
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Hematol., 17 March 2023
Sec. Blood Cancer
Volume 2 - 2023 | https://doi.org/10.3389/frhem.2023.1127322
 Ascanio Tridente1,2†
Ascanio Tridente1,2† Nina C. Dempsey2*†
Nina C. Dempsey2*† Mai Khalifa3
Mai Khalifa3 Jack Goddard3
Jack Goddard3 Katy Shuker4
Katy Shuker4 Joni Hall4
Joni Hall4 Youssef Sorour3
Youssef Sorour3 Josh Wright3
Josh Wright3 Stephen Webber4
Stephen Webber4 Gary H. Mills4‡
Gary H. Mills4‡ John A. Snowden3,5‡
John A. Snowden3,5‡Background: Critical care (CC) admission has traditionally been viewed as likely to result in a poor outcome for hematological malignancy (HM) patients. Such a view can have implications for decisions surrounding CC admission. Recent studies have challenged this poor prognostication, however, there still remains limited data to support CC admission and escalation decisions and to elucidate risk factors which independently predict short- and longer-term survival outcomes.
Methods: We retrospectively analyzed a large cohort of adult HM patients (n=437) admitted to CC over a sixteen-year period, with the specific aim of identifying risk factors present at CC unit admission that could help to predict outcome. We assessed all-cause mortality at CC discharge (CC mortality, primary outcome) and at further time points (hospital discharge and 12-months post-discharge from CC). Single variable and multivariate analyses were performed to identify independent predictors of outcome.
Results: CC unit and hospital mortality rates were 33.4% (146 patients) and 46.2% (202 patients) respectively. At six-month and one-year follow-up, mortality increased to 59.5% and 67.9% respectively. At single variable adjusted regression analysis, eight factors were associated with CC mortality: APACHE II score, the number of organs supported, requirement for continuous renal replacement therapy (CRRT), cardiovascular support, or respiratory support (invasive and non-invasive), the ratio between arterial partial pressure of oxygen (PaO2) and the inspired oxygen concentration (FiO2) (P/F ratio) on CC admission, and the lowest P/F ratio during CC admission. However, only three factors showed independent predictive capacity for CC outcome at multivariate logistic regression analysis; APACHE II score on admission, requirement for ventilation and lowest P/F ratio.
Conclusion: One third of HM patients admitted to CC died on the unit and, following admission to CC, approximately one-third of HM patients survived over 1 year. Our data show that, while a diagnosis of HM should not preclude admission of patients who might otherwise benefit from CC support, the prognosis of those with a high APACHE II score upon admission, or those requiring IMV remains poor, despite considerable advances in IMV techniques.
Patients with hematological malignancies (HM) have traditionally been perceived as poor candidates for Critical Care (CC), based on significantly poorer survival rates described for this cohort in comparison with non-HM patients requiring CC (1–3). Historically, CC survival for HM patients has been reported at approximately 20%, and as low as 5% or less in some studies (2, 3). Such findings may influence individual clinical decision making, with potential reluctance to admit HM patients to CC units, which is reflected in published guidelines from the American College of Critical Care Medicine regarding CC admission, discharge and triage decisions (4). However, more recent publications suggest that HM patients have benefitted from improved intensive care support during the last decade (5–7), and in response to this, the British Society of Haematology (BSH) guidance on management of the critically unwell patients with a HM emphasizes the need for access to CC for this cohort (8).
When exploring the factors potentially associated with patient outcome, numerous variables have been implicated but not all are reported consistently across the literature. The most consistent factors include the need for mechanical ventilation (MV), the presence of two or more organ failures and severe sepsis (1, 7, 9–11). In 2009, Hampshire et al. reviewed over 500,000 admissions of patients with HM to 178 Intensive Care Units (ICU) across England, Wales and Northern Ireland (12). This report showed that the Acute Physiology and Chronic Health Evaluation II (APACHE II) score was a more accurate predictor of mortality when compared with the Simplified Acute Physiology Score (SAPS) and the Intensive Care National Audit and Research Centre (ICNARC) model, but underestimated mortality at the lower end of the prediction range. Other smaller single-center studies have suggested an association between APACHE II scores and mortality in patients with HM admitted to CC (1, 2, 13–15). This is in contrast to other cancer types in which traditional physiological scores do not perform well as predictive models (16–18). Given the conflicting reports regarding HM patient outcomes in CC and the need to identify reliable prognostication strategies for this patient group in the CC setting (19), we retrospectively studied a large cohort of HM patients admitted to CC over a period of sixteen years with the specific aim of identifying risk factors present at CC unit admission that could help to predict outcome.
This study was performed at Sheffield Teaching Hospitals (STH) NHS Foundation Trust. STH has a two-site CC department, comprising of 36 CC beds at the Northern General Hospital site and 8 beds at the Royal Hallamshire Hospital site, contiguous with inpatient, day-case and outpatient hematology and hemopoietic stem cell transplant (HSCT) facilities. The CC is a tertiary medical-surgical unit, with approximately 3400 admissions per year.
Following ethical approval from the Human Research Authority (HRA; IRAS number 290345), data were collected on all Hematological Malignancy (HM) patients 18 years or over and admitted to STH CC between 1st September 2002 and 31 July 2018. Patients with non-malignant hematology diagnoses, including those who had received a transplant for a non-malignant diagnosis were excluded. Only the first admission data were included; repeat admissions were excluded from analysis.
Patient baseline data, including demographics, HM diagnosis, APACHE II and clinical frailty scores, the nature of organ support during the CC stay (i.e. requirement for invasive MV (IMV), non-invasive ventilation (NIV), continuous renal replacement therapy (CRRT), and cardiovascular system (CVS) with vasopressors and inotropes) and results from relevant laboratory tests were retrospectively collected from the medical records, hospital information systems and CC charts.
