- 1Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, United States
- 2Division of Oncology, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States
- 3Division of Bone Marrow Transplantation and Immune Deficiency, Cancer and Blood Diseases Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States
- 4Kids Cancer Center, Sydney Children’s Hospital, Randwick, Randwick, NSW, Australia
Background: Tisagenlecleucel (tisa-cel) is increasingly being used in hematopoietic stem cell transplantation (HSCT)-naive patients. Outcomes for HSCT patients following chimeric antigen receptor (CAR) T-cell therapy demonstrate low relapse rates; however, a significant number of patients who receive tisa-cel can maintain remission without an HSCT. Multiple factors are considered when choosing whether or not to proceed with HSCT.
Methods: We retrospectively reviewed 31 patients who had received tisa-cel at our institution and who were transplant naive at the time of infusion. The aim was to determine the rate and timing of consolidative HSCT, factors that led to HSCT, and overall survival.
Results: Three of the 31 patients were non-responders to tisa-cel and ultimately died of disease. Twelve of the 28 responders remain alive with no evidence of disease (NED) without subsequent therapy. Of these patients, 5 of the 12 had isolated extramedullary acute lymphoblastic leukemia (ALL) (CNS, n = 4; testes, n = 1) and 2 of the 12 had Down syndrome, so no transplantation was planned. In the remaining 5 of 12 patients, close monitoring for signs of relapsed ALL, using serial next-generation sequencing (NGS) minimal residual disease (MRD) and lymphocyte subpopulation measurements, was performed. Owing to continued negative findings, no HSCT was chosen. Ultimately, 43% (12 of 28) of responders proceeded to HSCT, with three receiving tisa-cel as a planned bridge to HSCT as a result of CD22 negativity and/or provider preference (two patients survived with NED); three proceeded to HSCT as a result of early loss of B-cell aplasia (BCA) (all survived with NED); and six had salvage HSCT following relapse (three patients survived with NED and one patient was alive in relapse). Three of the 28 patients died following relapse post CAR T-cell therapy without HSCT. The final patient had an isolated extramedullary soft tissue CD19+ relapse 1 year post tisa-cel treatment, and is now NED without HSCT and persistent BCA.
Conclusion: Close monitoring of NGS results and BCA, as well as consideration of the site of the disease, can spare a subset of patients HSCT with the maintenance of leukemia-free remission, while still allowing for later HSCT in others. In our cohort, only a small subset of patients was unable to proceed to HSCT following relapse post-CAR T-cell therapy.
1 Introduction
Chimeric antigen receptor (CAR) T cells have revolutionized therapy for children and young adults with relapsed, refractory B-cell precursor acute lymphoblastic leukemia (B-ALL). Complete remission rates of 81%–90% are seen in patients with severe chemotherapy-refractory disease, many of whom have previously undergone hematopoietic stem cell transplantation (HSCT) (1, 2). Furthermore, enduring remissions were observed without further therapy in this population of patients, who were previously believed to be incurable (3). Since its US FDA approval, tisagenlecleucel, a CD-19-directed CAR T-cell therapy, has been more widely available to patients, with many being treated earlier in their relapse or refractory course. Follow-up data from these clinical trials have demonstrated continued durable remissions in about half of the patients who achieve remission with this CAR T-cell product (3). Accordingly, in real-world practice, there is increasing use of CAR T-cell therapy in patients who are HSCT naive, in some cases as a way to achieve minimal residual disease (MRD) -negative remission status prior to HSCT and in other cases as a potential definitive therapy (4, 5). The possibility of decreased relapse rates has been observed when CAR T-cell remission is followed by consolidative HSCT (6). However, a blanket approach using consolidative transplantation will cause additional toxicity in some patients who could maintain a long-term remission with CAR T-cell therapy as definitive treatment. Importantly, the data available to guide treatment decisions are very heterogeneous, with varied patient populations, CAR T-cell products, and institutional preferences. In the absence of a randomized trial, we have reviewed outcomes following CAR T-cell infusion in transplant-naive patients treated at our center to further understand factors that lead to the use or avoidance of HSCT. In particular, we have examined the feasibility of HSCT at a later date if consolidative HSCT was not initially considered.
2 Materials and methods
We performed a retrospective review of relapsed or refractory B-ALL patients treated with tisagenlecleucel at Cincinnati Children’s Hospital Medical Center (CCHMC) between 2017 and 31 December 2021. We excluded patients who had had a prior stem cell transplant (SCT) from this analysis.
This study was approved by CCHMC’s Institutional Review Board. A comprehensive review of disease status and patient characteristics, toxicities, and patient outcomes was performed. Cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) were graded according to American Society for Transplantation and Cellular Therapy (ASTCT) consensus guidelines (7). Remission status was measured at day 28 post CAR T-cell infusion by bone marrow MRD using multiparametric flow cytometry. Next-generation sequencing determination of minimal residual disease (NGS-MRD) was performed using the commercially available clonoSEQ MRD® assay (Adaptive Biotechnologies, Seattle, WA, USA) (8–10). NGS-MRD evaluation required prior detection of baseline dominant clones from diagnostic marrow samples, and was not available for all patients. Morphologic CNS status was obtained via lumbar puncture at day 28. Patients had, at least, monthly evaluations of remission status, as measured by complete blood cell counts as well as measurements of B-cell aplasia (BCA) via peripheral blood lymphocyte subpopulations. B-cell recovery was defined as > 1% CD19+ cells on peripheral blood lymphocyte analysis. This was repeated on a confirmatory sample at least 1 week later. The data cut-off date for post CAR T-cell therapy outcomes was 31 December 2022.
