
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Hematol., 14 November 2022
Sec. Immunobiology and Immunotherapy
Volume 1 - 2022 | https://doi.org/10.3389/frhem.2022.967156
This article is part of the Research TopicEditors' Showcase: Immunobiology and ImmunotherapyView all 4 articles
Relapsed/refractory (R/R) mantle cell lymphoma (MCL) with primary drug resistance to Bruton tyrosine kinase inhibitor and mutated TP53 responds poorly to conventional treatments. Chimeric antigen receptor (CAR) T cell therapy has emerged as one of the most effective treatments for R/R B cell lymphoma. However, no reports of CD5 CAR T cell treatment for MCL have been reported. In this paper, we report a R/R MCL patient with primary drug resistance to BTK inhibitors and TP53 mutation enrolled in a human CD5 CAR T cell trial. Remission of the primary disease was observed half a month after CAR T cell infusion. However, ascites was observed 2 weeks later. Flow cytometry suggested disease progression and immunophenotypic transformation. CD5 in CAR T cells turned negative and the expression of CD38 was enhanced. The patient was treated with a combination of daratumumab and Gemox (gemcitabine + oxaliplatin), abdominal distension and pain were markedly reduced, and ascites disappeared. We report the first case of human CD5 CAR T cell treatment for a patient with R/R MCL, providing insight on treatment strategies for such patients.
Mantle cell lymphoma (MCL) is an incurable mature B-cell lymphoma that accounts for 2–6% of all non-Hodgkin lymphomas in Asian populations, combining the unfavorable clinical features of aggressive and indolent lymphomas (1, 2). Chromosomal translocation of t (11;14) (q13;q32) and overexpression of cyclin D1 (CCND1) are characteristic. Despite the emergence of new targeted drugs, such as Bruton tyrosine kinase inhibitors, that have markedly improved the outcomes of patients with relapsed/refractory (R/R) MCL, it remains incurable. Approximately one-third of the patients have primary drug resistance while others appear to develop acquired resistance. (3) MCL with primary drug resistance to BTK inhibitors, or those with disease progression during BTK inhibitor treatment, have an extremely poor prognosis, with an objective response rate (ORR) as low as 25% and a median overall survival (OS) of only 6 months after salvage treatment (4). Additionally, patients presenting with primary drug resistance to BTK inhibitor and the TP53 mutation respond poorly to conventional treatments, including bone marrow transplantation; therefore, new treatments are needed for this subset of patients. Chimeric antigen receptor (CAR) T cell therapy has emerged as one of the most effective treatments for R/R B cell lymphoma (5). In the ZUMA-2 trial, 68 patients with R/R MCL underwent CAR T cell treatment, ORR was 93%, and complete remission rate was 67%, with estimated 12-month progression-free survival and OS rates of 61% and 83%, respectively (6). The US Food and Drug Administration has achieved a milestone by approving brexu-cel for R/R MCL immunotherapy; however, brexu-cel is currently not listed in China. CD5 is constitutively expressed in normal T cells and is present in 85% of T-cell malignancies (7). It is also frequently expressed in some B-cell malignancies (8, 9). A previous study evaluating CD5 CAR T-cell treatment of R/R T cell lymphoma/leukemia achieved a 44% ORR with a satisfactory safety profile (7), consistent with the findings of several other investigations. However, to our knowledge, there have been no reports of CD5 CAR T-cell treatment for MCL. Here, we report on the first case of human CD5 CAR T-cell treatment for a 36-year-old patient with R/R MCL who experienced rapid tumor recurrence due to immunophenotypic transformation. We present this report with the goal of providing information on treatment strategies for patients with a similar disease presentation.
A 36-year-old man was diagnosed with stage IVA MCL (Ann Arbor staging) at the local hospital in November 2019. The international prognostic index score of MCL was 2, and bone marrow infiltration was observed. Immunohistochemistry indicated CD20+, CD5+, cyclin D1+, and Ki-67 (30%+). Next-generation sequencing (NGS) indicated TP53 c.524G>A of 54.50%, KMT2D c.6010C>T of 29.10% and NOTCH1 c.5244C>G of 10.14%. The patient underwent standard-dose RCHOP/RDHAP chemotherapy and autologous hematopoietic stem cell transplantation. Disease relapse was noted in January 2021, with disease progression after receiving ibrutinib and standard dose RBAC 500 chemotherapy. The clinical treatment timeline of this patient is shown in Figure 1A.
