- 1Department of Obstetrics & Gynecology, Women’s Wellness and Research Center, Doha, Qatar
- 2Clinical Department, College of Medicine, Qatar University, Doha, Qatar
- 3Department of Hematology, Hamad Medical Corporation, Doha, Qatar
- 4Internal Medicine department, Hamad Medical Corporations, Doha, Qatar
Immune thrombocytopenia (ITP) affects 0.1 to 1 per 1000 pregnancies and severe ITP, with platelet counts less than 10,000/µL, is difficult to manage. Two pregnant patients with ITP who were successfully treated with thrombopoietin receptor agonists (TPO-RA) at a tertiary institution are discussed. The first patient had chronic ITP, achieved complete remission with eltrombopag, but was resistant to intravenous immunoglobulin (IVIG) and steroids in her second pregnancy. Romiplostim was effective, and she had an uneventful cesarean section. The second patient responded well to eltrombopag at 35 weeks of gestation and had a vaginal delivery. ITP in pregnancy is managed based on clinical expertise, and TPO-RA use during pregnancy is largely from case reports. For severe ITP, eltrombopag or romiplostim from around 34 weeks can be used if other treatments fail, with a goal of achieving a platelet count of over 80,000/µL. The mother’s response to medication may vary in different pregnancies. Induction of labor may be appropriate in some cases.
Introduction
Thrombocytopenia is a condition characterized by a low platelet count, which is defined as below the normal range of 150,000/µL. In 7-12% of pregnant women, there is a decrease in platelet counts, especially during the third trimester (1). Gestational thrombocytopenia can occur if the initial level of platelets is in the lower normal range, and a further drop can lead to gestational thrombocytopenia. Platelet counts are slightly lower in twin pregnancies than in singleton pregnancies (1). In addition to gestational thrombocytopenia, which accounts for 75% of all causes of thrombocytopenia in pregnancy, there are numerous other causes. These include Microangiopathic hemolytic anemia (MAHA), antiphospholipid antibody syndrome, systemic lupus erythematosus, type 2b von Willebrand disease, viral infections, particularly hepatitis B and C virus, cytomegalovirus, human immunodeficiency virus, Epstein-Barr virus, various drugs, and severe vitamin B12 deficiency (2, 3).
Idiopathic thrombocytopenic purpura (ITP) is a clinically significant cause of thrombocytopenia in pregnancy, occurring in 1/10,000 to 1/1000 pregnancies (4, 5). While it accounts for only 3% of all cases of thrombocytopenia in pregnancy, it can have adverse fetal and maternal outcomes. ITP is the most common cause of platelet counts below 50,000/µL in the first and second trimesters (6). Antiplatelet antibodies that cause thrombocytopenia in the mother can also cross the placenta and cause fetal thrombocytopenia, increasing the risk of intracranial hemorrhage at delivery (7, 8). Two-thirds of ITP cases are diagnosed before pregnancy, but one-third are diagnosed during antenatal visits through a complete blood count. However, distinguishing between mild cases of ITP and severe cases of gestational thrombocytopenia can be challenging (4, 9).
Patients with ITP can have variable platelet counts due to the disease. Severe ITP is defined as platelet counts below 50,000/µL and occurs in a small fraction of pregnancies, estimated to be around 0.83/10,000 maternities, but it is of particular concern. Severe postpartum hemorrhage risks increase for the mother, and there is a historical fear of neonatal thrombocytopenia and intracranial hemorrhage (8).
When treating ITP during pregnancy, optimal treatment options are limited by the presence of the fetus. Some medications that are effective outside of pregnancy are contraindicated during pregnancy, while the safety of others is unknown. To address this issue, medications used to treat ITP during pregnancy are classified based on their safety profile and intended benefits to the mother in keeping platelet counts at appropriate levels (10). The first-line treatments are oral prednisolone, intravenous immunoglobulin (IVIG), or a combination of both. High-dose intravenous methylprednisolone (with or without IVIG) is used as a second-line therapy for patients resistant to oral prednisone and IVIG. These medications have side effects and variable success rates in raising platelet counts (11). Splenectomy is rarely indicated in pregnancy. Teratogens such as mycophenolate mofetil, Cyclophosphamide, Vinca alkaloids, and Danazol are contraindicated in pregnancy (8, 11).