Statistical analysis
Event-free survival (EFS) was calculated by the Kaplan–Meier method, and the Mantel–Cox test was used to evaluate significance. An event was defined as a relapse of the disease, a second malignancy, or death. Relapse was defined as the development of leukemic blasts in the CNS or detectable recurrence of disease by flow cytometry. HSCT was not considered an event. The Kaplan–Meier method was also used to determine overall survival. Statistical analysis was performed using Fisher’s exact text.
3 Results
3.1 Patients
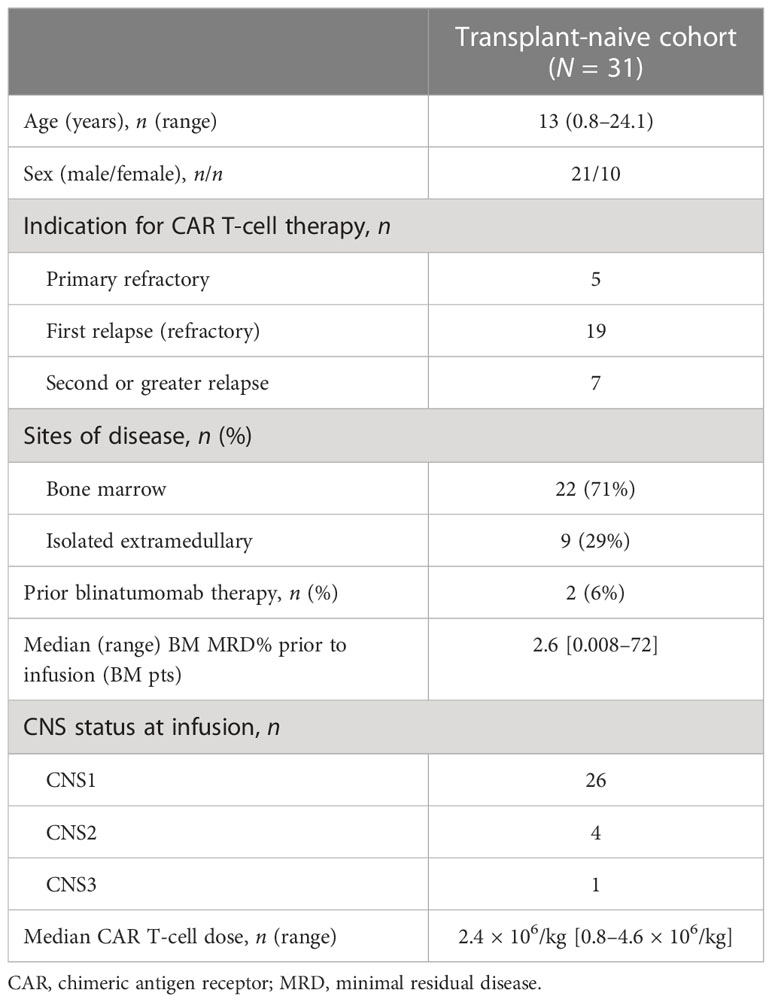
Between 2014 and 2021, 41 patients with refractory or multiply relapsed B-ALL were treated at our institution. Thirty-one of the 41 (76%) had not undergone previous HSCT and were included in this study (Table 1). The median age at the time of tisagenlecleucel infusion was 13.0 years [range 0.9–24.1 years]. The male-to-female ratio was 21:10. Three patients in our cohort had Down syndrome ALL (DS-ALL). Nine patients (29.0%) had isolated extramedullary disease (isolated CNS, n = 8; isolated testicular disease, n = 1), and the remaining 24 (71.0%) had bone marrow involvement. The median disease burden by MRD at the last evaluation prior to infusion in patients with marrow disease was 2.4% (range 0.008%–72%). Three patients with isolated CNS leukemia were CNS2 at the time of infusion, and the remaining five were in CNS remission prior to infusion. One patient had a combined marrow and CNS relapse and was CNS3 at the time of infusion. The median CAR T-cell dose was 2.3 × 106/kg (range 0.8–4.6 × 106/kg). A total of 26 patients were treated with commercial tisagenlecleucel. Four patients were treated with the same product on a single patient investigational new drug (IND) or managed access program for failure to meet commercial release parameters [apheresis product > 9 months (n = 1), viability < 80% (n = 2), and retention of beads (n = 1)]. One patient was treated in accordance with the Children’s Oncology Group AALL1721 protocol investigating the use of Kymriah® for patients with National Cancer Institute (NCI) high-risk ALL and residual MRD at the end of up-front consolidation chemotherapy.
