- 1Division of Hematology, Department of Internal Medicine, The Ohio State University, Columbus, OH, United States
- 2College of Medicine, The Ohio State University, Columbus, OH, United States
Background: Allogeneic stem cell transplant (allo-SCT) plays a key role in the treatment of patients with both acute myeloid leukemia (AML) and myelodysplastic (MDS). Outcomes of allo-SCT have improved with optimization of transplant practices. We sought to evaluate trends in survival in AML and MDS patients undergoing allo-SCT at our institution from 1984 to 2018.
Methods: A retrospective analysis of 900 consecutive AML and MDS patients undergoing allo-SCT was performed. Patients were divided by year of transplant for analysis. Primary endpoints were progression free survival (PFS) and overall survival (OS). Secondary endpoints included non-relapse mortality (NRM), graft-versus-host disease (GVHD), GVHD-free relapse free survival (GRFS), and transplant complications.
Results: We found a significant improvement in survival from 1984 to 2018 with 5-year PFS and OS improving from 17% to 49% and 17% to 53%, respectively (statistically significant difference since 2004; p<0.001). There was a significant difference in rates of grade II-IV aGVHD (p<0.001) and chronic GVHD at day +365 with cumulative incidence of both highest from 2014-2018, however, NRM improved across the years with 5- year NRM decreasing from 45% to 21%. Rates of pulmonary infections, hemorrhagic cystitis, veno-occlusive disease, and fungal infections also decreased across the years (p<0.001).
Conclusions: We found a significant improvement in survival of AML and MDS patients undergoing allo-HCT over the past several decades. This likely reflects improvements in transplant practices and general supportive care. Post-transplant relapse remains the leading cause of transplant failure in this group.
Introduction
Allogeneic stem cell transplant (allo-SCT) continues to play a key role in the post-remission therapy for many acute myeloid leukemia (AML) patients due to high rates of efficacy and sustained remissions as compared to alternate therapies (1). For those patients with relapsed/refractory AML and those with high-risk myelodysplastic syndrome (MDS), it is the sole curative option (2, 3). The utilization of allo-SCT has increased over time, likely due to the growth of unrelated donor registries, improved outcomes in unrelated donor transplants, and increased numbers of allo-HCT in patients >60 years old with the introduction of reduced intensity conditioning (RIC) regimens (4). However, allo-SCT transplant itself is not without risk and obstacles for successful transplant remain including risk for relapse of underlying disease, graft versus host disease (GVHD), and infectious complications (5).
Prior retrospective studies have demonstrated an improvement in outcomes of allo-SCT over the years, with decreased mortality from infections, GVHD, and toxicity (5, 6). Such findings were also confirmed in a previously published retrospective analysis from our Institution including all patients undergoing allo-SCT from 1984 to 2018 for all hematologic malignancies (7). Factors that have been thought to contribute to this improvement in outcomes include modifications in transplant conditioning regimens, supportive care, and earlier recognition of transplant complications. In this study we sought to evaluate the major changes in practice during transplant as well as trends in outcomes in AML and MDS patients undergoing allo-SCT at the Ohio State University from 1984-2018.
Methods
Data collection and study population
We performed a retrospective sub-analysis of a larger cohort of all adult AML and MDS patients who received their first allo-HCT at the Ohio State University from January 1st, 1984 to December 31st, 2018. Patients were identified through the blood and marrow transplant registry. All data was verified via medical chart review. A total of 900 patients were included in the analysis, 705 patients with AML and 195 with MDS. The patients were divided into seven groups based on year of transplant for analysis using five-year increments: group 1 included 1984-1988, group 2 1989-1993, group 3 1994-1998, group 4 1999-2003, group 5 2004-2008, group 6 2009-2013, and group 7 2014-2018.
Endpoints
The primary aim of our study was to assess trends in outcomes in allo-HCT across the years from 1984 to 2018. Progression free survival (PFS) and overall survival (OS) were the main primary endpoints with those patients who were not progressed being censored at the last assessment date for PFS, and those patients who were living being censored at last follow-up for OS. Secondary endpoints included non-relapse mortality (NRM), acute and chronic GVHD, GVHD-free relapse free survival (GRFS), and transplant complications including infections, hemorrhagic cystitis, VOD, and pulmonary complications. Relapse was defined as recurrence of disease at any site. The Glucksberg grade was used for grading of acute GVHD (aGVHD) and standard definitions of chronic GVHD (cGVHD) were used (8). GVHD-free relapse free survival was defined as the absence of grade III-IV a GVHD and cGVHD requiring systemic treatment, relapse, and death.
Statistical analysis
The Kruskal-Wallis test for continuous variables and chi-squared or Fisher’s exact test for categorical variables was used to compare patient, disease, and transplant related characteristics among the seven groups. The Kaplan-Meier method was used to calculate probabilities of OS, PFS, and GRFS and they were compared using the log-rank test. Gray’s test, accounting for competing risks, was utilized to estimate cumulative incidence rates for relapse, NRM, and acute and chronic GVHD. The competing risks for GVHD were relapse and death; the competing risk for relapse was death; and the competing risk for NRM was disease progression or relapse. A p-value of<0.05 was considered statistically significant. Analyses were performed using Stata 16 (College Station, Texas).