The difficulty arises when these medications fail to increase the platelet count, particularly near the delivery time. The mothers are at high risk of life-threatening bleeding at this stage, especially if their platelet count is critically low. Recently, there has been increasing interest in using TPO-RA to manage these refractory cases during pregnancy. Outside of pregnancy, hematologists have used them successfully for over a decade, and they have decreased splenectomy rates among these patients (12).
However, data regarding these medications’ fetal safety profiles is limited. Currently, case reports make up a significant portion of our understanding of the unwanted side effects on the fetus and the maternal response during pregnancy, which can differ from non-pregnant women. Due to the rarity of severe ITP resistant to first-line treatments, conducting randomized controlled trials may not be feasible to compare treatments and provide more definitive evidence (13). We hope that our case report contributes to the existing evidence.
We present two case reports of patients treated at our institution with two different TPO-RA: Eltrombopag and Romiplostim. We also demonstrate that the same patient responds differently to medications during different pregnancies. Additionally, we performed a literature review on existing studies using TPO-RA in pregnancy.
Case scenarios
Case 1
Our first patient was a 28-year-old nulliparous woman with a history of chronic immune thrombocytopenia (ITP). Prior to her pregnancy, she was known to be ‘steroid-resistant’ and only achieved complete remission on eltrombopag. During the first trimester, she presented with body bruises and a platelet count of 15,000/µL. She had regular follow-ups and planned admissions every 2-3 weeks for intravenous immunoglobulin (IVIG) to maintain her platelets over 20,000/µL. Despite not responding to steroids before her pregnancy, she responded to a combination of steroids and IVIG during pregnancy. A multidisciplinary team meeting was held to plan for her labor and delivery, and a planned labor induction at 38 weeks was performed. The labor and delivery were carefully planned, and she delivered by cesarean section due to suspected fetal compromise during the second stage of labor. She was discharged in good condition a few days after delivery. After her pregnancy, she was started on Eltrombopag, which she responded well to, but it was discontinued as soon as she became pregnant again.
We had the opportunity to manage the same patient in her second pregnancy. Interestingly, she was resistant to both IVIG and steroids, as well as a combination of both. Various steroids were attempted, including prednisolone, a short course of dexamethasone, and a combination with IVIG. Despite these interventions, her platelet count dropped to 8,000/µL, and she did not respond as effectively to these medications as she did in her first pregnancy. An MDT was held, and Romiplostim was initiated at 34 weeks, with weekly administration until delivery. She responded well to Romiplostim, and due to her low-lying placenta, she underwent cesarean section at 38 weeks.
Case 2
Our second patient was a primigravida with a history of ITP since 2018. Prior to her pregnancy, she was resistant to steroids and was started on Eltrombopag. She was referred to our institution at eight weeks gestation with a critically low platelet level of 13,000/µL detected during a routine pregnancy checkup. However, she did not have any episodes of bleeding, ecchymosis, or other bleeding manifestations.
She was regularly followed up in the high-risk antenatal and specialized hematology clinics. At 24 weeks, she was started on IVIG and steroids, but the steroid dose was tapered at 28 weeks due to a lack of response and rising blood sugars. Despite these interventions, her platelet count remained around 25,000/µL with no further improvement. Following MDT, the patient was offered Eltrombopag at 35 weeks gestation. She initially received a dose of 25 mg, followed by an increase to 50 mg after one week, resulting in a platelet count of 51,000/µL. She remained asymptomatic throughout and was planned for a labor induction at 38 weeks. However, at 37 + 2 weeks, she presented with labor pains, and her platelet count was 62,000/µL. Fortunately, she progressed well in labor, had a vaginal delivery, and was discharged with good maternal and fetal outcomes. Her postpartum period was uneventful.
