
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hematol., 07 March 2023
Sec. Immunobiology and Immunotherapy
Volume 2 - 2023 | https://doi.org/10.3389/frhem.2023.1135261
 Kunapa Iam-arunthai1
Kunapa Iam-arunthai1 Supat Chamnanchanunt1,2*
Supat Chamnanchanunt1,2* Pravinwan Thungthong1
Pravinwan Thungthong1 Chajchawan Nakhahes1
Chajchawan Nakhahes1 Tawatchai Suwanban1
Tawatchai Suwanban1 Ponlapat Rojnuckarin3
Ponlapat Rojnuckarin3Purpose: Recent studies suggested that adding other agents to corticosteroids as a first-line treatment for immune thrombocytopenia (ITP) could improve outcomes. However, combination regimens may increase side effects and costs. To determine clinical factors associated with responses to the first-line steroid at 1 month.
Materials and methods: We retrospectively reviewed the medical records of patients with ITP aged ≥ 18 years, who were treated at Rajavithi Hospital between 2012 and 2020. Clinical data, laboratory results, treatment regimens, and responses to therapy were analyzed.
Results: Of the 226 patients, 76.6% were female. The mean age was 46.5 ± 18.1 years, and the median follow-up duration was 40 months. The proportion of chronic ITP was 97.3%. The complete response and response rates to first-line therapy were 65.5% and 88.9%, respectively. The age over 26 years, presentation clinically non-significant bleeding and a difference in platelet count of >50 x 109/L between days 1 and 7 after initial treatment were associated with the response to first-line treatment (adjusted odds ratio [OR] 5.09, 95% confidence interval [CI] 1.50-17.28, p = 0.009); OR 5.87, 95%CI 1.19-28.91, p = 0.029, and OR 3.60, 95%CI 1.10-11.73, p = 0.034, respectively. Younger patients and a difference in platelet count between day 1 and 7 ≤ 50 x 109/L were more likely to require second-line treatments. There were significant increases in the median platelet counts after prescribing azathioprine (baseline vs. 3 months, p = 0.001), cyclophosphamide (baseline vs. 6 months, p = 0.021), or danazol (baseline vs. 12 months, p = 0.039).
Conclusion: Adult, severity of bleeding at presentation, and rapid platelet increases within 1 week were related to excellent corticosteroid responses in ITP patients. These patients may not need combination regimens.
Immune thrombocytopenia (ITP) is an acquired autoantibody-mediated bleeding disorder that results from the accelerated destruction and impaired production of platelets. Consequently, low platelet counts are responsible for variable severities of bleeding (1, 2). Immune abnormalities in T-lymphocytes may also play an essential role in the cellular and molecular mechanisms underlying ITP. The incidence of ITP is approximately 3.3/100,000 per year in adults with female predominance. The aim of treatment is to achieve a balance between the prevention of major or life-threatening bleeding events and a good quality of life with minimal toxicities from therapy (3, 4). The first-line ITP treatments are corticosteroids, intravenous immunoglobulin (IVIG), or anti-D immunoglobulin depending on the patient profiles, as well as the discretions of the physicians (5–7). Corticosteroids are, by far, the preferred first-line treatment because the ability to rapidly decrease platelet clearance and increase platelet production (8–11). Recently, glucocorticoids in combination with other immunosuppressive medications have emerged to become first-line treatments to gain high and sustained treatment responses (12–14). However, these regimens may be related to inferior quality of life as well as higher costs (13). Selection of a subgroup of patients who likely have poor responses to glucocorticoid-only regimens will be helpful in clinical practice.
