- 1Division of Hematology, Department of Internal Medicine, Comprehensive Cancer Center, The Ohio State University, Columbus, OH, United States
- 2Department of Pathology, Comprehensive Cancer Center, The Ohio State University, Columbus, OH, United States
- 3Department of Internal Medicine, Mount Carmel Health, Columbus, OH, United States
- 4Division of Hematology and Medical Oncology, Cancer Institute, West Virginia University, Morgantown, WV, United States
Older patients with acute lymphoblastic leukemia (ALL) have a poor prognosis, with a 5-year overall survival rate of only 10%–20%. This is attributable to patient comorbidities, poor performance status, and high-risk disease biology. The prognosis for patients with relapsed/refractory (R/R) disease remains poor, particularly for patients who are not candidates for therapies targeting CD19 or CD22. Additional treatment options are needed for these patient populations. The patient presented here is a 76-year-old man diagnosed with precursor B-cell ALL with aberrant expression of myeloid markers and lacking significant CD19 or CD22 expression. A 3-year remission was achieved with one cycle of CVP (cyclophosphamide, vincristine, and prednisone) followed by 22 months of maintenance DOMP (dexamethasone, vincristine, methotrexate, and 6-mercaptopurine) prior to relapse. He was then treated with one cycle of salvage CVP, which was complicated by a stroke resulting in hemiparesis. Next-generation sequencing (NGS) was performed on the relapsed bone marrow, which revealed the presence of an R132H mutation in the isocitrate dehydrogenase 1 (IDH1) gene. He was subsequently treated with the IDH1 inhibitor ivosidenib and remained in a second remission for nearly 1 year. IDH1 mutations are present in up to 14% of acute myeloid leukemia (AML) cases but are also seen more rarely in ALL, particularly in cases involving aberrant expression of myeloid markers. Ivosidenib has demonstrated efficacy in patients with IDH1-mutated AML but has not been extensively studied in other hematologic malignancies. This case demonstrates the role of NGS in revealing treatment options in patients with otherwise limited available therapies.
Introduction
Acute lymphoblastic leukemia (ALL) most commonly occurs in children and younger adults; only 10%–15% of cases are seen in adults aged 65 years or older. The prognosis for older adults with ALL remains poor, with a 5-year overall survival (OS) of 10%–20% (1–5). Factors contributing to this poor prognosis include the presence of significant comorbidities, poor performance status, and high-risk genetic features (1, 2, 4). Relapsed or refractory (R/R) ALL has also traditionally been associated with a very poor prognosis, with median OS ranging from 2 to 6 months and 5-year survival rates of less than 10% (6–10). Recently, blinatumomab and CD19-directed chimeric antigen receptor (CAR) T-cell therapies have improved the outlook for patients with R/R CD19+ disease, while inotuzumab ozogamicin is an effective option for those with CD22+ disease (11, 12). However, treatment options remain limited for patients lacking these markers.
The patient presented here is a 76-year-old man diagnosed with Philadelphia chromosome-negative Ph(-) precursor B-cell ALL (B-ALL) that was found to harbor a point mutation in the isocitrate dehydrogenase 1 (IDH1) gene at relapse. A second complete remission lasting nearly 1 year was achieved with one cycle of salvage cyclophosphamide, vincristine, and prednisone (CVP) followed by ivosidenib monotherapy. This case demonstrates the utility of next-generation sequencing (NGS) in revealing treatment options in patients with limited available therapies.
Case description
A 76-year-old man without a significant past medical history presented to his primary care physician with the principal complaint of worsening dyspnea on exertion and fatigue. Laboratory tests revealed pancytopenia with 10% circulating blasts and he was admitted for further management. Vital signs on admission were normal, and physical examination was unremarkable.