Discussion
When treating immune thrombocytopenia (ITP) during pregnancy, the main goal is to achieve a safe platelet count that minimizes the risk of maternal bleeding, rather than normalizing the count. Platelet counts should be monitored regularly during pregnancy, with more frequent monitoring if the count drops or is low (6, 14). Treatment decisions should be based on maternal indications, as there is no evidence that current medications can improve fetal thrombocytopenia or neonatal outcomes (14). The minimum safe platelet count varies by gestational stage, with a target of over 20,000-30,000/µL reasonable during early pregnancy if no invasive procedures are planned and the patient is asymptomatic. Intravenous immunoglobulin (IVIG) can be administered every two to three weeks to raise the platelet count more than 10,000/µL, but aiming for higher levels may be unrealistic (11). In practice, despite administering IVIG every two weeks, the platelet count could only be increased to just over 20,000/µL, which is considered safe.
In the weeks leading up to delivery (from 34 weeks), it is important to maintain a platelet count of over 50,000/µL to reduce the risk of complications during childbirth. While some sources suggest a minimum count of 40,000 for vaginal delivery and 50,000 for cesarean section, it is recommended to aim for a count of at least 50,000 for both types of delivery (3, 11, 15). However, real-world situations can be more complex than these guidelines suggest. The evidence supporting the minimum platelet count (50,000/µL) is often based on studies from other medical procedures, these levels represent the minimum for elective non-neuraxial surgery (16). Pregnancy and labor present unique challenges; for instance, many mothers require epidurals for pain relief, which can further complicate platelet count management (3, 8).
Individualized care is crucial in managing platelet counts for pregnant women with severe ITP. The ideal goal is to achieve a level of over 80,000/µL closer to delivery, providing a safe threshold for any mode of delivery and allowing for pain relief during labor. Cesarean sections may result in more blood loss than vaginal deliveries, and pregnancy-related procedures may differ from other operations. However, achieving this level can be challenging but should be the goal whenever possible. Considering these goals, our first case (in her first pregnancy) responded well to steroids combined with regular IVIG, even though she did not respond to steroids before becoming pregnant. Therefore, a vital practice point is to test these medications during pregnancy. The maternal response during pregnancy can differ from outside of pregnancy.
Interestingly, the same patient did not respond to either IVIG or steroids in her subsequent pregnancy, providing another interesting perspective that maternal response can differ between pregnancies. At around 32 weeks, An MDT consisting of obstetricians, hematologists, intensivists, anesthesiologists, and neonatologists gathered because her platelets had dropped to 8,000/µL, and she did not respond to IVIG and steroids as she had in her first pregnancy. We offered the patient the TPO agonist Romiplostim, and she responded favorably to the treatment. There were no reported side effects on the mother or fetus.
In our second case, there was no response to regular IVIG, and the platelet count remained around 25,000/µL with no further improvement. Following an MDT meeting, the patient was offered Eltrombopag at 35 weeks gestation. Initially, she received a treatment of 25 mg, which was followed by a one-week increase to 50 mg, after which her platelet count reached 51,000/µL. When she presented with labor pains at 37 + 2 weeks, her platelet count had increased to 62,000/µL. She progressed well during labor, had a vaginal delivery, was discharged with good maternal and fetal outcomes, and had an uneventful postpartum period.
There is limited data on the use of TPO receptor agonists such as Romiplostim or Eltrombopag in pregnancy, and most of the available data comes from case reports (17–31). Both medications can cross the placenta and potentially cause unwanted side effects to the fetus. During pregnancy, they are considered category C drugs according to the Food and Drug Administration (FDA) (32, 33). The Electronic Medicines Compendium (EMC) notes that in animal studies, Romiplostim crosses the placenta and can cause fetal thrombocytosis, post-implantation loss, and increased fetal and perinatal mortality in pups. On the other hand, high doses of Eltrombopag were associated with low birth weight in rabbits and rats. It should be noted that very high doses were used for prolonged periods in these animal studies (34, 35).
