- 1School of Pharmacy, Henan University, Kaifeng, China
- 2Department of Pharmacy, Zhengzhou Shuqing Medical College, Zhengzhou, China
Background: Pharmaceutical management is a new frontier subject between pharmacy, law and management, and related research involves the whole process of drug development, production, circulation and use. With the development of medical systems and the diversification of patients’ drug needs, research in the field of pharmaceutical management is becoming increasingly abundant. To clarify the development status of this field, this study conducted a bibliometric analysis of relevant literature in the field based on the knowledge graph method for the first time and explored the evolutionary trends of research hotspots and frontiers.
Methods: Literature was obtained from the Web of Science Core Collection database. CiteSpace 6.2.R4 (Advanced), VOSViewer, Scimago Graphica, Pajek and the R programming language were used to visualize the data.
Results: A total of 12,771 publications were included in the study. The publications in the field of pharmaceutical management show an overall increasing trend. In terms of discipline evolution, early research topics tended to involve the positioning of pharmacists and pharmaceutical care and the establishment of a management system. From 2000 to 2005, this period tended to focus on clinical pharmacy and institutional norms. With the development of globalization and the market economy, research from 2005 to 2010 began to trend to the fields of drug markets and economics. From 2010 to 2015, research was gradually integrated into health systems and medical services. With the development of information technology, after 2015, research in the field of pharmaceutical management also began to develop in the direction of digitalization and intelligence. In light of the global pandemic of COVID-19, research topics such as drug supply management, pharmaceutical care and telemedicine services under major public health events have shown increased interest since 2020.
Conclusion: Based on the knowledge mapping approach, this study provides a knowledge landscape in the field of pharmaceutical management research. The results showed that the reform of pharmacy education, the challenge of drug management under the COVID-19 pandemic, digital transformation and the rise of telemedicine services were the hot topics in this field. In addition, the research frontier also shows the broad prospects of the integration of information technology and pharmaceutical management, the practical value of precision pharmaceutical services, the urgent need of global drug governance, and the ethical and legal issues involved in the application of artificial intelligence technology in drug design, which points out the direction for the future development of pharmaceutical practice.
1 Introduction
In the era of information explosion, the accumulation and dissemination of scientific knowledge has accelerated unprecedentedly. In the face of massive literature, how to effectively mine, understand and visualize the knowledge structure has become an important topic of academic research, and scientific knowledge mapping technology has become an important means to solve this problem (1). Research on scientific knowledge mapping originated in the field of information visualization, which mainly reveals the structure, evolution and cross-fertilization process of scientific knowledge by mining the intrinsic connections and dynamic evolution of literature data (2). With the rapid development of big data and artificial intelligence technology, the application scope of these methods are constantly expanding (3). In particular, the emergence of tools such as CiteSpace (2), VOSviewer (2), Pajek (4), and R-tool (5) has led to the widespread application of scientific knowledge mapping technologies in many disciplines, which provides new perspectives for researchers to understand in depth the development of disciplines and cutting-edge dynamics.
As a new interdisciplinary discipline, pharmaceutical management aims to ensure the safety, effectiveness, economy and rationality of public medication through the supervision of the whole life cycle of drugs. With the development of the pharmaceutical industry and changes in the policy environment, research in the field of pharmaceutical management has increased, and research hotspots and trends are constantly changing. Therefore, sorting out the evolutionary trends of research topics in the field of pharmaceutical management from the massive literature is highly important, as is clarifying the current research hotspots and frontiers, which are important for promoting the theoretical and practical development of the field. At present, scholars have used the scientific knowledge mapping method to explore the development trends of “pharmacoeconomics” (6) and “pharmaceutical care” (7), but no research has been conducted on trends in the field of pharmaceutical management. Therefore, to fill this gap, the purpose of this study is to analyze the relevant literature in the field of pharmaceutical management through the use of the scientific knowledge mapping method to identify research trends in this area and to solve the following problems:
Q1: What is the global development trend in the field of pharmaceutical management based on the information from published literature?
Q2: What are the most prolific and influential countries/regions and institutions in this field?
Q3: What are the main research directions and hotspots? How did they change over time?
Q4: What are the current research frontiers in the field? What are the potential hotspots for the future?
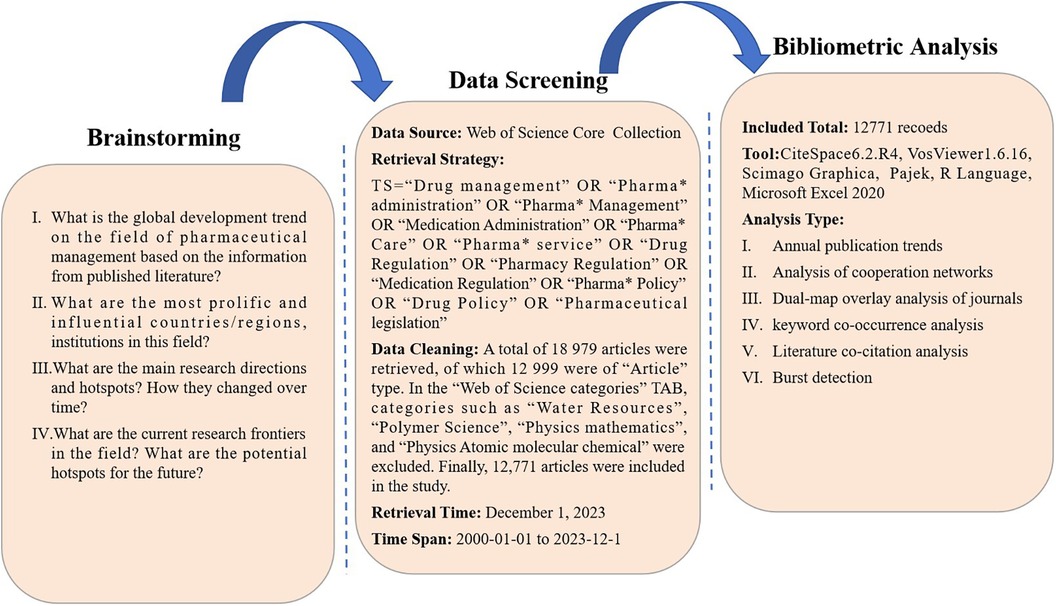
2 Methods
2.1 Data sources and retrieval strategies
The method used for data analysis is closely related to the data composition. In addition to classifying and indexing an academic paper according to its own database, indexing databases usually include all the relevant literature except for the text. Considering the integrity of the data structure and the level of the paper, this study selected the Web of Science Core Collection (WoSCC) as the data source.
The retrieval criteria were as follows: (TS = “Drug Management” OR “Pharma* Administration” OR “Pharma* Management” OR “Medication Administration” OR “Pharma* Care” OR “Pharma* Service” OR “Drug Regulation” OR “Pharmacy Regulation” OR “Medication Regulation” OR “Pharma* Policy” OR “Drug Policy” OR “Pharmaceutical legislation”). To capture as many data sources as possible, the wildcard character (*) that could be substituted for any other characters and allows variable endings of keywords was used.
2.2 Data collection and analysis
All the literature data for this study were downloaded independently from the Web of Science Core Collection database on December 1, 2023, by the author (JK Shen). The process of cleaning the literature data is shown in Figure 1. The bibliometric analysis in this paper included academic community analysis, dual-map overlay analysis of journals, keyword co-occurrence analysis, literature co-citation analysis and burst detection (8).
Visualization of the academic community analysis by VOSviewer and Scimago Graphica; visualization of dual-map overlay analysis of journals by VOSviewer and Pajek; and visualization of keyword co-occurrence analysis by VOSviewer, Pajek and the R programming language. Literature co-citation analysis and burst detection were visualized by CiteSpace 6.2.R4 (Advanced).
3 Results
3.1 Annual publication trends and dual-map overlay analysis
The number of annual publications in the field of pharmacy administration in the WoSCC database is shown in Figure 2. The time span of the literature was from 2000 to 2023. Overall, publications in this field showed a steady growth trend, with a peak of 1,335 articles published in 2021 and a total of 12,771 articles published in the past 24 years.
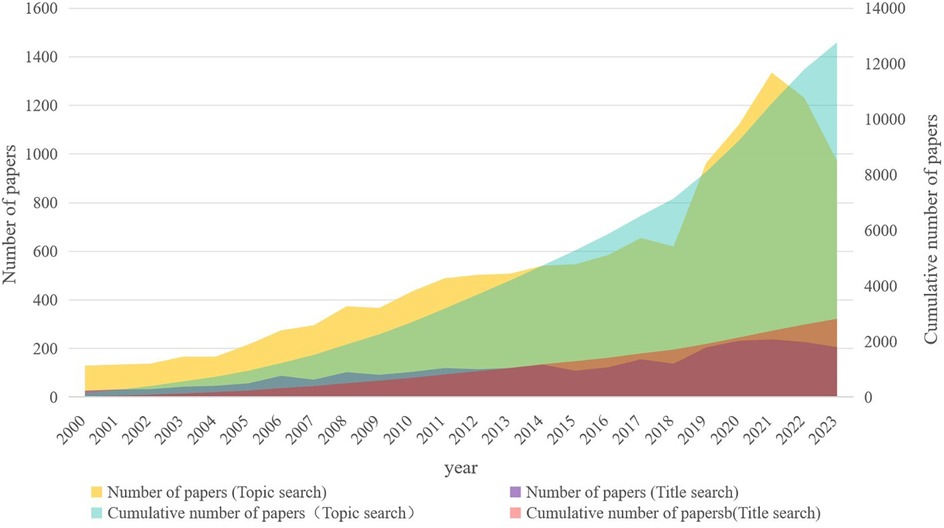
Figure 2 Publication trends of papers in WoSCC database. The publication of papers first started in 2000 and reached a peak of 1,335 in 2021.
Based on the Web of Science subject classification standard, all the literature was analyzed via dual-map overlay analysis. The visualization of the result was achieved through VOSviewer and Pajek. The results revealed 12,771 papers distributed across 175 different fields, as shown in Figure 3. The literature in this field is primarily distributed across two major disciplinary groups: Biology and Medicine, Psychology and Social Sciences. Among the subfields, the most widely distributed areas include Pharmacology & Pharmacy, Health Care Sciences Services, Public Environmental Occupational Health, Substance Abuse, Nursing, Health Policy Services, Medicine General Internal, Psychiatry, Education Scientific Disciplines and Medical Informatics.