A bone marrow biopsy was carried out on the day of admission, and differential analysis of the bone marrow aspirate revealed 85% blasts. Erythropoiesis and granulopoiesis were markedly decreased, with adequate megakaryopoiesis. No significant dysplasia was seen. Blasts accounted for 80.1% of cells on flow cytometric analysis and expressed CD34, HLA-DR, and CD38. The blasts were nearly all positive for terminal deoxynucleotidyl transferase (TdT) and mostly positive for CD7, CD13, CD33, and the B-cell marker CD79a. A subpopulation (17%) demonstrated low levels of expression of the B-cell marker CD19. The blasts were negative for myeloperoxidase (MPO) and for definitive T-cell markers. Tests for other B-cell markers, including CD10, CD20, and CD22, were also negative (Figure 1). Cytogenetic analysis demonstrated a diploid karyotype. PCR analysis to detect the BCR-ABL1 translocation was negative. No additional genetic analysis, including testing for the Ph-like signature or NGS, was performed at this time. Based on a hematopathology review, the patient was diagnosed with a Ph(-) acute leukemia expressing both B-lymphoid and myeloid markers. Classification as Ph(-) B-ALL was favored at the time given the strong expression of TdT and CD79a. Based on the European Group for Immunological Classification of Leukemia (EGIL) scoring system, 2.5 points were assigned for B lineage based on the expression of CD79a (2 points) and TdT (0.5 points). Only 2 points were assigned for myeloid lineage based on the expression of CD13 and CD33 (1 point each). The blasts lacked expression of other myeloid markers, including MPO, CDw65, CD14, CD15, CD64, and CD117. Therefore, the leukemia did not reach the scoring threshold of >2 points for myeloid lineage required to meet the classification of a biphenotypic acute leukemia according to this scoring system (13).