So far, data from most case reports on pregnant women have been promising, with minor safety concerns for mothers or newborns (11). In a study of 31 patients with platelets less than 30,000/µL, resistant to both IVIG and steroids, TPO-RA was well tolerated by patients and was not associated with adverse effects in newborns. The overall response rate to TPO-RA was around 74% (36). A recent multicenter study on 15 patients showed no severe adverse effects, and the treatment seemed safe for the mother, fetus, and neonate. Furthermore, as opposed to animal studies, TPO-RA did not significantly impact fetal thrombopoiesis. Thrombocytosis was observed in only one neonate. There were no differences between Romiplostim and Eltrombopag in the study (17).
Moreover, according to a case study, Eltrombopag has been used successfully during the first and even the second trimester (37). However, one concern about starting TPO-RA too early in pregnancy is the unknown effects on the fetus. For instance, one case report showed fetal supraventricular tachycardia and preeclampsia after using Eltrombopag. Although causation is difficult to prove, this necessitated delivery of the patient at 30 weeks (25). Additionally, a literature review suggested a link between Eltrombopag and low birth weight if administered during the first or second trimesters. This finding is, however, limited by the fact that the use of Eltrombopag was confounded by the use of multiple other medications, variable patient factors, and a small sample size (38).
Based on the limited data available, a reasonable and pragmatic approach is to administer the smallest possible dose of TPOs for the shortest duration, late in pregnancy, until more is known about their effects on the mother and fetus (11). In refractory cases where all other treatments have failed, initiating TPOs around 34 weeks helps to achieve safe platelet levels closer to delivery. In our patients, IVIG therapy was administered every two weeks to maintain a safe platelet count closer to delivery.
Regarding the delivery mode, this should be based on obstetric considerations, as there is no evidence that a cesarean section is safer for the fetus with thrombocytopenia. Furthermore, a vaginal delivery is safer for the mother (6). We induced the first case in her first pregnancy and offered induction for the second case. The platelets were critically low in both patients and significantly fluctuated between IVIG treatments. IVIG can provide a relatively rapid but transient increase in platelet counts (10).
We found little information in our search on whether to induce labor in these patients or await spontaneous delivery. In the studies that addressed this, the authors suggest that labor induction in these cases is indicated only for obstetric reasons (39). However, individualized care is essential in these cases. In some patients, induction of labor is necessary to schedule delivery at a time when platelet levels are over 50,000/µL, especially if levels fluctuate significantly between treatment cycles. In a prospective cohort study conducted in the United Kingdom (UK) with data collected from the national UK Obstetric Surveillance System (UKOSS), the authors found more than triple the rates of induction of labor in cases with severe ITP (38%) compared to the national rate of 13.2% (8).
If a patient goes into labor with platelets below 50,000/µL, platelet transfusions should be administered, and additional platelets should be kept on standby. Checking the availability of platelets during the planning phase is essential, including availability on weekends. Platelet transfusions should be given during established labor, rather than very early in labor. Due to the immune response, transfused platelets may be quickly destroyed, leading to transient effects (7), and platelet transfusions alone may not be effective. Considering the administration of IVIG to these patients can help achieve adequate platelet counts.