Figure 3 Dual-map overlay analysis of journals of pharmaceutical management fields. The literatures are mainly distributed in two subject clusters of “Biology and Medicine” and “Psychology and Social Sciences”.
3.2 Analysis of national and institutional cooperation
There are many forms of scientific research cooperation, and the scientific research cooperation mentioned in this article refers to the countries or scientific research units that appear at the same time in an article; thus, we determined that there is a cooperative relationship between them (9). The analysis helps us understand the core research groups and cooperation in this field, which can provide a reference for the introduction of academic resources or subject cooperation in the future. We visualized this cooperative relationship through the Scimago Graphica tool.
Figure 4 shows the national cooperation network of papers in this field. The size of the nodes in the figure represents the number of publications, the color represents the total link strength, and the lines between nodes represent the cooperative relationships. “Total link strength” is a key indicator used to quantify the total strength of the connection between specific nodes in the network, such as authors, countries, institutions, etc., and other nodes. The larger the value, the closer the cooperation relationship. According to the results, these studies involved 166 countries or regions, among which the United States ranked first in the world in terms of the number of publications (n = 4,557, 35.68%) and citations (n = 106,685), and formed a radiation to other countries and regions, which had an important academic influence in the field of pharmaceutical management research. The other countries with more than 300 publications were England (1,343, 10.52%), Canada (943, 7.38%), Australia (908, 7.11%), China (671, 5.25%), Spain (623, 4.88%), Brazil (545, 4.27%), the Netherlands (480, 3.76%), Germany (436, 3.41%), France (409, 3.20%) and Italy (375, 2.94%). Overall, research in the field of pharmaceutical management has formed a close and extensive cooperation network around the world.
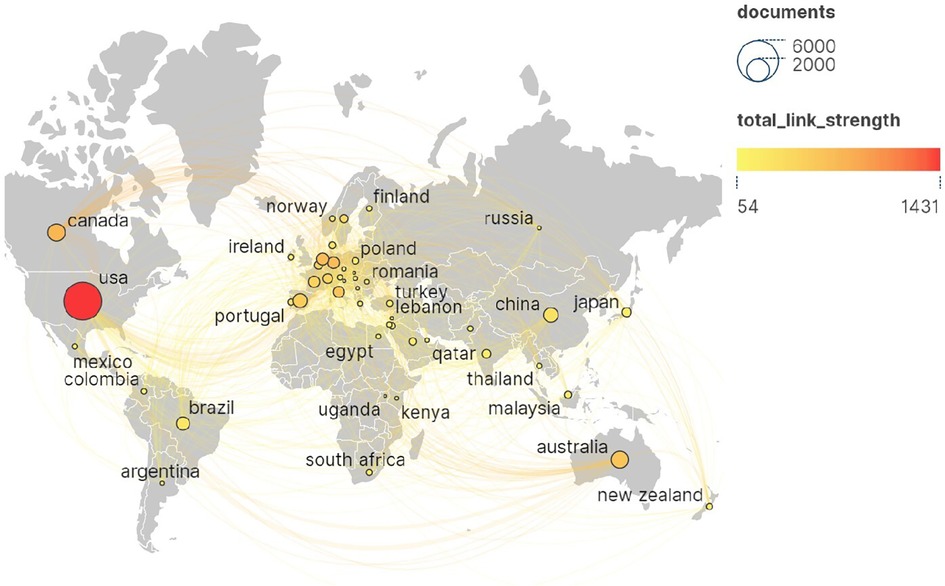
Figure 4 National collaborative network for papers in the field of pharmaceutical management. The United States ranked first in the world in terms of the number of publications (n = 4,557, 35.68%) and citations (n = 106,685). The size of the nodes in the figure represents the number of publications, the color represents the total link strength, and the lines between nodes represent the cooperative relationships. “Total link strength” is a key indicator used to quantify the total strength of the connection between specific nodes in the network and other nodes, with greater value indicating closer cooperation.
Figure 5 shows the network of institutional collaborations for papers in this field. According to the results, Univ Toronto ranked first in terms of the number of publications (n = 242) and citations (c = 8,973) among all the institutions, indicating its high academic influence in this field. The following institutions are included: the University of Sydney (n = 193, c = 3,253), the University of British Columbia (n = 158, c = 5,133), the University of North Carolina (n = 128, c = 2,360), Monash University (n = 126, c = 2,037), the University of Colorado (n = 120, c = 3,480), the University of Washington (n = 118, c = 4,144), the University of Pittsburgh (n = 118, c = 2,608) and the University of California San Francisco (n = 115, c = 4,254), most of which can be found in the United States.

Figure 5 Institutional collaboration network for papers in the field of pharmaceutical management. Univ Toronto ranked first in terms of the number of publications (n = 242) and citations (c = 8,973) among all the institutions. The size of the nodes in the figure represents the number of publications, the color represents the total link strength, and the lines between nodes represent the cooperative relationships. “Total link strength” is a key indicator used to quantify the total strength of the connection between specific nodes in the network and other nodes, with greater value indicating closer cooperation.
3.3 Keyword co-occurrence analysis
Keywords are often regarded as highly condensed versions of a paper and can reflect the main object and core issues of a scholar's research. Through keyword co-occurrence analysis, we identified the core keywords in this field and their positions in the network and subsequently revealed the current research hotspots (10). The results were visualized by VOSviewer (Figure 6) and R-bibliometrix (Figure 7).
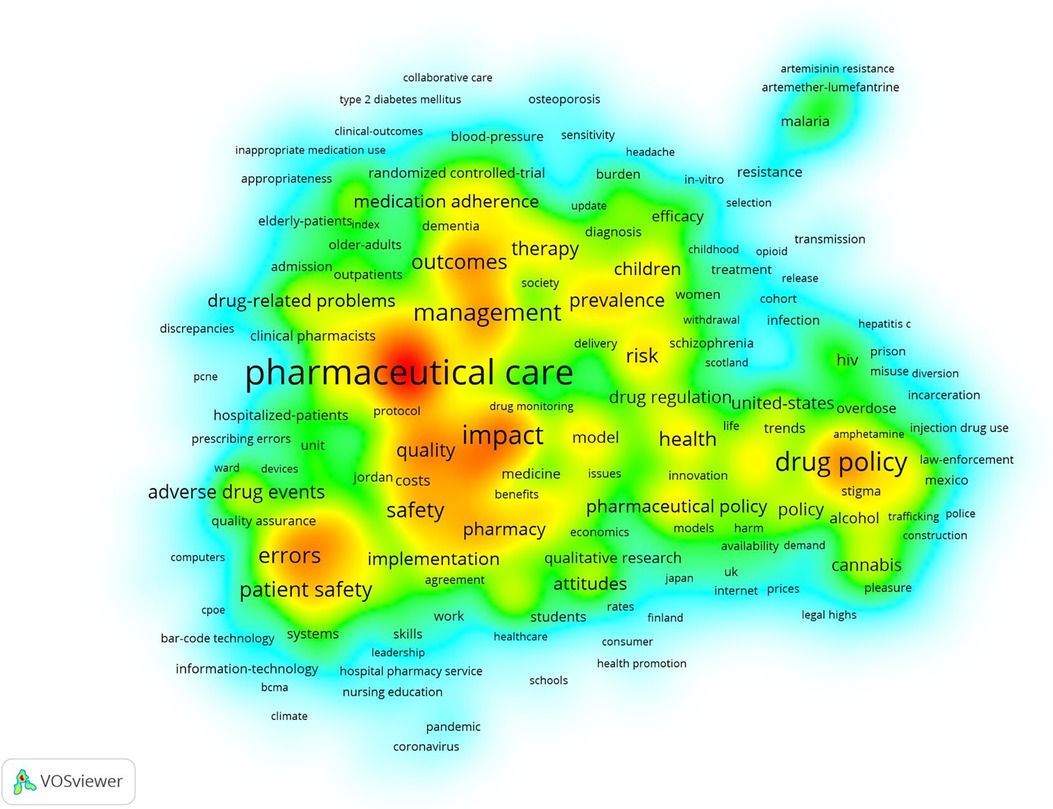
Figure 6 Keyword frequency density distribution of papers in the field of pharmaceutical management. Keywords that tend to occur in the middle of yellow areas have a higher co-occurrence frequency, while the green areas at the edge have the opposite trend. Keywords such as “pharmaceutical care”, “drug policy”, “medication errors”, “patient safety”, “adherence”, “pharmacy”, “interventions”, “outcomes”, “costs”, “prevalence” and “adverse drug events” had high co-occurrence frequencies.
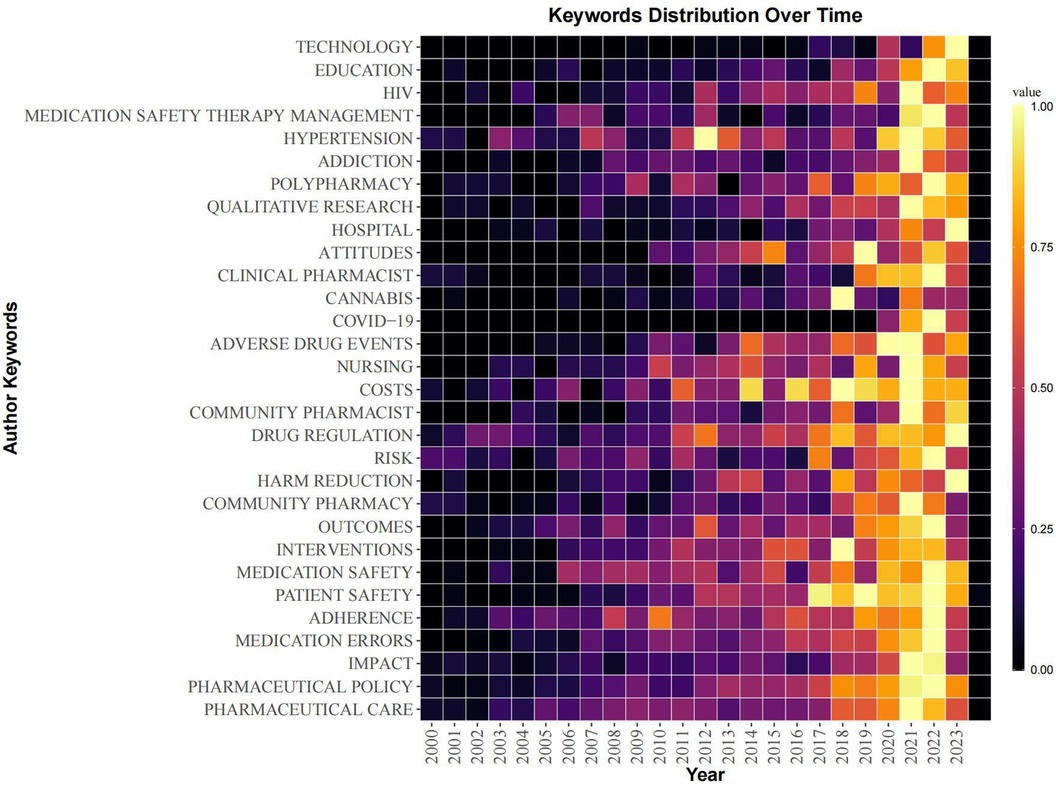
Figure 7 Author keywords heat map of papers in the field of pharmaceutical management. The figure shows the trend of the popularity of author keywords over time, and the color of the square represents the frequency of author keywords. The larger the value, the greater the attention of the keyword at this stage.
