- Struttura Complessa (S.C.) di Ematologia e Centro Trapianti di Midollo Osseo (C.T.M.O.) - Ospedale Oncologico di Riferimento Regionale "A.Businco", Cagliari, Italy
Background: Non-Hodgkin’s lymphoma (NHL) and Hodgkin’s lymphoma (HL) are two of the most common hematologic diseases that require an infusion of immunochemotherapies in conjunction with radiotherapy, often in an outpatient setting. For relapsed/refractory disease, autologous peripheral hematopoietic stem cell transplantation and sometimes allogeneic transplantation (HSCT) are considered standard treatment options. Recently, chimeric antigen receptor (CAR) T cells and bispecific antibodies have emerged as an important and effective option for the treatment of relapsed/refractory patients. These medical approaches deserve effective, safe, and durable vascular access, especially for the ambulatory population undergoing discontinuous treatment associated with high rates of complications and life-threatening toxicities. Peripherally inserted central catheters (PICCs) are vascular devices with an intermediate-to-long-term lifespan that are inserted ultrasonically into a peripheral brachial vein. Their ease of insertion by trained nurses and low rate of catheter-related infectious and thrombotic complications make them ideal devices for treating oncology and hematology patients.
Purpose: In this study, we aim to demonstrate that PICCs are an essential tool for the treatment of HL and NHL patients in terms of efficiency and safety
Methods and results: From March 2007 to June 2020, 316 PICC implantations were performed by our PICC team in 276 HL patients and 363 PICC in 322 NHL patients. The total lifespan of the PICCs was 50,660 days in HL and 43,919 days in NHL patients. Most PICCs were removed at the end of therapy, and the rate of mechanical complications was low. Only one and four episodes of confirmed PICC-related catheter-related bloodstream infections (CRBSIs) (0.3%; 0.02/1,000 days/PICC and 1.2%; 0.07/1,000 days/PICC) were recorded in HL and NHL patients, respectively. There were only 11 (3.6%; 0.25/1,000 days/PICC) and nine (2.6%; 0.17/1,000 days/PICC) episodes of symptomatic PICC-related thrombotic complications in HL and NHL patients, respectively, without removal.
Conclusion: Our data indicate that the PICC can be considered the device of choice for treating HL and NHL patients because it is easy to insert, safe to use, long-lasting, and has a low complication rate, especially in the outpatient setting.
Introduction
Non-Hodgkin lymphoma (NHL) and Hodgkin lymphomas (HL) are two of the most common hematologic malignancies. NHLs are the most common hematologic tumor worldwide, accounting for 4.3% of cancer diagnoses in the United States (1). On the other hand, HL accounted for 0.4% of all newly reported cancer cases worldwide in 2020 (2). These two lymphoid malignancies are characterized by distinct subtypes that differ in morphological and biological features, which in turn are associated with different epidemiology, clinical manifestations, and treatment. Despite the different therapeutic approaches, treatments for NHL (3–5) and HL (6–8) patients require the administration of immunochemotherapy and radiotherapy with different schedules (depending on histotype, stage, age, and fitness), often on an outpatient basis. Autologous (9–11) and allogeneic peripheral hematopoietic stem cell transplantation (12, 13) (HSCT) may also be an important option for both populations, especially in relapsed/refractory (R/R) patients. Recently, chimeric antigen T-cell therapy (14–17) and bispecific antibodies (18) have shown high efficacy in R/R NHL patients and have been included in algorithms for the treatment of patients with mantle cell lymphoma (MCL) and diffuse large B-cell lymphoma (DLBCL).
The treatment of oncohematologic patients requires durable, safe, and reliable vascular access. In particular, these patients are characterized by an immunosuppressive status and cytopenias related to underlying diseases and treatment complications. In addition, safe and easy-to-use venous access is essential for chemotherapy administration, blood and platelet transfusions, parenteral nutrition, supportive care, and autologous and allogeneic peripheral stem cell transplantation. Centrally inserted central catheters (CICCs) have been the gold standard for the treatment of oncohematologic patients for many years, but can often cause problems during implantation and removal. The insertion of CICCs can lead to complications (arterial puncture, hematoma, hemothorax, and pneumothorax) in approximately 6% to 19% of cases and is associated with a higher incidence of complications than other devices, such as peripherally inserted central catheters (PICCs) (19). The use of PICCs has increased over the years in hematology departments because PICCs are easier to insert, can be deployed by trained nurses without the need for an operating room, and are easier to remove than tunneled or fully implanted devices, which increases manageability when complications occur (20). In addition, medium-to-long-term use allows discontinuous use, especially in day-case care, and the latest devices with multiple lumens and larger diameters (power PICC) make these catheters suitable and safe for complex procedures, such as autologous and allogeneic hematopoietic peripheral stem cell transplantation. Thrombosis and infection are the most commonly reported PICC-related complications. Although data from retrospective and prospective studies seem to indicate a higher incidence of complications (particularly thromboembolic events) associated with the use of PICCs, recent clinical evaluations have shown that management is safer in terms of the incidence of systemic infection and thrombosis, even in the transplant setting (19, 21–23).