Patients who do not respond to corticosteroids or those with steroid dependence are subjected to second-line treatments including splenectomy, anti-CD20 antibody (rituximab), thrombopoietin-receptor agonists (TPO-RAs), or immunosuppressive agents (15–19). The aim of second-line treatments is to maintain platelet counts that can prevent severe bleeding complications (18, 20, 21). Splenectomy may have unpredictable platelet responses and be associated with infections and thromboembolism. Accessibility to expensive medicines, such as anti-CD20 antibodies and TPO-RAs, significantly impacts physician’s decision-making in the real-world setting (18). The selection of specific second-line regimens for adult patients with ITP is complex and should be tailored for each patient. The definitive guidelines for physicians are lacking due to the absence of data directly comparing among second-line treatment outcomes. Information on response rates and side effects of second-line treatment in real-world practice is needed, especially in limited-resource countries. The present study aimed to investigate the clinical factors that predicted the response to corticosteroid treatment in adult ITP patients and to explore the platelet response outcomes following various second-line regimens.
This retrospective cohort study was based on the medical record review of adult patients aged ≥ 18 years diagnosed as ITP with no secondary cause of thrombocytopenia at the Rajavithi Hospital in Bangkok, Thailand, between 2012 and 2020. Patients with primary ITP, which was defined as platelet count of < 100 x 109/L in the absence of other causes of thrombocytopenia were eligible. The following information was collected: demographic characteristics, dates of diagnosis, comorbidities, bleeding severity, and platelet counts at baseline, at one week and one month after treatment and every 3 months during follow-up. Lost to follow-up patients, the data was excluded. This study was approved by the Ethics Committee of the Rajavithi Hospital (Number 111/2563).
ITP cases from the time of diagnosis to 3 months were considered acute, those diagnosed at 3 months after receiving first-line treatment until 12 months were considered persistent, and those diagnosed after receiving treatments and still thrombocytopenia for over 12 months were considered chronic. Glucocorticoid-only regimens were defined as either dexamethasone or prednisolone alone. Dexamethasone regimen was 40 mg daily for up to 4 days before switching to prednisolone orally. Prednisolone was started at a daily dose of 1 mg. per kilogram for weeks and subsequently decreased the doses to maintain platelets at a response level. If platelets count did not respond to glucocorticoid-only regimen after one month, patients were shifted to glucocorticoid combination regimens (prednisolone ranged between 0.5 to 60 mg daily and other immunosuppressive drugs) in order to achieve a platelet response level.
We compared the treatment outcomes and side effects of among the three treatment groups. The definitions of treatment responses were based on the criteria of the International Working Group (IWG) on ITP (22) as follows. Complete response (CR) was platelet counts of ≥ 100 x 109/L in two measurements performed 7 days apart and no bleeding events; partial response (PR) were platelet counts between 30 - 99 x 109/L or 2-fold increase in platelet counts from baseline based on two measurements performed 7 days apart and no bleeding events; and no response (NR) was a platelet count of <30 x 109/L or <2-fold increase in platelet counts from baseline or the presence of bleeding events. Platelet counts were measured on two occasions more than 7 days apart. The response in this study was defined as a complete or partial response at one month after the treatment initiation.
Bleeding events before and after each treatment regimen were evaluated. Bleeding events were characterized based on the ITP bleeding scale (23) before treatment and at 1, 3 and 6 months after treatment initiation. The bleeding scale, which is based on bleeding events at different sites, was used to grade bleeding severity as follows: 0 no bleeding,1 mild to moderate bleeding; and 2 significant bleeding, as well as skin or non-skin bleeding.
Categorical variables were presented as numbers and percentages, and continuous variables were presented as means with standard deviations for normally distributed data, and as medians with interquartile ranges (IQR) for non-normally distributed data. Univariate analysis was performed using the Chi-square test and multivariate analysis was performed using logistic regression. All data were analyzed using SPSS program version 22.0(IBM). A p-values of ≤0.05 were considered statistically significant.
The study comprised 226 adult patients with ITP. The majority of the patients were female (76.6%) and the mean age was 46.5 ± 18.1 years (Table 1). Metabolic disorders were the most common comorbidities including hypertension (51.1%), dyslipidemia (43.6%), and type 2 diabetes mellitus (41.5%). The median platelet count at presentation was 14 x 109/L (range 1-96 x 109/L). The antinuclear antibody (ANA) test was positive in 40 (17.7%) patients. The most common clinical presentations were grade 2 non-skin bleeding (55.8%, n= 126), grade 2 skin bleeding (24.3%, n= 55).