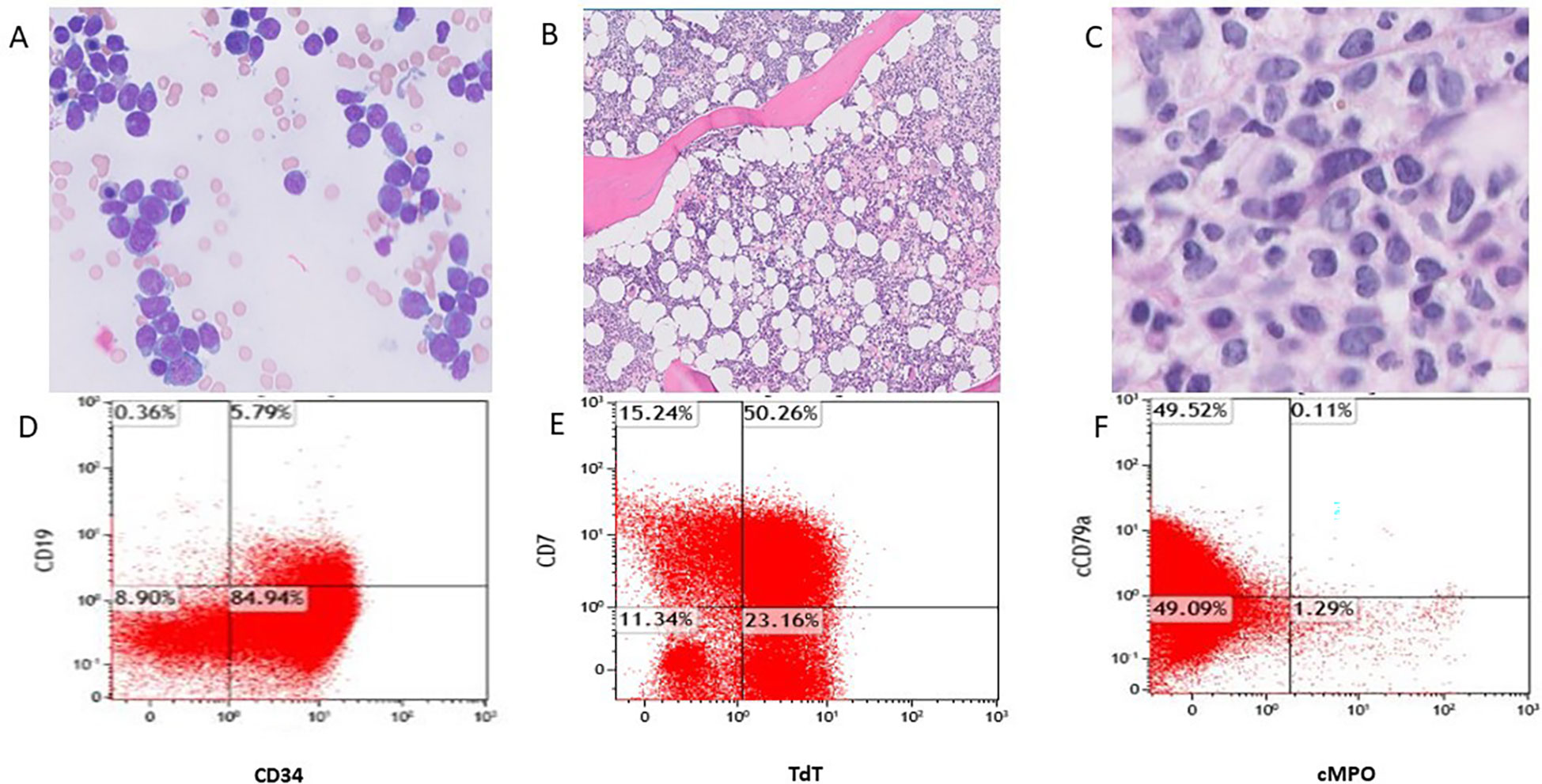
Figure 1 Bone marrow aspirate smear (A, Wright-Giemsa stain, original magnification 1000x) shows blasts, and Bone marrow core biopsy (B, original magnification 40x) shows hypercellularity and increased blasts (C, original magnification 50x). Flow cytometric analysis (D-F) shows the blasts co-expressing CD34+/CD19+, TdT+/CD7+, partially CD79a and negative for MPO.
The patient was treated with CVP (cyclophosphamide 750 mg/m2 on day 1, vincristine 2 mg on day 1, and prednisone 100 mg on days 1–5 of a 21-day cycle) as induction chemotherapy. A repeat bone marrow biopsy after cycle 1 of CVP was 40% cellular with trilineage hematopoiesis, erythroid predominance with 30% ring sideroblasts, and no morphologic or immunophenotypic evidence of leukemia. Maintenance therapy with DOMP (dexamethasone 10 mg/m2 on days 1–5, 29–33, and 57–61; vincristine 2 mg on days 1, 29, and 57; methotrexate 20 mg/m2 weekly; and 6-mercaptopurine 60 mg/m2 daily) in 84-day cycles was then instituted. Therapy was discontinued after the completion of eight cycles, as the patient developed vertebral osteomyelitis caused by methicillin-resistant Staphylococcus aureus (MRSA), which responded well to antibiotics. A repeat bone marrow biopsy 3 months after discontinuing maintenance therapy demonstrated dysgranulopoiesis and marked dysmegakaryopoiesis, with no evidence of leukemia.