The primary outcome was CC unit mortality (from all causes). Secondary outcomes were hospital mortality and 12-month mortality.
All of the statistical analyses were performed using STATA 15 (StataCorp, Lakeway Drive, College Station, Texas 77845 USA). Data are presented as mean values (and standard deviation, SD), or as medians (with interquartile range, IQR), as appropriate. To evaluate factors associated with mortality, univariate and multivariate logistic regression analyses were performed, adjusting for age, gender and hematological diagnosis. Univariate association filtering was relied upon to select variables to feed into the multivariate model, as commonly performed purposeful selection methodology (20, 21). A p-value cut off of 0.05 was used for statistical significance. Statistical interaction was examined using Wald.
A total of 437 HM patients were admitted on to CC during the period examined (2002-2018) and met the inclusion criteria for the study. Patients’ characteristics are described in Table 1. The most common hematological diagnoses on admission were Acute Myeloid Leukemia (AML) in 108 (24.8%) cases, non-Hodgkin Lymphoma (NHL) in 98 (22.5%) and Multiple Myeloma (MM) in 70 (16.1%) patients. The median APACHE II score across the 437 patients on admission was 21 (IQR 17-25). A total of 185 (42.5%) patients had previously undergone HSCT, of which 60 cases (32.4%) were allogeneic HSCT recipients. Clinical frailty score data were available for 225 of the patients.
With regards to CC treatments, 369 patients (84.4%) had at least one organ supported, while 142 patients (32.5%) had two or more. Fifty-eight patients (13.3%) received CRRT, 226 (51.7%) CVS support, 100 (22.9%) IMV and 191 (43.7%) NIV. Unit and hospital mortality rates were 33.4% and 46.2% respectively. At three-month, six-month and one-year follow-up, all causes mortality was 56.1%, 59.5% and 67.9% respectively.
Univariate logistic regression analysis, adjusted by age and gender, was conducted to determine predictors of CC outcome with CC unit mortality being the primary endpoint. Eight factors were associated with CC unit mortality (Table 2): Admission APACHE II (Odds Ratio (OR)= 0.93, 95% confidence interval (CI)=0.89-0.96, p <0.001), the number of organs supported (OR=0.36, 95% CI=0.28-0.47, p<0.001), CRRT (OR=0.42, 95% CI=0.24-0.75, p<0.01), CVS support (OR=0.32, 95% CI=0.21-0.49, p<0.001), IMV (OR=0.11, 95% CI=0.07-0.18, p<0.001), NIV (OR=0.6, 95% CI=0.40-0.90, p<0.05), P/F ratio (ratio between arterial partial pressure of oxygen (PaO2) and the inspired oxygen concentration (FiO2)) (OR=1.07, 95%CI=1.05-1.95, p < 0.001) and the lowest P/F ratio recorded during CC stay (OR=1.16, 95% CI=1.10-1.21, p<0.001). There was no statistical interaction between use of NIV and lowest P/F ratio (Wald Chi-Squared Test p=0.81).
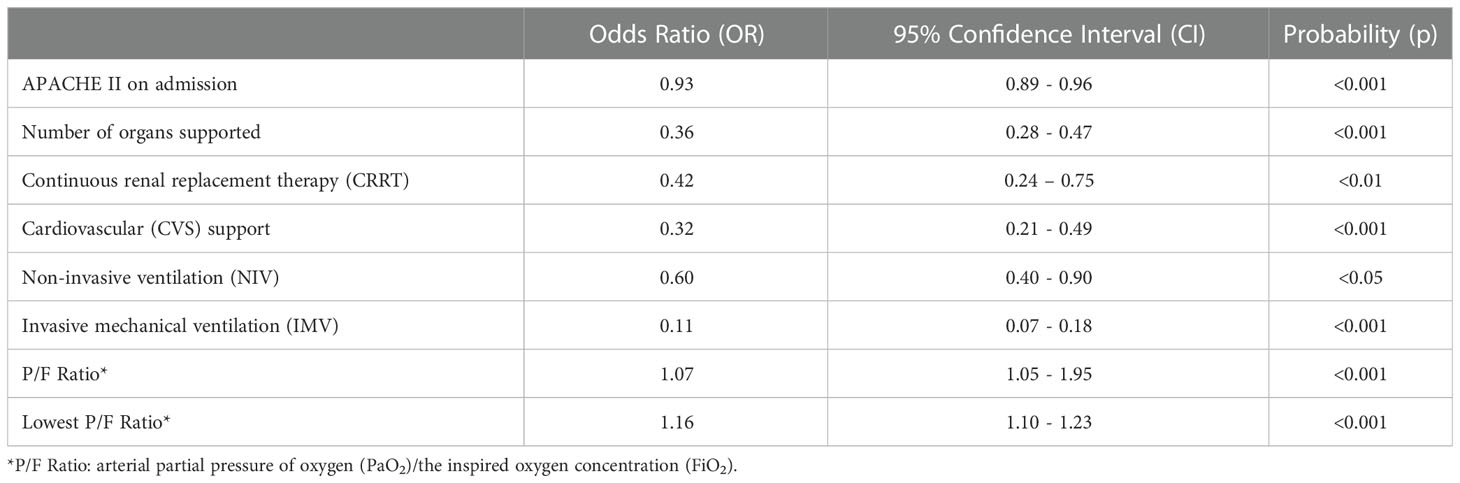
Table 2 Significant predictors of critical care unit outcome, at univariate logistic regression analysis.
The HM diagnosis, past history of HSCT and the CFS did not have any effect on unit outcome (Supplementary Material, Table S1) at univariate analysis. Additional physiological variables assessed, including neutrophil count, creatinine and bilirubin levels upon admission to the CC unit were also not associated with patient outcome (Supplementary Material, Table S1).