Figure 6 shows the distribution of the keyword frequency density of papers in the field of pharmaceutical management. In the figure, keywords that tend to occur in the middle of yellow areas have a higher co-occurrence frequency, while the green areas at the edge have the opposite trend. The results showed that keywords such as “pharmaceutical care”, “drug policy”, “medication errors”, “patient safety”, “adherence”, “pharmacy”, “interventions”, “outcomes”, “costs”, “prevalence” and “adverse drug events” had high co-occurrence frequencies.
Although this study hopes to describe the distribution of hot topics in the field of pharmaceutical administration based on the distribution of keyword word frequency, there are still some limitations in the processing of keyword co-occurrence analysis. Specifically, because the keywords in the literature are subjectively marked by the authors, there are more synonyms, such as “drug policy” and “drug management policy”. In view of the large number of keyword nodes, manual merging and standardization of them one by one is not only very cumbersome in operation, but also may introduce new subjective judgment errors. Therefore, this study did not merge all synonyms, but retained the diversity of the original data. This approach may result in a slightly fragmented representation of some research topics in the co-occurrence network and may slightly underestimate the actual attention paid to some core topics. Although the preliminary analysis shows that these subtle keyword differences have limited impact on the overall research hotspot identification and will not fundamentally change the main findings of the study, it is still necessary to consider the use of more advanced natural language processing technology to optimize the keyword processing process in future studies to improve the accuracy and consistency of the analysis.
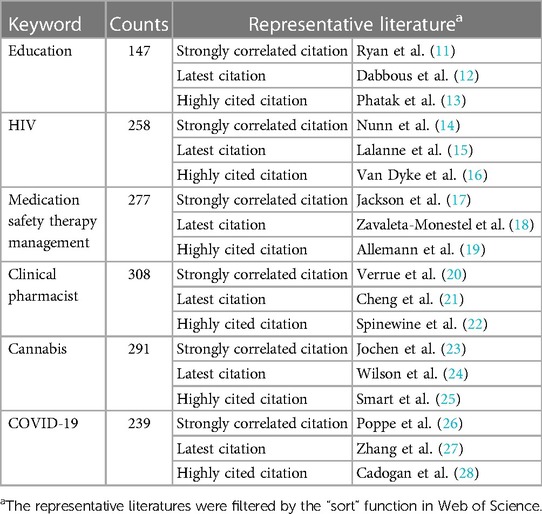
In addition, in order to reflect the changing trend of keyword popularity over time, we plotted author keyword heat map based on R-bibliometrix to detect the hot research topics in recent years. Because the information provided by the keywords of the literature is relatively limited, the results on research hotspots provided by a single analysis may also be biased, so this analysis can also be mutually corroborated and supplemented with the results of subsequent literature co-citation analysis. As shown in Figure 7, the keywords “education”, “HIV”, “medication safety therapy management”, “clinical pharmacist”, “cannabis” and “COVID-19” have shown high heat in recent years.
Since the information provided by the keywords is relatively limited, the literature under the keyword nodes is used to reflect the specific content of the keywords. In addition, the number of literature under some nodes is huge, so we filtered the strongly relevant literature, latest literature and highly cited literature as the representative documents under the author keyword nodes through the “sort” tab of Web of Science. The author keyword node information is shown in Table 1. There were 147 papers under the “education” node, among which the most relevant literature was on clinical pharmacist education in China (11). The latest literature is an investigation of the effect of implementing instructional games on the learning outcomes of a pharmacy practice course (12). The highly cited literature involved a study on the positive impact of pharmacists in the transitional care process of high-risk patients (13). Under the “HIV” node, the most relevant literature reported Brazil's efforts and contributions in changing the global specification of essential medicines and its experience and lessons in the treatment of AIDS (14). Recent literature under this node reports the potential positive impact of drug consumption rooms on reducing high risk behaviors of drug users for human immunodeficiency virus (HIV) and hepatitis C (15). The highly cited literature is a clinical study of adherence to highly active antiretroviral therapy (HAART) in children (16).
In the node “medication safety therapy management”, the most relevant literature designed questionnaires from the preferred terms related to pharmaceutical care, service nature, perceived benefits and barriers and discussed the construction of the pharmaceutical care framework (17). The most highly cited publication was the definition of PCNE for pharmaceutical care (19). Recent literature provides insight into the practices of clinical pharmacists in pharmaceutical care in the Latin American region (18). In the “Clinical Pharmacist” node, the most relevant literature was a study on drug management in nursing homes, highlighting the contribution of clinical pharmacists in reducing the medication error rate (20). The latest literature introduces clinical pharmacists in central nervous system infection (CNSI) for the treatment of infections in patients with positive effects (21). The highly cited literature was a randomized controlled trial that explored the impact of a collaborative approach on prescription quality in geriatric inpatients (22).
In the “cannabis” node, the most relevant literature involved the study of policy making for medical cannabis management in schools (23). The latest literature explores the legalization of prescription use of medical cannabis in the UK (24). The highly cited literature explores the question of how cannabis policy in the United States affects substance use and provides early evidence on the effects of cannabis legalization on cannabis use, cannabis use disorders, and other substance use (25). In the “COVID-19” node, the most relevant literature explored the impact of nondrug policy interventions on COVID-19 transmission in three Colombian cities (26). The latest literature is a cross-sectional survey of patients with fever on their knowledge, attitudes and behaviors toward over-the-counter antipyretics (OTC) (27). The highly cited literature discussed the role and activities of community pharmacists in the public health crisis, with special emphasis on their contribution to the prevention of COVID-19 (28).
3.4 Literature co-citation analysis
Co-citation analysis is a quantitative method in which cocitation information is used for in-depth mining to reveal the knowledge structure and internal relationships of a subject domain. Because the knowledge base of a certain field is precisely based on the cocited literature, some cited studies with high citation and high mediation centrality also constitute the core knowledge base of the field. The mapping of cited literature to cited literature also reflects the mapping of the knowledge base to the research frontier. In addition, the cocitation relationship of literature often involves a dynamic process, so co-citation analysis can capture the development and evolution of this field (29). The visualization of the result is based on CiteSpace 6.2.R4 (Advanced).
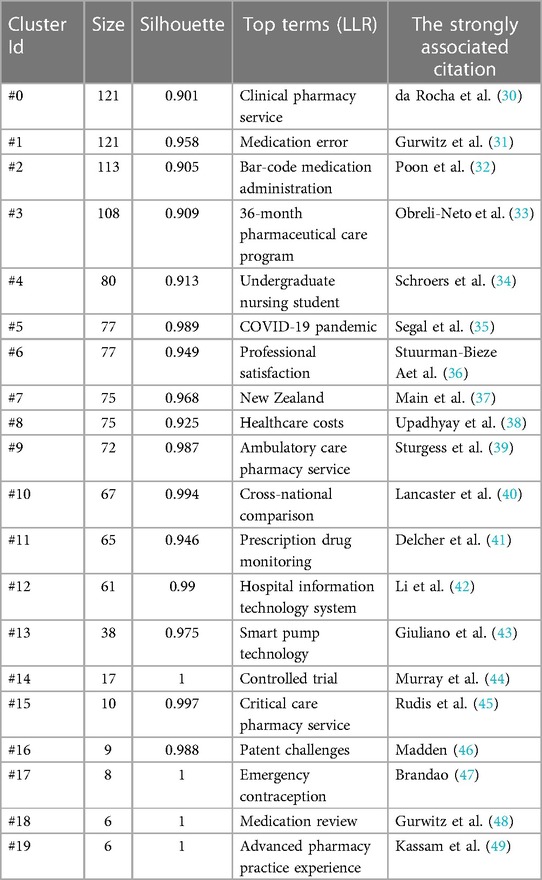
Based on CiteSpace6.2.R4 (Advanced), the literature co-citation network was obtained (Figure 8), and the top 20 clusters were: “clinical pharmacy service” (N = 121, S = 0.901), “medication error” (N = 121, S = 0.958), “bar-code medication administration” (N = 113, S = 0.905), “36-month pharmaceutical care program” (N = 108, S = 0.909), “undergraduate nursing student” (N = 80, S = 0.913), “covid-19 pandemic” (N = 77, S = 0.989), “professional satisfaction” (N = 77, S = 0.949), “New Zealand” (N = 75, S = 0.968), “healthcare costs” (N = 75, S = 0.925), “ambulatory care pharmacy service” (N = 72, S = 0.987), “cross-national comparison” (N = 67, S = 0.994), “prescription drug monitoring” (N = 65, S = 0.946), “hospital information technology system” (N = 61, S = 0.990), “smart pump technology” (N = 38, S = 0.975), “controlled trial” (N = 17, S = 1), “critical care pharmacy service” (N = 10, S = 0.997), “patent challenges” (N = 9, S = 0.988), “emergency contraception” (N = 8, S = 1), “medication review” (N = 6, S = 1) and “advanced pharmacy practice experience” (N = 6, S = 1). The cluster labels in this paper were extracted by the Log likelihood ratio (LLR) algorithm. The N value is the capacity of each cluster, the silhouette (S value) is used to measure the homogeneity between nodes in each cluster, and an S value greater than 0.7 indicates that the clustering result is convincing.