3.2 Response and toxicity
All patients had bone marrow evaluations and lumbar punctures at day 28 post-CAR T-cell infusion. Twenty-eight of the 31 (90%) patients achieved remission, which was defined as a negative MRD, as determined by flow cytometric analysis, and no evidence of CNS or extramedullary leukemia. Three patients were non-responders, as determined by flow cytometric analysis: two had persistent CD19+ ALL and one patient with KMT2A-r ALL had a lineage switch to acute myeloid leukemia. All three died of refractory leukemia. In addition, NGS-MRD data were available for 15 of the 31 (48%) patients, 11 of whom were negative. Two of the four positive NGS-MRD patients also had disease detected by flow MRD/morphology, and were classified in the group of non-responders noted above. One patient had positive NGS-MRD results, with negative flow cytometry results, and suffered a frank relapse within 1 month post CAR T-cell therapy. The fourth patient, who had primary refractory disease, had a clone detectable below the limits of reporting, and proceeded to planned HSCT and remains in remission. HSCT was planned for this patient prior to this result and was chosen as a therapy because of the primary physician and family preference.
Toxicity was manageable, with 61.1% of patients having any grade of CRS, which was grade 3 or 4 in five cases (16.1%). Seven patients (19.4%) had any grade of ICANS, with five (13.9%) experiencing grade 3 or 4. Tocilizumab was given to 10 patients. Three patients were given dexamethasone for grade 4 ICANS. No treatment-related mortality was observed. One patient developed a disseminated Fusarium infection; however, this patient had been heavily pretreated and was neutropenic prior to starting lymphodepleting chemotherapy. No other bacterial or fungal infections were reported.
3.3 Outcomes post CAR T-cell therapy-mediated remission
Clinical pathways following CAR T-cell infusion are shown in Figure 1. Three patients (6.5%) experienced treatment failure and ultimately died of disease. Three patients (6.5%) proceeded directly to planned consolidative HSCT, leaving 25 of the 31 patients (81%) undergoing surveillance post-CAR T-cell therapy. Two of the three patients who proceeded directly to HSCT remained disease-free after HSCT, whereas the third, who had not received prior inotuzumab ozogamicin, died of sinusoidal obstructive syndrome (SOS) post transplantation. High-risk features for CAR T-cell therapy failure and/or limited salvage options led to the use of consolidative transplantation in the three patients. Two of the three patients had high disease burden (8.8% and 72%) prior to CAR T-cell therapy, and one patient had previously failed blinatumomab treatment. The other patient had chemorefractory marrow and CNS3 disease, and was noted to be CD22 negative.
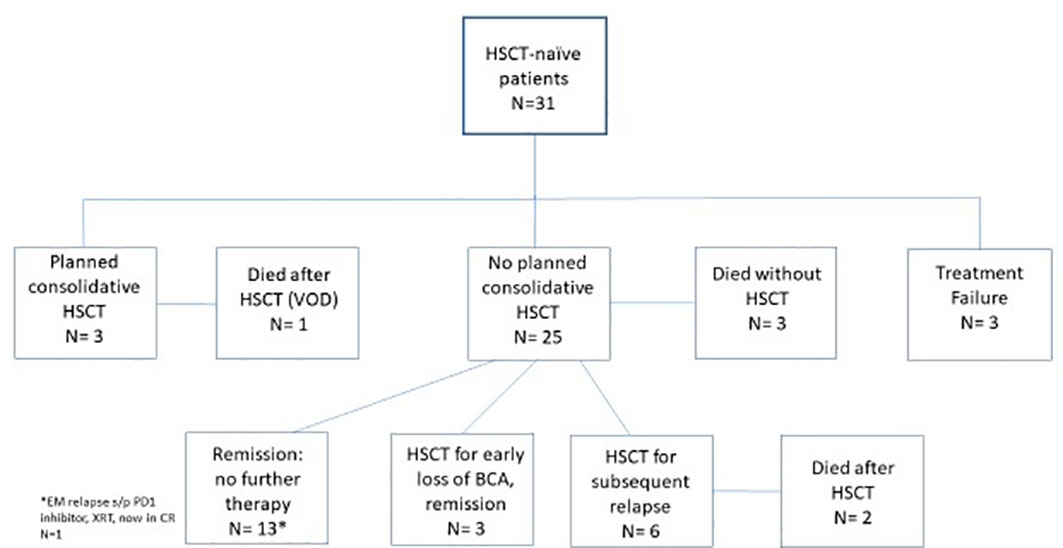
Figure 1 Clinical course and outcome.This flow diagram shows the distribution of patients following CAR T-cell therapy: no remission, bridge directly to HSCT, no further therapy, HSCT at time of B-cell recovery, relapse followed by salvage HSCT, and death after relapse without HSCT. CAR, chimeric antigen receptor; HSCT, hematopoietic stem cell transplantation.