APACHE II score on admission, requirement for IMV and lowest P/F ratio were all retained as independently statistically significant predictors of CC unit outcome in the multivariate logistic regression model (Table 3). The odds ratios from the multivariate analysis reveal that for every incremental increase in APACHE II score upon CC admission, there is a 12% decrease in the odds of CC unit survival. For every incremental increase in the lowest P/F ratio recorded, there is a 13% increase in the odds of survival, while the requirement for IMV results in an 88% decrease in the odds of survival on the CC unit.
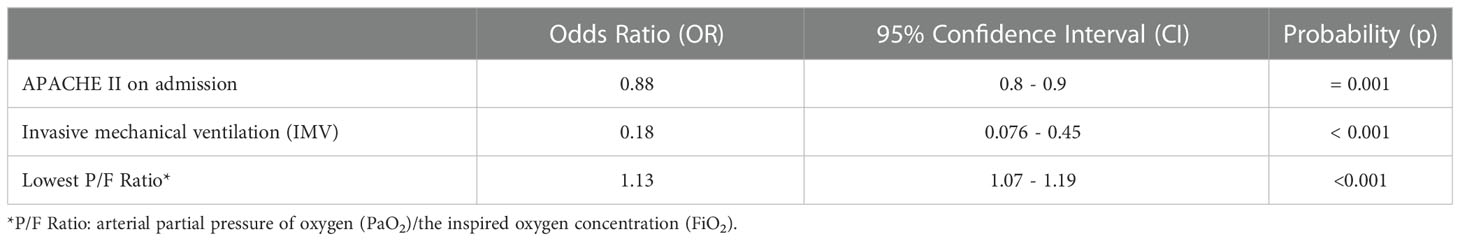
Table 3 Independent predictors of critical care unit outcome, by multivariate logistic regression analysis.
Univariate logistic regression analysis was also conducted to determine predictors of overall hospital outcome and 12-month survival. Of all the parameters measured, only the lowest P/F Ratio was able to help to predict hospital outcome (OR=1.15, 95%CI=1.02-1.30, p = 0.024). No independent predictors of 12-month mortality were identified in the multivariate model.
This retrospective cohort study of critically ill patients with HM examines characteristics and outcomes over a period of 16 years, given recent evolutions in the treatment of this patient group. Critical Care mortality was 33.4%. Three factors were able to predict unit outcome; requirement for IMV, lowest P/F ratio and APACHE II score, which is the only one that can be used entirely a priori.
The recent advances in treatments for HM over the last few decades have translated into significantly improved survival rates. This continually improving prognosis increasingly justifies the rationale to provide CC support to many HM patients through life-threatening, but potentially reversible, complications of their disease and often complex, intensive, and high-cost treatments (including HSCT), which have extended to older and more frail patients.
It has been historically reported that HM patients requiring CC support have a limited survival. Numerous recent studies have attempted to determine whether, with advances in CC and better working practices, such as closer liaison and collaboration between Hematologists and Intensivists, this doctrine remains valid. Indeed, there are several reports of similar long-term outcomes in sub-groups of HM patients to non-HM patients following CC support (6, 22, 23) and evidence that better survival is associated with earlier CC admission (6), although these findings are not consistent throughout recent literature and therefore controversy still persists regarding the benefits of CC for sub-groups of HM patients (24, 25). Of note, our present study found no significant change or detectable trends in patients’ baseline demographic characteristics, acute physiological derangement or outcomes over the sixteen-year study period. However, it remains important that CC resource is provided efficiently and maximized in those patients with achievable survival. The identification of predictive factors is valuable in aiding clinical decision-making around CC admission, for informing individualized escalation of care decisions or referral to palliative care services, and also for counselling patients and their families regarding expectations of short, medium and long-term outcomes in this specialized group of patients whose HM diagnosis carries a prognosis in itself.
Our study found that the unit mortality of this cohort increased from 33.4% to 46.25% at hospital discharge. Furthermore, three-, six- and twelve-month mortality was 56.1%, 59.5% and 67.9% respectively overall. These figures are in line with the most recent similar studies reporting outcomes of HM patients requiring CC support (6, 7). Although a number of factors were identified to have prognostic utility at univariate analysis, only three factors were retained as independent predictors of unit outcome at multivariate analysis; requirement for IMV, the lowest P/F ratio and the APACHE II score. Only the APACHE II score can be used entirely a priori to help with early decision making relating to CC escalation, whilst requirement for IMV and lowest P/F ratio are strictly interconnected variables, which may reflect deterioration of patients from baseline CC admission. Therefore the detection of such deterioration, particularly in the wider context of progression in multi-organ dysfunction, may prompt the treating clinician to reassess management decisions, with consideration to response to the therapies administered. Although the APACHE II score has shown strong predictive power, early decision making should be made on an individualized patient basis, weighing the potential benefits against the burden of treatment, and taking the patient’s wishes into account.
The prognostic value of requirement for IMV support has been reported by numerous studies (1, 7, 9–11, 13) and has been attributed to various factors including the lack of effective treatment for several non-infectious pulmonary complications, concomitant severe pulmonary dysfunction, nosocomial infections such as ventilator-associated pneumonitis and the possibility of delayed diagnosis and management, including delayed endotracheal intubation and initiation of IMV, which can lead to multi-organ failure (26, 27). Acute respiratory failure ARF is one of the most important reasons for HM patient admission to CC (28). Requirement for IMV in HM patients has also been implicated as the cause of the high mortality rates (29–31).