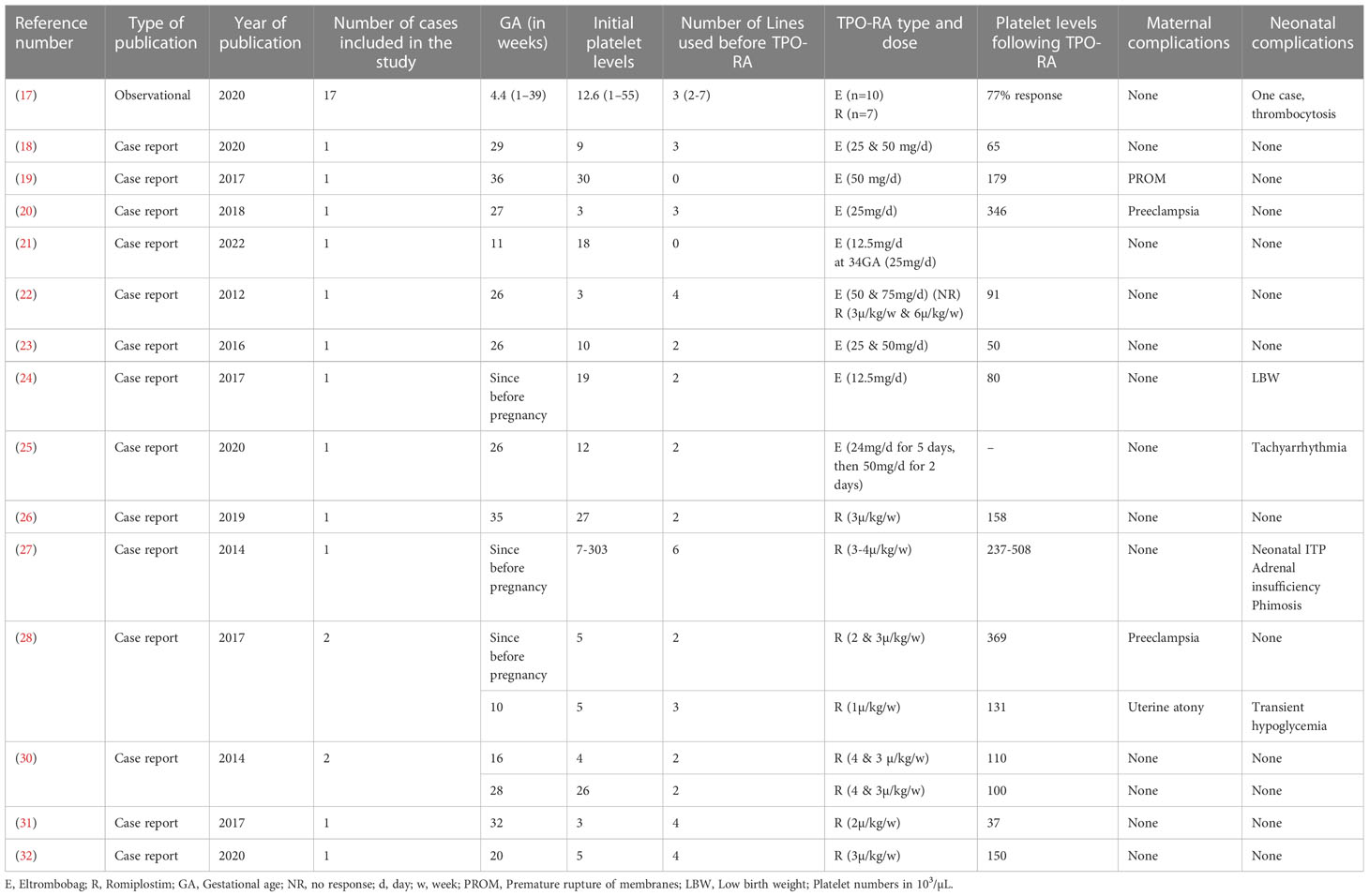
We conducted a literature review for relevant studies on TPO agonists during pregnancy (see Table 1) using electronic databases such as PubMed, Google Scholar, and Scopus. The search was limited to the English language, and we found one observational study along with several case series and case reports. The earliest case report was from 2012, and the most recent was from 2022. In total, we identified thirty-three studies. We extracted baseline information from these studies, including gestational age, initial platelet levels, the number of prior treatment modalities, the TPO used, platelet levels after TPO administration, and any complications for the mother and baby.
We compiled the results of these studies in Table 1. The reported gestational weeks with low platelet counts ranged from the beginning of pregnancy to late in the third trimester. This wide range was demonstrated in a study involving 17 patients, with platelet counts ranging from 1 week to 39 weeks of gestation. The median platelet count was 15,000/µL, and 17 patients (51%) received Eltrombopag tablets, while the remaining 49% received Romiplostim injections. Prior to using TPO agonists, an average of two different medications had been tried and failed.