The HL and NHL patients represent the perfect model of a population that can benefit from the advantages of PICC use because of the characteristics of the disease, outpatient treatment in most cases, and the complexity of transplantation and new cellular therapies.
In our study, we analyzed the efficacy and safety of PICCs in the treatment and support of patients with NHL and HL, for whom we consider these devices to be the gold standard for vascular access in the diagnostic-therapeutic program.
Materials and methods
Since its inception in 2007, a PICC team consisting of a hematology physician and three dedicated nurses has conducted a prospective study in our medical oncology department to evaluate the complication rate and utility of the PICC system in hematology clinical practice, particularly in HL and NHL patients. Inclusion criteria included all HL and NHL patients who required a program of immunochemotherapy, supportive care, and HSCT as inpatients or outpatients. All PICC insertions, both elective and urgent procedures, were performed in a dedicated interventional surgical facility within the hematology center using aseptic techniques and only occasionally at the patient’s bedside when the patient was hospitalized. The PICC devices used in clinical practice were Groshong® PICC monolumen 4 French BARD® (BARD® INC, CA, USA), PICC bilumen 5 French Vygon® (Vygon®, Ecouen, France), PowerPICC monolumen 4 French Vygon® (Vygon®, Ecouen, France), PowerPICC monolumen 4 French Medcomp® (Medcomp®, PA, USA), and PowerPICC bilumen 5 French Medcomp® (Medcomp®, PA, USA).
Patients were informed about the procedure and its potential complications. Written informed consent was obtained for catheter insertion and the use of data for scientific purposes.
A complete blood count and coagulation test were performed on all patients. The platelet count (PLT) threshold considered safe for PICC insertion was 10 x 10 (9)/L. Below this threshold, patients received a concentrated PLT infusion before the procedure. A low white blood cell (WBC) count was not evaluated as a contraindication for insertion. In all patients, the vascular anatomy of the arms had been previously assessed by ultrasound. After echo-scan evaluation of the adequate caliber of the vein, the characteristics of blood flow, and the presence of thrombotic features, the vessels most commonly used for implantation were the basilic veins (mostly in the right arm), followed by the brachial and cephalic veins, in order of preference. A local anesthetic (lidocaine 2%) was injected into the subcutaneous tissue at the site of venipuncture. A 21–22-gauge needle was systematically inserted into the anterior vein wall under color Doppler ultrasound guidance and inserted into the vessel until blood return was observed. The next step was to insert a metallic 0.018-inch guidewire into the vein. The site of venipuncture was then enlarged with a scalpel blade, and a micro-introducer was inserted over the guidewire. The PICC was inserted into the micro-introducer until the calculated length was achieved. To prevent early misplacement in 2019, the correct tip position (near the cavo-atrial junction) was confirmed during insertion by real-time intracavitary ECG guidance. A standard chest radiograph was performed in all patients to rule out malposition. In case of mediastinal volume, midline placement was preferred to start therapy because it compresses the deep veins, which often leads to thrombotic complications, followed by PICC insertion if the mass could be reduced. None of the PICCs were sutured and they were fixed with an adhesive dressing (StatLock®; Bard, Murray Hill, NJ). Every 10 days, outpatients received routine PICC site care, puncture site cleaning, and set change in a dedicated room assigned by the PICC team. In case of hospitalization, the drugs were administered in the hematology ward. Additionally, the PICC team checked for the occurrence of PICC-related complications (local infections, hematomas, and mechanical and thrombotic complications) during medication until the removal of the device or patient death. All interventions were recorded. Most catheters were removed by the PICC team. The main criteria for device removal were bacteremia or fungemia related to the PICC, catheter obstruction or dislocation, or malfunction. In cases of thrombotic complications, the PICC was removed only if recanalization of the veins was demonstrated by echo-Doppler after appropriate low-molecular-weight heparin (LMWH) therapy.
The following definitions were used to capture the complications associated with PICCs.
CRBSIs were defined according to the criteria of the CDC’s National Surveillance System for Nosocomial Infections in Atlanta, USA (24). Specifically, a definitive diagnosis of CRBSI requires the detection of the same organism growing in at least one percutaneous blood culture and catheter tip culture, or that two blood samples (from the catheter port and the peripheral vein) meet CRBSI criteria for quantitative blood cultures or differential time to positivity (DTP). An association between thrombosis-related catheter complications (CRT) and CRBSIs (CRBSIs + CRT) was considered when the CRBSI was associated with vascular occlusion requiring combined anticoagulant and antibiotic therapy.