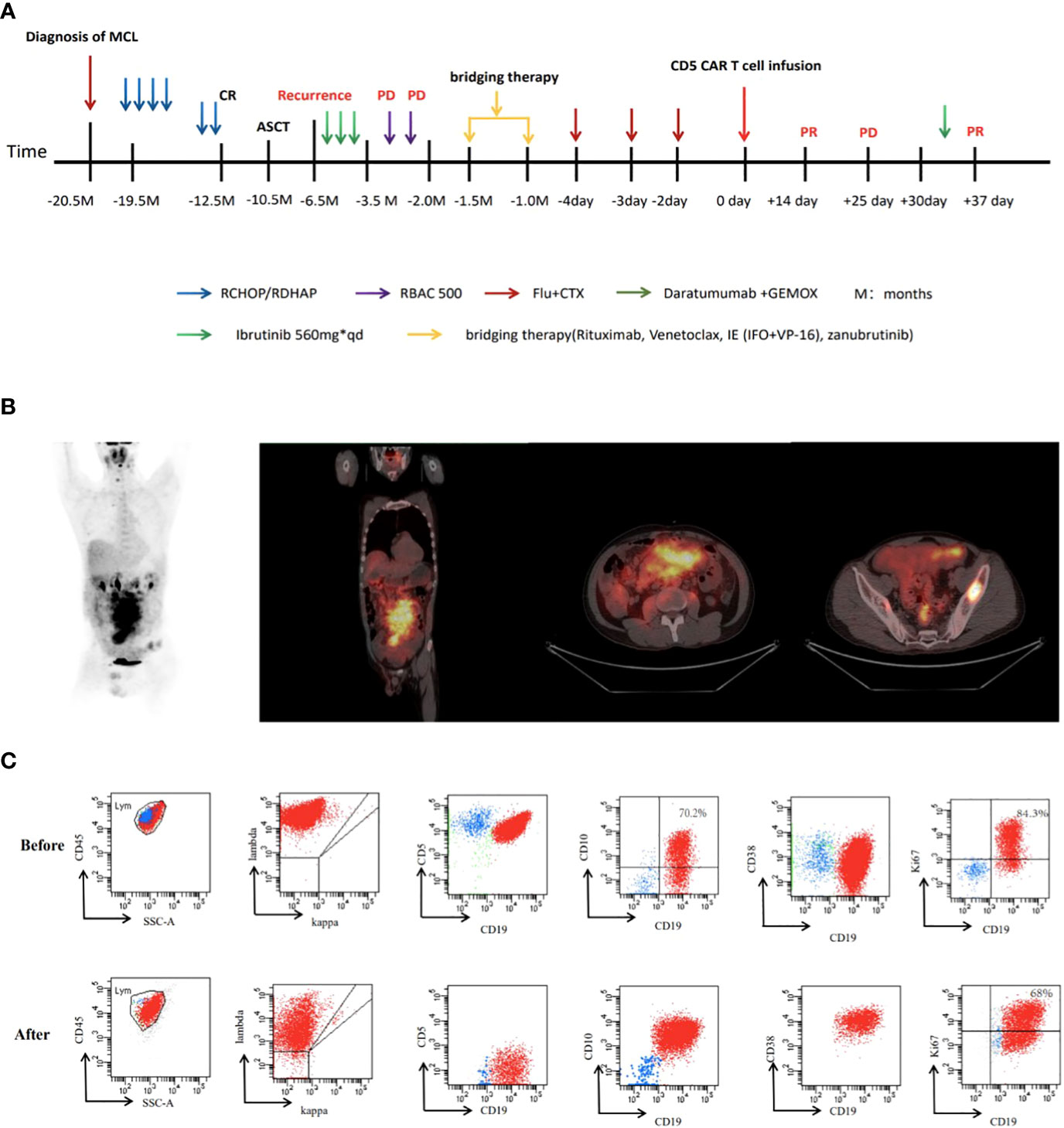
Figure 1 (A)Timeline of key clinical events in this case. (B) The positron emission tomography-CT scan images of this patient before CAR T. (C) flow cytometry analysis before and after CAR T.
