
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Health Serv. , 19 January 2024
Sec. Implementation Science
Volume 3 - 2023 | https://doi.org/10.3389/frhs.2023.1310694
This article is part of the Research Topic Learning for Action in Policy Implementation View all 12 articles
 Arianna Rubin Means1,2*
Arianna Rubin Means1,2* Kellie List1
Kellie List1 Amy Roll1,2
Amy Roll1,2 Marie-Claire Gwayi-Chore1,2
Marie-Claire Gwayi-Chore1,2 Shawn Dolley3
Shawn Dolley3 Holger J. Schünemann4,5
Holger J. Schünemann4,5 Thea C. Norman6
Thea C. Norman6 Judd L. Walson1,2,7
Judd L. Walson1,2,7
Introduction: Soil-transmitted helminths (STH) are parasitic worms that infect nearly a quarter of the world's population, particularly those living in communities without access to adequate water, sanitation, and housing. Emerging evidence suggests that it may be possible to interrupt transmission of STH by deworming individuals of all ages via community-wide MDA (cMDA), as opposed to only treating children and other focal populations. Transitioning from a policy of STH control to STH elimination in targeted areas would require a fundamental shift in STH policy and programming. This policy change would require updated guidance to support countries as they adapt their current approaches for STH surveillance, supply chain management, community mobilization, and core programmatic activities in pursuit of STH elimination. There is an opportunity to engage with key stakeholders, such as program implementers and implementation partners, to understand what evidence they need to confidently adopt a new policy guideline and to deliver guideline adherent management at scale.
Methods: We aimed to engage with STH stakeholders to develop a Target Policy Profile (TPoP), a single document that describes optimal characteristics and evidence requirements that STH stakeholders prioritized in future potential STH transmission interruption efforts. Steps in TPoP development included a scoping review and key informant interviews (KIIs), which were used to design a two-stage Delphi technique to identify and verify TPoP components.
Results: The scoping review resulted in 25 articles, and 8 experts participated in KII's. Twenty respondents completed the first Delphi survey and 10 respondents completed the second. This systematic effort resulted in a net of 3 key information domains (background/context, clinical considerations, and implementation considerations) encompassing 24 evidence categories (examples include evidence regarding safety and adverse events, implementation feasibility, or evidence dissemination). For each evidence category, STH stakeholders reviewed, endorsed, or revised a range of options for how the evidence could be presented.
Discussion: This information can be used by guideline committees or global policy makers prior to convening guideline advisory groups. The TPoP tool may also speed the process of stakeholder consensus building around guidelines, accelerating progress towards implementing evidence-based policy at scale.
Soil-transmitted helminths (STH) are intestinal parasitic worms that infect nearly a quarter of the world's population, particularly those living in communities without access to adequate water, sanitation, and housing (1). When individuals have heavy-to-moderate intensity infections with STH, they may experience adverse outcomes such as diarrhea, weakness, malnutrition and impaired growth in children, and chronic anemia in women of reproductive age (WRA) (2). The current standard-of-care for controlling STH-associated morbidities in current WHO guidelines includes annual or bi-annual preventive chemotherapy delivered via mass drug administration (MDA), which requires large-scale delivery of deworming medications to all eligible pre-school and school-age children and WRA living in at-risk areas. MDA for STH control is often delivered via school-based delivery platforms (i.e., school-based MDA) that engage both teachers and volunteer community drug distributors (CDDs) as the primary implementers for reaching pre-school and school-age children (3, 4).