Results
Patient, disease, and transplant characteristics
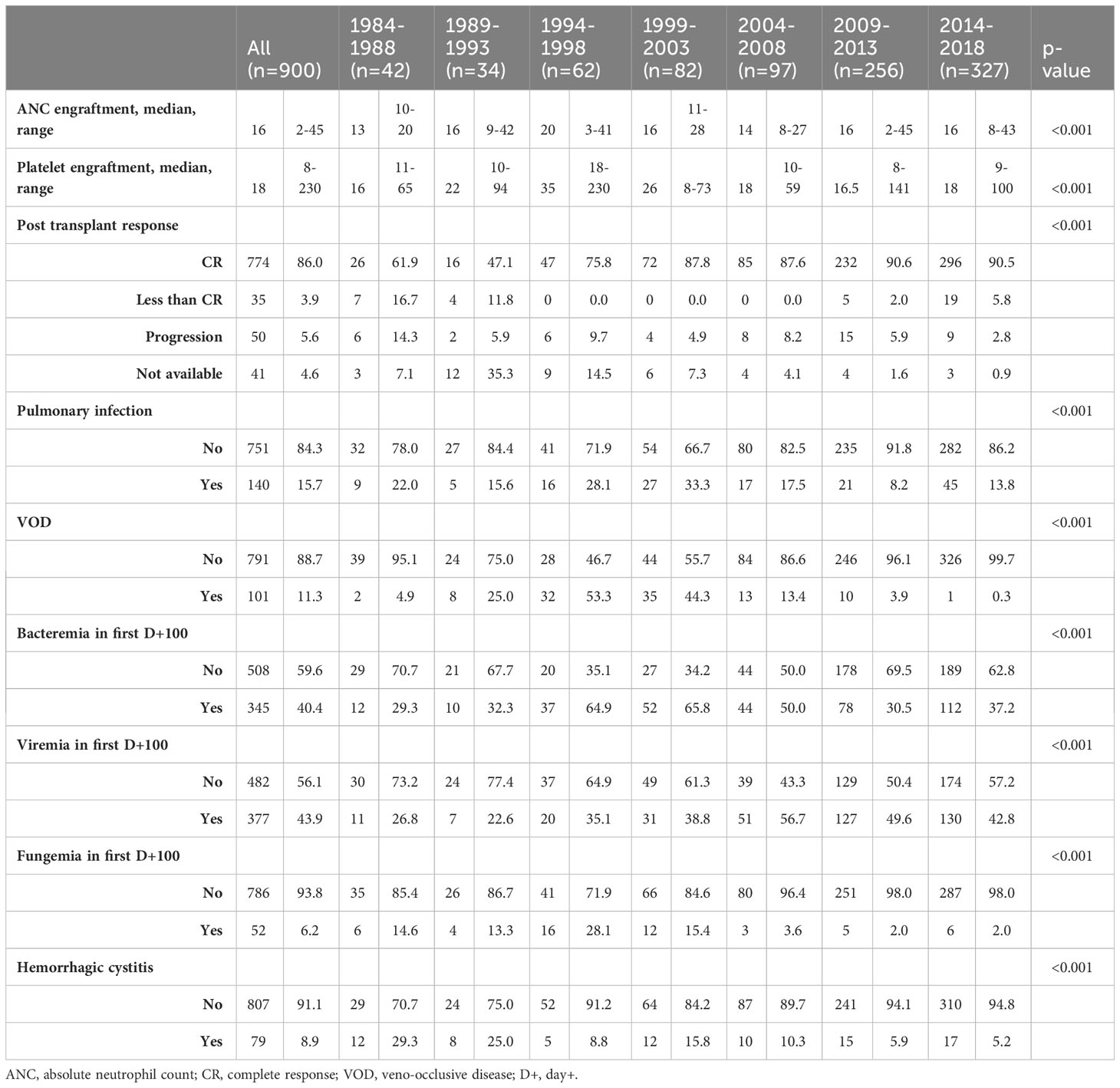
The cohort included 900 patients, 705 patients with AML and 195 with MDS (Table 1 & Supplementary Table 1). Table 1 shows an overview of patient, disease, and transplant characteristics. Median age at the time of transplant was 52.0 years and 55.6% of patients were male. There was a significant increase in age at time of transplant seen across the years with median age 29.5 years from 1984-1988 and 59.0 years from 2014-2018 (p<0.001). Conditioning regimen intensity was defined based on the Center for International Blood and Marrow Transplantation (CIBMTR) research definitions for myeloablative (MA) versus non-MA and reduce-intensity conditioning (RIC) (9). The majority of patients underwent MA conditioning regimens with 57.6% undergoing MA and 42.4% RIC. There was also a significant increase in the use of RIC conditioning regimens over time, with 0% RIC regimens from 1984-1998 and 45.9% from 2014-2018 (p<0.001). There was a significant increase in the use of peripheral blood stem cell source, with 0% from 1984-2018 to 77.1% from 2014-2018 (p<0.001). GVHD prophylaxis varied over time, with all of patients receiving a Cyclosporine based prophylaxis in 1989-1993 while 91.7% of patients received a Tacrolimus based regimen from 2014-2018 (p<0.001).
