- 1Department of Internal Medicine, University of Missouri Kansas City, Kansas City, MO, United States
- 2Division of Hematological Malignancies and Cellular Therapeutics, University of Kansas Medical Center, Kansas City, KS, United States
- 3Internal Medicine Department, University of Kansas Cancer Center, Kansas City, KS, United States
- 4Department of Pathology, University of Kansas Medical Center, Kansas City, KS, United States
- 5Division of Hematology and Bone Marrow Transplantation, University of Rochester Medical Center, Rochester, NY, United States
The utilization of hematopoietic stem cell transplantation (HSCT) has been rapidly growing due to multiple factors, including better availability of donors and improved supportive care. Hyperbaric oxygen has been associated with the improvement of hematopoietic stem cell (HSC) homing at the time of transplant through lowering erythropoietin levels in preclinical studies. We studied the role of hyperbaric oxygen (HBO) in the enhancement of engraftment of HSC when utilized prior to umbilical cord HSCT and autologous HSCT in two pilot clinical trials with excellent safety profiles. In these two pilot studies, we observed an uncommon phenomenon post-transplant, particularly a significant peripheral blood lymphocytosis and lymphocyte infiltration of different tissues in 3/34 of HBO-treated patients. This peripheral blood lymphocyte expansion was associated with various clinical manifestations that can be confused with infections, inflammatory conditions, or disease relapse. We hypothesize that this observation is related to different immune reconstitution dynamics related to the use of HBO. While the incidence is ~9%, this may have implications as HBO is being investigated in larger clinical trials. This case series highlights the clinical presentation, course, outcome, and potential implications of this significant rise in lymphocytes when utilizing HBO before HSCT.
Introduction
Hematopoietic stem cell transplant (HSCT) is the mainstay in treating many types of malignancies and other benign conditions (1, 2). Graft failure and delayed engraftment post-HSCT increase the risk for mortality and significant morbidity following HSCT in general and following allogeneic HSCT in particular (3, 4). The readily available biobank-stored umbilical cord (UC) is a great source of hematopoietic stem cells (HSCs) (5, 6). However, the relatively small number of HSC in one UC unit may cause insufficient HSC expansion, leading to higher rates of graft failures and delayed engraftment post-UC-HSCT compared to bone marrow and mobilized peripheral HSC source (7, 8). The delayed engraftment of both neutrophils and platelets leads to life-threatening infections and bleeding in addition to the increased burden of transfusion requirement compared to other sources of hematopoietic stem cells (8–10). However, researchers have been developing strategies to increase the number of hematopoietic stem cells in UC units either in vivo or ex vivo (11). Preclinical experiments demonstrated that hyperbaric oxygen (HBO) treatment before the HSCT could improve homing of HSC in vivo during the transplant process and speed up neutrophil and platelet engraftment, reducing the risk of infections and bleeding during the critical period early post-HSCT (12, 13). The same technique can be applied in autologous hematopoietic stem cell transplant with the intent to improve engraftment and to decrease hospitalization length. Moreover, HBO has shown the potential to boost post-HSCT immune reconstitution with faster B lymphocytes and NK cells in patients undergoing UC-HSCT which may have implications in reducing disease relapse and non-relapse mortality due to infections (14). Early-phase clinical trials using HBO in both UC-HSCT and autologous HSCT have been conducted and showed safety and a signal of improved efficacy leading to ongoing larger clinical trials (15, 16). Here, we highlight three cases from two early-phase clinical trials utilizing HBO with unique patterns of early lymphocytosis and associated temporary clinical manifestations and potential implications for long-term outcomes. Informed consent was obtained from all patients before enrolling them in the clinical trial. On the day of HSCT, oxygen was compressed to 2.5 atmosphere absolutes in a monoplace hyperbaric chamber (Model 3200/3200R; Sechrist Industries, Anaheim, CA 92807, USA). The patients were placed in the chamber for a total of 120 min, breathing HBO for 90 min with 10-min breaks of room air every 10 min. The autologous HSC infusion was initiated 6 h after HBO therapy. All three patients received HBO therapy between 2012 and 2013.