In addition, in our study the requirement for NIV was associated with poor CC unit outcome at univariate analysis, but not independently retained within the final multivariate model. IMV in HM patients in particular, has previously been shown to carry a significantly worse prognosis than the use of NIV (32). Patients with neutropenia or the immunocompromised appear at higher risk when intubated and ventilated, and so NIV has been recommended by some in this situation (33, 34) and continuous positive airway pressure (CPAP) may reduce the evolution of acute lung injury in patients with HM (35). In some situations, this is better tolerated by CPAP helmet (36). While IMV may be associated with a worse outcome in ARF in this group (37) (37, 38, 39), some studies have not supported such a conclusion (37, 37, 40, 41, 42, 43). In the present study, we could not properly compare outcomes between the IMV and NIV groups. However, IMV does seem to be associated with a poorer outcome, perhaps for the reasons outlined above. There are many limiting factors present in our study and in others that have gathered data on the use of NIV in HM patients, and drawing definitive conclusions is difficult; for example, patients may be intubated before CC admission, and, in some cases, data on whether patients have received NIV prior to CC admission is unclear (although in our institution, this is unlikely to happen). Some of these outcomes may be due to, or worsened by, poor timing of invasive ventilation. This may, in some cases, be related to the logistics of where the NIV took place, especially when ward based. If NIV is failing, then prompt and timely intubation and ventilation is important in all areas of use including the hematology ward (29). Early treatment of the underlying etiology for the ARF is also very important. A definitive comparison of NIV with IMV is difficult because of several factors, including the need to achieve equipoise, timing of escalation from NIV to IMV where both are used and the use of IMV in many patients who are failing with NIV. In addition, some patients who are immediately intubated and ventilated without a trial of NIV are those where their level of deterioration was such that NIV was not practicable and so will be more severely ill than those who were able to establish themselves on NIV. In this study nearly twice as many patients received NIV than IMV and some NIV patients transferred to IMV. Some patients have treatment limited to NIV ventilation, which further complicates clinical comparisons. In our study treating clinicians generally perceived a benefit in avoiding sedation and introducing an artificial airway with the inevitable loss of a natural cough and a less natural expansion pattern of the lungs, where feasible in the first instance. The observational nature of our study therefore must be taken into account when interpreting our data in relation to NIV and IMV outcomes. A potential limitation of the current study is that it cannot elucidate the exact decision making surrounding the process of transitioning from NIV to IMV, and over what time interval (or whether such timing was optimal or otherwise). The reason for this limitation relates to the fact that such decisions are based on the best clinical judgement of the treating intensivist, which is a process known to be highly variable according to the existing literature, even in presence of pre-defined criteria in the tightly controlled set-up of randomized clinical trials (44, 45).
In the present cohort the lowest P/F ratio was found to independently predict unit and hospital outcome. For every incremental increase in the lowest P/F ratio recorded, there is a 13% increase in the odds of CC unit survival, and a 15% increase in the odds of hospital survival. The lowest P/F ratio is the only factor found to predict hospital outcome in this study. The P/F ratio expresses the severity of hypoxemia and indicates the trend in (or progression of) respiratory failure. Our work confirms findings of other authors who have also shown the strength of this ratio as an independent factor associated with CC mortality in HM and non-HM patients admitted to CC (46–48), but is the first to show its utility as a prognostic factor of overall hospital mortality in HM patients.
In line with a large number of other studies (1, 10, 14, 15, 49, 50), the APACHE II score, a well-established and validated prognostic tool for providing initial risk stratification for severely ill hospitalized patients, was found to be strongly associated with unit outcome at multivariate analysis. To date, the authors are not aware of a disease specific scoring system which caters for the needs to prognosticate HM patients admitted to Critical Care. Such a scoring system, once developed, may add further precision to the estimate of likely outcomes, can support ethical decision making and underpin clinical team discussions and decision making (19, 51). Interestingly, frailty did not appear to have any impact on CC unit, or hospital outcome. In previous studies, frailty has been associated with poor therapeutic response, increased toxicity, and worse survival for HM patients with blood cancers (52, 53). Frailty in patients admitted to CC has been well studied and its presence has been associated with negative CC outcomes and increased hospital mortality. However, data on frailty in HM patients specifically requiring CC support is very limited. The median age of patients in our study was 55 (IQR 46-67), and is significantly lower than that seen in studies looking at frailty in HM patients overall (52, 53), which may account for the lack of prognostic value of frailty seen here.
Organ failure as an overall clinical entity has previously been shown to be a useful prognostic factor in both non-cancer patients (54), cancer patients (55) and HM patients (6, 9, 12) admitted to CC. Furthermore, several studies have demonstrated that the number of dysfunctional organs has strong predictive value (9, 12, 47, 54, 56, 57). In the present study, univariate analyses showed that the number of organs requiring support was a predictor of CC mortality. Univariate analyses also showed that requirement for CRRT, CVS and respiratory support were all predictors of outcome. However, at multivariate logistic regression analysis, organ support was not an independent predictor of unit or hospital outcome. It would seem that, at least in our cohort, the nature of the organ failure (in particular, whether it is respiratory failure or not) is of much greater importance than the presence of any organ failure in predicting outcome.
Interestingly, our analysis also revealed that the diagnostic classification of HM appeared to have no impact on the outcome of these patients. Conflicting reports are available on this aspect within the literature; in agreement with the present findings, several studies have found patient outcome to be unrelated to HM diagnosis (15, 46, 58–60). In contrast, several studies have found an increased risk of death with specific HM diagnoses, such as HL (12), AML (60, 61) and NHL (61). However, even amongst those studies who report that the individual diagnosis is important in determining patient outcome, the relevant HM diagnoses vary between studies. HM is a very broad category of diseases both chronic and acute in nature. Disease status included in this study and in others are highly variable and can be newly diagnosed, in remission and those with relapsed or progressive disease. Hence, finding associations between specific diagnoses and outcomes is hampered by several potential cofounding variables. Furthermore, trends in variables across time may need to be considered when building prediction scores or algorithms (62). In studies in which outcomes were compared between patients in remission and those in relapse, mortality was found to be lower in those patients in remission (6, 63). These questions may be answered in more disease-specific analyses, which, given the relatively rare incidence of each specific HM, would require larger adequately powered studies.
