ LBCL treated with CHOP or CHOP-like regiments, revealing a 5-year OS rate of 25% and a median survival of 18 months. Pan et al. (1) reported 26 cases of ALK+ LBCLs. Six patients with stage III/IV received CHOP or CHOP-like treatment, four achieved partial response (PR), but only one achieved CR (1). The results obtained from using CHOP or CHOP-like regimens for treating ALK+ LBCLs were disappointing. Therefore, we aim to explore novel therapeutic approaches for these patients.
ALK+ LBCL commonly displays plasmablastic morphology and usually plasma cell immunophenotype (positive for CD38 and CD138). Plasma cell tumor chemotherapy scheme such as proteasome inhibitors and immunomodulatory agents may be effective for ALK+ LBCL. Plasmablastic lymphoma (PBL) is another aggressive CD20-negative DLBCL with plasmacytic differentiation. Previous studies have demonstrated the efficacy of bortezomib in combination with EPOCH regimen for PBL, resulting in a complete response rate exceeding 90% and a 5-year OS rate of 65% (9). A case report showed the encouraging result of lenalidomide in combination with bortezomib in PBL (10). A single case of relapsed ALK+ LBCL exhibited a transient response when treated with lenalidomide (8). Consequently, we administered myeloma-like therapy consisting of bortezomib, lenalidomide, dexamethasone (VRD), and cyclophosphamide as the first-line treatment for this patient.
ALK is a receptor of tyrosine kinase. ALK translocations associated with overexpression of ALK proteins, leading to aberrant constitutive autophosphorylation and activation of ALK kinase, which will trigger signal cascades such as JAK, PI3K, RAS, and PLC-γ pathways that promote cell proliferation, invasion, and migration (11). Small-molecule tyrosine kinase inhibitors targeting ALK have been reported to be effective for ALK-positive tumors. Crizotinib is the first-generation ALK inhibitor, which inhibits ALK, ROS1, and MET kinase. The second-generation ALK inhibitors, such as allectinib, ceritinib, brigatinib, and entrectinib, are reported to be effective against crizotinib-resistant cancers (3). Compared to crizotinib, alectinib showed superior efficiency and safety for ALK-positive NSCLC, with improved 12-month event-free survival rates (4). In a phase II trial for relapsed or refractory ALK-positive ALCLs, alectinib achieved complete response in 60% of patients (6/10) and partial response in 20% of patients (5). Lorlatinib is a third-generation ALK inhibitor targeting ALK and ROS1. It has been reported to overcome secondary resistance caused by mutations in the ALK gene. A phase II clinical trial demonstrated substantial systemic and intracranial activity of lorlatinib in treatment-naïve patients with ALK-positive NSCLC as well as those who had progressed on other ALK inhibitors (12).
ALK inhibitors have also been reported for the treatment of ALK+ LBCLs.
Although crizotinib has demonstrated efficacy in ALK+ LBCLs, the duration of response was found to be very short, and the survival time was shorter than 6 months in all cases (1, 13, 14). A recent study reported encouraging results of the second and third generations of ALK inhibitors for ALK+ LBCL (6). Lorlatinib and alectinib induced rapid tumor regression in ALK+ LBCL patient-derived xenograft tumor models (6). Furthermore, four relapsed/refractory ALK+ LBCL received alectinib at a dose of 600 mg orally twice daily. All patients achieved treatment response. One patient achieved CR and three achieved PR. The CR patient later experienced disease progression but regained CR after receiving lorlatinib. Two PR patients attained CR following allogeneic HSCT and allogeneic HSCT combined with daratumumab, respectively. Based on the aforementioned literature review, we recommended the third-generation ALK inhibitor, lorlatinib, for the ALK+ LBCL patient. VRD combined with lorlatinib for our patient resulted in complete remission with good tolerability.