The patient remained in remission for an additional 11 months but was diagnosed with relapse after surveillance laboratories demonstrated new leukopenia with neutropenia. Bone marrow biopsy revealed recurrent B-ALL, with flow cytometric analysis notable for 11% blasts with a similar immunophenotype to the blasts detected at diagnosis. The marrow was hypercellular (60%) with dysmegakaryopoiesis, mild dyserythropoiesis, and 6% blasts on morphologic assessment of the aspirate smear. Cytogenetic analysis again demonstrated a diploid karyotype. The decision was made to re-treat the patient with CVP as salvage therapy, given the long duration of remission (approximately 3 years) achieved with CVP followed by DOMP at the time of initial diagnosis. Following re-induction with CVP, the patient suffered a cerebrovascular accident, resulting in left-sided hemiparesis. Given the presence of myeloid antigens on the leukemic blasts, a myeloid NGS panel was performed on the relapsed bone marrow, which detected mutations in isocitrate dehydrogenase 1 (IDH1) R132H, with a variant allele frequency (VAF) of 43%, and serine- and arginine-rich splicing factor 2 (SRSF2) P95_R102del, with a VAF of 32%. Details regarding the NGS panel methodology and results can be found in the Supplementary Material. In light of the IDH1 mutation, the decision was made to discontinue CVP after one cycle and begin therapy with 500 mg daily of the IDH1 inhibitor ivosidenib. Pancytopenia developed during the first few months of treatment with ivosidenib, but blood counts subsequently normalized, except for very mild anemia. The patient underwent a repeat bone marrow biopsy approximately 3 months later, which showed no morphologic or immunophenotypic evidence of persistent leukemia.
The patient responded well to treatment for 10 months, then developed worsening pancytopenia with circulating blasts. A bone marrow biopsy revealed dyserythropoiesis and dysgranulopoiesis with 35% blasts. The blasts had a different immunophenotype than the original blast population: they were notable for the loss of B-cell markers CD79a and CD19 and were more consistent with a myeloid immunophenotype expressing HLA-DR, CD13, CD34, CD33, CD38, CD58, CD81, CD7, CD117 (50%), and CD4. Cytogenetics revealed an abnormal clone of cells with trisomy 21, and NGS revealed the previously identified mutations in IDH1 (VAF 41.9%) and SRSF2 (VAF 39.8%) along with an inactivating RUNX1 (RUNX family transcription