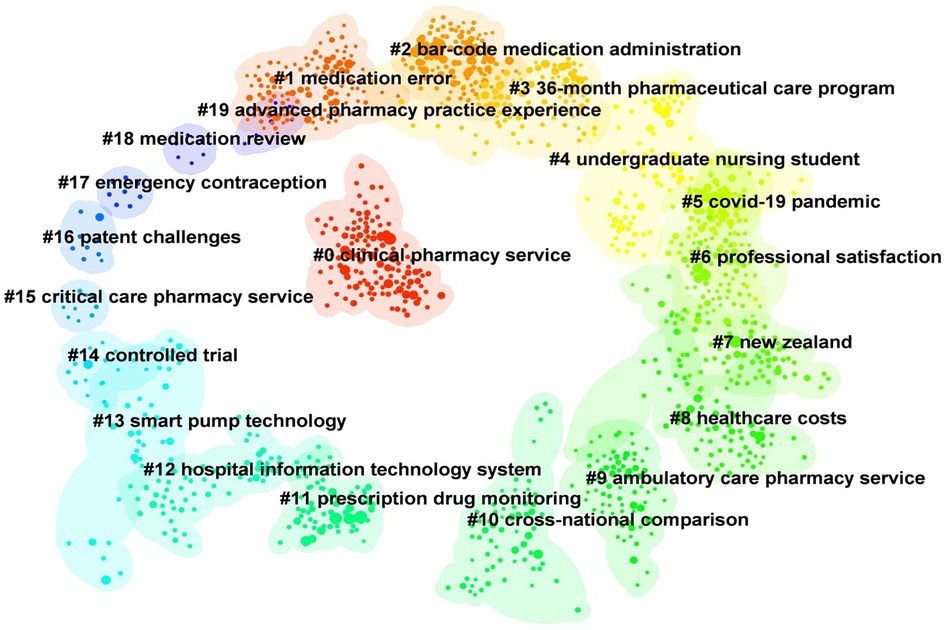
Figure 8 The literature co-citation network in the field of pharmacy management. Each color represents a different cluster. The top 20 cluster themes are: “clinical pharmacy service”, “medication error”, “bar-code medication administration”, “36-month pharmaceutical care program”, “undergraduate nursing student”, “COVID-19 pandemic”, “professional satisfaction”, “New Zealand”, “healthcare costs”, “ambulatory care pharmacy service”, “cross-national comparison”, “prescription drug monitoring”, “hospital information technology system”, “smart pump technology”, “controlled trial”, “critical care pharmacy service”, “patent challenges”, “emergency contraception”, “medication review” and “advanced pharmacy practice experience”.
By using the “cluster explore” function in CiteSpace, we obtained the citation information of the cocited networks (Table 2). In the “clinical pharmacy service” cluster, the strongly associated citation analyzed the clinical pharmacy service antitumor therapy interventions in a public tertiary hospital in Brazil through a retrospective study and evaluated the clinical significance and economic impact of clinical pharmacy services (30). In the “medication error” cluster, the strongly associated citation was a study on the incidence and preventability of adverse drug events in outpatient elderly patients (31). In the “bar-code medication administration” cluster, the strongly associated citation introduces the important role of barcode verification technology in improving medication safety (32). In the “36-month pharmaceutical care program” cluster, the strongly associated citation explored the effects of a 36-month medication care program on medication adherence in older patients with diabetes and hypertension (33).
In the “undergraduate nursing student” cluster, the strongly associated citation is a descriptive statistical study on clinical nursing students at a private university in the Midwest of the United States, aiming to understand undergraduate nursing students’ experience in skill application in clinical practice, supervision quality and feedback (34). In the “COVID-19 pandemic” cluster, the strongly associated citation explored the experience and limitations of clinical pharmacists in Washington State in conducting telemedicine for chronic disease and cancer patients in the context of the COVID-19 pandemic (35). In the “professional satisfaction” cluster, the strongly associated citation is a study on clinical drug care interventions, which describes the drug care process tailored to the individual problems of patients in the randomized controlled trial intervention group and discusses the satisfaction of pharmacists with pharmaceutical care methods (36). In the “New Zealand” cluster, the strongly associated citation explores the policy mechanisms behind New Zealand's remarkable achievement in cost control of public pharmaceutical expenditure, which contrasts with most other advanced economies (37).
In the “healthcare costs” cluster, the strongly associated citation reported the positive impact of a pharmacist's supervised intervention on the direct medical cost burden of patients with newly diagnosed diabetes through a nonclinical randomized controlled trial approach (38). In the “ambulatory care pharmacy service” cluster, the strong association citation introduced the positive role of pharmaceutical care in community pharmacies in improving the medication compliance of elderly patients (39). In the “cross-national comparison” cluster, the strongly associated citation was a cross-country comparison between the United Kingdom and Australia in terms of “drug use problems” (40). In the “prescription drug monitoring” cluster, the strongly associated citation introduced the significant impact of Florida's prescription drug monitoring program (PDMP) in reducing oxycodone deaths in the state (41).
In the “hospital information technology system” cluster, the strongly associated citation introduced the application of computer information technology in hospital clinical nursing work. Based on the Internet of Things (IoT), this study optimized the infusion process through a reasonable and effective outpatient and emergency infusion drug management system, realized closed-loop drug management, and greatly improved the efficiency and safety of infusion (42). In the “smart pump technology” cluster, the strongly associated citation introduced the positive role of intravenous (IV) smart pumps in reducing dose errors and drug administration error rates, identifying system errors, and identifying nurse training needs. These findings show the broad application prospects of medical information technology in the field of improving clinical patient satisfaction and drug safety (43). In the “controlled trial” cluster, the strongly associated citation was a randomized trial study of pharmacist intervention to improve medication adherence in patients with heart failure (44). In the “critical care pharmacy service” cluster, the strongly associated citation was a position paper on critical care pharmacy services, and the article identified and described the scope of practice of acute critical pharmacy pharmacists and acute critical pharmacy services (45).
In the “patent challenges” cluster, the strongly associated citation introduced the interaction between intellectual property rights and the pharmaceutical regulatory system. The article identifies problems with the current pharmaceutical regulatory system operating independently of the intellectual property system and describes the attempts and experiences of many regulators in developed countries to ensure a compromise between the rights of generic companies and intellectual property owners by incorporating safeguards, such as patent linkage and data protection, into the regulatory framework (46). In the “emergency contraception” cluster, the strongly associated citation was a social survey study on the issue of pharmacists’ and sales clerks’ positions on emergency contraception, which discussed pharmacists’ views on the commercialization of emergency contraception in Brazil and on the issue of pharmaceutical care for emergency contraceptive users (47). In the “medication review” cluster, the strongly associated citation was a study on adverse drug events, which focused on the incidence and prevention of adverse drug events in outpatient care for the elderly (48). In the cluster of “advanced pharmacy practice experience”, the strongly associated citation is a study on the cultivation of pharmaceutical care ability. This paper focuses on the nature and degree of learning opportunities that pharmacy students have in advance in the community (49).
Based on the literature co-citation network, we constructed a citation distribution map of the time series (Figure 9). According to the results, these citations from 1995 to 2005 were mainly distributed in the “medication error”, “professional satisfaction”, “ambulatory care pharmacy service”, “critical care pharmacy service” and “advanced pharmacy practice experience” clusters, which were the hot research directions in this period. From 2005 to 2015, these citations were distributed mainly among “barcode medication administration”, “36-month pharmaceutical care program”, “healthcare costs”, “cross-national comparison”, “prescription drug monitoring”, “patent challenges” and “emergency contraception”. From 2015 to 2023, these citations were distributed mainly in the “clinical pharmacy service”, “undergraduate nursing student”, “COVID-19 pandemic”, “New Zealand”, “hospital information technology system” and “smart pump technology” clusters and are hot topics.
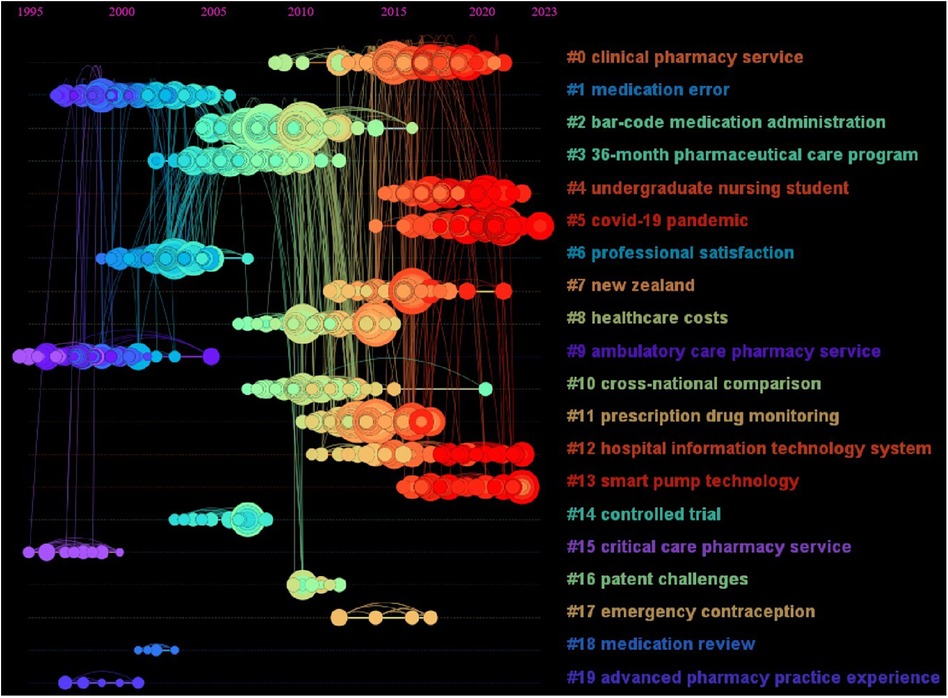
Figure 9 Time zone map of the literature co-citation network. The time zone map reflects the distribution of all cited literature nodes in each cluster in the time dimension, and then maps the evolution trend of hot topics in the field.
