- 1Sangath, Bhopal, India
- 2Department of Global Health and Social Medicine, Harvard Medical School, Boston, MA, United States
- 3Department of Public Health Leadership and Practice at Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Background: Upwards of ninety percent of individuals living with depression in India do not have access to evidence-based treatments, especially in rural areas. Integrating these treatments into primary care is essential for bridging this care gap. This trial aims to evaluate whether a remote coaching implementation support strategy, referred to as Enhanced Implementation Support, is superior to routine support, referred to as Routine Implementation Support, in supporting the delivery of collaborative depression care in rural primary care centers.
Methods: Employing a cluster-randomized hybrid type-III implementation trial design, 14 primary care facilities in Sehore district, Madhya Pradesh, will implement a collaborative depression care package based on the WHO's mhGAP program. Facilities will be randomized to either Enhanced Implementation Support or the Routine Implementation Support control condition. Enhanced Implementation Support consists of remote coaching and technical assistance, supplemented with in-person visits, and guided by the Plan-Do-Study-Act implementation cycles. The primary implementation outcome is the proportion of outpatients screened for depression by facility staff, with secondary outcomes including the proportions of outpatients who screen positive for depression, are referred to the medical officer, and initiate treatment. Secondary patient outcomes include proportion of patients who achieve reduction in depression symptom severity at 3-month follow up. Acceptability, feasibility, and fidelity of the depression care package will be assessed through routine observations collected during field visits, facility audits, and qualitative exit interviews with facility staff. Costs of delivering the Enhanced Implementation Support strategy will also be estimated.
Discussion: This trial can inform efforts to integrate depression care in rural primary care facilities in a low-resource setting, and illuminate whether external coaching support is superior relative to existing implementation support for achieving these goals.
Trial Registration: NCT05264792.
Introduction
Mental disorders pose a serious and growing challenge to health systems, with depression representing the leading cause of disability due to mental illness worldwide (1, 2). In India, depression affects over 50 million people, and is correlated with suicide (3) and ischemic heart disease (4). Evidence-based clinical interventions exist for depression (5); however, the gap between those who need and receive treatment, referred to as the care gap (6), is alarming, with upwards of 90% of individuals not having access to care in rural India (7–9).
Integrating evidence-based treatments for depression into primary care represents an essential priority for bridging this care gap in low-income and middle-income countries (LMICs) such as India (10–12). There are multiple barriers to the successful implementation of mental health services into routine care settings in LMICs related to legislation and policy, financing and resources, organization and planning, and ensuring necessary workforce capacity (13–15). In India, government efforts to integrate evidence-based depression care into routine care settings have faced challenges due to suboptimal organization and planning (13), emphasis on top-down “one size fits all” approaches to service delivery, limited attention to collaborative care models, and inadequate training and support for primary care personnel (16, 17). Efforts to overcome these challenges have demonstrated success through the use of lay health counsellors (18) and have resulted in high follow-up rates, and early remission and recovery among patients (14). However, significant limitations persist, including insufficient engagement of community-level health workers, low utilisation of evidence-based psychological interventions, and inability to sustain delivery of these programs due to overreliance on external resources and few available specialized providers (14, 16).
Novel approaches are needed to support frontline health workers within existing government primary health care facilities to ensure uptake and sustained delivery of depression care. Drawing from the implementation science literature, there is mounting evidence showing that “implementation strategies” can facilitate the integration of proven interventions into routine practice (19–22), including in LMICs (22). Implementation strategies refer to techniques, often guided by a theory or framework, to enable the adoption and implementation of an evidence-based clinical intervention in practice (23, 24). While there has been an increasing emphasis on examining the integration, acceptability, feasibility and cost of treating depression in various settings in India (22–25), there remains a paucity of studies employing rigorous randomized controlled designs, and few that have evaluated use of a comprehensive implementation strategy at the primary care level.
This trial seeks to address this knowledge gap through use of a cluster-randomized controlled superiority trial design to evaluate whether a “remote coaching implementation support strategy” is superior when compared to “routine implementation support” in facilitating the delivery of collaborative depression care in primary care facilities. Successful delivery of depression care will be defined by increased rates of screening for depression (i.e., primary implementation outcome) and detection of depression cases and initiation of treatment (i.e., secondary implementation outcomes) in the participating primary care facilities. Costs of the implementation support strategy will also be assessed, as well as secondary patient-level clinical and functional outcomes. This trial builds on recent health system-level changes in India, where the screening and management of non-communicable diseases (NCD) now form part of essential primary care services, yielding an opportunity for integrating depression care (26). This trial will employ routine health facility cadres, such as the auxiliary nurse midwife (ANM) and nurses, primarily for depression screening, and the medical officer (MO) for diagnosis, treatment and referral of cases (either to the District Mental Health Program for specialist-delivered care, or to a brief psychosocial intervention for depression delivered by a trained community health worker, referred to as an Accredited Social Health Activist), and employ routine data collection at the facility-level.
Study objectives and hypotheses
The objective of this hybrid type III cluster-randomized controlled superiority trial is to evaluate whether a remote coaching implementation support strategy, referred to as “Enhanced Implementation Support”, is superior when compared to routine support, referred to as “Routine Implementation Support”, in facilitating the delivery of depression care in primary care facilities in rural India. This will be ascertained by measuring the proportion of outpatients screened for depression, number of cases of depression detected, and number of patients referred to the MO and initiated on treatment at the participating primary care facilities. It is hypothesized that Enhanced Implementation Support will be superior to Routine Implementation Support in increasing the proportion of outpatients screened for depression using the two-item Patient Health Questionnaire (PHQ-2) (27) by ANMs/nurses. It is also hypothesized that Enhanced Implementation Support will be superior to Routine Implementation Support in increasing the number of cases of depression detected, and number of patients referred to the MO and initiated on treatment.
Methods
Trial design
This trial will employ a two-arm hybrid type III cluster-randomized controlled superiority trial design (28). Each cluster, or “Primary Health Center (PHC)”, is the unit of randomization, with equal allocation of clusters between arms.
Trial setting
Since 2011, Sangath has worked closely with the Ministry of Health and Family Welfare, Government of Madhya Pradesh, resulting in the establishment of a Memorandum of Understanding and a significant track record in the region, which serves as the foundation for this project (14, 29). This trial will be implemented in government-run rural PHCs of Sehore district, Madhya Pradesh. Madhya Pradesh has a population over 72 million, of which nearly 73% live in rural areas (30), and is ranked among the lowest on the Human Development Index (31, 32) compared to other Indian states. Sehore district has a population of 1.31 million (33), with a total of 25 PHCs (34). Each PHC serves about 20,000–30,000 people as per the Indian Public Health Standard Guidelines. PHCs were selected as the setting for this trial given ongoing roll out of the Ayushman Bharat program, which will upgrade these facilities to “Health and Wellness Centers” aimed at serving as the national platform for delivery of comprehensive NCD care including mental health screening, diagnosis, treatment and referral (35). PHCs offer outpatient services, typically for 6 h each day with an expected caseload of 40 outpatients. Mental health services are not currently provided through PHCs, and the initiation of mental health services under the Ayushman Bharat program has not yet started in the study setting as of the trial launch (29).