Similar to HM diagnosis, our analysis suggests that the neutrophil count, creatinine and bilirubin levels at time of admission to the CC unit were not important in determining patient outcome. Other studies have also failed to confirm them as consistent prognostic factors for HM patients requiring CC support, with some showing predictive (64) and others non-predictive (10, 13) value. It is hypothesized that, at the stage at which organ support is required for HM patients, classically used predictors of mortality including hematological and biochemical parameters, as well as HM diagnosis and also previous HSCT have lost their significance. Furthermore, since our group of HM patients was heterogeneous, assessing the predictive value of such specific disease- or treatment-related factors (such as HM diagnosis, HSCT or neutropenia) is challenging in a retrospective study (65). These aspects and other important variables, including frailty assessments and patient-reported outcome measures (PROMs) to reflect long-term health-related quality-of-life, recovery of physical and mental functioning and support health economic appraisals, could be integrated into well-designed prospective studies to determine the long-term value of CC support in HM patients.
This study adds significant value to the literature since many conclusions have previously been drawn from much smaller cohorts (1, 2, 7, 9–11, 13–15, 32, 50, 60, 64, 65). While a diagnosis of HM should not preclude admission of patients who might otherwise benefit from CC support, the prognosis of those with a high APACHE II score upon admission, or those with deteriorating P/F ratio or requiring IMV remains particularly poor. Other variables such as neutropenia, specific HM diagnosis and previous HSCT did not show significance in our patient population and are likely to be of limited reliability as a basis for CC admission and management decision-making in our cohort. The value of NIV in HM patients suffering from acute respiratory failure warrants further investigation, ideally via a randomized controlled trial.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Health Research Authority (HRA) (IRAS number 290345). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
All the authors contributed to the revision of the intellectual content of the manuscript. JS, SW and GM had substantial contribution to the design of the study. MK, JG, KS, JH, YS, and JW were involved in acquisition of the data. AT performed data cleaning, devised the analysis plan and conducted all statistical data analyses, data interpretation, contributed to initial drafting, version control and revising of manuscript. ND was responsible for the data cleaning and interpretation, drafting of the manuscript, contributed to version control and revision of the manuscript. All authors contributed to the article and approved the submitted version.
We acknowledge the Critical Care and Hematology teams who provided collaborative care for patients during the time period of this retrospective study. We acknowledge the support of data managers, Sister Joanne Pons and Sister Sheila Reynolds.
JS declares honoraria for educational events from Jazz, Gilead, Janssen, for advisory board membership from Medac, and for trial IDMC membership from Kiadis Pharma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhem.2023.1127322/full#supplementary-material
HM, Hematological Malignancy; CFS, Clinical Frailty Score; SAPS, Simplified Acute Physiology Score; APACHE II, Acute Physiology and Chronic Health Evaluation II; ICNARC, Intensive Care National Audit and Research Centre; AML, Acute Myeloid Leukemia; NHL, Non-Hodgkin Lymphoma; MM, Multiple Myeloma; ALL, Acute Lymphoblastic Leukemia; MDS, Myelodysplastic Syndrome, CLL, Chronic Lymphocytic Leukemia; CML, Chronic Myeloid Leukemia; HL, Hodgkin Lymphoma; CRRT, Continuous renal replacement therapy; CVS, Cardiovascular; IMV, Invasive mechanical ventilation; NIV, Non-invasive ventilation; IQR, Interquartile Range; OR, Odds Ratio.
1. Hill QA, Kelly RJ, Patalappa C, Whittle AM, Scally AJ, Hughes A, et al. Survival of patients with hematological malignancy admitted to the intensive care unit: Prognostic factors and outcome compared to unselected medical intensive care unit admissions, a parallel group study. Leukemia Lymphoma (2012) 53(2):282–8. doi: 10.3109/10428194.2011.614705
2. Lloyd-Thomas AR, Wright I, Lister TA, Hinds CJ. Prognosis of patients receiving intensive care for lifethreatening medical complications of haematological malignancy. Br. Med. J. (Clin Res. Ed) (1988) 296(6628):1025–9. doi: 10.1136/bmj.296.6628.1025
3. Wright JC, Plenderleith L, Ridley SA. Long-term survival following intensive care: Subgroup analysis and comparison with the general population. Anaesthesia (2003) 58(7):637–42. doi: 10.1046/j.1365-2044.2003.03205.x
4. Guidelines for intensive care unit admission, discharge, and triage. Task force of the American college of critical care medicine, society of critical care medicine. Crit. Care Med. (1999) 27(3):633–8.