Given the poor outcomes observed in ALK+ LBCLs treated with commonly used regimens, we employed VRD, cyclophosphamide, and a third-generation ALK inhibitor to achieve durable complete remission with excellent safety in this patient with ALK+ LBCL. However, it should be noted that this report represents a single case study. Treatment strategies for this rare and poor prognostic disease are worthy of further investigation.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical approval was not required for the study involving human samples in accordance with the local legislation and institutional requirements because [reason ethics approval was not required]. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
DL: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. LX: Funding acquisition, Writing – review & editing. HuW: Writing – review & editing. PL: Writing – review & editing. HaW: Writing – review & editing. ZL: Conceptualization, Formal analysis, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported in part by the National Natural Science Foundation of China (82200224), the Natural Science Foundation of Shandong Province (ZR2021MH072), and China Postdoctoral Science Foundation (2023M732124).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Pan Z, Hu S, Li M, Zhou Y, Kim YS, Reddy V, et al. ALK-positive large B-cell lymphoma: A clinicopathologic study of 26 cases with review of additional 108 cases in the literature. Am J Surg Pathol. (2017) 41:25–38. doi: 10.1097/pas.0000000000000753
2. Laurent C, Do C, Gascoyne RD, Lamant L, Ysebaert L, Laurent G, et al. Anaplastic lymphoma kinase–positive diffuse large B-cell lymphoma: A rare clinicopathologic entity with poor prognosis. J Clin Oncol. (2009) 27:4211–6. doi: 10.1200/jco.2008.21.5020
3. Liu Y-M, Kuo C-N and Liou J-P. Anaplastic lymphoma kinase inhibitors: an updated patent review (2014–2018). Expert Opin Ther Patents. (2020) 30:351–73. doi: 10.1080/13543776.2020.1738389
4. Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. (2017) 377:829–38. doi: 10.1056/NEJMoa1704795
5. Fukano R, Mori T, Sekimizu M, Choi I, Kada A, Saito AM, et al. Alectinib for relapsed or refractory anaplastic lymphoma kinase-positive anaplastic large cell lymphoma: An open-label phase II trial. Cancer Sci. (2020) 111:4540–7. doi: 10.1111/cas.14671
6. Soumerai JD, Rosenthal A, Harkins S, Duffy J, Mecca C, Wang Y, et al. Next-generation ALK inhibitors are highly active in ALK-positive large B-cell lymphoma. Blood. (2022) 140:1822–6. doi: 10.1182/blood.2022015443
7. Laurent C, Do C, Gascoyne RD, Lamant L, Ysebaert L, Laurent G, et al. Anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma: a rare clinicopathologic entity with poor prognosis. J Clin Oncol. (2009) 27:4211–6. doi: 10.1200/jco.2008.21.5020
8. Castillo JJ, Beltran BE, Malpica L, Marques-Piubelli ML, Miranda RN. Anaplastic lymphoma kinase-positive large B-cell lymphoma (ALK + LBCL): a systematic review of clinicopathological features and management. Leukemia Lymphoma. (2021) 62:2845–53. doi: 10.1080/10428194.2021.1941929
9. Castillo JJ, Guerrero-Garcia T, Baldini F, Tchernonog E, Cartron G, Ninkovic S, et al. Bortezomib plus EPOCH is effective as frontline treatment in patients with plasmablastic lymphoma. Br J haematology. (2019) 184:679–82. doi: 10.1111/bjh.15156
10. Marrero WD, Cruz-Chacón A, Castillo C, Cabanillas F. Successful use of bortezomib-lenalidomide combination as treatment for a patient with plasmablastic lymphoma. Clin lymphoma myeloma leukemia. (2018) 18:e275–7. doi: 10.1016/j.clml.2018.04.011
11. Aubry A, Galiacy S and Allouche M. Targeting ALK in cancer: therapeutic potential of proapoptotic peptides. Cancers. (2019) 11(3):275. doi: 10.3390/cancers11030275
12. Solomon BJ, Besse B, Bauer TM, Felip E, Soo RA, Camidge DR, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. (2018) 19:1654–67. doi: 10.1016/s1470-2045(18)30649-1
13. Mehra V, Pomplum S, Ireland R, Yallop D, Devereux S, Marcus R, et al. ALK-positive large B-cell lymphoma with strong CD30 expression; a diagnostic pitfall and resistance to brentuximab and crizotinib. Histopathology. (2016) 69:880–2. doi: 10.1111/his.13002
Keywords: ALK positive large B-cell lymphoma, lorlatinib, bortezomib, lenalidomide, case report
Citation: Liu D, Xing L, Wang H, Li P, Wei H and Li Z (2024) Case report: A patient with ALK-positive large B-cell lymphoma benefited from myeloma-like treatment combined with the ALK inhibitor lorlatinib. Front. Hematol. 3:1334577. doi: 10.3389/frhem.2024.1334577
Received: 07 November 2023; Accepted: 19 February 2024;
Published: 06 March 2024.
Edited by:
Alejandro Martín García-Sancho, University Hospital of Salamanca, SpainReviewed by:
Norma Carmen Gutiérrez, University of Salamanca Health Care Complex, SpainCopyright © 2024 Liu, Xing, Wang, Li, Wei and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zengjun Li, emVuZ2p1bmxpQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.