Study procedures
In preparation for this trial, PHCs were identified and recruited, and facility staff were trained in the delivery of the depression care package. The PHCs were then randomized to the Enhanced Implementation Support or Routine Implementation Support strategies. This was followed by a 4-month embedding period from May-August 2022 to allow participating facilities to begin delivering depression care, and to ensure data collection procedures could be tested across both intervention and control facilities. For intervention facilities, the embedding period offered the opportunity to train staff in the Enhanced Implementation Support strategy protocol, and to allow sufficient uptake of the strategy ahead of the trial launch. The embedding period also made it possible to understand potential barriers at the facility or system-level, modify the study procedures, and make revisions as needed to the Enhanced Implementation Support remote coaching protocol. The active intervention phase will then last 12-months, followed by a 6-month period for continued data collection from the PHCs.
Facility (“cluster”) eligibility and recruitment
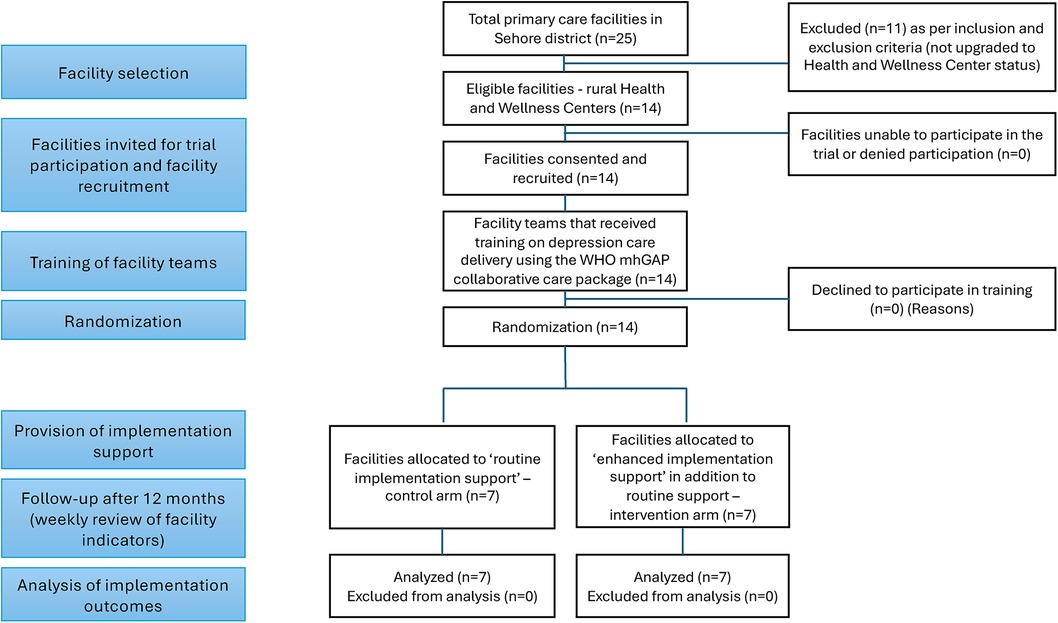
Of the total PHCs, 16 have been upgraded to “Health and Wellness Centers”, of which 14 are rural and serve as the setting for this trial. These rural PHCs also have a linked Accredited Social Health Activist (ASHA), a cadre of frontline health worker that serves as the link between the PHC and the community, and who will be available to deliver a brief psychosocial intervention for depression, called the Healthy Activity Program (HAP) (36), following previous district-wide training efforts (29, 37). Through consultation with the Ministry of Health and Family Welfare and officials from the National Health Mission, Government of Madhya Pradesh, and the District Chief Medical Health Officer (CMHO), these 14 facilities were invited and enrolled in the trial. The teams at each PHC were briefed on the study details and trained on the depression care package. The characteristics of each PHC were documented, including: average number of adult outpatient attendees per week; size of catchment area population; staff availability and turnover; number of Sub-Health Centres linked to the PHC; distance to nearest higher-level health facility for referrals; and status of the NCD programming, including format and frequency of data reporting, use of digital applications, and status/plans of integration of mental health services.
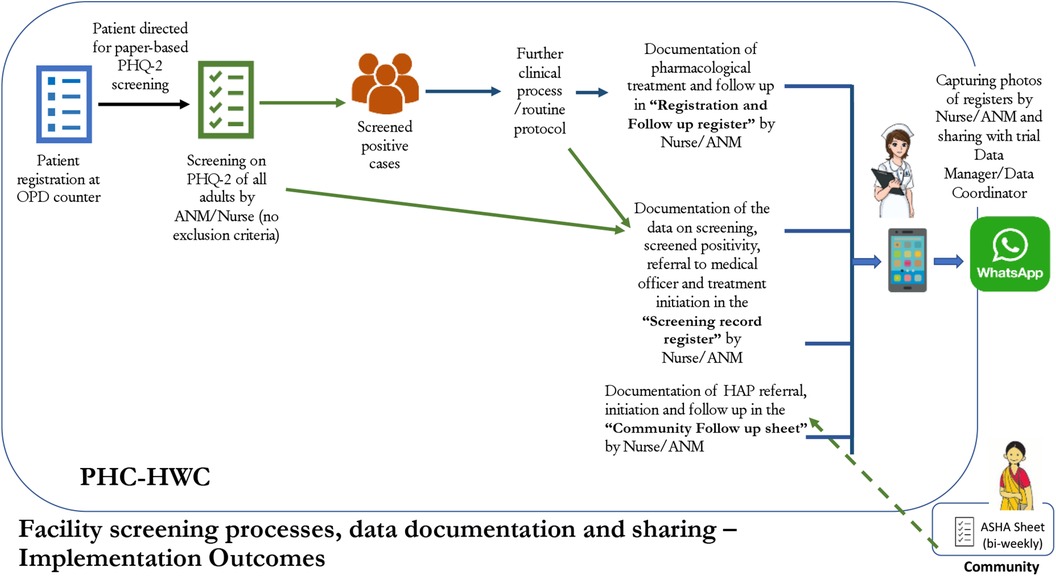
The teams at all 14 PHCs were trained on the World Health Organization's (WHO) mhGAP intervention guide (5), previously adapted for Sehore district (14). The MOs were trained through in-person and virtual modes, depending on their availability to attend in-person training, over eight days in the diagnosis, treatment and referral of positive cases of depression, including the option for referring patients to receive HAP delivered by ASHAs (29). The training for ANMs and nurses was conducted separately, as part of a 2-day in person training with instruction on screening of outpatients for depression, and referral and tracking of depression cases. Training for ANMs and nurses emphasized case detection and screening outpatients using the PHQ-2, and entering data into a “Screening Record Register” to track screening rates, refusal, patient willingness to be contacted by the research team (to participate if they screen positive), and referral to the MO and initiation of treatment. The PHQ-2 is a screening questionnaire for depression which comprises two items of the widely used and contextually validated PHQ-9. The PHQ-2 was selected to increase the quality and efficiency of screening conducted by ANMs/Nurses, and its use, instead of the full 9 item version, is supported by prior studies showing that the positive predictive value for the presence of “any depressive disorder” is 75% for a score of ≥3 (38). Further, the health system in India has planned to adopt the PHQ-2 for community-level depression screening (39). Each trial arm will have 7 PHCs, with estimated total staffing of 14 MOs (2 per facility), 14 nurses (2 per facility), and 7 ANMs (1 per facility), with some variation in staffing expected between facilities.
Participant recruitment
Adult outpatients (age ≥18 years) of any gender who screen positive for depression (PHQ-2 ≥ 3) at participating PHCs will be invited to enroll in this trial. Outpatients will be excluded if they have significant speech, hearing, language or cognitive impairment impacting their ability to provide informed consent and complete study assessments, those needing urgent medical or psychiatric attention, those not planning to stay in the study catchment area for at least three months (for participation in the clinical outcome assessment), or those who do not understand Hindi.