CRTs were defined as the occurrence of a thrombotic episode in the veins assessed by color flow Doppler ultrasonography when symptoms and signs (pain, tenderness, swelling or edema, or warmth) appeared. Direct visualization of endoluminal thrombotic material and the non-compressibility criterion were used to demonstrate the presence of thrombosis. Doppler imaging was used to obtain information on blood flow. Ultrasonography was not routinely performed in asymptomatic patients to monitor venous blood flow. CRT not associated with signs or symptoms was not considered a complication in our study. According to internal guidelines, the administration of LMWH in the case of CRT was based on the PLT count: the full dose of the drug (100 UI/kg twice) was administered when the platelet count was >50 x 109. The 50% dose was considered when the PLT value was between 50 x 109 and 20 x 109, and transfusion of platelet concentration was administered in the case of a PLT count <20 x 109.
Mechanical complications included obstruction, malfunction, rupture, and dislocation.
Statistical analysis was performed using R (25). If more than one PICC was used in a single patient, each was counted as a separate event in our analysis. The rate of infectious and thrombotic complications was expressed as absolute number/1,000 days/PICC.
Results
From March 2007 to June 2020, 316 PICC implantations were performed in 276 HL patients (156 men and 120 women) and 363 PICC in 322 NHL patients (178 men and 144 women). The mean age for HL was 36 years (range 16–85) and 58 (range 17–86) for NHL.
Indication
In HL, 305 (96.5%) patients were used for immunochemotherapy, two (0.6%) for supportive care, seven (2.2%) for autologous HSCT, one (0.3%) for allogeneic HSCT, and one (0.3%) for a tandem program of autologous and allogeneic HSCT. Similar data were observed in the NHL population: 333 PICC (91.7%) for immunochemotherapy courses, 13 (3.6%) for supportive care, 14 (3.8%) for autologous HSCT, and three (0.8%) for allogeneic HSCT.
148 (46.8%) and 255 (70.2%) PICCs were implanted in HL and NHL patients who had previously received chemotherapy, respectively.
Device Characteristics and Implantation Techniques
Of 316 PICCs implanted in HL patients, 184 (58.2%) were non-valved and 132 (41.8%) were valved. There were 200 polyurethane devices implanted (63.3%), of which 148 (74% polyurethane and 46.8% total PICC) were power PICCs. There were 116 (36.7%) silicone catheters. Only nine devices were double-lumen power PICCs (2.8% of the total). Intracavitary ECG guidance was used for implantation in 58 (18.4%) PICCs.
In the NHL population, 289 (79.6%) were non-valved and 74 (20.4%) were characterized by a valved system. 306 (84.3%) and 57 (15.7%) devices were made of polyurethane and silicone, respectively. There were 255 power PICCs (70.2% of the total and 83.3% of the polyurethane catheters) and 30 bilumen power PICCs (8.3% of total catheters). A total of 62 (17%) PICCs were inserted with intracavitary ECG guidance.
Outcome and Complications
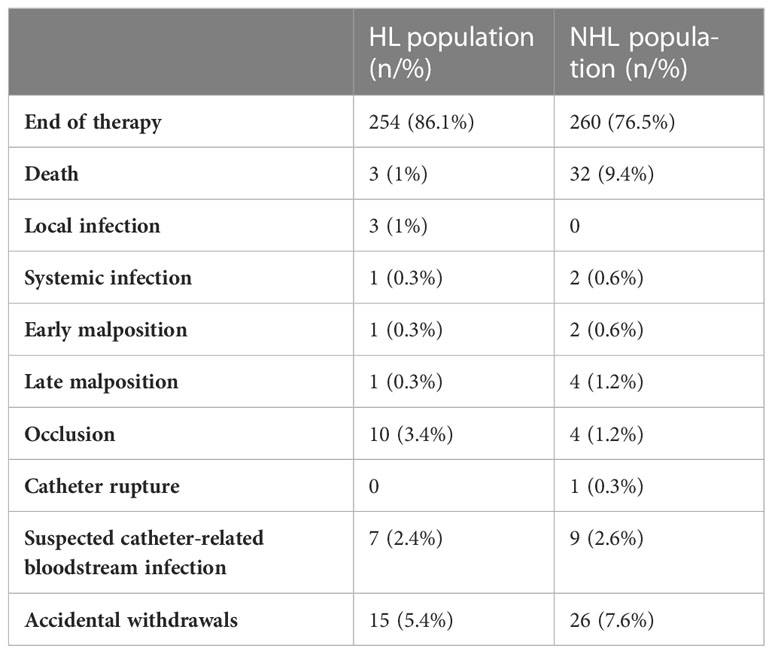
Twelve and 20 PICCs were lost during follow-up in the HL and NHL populations, respectively. Therefore, an analysis of duration and complications was performed in 304 and 343 devices, respectively. Nine of 304 PICCs (2.9%) in HL patients and 3 of 343 (0.9%) in NHL patients were still in situ and in use at the time of analysis. The total duration of PICC life was 50,660 days (median 157; range 1-828) in the HL population and 43,919 days (median 150; range 1-518) in the NHL patients. The reasons for the difference in the two populations are shown in Table 1.