After TPO treatment, the median platelet count was 131,000/µL, and the mean was 151,000/µL (we excluded the observational study as the increments were in percentages). Maternal complications observed were limited to two cases of preeclampsia, one case of premature rupture of membranes, and one case of uterine atony. In most cases, fetal outcomes were satisfactory. However, one newborn developed neonatal ITP, while another developed thrombocytosis. In addition, three neonates experienced hypoglycemia, tachyarrhythmia, and low birth weight.
Conclusion
Management of resistant cases of ITP in pregnancy is mainly derived from clinical expertise and expert consensus. Moreover, the majority of experience with TPO-RA during pregnancy comes from case reports. To prepare for delivery, the transient use of Eltrombopag or Romiplostim from around 34 weeks in refractory cases of severe ITP is a reasonable option when other treatments fail. An ideal aim is for a platelet count of over 80,000/µL. Induction of labor is appropriate in a subset of patients with severe disease. In different pregnancies, a mother’s response to medication can vary.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Research Center (MRC), Qatar. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Contribution: GS wrote the manuscript. SF performed literature search on TPO-RA and produced the table of results. GS, SE, SF, and MA edited and revised the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that this study received funding from Qatar National Library (QNL). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ITP, Immune thrombocytopenia; IVIG, intravenous immunoglobulin; CS, Caesarean section; MDT, Multidisciplinary team; TPO-RA, Thrombopoietin Receptor Agonists.
References
1. Reese JA, Peck JD, Deschamps DR, McIntosh JJ, Knudtson EJ, Terrell DR, et al. Platelet counts during pregnancy. N Engl. J. Med. (2018) 379(1):32–43. doi: 10.1056/NEJMoa1802897
2. Habas E, Rayani A, Alfitori G, Eldin Ahmed G, Elzouki ANY. Gestational thrombocytopenia: A review on recent updates. cureus (2022). Available at: https://www.cureus.com/articles/84295-gestational-thrombocytopenia-a-review-on-recent-updates.
3. ACOG practice bulletin no. 207: Thrombocytopenia in pregnancy. Obstet Gynecol (2019) 133(3):e181–93.
4. Myers B. Diagnosis and management of maternal thrombocytopenia in pregnancy. Br. J. Haematol. (2012) 158(1):3–15. doi: 10.1111/j.1365-2141.2012.09135.x
5. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am. J. Hematol. (2010). doi: 10.1002/ajh.21616
6. Cines DB, Levine LD. Thrombocytopenia in pregnancy. Blood (2017) 130(21):2271–7. doi: 10.1182/blood-2017-05-781971
7. Burrows RF, Kelton JG. Fetal thrombocytopenia and its relation to maternal thrombocytopenia. N Engl. J. Med. (1993) 329(20):1463–6. doi: 10.1056/NEJM199311113292005
8. Care A, Pavord S, Knight M, Alfirevic Z. Severe primary autoimmune thrombocytopenia in pregnancy: a national cohort study. BJOG Int. J. Obstet Gynaecol (2018) 125(5):604–12. doi: 10.1111/1471-0528.14697
9. Webert KE, Mittal R, Sigouin C, Heddle NM, Kelton JG. A retrospective 11-year analysis of obstetric patients with idiopathic thrombocytopenic purpura. Blood (2003) 102(13):4306–11. doi: 10.1182/blood-2002-10-3317
10. Gernsheimer T, James AH, Stasi R. How I treat thrombocytopenia in pregnancy. Blood (2013) 121(1):38–47. doi: 10.1182/blood-2012-08-448944
11. Provan D, Arnold DM, Bussel JB, Chong BH, Cooper N, Gernsheimer T, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. (2019) 3(22):3780–817. doi: 10.1182/bloodadvances.2019000812
12. Chaturvedi S, Arnold DM, McCrae KR. Splenectomy for immune thrombocytopenia: down but not out. Blood (2018) 131(11):1172–82. doi: 10.1182/blood-2017-09-742353
13. Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, et al. American Society of hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. (2019) 3(23):3829–66. doi: 10.1182/bloodadvances.2019000966
14. Stavrou E, McCrae KR. Immune thrombocytopenia in pregnancy. Hematol. Oncol. Clin. North Am. (2009) 23(6):1299–316. doi: 10.1016/j.hoc.2009.08.005
15. Myers B. Thrombocytopenia in pregnancy. Obstet Gynaecol (2009) 11(3):177–83. doi: 10.1576/toag.11.3.177.27502
16. Kaufman RM, Djulbegovic B, Gernsheimer T, Kleinman S, Tinmouth AT, Capocelli KE, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann. Intern. Med. (2015) 162(3):205–13. doi: 10.7326/M14-1589
17. Michel M, Ruggeri M, Gonzalez-Lopez TJ, Alkindi S, Cheze S, Ghanima W, et al. Use of thrombopoietin receptor agonists for immune thrombocytopenia in pregnancy: Results from a multicenter study. Blood (2020) 136(26):3056–61. doi: 10.1182/blood.2020007594
18. Leng Q, Wang W, Wang Y, Wu L. Treatment of severe thrombocytopenia associated with systemic lupus erythematosus in pregnancy with eltrombopag: A case report and literature review. J. Clin. Pharm. Ther. (2021) 46(2):532–8. doi: 10.1111/jcpt.13321
19. Favier R, De Carne C, Elefant E, Lapusneanu R, Gkalea V, Rigouzzo A. Eltrombopag to treat thrombocytopenia during last month of pregnancy in a woman with MYH9-related disease: a case report. AA Pract. (2018) 10(1):10–2. doi: 10.1213/XAA.0000000000000621
20. Ferreira IJMCF, Sousa F, Vasco EM, Areia ALF de A, Moura JPAS, Carda J, et al. Severe immune thrombocytopenia in pregnancy treated with eltrombopag – a case report. J. Gynecol Obstet Hum. Reprod. (2018) 47(8):405–8. doi: 10.1016/j.jogoh.2018.06.010
21. Shibata S, Misugi T, Nakane T, Hino M, Tachibana D. Use of eltrombopag for the first trimester pregnancy complicated with refractory idiopathic thrombocytopenic purpura: a case report and literature review. cureus (2022). Available at: https://www.cureus.com/articles/85448-use-of-eltrombopag-for-the-first-trimester-pregnancy-complicated-with-refractory-idiopathic-thrombocytopenic-purpura-a-case-report-and-literature-review.
22. Alkaabi J, Alkindi S, Riyami NA, Zia F, Balla L, Balla S. Successful treatment of severe thrombocytopenia with romiplostim in a pregnant patient with systemic lupus erythematosus. Lupus (2012) 21(14):1571–4. doi: 10.1177/0961203312463621
23. Purushothaman J, Puthumana K, Kumar A, Innah S, Gilvaz S. A case of refractory immune thrombocytopenia in pregnancy managed with elthrombopag. Asian J. Transfus Sci. (2016) 10(2):155. doi: 10.4103/0973-6247.177204
24. Suzuki N, Hiraga J, Hariyama Y, Takagi Y, Ohashi H, Kishigami Y, et al. A low birth weight infant with no malformations delivered by a primary immune thrombocytopenia patient treated with eltrombopag. Int. J. Hematol. (2018) 108(1):109–11. doi: 10.1007/s12185-017-2383-1
25. Weingarten SJ, Friedman D, Arora A. Eltrombopag use for refractory immune thrombocytopenia in pregnancy: a case report. Case Rep. Womens Health (2021) 29:e00281. doi: 10.1016/j.crwh.2020.e00281
26. Rosa María RN, Laura RL, Ángeles PB, Laura LB. Use of romiplostim during pregnancy as a rescue therapy in primary immune thrombocytopenia: literature review and case description. Platelets (2020) 31(3):403–6. doi: 10.1080/09537104.2019.1615613
27. Patil AS, Dotters-Katz SK, Metjian AD, James AH, Swamy GK. Use of a thrombopoietin mimetic for chronic immune thrombocytopenic purpura in pregnancy. Obstet Gynecol (2013) 122(2):483–5. doi: 10.1097/AOG.0b013e31828d5b56
28. Samuelson B T, Baumann Kreuziger L, Gernsheimer T. Use of romiplostim for refractory primary immune thrombocytopenia during pregnancy. clin obstet gynecol reprod med (2017). Available at: https://oatext.com/Use-of-romiplostim-for-refractory-primary-immune-thrombocytopenia-during-pregnancy.php.