All the procedures followed were in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. The clinical trial was approved by the institutional review board before the initiation of research and was registered at clinicaltrials.gov (NCT02087657).
Cases
Case 1
Our first case is a white male patient in his 60s who was diagnosed with high-risk IgG kappa multiple myeloma and underwent three autologous HSCTs. Initially, the patient started on induction chemotherapy with bortezomib and dexamethasone, followed by tandem autologous HSCT with high-dose melphalan for conditioning. He was on bortezomib maintenance and had disease progression 3 years after his second auto HSCT. Multiple myeloma went into partial remission with carfilzomib lenalidomide dexamethasone followed by a third auto HSCT. He enrolled in the HBO trial for his third auto HSCT and received high-dose chemotherapy with the BEAM regimen (BCNU, etoposide, cytarabine, melphalan). His early post-transplant course was complicated by high fevers, rigors, extensive skin rash, and atrial fibrillation with a rapid ventricular response (RVR) and hypotension. The patient was transferred to the ICU and required broad-spectrum empiric antibiotics. This raised suspicion of engraftment syndrome or sepsis, but there was no evidence of diarrhea or pulmonary edema which made engraftment syndrome less likely. Moreover, his acute illness was associated with a significant rise in his white blood cell count (WBC) with lymphocyte predominance (peaking at 90% of total WBC at 5.2k/μl) 10 days post-transplant (Figure 1A). Peripheral blood showed atypical lymphocytosis (Figure 1B), and flow cytometry revealed an atypical T-lymphocyte phenotype, with a CD4 to CD8 ratio of 1:1 and co-expression of CD15, CD38, and HLA-DR. CD15, CD38, and HLA-DR are all T-cell activation markers, and T-cell activation may explain the atypical morphology under a light microscope (17, 18). The expression of mature T-cell antigens CD2, CD5, and