Outcomes
PFS and OS
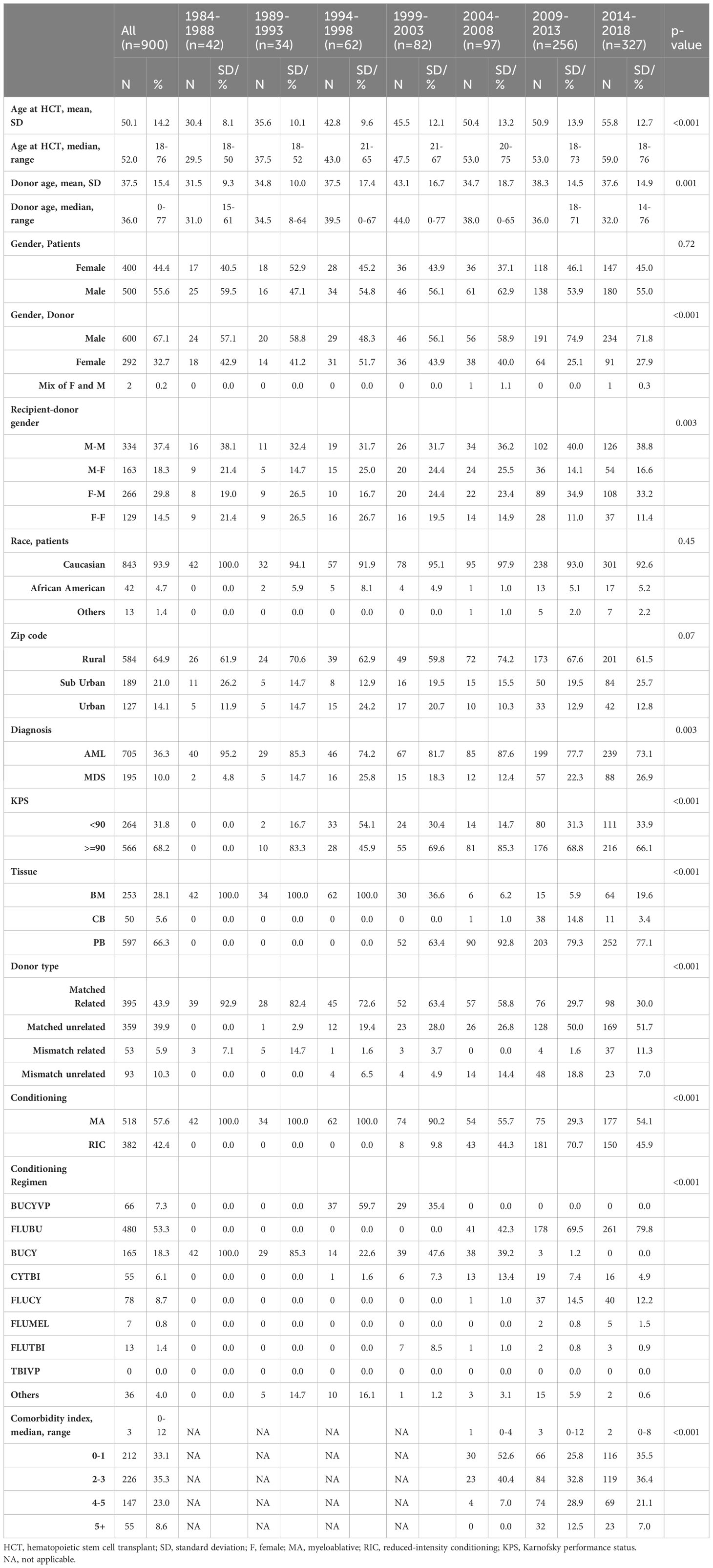
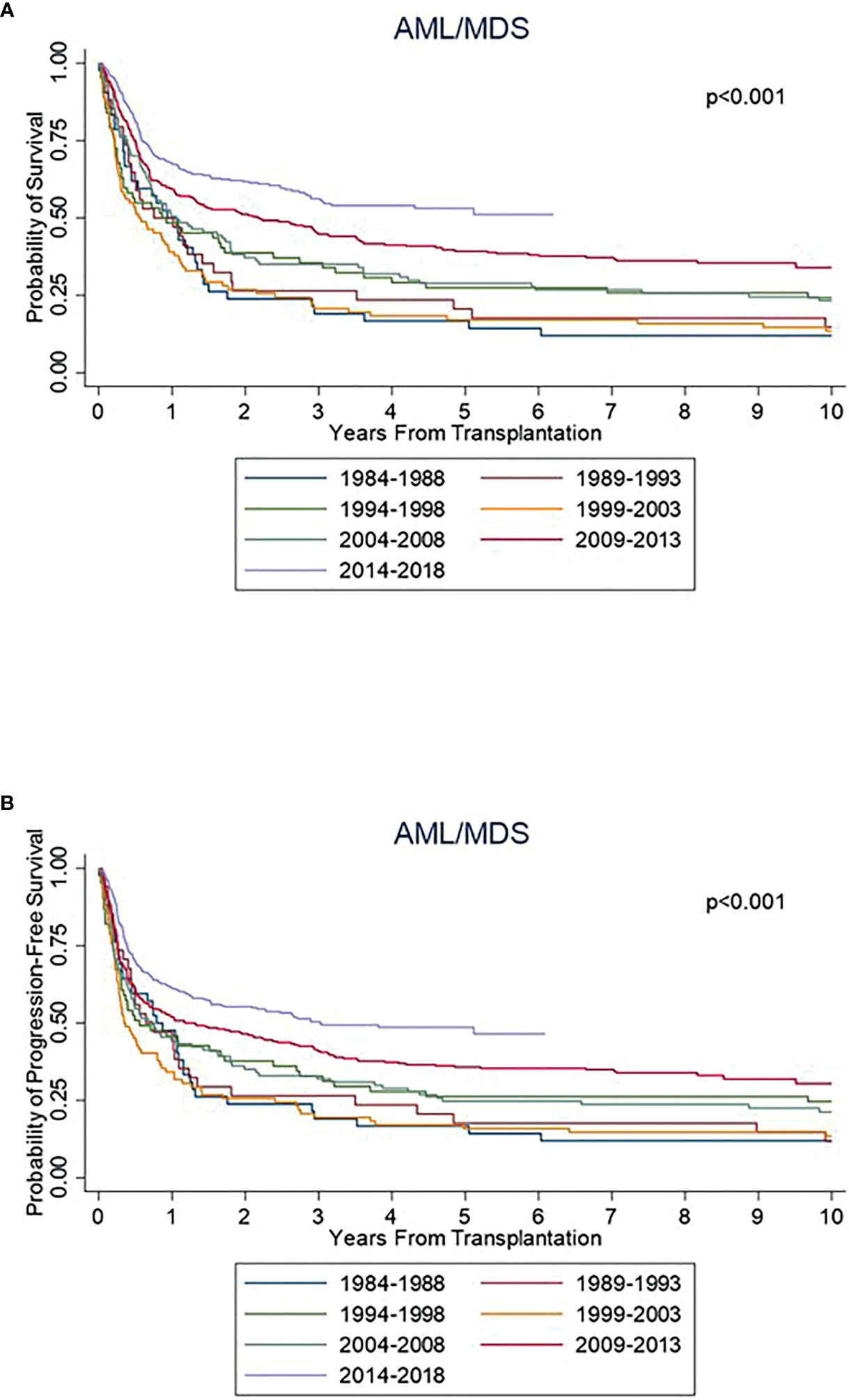
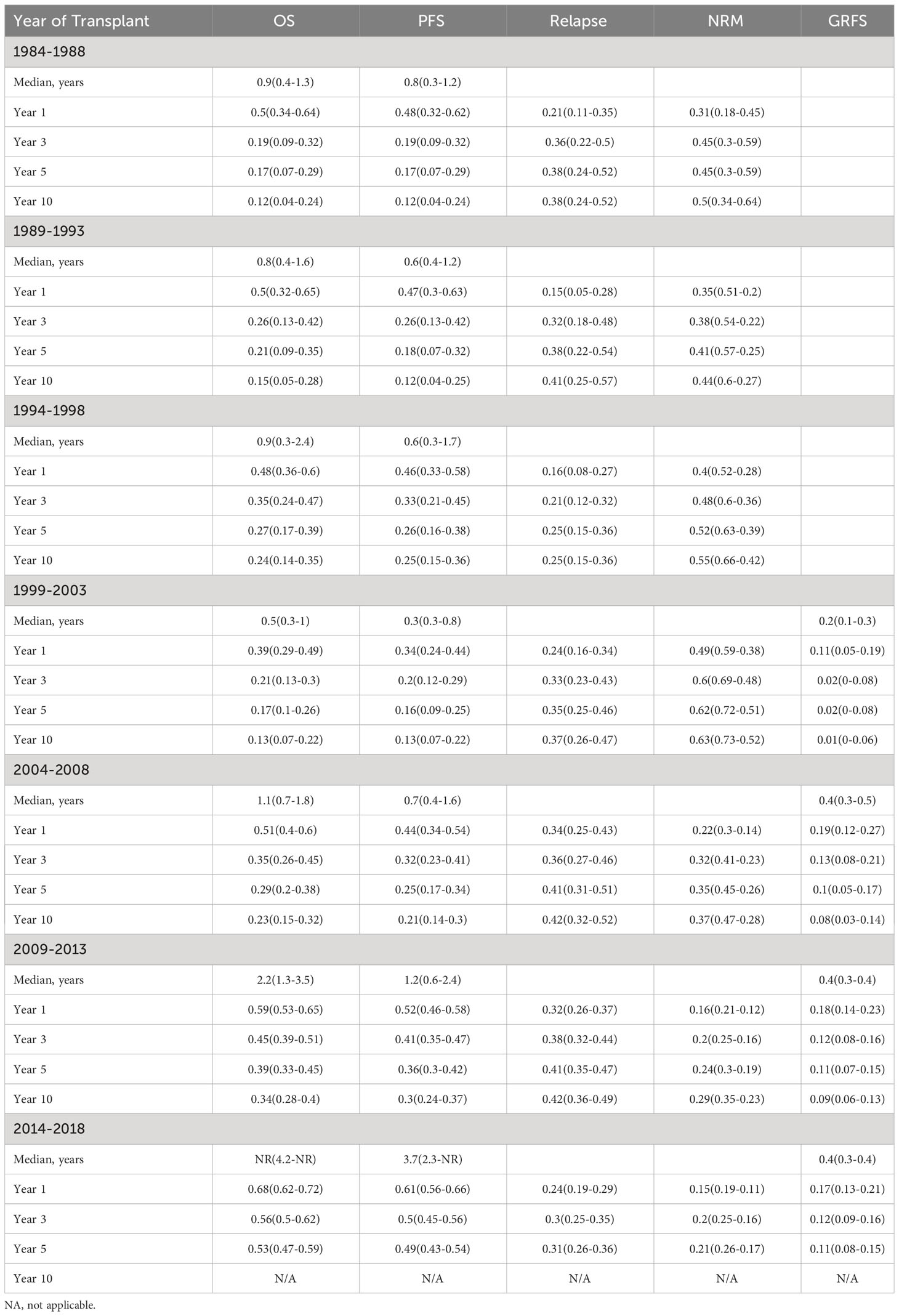
There was a statistically significant improvement in both PFS and OS from 1984 to 2018 (Figure 1). Median PFS improved from 0.8 years [95% confidence interval (CI) 0.3-1.2 years)] from 1984-1988 to 3.0 years (95% CI: 1.7-NR) from 2014-2018. Median OS improved from 0.9 years from 1984-1988 (95% CI: 0.4-1.3 years) to NR (95% CI: 3.0 years-NR) from 2014-2018. The 5-year PFS across the years was 17%, 18%, 26%, 16%, 25%, 36% and 49%, respectively, with a significant improvement seen since 2004. Similar improvements in PFS were seen at 10 years. The 5-year OS across the years was 17%, 21%, 27%, 17%, 29%, 39% and 53%, respectively, with a similar significant improvement seen since 2004. Similar improvements in OS were also seen at 10 years (Table 2).
NRM and relapse
The rate of NRM significantly improved across the years, with 1-year NRM of 31%, 35%, 40%, 49%, 22%, 16% and 15% and 5-year NRM at 45%, 41%, 52%, 62%, 35%, 24% and 21% respectively (Table 3). A significant improvement in NRM was seen since 2004 (p<0.001; Figure 2). There was no significant different in cumulative incidence of relapse over the years (p=0.07; Figure 2). One-year relapse rates were 21%, 15%, 16%, 24%, 34%, 32%, and 24% and 5-year relapse was 38%, 38%, 25%, 35%, 41%, 41%, and 31%, respectively (Table 2).