Focused analysis of patients who underwent surveillance identified that 12 of 25 (48%) remain in remission beyond 1 year without further leukemia-directed therapy, with a median duration of remission (DOR) of 32.4 months (range 12.4–54.1 months). Five of these patients had isolated extramedullary disease at a site of disease pre CAR T-cell therapy (CNS, n = 4; testicular, n = 1). Two patients with DS-ALL, who were not considered for empiric consolidative HSCT due to the high risk of toxicity, remained alive with NED. Five of the 12 patients had bone marrow-only disease, and have been followed by close serial monitoring of NGS-MRD results and BCA. These patients remain in initial CAR-induced remission. Owing to ongoing NGS-MRD negativity and/or at least 6 months of BCA these patients never underwent consolidative HSCT. An additional patient had persistent BCA and marrow NGS-MRD negativity at more than 1 year after infusion, but suffered an isolated breakthrough extramedullary soft tissue CD19+ relapse in the breast tissue. She was treated with radiation to the site and pembrolizumab. She was in her third continuous remission (CR) 5 months post-recurrence without HSCT. Nine patients (36%) in the post CAR T-cell surveillance cohort proceeded to consolidative HSCT. Three patients went to expedited HSCT at the time of early B-cell recovery (all at 3 months) and all remained alive with NED. Six patients out of 25 (24%) suffered a CD19+ relapse and achieved an additional remission post CAR T-cell therapy failure and proceeded to HSCT. Notably, four of the six patients had previously lost B-aplasia at 3–6 months post CAR T-cell therapy, but did not proceed directly to consolidative HSCT for various reasons [isolated CNS disease, DS-ALL, patient refusal for additional therapy, and religious beliefs (Jehovah’s Witness)] until after a subsequent relapse. Four patients remained alive post-HSCT following CAR T-cell therapy failure, with one unfortunately having an active relapse. Two patients who proceeded to HSCT following CAR T-cell therapy failure died: one from subsequent disease relapse and one from transplant-related infection toxicity. Three patients (12%) in the surveillance cohort died of relapsed ALL without proceeding to HSCT. One of the three patients with an isolated CD19-negative relapse had suffered neurologic comorbidities in up-front and relapsed therapy and chose not to pursue further therapy. The other two patients had significant previous medical history, as both had previously been treated for medulloblastoma and one had suffered from congenital mismatch repair deficiency (CMMRD). Both of these patients failed to attain a subsequent remission with conventional chemotherapy. Figure 2 is a swimmer plot that graphically represents the duration of follow-up/remission, as well as the timing of key events including the loss of BCA, relapse, transplantation, and death for all responding patients.

Figure 2 Swimmer plot of outcomes post CAR T-cell response. The y-axis represents individual record numbers of the 25 responding patients. The x-axis is time in days since tisa-cel infusion. The blue arrows depict the 17 patients who remain in continuous CR post tisa-cel, either with or without consolidative transplantation (marked by a square). The black arrows represent patients who have suffered either a post tisa-cel relapse or treatment-related death post transplant. Non-responders are not represented on this plot. The triangle represents time of loss of BCA. The circle represents time of relapse. X indicates time of death. CAR, chimeric antigen receptor; CR, continuous response.
3.4 B-cell aplasia
The duration of BCA was noted to be a predictor of outcome for our patients (Figure 3). For patients in our cohort who remain in remission without further therapy, 11 out of 12 (91.7%) maintained BCA longer than 6 months. The median duration of BCA for this group of patients has not been reached as 11 have ongoing BCA. This is in contrast to those patients who relapsed after the initial response, where only 2 out of 10 (20%) maintained BCA for longer than 6 months. Three additional responding patients, not in either of the previous categories, lost BCA at 3 months and proceeded directly to HSCT in MRD-negative remission. The median time from loss of BCA to day of stem cell infusion was 3 days (range 26–67 days). All remain in a continuous complete remission post HSCT.