Morbidity control programs using school-based MDA have been successful in many settings, however in the absence of continued treatment such programs may need to be continued indefinitely, or at least until major improvements in infrastructure and sanitation can be realized (5). Emerging evidence suggests that it may be possible to interrupt transmission of STH by deworming individuals of all ages via community-wide MDA (cMDA), as opposed to only treating children and other focal populations (6–8). A cMDA approach would reduce the presence of adult reservoirs of infection in the community and the risk of re-infection for individuals post-deworming (9). Field trials and observational studies are currently underway to determine definitively whether transmission interruption via cMDA is feasible (10, 11). While several similar neglected tropical disease (NTD) programs, such as lymphatic filariasis (LF), onchocerciasis, and trachoma programs currently target entire populations with treatment during MDA, transitioning from a policy of STH control to STH elimination in targeted areas would require a fundamental shift in STH programming. This policy change would require updated guidance to support countries as they adapt their current approaches for STH surveillance, supply chain management, community mobilization, impact assessment, and other core programmatic activities in pursuit of STH elimination.
The World Health Organization (WHO) has developed a rigorous process for creating, updating, and approving clinical, public health, and health policy guidelines (12, 13). Briefly, standard guidelines are produced following requests for guidance, often from endemic country governments, and typically following the release of promising new evidence or interventions. Once a guideline development or updating process is initiated, advisory groups develop questions and outcomes for the guidelines to address. These groups also prioritize which questions require systematic reviews of the evidence to inform subsequent recommendations. A guideline development group (GDG) composed of external experts appraises existing evidence summarized and assessed by an evidence review team using systematic review methodology and the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach (14). The guidelines also undergo multiple rounds of review prior to approval from the WHO Guidelines Review Committee (GRC). Many guidelines are also accompanied by operational guides to support country governments in implementing new recommendations. The WHO Handbook for guidelines requests Evidence to Decision (EtD) frameworks, such as the GRADE-EtD, to be used as tools for guideline panels to move from evidence to recommendations by considering and discussing evidence within the context of a list of key criteria, such as the “certainty of the evidence”, “balance of effects”, “cost”, “equity”, “feasibility”, and “acceptability” (15, 16).
Before initiating a guideline creation or updating process, there is an opportunity to engage with key stakeholders, such as program implementers and implementation partners (ex. non-governmental organizations, NGOs), to understand what evidence they need to confidently adopt a new policy guideline and to deliver guideline adherent management at scale. As increasing evidence is emerging suggesting the possibility of interrupting the transmission of STH and the recognition that a shift in future STH guidelines towards transmission interruption would require updates to future guidelines, we aimed to engage with STH stakeholders to develop a target policy profile (TPoP). The purpose of the TPoP is to systematically describe the optimal characteristics and requirements of evidence to include in clinical and operational guidelines for future potential STH transmission interruption efforts (17). A TPoP would in no way replace established WHO or national-level guideline development processes. Rather, findings from the TPoP could be used by stakeholders, including potentially a WHO steering group and GDG, prior to starting a guideline develop process in order to understand STH stakeholder priorities for guidance, and to consider what types of evidence would be most helpful to include in an updated STH guideline and/or accompanying operational guidance documents.
The objective of the TPoP development process was to describe optimal and minimally acceptable evidence desired by STH stakeholders in the context of guidance for potential STH transmission interruption efforts. Steps in TPoP development included a scoping review and key informant interviews (KIIs), which were used to design a two-stage Delphi technique to identify and verify TPoP components (Figure 1).
Scoping reviews can be conducted to clarify concepts and examine characteristics of a specific concept (18). Here we conducted a scoping review to understand categories of evidence that have been used to shape guidelines focused specifically on launching or scaling-up community-wide interventions, such as MDA for STH elimination. A list of search terms was developed to conduct online searches on PubMed, Google Scholar, Google, Global Health Database, PAIS Index, Scopus, and Web of Science databases (Supplementary Material S1). Abstracts were reviewed for relevance and full texts downloaded when appropriate. Upon identifying relevant texts, we also employed citation chaining and reviewed works cited for additional resources.
We used an Excel-based abstraction database to track articles included in the review. The spreadsheet included a summary of the article and descriptions of evidence that the article noted could or should inform guideline development. A single reviewer abstracted data and an additional author reviewed abstractions, referring to full text articles when necessary. We determined that we reached review saturation when no new or unique descriptions of evidence needed to inform guidelines emerged.