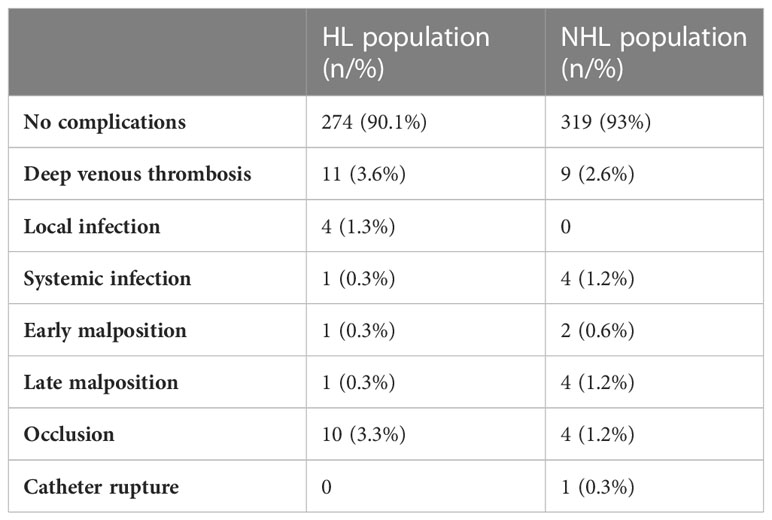
Of note, most PICCs in HL and NHL patients were removed at the end of therapy (86.1% vs. 76.5%), and none of the suspected catheter-related bloodstream infections were confirmed by microbiological testing. Only one case of rupture was recorded in an NHL patient with a silicone PICC. Catheter salvage was performed under angiographic guidance. Overall, the data were consistent between the populations despite the differences in therapeutic regimens. Complications recorded in both populations are described in Table 2.
No complications occurred in more than 90% of PICCs in either population. Only one and four episodes of confirmed PICC-related CRBSI (0.3%; 0.02/1,000 days/PICC and 1.2%; 0.07/1,000 days/PICC) were recorded in HL and NHL patients, respectively. The pathogens isolated in blood cultures and PICC tip analysis were Staphylococcus epidermidis, Staphylococcus hominis, Pseudomonas aeruginosa, and Candida albicans. All CRBSIs occurred during neutropenia associated with peripheral hematopoietic cell transplantation. The higher rate in NHL patients is probably related to the more frequent use of AutoHSCT in these patients. There were only 11 (3.6%; 0.25/1,000 days/PICC) and nine (2.6%; 0.17/1,000 days/PICC) episodes of symptomatic PICC-related thrombotic complications in HL and NHL patients, respectively, without removal. All CRT in NHL patients were observed in association with polyurethane PICCs, whereas two cases of thrombotic events in the HL population were associated with silicone devices. No difference in the frequency of CRT was found in association with sex, the presence of a valve, the use of an intracavitary ECG lead, and the number of lumens in either population. CRT occurred as a late complication in most patients (>100 days/PICC). No CRBSI + CRT episodes were reported.
Discussion
The importance of PICCs for the management of oncohematologic patients has increased over the past 20 years, as they represent a reliable central venous catheter characterized by ease of insertion with possible bedside intervention and low rates of mechanical, infectious, and thrombotic complications. In addition, the introduction of new materials and technical features (e.g. Power-PICC, multilumen devices, tunneled devices, and PORT) has improved the safety profile in this patient population, making these devices a reliable alternative to CICCs for the management of immunochemotherapy, supportive care (such as blood transfusions), and frequent blood sampling, injection of high-flow X-ray contrast agents (magnetic resonance imaging, computed tomography, and positron emission tomography) and, more recently, peripheral autologous (auto-HSCT) and allogeneic (allo-HSCT) hematopoietic stem cell transplantation in inpatient and outpatient settings (26–31).
In this prospective study, we investigated the efficacy and safety of PICCs for the treatment of patients with non-Hodgkin’s and Hodgkin’s lymphomas, who represent ideal populations for obtaining the best benefit from these devices, depending on the management characteristics. We started our activity with the silicone Groshong® PICC monolumen 4 French BARD® because of the lower risk of occlusion in discontinuous use due to the presence of the valve and the low rate of CRT. With the improvement of our bundle for the management of PICCs and the introduction of more resistant and biocompatible materials, we have moved to the use of non-valved polyurethane multilumen devices and power PICCs. These latter catheters proved to be more reliable, especially for more complex therapies. The choice of the best catheter depends on the therapeutic regimen (immuno-chemotherapy, peripheral hematopoietic stem cells, autologous and allogeneic transplantation, cellular therapies, and supportive care) and patient and disease characteristics (i.e., stage and presence of bulky masses). We prefer the power PICC and multilumen systems for long-term treatments, including peripheral stem cell transplantation.