The ANM/nurse will be the first point of contact for determining a patient's willingness to participate in the study. After screening on the PHQ-2, if a patient is screened positive (score ≥3), they are potentially eligible to participate in the clinical care component of the trial. Prior to referral to the MO, the ANM/nurse will mention the study to the patient and ask whether they are willing to be contacted to learn more (Figure 1). If the patient agrees, the ANM/nurse will record their name, address, and contact number to share with the study team. The study data manager will retrieve the data on patient willingness to be contacted during weekly review of facility screening activities, and share this data with a study research assistant. The research assistant will call the patient to introduce the study, and determine whether the patient is interested. If the patient assents, the research assistant will meet the patient within 7 days (up to a maximum of 2 weeks) at the patient's home or a mutually agreed location (e.g., PHC) to confirm eligibility, collect informed consent, and complete baseline assessment.
Interventions: implementation support strategies
Control condition: routine implementation support
All 14 PHCs will receive Routine Implementation Support, referring to the existing implementation strategy utilized by the health system for facilitating the roll out of NCD care services (Table 1). As part of Routine Implementation Support, mental health performance indicators will be integrated into existing NCD monitoring and recorded using a standardized Screening Record Register (Table 2). These indicators will be collated, reviewed and synthesized by the facility teams and the district team as part of existing NCD indicators. The district team is composed of the CMHO, District Program Manager, and the District Community Mobilizer & Monitoring and Evaluation Officer. Each facility shares monthly NCD performance indicators with the district team via email, followed by review and discussion over WhatsApp. The district team also visits the facilities on an as needed basis, and coordinates district-level meetings.
Intervention condition: enhanced implementation support
The seven PHCs randomized to the intervention arm will receive the Enhanced Implementation Support strategy consisting of individualized coaching support, in addition to the components of Routine Implementation Support described above. There is one lead coach and two support coaches, who are members of the research team with prior knowledge and experience working with the health system. No additional coaching staff will be involved in the enhanced implementation support activities, and there will be no coaching staff from the health system. Following a train-the-trainer model, the lead coach will receive a 2-day remote training offered by a member of the investigator team with expertise in implementation science. The training will consist of didactic sessions and roleplay exercises using a decision-making flowchart to guide the technical assistance coaching sessions with the facility teams. The lead coach will then train the support coaches in a 2-day in person training covering the same content and activities. The coach trainings will take place ahead of the trial launch. Drawing from the Institute of Healthcare Improvement's (IHI's) Breakthrough Series model, which recommends that collaboratives meet for a 6–15 month period to support organizations in making “breakthrough” improvements in quality (40, 41), a 12-month intervention period was selected for this trial.
The coaching protocol is guided by the Evidence-Based System for Innovation Support (EBSIS) framework (42, 43), which identifies four critical components for successful implementation of an intervention (i.e., the mhGAP-guided depression care package): (a) adequate training (e.g., training for ANMs/nurses, MOs); (b) appropriate tools at the facility-level to assess and address implementation challenges [e.g., quality improvement enabled using “Plan Do Study Act (PDSA)” cycles]; (c) regular technical assistance and support (e.g., the enhanced implementation support coaching); and (d) quality assurance followed by improvement activities to address implementation gaps. Figure 2 outlines the components of the Enhanced Implementation Support strategy.
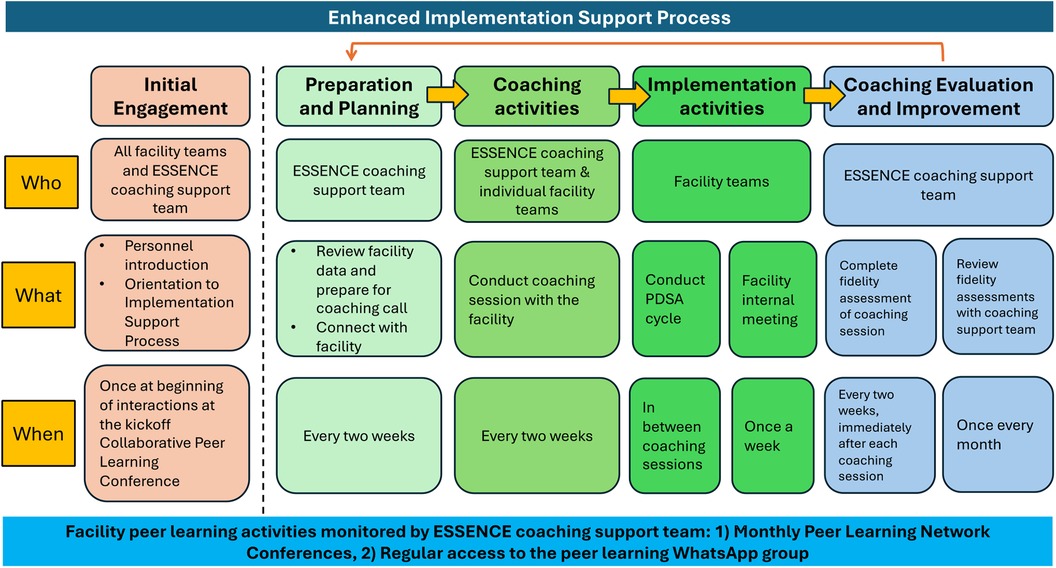
Figure 2. Overview of the enhanced implementation support coaching strategy for intervention arm facilities.
The remote technical assistance coaching sessions will be conducted every two weeks with the facility team over phone or videoconferencing platforms (i.e., Zoom). These coaching sessions will involve discussing with the facility team whether they met the performance target they set in the previous session, successes or challenges encountered since the last call, and outlining the plan for the next two weeks. In advance of each call, the coaches will review the facility-level data, which can help guide the coaching session. The calls will involve using the PDSA cycle approach, and offer an opportunity to review performance data, discuss barriers, and identify improvement targets. The coaches will moderate a WhatsApp group to allow follow up and to encourage the facility teams to share progress between coaching calls. There is also a peer learning component where the coaches will host monthly virtual meetings where all the facility teams can exchange challenges, successes, best practices, and lessons learned to collectively problem solve and guide improvement (41, 44).
While the goals of depression care will be based on current NCD program goals (e.g., 100% screening, 100% referral of screened positive cases to the MO), the performance “targets” mentioned under the coaching sessions, will be set during each call. These targets will be revised based on the PDSA cycles and implementation barriers that emerge at the level of each facility. As summarized in Table 2, indicators of successful integration of depression care will be derived from the screening record register, modeled after existing NCD care process indicators. Implementation metrics will be reviewed over the course of the trial, and the Enhanced Implementation Support strategy will intentionally be kept flexible to allow potential modifications, such as including more frequent contact with the facility teams or use of in-person facility visits, to facilitate implementation of the depression care package.
The coaches will complete self-report checklists after each coaching session to monitor fidelity to the coaching protocol. Coaching calls will be audio-recorded, and two members of the research team will listen to a random selection of audio recorded sessions and complete the same checklist to assess fidelity. Rated checklists will be discussed during weekly coach supervision to assess adherence to the coaching protocol and strategies to address implementation barriers. Process data for the coaching sessions will be tracked for each facility, including number of coaching sessions delivered, number and type of facility team members participating in the coaching sessions, number of action plans and PDSA worksheets prepared for each PHC, and time duration of each coaching session.