We sorted the evidence descriptions identified from the review into broad groupings informed by the WHO 2017 guidelines for preventative chemotherapy for STH and a prior TPoP developed for other initiatives (17, 19). These groupings were henceforth referred to as “evidence categories”. Categories of evidence that were similar to one another (e.g., overlapping definitions) were then refined into a single evidence category to be included in the TPoP. These evidence categories were then used to design interview guides for subsequent KIIs and develop the template for the first TPoP prototype.
To further refine categories of evidence ahead of the Delphi process, we conducted KII with STH stakeholders with expert knowledge on prior STH guideline development including WHO staff, technical experts, and country-level NTD program managers. We used a semi-structured interview guide to solicit information about the guideline updating and development process, TPoP specifications, and proposed categories of evidence. Fourteen individuals were purposively identified and invited to participate in interviews. An interviewer and notetaker were present during all interviews. All interviews took place over Zoom and were recorded following verbal consent. Key insights and highlights from the interviews were summarized using a matrix approach, deductively organized by proposed categories of evidence (20, 21). Newly proposed categories of evidence were inductively added to the matrix, as appropriate, and data summarized accordingly. Following interviews with key stakeholders, we undertook a second iteration of editing to incorporate stakeholder feedback into proposed TPoP categories of evidence.
Following KIIs, a two-round Delphi method was used to solicit feedback about the TPoP prototype and finalize the TPoP tool. The Delphi method includes iterative “rounds” in which experts are asked their opinion on a particular issue, and questions for each round are based in part on the findings from the previous round (22). We used a series of two REDCap-based surveys that were emailed to individuals who participated in the KIIs and additional STH and NTD policymakers and technical experts (N = 75 individuals invited in total). Invitees were sent one email reminder to participate and were not offered incentives to complete the surveys.
During the first Delphi survey, participants were presented with possible TPoP evidence categories (e.g., groupings of types of evidence that may be included in a future guideline) and asked to rate each evidence category on a 1–3 scale (23), with 1 being “not necessary” evidence for inclusion in a future guideline or policy, 2 being “desirable but not necessary” evidence for inclusion in a future guideline or policy, and 3 being “necessary” evidence for inclusion in a future guideline or policy. We calculated the mean score and the proportion of respondents indicating an evidence category was “necessary” for inclusion. Evidence categories with a mean score above 2.5 and proportion of “necessary” responses greater than or equal to 60% were deemed potentially important for inclusion in future STH guidelines and were included in the revised TPoP (third iteration). Participants were not asked to rate 15 of the proposed evidence categories, as these categories were deemed a priori as mandatory for inclusion because they are criteria within the EtD framework.
The purpose of the second survey was to incorporate feedback from the first survey regarding the evidence categories that should be addressed in future guidelines and define what “optimal” or “minimally acceptable” evidence would include within each category. Survey respondents were provided a brief overview of findings from the first Delphi survey, and then were asked to review minimally acceptable and optimal characteristics of potential evidence categories to include in a future STH guideline. “Optimal” characteristics represented ideal attributes of evidence while “minimally acceptable” characteristics described the necessary basic level of evidence to be included in future guidelines. For example, evidence regarding “surveillance” could range from minimally acceptable levels of “provides surveillance guidance that includes clear criteria (thresholds) for starting and stopping community-wide MDA” to optimal levels of “provides surveillance guidance that includes clear criteria (thresholds) for starting and stopping community-wide MDA, monitoring for recrudescence, and verifying transmission interruption. Additionally includes guidance for use of existing and new diagnostics, including drug resistance surveillance.”