The median life expectancy was 150 days for NHL patients and 157 days for the HL population. These data are consistent with the duration of chemotherapy in most patients and are associated with continued management by our PICC team using internal protocols. According to the guidelines of our vascular access center, more than 90% of PICCs were implanted in a dedicated clinic by trained nurses, avoiding the use of an operating room and operation room staff. This allowed for easier management of insertion and economic efficiency of the process. In 70.2% of NHL patients, PICCs were implanted at a relapsed/refractory stage with prior chemotherapy, which did not affect the ability to implant, and often the same PICC was used to perform auto- or allo-HSCT as part of the therapeutic regimen. No differences were observed in the difficulty of insertion or the occurrence of complications according to the arm or vein of implantation.
For HL and NHL, 86.1% and 76.5% of PICCs, respectively, were removed at the end of therapy, which is considered as the completion of first-line therapy or hematologic recovery after transplantation, and only less than 14% and 25% of PICCs, respectively, were removed because of complications or death. These data suggest high efficacy and safety as a supportive tool for the management of these populations.
Of note, we recorded a relatively high rate of accidental removal (more common in NHL patients at 7.6% of PICCs), likely due to the anchoring of the devices with a needle system. This procedure was preferred to avoid the inconvenience of using sutures and the risk of local infectious complications, but requires adequate patient education on the proper handling of the device at home in terms of arm movements.
Occlusion that does not respond to local therapy is another common cause of remission; in this case, it is mandatory to wash the device after each infusion and apply local therapy at the first sign of occlusion. None of the suspected CRBSIs in which the PICCs were removed were confirmed by microbiological testing.
Only one NHL patient ruptured a silicone PICC. Infectious and thrombotic events represent the most dangerous complications associated with central venous catheters. In particular, CRBSIs require immediate removal of the device and administration of parenteral antibiotic therapy, depending on the results of peripheral vein and device blood cultures and tip evaluation.
Several clinical prospective and retrospective experiences (32–35) reported an increased risk of complications from the use of PICCs in oncohematologic patients.
In our series, no complications occurred in more than 90% of PICCs (90.1% and 93% in HL and NHL patients, respectively). These results may be due to the management by a dedicated PICC team and the proper choice of catheter type according to the treatment program and disease and patient characteristics.
Despite data from several studies (32–38) showing a high incidence of CRBSIs in hematologic patients with PICC, in our evaluation, the rate of bloodstream infections was very low in the HL and NHL populations. These infections were detected during the transplant procedure and were associated with marked neutropenia. The rigorous application of CDC criteria for the detection of CRBSIs allowed us to exclude cases of bloodstream infections due to other causes, such as mucosal barrier injury. All PICCs were removed and systemic antibiotic therapy was initiated. This low incidence might be related to the outpatient treatment, short time period, and low degree of neutropenia in most patients.
Thrombotic events have been the major obstacle to the use of PICCs in clinical practice, especially in oncology. Cancer-related hypercoagulative status, reduced vessel lumen, difficult venipuncture due to blind access, and stiffness of materials were considered risk factors for the occurrence of CRT (38–40). The use of color Doppler ultrasound-guided implantation, new biocompatible materials, and proper selection of available vessels with an appropriate caliber have reduced these risks. Despite these improvements, the rate of CRT remains high in hematology (33–35, 41, 42). In addition, the management of CRT in oncohematologic patients can be challenging because of thrombocytopenia (43). At our center, we manage CRT according to the recommendations of the GAVeCeLT group (44, 45), and trial data show a relatively low incidence of CRT. This rate is consistent with data from reported clinical experience and studies. As described above, we did not detect a difference in the incidence of CRT associated with sex, use of intracavitary ECG guidance, device type (number of lumens, caliber, power PICC, material, and presence of a valve), vessels, and presence of massive disease. CRT showed late onset (>100 days/PICC), which is likely due to the long median life of the devices. No concurrent CRBSI and CRT episodes were reported. The presence of CRT affected the lifespan of PICCs used during this complication without tip occlusion. We removed the PICCs after LMWH therapy and documentation of partial recanalization of the veins. We did not take prophylactic measures to reduce the risk of CRT.
Conclusion
PICCs are an important tool for the management of oncohematologic patients.
They allow the safe and efficient administration of intensive treatments, even in the context of complex therapeutic programs, such as autologous and allogeneic peripheral hematopoietic stem cell transplantation. One of the most important advantages of PICCs is the possibility of safe discontinuous use in a day hospital regimen. HL and NHL patients represent the ideal population to benefit from the features of these devices. To our knowledge, this is the first study to evaluate the efficacy and safety of PICCs in clinical practice in these patient populations. Our data show that PICCs provide efficient treatment with a manageable safety profile for patients and nursing and medical staff. We reported low rates of infectious and thrombotic complications, with little impact on patient management. Importantly, the presence of a dedicated PICC team with a validated internal protocol has been shown to be essential for preventing complications and providing appropriate and early intervention when they occur.