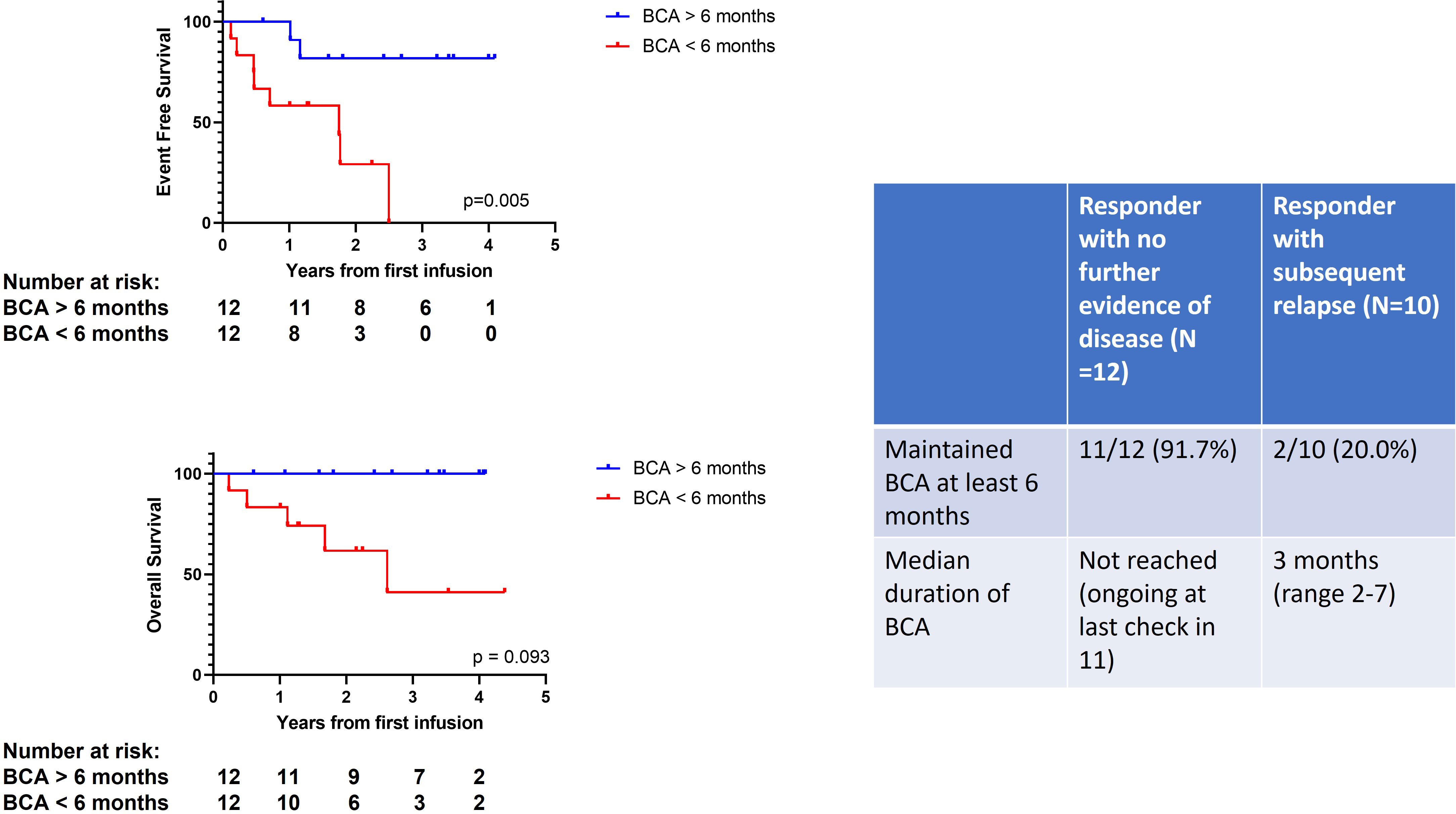
Figure 3 Duration of B-cell aplasia predicts outcome. The Kaplan–Meier curves on the left show EFS and OS according to B-cell aplasia (BCA) > 6 months (blue line) vs. BCA < 6 months (red line). BCA, B-cell aplasia; EFS, event-free survival; OS, overall survival.
3.5 Retrieval therapies following relapse post-CAR T-cell therapy remission
A total of 10 out of 25 patients suffered a relapse post CAR T-cell therapy remission during the post-CAR T-cell therapy surveillance period. Six of the patients were successfully salvaged with a combination of CAR T-cell reinfusion, investigational humanized CAR T cells, and/or inotuzumab and were able to undergo HSCT in an MRD-negative remission. (Table 2). Two of the patients received a reinfusion of tisagenlecleucel after CD19+ relapse. Patient 16 had no response to reinfusion, but ultimately achieved MRD-negative remission with inotuzumab and is in remission post HSCT. Patient 4, with a third isolated CNS relapse, had CR with re-establishment of BCA for 2 months with tisagenlecleucel reinfusion and then proceeded to humanized CAR T cells on a clinical trial as a path to HSCT. Two other patients (patients 5 and 7) had humanized CAR T cells on a clinical trial and, ultimately, proceeded to HSCT due to early loss of BCA. Inotuzumab was given to patients 2 and 11 to achieve MRD-negative remission prior to HSCT. Only one of the five patients who received inotuzumab as part of the salvage regimen had sinusoidal obstructive syndrome during transplantation, which resolved with treatment. Three of the 10 patients died without HSCT and one patient (patient 1) with isolated CD19-CNS relapse elected for only palliative therapy due to significant previous toxicities with up-front therapy. The other two patients who had secondary ALL post medulloblastoma (patients 9 and 22) suffered an aggressive relapse within 2 months of infusion and died of refractory leukemia.
Five patients received a reinfusion of tisagenlecleucel from the original manufactured batch. In addition to the two patients noted above who were reinfused for relapsed CD19+ disease, three others were given a reinfusion for early B-cell recovery at 3, 4, and 7 months. BCA was re-established in two patients, in one case for 1 month and in the other for 14 months. All three patients ultimately relapsed and were able to achieve MRD-negative remission with alternate salvage regimens and proceeded to HSCT, with one of three alive without disease.
4 Discussion
Our data show the clinical post-CAR T-cell pathway for transplant-naive patients with B-ALL following remission induced by tisagenlecleucel remains variable. Twelve patients, all of whom had at least 1 year of follow-up, had CAR T-cell treatment as a stand-alone therapy and are alive with no evidence of disease. Given that the median follow-up is 32 months for this group, transplantation will likely not be needed for the majority of these patients, as follow-up data from the global registration trial and real-world data show that most relapses occur in the first year after infusion (3–5). Five additional patients remain in continuous remission following CAR T-cell therapy and HSCT, two of whom two proceeded directly to HSCT; the others underwent HSCT at the time of early loss of BCA. Our data show that only a small number of patients will not be able to proceed to transplantation at a later date, with only 2 of 28 initially responding patients (7.1%) attempting but failing to achieve a subsequent remission following a relapse post CAR T-cell therapy. Even these two cases may be outliers, as both had secondary leukemias following an earlier treatment course for medulloblastoma. The risk of transplant morbidity and mortality has to be weighed against the known failure rate of CAR T-cell therapy. We examined the patient characteristics and criteria used to avoid or defer HSCT in a subset of patients.