Participants were asked if they agree or disagree with the proposed approaches to defining optimal and minimally acceptable characteristics of each evidence category. Participants were also provided space for qualitative reactions to each description of optimal and minimally acceptable evidence, if they chose to provide one. We identified “optimal” and “minimally acceptable” characteristics with particularly high approval and low approval. High approval was defined as 80% or more of respondents agreed with how an evidence category was characterized. Low approval was defined as 50% or fewer respondents disagree with how a category was characterized.
The study was approved by The Human Subjects Division at the University of Washington (STUDY00000180).
This project systematically engaged stakeholders to learn about the type and depth of information that they seek in future STH guidelines that might target the interruption of transmission of STH. The results of this analysis provide a range of approved “optimal” and “minimally acceptable” categories of evidence that may support implementers of future STH elimination guidelines or operational documents.
The scoping review search yielded 75 potential articles, 25 of which included relevant information about evidence needed to guide scale-up of community-wide interventions. These articles included 504 potential evidence categories. We grouped similar evidence categories and removed any duplicates. We further organized evidence categories into nine broad domains: background, evidence of effectiveness, intervention costs and benefits, contextual considerations, partnerships, implementation considerations, intervention/product details, existing use of the intervention, and dissemination. After this process, a total of 51 unique evidence categories were identified and included in the first iteration of the TPoP.
Fourteen individuals were invited to participate in KIIs and eight individuals ultimately participated (response rate of 57%). This included two individuals based at the WHO, four individuals who had been involved in previous relevant GDGs, and two individuals who had led national STH programs. Many key informants noted that evidence included in existing STH guidelines has been perceived as minimal and incomplete. KIIs noted that guidelines have included limited or no evidence related to program duration, outcome certification, feasibility, acceptability, and other aspects of implementation. They noted that this may be, in part, because the methods used to collect this evidence are not from randomized trials and therefore traditionally receive lower assessments of rigor using GRADE domains. There are also evidence gaps, such as the inclusion of cost of implementation data, that need to be addressed in future guidelines. Should there be a future policy shift, adding specific milestones for when a country might be eligible for cMDA will help motivate countries to move from control to elimination.
In addition to providing feedback about proposed evidence categories, key informants also provided feedback that coalesced into two additional main themes. First, many interviewees noted that guidelines will be most impactful if there are updates to how evidence is presented. For example, current STH guidance from the WHO is scattered across guidelines, technical manuals, and M&E plans, which poses challenges for implementers. Consolidating guidance and implementation information would make it easier for implementers to apply recommendations in their setting. Several respondents noted that guidelines should be simple but with sufficient detail needed to guide countries with STH programs of varying levels of maturity.
KIIs also noted that there may be opportunities to speed the evidence-to-recommendation process, even before guideline committees are convened. For example, trials can sign memoranda of understanding that allow their results to be pooled in systematic reviews as soon as they are available, parallel to the publishing of primary outcomes. The evidence-to-recommendation process would also be improved by engaging a more heterogenous mix of experts and linking STH evidence to evidence from other NTDs or even universal health coverage (UHC) endeavors.
Information from the KIIs helped refine the draft TPoP by reducing the number of proposed evidence categories from 51 to 41, across six refined domains and sub-domains, including: background and context, clinical considerations, and implementation considerations (including sub-domains of community considerations, distribution considerations, health system considerations, and partnership considerations.
Twenty individuals responded to the first Delphi survey (27% response rate). Four evidence categories were deemed unnecessary and removed from the TPoP based on a priori criteria described above: incentive systems, regulatory/legal context, public-private partnerships, and civil-society partnerships. The revised TPoP incorporated stakeholder feedback and included 37 evidence categories.
Ten individuals responded to the second Delphi survey (13% response rate). We identified higher agreement for “optimal” descriptions of evidence, as compared to the “minimally acceptable” descriptions of evidence. Thirty (81%) of the evidence categories had high approval of their proposed optimal characteristics. Meanwhile, only 13 (35%) of the evidence categories had high approval of their proposed minimally acceptable characteristics (Table 1, with category-levels of evidence presented in Supplementary Table S2).