In June 2021, the patient was admitted to our hospital for CAR T cell treatment. Positron emission tomography/computed tomography indicated multiple lymphadenopathies and elevated metabolic activity throughout the body (Figure 1B). The flow cytometry results of lymph node tissues indicated that 92% of the cells were large monoclonal abnormally mature B lymphocytes expressing CD19, CD22, CD5, CD20, and CD38 (partially weakly positive), with some expressing CD10 and Ki67 (73.3%+) (Figure 1C). NGS of the peripheral blood circulating tumor DNA (ctDNA) indicated that the TP53 c.524G>A (p. Arg175His) mutation was found in 33.70% of the cells, while the KMT2D c.6010C>T (p. Gln2004Ter) mutation was found in 17.00% of the cells (Figures 2A, B). The patient had a 46XY karyotype and was enrolled in a human CD5 CAR T-cell trial, with his informed consent, at the time of this case presentation. He received lymphodepletion chemotherapy with the FC regimen (fludarabine 30 mg/m2 and cyclophosphamide 500 mg/m2) on 29 July 2021, for 3 days (day 2 to day 4), followed by CAR T cell infusion at a dose of 2*106 cells/kg on 2 August 2021. The patient developed grade 1 cytokine release syndrome (CRS), with minimal elevations in interleukin-6 and ferritin concentrations (Figure 3A). The CAR T cells were well expanded (Figure 3B). The function of CD5-positive and CD5-negative T lymphocytes was monitored during treatment. The results showed that before CAR T cell infusion, CD5+IFN-γ+T lymphocytes accounted for 37.8% of the total T lymphocytes. After treatment with CAR T-cells, CD5-IFN-γ+T lymphocytes accounted