3.5 Burst detection
Burst detection is mainly used to mine keywords or cited literature with large frequency fluctuations over a period of time. Through burst detection, we can discover the evolutionary trend of the field of pharmaceutical management from macro to micro, from single to multiple, and review or predict which key technology branches will become hot topics of concern in the future. Based on CiteSpace 6.2.R4, we conducted a burst detection of keywords in these literatures. After detection, a total of 217 keywords were found to have citation bursts. To ensure the reliability of the detection results, this article uses the keyword mutation time as the basis for sorting, and the 25 nodes with the largest mutation intensity are selected.
The results are shown in Figure 10, in which “Begin” represents the start time of the keyword burst, “End” represents the year when the burst ended, and “Strength” represents the keyword burst intensity. The larger the value is, the larger the frequency fluctuation. The red bands represent the length of the burst. From the perspective of time series, the keywords “prevention”, “advanced pharmacy practice experience”, “adverse drug events”, “quality assurance”, “pharmaceutical care”, “resistance”, “community pharmacy”, “elderly patients”, “drug administration”, “chloroquine” and “community and ambulatory pharmacy” had higher burst intensities in the 2000–2010 phase. Keywords such as “information technology”, “national drug policy”, “meta analysis”, “systems”, “intensive care” and “smart pump technology” had high burst intensities during the period 2011–2019. Keywords such as “clinical pharmacist”, “saudi arabia”, “COVID-19”, “pharmacy education”, “telemedicine”, “computer-assisted decision making” and “barcoding” had high burst intensities from 2020 to 2023. It should be noted that the keyword “saudi arabia” here refers to the related pharmaceutical management policy (50), pharmaceutical care (51), pharmaceutical education (52) and other related research topics with a significant increase in frequency during this period.
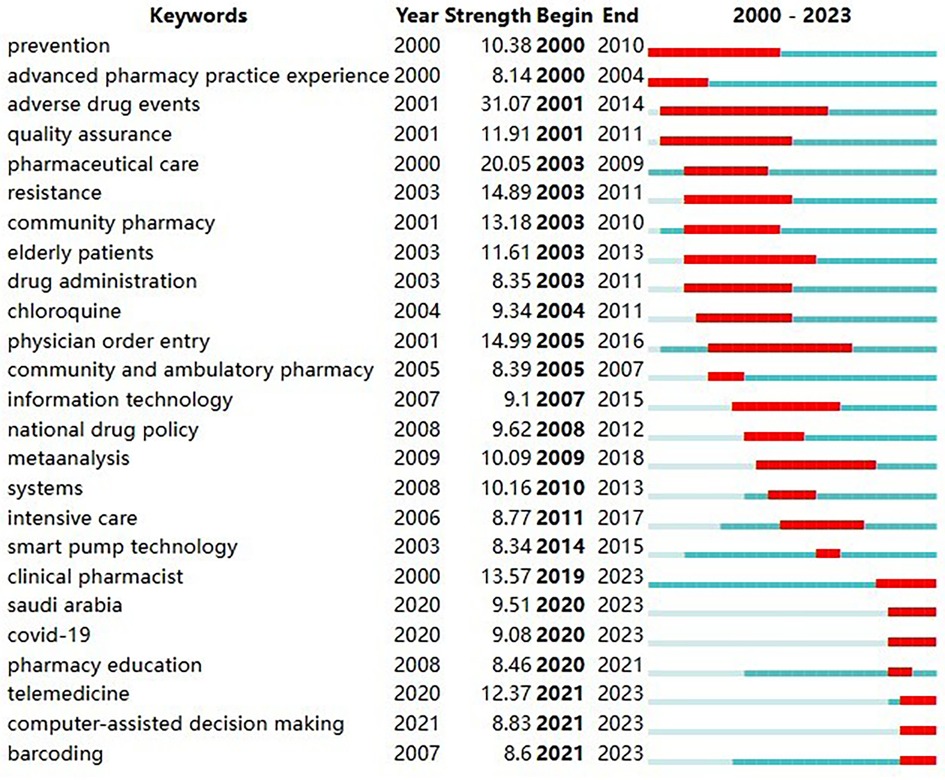
Figure 10 Top 25 keywords with the strongest citation bursts. The keywords “clinical pharmacist”, “Saudi arabia”, “COVID-19”, “pharmacy education”, “telemedicine”, “computer-assisted decision making” and “barcoding” have shown high burst intensity in recent years. “Begin” represents the start time of the keyword burst, “End” represents the year when the burst ended, and “Strength” represents the keyword burst intensity. The larger the value is, the larger the frequency fluctuation. The red bands represent the length of the burst.
Based on the “Burstness” function, a total of 247 references had citation bursts, and the 25 nodes with the largest burst intensity are displayed (Figure 11). From 2000 to 2009, research content with a high burst of citations focused on the positive impact of pharmacist reviews on reducing medication errors (53) and the contribution of community pharmacists to improving clinical outcomes and reducing healthcare costs (54, 55). From 2010 to 2019, the research content with a high burst of citations focused on the impact of electronic medication records (EMAR) and barcode medication administration (BCMA) technology on medication safety (32, 56, 57), the verification of the correlation between medication interruptions and the risk level of medication errors (58, 59), the positive impact of pharmacists on patient care (60) and chronic disease management (61), the definition of pharmaceutical services (19), and the prevalence, nature (62) and causes (63) of medication errors in health care settings. From 2020 to 2023, the research content with a high burst of citations focused on the role of pharmacists in reducing medical costs (64), the contribution of community pharmacists to the COVID-19 pandemic (28, 65, 66), the definition of polypharmacy (67), medication safety (68), medication error factors and improvement (69), and telepharmacy services (70).
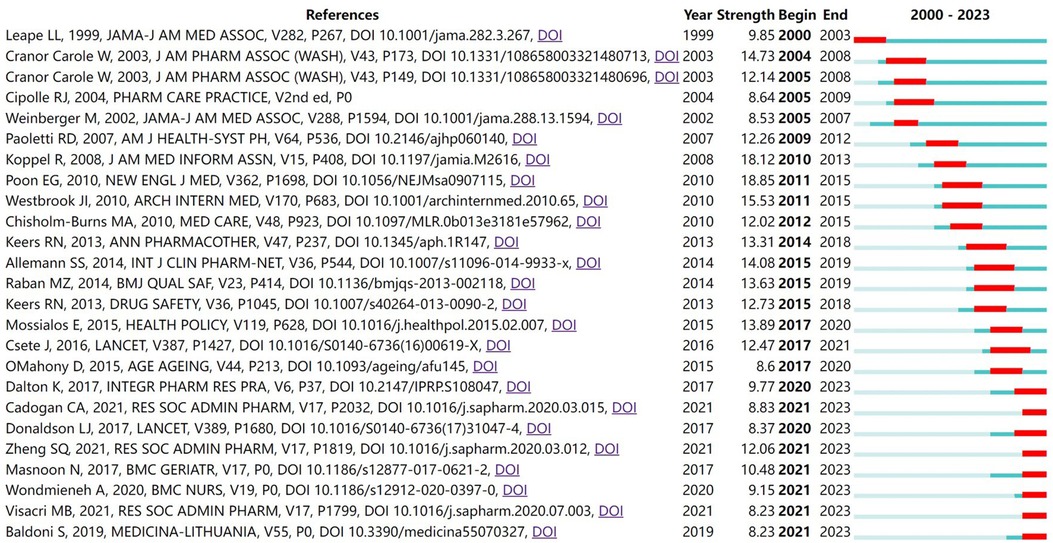
Figure 11 Top 25 references with the strongest citation bursts. “Begin” represents the start time of the reference burst, “End” represents the year when the burst ended, and “Strength” represents the reference burst intensity. The larger the value is, the larger the frequency fluctuation. The red bands represent the length of the burst.
4 Discussion
4.1 Answer for Q1: what are the trends in publications in the field of pharmaceutical management?
In the Web of Science Core Collection database, the publication of papers in the field of pharmacy management first started in 2000, with only 130, peaking at 1,335 in 2021 and then declining slightly, with a total of 12,771 publications accumulated during this period. According to the results of keyword co-occurrence analysis and literature co-citation analysis, the early studies mainly focused on the positioning of pharmacists and pharmaceutical care and the establishment of management system, which also provided a rich theoretical basis for subsequent practical research in the field of pharmaceutical management. With the intensification of global health challenges, the extensive application of information technology in the medical field and the continuous changes in the policy environment, pharmaceutical management research has ushered in a rich and diverse practical scenarios and theoretical exploration space, which has contributed to the significant growth of the literature in the field. Notably, these literatures were widely distributed in the two subject clusters of biology and medicine, psychology and social sciences, and the core involved pharmacology & pharmacy, health care science services, public environmental occupational health, substance abuse, nursing, health policy services, education science disciplines, and medical informatics.
To further evaluate the relativity of this growth phenomenon, we compared the publication trend of literature in fields close to pharmaceutical administration, including public health emergency management (71), global health management (72), pharmaceutical services (7), and pharmaceutical economics and policy research (6). The results showed that although the growth rate of literature publication in each field varied, it generally showed an upward trend similar to that in the field of pharmacy management. This shows that the growth of literature in the field of pharmaceutical administration is not an isolated phenomenon, but coincides with the booming background of the entire medical and health research field, which also reflects the general increase in attention to the related fields of drug regulation worldwide.
4.2 Answer for Q2: what are the most prolific and influential countries/regions and institutions in this field?
Generally, the annual publication volume and citation frequency of scholars or scientific research institutions are the easiest to quantify, and they are also the most intuitive indicators reflecting their academic influence (73). According to the map of national cooperation network in Figure 4, the United States ranks first in the world in terms of the number of publications (n = 4,557) and citations (n = 106,685), and radiates to Britain, Canada, Australia, China, Spain, Brazil, the Netherlands, Germany, France and Italy in terms of cooperation. Reflecting its significant academic influence in the field of pharmaceutical administration, it also means that the United States plays a key role in setting international drug standards, promoting transnational drug safety regulatory information exchange, and improving global access and quality of drugs, which plays a crucial role in the standardization and efficiency of global pharmaceutical practice. In addition, in terms of institutional cooperation network (Figure 5), the University of Toronto ranked first among all institutions in terms of the number of publications (n = 242) and citations (c = 8,973), and radiated to other institutions.