CD 7 and the lack of CD1a expression; a characteristic of T cells with normal antigen expression were observed (Figure 1C). There was a small population of T cells co-expressing CD4 and CD8 which can be seen in non-malignant T-cell proliferation. B cells were polyclonal with normal antigen expression, and there was no evidence of an increased plasma cell population.
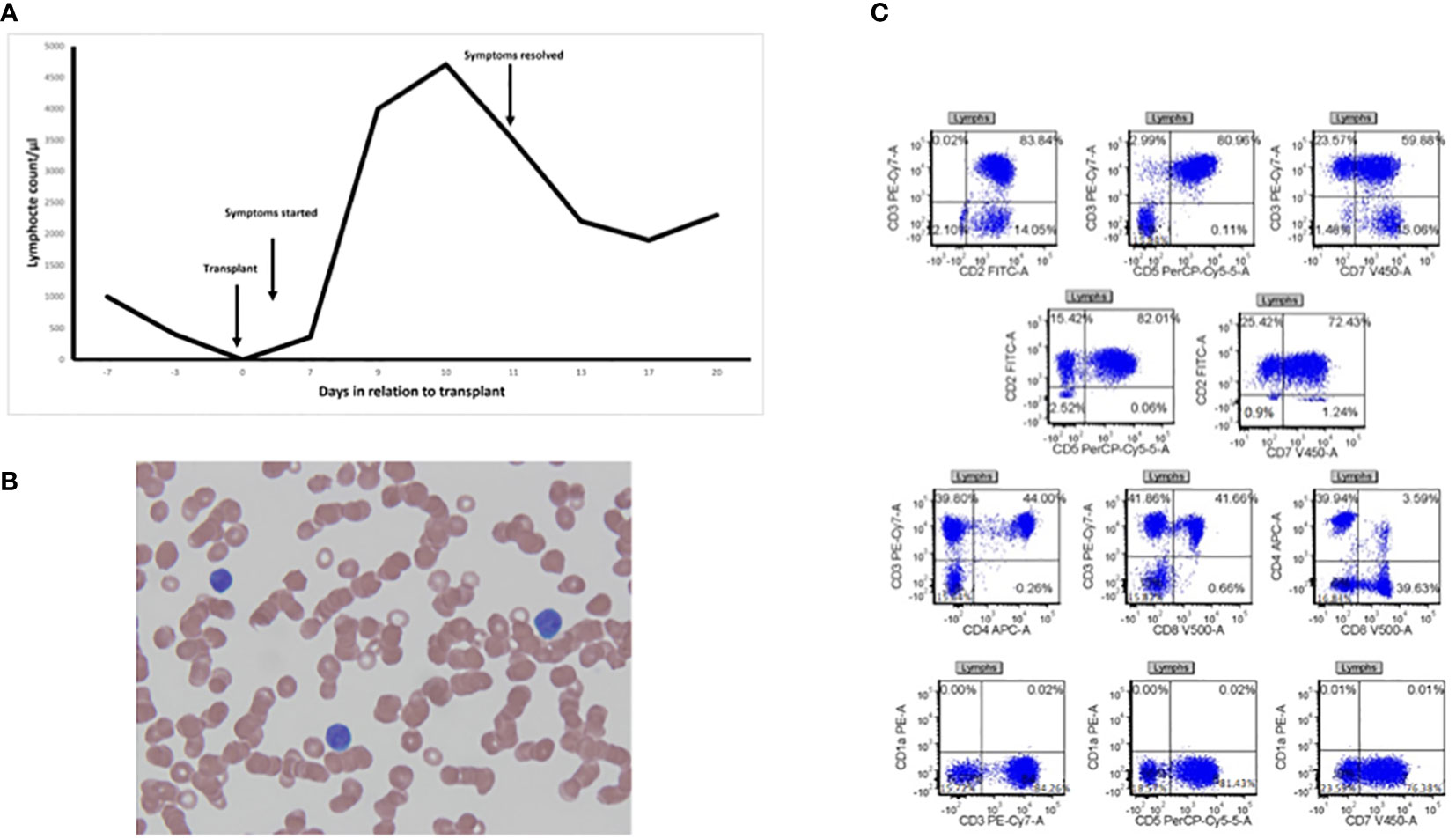
Figure 1 Lymphocytosis after engraftment. (A) Timeline for lymphocyte counts against days in relation to transplant marking when symptoms started and resolved (black arrows). (B) Peripheral smear from a patient drawn on day 9 post-transplant at ×100 magnification showing atypical lymphocytes. (C) Flow cytometry gating of lymphocytes showing atypical antigen expression.
There was also a small population of T cells co-expressing CD4 and CD8. There were no CD38 bright plasma cells and no evidence of an increased plasma cell population. All the workup including viral serology, urinalysis, and blood cultures was negative for any infectious etiology. The patient’s condition improved subsequently, coinciding with the decline of peripheral lymphocytosis spontaneously within a couple of days without the administration of any systemic steroids. As a result, the patient was discharged home safely. The patient achieved very good partial remission and was started on thalidomide maintenance that continued for 2 years and then stopped due to side effects. He was monitored off treatment until he had evidence of disease progression 4 years and 5 months from his third auto HSCT and was started on further treatment. This is the longest time he has been in remission, compared to 3 years in remission after tandem auto HSCT, which is very unusual after a third auto HSCT and may suggest a role of the observed early lymphocytosis in controlling his multiple myeloma. He remains alive more than 7 years after the third auto HSCT and 11 years from the initial diagnosis.