29. Decroocq J, Marcellin L, Le Ray C, Willems L. Rescue therapy with romiplostim for refractory primary immune thrombocytopenia during pregnancy. Obstet Gynecol (2014) 124(2):481–3. doi: 10.1097/AOG.0000000000000371
30. Harrington P, Nelson-Piercy C, Williamson C, Cooper N, Kesse-Adu R, Robinson S. Refractory severe immune thrombocytopenia in a twin pregnancy. Obstet Med. (2018) 11(1):35–8. doi: 10.1177/1753495X17709188
31. Chon AH, Chan R, Lee RH, Kwong K, Wertheimer FB, Weitz IC. Multidrug therapy for refractory immune thrombocytopenia in pregnancy. Obstet Gynecol (2020) 135(3):723–7. doi: 10.1097/AOG.0000000000003699
32. U.S. Food and Drug Administration. Amgen. nplate (romiplostim). USA: U.S. Food and Drug Administration. (2021). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125268s167lbl.pdf.
33. Promacta (eltrombopag). USA: U.S. Food and Drug Administration. (2021) Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022291s006lbl.pdf.
34. Novartis pharmaceuticals UK. revolade 25 mg film-coated tablets . Available at: https://www.medicines.org.uk/emc/product/7819/smpc.
35. Amgen. nplate 125 micrograms powder for solution for injection vial . Available at: https://www.medicines.org.uk/emc/product/9325/smpc.
36. Kong Z, Qin P, Xiao S, Zhou H, Li H, Yang R, et al. A novel recombinant human thrombopoietin therapy for the management of immune thrombocytopenia in pregnancy. Blood (2017) 130(9):1097–103. doi: 10.1182/blood-2017-01-761262
37. Sammartano V, Santoni A, Defina M, Ciofini S, Cencini E, Bocchia M. Efficacy and safety of eltrombopag during conception and first trimester of pregnancy in a case of refractory severe immune thrombocytopenia. Blood Coagul Fibrinolysis (2020) 31(6):416–8. doi: 10.1097/MBC.0000000000000936
38. Howaidi J, AlRajhi AM, Howaidi A, AlNajjar FH, Tailor IK. Use of thrombopoietin receptor agonists in pregnancy: A review of the literature. Hematol. Oncol. Stem Cell Ther. (2021), S1658387621000558. doi: 10.1016/j.hemonc.2021.05.004
Keywords: immune thrombocytopenia, TPO receptor agonists, Romiplostim, eltrombopag, platelet transfusion, maternal outcomes, fetal outcomes
Citation: Sayed G, ElKourashy SA, Alnajjar M, Mallahi NA and Fareed S (2023) Case Report: Thrombopoietin receptor agonists in resistant thrombocytopenia in pregnancy: a case series and review of literature. Front. Hematol. 2:1180156. doi: 10.3389/frhem.2023.1180156
Received: 05 March 2023; Accepted: 25 April 2023;
Published: 15 May 2023.
Edited by:
Emmanouil Nikolousis, European University Cyprus, CyprusReviewed by:
Serena Valsami, National and Kapodistrian University of Athens, GreeceMuhammad Saboor, University of Sharjah, United Arab Emirates
Copyright © 2023 Sayed, ElKourashy, Alnajjar, Mallahi and Fareed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shehab Fareed, c2hpaGFiZmFyZWVkQHlhaG9vLmNvbQ==
 Gamal Sayed1,2
Gamal Sayed1,2 Sarah A. ElKourashy
Sarah A. ElKourashy Mohammed Alnajjar
Mohammed Alnajjar