In our opinion, the results of the study suggest that PICCs must be considered the gold standard for vascular access management in these patients and should be considered an essential step in managing HL and NHL populations.
Furthermore, the establishment of a dedicated “PICC team” in a hematology center is essential for minimizing the risk of complications and improving the efficiency of these devices.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients provided a written consent for the implantation and use of the data for study.
Author contributions
DDer, SM, MS, DDes, SU, GL, DI, LA, MA, GLN contributed equally to the study. DDer written the paper and evaluated the data. MS and DDer reviewed the paper. All authors contributed to the article and approved the submitted version.
Funding
The payment will be performed by my institutional founding.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) [DD] declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Howlander N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER cancer statics review 1975-016. Natl. Cancer Inst. (2020).
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomatarm I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Coiffier B, Thieblemont C, Van Den Neste E, Lapeu I, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the groupe d’Etudes des lymphomes de l’Adulte. Blood (2010) 116(12):2040–5. doi: 10.1182/blood-2010-03-276246
4. Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, et al. Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl. J. Med. (2017) 377(14):1331–44. doi: 10.1056/NEJMoa1614598
5. Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet (9873) 2013:381. doi: 10.1016/S0140-6736(12)61763-2
6. Younes A, Connors JM, Park SI, Fanale M, O'Meara MM, Hunder NN, et al. Brentuximab vedotin combined with ABVD or AVD for patients with newly diagnosed hodgkin's lymphoma: a phase 1, open-la- bel, dose-escalation study. Lancet Oncol. (2013) 14(13):1348–56. doi: 10.1016/S1470-2045(13)70501-1
7. Canellos GP, Rosenberg SA, Friedberg JW, Lister TA, Devita VT. Treatment of Hodgkin lymphoma: a 50-year perspective. J. Clin. Oncol. (2014) 32(3):163–8. doi: 10.1200/JCO.2013.53.1194
8. National Comprehensive Cancer Network. Hodgkin Lymphoma (Version 2.2020) (2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf (Accessed November 2, 2020).
9. Philip T, Armitage JO, Spitzer G, Chauvin F, Jagannath S, Cahn P, et al. High-dose therapy and autologous bone marrow transplantation after failure of conventional chemotherapy in adults with intermediate-grade or high-grade non-hodgkin’s lymphoma. N Engl. J. Med. (1987) 316(24):1493–8. doi: 10.1056/NEJM198706113162401
10. Linch DC, Winfield D, Goldstone AH, Moir D, Hancock B, McMillan A, et al. Dose intensification with autologous bone-marrow transplantation in relapsed and resistant hodgkin's disease: results of a BNLI randomised trial. Lancet (8852) 1993:341. doi: 10.1016/0140-6736(93)92411-l
11. Lazarus HM, Rowlings PA, Zhang MJ, Vose JM, Armitage JO, Bierman PJ, et al. Autotransplants for hodgkin's disease in patients never achieving remis- sion: a report from the autologous blood and marrow transplant registry. J. Clin. Oncol. (1999) 17(2):534–45. doi: 10.1200/JCO.1999.17.2.534
12. Faisal MS, Hanel W, Voorhees T, Li R, Huang Y, Khan A, et al. Outcomes associated with allogenic hematopoietic stem cell transplantation for relapsed and refractory Hodgkin lymphoma in the era of novel agents. Cancer Med. (2023) 12(7):8228–37. doi: 10.1002/cam4.5631
13. Dreger P. Allogeneic stem cell transplant in non-Hodgkin lymphomas: still an indication? Hematol. Oncol. (2021) 39 Suppl 1:100–3. doi: 10.1002/hon.2845
14. Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large b-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. (2019) 20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7
15. Jacobson CA, Hunter BD, Redd R, Rodig SJ, Chen P-H, Wright K, et al. Axicabtagene ciloleucel in the non-trial setting: outcomes and correlates of response, resistance, and toxicity. J. Clin. Oncol. (2020) 38(27):3095–106. doi: 10.1200/JCO.19.02103
16. Nastoupil LJ, Jain MD, Feng L, Spiegel JY, Ghobadi A, Lin Y, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large b-cell lymphoma: results from the US lymphoma CAR T consortium. J. Clin. Oncol. (2020) 38(27):3119–28. doi: 10.1200/JCO.19.02104