Case 2
Our second case is a white male patient in his 30s, who was diagnosed with refractory nodular sclerosis Hodgkin lymphoma and received multiple lines of treatment, including doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) for two cycles with a partial response, followed by bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPP), and finally brentuximab. His disease was at a partial response, and he proceeded with autologous peripheral SCT under the HBO protocol, around 7 months from the original diagnosis. The patient received a BEAM conditioning regimen. His post-transplant course was complicated by neutropenic fever, and he engrafted on day 14. Interestingly, on day +75 post-transplant, he developed marked lymphocytosis (the lymphocyte percentage peaked at 88% from a total WBC of 6.7k/μl) that was associated with bilateral eye pain, redness, and visual changes diagnosed as bilateral anterior uveitis. Infections, autoimmune disease, and relapse of lymphoma were the top differential diagnoses. A flow cytometry analysis from his right eye aqueous humor did show lymphocytes comprising 13% of the total events. Most were T cells with a normal CD4:CD8 ratio and normal antigen expression. B cells were absent, and there was no immunophenotypic evidence of lymphoma. Cerebrospinal fluid (CSF) analysis was significant for leukocytosis with a total WBC of 55; lymphocytes represented 90% of the total events. CSF flow cytometry revealed lymphocytes comprising 97% of the total events. The majority were T cells with a normal CD4:CD8 ratio and normal antigen expression. B cells were absent and there was no immunophenotypic evidence of non-Hodgkin lymphoma. The patient was treated with local steroid eye drops, and his eye symptoms started to improve and completely resolved within 2 weeks. All the infectious work at that point came back negative. The lymphocytosis resolved in 2 weeks. PET scan on day 100 showed complete metabolic remission for the first time since his diagnosis. However, he had evidence of relapse on imaging 3 months later, and a decision was made to proceed with reduced intensity conditioning allogeneic HSCT. He had localized disease recurrence 6 months after allogeneic HSCT and received brentuximab and donor lymphocyte infusion. He achieved a response, but he passed away from GVHD complications after 1 year and 4 months from allogeneic HSCT.
Case 3
Our third case is a white female patient in her 50s with hepatosplenic T-cell lymphoma. The patient received four cycles of cyclophosphamide, doxorubicin, vincristine, dexamethasone, mesna, methotrexate, and leucovorin (hyper-CVAD), followed by a single-unit UC-HSCT in the first complete remission on the HBO protocol. She underwent a non-myeloablative preparative regimen of fludarabine, cyclophosphamide, and total body irradiation. Her post-transplant course was complicated by stage II graft versus host disease in the gut and skin, which responded to steroid treatment with a complete response. The patient achieved neutropenic engraftment on day 25, and peripheral blood chimerism testing on day 30 showed 99% donor cells. Follow-up at day 100 post-transplant revealed complete remission of the disease. However, on day +147 post-transplant, her CBC indicated persistent peripheral lymphocytosis, ranging between 42% and 64% of the total WBC, which persisted for several months. Lymphocytosis was not associated with any systemic manifestations throughout. Suspicion of relapse of the lymphoma led to bone marrow aspiration and biopsy as well as bone marrow flow cytometry showing that majority of the lymphocytes were T cells with a mildly decreased CD4:CD8 ratio (0.9:1) and normal antigen expression with no evidence of malignant cells. Repeated bone marrow biopsy and flow cytometry were completed 2 months later to rule out lymphoma again, showing similar results. Viral serology was negative for CMV and EBV. Repeated imaging showed that the patient was in complete remission and was still in remission until her last follow-up 3 years after HSCT; thereafter, the patient was completely lost to follow-up.
Discussion
Studies have shown the effect of erythropoietin on HSC differentiation as erythropoietin induced HSC differentiation into erythroid progenitors and reduced myeloid differentiation, which increased the risk of delayed homing and engraftment failure (19). Preclinical experiments in mice showed an improvement in the kinetics and engraftment when using HBO to decrease erythropoietin levels before transfusing UC CD34+ cells (12). Aljitawi et al. conducted the first pilot trial investigating the role of HBO prior to UC-HSCT in humans (15). The median time for neutrophil recovery was lower in the population treated with HBO before UC-HSCT compared to the control group (14 vs. 20.5 days, p = 0.11). Interestingly, the survival rate at 6-month follow-up was higher in the HBO group compared to the control group (100% vs. 76%, respectively; 95% CI, 50.1–79.4%, p < 0.0001). There was a significant faster recovery of B cells as well as a trend toward a faster recovery of NK cells. Another pilot clinical trial investigated the effect of HBO pretreatment in patients receiving autologous hematopoietic stem cells (20). In the 19 patients who completed the HBO therapy, the median time for neutrophil and platelet recovery in the HBO arm was 1 and 2 days, respectively, earlier than the matched historical controls (16).