Many respondents qualitatively responded that the “minimally acceptable” descriptions of evidence were too basic and, in many cases, the “optimal” levels of evidence should be considered the only option (e.g., that only the presented optimal characteristics of evidence would be acceptable in future guidelines). Other qualitative responses include that narrative reviews may be just as helpful as systematic reviews for certain evidence categories and could in fact speed the evidence-to-recommendations process, that the presentation of systematic reviews can be confusing content in guidelines and presentation should be simplified, and that stakeholders value very clear and concise recommendations/guidance. Lastly, several respondents noted that the guidelines should focus on endemic countries as the target users and recommendations should be accompanied with detailed information on best practices for operationalizing the recommendations.
Based upon these responses, we updated 30 optimal and/or minimally acceptable characteristics of evidence across 24 evidence categories (65% of all evidence categories), most of which were minor adaptations to include respondent clarifications and preferences (Table 2).
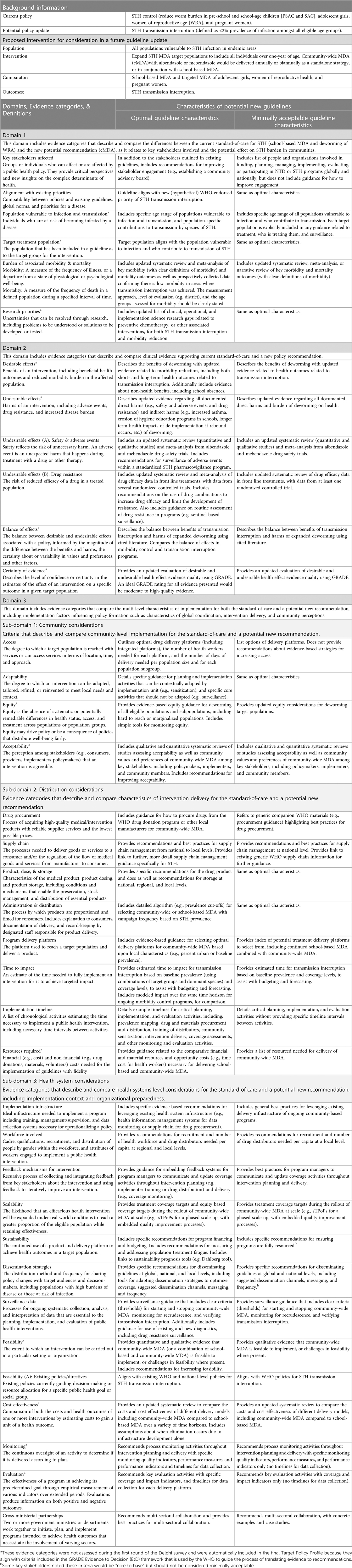
Table 2. Target policy profile, including optimal and minimally acceptable characteristics of evidence.
With new evidence regarding the feasibility of achieving STH transmission through cMDA emerging in the near future, there may be opportunity to revisit guideline content and scope in future updates. This study included a participatory approach to soliciting and incorporating feedback from key STH stakeholders in planning for such possible updates. Following multiple rounds of stakeholder engagement, we created a final TPoP that includes categories of evidence and characteristics of evidence that may be useful in introducing and implementing future STH policies.