Several patient or disease characteristics that have emerged since the broader use of tisagenlecleucel often guide a watchful waiting versus a consolidative HSCT approach. Optimal management of an isolated CNS relapse has been challenging, with a variety of strategies being adopted, including cranial irradiation combined with HSCT, a long, intensive chemotherapy backbone, or a less intense chemotherapy regimen (11, 12). Attempts to reduce chemotherapy backbone have yielded less favorable results (13). CAR T cells have been shown to effectively track into the CNS, and recent data have demonstrated encouraging outcomes when CAR T-cell therapy is used for isolated CNS relapse (14, 15). The data presented here are comparable to those that have been previously published. Given the favorable data with CAR T-cell therapy in this population, and the questionable benefit that an allograft offers these patients, we often use tisagenlecleucel as a stand-alone therapy for iCNS relapse. Patients with DS-ALL have a higher risk of relapse, and management of relapsed disease has been very challenging, not only due to chemorefractory disease, but also due to excess toxicity and relapse following HSCT (16). The 3-year overall survival in a cohort of DS patients undergoing HSCT is only 24%. Fortunately, toxicity and treatment outcomes for patients with DS-ALL undergoing CAR T-cell therapy are comparable to those seen in their non-DS counterparts (17). Given the higher risk of transplant-associated toxicities and relapse, consolidative HSCT is not routinely employed for children and young adults with DS-ALL. For patients with genetic conditions (such as CMMRD or Li–Fraumeni syndrome), very young age or previous organ or infectious comorbidity that escalates the risk of toxicity from a TBI-based HSCT, CAR T-cell therapy can potentially offer an opportunity to avoid or defer HSCT. Furthermore, religious reasons and a strong patient preference can also lead to avoidance or deferral of consolidative transplantation. In our cohort, isolated CNS disease (n = 7), Down syndrome (n = 3), cancer predisposition syndrome (n = 2), young age (n = 1), religious beliefs (Jehovah’s Witness; n = 1), and the patient’s refusal of HSCT (n = 1) were factors that guided adopting a watchful waiting approach in 15 of our patients.
In contrast, data are also emerging to suggest that a subset of treatment and disease factors increase risk of eventual CAR T-cell failure, leading to increased use of consolidative transplantation up front for these patients. Even if negative by flow cytometry, NGS-MRD > 0 at day 28 is a significant risk factor for relapse and remains an independent predictor of relapse at 3 months, with an increased hazard ratio (HR 12.0) (10). Another group of patients with an unacceptably high risk of relapse is patients who have previously failed to respond to blinatumomab. These patients tend to have a lower response rate to CD19+ CAR T-cell therapy and a markedly lower EFS at 6 months (27.3%, compared with 66.9% for responders or 72% for blinatumomab-naive patients) (18). This was the rationale for bridging directly to transplantation in one patient with blinatumomab failure and a high disease burden. This patient unfortunately had SOS during transplantation and died of this complication. The prior use of blinatumomab in this cohort was notably low, with only one other patient having received and responded to blinatumomab and had CD19+ relapse 12 months post-CAR T-cell therapy. The absence of immunotherapy targets, including CD22, causes concern for the ability to salvage chemotherapy-refractory patients post-CAR relapse, especially in the setting of a CD19 antigen loss at relapse. For this reason, we chose to proceed to HSCT in a chemotherapy-refractory patient whose leukemia failed to exhibit CD22. Inotuzumab was utilized to achieve MRD remission prior to HSCT in several of our patients who relapsed post-CAR T-cell treatment, as well as 30% of salvage attempts post-CAR relapse in the Pediatric Real World CART Consortium (PRWCC) (19). A high disease burden at the time prior to tisagenlecleucel treatment is associated with inferior outcomes in the PWRCC; however, as many patients did not proceed directly to HSCT in that cohort, it is unclear whether or not proceeding to HSCT will abrogate that risk. The presence of a refractory high disease burden of 72% marrow blasts was a factor in selecting HSCT for one of our patients.
We monitored monthly lymphocyte subpopulations to identify B-cell recovery for the 25 transplant-naive patients at our center who did not bridge to HSCT. Early B-cell recovery (< 6 months) has been consistently shown to be associated with a risk of relapse, and has been used to recommend HSCT over continued monitoring. Follow-up data from the ELIANA trial showed the median DOR for patients with BCA < 6 months not undergoing SCT to be 12 months, whereas the median DOR of remission for BCA persisting longer than 6 months was not reached (3). Three of our patients with early loss of BCA proceeded to transplantation and remain in remission. In the PWRCC, 15 of the 25 relapsed patients had a loss of BCA preceding relapse by a median of 84 days prior (19). The majority of patients lost BCA prior to 6 months, providing a potential opportunity to avoid relapse by an expedited transplant. Our data also support the risk of relapse with early B-cell recovery, as loss of BCA prior to 6 months was seen in four of our patients who ultimately relapsed. These patients did not proceed directly to HSCT with loss of BCA due to patient-specific factors. These factors include patient refusal, religious beliefs, underlying Down syndrome, and isolated CNS disease. More recently, we have increased the frequency and number of patients who are followed using NGS-MRD results. We perform NGS-MRD on marrows at day 29 as well as at 3, 6, 9, and 12 months post-CAR T-cell therapy, as well as monthly using peripheral blood during the intervening months. Any recurrence of MRD by NGS warrants confirmation and often therapeutic intervention.