for 78.1% of the total T lymphocytes (Figure 3C), and the positive rate of IFN-γ had increased significantly, indicating that the cytotoxic function of T cells was majorly enhanced. CD5 has been reported to be a negative regulator of T cell receptor signaling and has a protective effect on autoimmunity (10, 11). CD5 deletion can enhance the antitumor effect of the CAR T cell-targeted antigens (12, 13). The changes in T cell function in our patient treated with CD5 CAR T cells are consistent with the results reported in the literature.
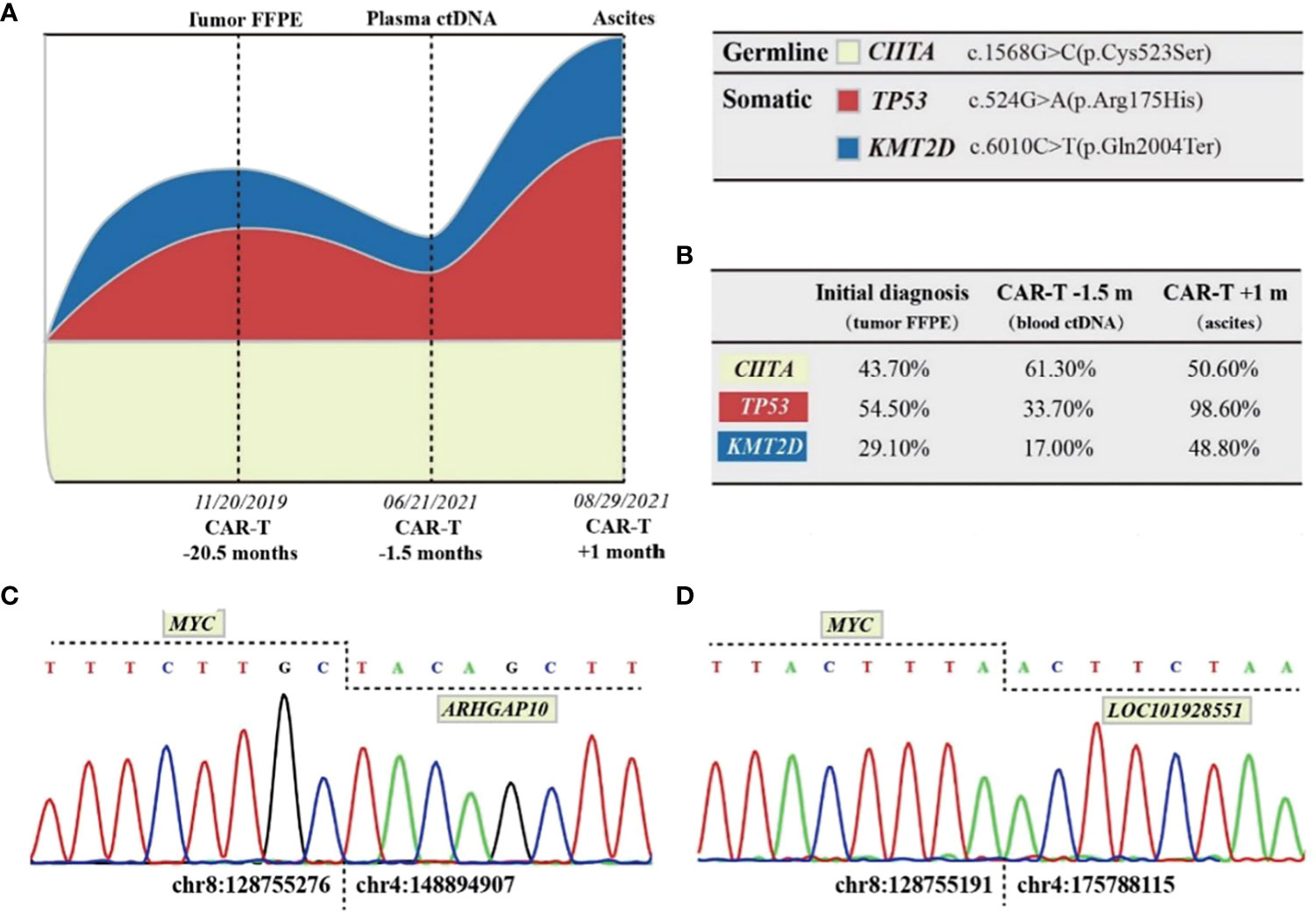
Figure 2 DNA high-throughput sequencing results and analysis. (A) Schematic models of evolutionary progression before and after CAR T cell infusion. Germline clones, primary dominant clones, and secondary dominant clones are represented in creamy white, red, and blue, respectively. (B) References and VAFs of DNA mutations investigated by NGS. An NGS panel with 129 target genes was employed for analysis of the DNA samples. (C) Partial sequence chromatograms of the gDNA amplified fragment showing the junction position of the MYC and ARHGAP10 genes (vertical dotted line). In the MYC-ARHGAP10 fusion gene, intergenic region of MYC (chr8:128755276) was fused in frame with intronic region of ARHGAP10 (chr4:148894907). (D) Partial sequence chromatograms of the gDNA amplified fragment showing the junction position of the MYC and LOC101928551 genes (vertical dotted line). In the MYCLOC101928551 fusion gene, intergenic region of MYC(chr8:128755191) was fused in frame with ncRNA intronic region of LOC101928551 (chr4:175788115).