17. Denlinger N, Bond D, Jaglowski S. CAR T-cell therapy for b-cell lymphoma. Curr. Probl Cancer (2022) 46(1):100826. doi: 10.1016/j.currproblcancer.2021.100826
18. Bock AM, Nowakowski GS, Wang Y. Bispecific antibodies for non-Hodgkin lymphoma treatment. Curr. Treat Options Oncol. (2022) 23(2):155–70. doi: 10.1007/s11864-021-00925-1
19. McGee DC, Gould MK. Preventing complication of central venous catheterization. N Engl. J. Med. (2003) 348:1123–33. doi: 10.1056/NEJMra011883
20. Lamperti M, Bodhenam AR, Pittirutti M, Blaivas M, Augoustides JG, Elbarbary M, et al. International evidence-based recommendations on ultra-sound vascular access. Intensive Care Med. (2012) 3:1105–17. doi: 10.1007/s00134-012-2597-x
21. Bellesi S, Chiusolo P, De Pascale G, Pittirutti M, Scoppettuolo G, Metafuni E, et al. Peripherally inserted central catheters (PICCs) in the management of onchoematological patients submitted to autologous stem cell transplantation. Support Care Cancer (2013) 21:531–535. doi: 10.1007/s00520-012-1554-0
22. Hashimoto Y, Fukuta T, Maruyama J, Omura H, Tanaka T. Experience of peripherally inserted central venous catheter in patients with hematologic diseases. Intern. Med. (2017) 56:389–93. doi: 10.2169/internalmedicine.56.7625
23. Sakai T, Kohda K, Konouma Y, Hiraoka Y, Ichikawa Y, Ono K, et al. A role of peripherally inserted central venous catheters in the prevention of catheter-related blood stream infections in patients with hematological malignancies. Intern. Med. (2014) 56:389–93. doi: 10.1007/s12185-014-1677-9
24. Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O’Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infections: 2009 updated by the infectious diseases society of America. Clin. Infect. Dis. (2009) 49:I–45. doi: 10.1086/599376
25. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (2020). Available at: https://www.R-project.org/.
26. Meriggiò E, Iori AP, Micozzi A, Chistolini A, Latagliata R, Berneschi P, et al. Peripherally inserted central catheters in allogeneic hematopoietic stem cell transplant recipients. Support Care Cancer (2020) 28(9):4193–9. doi: 10.1007/s00520-019-05269-z
27. Shih YH, Teng C-LT, Chen T-C, Chang K-H, Chen M-H. Dual-lumen power injectable peripherally inserted central catheters in allogeneic hematopoietic stem cell transplantation: a prospective observational study. J. Clin. Nurs. (2022) 31(11-12):1654–61. doi: 10.1111/jocn.16020
28. Garcès-Carrasco AM, Santacatalina-Rog E, Carretero-Màrquez C, Martinèz-sabater A, Balanguer-Lòpez E. Complications associated with peripherally inserted central catheters (PICC) in people undergoing autologous hematopoietic stem cell transplantation (HSCT) in home hospitalization. Int. J. Environ. Res. Public Health (2023) 20(3):1704. doi: 10.3390/ijerph20031704
29. Cornillon J, Martignoles JA, Tavernier-Tardy E, Gire M, Martinez P, Tranchan C, et al. Prospective evaluation of systematic use of peripherally inserted central catheters (PICC lines) for the home care after allogeneic hematopoietic stem cells transplantation. Support Care Cancer (2017) 25(9):2843–7. doi: 10.1007/s00520-017-3699-3
30. Harter C, Ostendorf T, Bach A, Egerer G, Goldsmith H, Ho AD. Peripherally inserted central venous catheters for autologous blood progenitor cell transplantation in patients with haematological malignancies. Support Care Cancer (2003) 11(12):790–4. doi: 10.1007/s00520-003-0517-x
31. Bellesi S, Chiusolo P, De Pascale G, Pittiruti M, Scoppettuolo G, Metafuni E, et al. Peripherally inserted central catheters (PICCs) in the management of oncohematological patients submitted to autologous stem cell transplantation. Support Care Cancer (2013) 21(2):531–5. doi: 10.1007/s00520-012-1554-0
32. Fracchiola NS, Todisco E, Bilancia A, Gandolfi S, Orofino N, Guidotti F, et al. Clinical management of peripherally inserted catheter compared to conventional central venous catheters in patients with hematological malignancies: a large multicenter study of REAL GROUP (Rete ematologica lombarda- Lombardy hematologic network Italy). Am. J. Hematol. (2017) 92:E656–9. doi: 10.1002/ajh.24903
33. Scrivens N, Sabri E, Bredeson C, McDiarmid S. Comparison of complication rates and incidences associated with different peripherally inserted catheters (PICC) in patients with hematological malignancies: a retrospective cohort study. Leuk Lymphoma (2020) 61(1):156–64. doi: 10.1080/10428194.2019.1646908