Clinical trials and real-world data for our current FDA-approved CD19 CAR T-cell therapy, tisagenlecleucel, show that approximately half of the patients can maintain a CAR T-cell-mediated remission without other therapies (1, 3–5). This study illustrates the real-world individualized factors that led to the decisions whether or not to pursue consolidative transplantation following CAR T therapy and why a one size fits all approach will not work. The goal is ultimately to avoid transplant toxicity in those who do not need it, to minimize the number of patients who relapse, and, importantly, to identify those who relapse and who are unlikely to be salvaged and get a second opportunity to proceed to HSCT in MRD-negative remission. The risk of relapse, which is inherent to this therapy, must be balanced against the morbidity and mortality risk of HSCT that accompanies the reduction in relapse it may offer (6). Patients and their families, as well as their physicians, have a variable tolerance for the risk of relapse that accompanies watchful waiting that may be abrogated by proceeding directly to HSCT. A well-designed randomized clinical trial would be helpful to provide additional data for these patients. However, the design of such a trial remains a significant challenge because, illustrated in this paper, many individual factors already contribute to the selection or omission of consolidative transplantation. In settings where pretreatment of a disease or patient characteristics do not strongly influence the choice to proceed to allogeneic transplant, informed consent between families and their physicians is needed to adopt a post-CAR T-cell treatment strategy. Future widespread use of humanized CAR or an improved CAR with persistence that improves disease-free survival for CAR may allow more comfort in a watchful waiting approach (20).
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to christine.phillips@cchmc.org.
Ethics statement
The studies involving human participants were reviewed and approved by Cincinnati Children’s Hospital Medical Center. Written informed consent for participation was not provided by the participants’ legal guardians/next of kin because: retrospective review, exempt.
Author contributions
CP and JR contributed to the design and conception of the study. CP wrote the manuscript. JR and TG performed the statistical analysis and designed figures/tables. All authors participated in the care, management, and treatment decisions of the patients. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank the staff of the Regenerative Medicine and Cellular Therapies Division at Hoxworth Blood Center for their critical aid in managing cellular products. The authors also gratefully acknowledge the patients treated and their families, as well as the CAR-T and Immunotherapy Team within the Cancer and Blood Diseases Institute of Cincinnati Children’s Hospital Medical Center.
Conflict of interest
CP has served on the advisory board for Novartis. SD serves on the scientific advisory board for Allovir, and is an advisor for Anthem and Rocket Pharma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with b-cell lymphoblastic leukemia. New Engl. J. Med. (2018) 378:439–48. doi: 10.1056/NEJMoa1709866
2. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. New Engl. J. Med. (2014) 371:1507–17. doi: 10.1056/NEJMoa1407222.
3. Laetsch TW, Maude SL, Rives S, Hiramatsu H, Bittencourt H, Bader P, et al. Three-year update of tisagenlecleucel in pediatric and young adult patients with Relapsed/Refractory acute lymphoblastic leukemia in the ELIANA trial. J. Clin. Oncol. (2023) 41(9):1664–9. doi: 10.1200/JCO.22.00642
4. Pasquini MC, Hu ZH, Curran K, Laetsch T, Locke F, Rouce R, et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. (2020) 4:5414–24. doi: 10.1182/bloodadvances.2020003092
5. Schultz LM, Baggott C, Prabhu S, Pacenta HL, Phillips CL, Rossoff J, et al. Disease burden affects outcomes in pediatric and young adult b-cell lymphoblastic leukemia after commercial tisagenlecleucel: a pediatric real-world chimeric antigen receptor consortium report. J. Clin. Oncol. (2022) 40:945–55. doi: 10.1200/JCO.20.03585
6. Shah NN, Lee DW, Yates B, Yuan CM, Shalabi H, Martin S, et al. Long-term follow-up of CD19-CAR T-cell therapy in children and young adults with b-ALL. J. Clin. Oncol. (2021) 39:1650–9. doi: 10.1200/JCO.20.02262
7. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood marrow transplantation: J. Am. Soc. Blood Marrow Transplant. (2019) 25:625–38. doi: 10.1016/j.bbmt.2018.12.758
8. Ching T, Duncan ME, Newman-Eerkes T, McWhorter MME, Tracy JM, Steen MS, et al. Analytical evaluation of the clonoSEQ assay for establishing measurable (minimal) residual disease in acute lymphoblastic leukemia, chronic lymphocytic leukemia, and multiple myeloma. BMC Cancer (2020) 20:612. doi: 10.1186/s12885-020-07077-9