During the second of a two-cycle Delphi survey, participants were asked not only to provide a final endorsement of evidence categories to include in future guidelines and/or associated operational materials, but also to provide feedback on the range of evidence characteristics that could be included. We found that most of the proposed “optimal characteristics” of evidence were approved by survey respondents (Table 1). In contrast, only about one-third of “minimally acceptable characteristics” of evidence were approved by survey respondents and respondents often thought the minimal levels of proposed evidence would be insufficient for future guidelines. This highlights that stakeholders generally sought more detailed guidance. The evidence categories that consistently had lowest approval reflect topics of ongoing controversy within STH literature. For example, the evidence categories of burden of associated morbidity and mortality had low approval of both optimal and minimally acceptable proposed levels of evidence. This may reflect ongoing controversies around the burden of STH-associated morbidities and methods used to detect STH-associated outcomes (24). We also observed lower approval of evidence regarding desirable effects and undesirable effects related to drug resistance and adverse events, for optimal and minimally acceptable characteristics of evidence. This may reflect mixed perceptions of the risks of clinically relevant resistance to deworming medications in humans or adverse events, and simultaneous recognition that cMDA would increase drug pressure and the number of adverse events as more people are treated (25, 26).
A TPoP would be useful for guideline committees or global policy makers prior to convening guideline advisory groups. Because the TPoP incorporates stakeholder priorities, global policy makers can use it to assess where existing evidence falls within identified minimally acceptable and optimal ranges, where there are gaps in evidence that need to be addressed prior to guideline updating, what questions should be answered within a guideline update, and if there are other criteria that could be added to EtD frameworks used during the evidence-to-recommendation process. In particular, the “implementation considerations” such as program delivery platform and time till impact that were proposed in the TPoP may be valuable additions to the EtD. The implementation considerations domain, including sub-domains of community, distribution, and health systems considerations, was highly endorsed during KIIs and the first Delphi round. In the second Delphi survey only two of 25 evidence categories in this domain received low approval endorsements (both for proposed minimally acceptable levels of evidence). This highlights that evidence about implementation is highly valued by guideline stakeholders, including both guidance for how to operationalize guidelines but also rigorous evidence regarding best practices for implementers.
Target product profiles (TPPs) have long been used as planning tools to guide the development of new technologies to ensure that they meet necessary design specifications (27). A TPoP could similarly be used during early policy development as a collaborative approach to understanding stakeholder priorities. A similar initiative was undertaken to identify vaccine-related evidence anticipated to facilitate global policy recommendations (28). The Evidence Considerations for Vaccine Policy (ECVP) initiative, based on a tool developed by the Bill & Melinda Gates Foundation called the Target Policy Profile, developed a tool to identify the anticipated clinical trial and observational data or evidence that could support WHO and/or policy decision making for new vaccines (17). Like the ECVP, the STH TPoP does not preclude or supersede independent guideline convenings or GRADE-based recommendations.
While this study used a series of participatory approaches to generate robust information about evidence that could inform policy and guidelines, there are a number of limitations to using participatory approaches like a Delphi technique. For example, this approach does not include live conversations, which may limit generation of new and creative ideas. We also did not have a third round of Delphi surveys for participants to verify final amendments to TPoP category descriptions. In addition, the study had a relatively low sample of engaged experts and participation rates were not optimal. The degree to which these findings are generalizable is influenced by the perspectives and positionality of the included experts. However, because the STH community is relatively small, we feel confident that a small sample size of key experts can have a deep understanding of STH implementer experiences and important insights into the challenges at hand. Finally, the formative scoping review in this study was used to map a body of literature and was therefore not systematic; a systematic approach to synthesizing evidence about factors influencing evidence uptake for community-based interventions may also be useful in the future to ensure that new guidelines are successfully implemented. Despite these limitations, the systematic approach undertaken in this study provided the opportunity to garner feedback and ideas from a heterogenous mix of STH stakeholders to co-envision next steps for STH guidance.
We developed a TPoP using participatory methods to guide decision makers as they consider updating STH guidelines, including for guidelines to support a potential transition from STH control to STH transmission interruption. The TPoP reflects areas of evidence, ranging from clinical to pragmatic implementation evidence, that are important to a wide array of STH stakeholders and can be used to craft guidelines and operational materials that are appropriate and useful for guiding future implementation at scale.
The datasets presented in this article are not readily available because the raw datasets may contain identifiable information such as the participants place of work or past experiences. Redacted datasets are available upon request. Requests to access the datasets should be directed toZGV3b3JtM0B1dy5lZHU=.