HBO exposure in preclinical trials led to leukocyte and lymphocyte depletion. In addition, HBO exposure induced abnormalities in other lymphoid organs, such as the spleen and lymph nodes (21), which encouraged scientists to study HBO exposure in humans further. In clinical studies, HBO therapy caused a significant reduction in the CD4:CD8 ratio and a decrease in lymphatic tissue proliferation, with a concomitant increase in neutrophil activation. However, it did not affect the total number of T cells, B cells, or natural killer cells (22, 23). Such changes were utilized for the purpose of increasing recovery and decreasing graft failure after organ transplant (24). In contrast, UC-HSCs were found to inhibit the proliferation of lymphocytes and the production of interleukin-2 (IL-2) in vitro (25). Patients who underwent UC-HSCT were found to have slower lymphocyte recovery in general and T cell in particular compared to HSCT from matched donors (26, 27). The patterns of lymphocyte recovery can be attributed to the effects of HBO on the cytokine and hormonal milieu in the microenvironment as HBO was administered prior to HSC infusion.
Interestingly, we observed differences in the three cases involved in our pilot clinical trial that studied the effects of HBO therapy on engraftment when given prior to UC-HSCT and autologous HSCT. The incidence of this unique pattern of lymphocytosis remains low (3/34). Despite the low incidence rate (8.8%), it is important to recognize and highlight it since this might affect early identification and treatment, especially if associated with significant symptoms such as a rash that can be confused with infections, graft versus host disease, drug reactions, or even malignant disease relapse which adds another layer of complexity to the care of patients post-HSCT. The onset of lymphocytosis after HBO exposure was also variable and ranged between days such as in one multiple myeloma patient and a few months such as in two lymphoma cases (Table 1). We assume that these findings occur as part of a change in the inflammatory milieu and immune reconstitution and not as a new transformation to lymphoma or other malignant conditions, as there was no evidence for malignant transformation to lymphoma in the blood, bone marrow, or other body fluid by morphology or flow cytometry analysis. All the episodes of lymphocytosis subsided spontaneously without a specific systemic lymphodepleting treatment such as high-dose steroids. The long-term outcome varied among these three cases; nonetheless, prolonged remission states were observed in two of the three cases in a high risk of relapse setting. This may suggest a beneficial effect on lymphocyte recovery in this small case series. To our knowledge, this is the first published case series documenting this unique pattern for lymphocyte recovery after utilizing HBO therapy prior to HSCT. However, it is important to recognize this phenomenon as larger-scale clinical trials (NCT03739502, NCT03398200, NCT03964506) are ongoing to test the efficacy of HBO employment further to improve engraftment in recipients of UC-HSCT and autologous HSCT. The ongoing clinical trials may provide insights into the correlation between HBO and lymphocyte recovery and the impact of this association, if present, on long-term outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the University of Kansas Research Center. The patients/participants provided their written informed consent to participate in this study.