factor 1) mutation (VAF 41.7%). This was felt to represent either the evolution of the original acute leukemia or the development of a new therapy-related AML. The patient was treated with salvage azacitidine plus venetoclax, given the high complete remission rates previously observed in IDH1-mutated AML patients treated with this combination (14). Persistent disease was detected after two cycles of therapy. The patient decided to forgo additional treatment with ivosidenib or other salvage regimens since he did not feel that receiving more leukemia-directed therapy was in line with his goals of care. He subsequently decided to transition to hospice care and died 2 months later. A timeline of events for this case is presented in Figure 2.
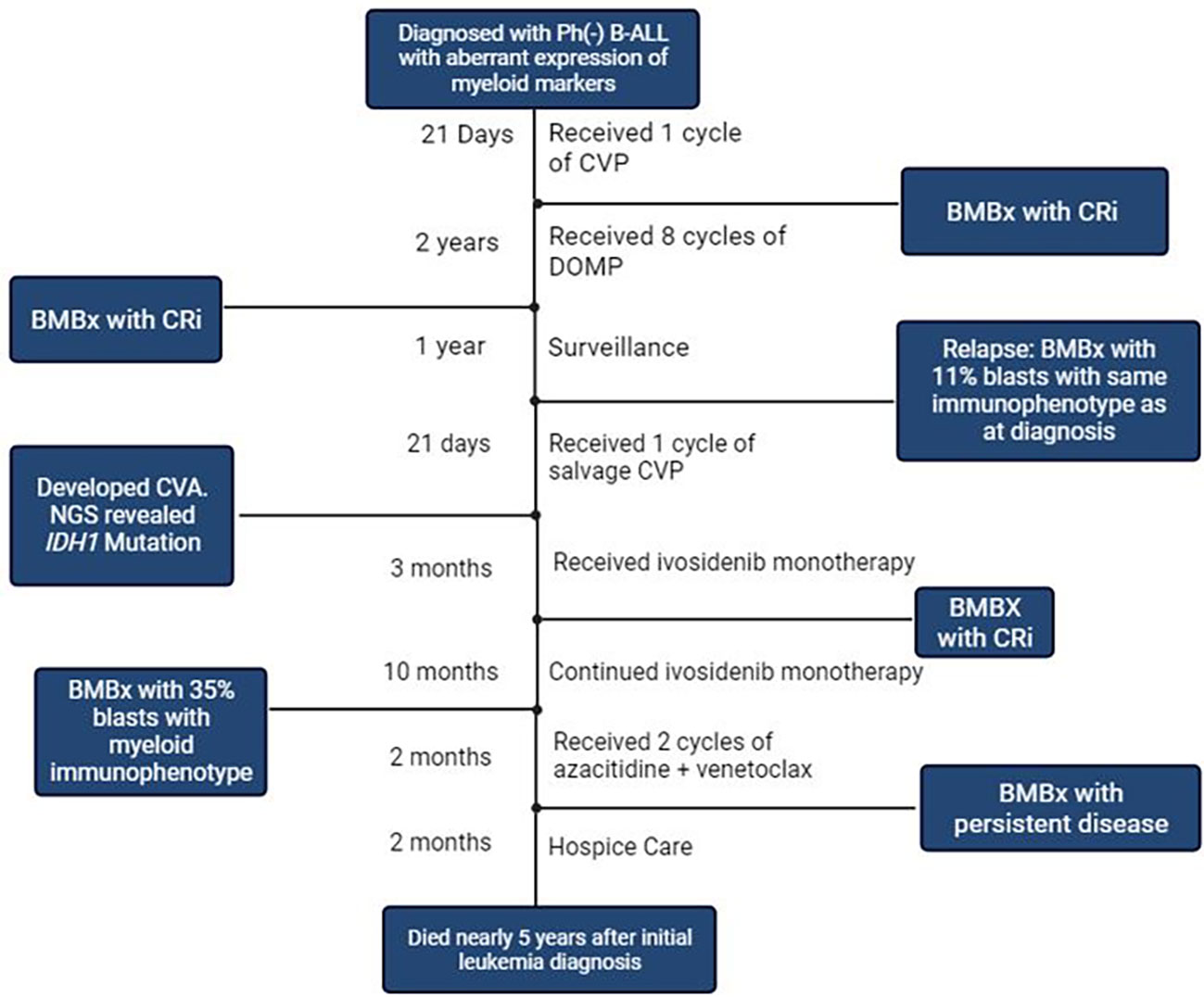
Figure 2 Timeline of Events. Ph: Philadelphia; B-ALL: B Cell Acute Lymphoblastic Leukemia; CVP, Cyclophosphamide, Vincristine, Prednisone; BMBx, Bone marrow Biopsy; Cri, Complete Response with incomplete count recovery; DOMP, Dexamethasone, Vincristine, Methotrexate, 6- Mercaptopurine; CVA, Cerebrovascular Accident; NGS, Next Generation Sequencing; IDH1, Isocitrate Dehydrogenase 1. Created with BioRender.com.
Discussion
Mutations in IDH1 occur in 7%–14% of AML cases and have also been seen in a variety of other malignancies. In AML, these mutations affect arginine residue 132 (R132) and interfere with hematopoietic differentiation by increasing the production of 2-hydroxyglutarate.
2-Hydroxyglutarate induces a state of DNA hypermethylation through inhibition of the DNA methyltransferase enzyme encoded by the TET2 (tet methylcytosine dioxygenase 2) gene (15–19). The IDH1 inhibitor ivosidenib has demonstrated efficacy in patients with IDH1-mutated AML in both R/R and frontline settings (20–22). IDH1 mutations are known to occur less frequently in ALL, and IDH1-mutated ALL blasts often display aberrant expression of myeloid markers (23–25). Ivosidenib has not been extensively studied in patients with IDH1-mutated ALL.
The patient presented in this case study had limited treatment options for relapsed leukemia owing to his age and lack of significant CD19 or CD22 expression, and he was able to achieve a second remission of nearly 1 year with one cycle of salvage CVP followed by ivosidenib monotherapy. This case demonstrates the potential of NGS to reveal additional therapies, particularly in the context of relapsed disease or restricted care options.