Transplant complications
A summary of transplant complications is shown in Table 4. Overall, 15.7% of patients were noted to have pulmonary infections post-transplant, 11.3% of patients experienced VOD, 8.9% hemorrhagic cystitis, 40.4% of patients had bacteremia in D+100, 43.9% had viremia, and 6.2% had fungemia. From 1984-1988, rates of pulmonary infection were 22%, which improved to 13.8% from 2014-2018 (p<0.001). Rates of VOD improved from 4.9% to 0.3% (p<0.001); rates of hemorrhagic cystitis improved from 29.3% to 5.2% (p<0.001); and rates of fungemia improved from 14.6% to 2% (p<0.001). There was a trend toward increased rates of bacteremia and viremia over time, with rates of bacteremia increasing from 29.3% from 1984-1988 to 37.2% from 2014-2018 (p<0.001) and rates of viremia increasing from 26.8% to 42.9% (p<0.001).
GVHD and GRFS
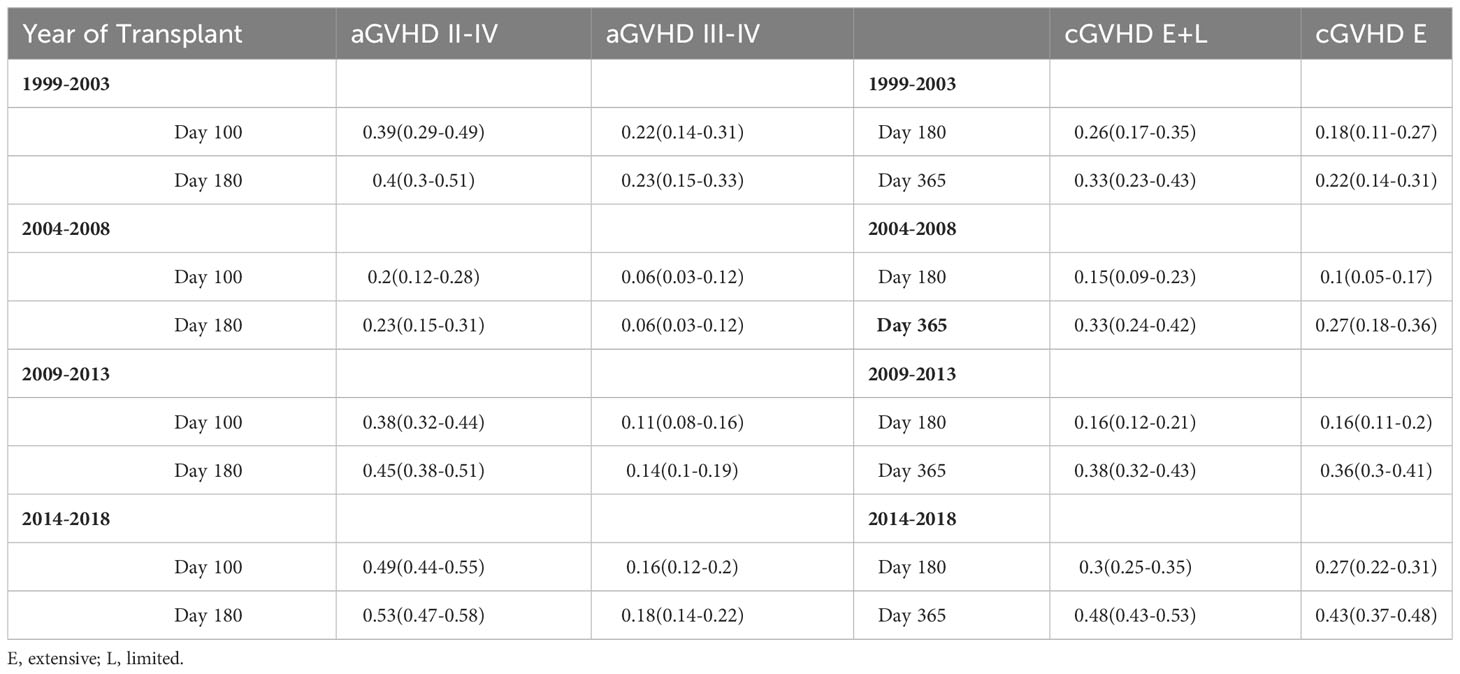
Complete GVHD data was available starting with the 1999-2003 group. There was a significant difference seen in rates of grade II-IV aGVHD between the groups (p<0.001), with a cumulative incidence at day +180 of 40% from 1999-2003, 23% from 2004-2008, 45% from 2009-2013, and 53% from 2014-2018 (Figure 3). Rates of grade III-IV aGVHD at day +180 were 23%, 6%, 14%, and 18%, respectively, with the highest rate seen from 1999-2003 and 2014-2018. Rates of cGVHD also varied between the groups (p=0.002) with cumulative incidence at day +365 of 33% from 1999-2003 and 2004-2008, 38% from 2009-2013, and 48% from 2014-2018. Incidence of extensive cGVHD at day +365 increased across the years at 22%, 27%, 36% and 43%, respectively (p<0.001; Table 3). Median GRFS was 0.2, 0.4, 0.4, and 0.4 years across the years (Figure 2). There was a significant different in GRFS across the years with 1-year GRFS 11%, 19%, 18%, and 17% and 5-year GRFS 2%, 10%, 11%, and 11%, respectively (Table 3).