All ITP patients received at least one first-line treatment, including prednisolone (n= 155, 68.6%), dexamethasone (n= 52, 23.0%), methylprednisolone (n= 15, 6.6%), and first-line splenectomy (n=1, 0.4%) prior to second-line treatments. Consequently, 148 (65.5%) patients achieved complete response to the first-line treatment. However, 59 (26.1%) patients required a second-line treatment including azathioprine (n= 34, 57.6%), cyclophosphamide (n= 27, 45.8%), danazol (n= 21, 35.6%), or splenectomy (n= 6, 9.5%). Of 9% patients had a partial response but did not received further treatment.
Chronic ITP was diagnosed in 220 patients (97.3%). Five (2.2%) patients expired. The most common cause of death was infection (3), followed by fatal bleeding (1) and cancer (1). The median follow-up duration was 40.0 (range 0.1-381.0) months in the overall cohort. Adverse events occurred in 31% of the adult patients with ITP including major bleeding, hyperglycemia, and infections. The cause of death were infection (3), cancer (1), and bleeding event (1).
As shown in Table 2, univariate analysis revealed that the age (> 26 year-old), bleeding (grade 2 non-skin) at clinical presentation and a difference in platelet counts between day 1 and 7 of ≤ 50 x 109/L were significantly associated with treatment response (p= 0.025, 0.018, and 0.045 respectively). In contrast, sex, comorbidities, initial complete blood count, ANA positivity and disease classifications were not significantly associated with treatment response.
Upon multivariate analysis, the patients age >26 years (odds ratio (OR), 5.09, 95% confidence interval (CI) 1.50, 17.28, p= 0.009), clinical bleeding (grade 1 non-skin and grade 2 skin) presentation (OR, 5.87, 95% CI, 1.19, 28.91, p= 0.029), and difference in platelet counts between day 1 and 7 over 50 x 109/L (OR, 3.60, 95% CI, 1.10, 11.73, p = 0.034) were statistically associated with initial treatment response in ITP patients (Table 3).
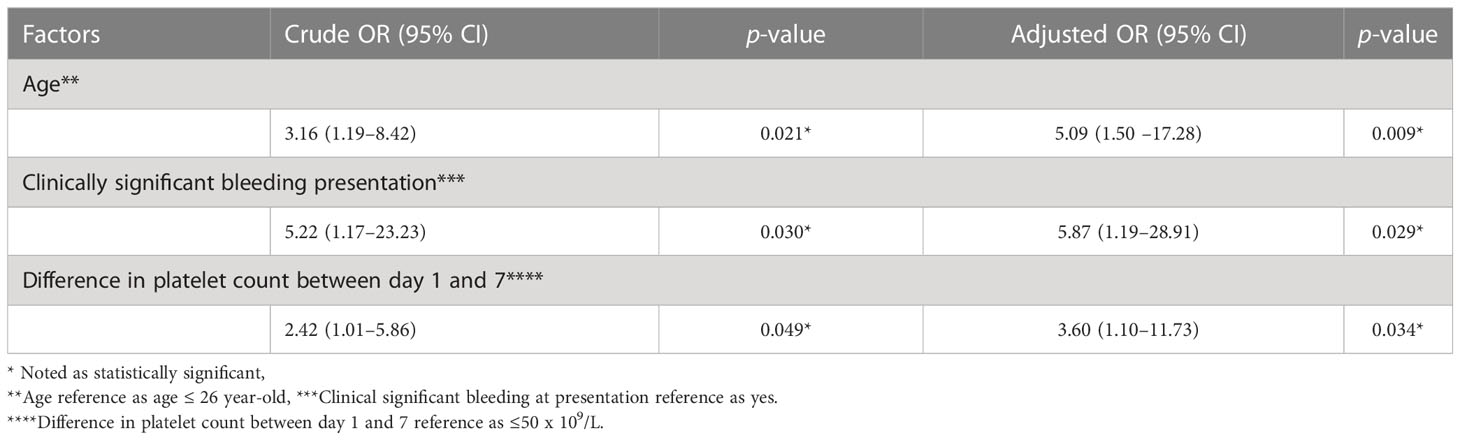
Table 3 Multivariate logistic regression for response to treatment among patients with immune thrombocytopenia.