There are unique aspects to this case that should be considered. It is possible that the patient had underlying myelodysplastic syndrome (MDS) prior to the ALL diagnosis that may have been masked by the near-complete leukemic involvement of the bone marrow. The dysplastic findings seen on bone marrow biopsies during the course of therapy and the presence of additional myeloid mutations seen on NGS are suggestive of an underlying clonal myeloid disorder. However, these findings could also represent therapy-related changes, and laboratory values prior to the leukemia diagnosis are not available to determine if pre-existing cytopenias were present. If the patient did have concurrent MDS, the IDH1 mutation could have been present in the ALL, MDS, or both. Though rare, ALL and MDS can be present simultaneously and can be clonally related (26). Baseline samples were not available for NGS after the IDH1 mutation was detected at relapse, which would have helped to further clarify the clonal evolution of the disease. This also raises the question of whether the original leukemia truly represented a mixed-phenotype leukemia as opposed to Ph(-) B-ALL. Based on the updated 2022 World Health Organization (WHO) classification, the acute leukemia present at diagnosis in this case does not strictly meet B lineage criteria. In the context of weak (<50%) CD19 expression, B lineage criteria would require two or more of the following to be strongly expressed: CD10, CD22, or CD79a. Of these three markers, only CD79a was expressed. The acute leukemia in this case also does not meet myeloid lineage criteria (lack of MPO expression or expression of monocytic markers CD11c, CD14, CD65, or lysozyme) or T lineage criteria (no surface or cytoplasmic CD3 expression). Based on this updated classification system, a diagnosis of acute leukemia of ambiguous lineage (ALAL) not otherwise specified (NOS) would be favored if the patient was diagnosed today (27). Notably, the 2022 WHO system also stresses the importance of molecular classification. NGS was not performed at diagnosis, but IDH1 and SRSF2 mutations were present at both the first and second relapse and may have been stable mutations from the initial neoplastic clone. Neither of these mutations is AML defining, but they are more commonly associated with myeloid neoplasms, with SRSF2 now defined as a myelodysplasia-related gene mutation. This is a further indication of a myeloid component in the disease process, as is the eventual development of AML. It is also notable that a bone marrow biopsy was not carried out following treatment with salvage CVP to determine whether or not remission was achieved prior to starting ivosidenib. Despite this, the fact that the patient remained in a second remission for nearly 1 year on targeted ivosidenib monotherapy remains significant.
This case represents an example of NGS being used to select a targeted therapy in a patient in whom available treatments were otherwise limited. This strategy is being increasingly adopted to expand treatment options for patients with hematologic malignancies as our understanding of molecular pathogenesis increases, along with the development of therapies that target specific molecular derangements. A new targeted therapy is often initially validated in the context of a molecular alteration that is known to occur in one specific disease. Expanding the targeted therapy to other diseases requires additional study to determine if the molecular alteration remains predictive of response in different disease states. This patient’s clinical course raises the question of whether ivosidenib may have therapeutic efficacy in IDH1-mutated acute leukemias other than AML, including mixed-phenotype acute leukemias, ALAL, or ALL. Further studies would be needed to establish IDH1 mutations as a predictive biomarker for response to ivosidenib in these disease states prior to recommending routine IDH1 mutation analysis or ivosidenib therapy in this patient population. This could be done with basket trials for ivosidenib in IDH1-mutated acute leukemias.
Patient perspective
The following perspective was provided by the patient’s daughter, who was a primary source of support for the patient throughout his treatment:
“He tolerated the ivosidenib therapy well. This medication did not cause any significant complications, and he felt well overall while taking it. This medication was more tolerable than chemotherapy, and he appreciated being on a targeted therapy that was easier to take and caused fewer side effects.”