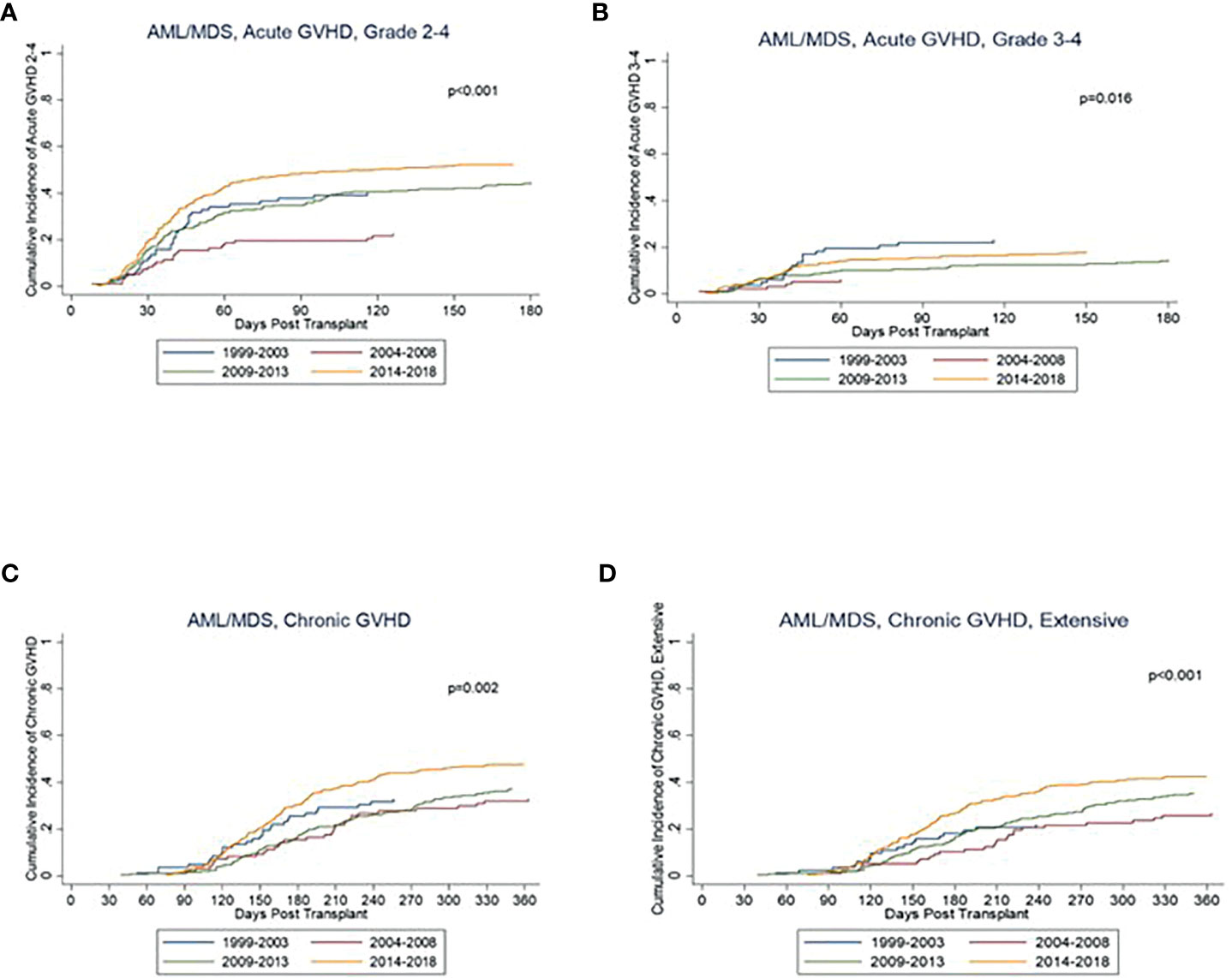
Figure 3 Kaplan Meier curves for GVHD including (A) acute GVHD grade 2-4, (B) acute GVHD grade 3-4, (C) chronic GVHD, and (D) chronic GVHD, extensive.
Discussion
Our study showed a significant improvement in survival in AML and MDS patients undergoing allo-SCT over the past several decades. This is a sub-analysis of our previously published study showing improved PFS and OS across years in patients with hematologic malignancies undergoing allogenic transplantation (7). In this study our findings are consistent with several other retrospective studies that have evaluated trends in allo-HCT survival outcomes over the years (6, 10–13). We demonstrated an improvement in 5-year PFS from 17% to 49% and an improvement in OS from 12% to 53%. We observed that there was worsening of PFS and OS in the years 1999-2003. This could be related to an increase in NRM as shown in Table 2.
There are several key changes in our transplant practice that likely led to these improved outcomes. For instance, on one hand there was an increase in the use of RIC conditioning regimens as compared to MA regimens, which allowed patients with co-morbid conditions as well as older patients to be treated with RIC regimens. These RIC regimens have been shown to have lower toxicity profiles overall and consequently are less likely to lead to organ damage as compared to MA regimens without any significant overall reduction in efficacy (14). The change in GVHD prophylaxis over time may also have led to lower rates of grade II-IV aGVHD and extensive cGVHD. We saw a shift toward the use of Tacrolimus based regimens in place of Cyclosporine based ones. These regimens have been shown to have lower rates of both grade II-IV aGVHD and extensive cGVHD in HLA-matched sibling and unrelated donor transplants (15, 16).
Transplant complications also decreased over time with a significant decrease in pulmonary infections, VOD, hemorrhagic cystitis, and infections complications including bacteremia, viremia, and fungemia in the first day +100. Lower rates of infectious complications likely reflect the use of routine fungal prophylaxis for high risk patients, implementation of CMV prophylaxis for patients when indicated, use of less intensive preparative regimens, and lower doses of prednisone as well as development of alternate agents to treat aGVHD. While there was an increased rate of VOD in the years 1994-2003 likely due to myeloablative regimen and increase in diagnostic criteria, the lower rates of VOD were noticeable in the years after due to decreased utilization of aggressive conditioning regimens, as MA conditioning regimens have been shown have a higher risk of VOD as compared to RIC regimens (17). The introduction of defibrotide also likely led to a reduced mortality in those patients with VOD, as this has been shown to have a significant impact on outcomes in these patients (18).