This cooperation pattern is not only a reflection of academic influence, but also a vindicator of the dynamic evolution of the global drug regulatory system. These data underscore the importance of stronger international cooperation, particularly to facilitate rapid response mechanisms and accelerate the development and distribution of vaccines and therapeutics in the face of global public health challenges, such as COVID-19. In the future, the research in the field of pharmaceutical administration should pay more attention to interdisciplinary and cross-border cooperation, and promote the continuous improvement of the global drug regulatory system based on scientific evidence.
In this study, the analysis of cooperation in the field of pharmacy management mainly focused on the description and analysis of the number of publications jointly published by countries or institutions. A notable limitation is that we failed to take into account the distribution of the number of HEIs within countries or the effect of the size of individual institutions on the output of research outcomes. For example, although the United States leads in total publications and the University of Toronto stands out among institutions, the international collaborative impact rankings might look different if we could obtain and analyze data on the number of universities or colleges in each country and the size of their corresponding research departments. Because of practical difficulties in obtaining such detailed data, we were not able to include such adjustment factors directly in our analyses, which constitutes a limitation of the study. Future research could explore how these variables can further refine and influence the analysis of the international cooperation landscape through more extensive data integration.
4.3 Answer for Q3: what are the main research directions and hotspots? how did they change over time?
Through keyword co-occurrence analysis (Figure 6) and literature co-citation analysis (Figure 8), we explored the distribution of the core research topics of papers in the field of pharmaceutical management from the perspectives of keywords and cited literature. The findings can be summarized as follows: pharmacy services and clinical pharmacists, development and implementation of drug policies and regulations, drug utilization evaluation, medical insurance and drug cost control, medication safety and risk management, drug information management and technology application, pharmacoeconomics and drug evaluation, education and training of pharmacy students, career satisfaction of pharmacists, pharmaceutical intellectual property protection, and drug review.
As shown in the co-cited network time zone map (Figure 9), early pharmacy management research (1995–2000) focused on the positioning of pharmacists and pharmacy services and the establishment of management systems. The research content covered the important role of pharmaceutical services in clinical practice, the positioning of pharmacists and pharmaceutical education. From 2000 to 2005, research was conducted mainly in clinical pharmacy and institutional regulation, with research topics covering pharmacists’ professional satisfaction, clinical medication guidance and clinical trials. With the development of globalization and the market economy, pharmaceutical administration research began to shift to the field of drug marketing and economics from 2005 to 2010. The research topics included drug market competition, drug intellectual property protection, drug consumption behavior, drug cost‒benefit analysis, medical insurance, etc. In 2010–2015, pharmaceutical management research was gradually integrated into the field of health systems and healthcare delivery, with research topics including healthcare quality and safety (e.g., medication safety), healthcare service delivery, and patient rights protection. With the rapid development of digital technology, research in the field of pharmacy management has also begun to explore the direction of digitalization and intelligence since 2015, and the research topics include digital drug management, intelligent medical services, and telemedicine. In view of the COVID-19 global pandemic, the research hotspots in 2000–2023 have focused mainly on the research directions of drug supply management, pharmacy services and telemedicine services under major public health events.
4.4 Answer for Q4: what are the current research frontiers in the field? what are the potential hotspots for the future?
Through the burst detection of keywords (Figure 10) and co-cited literature (Figure 11), we found that the topics of telepharmacy services, information technology, computer-assisted decision making, pharmacy education, and cross-national comparisons of pharmacy policies showed a high burst intensity. Therefore, it is predicted that future research in the field may focus on the following areas.
I. Information technology and pharmacy: With the development of information technology, the application of big data, artificial intelligence and other technologies in the medical field will further increase. Pharmacy management will focus more on utilizing these technologies to improve the efficiency and quality of medical management. For example, big data are used for the monitoring, analysis and management of adverse drug reactions (74–76), and artificial intelligence is used for prescription reviews and medication reminders (77, 78).
II. Precision pharmacy: Precision Pharmacy is an emerging field of multidisciplinary crossover between pharmacy management and clinical pharmacy, bioinformatics and other disciplines. It aims to provide patients with personalized and precise drug use solutions based on their genome, phenotype and other information. In the future, more in-depth research on precision pharmacies, including research on pharmacogenomics, precision drug use decision-making, and precision clinical trials, will be performed (79).
III. Global Pharmaceutical Governance: With the deepening of globalization and international exchanges, global pharmaceutical governance has become an important topic in pharmaceutical management. This includes comparative research on international drug regulation, transnational drug circulation and trade rules (80, 81). In addition, with the deepening of the Belt and Road Initiative, the study of drug regulatory systems and policies of countries along the route will also become a priority.
IV. Pharmaceutical ethics and legal issues: With the development of pharmaceutical science and technology, pharmaceutical ethical and legal issues have become increasingly prominent. In the future, ethical and legal research on these issues, such as gene editing in the field of pharmaceutical research and artificial intelligence-assisted decision-making, will become popular (82, 83). In addition, research on the protection of pharmaceutical intellectual property rights (84) and data privacy (85) will also become a focus of attention.
5 Conclusion
Based on the macro exploration of bibliometric analysis, this study revealed the research pattern and development trend in the field of pharmacy management, highlighting the importance of this field in addressing complex and changing health challenges. The trend of diversification of research topics, It covers the optimization of clinical pharmacists and pharmaceutical services, efficient evaluation of drug utilization, fine control of medical insurance and drug costs, strengthening of drug safety and risk management system, innovation and application of drug information management technology, deepening of pharmacoeconomic evaluation, expansion of drug intellectual property protection strategies, and continuous improvement of pharmaceutical education and regulatory framework. These constitute the core pillars of pharmaceutical administration research.
Current research focuses on the modernization transformation of pharmaceutical education, especially in the training of future pharmaceutical professionals. The comprehensive drug management strategy in the context of COVID-19 highlights the key role of pharmaceutical management in public health emergency response; The development and application of digital drug management system is leading the practice of pharmacy management into a new era of intelligence; And the expansion of telemedicine services has opened up a new way to improve the accessibility of medical services for patients. These hot topics not only reflect the characteristics of pharmaceutical management research keeping pace with The Times, but also provide scientific basis and guidance for the current pharmaceutical practice.
Looking forward to the future, research front pointing in the direction of information technology and drug management innovation mode of depth fusion, such as precision of pharmaceutical care, heralds the arrival of the era of personalized medicine; Global drug management system construction and cooperation, to ensure global drug supply security, promote health equity have far-reaching significance; Under the background of the rapid development of artificial intelligence technology, the ethical and legal considerations of drug design have become a new issue that needs to be solved urgently, which requires the interdisciplinary integration of pharmaceutical administration research to meet the challenges of science and technology ethics.
6 Limitations
Due to the limitations of the overall structure and length of the article, this study only provides a macro overview of the field of pharmacy management, without deeper discussion of specific subtopics. We encourage scholars to select the subfields of interest for subsequent thematic research according to the outline outlined in this study, and we also plan this as part of future personal research plans.
Author contributions
JS: Conceptualization, Data curation, Formal Analysis, Investigation, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. SW: Conceptualization, Investigation, Software, Visualization, Writing – review & editing. JG: Formal Analysis, Investigation, Software, Writing – review & editing. SX: Conceptualization, Data curation, Investigation, Methodology, Software, Supervision, Writing – review & editing. ML: Conceptualization, Formal Analysis, Project administration, Supervision, Writing – review & editing. DW: Conceptualization, Formal Analysis, Project administration, Writing – review & editing. LL: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the Higher Education Research Program of Henan Higher Education Society, “Innovation and Practice of Pharmaceutical Management Curriculum System for Pharmacy Majors Based on Case Analysis in the Context of New Medical Science” (2021SXHLX038).