Outcome measures
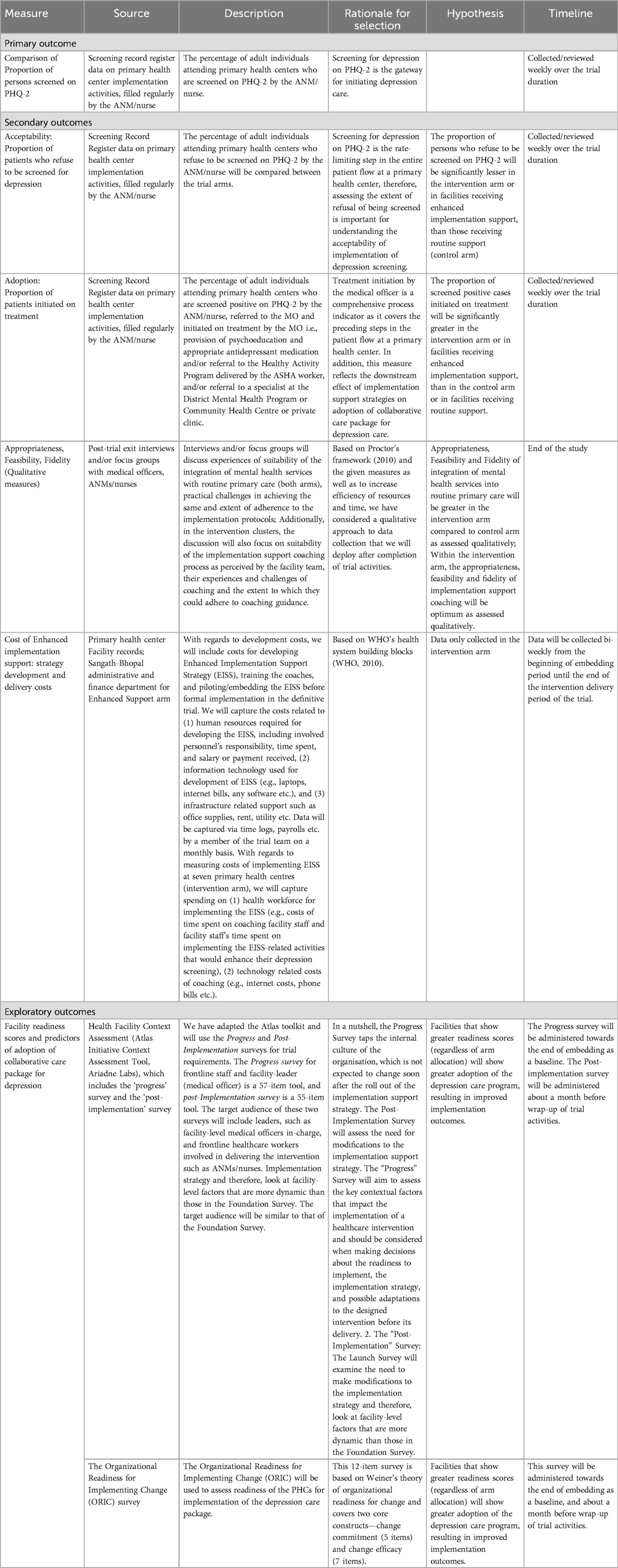
Primary implementation outcome
Table 3 lists the study implementation outcomes. For the primary implementation outcome, the proportions of outpatients screened on the PHQ-2 by the ANM/nurse will be compared between study arms. The denominator for the proportion of outpatients screened will be calculated as the total number of adult outpatients for the NCD care program attending the PHCs during the trial period. ANMs/nurses will use a Screening Record Register to document the number of patients screened. The facilities will send the screening data to the data manager on a weekly basis.
Secondary implementation outcomes
As outlined in Table 3, secondary implementation outcomes will be guided by the heuristic defined by Proctor et al, 2010 (19), and will be assessed using facility-level administrative data and the indicators captured in the screening record register (Table 2), and supplemented with qualitative semi-structured interviews with the facility teams. Implementation metrics will include number of patients who refuse screening, number of patients who screen positive for depression, number of screen positive patients who are referred to the MO and number of patients who are initiated on treatment. Treatment initiation following referral to the MO is an important adoption outcome, and can involve provision of psychoeducation, prescription of antidepressant medication, referral to the brief psychosocial intervention (i.e., HAP) delivered by ASHAs, and/or referral to a specialist at the District Mental Health Program, Community Health Centre or private clinic.
The costs of delivering the Enhanced Implementation Support strategy in the intervention arm PHCs will be collected and categorized into the six building blocks of a health system based on the WHO's health system framework (45). This will include spending on health workforce for participating in the Enhanced Implementation Support strategy activities, such as time required to moderate (for the coaches) and participate (for the ANM/nurse) in the coaching sessions; and information technology, such as internet costs or phone bills for engaging in the coaching sessions. This data will be captured via time logs, payroll, and expense reports. Facility staff salaries will be collected from the National Health Mission reports on salary range for various health personnel in public health facilities. To clarify, the cost analysis will not evaluate the cost-effectiveness or involve a comparison of costs between the study arms because we are not able to collect cost data from the control arm given the irregular or need-based format of routine district team's support to the clinics for delivering depression care (refer, “Routine implementation support”), and because there is no additional implementation support being provided by our team for which the costs could be collected. We will be assessing only the cost of delivery of Enhanced Implementation Support strategy among the intervention arm clinics.
Qualitative and observational data collection
At the end of the trial, qualitative interviews with facility teams, including ANMs/nurses and MOs, will be used to assess acceptability and feasibility of implementing the mhGAP depression care package across facilities in both arms. These interviews will focus on understanding experiences delivering depression care in both the intervention and control facilities, and to determine whether there may have been differing experiences in facilities receiving the Enhanced Implementation Support relative to control facilities receiving the Routine Implementation Support. The research team will also visit the facilities in-person to further collect observational and qualitative data about facility-level characteristics that may affect implementation of depression care, such as: facility staffing characteristics including workload, leaves, transfers and new appointments; the extent of dedicated physical space and the privacy of this space for conducting depression screening in the clinic; facility staff perceptions about asking questions included in depression screening, views about potential stigma, and about the materials posted in the clinics such as informational posters; MO attendance and their involvement in depression care activities; and screening rates of comparable NCD care programming. These visits will be coordinated in advance with the facility teams.
Secondary patient outcomes
Table 4 lists patient outcomes that will be assessed at 3 months after enrolment. The proportion of enrolled patients with scores <5 on the nine-item Patient Health Questionnaire (PHQ-9), indicating remission (46), and functional outcomes using the WHO Disability Assessment Schedule (WHODAS 2.0) (47), and symptoms of anxiety using the Generalized Anxiety Disorder scale (GAD-7) (48), will be collected. A 2-week window for collection of follow up assessments from patients will be used to accommodate scheduling and logistics.
Exploratory outcomes
The Organizational Readiness for Implementing Change (ORIC) (49) will be used to assess readiness of the PHCs for implementing the depression care package. This 12-item survey is based on Weiner's theory of organizational readiness for change and covers two core constructs—change commitment (5 items) and change efficacy (7 items). The Atlas Initiative Toolkit will be used to assess facility-specific implementation factors (50). The toolkit includes two surveys, beginning with the Progress Survey followed by the Post-Implementation Survey. The surveys are based on an organizational readiness heuristic, abbreviated as R = MC2 (51), which defines organizational readiness for an innovation (R) as a function of three components: motivation to implement an innovation (M), the general capacities of an organization (C), and the innovation-specific capacities needed for a particular innovation (C). The surveys were translated and adapted for use in Madhya Pradesh, India, and will be collected from the MOs and ANMs/nurses involved in delivering depression care. The Progress Survey will be collected before trial launch to assess contextual factors that may impact the readiness to implement depression care. This survey captures the internal culture of the facility, which is not expected to change soon after initiating the Enhanced Implementation Support strategy. The Post-Implementation Survey will be collected from the same facility staff about one month before the end of the 12-month active intervention phase to assess the need for modifications to the implementation strategy.