While other transplant complications decreased over the years, there was a trend toward an increase in rates of grade II-IV aGVHD as well as extensive cGVHD. This trend in the setting of improved outcomes may reflect several factors. For one, detection of aGVHD grade II was higher in these groups, mostly due to clinical determination of upper GI GVHD (versus medication induced upper GI symptoms). The fact that there were increasing rates of aGVHD but improved outcomes overall may also reflect the graft-versus-leukemia (GVL) effect, with patients with GVHD potentially having higher rates of survival due to the GVL effect (19). Higher rates of GVHD may also be a least partially related to the increase in peripheral blood stem cell as a source in place of bone marrow, as prior studies have suggested a higher incidence in grade III-IV aGVHD as well as extensive cGVHD in patients undergoing allo-HCT with peripheral blood source (20–23). A prior CIMBTR analysis from 2015 also showed an up-trend in rates of cGVHD over time independent of stem cell source, which suggests that there may be other factors contributing to a trend toward an increase in cGVHD over time (24).
We also found a significant increase in the average age for patients with AML and MDS undergoing allo-HCT, with an increase from 30.4 years old from 1984-1988 to 55.8 years old from 2014-2018. This is in line with CIBMTR data, which has shown an increase in allo-HCT utilization in older patients over time with patients aged 65 and older representing 26% of patients undergoing allo-HCT in 2019 in the United States (25). The principle reason for this increase is likely the expansion of RIC regimens, which has allowed older patients who are traditionally thought to be at higher risk of treatment related morbidity and mortality as compared to their younger counterparts to safely undergo allo-HCT. The impacts of age on outcomes of allo-HCT as well as the safest age cutoff for allo-HCT are ongoing topics of research, as prior studies have shown variable findings (26–29).
The primary limitation of our study is the nature of its retrospective design. Information on rates of acute and chronic GVHD were missing prior to 1999 so trends in GVHD were unable to be assessed during this time period. Impact of disease specific factors was also unable to be evaluated, as this information was not available during the early transplant years.
Overall, while outcomes post allo-HCT have improved significantly over time in AML and MDS patients, post-transplant relapse unfortunately remains a significant cause of transplant failure in this group (30, 31). Preventing post-transplant relapse is one of the primary targets of continued research today.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by The Institutional Review Board at The Ohio State University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
AS: Data curation, Writing- original draft, Writing- review & editing. JJ: Data curation, Writing- review & editing. QZ: Writing- review & editing, Formal analysis, Methodology. PE: Writing- review & editing, Data curation. DB: Writing- review & editing. SV: Writing- review & editing. SJ: Writing- review & editing. AM: Writing- review & editing. HC: Writing- review & editing. KL: Writing- review & editing. JB: Writing- review & editing. SW: Writing- review & editing. NG: Writing- review & editing. WB: Writing- review & editing. SP: Writing- review & editing. YE: Writing- review & editing, Conceptualization, Supervision. NS: Conceptualization, Writing- original draft, Writing- review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2023.1274649/full#supplementary-material
References
1. Heuser M, Ofran Y, Boissel N, Brunet Mauri S, Craddock C, Janssen J, et al. Acute myeloid leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol (2020) 31(6):697–712. doi: 10.1016/j.annonc.2020.02.018
2. de Witte T, Bowen D, Robin M, Malcovati L, Niederwieser D, Yakoub-Agha I, et al. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood (2017) 129(13):1753–62. doi: 10.1182/blood-2016-06-724500
3. Gyurkocza B, Lazarus HM, Giralt S. Allogeneic hematopoietic cell transplantation in patients with AML not achieving remission: potentially curative therapy. Bone Marrow Transplant (2017) 52(8):1083–90. doi: 10.1038/bmt.2017.8
4. D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current use of and trends in hematopoietic cell transplantation in the United States. Biol Blood Marrow Transplant (2020) 26(8):e177–82. doi: 10.1016/j.bbmt.2020.04.013
5. Styczynski J, Tridello G, Koster L, Iacobelli S, van Biezen A, van der Werf S, et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant (2020) 55(1):126–36. doi: 10.1038/s41409-019-0624-z
6. Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med (2010) 363(22):2091–101. doi: 10.1056/NEJMoa1004383
7. Jiang J, Sigmund AM, Zhao Q, Elder P, Benson DM, Vasu S, et al. Longitudinal survival outcomes in allogeneic stem cell transplantation: an institutional experience. Cancers (Basel) (2022) 14(22). doi: 10.3390/cancers14225587
8. Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation (1974) 18(4):295–304. doi: 10.1097/00007890-197410000-00001
9. Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant (2009) 15(12):1628–33. doi: 10.1016/j.bbmt.2009.07.004
10. Remberger M, Ackefors M, Berglund S, Blennow O, Dahllof G, Dlugosz A, et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant (2011) 17(11):1688–97. doi: 10.1016/j.bbmt.2011.05.001
11. Giebel S, Labopin M, Holowiecki J, Labar B, Komarnicki M, Koza V, et al. Outcome of HLA-matched related allogeneic hematopoietic stem cell transplantation for patients with acute leukemia in first complete remission treated in Eastern European centers. Better results in recent years. Ann Hematol (2009) 88(10):1005–13. doi: 10.1007/s00277-009-0719-5
12. Horan JT, Logan BR, Agovi-Johnson MA, Lazarus HM, Bacigalupo AA, Ballen KK, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol (2011) 29(7):805–13. doi: 10.1200/JCO.2010.32.5001
13. McDonald GB, Sandmaier BM, Mielcarek M, Sorror M, Pergam SA, Cheng GS, et al. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: comparing 2003-2007 versus 2013-2017 cohorts. Ann Intern Med (2020) 172(4):229–39. doi: 10.7326/M19-2936
14. Warlick ED, DeFor TE, Bejanyan N, Holtan S, MacMillan M, Blazar BR, et al. Reduced-intensity conditioning followed by related and unrelated allografts for hematologic Malignancies: expanded analysis and long-term follow-up. Biol Blood Marrow Transplant (2019) 25(1):56–62. doi: 10.1016/j.bbmt.2018.07.038
15. Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood (2000) 96(6):2062–8.
16. Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood (1998) 92(7):2303–14.
17. Carreras E, Diaz-Beya M, Rosinol L, Martinez C, Fernandez-Aviles F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant (2011) 17(11):1713–20. doi: 10.1016/j.bbmt.2011.06.006
18. Richardson PG, Riches ML, Kernan NA, Brochstein JA, Mineishi S, Termuhlen AM, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood (2016) 127(13):1656–65. doi: 10.1182/blood-2015-10-676924
19. Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood (2008) 112(12):4371–83. doi: 10.1182/blood-2008-03-077974
20. Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med (2012) 367(16):1487–96. doi: 10.1056/NEJMoa1203517
21. Anderson D, DeFor T, Burns L, McGlave P, Miller J, Wagner J, et al. A comparison of related donor peripheral blood and bone marrow transplants: importance of late-onset chronic graft-versus-host disease and infections. Biol Blood Marrow Transplant (2003) 9(1):52–9. doi: 10.1053/bbmt.2003.50000
22. Stem Cell Trialists’ Collaborative, G. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic Malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol (2005) 23(22):5074–87.
23. Lee SJ, Logan B, Westervelt P, Cutler C, Woolfrey A, Khan SP, et al. Comparison of patient-reported outcomes in 5-year survivors who received bone marrow vs peripheral blood unrelated donor transplantation: long-term follow-up of a randomized clinical trial. JAMA Oncol (2016) 2(12):1583–9. doi: 10.1001/jamaoncol.2016.2520
24. Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant (2015) 21(2):266–74. doi: 10.1016/j.bbmt.2014.10.021
25. Phelan R, Arora M, Chen M. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides. (2020).
26. Pohlen M, Groth C, Sauer T, Gorlich D, Mesters R, Schliemann C, et al. Outcome of allogeneic stem cell transplantation for AML and myelodysplastic syndrome in elderly patients (60 years). Bone Marrow Transplant (2016) 51(11):1441–8. doi: 10.1038/bmt.2016.156
27. Rashidi A, Ebadi M, Colditz GA, DiPersio JF. Outcomes of allogeneic stem cell transplantation in elderly patients with acute myeloid leukemia: A systematic review and meta-analysis. Biol Blood Marrow Transplant (2016) 22(4):651–7. doi: 10.1016/j.bbmt.2015.10.019
28. Ringden O, Boumendil A, Labopin M, Canaani J, Beelen D, Ehninger G, et al. Outcome of allogeneic hematopoietic stem cell transplantation in patients age >69 years with acute myelogenous leukemia: on behalf of the acute leukemia working party of the european society for blood and marrow transplantation. Biol Blood Marrow Transplant (2019) 25(10):1975–83. doi: 10.1016/j.bbmt.2019.05.037
29. Muffly L, Pasquini MC, Martens M, Brazauskas R, Zhu X, Adekola K, et al. Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood (2017) 130(9):1156–64. doi: 10.1182/blood-2017-03-772368
30. Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant (2015) 21(3):454–9. doi: 10.1016/j.bbmt.2014.11.007
31. Schmid C, de Wreede LC, van Biezen A, Finke J, Ehninger G, Ganser A, et al. Outcome after relapse of myelodysplastic syndrome and secondary acute myeloid leukemia following allogeneic stem cell transplantation: a retrospective registry analysis on 698 patients by the Chronic Malignancies Working Party of the European Society of Blood and Marrow Transplantation. Haematologica (2018) 103(2):237–45. doi: 10.3324/haematol.2017.168716
Keywords: acute myeloid leukemia, allogenic transplantation, overall survival, progression-free survival, graft-versus-host disease
Citation: Sigmund AM, Jiang J, Zhao Q, Elder P, Benson DM, Vasu S, Jaglowski S, Mims AS, Choe H, Larkin K, Brammer JE, Wall SA, Grieselhuber N, Basem W, Penza S, Efebera YA and Sharma N (2023) Improvement in survival of acute myeloid leukemia and myelodysplastic syndrome patients following allogeneic transplant: a long-term institutional experience. Front. Hematol. 2:1274649. doi: 10.3389/frhem.2023.1274649
Received: 08 August 2023; Accepted: 13 September 2023;
Published: 03 October 2023.
Edited by:
Francesco Di Raimondo, University of Catania, ItalyReviewed by:
Francesco Onida, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyRaffaele Palmieri, University of Rome Tor Vergata, Italy
Copyright © 2023 Sigmund, Jiang, Zhao, Elder, Benson, Vasu, Jaglowski, Mims, Choe, Larkin, Brammer, Wall, Grieselhuber, Basem, Penza, Efebera and Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nidhi Sharma, TmlkaGkuU2hhcm1hQG9zdW1jLmVkdQ==
†Present address: William Basem, OhioHealth, Blood and Marrow Transplant and Cellular Therapy, Columbus, OH, United States
Yvonne A. Efebera, OhioHealth, Blood and Marrow Transplant and Cellular Therapy, Columbus, OH, United States
 Audrey M. Sigmund
Audrey M. Sigmund Justin Jiang2
Justin Jiang2 Qiuhong Zhao
Qiuhong Zhao Alice S. Mims
Alice S. Mims Hannah Choe
Hannah Choe Jonathan E. Brammer
Jonathan E. Brammer Sarah A. Wall
Sarah A. Wall Nicole Grieselhuber
Nicole Grieselhuber Nidhi Sharma
Nidhi Sharma