Author contributions
ME, HA, and LS: study design and writing of the manuscript. SA, AS, DZ, and JM: manuscript writing and review. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, et al. Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American society for blood and marrow transplantation. Biol. Blood Marrow Transplant. (2015) 21(11):1863–9. doi: 10.1016/j.bbmt.2015.07.032
2. Copelan EA. Hematopoietic stem-cell transplantation. N Engl. J. Med. (2006) 354(17):1813–26. doi: 10.1056/NEJMra052638
3. Mital M, Curtis A, Spencer V, Barge D, Skinner R. Delayed engraftment and mixed chimerism after HLA-identical sibling donor BMT in fanconi anaemia. Bone marrow Transplant. (1999) 24(2):201–4. doi: 10.1038/sj.bmt.1701855
4. Lutfi F, Skelton WP IV, Wang Y, Rosenau E, Farhadfar N, Murthy H, et al. Clinical predictors of delayed engraftment in autologous hematopoietic cell transplant recipients. Hematol/Oncol Stem Cell Ther. (2020) 13(1):23–31. doi: 10.1016/j.hemonc.2019.08.003
5. Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc. Natl. Acad. Sci. (1989) 86(10):3828–32. doi: 10.1073/pnas.86.10.3828
6. Wagner JE, Gluckman E. Umbilical cord blood transplantation: the first 20 years. In: Seminars in hematology. WB Saunders (2010) 47(1):3–12.
7. Ruggeri A, Labopin M, Sormani MP, Sanz G, Sanz J, Volt F, et al. Engraftment kinetics and graft failure after single umbilical cord blood transplantation using a myeloablative conditioning regimen. Haematologica (2014) 99(9):1509–15. doi: 10.3324/haematol.2014.109280
8. Gupta AO, Wagner JE. Umbilical cord blood transplants: Current status and evolving therapies. Front. Pediatr. (2020) 8:570282. doi: 10.3389/fped.2020.570282
9. Narimatsu H, Matsumura T, Kami M, Miyakoshi S, Kusumi E, Takagi S, et al. Bloodstream infection after umbilical cord blood transplantation using reduced-intensity stem cell transplantation for adult patients. Biol. Blood Marrow Transplant. (2005) 11(6):429–36. doi: 10.1016/j.bbmt.2005.01.010
10. Fuchs EJ, O’Donnell PV, Eapen M, Logan B, Antin JH, Dawson P, et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: the BMT CTN 1101 trial. Blood (2021) 137(3):420–8. doi: 10.1182/blood.2020007535
11. Pineault N, Abu-Khader A. Advances in umbilical cord blood stem cell expansion and clinical translation. Exp. Hematol. (2015) 43(7):498–513. doi: 10.1016/j.exphem.2015.04.011
12. Aljitawi OS, Xiao Y, Eskew JD, Parelkar NK, Swink M, Radel J, et al. Hyperbaric oxygen improves engraftment of ex-vivo expanded and gene transduced human CD34+ cells in a murine model of umbilical cord blood transplantation. Blood Cells Molecules Dis. (2014) 52(1):59–67. doi: 10.1016/j.bcmd.2013.07.013
13. Mina A, Aljitawi OS. Use of hyperbaric oxygen in hematopoietic cell transplantation to aid post-transplant recovery. J Comp Eff Res(2019) 9(3):149–53. doi: 10.2217/cer-2019-0193
14. Mina A, Shune L, Abdelhakim H, Lin TL, Ganguly S, Baran A, et al. Long-term results of a pilot study evaluating hyperbaric oxygen therapy to improve umbilical cord blood engraftment. Ann. Hematol. (2019) 98(2):481–9. doi: 10.1007/s00277-018-3532-1
15. Aljitawi OS, Paul S, Ganguly A, Lin TL, Ganguly S, Vielhauer G, et al. Erythropoietin modulation is associated with improved homing and engraftment after umbilical cord blood transplantation. Blood J. Am. Soc. Hematol. (2016) 128(25):3000–10. doi: 10.1182/blood-2016-05-715292