5. van Vliet M, Verburg IW, van den Boogaard M, Keizer NF, Peek N, Blijlevens NM, et al. Trends in admission prevalence, illness severity and survival of haematological patients treated in Dutch intensive care units. Intensive Care Med. (2014) 40(9):1275–84. doi: 10.1007/s00134-014-3373-x
6. Azoulay E, Mokart D, Pène F, Lambert J, Kouatchet A, Mayaux J, et al. Outcomes of critically ill patients with hematologic malignancies: Prospective multicenter data from France and Belgium–a groupe de recherche respiratoire en réanimation onco-hématologique study. J. Clin. Oncol. (2013) 31(22):2810–8. doi: 10.1200/JCO.2012.47.2365
7. Al-Zubaidi N, Shehada E, Alshabani K, ZazaDitYafawi J, Kingah P, Soubani AO. Predictors of outcome in patients with hematologic malignancies admitted to the intensive care unit. Hematology/Oncol Stem Cell Ther. (2018) 11(4):206–18. doi: 10.1016/j.hemonc.2018.03.003
8. Wise MP, Barnes RA, Baudouin SV, Howell D, Lyttelton M, Marks DI, et al. Guidelines on the management and admission to intensive care of critically ill adult patients with haematological malignancy in the UK. Br. J. Haematol (2015) 171(2):179–88. doi: 10.1111/bjh.13594
9. Bird GT, Farquhar-Smith P, Wigmore T, Potter M, Gruber PC. Outcomes and prognostic factors in patients with haematological malignancy admitted to a specialist cancer intensive care unit: A 5 yr study. Br. J. Anaesthesia (2012) 108(3):452–9. doi: 10.1093/bja/aer449
10. Liu J, Cheng Q, Yang Q, Li X, Shen X, Zhang L, et al. Prognosis-related factors in intensive care unit (ICU) patients with hematological malignancies: A retrospective cohort analysis in a Chinese population. Hematology (2015) 20(9):494–503. doi: 10.1179/1607845414Y.0000000216
11. Ferrà C, Marcos P, Misis M, Morgades M, Bordejé ML, Oriol A, et al. Outcome and prognostic factors in patients with hematologic malignancies admitted to the intensive care unit: A single-center experience. Int. J. Hematol. (2007) 85(3):195–202. doi: 10.1532/IJH97.E0625
12. Hampshire Peter AP, Welch CA, McCrossan LA, Francis K, Harrison DA. Admission factors associated with hospital mortality in patients with haematological malignancy admitted to UK adult, general critical care units: A secondary analysis of the ICNARC case mix programme database. Crit. Care (2009) 13(4):R137. doi: 10.1186/cc8016
13. Geerse DA, Span LF, Pinto-Sietsma SJ, van Mook WN. Prognosis of patients with haematological malignancies admitted to the intensive care unit: Sequential organ failure assessment (SOFA) trend is a powerful predictor of mortality. Eur. J. Intern. Med. (2011) 22(1):57–61. doi: 10.1016/j.ejim.2010.11.003
14. Thakkar SG, Fu AZ, Sweetenham JW, McIver ZA, Mohan SR, Ramsingh G, et al. Survival and predictors of outcome in patients with acute leukemia admitted to the intensive care unit. Cancer (2008) 112(10):2233–40. doi: 10.1002/cncr.23394
15. Owczuk R, Wujtewicz MA, Sawicka W, Wadrzyk A, Wujtewicz M. Patients with haematological malignancies requiring invasive mechanical ventilation: Differences between survivors and non-survivors in intensive care unit. Support Care Cancer (2005) 13(5):332–8. doi: 10.1007/s00520-004-0750-y
16. Soares M, Fontes F, Dantas J, Gadelha D, Cariello P, Nardes F, et al. Performance of six severity-of-illness scores in cancer patients requiring admission to the intensive care unit: A prospective observational study. Crit. Care (2004) 8(4):R194. doi: 10.1186/cc2870
17. Cooksley T, Kitlowski E, Haji-Michael P. Effectiveness of modified early warning score in predicting outcomes in oncology patients. Qjm (2012) 105(11):1083–8. doi: 10.1093/qjmed/hcs138
18. Divatia J, Priya V, Ranganathan P, Chidrawar S. Evaluation of APACHE II and the ICU cancer mortality model in an Indian cancer hospital ICU. Crit. Care (2006) 10(1):P404.
19. Tridente A, Bion J, Mills GH, Gordon AC, Clarke GM, Walden A, et al. Derivation and validation of a prognostic model for postoperative risk stratification of critically ill patients with faecal peritonitis. Ann. Intensive Care (2017) 7(1):96–6. doi: 10.1186/s13613-017-0314-1
20. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol. Med. (2008) 3(1):17. doi: 10.1186/1751-0473-3-17
21. Tridente A, Clarke GM, Walden A, McKechnie S, Hutton P, Mills GH, et al. Patients with faecal peritonitis admitted to European intensive care units: an epidemiological survey of the GenOSept cohort. Intensive Care Med. (2014) 40(2):202–10. doi: 10.1007/s00134-013-3158-7
22. Zuber B, Tran TC, Aegerter P, Grimaldi D, Charpentier J, Guidet B, et al. Impact of case volume on survival of septic shock in patients with malignancies. Crit. Care Med. (2012) 40(1):55–62. doi: 10.1097/CCM.0b013e31822d74ba
23. Azoulay E, Lemiale V, Mokart D, Pène F, Kouatchet A, Perez P, et al. Acute respiratory distress syndrome in patients with malignancies. Intensive Care Med. (2014) 40(8):1106–14. doi: 10.1007/s00134-014-3354-0
24. Oeyen SG, Benoit DD, Annemans L, Depuydt PO, Belle Van SJ, Troisi RI, et al. Long-term outcomes and quality of life in critically ill patients with hematological or solid malignancies: A single center study. Intensive Care Med. (2013) 39(5):889–98. doi: 10.1007/s00134-012-2791-x