Comparison with the glucocorticoid-only group, patients with younger ages, higher initial platelet counts, and differences in platelet counts between day 1 and 7 of ≤ 50 x 109/L were more likely to require combination regimens (p=0.043, 0.034, and 0.047 respectively) (Table 4). Durations of treatments and adverse events were similar between the two groups.
We also determined the time to treatment response in patients who received second-line treatments. In patients receiving azathioprine, the median platelet counts at baseline and at 3, 6, and 12 months after treatment initiation were 29 x 109/L (interquartile range; [IQR] 16-61 x 109/L), 66 x 109/L (IQR 37-130 x 109/L), 63 x 109/L (IQR 25-102 x 109/L), and 60 x 109/L (IQR 49-104 x 109/L), respectively. The platelet count significantly increased at 3 months after azathioprine (p= 0.001) (Figure 1A).
In patients who received cyclophosphamide, the median platelet counts at baseline, at 3 months, and 6 months after treatment initiation were 23 x 109/L (IQR 12-48 x 109/L), 70.5 x 109/L (IQR 9.5-121.5 x 109/L), and 74.5 x109/L (IQR 17-175 x 109/L), respectively. The median platelet count was significantly higher at 6 months after cyclophosphamide initiation compared to the baseline (p= 0.021) (Figure 1B).
In patients who received danazol the second-line treatment, the median platelet counts at baseline, and at 3, 6, 12 months after treatment were 20 x 109/L (IQR 12-35 x 109/L), 41 x 109/L (IQR 20-143 x 109/L), 68 x 109/L (IQR 12.5-117 x 109/L), and 47 x 109/L (IQR 26-97 x 109/L), respectively. There was a significant increase in the median platelet count between baseline and at 12 months after danazol treatment (p= 0.039) (Figure 1C).
Five patients who received eltrombopag, 2 patients developed CR, 1 PR, and the rest with NR. In patients who received eltrombopag, the median platelet counts at baseline, and 1, 3, 6 months after treatment were 173 x 109/L (IQR 28-319 x 109/L), 123 x 109/L (IQR 51-196 x 109/L), and 20 x 109/L, respectively. For rituximab, two patients showed CR, one with PR, and one with NR. In patients who received rituximab, the median platelet counts at baseline, and 1, 2, 3 weeks after treatment were 108 x 109/L (IQR 5-266 x 109/L), 72 x 109/L (IQR 10-160 x 109/L), and 91 x 109/L (IQR 20-162 x 109/L), respectively.
In the present study, clinical factors predicting responses to the first-line treatment at 1 month were adult age (>26 year-old), presenting with grade 1 non-skin and grade 2 skin bleeding, and rapid rising platelet counts within 1 week of treatment initiation. The odds ratios were more than five times in the former two factors and over three times for the latter. We observed may be one pathway corresponding to immunosuppressive treatment observed in adult age. One may postulate that the age above 26 year-old representing adult’s immune systems. These factors might be considered as the early predictors that can aid in the identification of patients who might respond well to treatment versus those who might progress to refractory ITP. The patients without these factors should be considered for a glucocorticoid combination regimen, such as steroid plus mycophenolate mofetil or all-trans retinoic acid, as the first-line treatment because it showed good outcomes with lower risks of refractory or relapsed disease (13, 14). Although combined regimens yield lower quality of life, they may be more beneficial in high-risk patients.