Acknowledgments
We are grateful to all the study participants for their cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Linnenluecke MK, Marrone M, Singh AK. Conducting systematic literature reviews and bibliometric analyses. Aust J Manag. (2020) 45(2):175–94. doi: 10.1177/0312896219877678
2. Zhu WJ, Yuan NJ, Wan CM, Huang MY, Fang SY, Chen M, et al. Mapping the scientific research on bipolar disorder: a scientometric study of hotspots, bursts, and trends. J Affect Disord. (2023) 340:626–38. doi: 10.1016/j.jad.2023.08.069
3. Asemi A, Ko A, Asemi A. Infoecology of the deep learning and smart manufacturing: thematic and concept interactions. Library Hi Tech. (2022) 40(4):994–1012. doi: 10.1108/lht-08-2021-0252
4. Wang NM, Tang GW, Jiang B, He ZW, He QD. The development of green enterprises: a literature review based on VOSviewer and Pajek. Aust J Manag. (2023) 48(2):204–34. doi: 10.1177/03128962211035470
5. Aria M, Cuccurullo C. Bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. (2017) 11(4):959–75. doi: 10.1016/j.joi.2017.08.007
6. Liu Y, Bo ZY, Liu D, Diao S, Yang CS, Li HL, et al. Trends and frontiers of research on pharmacoeconomics from 2012 to 2021: a scientometric analysis. Ann Transl Med. (2022) 10(6):1–13. doi: 10.21037/atm-22-1050
7. Zhang YN, Yao J, Li WN, Wang H. Global research trends and hotspots in pharmaceutical care: a bibliometric analysis and visualisation using CiteSpace and VOSviewer. Eur J Hosp Pharm. (2023):1–9. doi: 10.1136/ejhpharm-2022-003617. [Epub ahead of print]
8. Yu DJ, Xu ZS, Pedrycz W, Wang WR. Information sciences 1968–2016: a retrospective analysis with text mining and bibliometric. Inf Sci (Ny). (2017) 418:619–34. doi: 10.1016/j.ins.2017.08.031
9. Sahin K, Candan G. Scientific productivity and cooperation in Turkic world: a bibliometric analysis. Scientometrics. (2018) 115(3):1199–229. doi: 10.1007/s11192-018-2730-x
10. Qin JH, Wang JJ, Ye FY. A metric approach to hot topics in biomedicine via keyword co-occurrence. J Data Inform Sci. (2019) 4(4):13–25. doi: 10.2478/jdis-2019-0018
11. Ryan M, Shao H, Yang L, Nie XY, Zhai SD, Shi LW, et al. Clinical pharmacy education in China. Am J Pharm Educ. (2008) 72(6):1–7. doi: 10.5688/aj7206129
12. Dabbous M, Sakr F, Safwan J, Akel M, Malaeb D, Rahal M, et al. Instructional educational games in pharmacy experiential education: a quasi-experimental assessment of learning outcomes, students’ engagement and motivation. BMC Med Educ. (2023) 23(1):1–9 doi: 10.1186/s12909-023-04742-y
13. Phatak A, Prusi R, Ward B, Hansen LO, Williams MV, Vetter E, et al. Impact of pharmacist involvement in the transitional care of high-risk patients through medication reconciliation, medication education, and postdischarge call-backs (IPITCH study). J Hosp Med. (2016) 11(1):39–44. doi: 10.1002/jhm.2493
14. Nunn A, Da Fonseca E, Gruskin S. Changing global essential medicines norms to improve access to AIDS treatment: lessons from Brazil. Glob Public Health. (2009) 4(2):131–49. doi: 10.1080/17441690802684067
15. Lalanne L, Roux P, Donadille C, Madrid LB, Célerier I, Chauvin C, et al. Drug consumption rooms are effective to reduce at-risk practices associated with HIV/HCV infections among people who inject drugs: results from the COSINUS cohort study. Addiction. (2023)119(1):180–99. doi: 10.1111/add.16320
16. Van Dyke RB, Lee S, Johnson GM, Wiznia A, Mohan K, Stanley K, et al. Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have human immunodeficiency virus infection. Pediatrics. (2002) 109(4):1–11. doi: 10.1542/peds.109.4.e61
17. Jackson J, Mishra P, Caliph S. A scoping study of the medication therapy related pharmacist services in the western pacific region. Int J Pharm Pract. (2022) 30(6):576–9. doi: 10.1093/ijpp/riac068
18. Zavaleta-Monestel E, Serrano-Arias B, Milano-Gil A, Sanchez-Solis C, Arroyo-Monterrosa DA, Munoz-Pichuante D, et al. Insights into clinical pharmacy practice in Latin America. J Am Coll Clin Pharm. (2023) 6(10):1103–6 doi: 10.1002/jac5.1854
19. Allemann SS, van Mil JWF, Botermann L, Berger K, Griese N, Hersberger KE. Pharmaceutical care: the PCNE definition 2013. Int J Clin Pharm. (2014) 36(3):544–55. doi: 10.1007/s11096-014-9933-x
20. Verrue CL, Mehuys E, Somers A, Van Maele G, Remon JP, Petrovic M. Medication administration in nursing homes: pharmacists contribution to error prevention. J Am Med Dir Assoc. (2010) 11(4):275–83. doi: 10.1016/j.jamda.2009.10.013
21. Cheng J, Dang CD, Li X, Wang JJ, Huang X, Li Y, et al. The participation of clinical pharmacists in the treatment of patients with central nervous system infection can improve the effectiveness and appropriateness of anti-infective treatments: a retrospective cohort study. Front Pharmacol. (2023) 14:1–9. doi: 10.3389/fphar.2023.1226333
22. Spinewine A, Swine C, Dhillon S, Lambert P, Nachega JB, Wilmotte L, et al. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. J Am Geriatr Soc. (2007) 55(5):658–65. doi: 10.1111/j.1532-5415.2007.01132.x
23. Jochen A, Holben D. School nurse perspectives of medical Cannabis policy in K-12 schools: an exploratory descriptive study. J Sch Nurs. (2022):1–12. doi: 10.1177/10598405221136288. [Epub ahead of print]
24. Wilson HB, McGrath LM. “It’s a big added stress on top of being so ill”: the challenges facing people prescribed cannabis in the UK. Int J Drug Policy. (2023) 122:1–9. doi: 10.1016/j.drugpo.2023.104220
25. Smart R, Pacula RL. Early evidence of the impact of cannabis legalization on cannabis use, cannabis use disorder, and the use of other substances: findings from state policy evaluations. Am J Drug Alcohol Abuse. (2019) 45(6):644–63. doi: 10.1080/00952990.2019.1669626
26. Poppe A, Maskileyson D. The effect of non-pharmaceutical policy interventions on COVID-19 transmission across three cities in Colombia. Front Public Health. (2022)10:1–9. doi: 10.3389/fpubh.2022.937644
27. Zhang Y, Liang SC, Zhu T. Knowledge, attitudes, and practices toward over-the-counter antipyretics among fever patients: a cross-sectional study in the context of a policy change KAP of OTC antipyretics. Front Public Health. (2023) 11:1–11. doi: 10.3389/fpubh.2023.1267171
28. Cadogan CA, Hughes CM. On the frontline against COVID-19: community pharmacists’ contribution during a public health crisis. Res Social Adm Pharm. (2021) 17(1):2032–5. doi: 10.1016/j.sapharm.2020.03.015
29. Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. (2021) 133:285–96. doi: 10.1016/j.jbusres.2021.04.070
30. da Rocha C, Carlotto J, Zanis Neto J. Analysis of the interventions in antineoplastic therapy by a clinical pharmacy service at a tertiary hospital in Brazil. J Oncol Pharm Pract. (2022) 28(5):1049–55. doi: 10.1177/10781552211017650
31. Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. (2003) 289(9):1107–16. doi: 10.1001/jama.289.9.1107
32. Poon EG, Keohane CA, Yoon CS, Ditmore M, Bane A, Levtzion-Korach O, et al. Effect of bar-code technology on the safety of medication administration. N Engl J Med. (2010) 362(18):1698–707. doi: 10.1056/NEJMsa0907115
33. Obreli-Neto PR, Guidoni CM, de Oliveira Baldoni A, Pilger D, Cruciol-Souza JM, Gaeti-Franco WP, et al. Effect of a 36-month pharmaceutical care program on pharmacotherapy adherence in elderly diabetic and hypertensive patients. Int J Clin Pharm. (2011) 33(4):642–9. doi: 10.1007/s11096-011-9518-x
34. Schroers G, Shrikanth S, Pfieffer J. Undergraduate nursing student experiences in American clinical learning environments: a descriptive study. Nurse Educ Today. (2023) 129:1–9. doi: 10.1016/j.nedt.2023.105895
35. Segal EM, Alwan L, Pitney C, Taketa C, Indorf A, Held L, et al. Establishing clinical pharmacist telehealth services during the COVID-19 pandemic. Am J Health Syst Pharm. (2020) 77(17):1403–8. doi: 10.1093/ajhp/zxaa184
36. Stuurman-Bieze AGG, de Boer WO, Kokenberg M, Hugtenburg JG, de Jong-van den Berg LTW, Tromp T. Complex pharmaceutical care intervention in pulmonary care—part A. The process and pharmacists’ professional satisfaction. Pharm World Sci. (2005) 27(5):376–84. doi: 10.1007/s11096-005-7112-9
37. Main B, Csanadi M, Ozieranski P. Pricing strategies, executive committee power and negotiation leverage in New Zealand’s containment of public spending on pharmaceuticals. Health Econ Policy Law. (2022) 17(3):348–65. doi: 10.1017/s1744133122000068
38. Upadhyay DK, Ibrahim MIM, Mishra P, Alurkar VM, Ansari M. Does pharmacist-supervised intervention through pharmaceutical care program influence direct healthcare cost burden of newly diagnosed diabetics in a tertiary care teaching hospital in Nepal: a non-clinical randomised controlled trial approach. Daru. (2016) 24:1–10. doi: 10.1186/s40199-016-0145-x
39. Sturgess IK, McElnay JC, Hughes CM, Crealey G. Community pharmacy based provision of pharmaceutical care to older patients. Pharm World Sci. (2003) 25(5):218–26. doi: 10.1023/a:1025860402256
40. Lancaster K, Duke K, Ritter A. Producing the “problem of drugs”: a cross national-comparison of “recovery” discourse in two Australian and British reports. Int J Drug Policy. (2015) 26(7):617–25. doi: 10.1016/j.drugpo.2015.04.006
41. Delcher C, Wagenaar AC, Goldberger BA, Cook RL, Maldonado-Molina MM. Abrupt decline in oxycodone-caused mortality after implementation of Florida’s prescription drug monitoring program. Drug Alcohol Depend. (2015) 150:63–8. doi: 10.1016/j.drugalcdep.2015.02.010
42. Li L, Liang R, Zhou YM. Design and implementation of hospital automatic nursing management information system based on computer information technology. Comput Math Methods Med. (2021) 2021:1–11. doi: 10.1155/2021/1824300
43. Giuliano KK, Mahuren RS, Balyeat J. Data-based program management of system-wide IV smart pump integration. Am J Health Syst Pharm. (2023) 81:1–7. doi: 10.1093/ajhp/zxad245