25. Krok-Schoen JL, Fisher JL, Stephens JA, Mims A, Ayyappan S, Woyach JA, et al. Incidence and survival of hematological cancers among adults ages ≥75 years. Cancer Med. (2018) 7(7):3425–33. doi: 10.1002/cam4.1461
26. Mokart D, Lambert J, Schnell D, Fouché L, Rabbat A, Kouatchet A, et al. Delayed intensive care unit admission is associated with increased mortality in patients with cancer with acute respiratory failure. Leuk Lymphoma (2013) 54(8):1724–9. doi: 10.3109/10428194.2012.753446
27. Kwak YG, Lee SO, Kim HY, Kim YK, Park ES, Jin HY, et al. Risk factors for device-associated infection related to organisational characteristics of intensive care units: findings from the Korean nosocomial infections surveillance system. J. Hosp. Infection (2010) 75(3):195–9. doi: 10.1016/j.jhin.2010.01.014
28. Cheng Q, Tang Y, Yang Q, Wang E, Liu J, Li X. The prognostic factors for patients with hematological malignancies admitted to the intensive care unit. Springerplus (2016) 5(1):2038. doi: 10.1186/s40064-016-3714-z
29. Adda M, Coquet I, Darmon M, Thiery G, Schlemmer B, Azoulay E. Predictors of noninvasive ventilation failure in patients with hematologic malignancy and acute respiratory failure. Crit. Care Med. (2008) 36(10):2766–72. doi: 10.1097/CCM.0b013e31818699f6
30. Khassawneh BY, White P Jr, Anaissie EJ, Barlogie B, Hiller FC. Outcome from mechanical ventilation after autologous peripheral blood stem cell transplantation. Chest (2002) 121(1):185–8. doi: 10.1378/chest.121.1.185
31. Liu J, Bell C, Campbell V, DeBacker J, Tamberg E, Lee C, et al. Noninvasive ventilation in patients with hematologic malignancy. J. Intensive Care Med. (2017) 34(3):197–203. doi: 10.1177/0885066617690725
32. Rabbat A, Chaoui D, Montani D, Legrand O, Lefebvre A, Rio B, et al. Prognosis of patients with acute myeloid leukaemia admitted to intensive care. Br. J. Haematol (2005) 129(3):350–7. doi: 10.1111/j.1365-2141.2005.05459.x
33. Antonelli M, Conti G, Rocco M, Bufi M, Blasi De RA, Vivino G, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl. J. Med. (1998) 339(7):429–35. doi: 10.1056/NEJM199808133390703
34. Gristina GR, Antonelli M, Conti G, Ciarlone A, Rogante S, Rossi C, et al. Noninvasive versus invasive ventilation for acute respiratory failure in patients with hematologic malignancies: A 5-year multicenter observational survey. Crit. Care Med. (2011) 39(10):2232–9. doi: 10.1097/CCM.0b013e3182227a27
35. Squadrone V, Massaia M, Bruno B, Marmont F, Falda M, Bagna C, et al. Early CPAP prevents evolution of acute lung injury in patients with hematologic malignancy. Intensive Care Med. (2010) 36(10):1666–74. doi: 10.1007/s00134-010-1934-1
36. Principi T, Pantanetti S, Catani F, Elisei D, Gabbanelli V, Pelaia P, et al. Noninvasive continuous positive airway pressure delivered by helmet in hematological malignancy patients with hypoxemic acute respiratory failure. Intensive Care Med. (2004) 30(1):147–50. doi: 10.1007/s00134-003-2056-9
37. Azoulay E, Alberti C, Bornstain C, Leleu G, Moreau D, Recher C, et al. Improved survival in cancer patients requiring mechanical ventilatory support: Impact of noninvasive mechanical ventilatory support. Crit. Care Med. (2001) 29(3):519–25. doi: 10.1097/00003246-200103000-00009
38. Azoulay É, Thiéry G, Chevret S, Moreau D, Darmon M, Bergeron A, et al. The prognosis of acute respiratory failure in critically ill cancer patients. Med. (Baltimore) (2004) 83(6):360–70. doi: 10.1097/01.md.0000145370.63676.fb
39. Azoulay E, Mokart D, Lambert J, Lemiale V, Rabbat A, Kouatchet A, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am. J. Respir. Crit. Care Med. (2010) 182(8):1038–46. doi: 10.1164/rccm.201001-0018OC
40. Molina R, Bernal T, Borges M, Zaragoza R, Bonastre J, Granada RM, et al. Ventilatory support in critically ill hematology patients with respiratory failure. Crit. Care (2012) 16(4):R133. doi: 10.1186/cc11438
41. Azoulay E, Lemiale V. Non-invasive mechanical ventilation in hematology patients with hypoxemic acute respiratory failure: A false belief? Bone Marrow Transplant. (2012) 47(4):469–72. doi: 10.1038/bmt.2011.232
42. Wermke M, Schiemanck S, Höffken G, Ehninger G, Bornhäuser M, Illmer T. Respiratory failure in patients undergoing allogeneic hematopoietic SCT–a randomized trial on early non-invasive ventilation based on standard care hematology wards. Bone Marrow Transplant. (2012) 47(4):574–80. doi: 10.1038/bmt.2011.160
43. Truwit JD, Bernard GR. Noninvasive ventilation–don't push too hard. N Engl. J. Med. (2004) 350(24):2512–5. doi: 10.1056/NEJMe048049
44. Peñuelas Ó, Esteban A. Noninvasive ventilation for acute respiratory failure: The next step is to know when to stop. Eur. Respir. J. (2018) 52(2):1801185. doi: 10.1183/13993003.01185-2018
45. Tulaimat A. Examining the intubation decision in randomised clinical trials. Eur. Respir. J. (2021) 57(3):2100051. doi: 10.1183/13993003.00051-2021
46. El motlb EA, El-Deeb A. Mortality association factors in hematologic cancer patients requiring mechanical ventilation for more than one day in a developing country. A prospective cohort study. Egyptian J. Crit. Care Med. (2017) 5(3):77–82. doi: 10.1016/j.ejccm.2017.11.001