The majority of the patients in the present study were female consistent with the results of a study by Abrahamson et al. (22), and other three studies from Thailand (8, 24, 25). Generally adult ITP is more common in females (15, 26, 27). The mean age of the current cohort was younger than those of European patients (10). Similar to the findings of Neunert et al., our mean age of female patients was lower than that of the male patients (11). Metabolic disorders including hypertension, dyslipidemia, and type 2 diabetes mellitus were the most common comorbidities in the current cohort. Furthermore, 17.7% of patients were ANA-positive which was showed to relate with poor outcomes and high disease severity as reported by Grimaldi-Bensouda et al., and Vantelon et al. (28, 29),. In this study, 5 ANA patients developed systemic lupus erythematosus (SLE) at 40 months later (range 24-60 months). Therefore, ANA positivity might be a risk factor for the development of SLE in ITP.
In the present study, grade 2 bleeding was a common finding, similar to those of previous studies from Thailand and other countries reporting moderate bleeding events at presentation (8, 15, 24, 30). Three patients in the present study had life-threatening bleeding that required immediate managements at presentation, highlighting that life-threatening hemorrhage is uncommon (15). The mortality rate was only 2.2%, which is comparable to the rate of lower 5% reported by Depre et al. (16). Moreover, the cause of death was more commonly from treatment-related complications, such as super imposed infection (8, 11, 17).
The treatment regimen selection depends on the physician’s experience, guidelines, patient affordability and preference. In our study, corticosteroids were the most commonly administered first-line regimen because of the high efficacy and low cost. However, long-term corticosteroids are associated with several complications, including hyperglycemia, osteoporosis, hormonal disturbances, hypertension, and opportunistic infections. The second choice first-line treatment is intravenous immunoglobulin, which shows fast response and a good safety profile, but the cost is high with transient effects. We found good responses to corticosteroid in agreement with several studies recommending corticosteroids as the first-line treatment (15–17, 24). Short duration of high-dose dexamethasone might be consider to evaluate rapid response as new recommendation (31). Certainly, our patients received a regimen of dexamethasone with switching to prednisolone later. Only eight patients underwent splenectomy, which was lower than reported in other studies (range 13.5%-57.0%) (8, 20). One explanation is the higher risks, such as surgical complications and overwhelming infections. The majority of our patients developed chronic ITP, similar to the reports by Makruasi et al., and Cantoni et al. (8, 18),. On contrary, Neylon et al. found that remission was achieved after first-line treatment in more than 50% of the patients when both medications and surgical interventions were used (26).
Adult patients with ITP who are unresponsive to first-line treatment received second-line treatments to achieve safe platelet levels. Thirty-eight patients with ITP relapsed after complete remission after received corticosteroid and then those received combination therapy (corticosteroids and azathioprine).The currently used agents are TPO-RAs which exhibit good efficacy and safety in patients with refractory ITP. However, they are associated with high costs prohibiting accesses in limited resource countries (32, 33). Therefore, more affordable therapeutic approaches, such as immunosuppressive agents, are preferred for refractory ITP in Thailand (34). The times to responses of these second-line medicines remain to be determined (33, 35). In this study, all three immunosuppressive drugs used as a second-line could lead to increases in platelet counts at 3, 6, and 12 months compared with those of baseline. Notably, responses were observed at 3 and 6 months after starting azathioprine, and cyclophosphamide, respectively, whereas a slower response time of 12 months was observed in danazol. This finding might aid to define patients who do not respond to these second-line treatments. For nine patients were treated with new medication. Patients who treated with rituximab or TPO mimetics (eltrombopag) demonstrated 50% achieved complete remission. This study has some limitations. First, this was a retrospective study performed at a single institution. Furthermore, clinical data were obtained solely from archived medical records, and most of the patients were referred from primary or secondary care centers. Future studies including larger cohort are warranted to confirm the findings of this study.