Sample size estimation
Based on prior outpatient footfall data from participating PHCs, it is estimated that roughly 178 adult outpatients will attend each PHC per month; thus, over a 12-month period of delivery of the Enhanced Implementation Support strategy, there will be approximately 14,994 patients attending facilities in each arm. Assuming about 10% of these patients [based on prior case detection data in the region (14)] will refuse screening or will be excluded at the discretion of the ANM/nurse, the resulting estimates work out to about 13,494 outpatients available for screening in each arm. This sample size will allow us to detect a 15% difference in the proportion of PHQ-2 screenings between arms, assuming that 10% of patients are screened in the Routine Implementation Support Arm, at 80% power, an inter-cluster coefficient of variation of 0.5 (calculated from background facility data on adult outpatient footfall and existing NCD screening rates) and an intra-cluster correlation (ICC) of 0.05 (52).
Proportions (and not numbers of screenings) will be used to account for the between-facility variation in outpatient footfall.
The earlier PRIME study conducted in the same region achieved a 12% depression screening rate in its facility detection survey component (14), and assuming an additional contribution by the various components of enhanced implementation support (i.e., fortnightly coaching calls, WhatsApp support and monthly peer-learning conferences) to further increase the depression screening rate, we will hypothesize a 25% screening rate in the intervention arm, or a 15% between-arm difference in screening rates. We have referred the formula for calculating sample size for parallel cluster randomized controlled trials with fixed number of clusters (n = 14 in this study) by Hemming et al. 2020 (52).
Facility randomization
As outlined in the CONSORT diagram in Figure 3, PHCs will be randomized to the “Enhanced Implementation Support” intervention or “Routine Implementation Support” control condition using 1:1 random allocation. Stata statistical software will be used to prepare the allocation table, to assign and monitor the allocation of PHCs. Prior studies have shown that facility size may affect implementation of mental health services, as large facilities may have more capacity for flexibly utilising resources during implementation of a new evidence-based practice compared to small facilities (53). Therefore, facilities will be stratified by number of monthly outpatient attendees, as this variable can serve as a proxy for facility size, and furthermore, busier clinics may face distinct implementation challenges compared to quieter clinics (e.g., a private location for screening). Drawing from facility characteristics, and based on probability proportional to facility size sampling, the expected contributions (percentages) by each facility per arm per month was calculated. These details will be used to define the strata as either “high” or “low” patient footfall facilities, to ensure balance in facility size between arms.
Blinding
It will not be possible to blind the PHC staff to arm allocation, and the research team will also not be blind to arm allocation. For patients who enrol, they will not be informed about the allocation of their respective facility, and it is unlikely that they would become aware of arm allocation. Study outcome assessors collecting outcomes from patients, and the statistician who will analyze the final outcome data will be blinded to arm allocation. There will be complete separation between the team members involved in delivering the enhanced implementation support intervention, and those involved in administering the outcome assessments; for example, the implementation support team will be based in Sangath Bhopal office and outcome assessors will work from the district office (rural), closer to the study population.
Statistical analysis
All analyses of implementation outcomes will be intention-to-treat comparisons between arms. Generalized linear regression models with a log link will be used to compare facility-level implementation outcomes, including the primary outcome of proportion of patients screened on the PHQ-2. ANCOVA analyses will be conducted for comparing secondary implementation outcomes between arms. Time by study arm interactions will be explored using a growth curve model to inspect non-linear trends in the progress of the screening, number of patients who screen positive, referral to MO and treatment initiation. Descriptive statistics will be used for comparing exploratory measures of facility readiness and context assessment between arms. The process indicators from the PDSA coaching sessions will be used for descriptive analysis, to quantitatively assess fidelity to the protocol for Enhanced Implementation Support. Logistic regression models will be used to compare the proportion of patients who remit on the PHQ-9, defined as score <5, between arms at 3-month follow-up. Possible covariates will be entered into the models, including age or gender of the patients, as well as adjusting for cluster effects. Linear regression models will be used to assess differences in the change in disability (WHODAS 2.0) and anxiety (GAD-7) outcomes at 3-month follow up, with baseline values as covariates to adjust for any baseline inter-cluster differences. Stata version 17.0 will be used for all statistical analyses and p-values <0.5 will be considered statistically significant.
We will use thematic analysis with a mix of deductive and inductive approaches for coding the transcribed data from qualitative interviews (e.g., post-trial clinic staff interviews) and generating the themes [Braun & Clarke, 2006 (54)]. Thematic analysis will flexibly allow us to include both a priori or pre-existing themes from the interview guide, as well as “inductive” themes that will emerge during the analysis. An independent researcher (RS) will develop the initial codes reflecting important areas that we will aim to explore, before reviewing the transcripts and further developing the codes. After independently coding the transcripts, the researcher will refine the codes using inductive/emerging themes, and after consultation with the wider team of academic researchers with expertise in qualitative methods (JN, VP, AB, RR, APB), subsequent iterations will be made to the coding structure i.e., by adding new codes, deleting redundant codes, and integrating the overlapping codes. We will organize consensus meetings to resolve disagreements, such as on the classification of themes.
Ethical considerations
Institutional Review Boards (IRB) at Harvard Medical School, United States and Sangath, India have approved all study procedures. Additional approval was obtained from the Government of India's Health Ministry Screening Committee, housed at the Indian Council of Medical Research. Written informed consent will be mandatory for enrolling patients in this trial. Participation in the study is completely voluntary and patients can decline participation or withdraw at any time without any consequence to their care at the PHCs. The confidentiality of participants will be protected using unique study ID numbers, and by separating study data from any identifiable data. An independent Data and Safety Monitoring Board (DSMB) will examine accumulating data to assure protection of participants’ safety and data integrity throughout the trial. The research team will submit regular progress reports, including serious adverse event (SAE) reporting, CONSORT flow charts, and baseline characteristics of enrolled participants across study arms to the DSMB. The DSMB will meet before the start of the trial, at the trial mid-point, and at the end of the study to review the statistical analysis plan. The team will maintain a Regulatory Binder containing all required regulatory documents that will be available at any time for study audit. Regulatory files will be checked at the research site for compliance prior to initiation of the trial, throughout the trial, and at trial closure. Study investigators will verify that study procedures are followed and that study staff are trained and able to conduct the protocol appropriately.