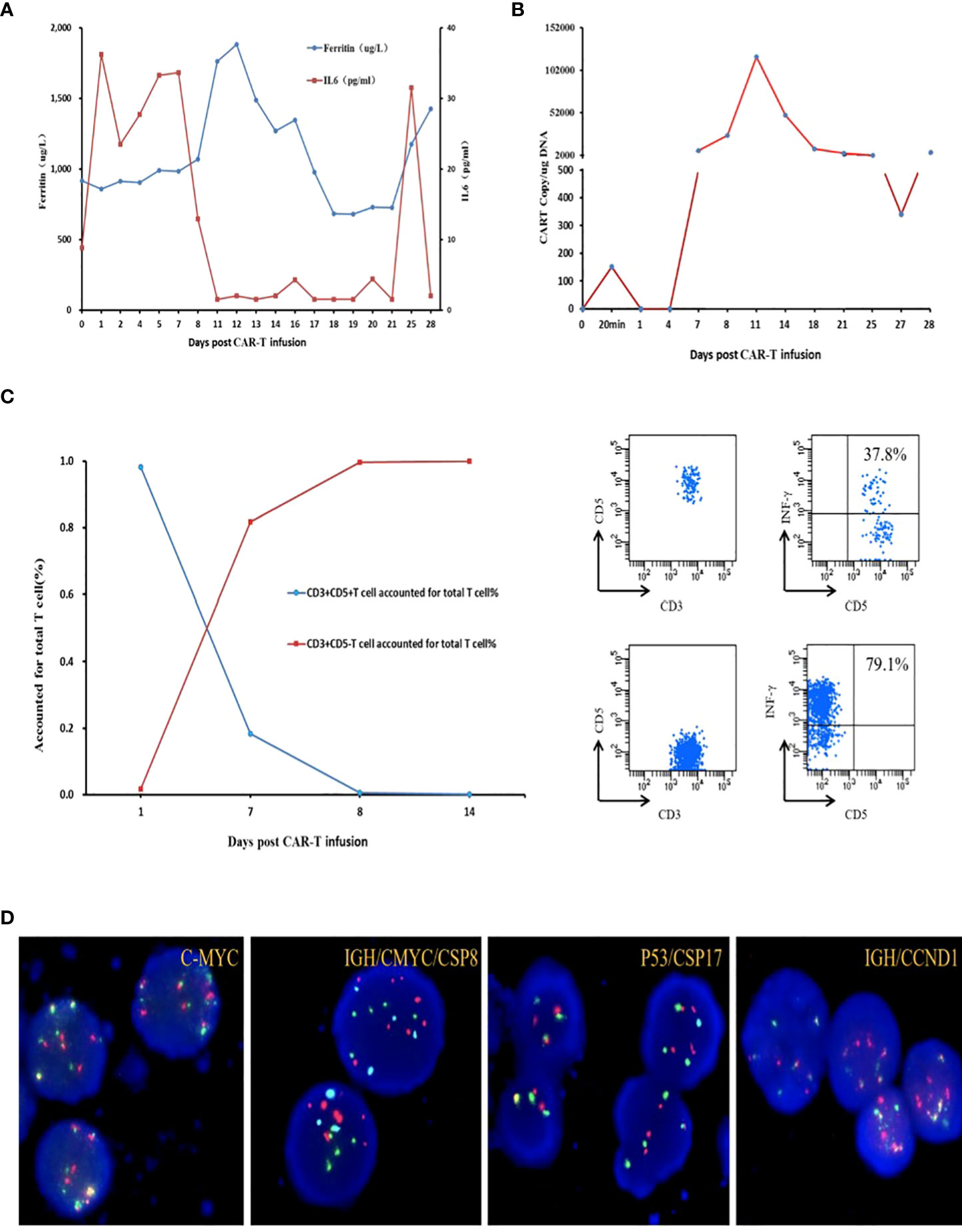
Figure 3 (A) Levels of IL-6 and ferritin after CAR T cell therapy. (B) CAR-5 transgene copy numbers detected by ddPCR. (C) CD3+ CD5+/CD5-T cell accounted for total T cell%; INF-γ secreted by CD5 positive T lymphocytes before infusion accounted for 37.8% of the total T lymphocytes secreted; INF-γ secreted by CD5-negative T lymphocytes accounted for 79.1% of the total T lymphocytes secreted after infusion. (D) Fluorescence in situ hybridization of ascites C-MYC (8q24) break apart probe, IGH/CMYC/CSP8t(8;14), P53/CSP17 (17p13), GH/CCND1t(11;14).