Adult age, clinical bleeding at presentation, and rising platelet counts within 7 days of treatment were early predictive factors for steroid-responsive ITP. Absence of these factors may suggest the need of an early second-line treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Rajavithi Hospital (Number 111/2563). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
KI-A and SC reviewed the literature and drafted the manuscript. KI-A, SC, PT, CN, and TS collected the data. KI-A and SC performed statistical analysis. KI-A, SC, PT, CN, TS, and PR reviewed and edited the final draft. All authors contributed to the article and approved the submitted version.
This study was supported by a research grant from Rajavithi Hospital, the Multicenter Research Grant from the Thai Society of Hematology, and Specific League Funds from Mahidol University.
The authors thank Lertnapa Lertum, Supasri Keawpai, and Chanjira Sae-Lim for supporting our adult immune thrombocytopenia patients, and Wannakorn Homsuwan, Associate Professor Dusit Sujirarat for suggesting and confirming data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am. J. Hematol. (2010) 85:174–80. doi: 10.1002/ajh.21616
2. Rodeghiero F, Besalduch J, Michel M, Provan D, Grotzinger K, Thompson G. Treatment practices in adults with chronic immune thrombocytopenia–a European perspective. Eur. J. Haematol. (2010) 84:160–8. doi: 10.1111/j.1600-0609.2009.01361.x
3. Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood (2010) 115:168–86. doi: 10.1182/blood-2009-06-225565
4. Harrison CN, Bareford D, Butt N, Campbell P, Conneally E, Drummond M, et al. Guideline for investigation and management of adults and children presenting with a thrombocytosis. Br. J. Haematol. (2010) 149:352–75. doi: 10.1111/j.1365-2141.2010.08122.x
5. An Z-Y, Wu Y-J, He Y, Zhu X-L, Shi H-X, Wang C-C, et al. Tacrolimus plus high-dose dexamethasone versus high-dose dexamethasone alone as first-line treatment for adult immune thrombocytopenia: The phase 2, open label, randomized trial (TARGET 020). Blood (2021) 138:13. doi: 10.1182/blood-2021-152111
6. Colunga-Pedraza PRR, Bustillos Muñoz M, de la Garza F, Gomez-De Leon A, Coronado-Alejandro EU, Gomez Gomez ET, et al. Romiplostim, low-dose rituximab and high-dose dexamethasone combination in newly diagnosed immune thrombocytopenia: Another "Total therapy" pilot study. Blood (2021) 138:1014. doi: 10.1182/blood-2021-149769
7. Matzdorff A, Binder M, Nimmerjahn F, Meyer O, Rummel MJ, Tesanovic T, et al. A phase II study to investigate the efficacy and safety of eltrombopag in combination with dexamethasone as first-line treatment in adult patients with newly diagnosed primary ITP (XPAG-ITP). Blood (2020) 136:36–7. doi: 10.1182/blood-2020-134318
8. Makruasi N, Phukiat N. Steroid responsiveness in adults with primary immune thrombocytopenia: a single center study. J. Med. Assoc. Thai. (2017) 100:S69–77.
9. Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood (2017) 129:2829–35. doi: 10.1182/blood-2017-03-754119
10. Michel M, Suzan F, Adoue D, Bordessoule D, Marolleau JP, Viallard JF, et al. Management of immune thrombocytopenia in adults: a population-based analysis of the French hospital discharge database from 2009 to 2012. Br. J. Haematol. (2015) 170:218–22. doi: 10.1111/bjh.13415
11. Neunert C, Lim W, Crowther M, Cohen A, Solberg L Jr., Crowther MA, et al. The American society of hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood (2011) 117:4190–207. doi: 10.1182/blood-2010-08-302984
12. Sun L, Wang J, Shao L, Yuan C, Zhao H, Li D, et al. Dexamethasone plus oseltamivir versus dexamethasone in treatment-naive primary immune thrombocytopenia: a multicentre, randomised, open-label, phase 2 trial. Lancet Haematol. (2021) 8:e289–e98. doi: 10.1016/S2352-3026(21)00030-2
13. Bradbury CA, Pell J, Hill Q, Bagot C, Cooper N, Ingram J, et al. Mycophenolate mofetil for first-line treatment of immune thrombocytopenia. N Engl. J. Med. (2021) 385:885–95. doi: 10.1056/NEJMoa2100596
14. Huang QS, Liu Y, Wang JB, Peng J, Hou M, Liu H, et al. All-trans retinoic acid plus high-dose dexamethasone as first-line treatment for patients with newly diagnosed immune thrombocytopenia: a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Haematol. (2021) 8:e688–e99. doi: 10.1016/S2352-3026(21)00240-4
15. Cooper N, Kruse A, Kruse C, Watson S, Morgan M, Provan D, et al. Immune thrombocytopenia (ITP) world impact survey (iWISh): Patient and physician perceptions of diagnosis, signs and symptoms, and treatment. Am. J. Hematol. (2021) 96:188–98. doi: 10.1002/ajh.26045
16. Depre F, Aboud N, Mayer B, Salama A. Efficacy and tolerability of old and new drugs used in the treatment of immune thrombocytopenia: Results from a long-term observation in clinical practice. PloS One (2018) 13:e0198184. doi: 10.1371/journal.pone.0198184
17. Chang H, Tang TC, Hung YS, Li PL, Kuo MC, Wu JH, et al. Immune thrombocytopenia: Effectiveness of frontline steroids and comparison of azathioprine, splenectomy, and rituximab as second-line treatment. Eur. J. Haematol. (2018) 101:549–55. doi: 10.1111/ejh.13144
18. Cantoni S, Carpenedo M, Mazzucconi MG, De Stefano V, Carrai V, Ruggeri M, et al. Alternate use of thrombopoietin receptor agonists in adult primary immune thrombocytopenia patients: A retrospective collaborative survey from Italian hematology centers. Am. J. Hematol. (2018) 93:58–64. doi: 10.1002/ajh.24935
19. Zhang J, Liang Y, Ai Y, Xie J, Li Y, Zheng W. Thrombopoietin-receptor agonists for children with immune thrombocytopenia: a systematic review. Expert Opin. Pharmacother. (2017) 18:1543–51. doi: 10.1080/14656566.2017.1373091
20. Cuker A. Transitioning patients with immune thrombocytopenia to second-line therapy: Challenges and best practices. Am. J. Hematol. (2018) 93:816–23. doi: 10.1002/ajh.25092
21. Tsukune Y, Komatsu N. Management of adult chronic immune thrombocytopenia in Japan: Patient and hematologist perspectives from a multi-center cross-sectional questionnaire survey. Intern. Med. (2016) 55:2379–85. doi: 10.2169/internalmedicine.55.6407
22. Abrahamson PE, Hall SA, Feudjo-Tepie M, Mitrani-Gold FS, Logie J. The incidence of idiopathic thrombocytopenic purpura among adults: a population-based study and literature review. Eur. J. Haematol. (2009) 83:83–9. doi: 10.1111/j.1600-0609.2009.01247.x
23. Page LK, Psaila B, Provan D, Michael Hamilton J, Jenkins JM, Elish AS, et al. The immune thrombocytopenic purpura (ITP) bleeding score: assessment of bleeding in patients with ITP. Br. J. Haematol. (2007) 138:245–8. doi: 10.1111/j.1365-2141.2007.06635.x
24. Pirunsarn A, Kijrattanakul P, Chamnanchanunt S, Polprasert C, Rojnuckarin P. A randomized multicenter trial comparing low-dose prednisolone versus observation for prevention of recurrences in adult immune thrombocytopenia. Clin. Appl. Thromb. Hemost. (2018) 24:867–73. doi: 10.1177/1076029618764843
25. Wanachiwanawin W, Visudhiphan S, Pinankijagum A, Vatanavicharn S. Therapy of chronic idiopathic thrombocytopenic purpura in adults: experiences from Thailand. Southeast Asian J. Trop. Med. Public Health (1993) 24(1):71–5.