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
KS contributed to the literature review, manuscript preparation, figure preparation, and manuscript editing. WZ contributed to the figure preparation and manuscript editing. MB contributed to the manuscript preparation and editing. BB contributed to the manuscript preparation and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2023.1134837/full#supplementary-material
References
1. Dinmohamed AG, Szabo A, van der Mark M, Visser O, Sonneveld P, Cornelissen JJ. Improved survival in adult patients with acute lymphoblastic leukemia in the Netherlands: a population-based study on treatment, trial participation and survival. Leukemia (2016) 30(2):310–7. doi: 10.1038/leu.2015.230
2. Legrand O, Marie JP, Marjanovic Z, Cadiou M, Blanc C, Ramond S, et al. Prognostic factors in elderly acute lymphoblastic leukaemia. Br J Haematol (1997) 97(3):596–602. doi: 10.1046/j.1365-2141.1997.952909.x
3. Pulte D, Gondos A, Brenner H. Improvement in survival in younger patients with acute lymphoblastic leukemia from the 1980s to the early 21st century. Blood (2009) 113(7):1408–11. doi: 10.1182/blood-2008-06-164863
4. Moorman AV, Chilton L, Wilkinson J, Ensor H, Bown N, Proctor S. A population-based cytogenetic study of adults with acute lymphoblastic leukemia. Blood (2010) 115(2):206–14. doi: 10.1182/blood-2009-07-232124
5. Toft N, Schmiegelow K, Klausen T, Birgens H. Adult acute lymphoblastic leukaemia in denmark. a national population-based retrospective study on acute lymphoblastic leukaemia in Denmark 1998-2008. Br J Haematol (2012) 157(1):97–104. doi: 10.1111/j.1365-2141.2011.09020.x
6. Fielding AK, Richards S, Chopra R, Lazarus HM, Litzow M, Buck G, et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); An MRC UKALL12/ECOG 2993 study. Blood (2007) 109(3):944–50. doi: 10.1182/blood-2006-05-018192
7. Gökbuget N, Stanze D, Beck J, Diedrich H, Horst H-A, Hüttmann A, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood (2012) 120(10):2032–41. doi: 10.1182/blood-2011-12-399287
8. O'Brien S, Thomas D, Ravandi F, Faderl S, Cortes J, Borthakur G, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer (2008) 113(11):3186–91. doi: 10.1002/cncr.23919
9. Kantarjian HM, Thomas D, Ravandi F, Faderl S, Jabbour E, Garcia-Manero G, et al. Defining the course and prognosis of adults with acute lymphocytic leukemia in first salvage after induction failure or short first remission duration. Cancer (2010) 116(24):5568–74. doi: 10.1002/cncr.25354
10. Thomas DA, Kantarjian H, Smith TL, Koller C, Cortes J, O'Brien S, et al. Primary refractory and relapsed adult acute lymphoblastic leukemia: characteristics, treatment results, and prognosis with salvage therapy. Cancer (1999) 86(7):1216–30. doi: 10.1002/(SICI)1097-0142(19991001)86:7<1216::AID-CNCR17>3.0.CO;2-O
11. Kantarjian HM, DeAngelo DJ, Stelljes M, Liedtke M, Stock W, Gökbuget N, et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer (2019) 125(14):2474–87. doi: 10.1002/cncr.32116
12. Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med (2017) 376(9):836–47. doi: 10.1056/NEJMoa1609783
13. Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A. Proposals for the immunological classification of acute leukemias. European group for the immunological characterization of leukemias (EGIL). Leukemia (1995) 9(10):1783–6.
14. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med (2020) 383:617–29. doi: 10.1056/NEJMoa2012971
15. Ward PS, Patel J, Wise DR, Abdel-Wahab O, Bennett BD, Coller HA, et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell (2010) 17(3):225–34. doi: 10.1016/j.ccr.2010.01.020
16. Dang L, White DW, Gross S, Bennett B, Bittinger M, Driggers EM, et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature (2009) 462(7274):739–44. doi: 10.1038/nature08617
17. Figueroa ME, Abdel-Wahab O, Lu C, Ward PS, Patel J, Shih A, et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell (2010) 18(6):553–67. doi: 10.1016/j.ccr.2010.11.015
18. Montalban-Bravo G, DiNardo CD. The role of IDH mutations in acute myeloid leukemia. Future Oncol (2018) 14(10):979–93. doi: 10.2217/fon-2017-0523
19. Dang L, Su SM. Isocitrate dehydrogenase mutation and (R)-2-Hydroxyglutarate: From basic discovery to therapeutics development. Annu. Rev. Biochem. (2017) 86:305–31. doi: 10.1146/annurev-biochem-061516-044732
20. DiNardo CD, Stein EM, de Botton S, Roboz G, Altman J, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med (2018) 378(25):2386–98. doi: 10.1056/NEJMoa1716984
21. Roboz G, Dinardo C, Stein E, de Botton S, Mims A, Prince G, et al. Ivosidenib (IVO; AG-120) in IDH1-mutant newly-diagnosed acute myeloid leukemia (ND AML): Updated results from a phase 1 study. J Clin Oncol (2019) 37(15_suppl):7028–8. doi: 10.1200/JCO.2019.37.15_suppl.7028
22. Montesinos P, Recher C, Vives S, Zarzycka E, Wang J, Bertani G, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med (2022) 386:1519–31. doi: 10.1056/NEJMoa2117344
23. Andersson AK, Miller DW, Lynch JA, Lemoff AS, Cai Z, Pounds SB, et al. IDH1 and IDH2 mutations in pediatric acute leukemia. Leukemia (2011) 25(10):1570–7. doi: 10.1038/leu.2011.133
24. Abbas S, Lugthart S, Kavelaars FG, Schelen A, Koenders JE, Zeilemaker A, et al. Acquired mutations in the genes encoding IDH1 and IDH2 both are recurrent aberrations in acute myeloid leukemia: Prevalence and prognostic value. Blood (2010) 116(12):2122–6. doi: 10.1182/blood-2009-11-250878
25. Zhang Y, Wei H, Tang K, Lin D, Zhang C, Mi Y, et al. Mutation analysis of isocitrate dehydrogenase in acute lymphoblastic leukemia. Genet Test Mol Biomarkers (2012) 16(8):991–5. doi: 10.1089/gtmb.2011.0323
26. Xie W, Chen Z, Wang SA, Hu S, Li S, Miranda R, et al. Lymphoblastic leukemia following myelodysplastic syndromes or Myelodysplastic/Myeloproliferative neoplasms. J Leukemia Lymphoma (2019) 60(12):2993–3001. doi: 10.1080/10428194.2019.1605509
Keywords: case report, acute lymphoblastic leukemia, ivosidenib, targeted therapy, next-generation sequencing
Citation: Sahasrabudhe K, Zhao W, Berg M and Bhatnagar B (2023) Case report: Sustained complete remission with ivosidenib in a patient with relapsed, IDH1-mutated acute leukemia. Front. Hematol. 2:1134837. doi: 10.3389/frhem.2023.1134837
Received: 30 December 2022; Accepted: 16 February 2023;
Published: 10 March 2023.
Edited by:
Stefano Molica, Hull University Teaching Hospitals NHS Trust, United KingdomReviewed by:
Salvatore Perrone, Local Health Department of Latina, ItalyRaffaele Palmieri, University of Rome Tor Vergata, Italy
Copyright © 2023 Sahasrabudhe, Zhao, Berg and Bhatnagar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kieran Sahasrabudhe, S2llcmFuLlNhaGFzcmFidWRoZUBvc3VtYy5lZHU=
 Kieran Sahasrabudhe
Kieran Sahasrabudhe Weiqiang Zhao
Weiqiang Zhao Miriam Berg
Miriam Berg Bhavana Bhatnagar
Bhavana Bhatnagar