Reexamination of the efficacy using computed tomography on day 14 indicated PR. On day 25 (27 August 2021), the patient returned to the hospital due to abdominal distention and pain. Ultrasound showed massive ascites; ascitic fluid flow cytometry results indicated 90.6% of the cells were monoclonal abnormally mature large B lymphocytes that did not express CD5 or CD20 (Figure 1C). Fluorescence in situ hybridization suggested the patient’s abnormal cells were polyploid, with positive IgH/CCND1 fusion and +17 chromosomes (Figure 3D). NGS indicated TP53 c.524G>A (p. Arg175His) of 98.6%, KMT2D c.6010C>T (p. Gln2004Ter) of 48.8%, IgH-CCND1 rearrangement of 97.08%, MYC-LOC101928551 rearrangement of 61.87%, and MYC-ARHGAP10 rearrangement of 55.56%. There was no mutation in IGHV4-34 (Figures 2C, D). Considering the above-mentioned findings and the strong expression of CD38 in B-cell lymphoma cells in ascitic fluid, the patient was treated with a regimen of daratumumab + Gemox (gemcitabine + oxaliplatin) on 1 September 2021, and his abdominal distention and pain were significantly reduced, and ascites disappeared. Unfortunately, the patient gave up further treatment for his own reasons and was discharged from the hospital. The follow-up results indicated that the patient died after 2 months, and the specific cause of death was unknown.
Research to date indicates that CAR T cell therapy targeting CD5 has good prospects for clinical application, not only for the treatment of T cell malignancies but also for CD5-expressing B cell malignancies, such as MCL, diffuse large B cell lymphoma, chronic lymphocytic leukemia, and small lymphocytic lymphoma (14–16). Our medical center created CAR-T cells uniquely targeting CD5 for the treatment of CD5+ lymphoma and, to our knowledge, was the first center to use these cells in patients with R/R MCL. No severe infections occurred after treatment, CRS and CAR-T cell-relevant encephalopathy syndrome (CRES) were well controlled, CAR T cells expanded well, and the patient achieved PR 2 weeks after treatment. After 4 weeks, the patient developed an immunophenotypic transformation, with CD5 and CD20 becoming negative; however, the intensity of expression of CD10 and CD38 increased significantly.
CD5 CAR T cells can induce T cell dysplasia, and long-term T cell dysplasia increases the likelihood of infection in patients (17, 18). However, a recent clinical trial showed that CD5 CAR T cell treatment for lymphoma does not cause life-threatening immunodeficiency (4). There is currently no precedent for MCL treatment with CAR T cells targeting CD5. Several patients at our center with R/R lymphoma have undergone CD5 CAR T-cell treatment. We observed that the infection status of all patients after CD5 CAR T cell treatment did not differ significantly from that of patients who received CAR T cell treatments with other targets. After CAR T cell treatment of the current patient, IFNγ secretion was significantly increased in CD5-negative T lymphocytes. CD5-negative T lymphocytes may also serve some function, but more research is needed to elucidate differences in the functions of CD5+ and CD5- lymphocytes.
Immunophenotypic transformation of MCL during treatment is rare. Two groups of tumor cells have previously been detected simultaneously in a patient with double-hit MCL, both of which carried IgH-CCND1 rearrangements and TP53 mutations. CD19 +/CD10 + cells carried MYC/IGH rearrangements and NOTCH2 mutations, whereas CD19 +/CD10 cells did not. The rearrangement of MYC and a mutation in NOTCH2 were believed to have induced the immunophenotypic transformation of MCL cells in this patient (19). It has also been reported that EBV can drive the conversion of MCL to DLBCL (20). The concept of a double-hit lymphoma was first defined in 2011 as any lymphoma with a chromosomal breakpoint affecting the MYC/8q24 locus in combination with another recurrent breakpoint, usually t(14;18) (q32;q21) involving BCL2 but occasionally t(11;14) (q13;q32) involving CCND1 (21). MCL with MYC rearrangement fits this definition. However, because the World Health Organization (WHO) classification still defines these cases as MCL, these cases were designated double-hit MCL (19). In the present case, the patient had double-hit MCL with TP53 mutation and rearrangement of IgH-CCND1 and MYC, and an immunophenotypic transformation occurred after treatment with CAR T-cell treatment; the specific mechanism for this is unclear. In CAR T cell therapy, tumor antigen escape is a common cause of disease control failure (22). CD5+ lymphoma cells are sensitive to CD5 CAR T cells, and active tumor cells are killed after treatment with CAR T cells. CAR T cells persist in the patient’s body, and CD5+ tumor cells are inhibited and cannot proliferate. Additionally, MYC rearrangement and mutation of the TP53 gene can cause instability of the tumor genome. Silent CD5- lymphoma cells are reactivated through the mutation of the TP53 gene and rearrangement of CCND1 and MYC, leading to clinical recurrence. In particular, ctDNA NGS is effective in monitoring the process of clonal evolution.