26. Neylon AJ, Saunders PW, Howard MR, Proctor SJ, Taylor PR. Northern region haematology g. clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: a prospective study of a population-based cohort of 245 patients. Br. J. Haematol. (2003) 122:966–74. doi: 10.1046/j.1365-2141.2003.04547.x
27. Jaime-Perez JC, Aguilar-Calderon P, Jimenez-Castillo RA, Ramos-Davila EM, Salazar-Cavazos L, Gomez-Almaguer D. Treatment outcomes and chronicity predictors for primary immune thrombocytopenia: 10-year data from an academic center. Ann. Hematol. (2020) 99:2513–20. doi: 10.1007/s00277-020-04257-2
28. Grimaldi-Bensouda L, Nordon C, Michel M, Viallard JF, Adoue D, Magy-Bertrand N, et al. Immune thrombocytopenia in adults: a prospective cohort study of clinical features and predictors of outcome. Haematologica (2016) 101:1039–45. doi: 10.3324/haematol.2016.146373
29. Vantelon J, Godeau B, André C, Bierling P. Screening for autoimmune markers is unnecessary during follow-up of adults with autoimmune thrombocytopenic purpura and no autoimmune markers at onset. Thromb. Haemost. (2000) 83:42–5. doi: 10.1055/s-0037-1613754
30. Chotsampancharoen T, Sripornsawan P, Duangchoo S, Wongchanchailert M, McNeil E. Predictive factors for resolution of childhood immune thrombocytopenia: Experience from a single tertiary center in Thailand. Pediatr. Blood Cancer. (2017) 64:128–34. doi: 10.1002/pbc.26178
31. Wei Y, Ji XB, Wang YW, Wang JX, Yang EQ, Wang ZC, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood (2016) 127:296–302. doi: 10.1182/blood-2015-07-659656
32. Ghanima W, Gernsheimer T, Kuter DJ. How I treat primary ITP in adult patients who are unresponsive to or dependent on corticosteroid treatment. Blood (2021) 137:2736–44. doi: 10.1182/blood.2021010968
33. Janssens A, Selleslag D, Depaus J, Beguin Y, Lambert C. Primary immune thrombocytopenia in adults: Belgian recommendations for diagnosis and treatment anno 2021 made by the Belgian hematology society. Acta Clin. Belg. (2022) 77:470–83. doi: 10.1080/17843286.2021.1876310
34. Grace RF, Shimano KA, Bhat R, Neunert C, Bussel JB, Klaassen RJ, et al. Second-line treatments in children with immune thrombocytopenia: Effect on platelet count and patient-centered outcomes. Am. J. Hematol. (2019) 94:741–50. doi: 10.1002/ajh.25479
Keywords: primary immune thrombocytopenia, second-line treatment, adult immune thrombocytopenia, response rate, glucocorticoid combination regimens
Citation: Iam-arunthai K, Chamnanchanunt S, Thungthong P, Nakhahes C, Suwanban T and Rojnuckarin P (2023) Identification factors to adjust early combination regimens in adult primary immune thrombocytopenia: An 8-year data analysis. Front. Hematol. 2:1135261. doi: 10.3389/frhem.2023.1135261
Received: 31 December 2022; Accepted: 21 February 2023;
Published: 07 March 2023.
Edited by:
Emmanouil Nikolousis, European University Cyprus, CyprusReviewed by:
Gemlyn George, University of Colorado, United StatesCopyright © 2023 Iam-arunthai, Chamnanchanunt, Thungthong, Nakhahes, Suwanban and Rojnuckarin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Supat Chamnanchanunt, c3VwYXQuY2hhQG1haGlkb2wuYWMudGg=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.