34. Cortelezzia A, Fracchiolla NS, Maisonneuve P, Moia M, Luchesini C, Ranzi ML, et al. Central venous catheter-related complications in patients with hematological malignancies: a retrospective analysis of risk factors and prophylactic measures. Leuk Lymphoma (2003) 44(9):1495–501. doi: 10.3109/10428190309178770
35. Worth LJ, Seymour JF, Slavin MA. Infective and thrombotic complications of central venous catheters in patients with hematological malignancy: prospective evaluation of non-tunneled devices. Support Care Cancer (2009) 17(7):811–8. doi: 10.1007/s00520-008-0561-7
36. Gao T, Zhu X, Zeng Q, Li X, Luo M, Yu C, et al. Peripherally inserted central catheter-related bloodstream infections in patients with hematological malignancies: a retrospective 7-years single-center study. Am. J. Infect. Control (2022) 50(10):1171–7. doi: 10.1016/j.ajic.2022.01.016
37. Morano SG, Latagliata R, Girmenia C, Massaro F, Berneschi P, Guerriero A, et al. Catheter-associated bloodstream infections and thrombotic risk in hematologic patients with peripherally inserted central catheters (PICC). Support Care Cancer (2015) 23(11):3289–95. doi: 10.1007/s00520-015-2740-7
38. Ban T, Fujiwara SI, Murahashi R, Nakajima H, Ikeda T, Matsuoka S, et al. Risk factors for complications associated with peripherally inserted central catheters during induction chemotherapy for acute myeloid leukemia. Intern (2022) 61(7):989–95. doi: 10.2169/internalmedicine.8184-21
39. Al-Asadi O, Almusarhed M, Eldeeb H. Predictive risk factors of venous thromboembolism (VTE) associated with peripherally inserted central catheters (PICC) in ambulant solid cancer patients: retrospective single centre cohort study. Thromb. J. (2019) 17:2. doi: 10.1186/s12959-019-0191-y
40. Lin BX, Xu S. Risk factors of PICC-related venous thrombosis in breast cancer patients undergoing chemotherapy. Int. J. Gen. Med. (2021) 14:1337–41. doi: 10.2147/IJGM.S296178
41. Yue J, Zhang Y, Xu F, Mi A, Zhou Q, Chen B, et al. A clinical study of peripherally inserted central catheter-related venous thromboembolism in patients with hematological malignancies. Sci. Rep. (2022) 12(1):9871. doi: 10.1038/s41598-022-13916-5
42. Tran H, Arellano M, Chamsuddin A, Flowers C, Heffner LT, Langston A, et al. Deep venous thromboses in patients with hematological malignancies after peripherally inserted central venous catheters. Leuk Lymphoma (2010) 51(8):1473–7. doi: 10.3109/10428194.2010.481065
43. Scamuffa MC, Morano SG, Serrao A, Bruzzese A, Stocchi F, Santoro C, et al. PICC-related upper deep venous thrombosis in patients with hematological malignancies. management of anticoagulant therapy according to the platelet count. J. Thromb. Thrombolysis (2020) 49(3):426–30. doi: 10.1007/s11239-020-02040-8
44. Pinelli F, Balsorano P, Mura B, Pittiruti M. Reconsidering the GAVeCeLT consensus on catheter-related thrombosis, 13 years later. J. Vasc. Access (2021) 22(4):501–8. doi: 10.1177/1129729820947594
45. Annetta MG, Bertoglio S, Biffi R, Brescia F, Giarretta I, Greca A, et al. Management of antithrombotic treatment and bleeding disorders in patients requiring venous access devices: a systematic review and a GAVeCeLT consensus statement. J. Vasc. Access (2022) 23(4):660–71. doi: 10.1177/11297298211072407
Keywords: PICC catheters, NHL – non-Hodgkin lymphomas, Hodgkin lymphoma (HL), PICC complications, catheter related blood stream, catheter related thrombosis
Citation: Derudas D, Massidda S, Simula MP, Dessì D, Usai SV, Longhitano G, Ibba D, Aracu L, Atzori M and La Nasa G (2023) Peripherally inserted central catheter insertion and management in Hodgkin and non-Hodgkin lymphomas: a 13-year monocentric experience. Front. Hematol. 2:1171991. doi: 10.3389/frhem.2023.1171991
Received: 22 February 2023; Accepted: 17 May 2023;
Published: 20 June 2023.
Edited by:
Stefano Molica, Hull University Teaching Hospitals NHS Trust, United KingdomReviewed by:
Giuseppe Gentile, Sapienza University of Rome, ItalyClaudio Cartoni, Umberto 1 Hospital, Italy
Pasquale Niscola, Sant’Eugenio Hospital of Rome, Italy
Copyright © 2023 Derudas, Massidda, Simula, Dessì, Usai, Longhitano, Ibba, Aracu, Atzori and La Nasa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniele Derudas, ZGFuaWVsZWRlcnVkYXNAdGlzY2FsaS5pdA==; ZGFuaWVsZS5kZXJ1ZGFzQGFvYi5pdA==
 Daniele Derudas
Daniele Derudas Stefania Massidda
Stefania Massidda