In summary, our case report confirms that double-hit status and TP53 mutation are poor prognostic indicators of MCL. The TP53 mutation and the rearrangement of CCND1/MYC may cause instability of the tumor genome and induce transformation of the tumor immunophenotype, thereby leading to the escape of tumor antigens, which is the primary challenge in CAR T-cell therapy. Tumor genetic abnormalities should be carefully monitored during CAR T-cell therapy, and a rebiopsy is necessary to confirm tumor immunophenotype and any genetic changes.
The trial was approved by the ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
SH analyzed the data and wrote the manuscript. XM conducted the flow cytometry. MX conducted next-generation sequencing. ZC interpreted and analyzed the images of 18F-fluorodeoxyglucose PET/CT scan. SH, XZ, and JZ treated the patient. XZ and MX reviewed the manuscript and were in charge of the final approval of the manuscript. All authors read and approved the final manuscript.
This work was supported by funding from the National Natural Science Foundation of China awarded to MX (81770211); the National Key R&D Program of China (2021YFF0703704); Key R & D plan of Hubei Province (2020BCB021 and 2020BCB043); and the excellent young science foundation project of Tongji Hospital (2020YQ0012).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CAR, Chimeric antigen receptor; CCND1, Cyclin D1; CRS, Cytokine release syndrome; MCL, Mantle cell lymphoma; NGS, Next-generation sequencing; ORR, Objective response rate; OS, Overall survival; R/R, Relapsed/Refractory; DLBCL, Diffuse large b-cell lymphoma.
1. Miao Y, Cao L, Sun Q, Li XT, Wang Y, Qiao C, et al. Spectrum and immunophenotyping of 653 patients with b-cell chronic lymphoproliferative disorders in China: a single-centre analysis. Hematol. Oncol. (2018) 36:121–7. doi: 10.1002/hon.2461
2. Meng J, Chang C, Pan H, Zhu F, Xiao Y, Liu T, et al. Epidemiologic characteristics of malignant lymphoma in hubei, China: a single-center 5-year retrospective study. Medicine (2018) 97:e12120. doi: 10.1097/MD.0000000000012120
3. Hershkovitz-Rokah O, Pulver D, Lenz G, Shpilberg O. Ibrutinib resistance in mantle cell lymphoma: Clinical, molecular and treatment aspects. Br. J. Haematol. (2018) 181(3):306–19. doi: 10.1111/bjh.15108
4. Hanel W, Epperla N. Emerging therapies in mantle cell lymphoma. J. Hematol. Oncol. (2020) 13:79. doi: 10.1186/s13045-020-00914-1
5. Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. (2007) 25:579–86. doi: 10.1200/JCO.2006.09.2403
6. Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl. J. Med. (2020) 382:1331–42. doi: 10.1056/NEJMoa1914347
7. Hill LC, Rouce RH, Smith TS, Yang L, Srinivasan M, Zhang H, et al. Safety and antitumor activity of CD5 CAR T-cells in patients with relapsed/refractory T-cell malignancies. Blood (2019) 134:199–210. doi: 10.1182/blood-2019-129559
8. Jones NH, Clabby ML, Dialynas DP, Huang HJ, Herzenberg LA, Strominger JL. Isolation of complementary DNA clones encoding the human lymphocyte glycoprotein T1/Leu-1. Nature (1986) 323:346–349.21. doi: 10.1038/323346a0
9. Berland R, Wortis HH. Origins and functions of b-1 cells with notes on the role of CD5. Annu. Rev. Immunol. (2002) 20:253–300. doi: 10.1146/annurev.immunol.20.100301.064833