From the perspective of collecting mental health data in a rural setting, all participants’ baseline and endpoint assessment and consent records will be kept in password-protected computers/servers. All physical copies of documents will be kept in locked cabinets located inside the Sangath office. All data containing personal identifiers of participants will be delinked by removing all direct identifiers and assignment of a unique ID, combining all main indirect identifiers. We will train and conduct periodic refresher trainings of outcome assessors to manage situations of emotional distress that the participants may experience during the assessments and we will put in place, referral pathways to report distress such as to the medical officer in the primary health centre and the psychiatrist at the district hospital (note that the enhanced implementation support intervention is delivered to the facility teams and not the patients). This will also include management of reported instances of severe adverse events such as reported suicidal ideations/attempts, which the research team will come to know at the patient's three-month follow-up assessment. A study psychiatrist based at Sangath or a tertiary hospital in Bhopal will contact the participant within 24 h of receiving the SAE report, facilitated by the study team if required, to arrange a convenient time and place to complete a detailed assessment either by phone or face-to-face within 7 days, to assess relatedness to trial procedures, and offer any necessary intervention. Finally, we will make sure that outcome assessors receive refresher trainings on administrating informed consent in the rural setting, especially for situations such as reaching out to appropriate legally appointed representatives (family members) in cases of illiterate patients, to avoid sensitive situations where depression screen positivity is potentially disclosed to others in the participant's neighbouring community.
Trial management
The Senior Management Team, consisting of the principal investigators, site-principal investigator, training program leads, outcome evaluators, and data manager, will provide overall trial leadership and will meet weekly to review trial progress, participant recruitment, data collection, process indicators, and any safety concerns or other issues that may arise.
Discussion
Given the plans to integrate mental health care under the national rollout of the Ayushman Bharat program (29), the knowledge generated from this trial could potentially be advantageous for health systems to guide the implementation of the integration of evidence-based intervention for depression in primary care settings in rural India. The Enhanced Implementation Support strategy relies on widely available human resources and scalable strategies, and draws from existing implementation science studies employing “facilitators”, like the remote implementation support coaches delivering technical assistance to the facilities guided by the model for implementation (49, 55–62).
Limitations
This study has several limitations that should be considered. First, the intervention requires an external remote coaching team to support the facilities in delivering depression care, however, if scaled up, the health system may have staffing or resource challenges to appoint these cadres. This poses significant limitations to the potential for sustainability of the implementation support strategy that will be tested in this trial. Additionally, the remote coaching approach may face challenges due to poor connectivity or low bandwidth, as well as low digital literacy among facility staff. The insufficient support to primary care personnel under the existing district mental health program to deliver depression care (16, 17) is a case in point. Collaborative care models such as the program being rolled out as part of the ESSENCE project should therefore be complemented by strong community outreach to enable communities to seek mental health care and use the clinic-based care models (to enhance case-detection, treatment and follow-up rates), as also recommended by findings of earlier studies in the region such as PRIME (14). To do so, more participatory approaches to involve community members in developing an outreach model is essential, particularly after accounting for their cultural attitudes towards mental health. This will increase community engagement in the delivery of depression care at the clinic-level, which can potentially reduce the intensity of the required external implementation support or additional human resources appointed by the health system, and contribute to a sustained use of remote implementation support. In such a scenario, the external support maybe required for a lesser frequency than in this trial (e.g., monthly coaching calls instead of fortnightly).
Second, while prior formative work revealed an emerging use of technology by primary care personnel in the rural study context (e.g., WhatsApp for routine data reporting by clinic teams to the district team), we anticipate a number of implementation factors that may challenge the delivery of depression care in the clinics, such as staff transfers and re-appointments requiring fresh training, and substantial workload of various existing programs on the nurses and auxiliary nurse midwives that may reduce their engagement in delivering depression care. We want to highlight that since this is an implementation trial rolled out in a rural primary care setting in collaboration with the state government, we will have several opportunities to document these challenges as they influence the implementation outcomes such as depression screening, case-detection and treatment initiation (data on these will be collected on a periodic basis throughout the trial). However, it is important to note the potential limits to generalizability, given that this study will only be conducted in one district, which may not be representative of other low-resource settings in rural India or in other contexts globally. This further emphasizes the need to document any implementation challenges encountered over the course of this trial as a means to better understand the context and whether these insights also inform implementation of depression care in other settings globally. The use of post-trial exit interviews in facilities of both arms will help address these limits to generalizability by allowing us to examine the appropriateness, fidelity and feasibility of depression care delivery given the aforesaid implementation challenges.
Third, as the trial has a hybrid type-III design (28), there is less emphasis on patient outcomes compared to implementation outcomes and we will have limited scope within the trial design and timelines to collect data on long-term mental health outcomes or patient quality of life (though we are gathering disability data of the participants via WHODAS 2.0). Fourth, the role of ANMs/nurses in determining eligibility and willingness of the screened positive to participate in the trial may introduce a selection bias. We had considered the approach of integrating baseline and endpoint assessments of depression (through PHQ-9) in routine primary care activities at the facility, so that all screened positive patients across clinics of both arms can be evaluated for depression outcomes, which can also enhance between-arm comparability of patients. However, this approach involved challenges such as workloads on nurses, auxiliary nurse midwives and doctors to perform additional depression-related assessments in the clinic. We had also considered the alternative of community-based screening of depression via frontline health workers, such as phase-1 PHQ-2 screening at the village-level (by the ASHA) followed by phase-2 PHQ-9 screening at the clinic-level, but there were similar issues of substantial workload on frontline workers. Therefore, we acknowledge the limitation of a potential selection bias in relation to the assessment of our secondary patient outcomes.
Trial status
After the embedding phase, the trial launched on October 6th, 2022. The 12-month delivery of the Enhanced Implementation Support was completed on October 5th, 2023. The trial closeout was completed on April 24th, 2024, with primary data analyses to follow.
Ethics statement
The studies involving humans were approved by Harvard Medical School, United States, and Sangath, India. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
ABo: Conceptualization, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing, Formal Analysis. AS: Data curation, Methodology, Writing – review & editing. DT: Project administration, Supervision, Writing – review & editing. DC: Data curation, Methodology, Writing – review & editing. AK: Investigation, Methodology, Supervision, Writing – review & editing. RS: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. CL: Formal Analysis, Methodology, Writing – review & editing. RR: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Writing – review & editing. VP: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Writing – review & editing. ABh: Investigation, Project administration, Supervision, Writing – review & editing. JN: Conceptualization, Formal Analysis, Investigation, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Institute of Mental Health, United States, Grant number: 1U19MH113211; the funder played no role in the study design, collection, analysis and interpretation of data, writing of the report, and the decision to submit the manuscript for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vigo D, Thornicroft G, Atun R. Estimating the true global burden of mental illness. Lancet Psychiatry. (2016) 3(2):171–78. doi: 10.1016/S2215-0366(15)00505-2
2. Patel V, Saxena S, Lund C, Thornicroft G, Baingana F, Bolton P, et al. The lancet commission on global mental health and sustainable development. Lancet. (2018) 392(10157):1553–98. doi: 10.1016/S0140-6736(18)31612-X
3. Sagar R, Dandona R, Gururaj G, Dhaliwal RS, Singh A, Ferrari A, et al. The burden of mental disorders across the states of India: the global burden of disease study 1990–2017. Lancet Psychiatry. (2020) 7(2):148–61. doi: 10.1016/S2215-0366(19)30475-4
4. Manoj MT, Joseph KA, Vijayaraghavan G. Association of depression, anxiety, and stress with myocardial infarction: a case–control study. J Clin Prev Cardiol. (2018) 7(3):86–92. doi: 10.4103/JCPC.JCPC_39_17
5. World Health Organization. mhGAP Intervention Guide for Mental, Neurological and Substance use Disorders in non-Specialized Health Settings: Mental Health Gap Action Programme (mhGAP). Geneva: World Health Organization (2016).