16. Abdelhakim H, Bhatti S, Cantilena AR, Lin TL, Ganguly S, Singh AK, et al. Outcomes of autologous hematopoietic cell transplantation patients receiving hyperbaric oxygen therapy. Biol. Blood Marrow Transplant. (2017) 23(3):S131–2. doi: 10.1016/j.bbmt.2016.12.245
17. Jevremovic D, Olteanu H. Flow cytometry applications in the diagnosis of T/NK-cell lymphoproliferative disorders. Cytometry Part B: Clin. Cytometry (2019) 96(2):99–115. doi: 10.1002/cyto.b.21768
18. Gorczyca W, Weisberger J, Liu Z, Tsang P, Hossein M, Wu CD, et al. An approach to diagnosis of T-cell lymphoproliferative disorders by flow cytometry. Cytometry: J. Int. Soc. Analytical Cytology (2002) 50(3):177–90. doi: 10.1002/cyto.10003
19. Grover A, Mancini E, Moore S, Mead AJ, Atkinson D, Rasmussen KD, et al. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. J. Exp. Med. (2014) 211(2):181–8. doi: 10.1084/jem.20131189
20. Abdelhakim H, Shune L, Bhatti S, Cantilena AR, Baran A, Lin TL, et al. Results of the first clinical study in humans that combines hyperbaric oxygen pretreatment with autologous peripheral blood stem cell transplantation. Biol. Blood Marrow Transplant. (2019) 25(9):1713–9. doi: 10.1016/j.bbmt.2019.05.028
21. Hansbrough JF, Piacentine JG, Eiseman B. Immunosuppression by hyperbaric oxygen. Surgery (1980) 87(6):662–7.
22. Bitterman N, Bitterman H, Kinarty A, Melamed Y, Lahat N. Effect of a single exposure to hyperbaric oxygen on blood mononuclear cells in human subjects. Undersea Hyperbaric Med (1993) 20(3):197–204.
23. Brenner I, Shephard R, Shek P. Immune function in hyperbaric environments, diving, and decompression. Undersea Hyperbaric Med (1999) 26(1):27–39.
24. Al-Waili NS, Butler GJ, Petrillo RL, Carrey Z, Hamilton R. Hyperbaric oxygen and lymphoid system function: a review supporting possible intervention in tissue transplantation. Technol. Health Care (2006) 14(6):489–98. doi: 10.3233/THC-2006-14604
25. Zhao Y, Huang Z, Qi M, Lazzarini P, Mazzone T. Immune regulation of T lymphocyte by a newly characterized human umbilical cord blood stem cell. Immunol. Lett. (2007) 108(1):78–87. doi: 10.1016/j.imlet.2006.10.007
26. Elmariah H, Brunstein CG, Bejanyan N. Immune reconstitution after haploidentical donor and umbilical cord blood allogeneic hematopoietic cell transplantation. Life (2021) 11(2):102. doi: 10.3390/life11020102
Keywords: hyperbaric oxygen, hematopoietic stem cell transplant, umbilical cord, lymphocyte engraftment, multiple myeloma, lymphoma
Citation: Elsayed M, Abdelhakim H, Shune L, Abhyankar S, Singh A, Zhang D, McGuirk J and Aljitawi O (2022) Case Report: Unique patterns of lymphocyte recovery post-hematopoietic stem cell transplant associated with hyperbaric oxygen therapy: A case series. Front. Hematol. 1:1008363. doi: 10.3389/frhem.2022.1008363
Received: 31 July 2022; Accepted: 31 August 2022;
Published: 10 October 2022.
Edited by:
Maegan Capitano, Indiana University Bloomington, United StatesReviewed by:
Andrea M. Patterson, Indiana University School of Medicine, United StatesMariusz Z Ratajczak, University of Louisville Physicians, United States
Copyright © 2022 Elsayed, Abdelhakim, Shune, Abhyankar, Singh, Zhang, McGuirk and Aljitawi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haitham Abdelhakim, aGFiZGVsaGFraW1Aa3VtYy5lZHU=
 Marwa Elsayed
Marwa Elsayed Haitham Abdelhakim
Haitham Abdelhakim Leyla Shune2,3
Leyla Shune2,3 Da Zhang
Da Zhang Joseph McGuirk
Joseph McGuirk