47. Soares M, Salluh JI, Spector N, Rocco JR. Characteristics and outcomes of cancer patients requiring mechanical ventilatory support for >24 hrs. Crit. Care Med. (2005) 33(3):520–6. doi: 10.1097/01.CCM.0000155783.46747.04
48. Park DP, Welch CA, Harrison DA, Palser TR, Cromwell DA, Gao F, et al. Outcomes following oesophagectomy in patients with oesophageal cancer: a secondary analysis of the ICNARC case mix programme database. Crit. Care (London England) (2009) 13 Suppl 2:S1. doi: 10.1186/cc7868
49. Cuthbertson BH, Rajalingam Y, Harrison S, McKirdy F. The outcome of haematological malignancy in Scottish intensive care units. J. Intensive Care Soc. (2008) 9(2):135–40. doi: 10.1177/175114370800900208
50. McGrath S, Chatterjee F, Whiteley C, Ostermann M. ICU And 6-month outcome of oncology patients in the intensive care unit. Qjm (2010) 103(6):397–403. doi: 10.1093/qjmed/hcq032
51. Stiel S, Bertram L, Neuhaus S, Nauck F, Ostgathe C, Elsner F, et al. Evaluation and comparison of two prognostic scores and the physicians’ estimate of survival in terminally ill patients. Supportive Care Cancer (2010) 18(1):43–9. doi: 10.1007/s00520-009-0628-0
52. Buckstein R, Wells RA, Zhu N, Leitch HA, Nevill TJ, Yee KW, et al. Patient-related factors independently impact overall survival in patients with myelodysplastic syndromes: An MDS-CAN prospective study. Br. J. Haematol (2016) 174(1):88–101. doi: 10.1111/bjh.14033
53. Hamaker ME, Mitrovic M, Stauder R. The G8 screening tool detects relevant geriatric impairments and predicts survival in elderly patients with a haematological malignancy. Ann. Hematol. (2014) 93(6):1031–40. doi: 10.1007/s00277-013-2001-0
54. Ñamendys-Silva SA, Correa-García P, García-Guillén FJ, López-Basave HN, Montalvo-Esquivel G, Texcocano-Becerra J, et al. Organ dysfunction in critically ill cancer patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. Oncol. Lett. (2015) 9(4):1873–6. doi: 10.3892/ol.2015.2921
55. Darmon M, Thiery G, Ciroldi M, Porcher R, Schlemmer B, Azoulay É. Should dialysis be offered to cancer patients with acute kidney injury? Intensive Care Med. (2007) 33(5):765–72. doi: 10.1007/s00134-007-0579-1
56. Evison J, Rickenbacher P, Ritz R, Gratwohl A, Haberthür C, Elsasser S, et al. Intensive care unit admission in patients with haematological disease: Incidence, outcome and prognostic factors. Swiss Med. Wkly (2001) 131(47-48):681–6. doi: 10.4414/smw.2001.09801
57. Taccone FS, Artigas AA, Sprung CL, Moreno R, Sakr Y, Vincent JL. Characteristics and outcomes of cancer patients in European ICUs. Crit. Care (2009) 13(1):R15. doi: 10.1186/cc7713
58. Blot F, Guiguet M, Nitenberg G, Leclercq B, Gachot B, Escudier B. Prognostic factors for neutropenic patients in an intensive care unit: respective roles of underlying malignancies and acute organ failures. Eur. J. Cancer (1997) 33(7):1031–7. doi: 10.1016/S0959-8049(97)00042-7
59. Merz TM, Schär P, Bühlmann M, Takala J, Rothen HU. Resource use and outcome in critically ill patients with hematological malignancy: A retrospective cohort study. Crit. Care (2008) 12(3):R75. doi: 10.1186/cc6921
60. Bruennler T, Mandraka F, Zierhut S, Siebig S, Wrede C, Klebl F, et al. Outcome of hemato-oncologic patients with and without stem cell transplantation in a medical ICU. Eur. J. Med. Res. (2007) 12(8):323–30.
61. Massion PB, Dive AM, Doyen C, Bulpa P, Jamart J, Bosly A, et al. Prognosis of hematologic malignancies does not predict intensive care unit mortality. Crit. Care Med. (2002) 30(10):2260–70. doi: 10.1097/00003246-200210000-00014
62. Tridente A, Clarke GM, Walden A, Gordon AC, Hutton P, Chiche JD, et al. Association between trends in clinical variables and outcome in intensive care patients with faecal peritonitis: Analysis of the GenOSept cohort. Crit. Care (2015) 19(1):210. doi: 10.1186/s13054-015-0931-8
63. Aygencel G, Turkoglu M, Sucak Turkoz G, Benekli M. Prognostic factors in critically ill cancer patients admitted to the intensive care unit. J. Crit. Care (2014) 29(4):618–26. doi: 10.1016/j.jcrc.2014.01.014
64. Benoit DD, Vandewoude KH, Decruyenaere JM, Hoste EA, Colardyn FA. Outcome and early prognostic indicators in patients with a hematologic malignancy admitted to the intensive care unit for a life-threatening complication. Crit. Care Med. (2003) 31(1):104–12. doi: 10.1097/00003246-200301000-00017
Keywords: hematological malignancy, mortality, outcome, APACHE II, mechanical ventilation, organ support
Citation: Tridente A, Dempsey NC, Khalifa M, Goddard J, Shuker K, Hall J, Sorour Y, Wright J, Webber S, Mills GH and Snowden JA (2023) Predicting outcomes of hematological malignancy patients admitted to critical care. Front. Hematol. 2:1127322. doi: 10.3389/frhem.2023.1127322
Received: 19 December 2022; Accepted: 27 February 2023;
Published: 17 March 2023.
Edited by:
Prashant Ramesh Tembhare, Research and Education in Cancer, IndiaReviewed by:
Amol Kothekar, Tata Memorial Hospital, IndiaCopyright © 2023 Tridente, Dempsey, Khalifa, Goddard, Shuker, Hall, Sorour, Wright, Webber, Mills and Snowden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nina C. Dempsey, bi5kZW1wc2V5LWhpYmJlcnRAbW11LmFjLnVr
†These authors have contributed equally to this work and share first authorship
‡These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.