6. Pathare S, Brazinova A, Levav I. Care gap: a comprehensive measure to quantify unmet needs in mental health. Epidemiol Psychiatr Sci. (2018) 27(5):463–67. doi: 10.1017/S2045796018000100
7. Sagar R, Pattanayak RD, Chandrasekaran R, Chaudhury PK, Deswal BS, Singh RL, et al. Twelve-month prevalence and treatment gap for common mental disorders: findings from a large-scale epidemiological survey in India. Indian J Psychiatry. (2017) 59(1):46–55. doi: 10.4103/psychiatry.IndianJPsychiatry_333_16
8. Arvind BA, Gururaj G, Loganathan S, Amudhan S, Varghese M, Benegal V, et al. Prevalence and socioeconomic impact of depressive disorders in India: multisite population-based cross-sectional study. BMJ open. (2019) 9(6):e027250. doi: 10.1136/bmjopen-2018-027250
9. Gautham MS, Gururaj G, Varghese M, Benegal V, Rao GN, Kokane A, et al. The national mental health survey of India (2016): prevalence, socio-demographic correlates and treatment gap of mental morbidity. Int. J. Soc. Psychiatry. (2020) 66(4):361–72. doi: 10.1177/0020764020907941
10. Kokane A, Pakhare A, Gururaj G, Varghese M, Benegal V, Rao GN, et al. Mental health issues in Madhya Pradesh: insights from national mental health survey of India 2016. Healthcare. (2019) 7(2):53. doi: 10.3390/healthcare7020053
11. World Health Organization, & Research for International Tobacco Control. WHO report on the Global Tobacco Epidemic, 2008: The MPOWER Package. Geneva: World Health Organization (2008).
12. Patel V, Araya R, Chatterjee S, Chisholm D, Cohen A, De Silva M, et al. Treatment and prevention of mental disorders in low-income and middle-income countries. Lancet. (2007) 370(9591):991–1005. doi: 10.1016/S0140-6736(07)61240-9
13. Rathod S, Pinninti N, Irfan M, Gorczynski P, Rathod P, Gega L, et al. Mental health service provision in low-and middle-income countries. Health Serv Insights. (2017) 10:1–7. doi: 10.1177/1178632917694350
14. Shidhaye R, Baron E, Murhar V, Rathod S, Khan A, Singh A, et al. Community, facility and individual level impact of integrating mental health screening and treatment into the primary healthcare system in Sehore district, Madhya Pradesh, India. BMJ Glob Heal. (2019) 4(3):e001344. doi: 10.1136/bmjgh-2018-001344
15. Breuer E, De Silva M, Shidaye R, Petersen I, Nakku J, Jordans MJ, et al. Planning and evaluating mental health services in low- and middle-income countries using theory of change. Br J Psychiatry. (2016) 208:s55–62. doi: 10.1192/bjp.bp.114.153841
16. Roy S, Rasheed N. The national mental health programme of India. Int J Curr Med Appl Sci. (2015) 7(1):7–15.
17. Goel DS. Why mental health services in low- and middle-income countries are under-resourced, underperforming: an Indian perspective. Natl Med J India. (2011) 24(2):94–7.21668055
18. Patel V, Weiss HA, Chowdhary N, Naik S, Pednekar S, Chatterjee S, et al. Effectiveness of an intervention led by lay health counsellors for depressive and anxiety disorders in primary care in Goa, India (MANAS): a cluster randomised controlled trial. Lancet. (2010) 376(9758):2086–95. doi: 10.1016/S0140-6736(10)61508-5
19. Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. (2011) 38:65–76. doi: 10.1007/s10488-010-0319-7
20. National Institutes of Health. Dissemination and Implementation Research in Health. Bethesda, Maryland: National Institutes of Health (2010). Available online at: http://grants.nih.gov/grants/guide/pa-files/PAR-10-038.html (Accessed March, 7 2024)
21. Institute of Medicine (US). Committee on Comparative Effectiveness Research Prioritization. Initial National Priorities for Comparative Effectiveness Research. Washington DC: National Academies Press (2009).
22. Wagenaar BH, Hammett WH, Jackson C, Atkins DL, Belus JM, Kemp CG. Implementation outcomes and strategies for depression interventions in low-and middle-income countries: a systematic review. Glob Ment Health. (2020) 7:e7. doi: 10.1017/gmh.2020.1
23. Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implementation Sci. (2013) 8(1):1–1. doi: 10.1186/1748-5908-8-139
24. Powell BJ, Garcia K, Fernandez ME. Implementation strategies. In: Chambers D, Vinson C, Norton W, editors. Advancing the Science of Implementation Across the Cancer Continuum. New York: Oxford University Press (2019). p. 98–122.
25. Buttorff C, Hock R, Weiss H, Naik S, Araya R, Kirkwood B, et al. Economic evaluation of a task-shifting intervention for common mental disorders in India. Bull World Health Organ. (2012) 90:813–21. doi: 10.2471/BLT.12.104133
26. National Health Mission. Ayushman Bharat. New Delhi: National Health Mission (2018). Available online at: https://www.nhm.gov.in/New_Updates_2018/NHM_Components/Health_System_Stregthening/Comprehensive_primary_health_care/letter/Operational_Guidelines_For_CPHC.pdf (Accessed March, 7 2024)
27. Levis B, Sun Y, He C, Wu Y, Krishnan A, Bhandari PM, et al. Accuracy of the PHQ-2 alone and in combination with the PHQ-9 for screening to detect major depression: systematic review and meta-analysis. JAMA. (2020) 323(22):2290–300. doi: 10.1001/jama.2020.6504
28. Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. (2012) 50(3):217–26. doi: 10.1097/MLR.0b013e3182408812
29. Naslund JA, Tugnawat D, Anand A, Cooper Z, Dimidjian S, Fairburn CG, et al. Digital training for non-specialist health workers to deliver a brief psychological treatment for depression in India: protocol for a three-arm randomized controlled trial. Contemp Clin Trials. (2021) 102:106267. doi: 10.1016/j.cct.2021.106267
30. De Costa A, Diwan V. ‘Where is the public health sector?’: public and private sector healthcare provision in madhya pradesh. India. Health pol. (2007) 84(2-3):269–76. doi: 10.1016/j.healthpol.2007.04.004
31. Suryanarayana MH, Agrawal A, Prabhu KS. Inequality-adjusted human development index: states in India. Indian J Hum Dev. (2016) 10(2):157–75. doi: 10.1177/0973703016675793
32. Menon P, Deolalikar AB, Bhaskar A. India State Hunger index: Comparisons of Hunger Across States. International Food Policy Research Institute (2009).
33. District Sehore. Washington, DC: International Food Policy Research Institute. Available online at: https://sehore.nic.in/en/demography/ (Accessed March, 7 2024)
34. Rural Health Statistics 2020–2021. Available online at: https://main.mohfw.gov.in/sites/default/files/rhs20-21_1.pdf (Accessed March, 7 2024).
35. National Health Policy 2017. Ministry of Health and Family Welfare. New Delhi: Ministry of Health and Family Welfare (2017). Available online at: https://main.mohfw.gov.in/sites/default/files/9147562941489753121.pdf (Accessed March, 7 2024).