10. Bikah G, Carey J, Ciallella JR, Tarakhovsky A, Bondada S. CD5-mediated negative regulation of antigen receptor-induced growth signals in b-1 b cells. Science (1996) 274:1906–9. doi: 10.1126/science.274.5294.1906
11. Dalloul A. CD5: a safeguard against autoimmunity and a shield for cancer cells. Autoimmun Rev. (2009) 8:349–53. doi: 10.1016/j.autrev.2008.11.007
12. Raikar SS, Fleischer LC, Moot R, Fedanov A, Paik NY, Knight KA, et al. Development of chimeric antigen receptors targeting T-cell malignancies using two structurally different anti-CD5 antigen binding domains in NK and CRISPR-edited T cell lines. Oncoimmunology (2018) 7:e1407898. doi: 10.1080/2162402X.2017.1407898
13. Chun I, Kim KH, Chiang YH, Xie W, Lee YGG, Pajarillo R, et al. CRISPR-Cas9 knock out of CD5 enhances the anti-tumor activity of chimeric antigen receptor T cells. Blood (2020) 136:51–2. doi: 10.1182/blood-2020-136860
14. Miyazaki K, Yamaguchi M, Suzuki R, Kobayashi Y, Maeshima AM, Niitsu N, et al. CD5-positive diffuse large b-cell lymphoma: a retrospective study in 337 patients treated by chemotherapy with or without rituximab. Ann. Oncol. (2011) 22:1601–7. doi: 10.1093/annonc/mdq627
15. Zhao P, Li L, Zhou S, Qiu L, Qian Z, Liu X, et al. CD5 expression correlates with inferior survival and enhances the negative effect of p53 overexpression in diffuse large b-cell lymphoma. Hematol. Oncol. (2019) 37:360–7. doi: 10.1002/hon.2657
16. Wang HY, Zu Y. Diagnostic algorithm of common mature b-cell lymphomas by immunohistochemistry. Arch. Pathol. Lab. Med. (2017) 141:1236–46. doi: 10.5858/arpa.2016-0521-RA
17. Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl. J. Med. (2018) 378:449–59. doi: 10.1056/NEJMoa1709919
18. Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl. J. Med. (1999) 340:508–16. doi: 10.1056/NEJM199902183400703
19. Zhou J, Hu L, Zuo M, Zhou Y, Li G, Zhang X. An uncommon case of double-hit mantle cell lymphoma that demonstrates a transformation process. Am. J. Clin. Pathol. (2020) 153:49–57. doi: 10.1093/ajcp/aqz133
20. Terasawa T, Ohashi H, Utsumi M, Tsushita K, Kinoshita T, Nakamura S, et al. Case of Epstein–Barr virus-associated transformation of mantle cell lymphoma. Am. J. Hematol. (2003) 73:194–9. doi: 10.1002/ajh.10343
21. Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma EJ, et al. Double-hit b-cell lymphomas. Blood. (2011) 117(8):2319–31. doi: 10.1182/blood-2010-09-297879
Keywords: mantle cell lymphoma, CD5 CAR T cell treatment, immunophenotypic transformation, relapsed/refractory, double-hit
Citation: He S, Mao X, Cheng Z, Zhu X, Xiao M and Zhou J (2022) Immunophenotypic transformation in relapsed/refractory mantle cell lymphoma treated with human anti-CD5 chimeric antigen receptor T cells: A Case Report. Front. Hematol. 1:967156. doi: 10.3389/frhem.2022.967156
Received: 12 June 2022; Accepted: 08 September 2022;
Published: 14 November 2022.
Edited by:
Paul Austin Moss, University of Birmingham, United KingdomReviewed by:
Andrea Janikova, University Hospital Brno, CzechiaCopyright © 2022 He, Mao, Cheng, Zhu, Xiao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojian Zhu, emh1eGlhb2ppYW5AaHVzdC5lZHUuY24=; Min Xiao, eGlhb21pbkB0amgudGptdS5lZHUuY24=
†These two authors contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.