9. Wood B, Wu D, Crossley B, Dai Y, Williamson D, Gawad C, et al. Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric b-ALL. Blood (2018) 131:1350–9. doi: 10.1182/blood-2017-09-806521
10. Pulsipher MA, Han X, Maude SL, Laetsch TW, Qayed M, Rives S, et al. Next-generation sequencing of minimal residual disease for predicting relapse after tisagenlecleucel in children and young adults with acute lymphoblastic leukemia. Blood Cancer Discovery (2022) 3:66–81. doi: 10.1158/2643-3230.BCD-21-0095
11. Domenech C, Mercier M, Plouvier E, Puraveau M, Bordigoni P, Michel G, et al. First isolated extramedullary relapse in children with b-cell precursor acute lymphoblastic leukaemia: results of the cooprall-97 study. Eur. J. Cancer (2008) 44:2461–9. doi: 10.1016/j.ejca.2008.08.007
12. Hastings C, Chen Y, Devidas M, Ritchey AK, Winick NJ, Carroll WL, et al. Late isolated central nervous system relapse in childhood b-cell acute lymphoblastic leukemia treated with intensified systemic therapy and delayed reduced dose cranial radiation: a report from the children’s oncology group study AALL02P2. Pediatr. Blood Cancer (2021) 68:e29256. doi: 10.1002/pbc.29256
13. Brown PA, Ji L, Xu X, Devidas M, Hogan L, Bhatla T, et al. A randomized phase 3 trial of blinatumomab vs. chemotherapy as post-reinduction therapy in low risk (LR) first relapse of b-acute lymphoblastic leukemia (B-ALL) in children and Adolescents/Young adults (AYAs): a report from children’s oncology group study AALL1331. Blood (2021) 138:363–. doi: 10.1182/blood-2021-147946
14. Rubinstein JD, Krupski C, Nelson AS, O’Brien MM, Davies SM, Phillips CL. Chimeric antigen receptor T cell therapy in patients with multiply relapsed or refractory extramedullary leukemia. Biol. Blood marrow transplantation: J. Am. Soc. Blood Marrow Transplant. (2020) 26:e280–e5. doi: 10.1016/j.bbmt.2020.07.036
15. Fabrizio VA, Phillips CL, Lane A, Baggott C, Prabhu S, Egeler E, et al. Tisagenlecleucel outcomes in relapsed/refractory extramedullary ALL: a pediatric real world CAR consortium report. Blood Adv. (2022) 6:600–10. doi: 10.1182/bloodadvances.2021005564
16. Hitzler JK, He W, Doyle J, Cairo M, Camitta BM, Chan KW, et al. Outcome of transplantation for acute lymphoblastic leukemia in children with down syndrome. Pediatr. Blood Cancer (2014) 61:1126–8. doi: 10.1002/pbc.24918
17. Laetsch TW, Maude SL, Balduzzi A, Rives S, Bittencourt H, Boyer MW, et al. Tisagenlecleucel in pediatric and young adult patients with down syndrome-associated relapsed/refractory acute lymphoblastic leukemia. Leukemia (2022) 36:1508–15. doi: 10.1038/s41375-022-01550-z
18. Myers RM, Taraseviciute A, Steinberg SM, Lamble AJ, Sheppard J, Yates B, et al. Blinatumomab nonresponse and high-disease burden are associated with inferior outcomes after CD19-CAR for b-ALL. J. Clin. Oncol. (2021) 40:932–44. doi: 10.1200/JCO.21.01405
19. Schultz LM, Eaton A, Baggott C, Rossoff J, Prabhu S, Keating AK, et al. Outcomes after nonresponse and relapse post-tisagenlecleucel in children, adolescents, and young adults with b-cell acute lymphoblastic leukemia. J. Clin. oncology: Off. J. Am. Soc. Clin. Oncol. (2022), Jco2201076. doi: 10.1200/JCO.22.01076
20. Myers RM, Li Y, Barz Leahy A, Barrett DM, Teachey DT, Callahan C, et al. Humanized CD19-targeted chimeric antigen receptor (CAR) T cells in CAR-naive and CAR-exposed children and young adults with relapsed or refractory acute lymphoblastic leukemia. J. Clin. oncology: Off. J. Am. Soc. Clin. Oncol. (2021) 39:3044–55. doi: 10.1200/JCO.20.03458
Keywords: relapsed/refractory B-cell lymphoblastic leukemia, pediatric, chimeric antigen receptor (CAR) T-cell therapy, tisagenlecleucel, transplant naive.
Citation: Phillips CL, Krupski C, Khoury R, Dandoy CE, Nelson AS, Galletta TJ, Faulhaber A, Davies SM and Rubinstein JD (2023) Post CAR T-cell therapy outcomes and management in HSCT-naive patients: a single-center experience. Front. Hematol. 2:1151744. doi: 10.3389/frhem.2023.1151744
Received: 26 January 2023; Accepted: 06 April 2023;
Published: 10 May 2023.
Edited by:
Liora Schultz, Stanford University, United StatesReviewed by:
Dalia Salem, Mansoura University, EgyptSenthil Velan Bhoopalan, St. Jude Children’s Research Hospital, United States
Copyright © 2023 Phillips, Krupski, Khoury, Dandoy, Nelson, Galletta, Faulhaber, Davies and Rubinstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christine L. Phillips, Y2hyaXN0aW5lLnBoaWxsaXBzQGNjaG1jLm9yZw==
 Christine L. Phillips
Christine L. Phillips Christa Krupski1,3
Christa Krupski1,3 Adam S. Nelson
Adam S. Nelson Thomas J. Galletta
Thomas J. Galletta Angela Faulhaber
Angela Faulhaber Stella M. Davies
Stella M. Davies Jeremy D. Rubinstein
Jeremy D. Rubinstein