36. Patel V, Weobong B, Weiss HA, Anand A, Bhat B, Katti B, et al. The healthy activity program (HAP), a lay counsellor-delivered brief psychological treatment for severe depression, in primary care in India: a randomised controlled trial. Lancet. (2017) 389(10065):176–85. doi: 10.1016/S0140-6736(16)31589-6
37. Tugnawat D, Singh A, Anand A, Bondre A, Chandke D, Dhurve P, et al. ESSENCE: an implementation research program to scale up depression care in rural communities. Psychiatr Serv. (2024) 75(2):167–77. doi: 10.1176/appi.ps.202100223
38. Kroenke K, Spitzer RL, Williams JB. The patient health questionnaire-2: validity of a two-item depression screener. Med Care. (2003) 41:1284–92. doi: 10.1097/01.MLR.0000093487.78664.3C
39. Ransing R, Kukreti P, Raghuveer P, Mahadevaiah M, Puri M, Pemde H, et al. Development of a brief psychological intervention for perinatal depression (BIND-P). Asia Pac Psychiatry. (2021) 13(1):e12436. doi: 10.1111/appy.12436
40. McDermott R, Tulip F, Schmidt B, Sinha A. Sustaining better diabetes care in remote indigenous Australian communities. Br Med J. (2003) 327(7412):428–30. doi: 10.1136/bmj.327.7412.428
41. Schouten LM, Hulscher ME, Van Everdingen JJ, Huijsman R, Grol RP. Evidence for the impact of quality improvement collaboratives: systematic review. BMJ. (2008) 336(7659):1491–4. doi: 10.1136/bmj.39570.749884.BE
42. Wandersman A, Chien VH, Katz J. Toward an evidence-based system for innovation support for implementing innovations with quality: tools, training, technical assistance, and quality assurance/quality improvement. Am J Community Psychol. (2012) 50(3-4):445–59. doi: 10.1007/s10464-012-9509-7
43. Leeman J, Calancie L, Hartman MA, Escoffery CT, Herrmann AK, Tague LE, et al. What strategies are used to build practitioners’ capacity to implement community-based interventions and are they effective? A Systematic Review. Implementation Sci. (2015) 10(1):80. doi: 10.1186/s13012-015-0272-7
44. Aschbrenner KA, Pratt I S, Bond GR, Zubkoff L, Naslund JA, Jue K, et al. A virtual learning collaborative to implement health promotion in routine mental health settings: protocol for a cluster randomized trial. Contemp Clin Trials. (2019) 84:105816. doi: 10.1016/j.cct.2019.105816
45. Indicators AH. Monitoring the Building Blocks of Health Systems. Geneva, Switzerland: WHO Document Production Services (2010).
46. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
47. Üstün TB. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule WHODAS 2.0. Geneva: World Health Organization (2010).
48. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. (2006) 166(10):1092–7. doi: 10.1001/archinte.166.10.1092
49. Shea CM, Jacobs SR, Esserman DA, Bruce K, Weiner BJ. Organizational readiness for implementing change: a psychometric assessment of a new measure. Implementation Sci. (2014) 9(1):1–5. doi: 10.1186/1748-5908-9-7
50. Ariadne Labs. Atlas Initiative. Boston: Ariadne Labs. Available online at: https://www.ariadnelabs.org/areas-of-work/atlas-initiative/ (Accessed March 7, 2024)
51. Scaccia JP, Cook BS, Lamont A, Wandersman A, Castellow J, Katz J, et al. A practical implementation science heuristic for organizational readiness: R=MC2. J. Community Psychol. (2015) 43(4):484–501. doi: 10.1002/jcop.21698
52. Hemming K, Kasza J, Hooper R, Forbes A, Taljaard M. A tutorial on sample size calculation for multiple-period cluster randomized parallel, cross-over and stepped-wedge trials using the shiny CRT calculator. Int J Epidemiol. (2020) 49(3):979–95. doi: 10.1093/ije/dyz237
53. Regan J, Lau AS, Barnett M, Stadnick N, Hamilton A, Pesanti K, et al. Agency responses to a system-driven implementation of multiple evidence-based practices in children’s mental health services. BMC Health Serv Res. (2017) 17(1):1–4. doi: 10.1186/s12913-017-2613-5
54. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. (2006) 3(2):77–101. doi: 10.1191/1478088706qp063oa
55. Manyazewal T, Mekonnen A, Demelew T, Mengestu S, Abdu Y, Mammo D, et al. Improving immunization capacity in Ethiopia through continuous quality improvement interventions: a prospective quasi-experimental study. Infect Dis Poverty. (2018) 7(06):35–48. doi: 10.1186/s40249-018-0502-8
56. Wagenaar BH, Hirschhorn LR, Henley C, Gremu A, Sindano N, Chilengi R. Data-driven quality improvement in low-and middle-income country health systems: lessons from seven years of implementation experience across Mozambique, Rwanda, and Zambia. BMC Health Serv. Res. (2017) 17:65–75. doi: 10.1186/s12913-017-2661-x
57. Weiner BJ, Rohweder CL, Scott JE, Teal R, Slade A, Deal AM, et al. Using practice facilitation to increase rates of colorectal cancer screening in community health centers, North Carolina, 2012–2013: feasibility, facilitators, and barriers. Prev Chronic Dis. (2017) 14:E66. doi: 10.5888/pcd14.160454
58. Noël PH, Romero RL, Robertson M, Parchman ML. Key activities used by community based primary care practices to improve the quality of diabetes care in response to practice facilitation. Qual Prim Care. (2014) 22(4):211–19.
59. Margolis PA, Lannon CM, Stuart JM, Fried BJ, Keyes-Elstein L, Moore DE. Practice based education to improve delivery systems for prevention in primary care: randomised trial. BMJ. (2004) 328(7436):388. doi: 10.1136/bmj.38009.706319.47
60. Meropol SB, Schiltz NK, Sattar A, Stange KC, Nevar AH, Davey C, et al. Practice-tailored facilitation to improve pediatric preventive care delivery: a randomized trial. Pediatrics. (2014) 133(6):e1664–75. doi: 10.1542/peds.2013-1578
61. Miguel Esponda G, Hartman S, Qureshi O, Sadler E, Cohen A, Kakuma R. Barriers and facilitators for the implementation of mental health programmes in primary care in low-and middle-income countries: a systematic review. Lancet Psychiatry. (2019) 7(1):78–92. doi: 10.1016/S2215-0366(19)30125-7
Keywords: primary care, rural, depression, mental health, implementation, hybrid trial, implementation support strategy
Citation: Bondre AP, Singh A, Tugnawat D, Chandke D, Khan A, Shrivastava R, Lu C, Ramaswamy R, Patel V, Bhan A and Naslund JA (2024) Remote coaching for supporting the implementation of treatment for depression in primary care in Madhya Pradesh, India: protocol for a cluster randomized controlled trial. Front. Health Serv. 4:1477444. doi: 10.3389/frhs.2024.1477444
Received: 7 August 2024; Accepted: 9 September 2024;
Published: 24 September 2024.
Edited by:
Snehil Kumar Singh, UNICEF, MalawiReviewed by:
Dhananjay Srivastava, Consultant, Delhi, IndiaKrupal Joshi, All India Institute of Medical Sciences, India
Beverly Laher, Kamuzu University of Health Sciences, Malawi
Copyright: © 2024 Bondre, Singh, Tugnawat, Chandke, Khan, Shrivastava, Lu, Ramaswamy, Patel, Bhan and Naslund. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ameya P. Bondre, YW1leWEuYm9uZHJlQHNhbmdhdGguaW4=; John A. Naslund, Sm9obl9OYXNsdW5kQGhtcy5oYXJ2YXJkLmVkdQ==
 Ameya P. Bondre
Ameya P. Bondre Abhishek Singh1
Abhishek Singh1 Vikram Patel
Vikram Patel Anant Bhan
Anant Bhan John A. Naslund
John A. Naslund