44. Murray MD, Young J, Hoke S, Tu W, Weiner M, Morrow D, et al. Pharmacist intervention to improve medication adherence in heart failure—a randomized trial. Ann Intern Med. (2007) 146(10):714–25. doi: 10.7326/0003-4819-146-10-200705150-00005
45. Rudis MI, Brandl KM, Soc Critical Care Med Amer Coll C. Position paper on critical care pharmacy services. Crit Care Med. (2000) 28(11):3746–50. doi: 10.1097/00003246-200011000-00037
46. Madden EA. The interaction between intellectual property and drug regulatory systems: global perspectives. Idrugs. (2007) 10(2):116–20. PMID: 1728546417285464
47. Brandao ER. Pharmaceutical care for emergency contraception users. Saude E Sociedade. (2017) 26(4):1122–35. doi: 10.1590/s0104-12902017000003
48. Gurwitz JH, Field TS, Harrold LR, Rothschild J, Debellis K, Seger AC, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. (2003) 289(9):1107–16. doi: 10.1001/jama.289.9.1107
49. Kassam R. Evaluation of pharmaceutical care opportunities within an advanced pharmacy practice experience. Am J Pharm Educ. (2006) 70(3):1–10. doi: 10.5688/aj700349
50. Haseeb A, Faidah HS, Al-Gethamy M, Iqbal MS, Alhifany AA, Ali M, et al. Evaluation of antimicrobial stewardship programs (ASPs) and their perceived level of success at Makkah region hospitals, kingdom of Saudi Arabia. Saudi Pharm J. (2020) 28(10):1166–71. doi: 10.1016/j.jsps.2020.08.005
51. Alanazi S, Dekhaela S, Obaidy S, Mutairi F, Majid K, Mufrij H, et al. Assessment of employee engagement in pharmaceutical care service at King Abdulaziz medical city-central region (KAMC): a cross-sectional study. Saudi Pharm J. (2023) 31(5):765–72. doi: 10.1016/j.jsps.2023.03.017
52. Alfaifi S, Bridges S, Arakawa N. Developing pharmacists? Competencies in Saudi Arabia: a proposed national competency framework to support initial education and professional development. Curr Pharm Teach Learn. (2022) 14(10):1256–68. doi: 10.1016/j.cptl.2022.09.010
53. Leape LL, Cullen DJ, Clapp MD, Burdick E, Demonaco HJ, Erickson JI, et al. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. (1999) 282(3):267–70. doi: 10.1001/jama.282.3.267
54. Cranor CW, Bunting BA, Christensen DB. The Asheville project: long-term clinical and economic outcomes of a community pharmacy diabetes care program. J Am Pharm Assoc (Wash). (2003) 43(2):173–84. doi: 10.1331/108658003321480713
55. Weinberger M, Murray MD, Marrero DG, Brewer N, Lykens M, Harris LE, et al. Effectiveness of pharmacist care for patients with reactive airways disease—a randomized controlled trial. JAMA. (2002) 288(13):1594–602. doi: 10.1001/jama.288.13.1594
56. Paoletti RD, Suess TM, Lesko MG, Feroli AA, Kennel JA, Mahler JM, et al. Using bar-code technology and medication observation methodology for safer medication administration. Am J Health Syst Pharm. (2007) 64(5):536–43. doi: 10.2146/ajhp060140
57. Koppel R, Wetterneck T, Telles JL, Karsh BT. Workarounds to barcode medication administration systems: their occurrences, causes, and threats to patient safety. J Am Med Inform Assoc. (2008) 15(4):408–23. doi: 10.1197/jamia.M2616
58. Westbrook JI, Woods A, Rob MI, Dunsmuir WTM, Day RO. Association of interruptions with an increased risk and severity of medication administration errors. Arch Intern Med. (2010) 170(8):683–90. doi: 10.1001/archinternmed.2010.65
59. Raban MZ, Westbrook JI. Are interventions to reduce interruptions and errors during medication administration effective?: a systematic review. BMJ Qual Saf. (2014) 23(5):414–21. doi: 10.1136/bmjqs-2013-002118
60. Chisholm-Burns MA, Lee JK, Spivey CA, Slack M, Herrier RN, Hall-Lipsy E, et al. US pharmacists’ effect as team members on patient care systematic review and meta-analyses. Med Care. (2010) 48(10):923–33. doi: 10.1097/MLR.0b013e3181e57962
61. Mossialos E, Courtin E, Naci H, Benrimoj S, Bouvy M, Farris K, et al. From “retailers” to health care providers: transforming the role of community pharmacists in chronic disease management. Health Policy. (2015) 119(5):628–39. doi: 10.1016/j.healthpol.2015.02.007
62. Keers RN, Williams SD, Cooke J, Ashcroft DM. Prevalence and nature of medication administration errors in health care settings: a systematic review of direct observational evidence. Ann Pharmacother. (2013) 47(2):237–56. doi: 10.1345/aph.1R147
63. Keers RN, Williams SD, Cooke J, Ashcroft DM. Causes of medication administration errors in hospitals: a systematic review of quantitative and qualitative evidence. Drug Saf. (2013) 36(11):1045–67. doi: 10.1007/s40264-013-0090-2
64. Dalton K, Byrne S. Role of the pharmacist in reducing healthcare costs: current insights. Integr Pharm Res Pract. (2017) 6:37–46. doi: 10.2147/iprp.S108047
65. Zheng S-Q, Yang L, Zhou P-X, Li H-B, Liu F, Zhao R-S. Recommendations and guidance for providing pharmaceutical care services during COVID-19 pandemic: a China perspective. Res Social Adm Pharm. (2021) 17(1):1819–24. doi: 10.1016/j.sapharm.2020.03.012
66. Visacri MB, Figueiredo IV, Lima T. Role of pharmacist during the COVID-19 pandemic: a scoping review. Res Social Adm Pharm. (2021) 17(1):1799–806. doi: 10.1016/j.sapharm.2020.07.003
67. Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. (2017) 17(1):230. doi: 10.1186/s12877-017-0621-2
68. Donaldson LJ, Kelley ET, Dhingra-Kumar N, Kieny MP, Sheikh A. Medication without harm: Who’s third global patient safety challenge. Lancet. (2017) 389(10080):1680. doi: 10.1016/s0140-6736(17)31047-4
69. Wondmieneh A, Alemu W, Tadele N, Demis A. Medication administration errors and contributing factors among nurses: a cross sectional study in tertiary hospitals, Addis Ababa, Ethiopia. BMC Nurs. (2020) 19(1):1–9. doi: 10.1186/s12912-020-0397-0
70. Baldoni S, Amenta F, Ricci G. Telepharmacy services: present status and future perspectives: a review. Medicina (Kaunas). (2019) 55(7):1–12. doi: 10.3390/medicina55070327
71. Li J, Zhu YH, Feng JN, Meng WJ, Begma K, Zhu GP, et al. A comparative study of international and Chinese public health emergency management from the perspective of knowledge domains mapping. Environ Health Prev Med. (2020) 25(1):1–15. doi: 10.1186/s12199-020-00896-z
72. Wang MX, Liu P, Zhang R, Li Z, Li X. A scientometric analysis of global health research. Int J Environ Res Public Health. (2020) 17(8):1–19. doi: 10.3390/ijerph17082963
73. Watari T, Nakano Y, Gupta A, Kakehi M, Tokonami A, Tokuda Y. Research trends and impact factor on PubMed among general medicine physicians in Japan: a cross-sectional bibliometric analysis. Int J Gen Med. (2022) 15:7277–85. doi: 10.2147/ijgm.S378662
74. Baesso KCB, do Nascimento DZ, Soares AD, Schuelter-Trevisol F. Use of tracking drugs for the search of intra-hospital adverse reactions: a pharmacovigilance study. Farm Hosp. (2022) 46(3):146–51. doi: 10.7399/fh.13039
75. Akyon SH, Akyon FC, Yilmaz TE. Artificial intelligence-supported web application design and development for reducing polypharmacy side effects and supporting rational drug use in geriatric patients. Front Med (Lausanne). (2023) 10:1–16. doi: 10.3389/fmed.2023.1029198
76. Gordo C, Núñez-Córdoba JM, Mateo R. Root causes of adverse drug events in hospitals and artificial intelligence capabilities for prevention. J Adv Nurs. (2021) 77(7):3168–75. doi: 10.1111/jan.14779
77. Bu FJ, Sun H, Li L, Tang FM, Zhang XW, Yan JC, et al. Artificial intelligence-based internet hospital pharmacy services in China: perspective based on a case study. Front Pharmacol. (2022) 13:1–10. doi: 10.3389/fphar.2022.1027808
78. Li XH, Tan BR, Zheng JK, Xu XM, Xiao J, Liu YL. The intervention of data mining in the allocation efficiency of multiple intelligent devices in intelligent pharmacy. Comput Intell Neurosci. (2022) 2022:1–12. doi: 10.1155/2022/5371575
79. Naimat F, Fahrni ML, Purushothaman S, Ghani MNA, Chumnumwat S, Babar ZUD. Community pharmacists’ perceived value on precision medicine, desired training components, and exposure during pharmacy education: Malaysia’s experience. Front Pharmacol. (2022) 13:1–11. doi: 10.3389/fphar.2022.978141
80. Huttin CC. Global value chains and international pharmaceutical policy. Technol Health Care. (2020) 28(3):337–44. doi: 10.3233/thc-192103
81. Aqqad F, Meilianti S, John C, Koudmani D, Akel M, Bates I. Needs assessment of global pharmaceutical development goals: an explanatory mixed-methods study of 21 countries. Int J Pharm Pract. (2023) 2023:29–38. doi: 10.1093/ijpp/riad078
82. Fei BN, Zhao J, Li X, Tang YM, Qin GY, Zhang W, et al. Randomised parallel trial on the effectiveness and cost-effectiveness in screening gait disorder of silent cerebrovascular disease assisted by artificial intelligent system versus clinical doctors (ACCURATE-1): study protocol. BMJ Open. (2022) 12(3):1–6. doi: 10.1136/bmjopen-2021-055880
83. Gyngell C, Stark Z, Savulescu J. Drugs, genes and screens: the ethics of preventing and treating spinal muscular atrophy. Bioethics. (2020) 34(5):493–501. doi: 10.1111/bioe.12695
84. Gore C, Morin S, Rottingen JA, Kieny MP. Negotiating public-health intellectual property licensing agreements to increase access to health technologies: an insider’s story. BMJ Glob Health. (2023) 8(9):1–8. doi: 10.1136/bmjgh-2023-012964
Keywords: pharmaceutical management, mapping knowledge domain, co-occurrence analysis, co-citation analysis, burst detection
Citation: Shen J, Wei S, Guo J, Xu S, Li M, Wang D and Liu L (2024) Evolutionary trend analysis of the pharmaceutical management research field from the perspective of mapping the knowledge domain. Front. Health Serv. 4:1384364. doi: 10.3389/frhs.2024.1384364
Received: 9 February 2024; Accepted: 25 June 2024;
Published: 11 July 2024.
Edited by:
Chandra Dhakal, CDC Foundation, United StatesReviewed by:
Uttam Dhakal, Youngstown State University, United StatesKeshob Sharma, University of Georgia, United States
© 2024 Shen, Wei, Guo, Xu, Li, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Liu, nanyangliuling@163.com
 Junkai Shen
Junkai Shen Sen Wei2
Sen Wei2