The studies involving humans were approved by The Human Subjects Division at the University of Washington. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this research was determined to be minimal risk, and verbal consent was obtained from key informants as a result.
AM: Conceptualization, Methodology, Supervision, Writing – original draft. KL: Data curation, Investigation, Writing – review & editing. AR: Conceptualization, Data curation, Investigation, Writing – review & editing. MG: Data curation, Investigation, Methodology, Writing – review & editing. SD: Conceptualization, Writing – review & editing. HS: Conceptualization, Supervision, Writing – review & editing. TN: Conceptualization, Writing – review & editing. JW: Conceptualization, Methodology, Supervision, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This work was funded by the Bill & Melinda Gates Foundation (INV-017893).
We would like to acknowledge the contributions of individuals who participated in key informant interviews and Delphi technique surveys for their time and contributions to this work. We would also like to acknowledge the leaders of soil-transmitted helminth programs in endemic countries who work tirelessly to implement evidence-based recommendations.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhs.2023.1310694/full#supplementary-material
Supplementary Table S1
TPoP scoping review search terms.
Supplementary Table S2
Category-level findings from second round of Delphi technique.
1. WHO. Soil-Transmitted Helminth Infections. (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (Accessed June 21, 2023).
2. Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG. Soil-transmitted helminth infections. Lancet. (2018) 391(10117):252–65. doi: 10.1016/S0140-6736(17)31930-X
3. WHO. Preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. Geneva: World Health Organization (2017).
4. Macfarlane CL, Dean L, Thomson R, Garner P. Community drug distributors for mass drug administration in neglected tropical disease programmes: systematic review and analysis of policy documents. J Glob Health. (2019) 9(2). doi: 10.7189/jogh.09.020414
5. Anderson R, Truscott J, Hollingsworth TD. The coverage and frequency of mass drug administration required to eliminate persistent transmission of soil-transmitted helminths. Philos Trans R Soc B. (2014) 369(1645):20130435. doi: 10.1098/rstb.2013.0435
6. Clarke NE, Clements AC, Doi SA, Wang D, Campbell SJ, Gray D, et al. Differential effect of mass deworming and targeted deworming for soil-transmitted helminth control in children: a systematic review and meta-analysis. Lancet. (2017) 389(10066):287–97. doi: 10.1016/S0140-6736(16)32123-7
7. Pullan RL, Halliday KE, Oswald WE, Mcharo C, Beaumont E, Kepha S, et al. Effects, equity, and cost of school-based and community-wide treatment strategies for soil-transmitted helminths in Kenya: a cluster-randomised controlled trial. Lancet. (2019) 393(10185):2039–50. doi: 10.1016/S0140-6736(18)32591-1
8. Brooker SJ, Mwandawiro CS, Halliday KE, Njenga SM, Mcharo C, Gichuki PM, et al. Interrupting transmission of soil-transmitted helminths: a study protocol for cluster randomised trials evaluating alternative treatment strategies and delivery systems in Kenya. BMJ Open. (2015) 5(10):e008950. doi: 10.1136/bmjopen-2015-008950
9. Njenga SM, Mwandawiro CS, Muniu E, Mwanje MT, Haji FM, Bockarie MJ. Adult population as potential reservoir of NTD infections in rural villages of kwale district, coastal Kenya: implications for preventive chemotherapy interventions policy. Parasit Vectors. (2011) 4(1):1–6. doi: 10.1186/1756-3305-4-175
10. Ásbjörnsdóttir KH, Ajjampur SSR, Anderson RM, Bailey R, Gardiner I, Halliday KE, et al. Assessing the feasibility of interrupting the transmission of soil-transmitted helminths through mass drug administration: the DeWorm3 cluster randomized trial protocol. PLoS Negl Trop Dis. (2018) 12(1):e0006166. doi: 10.1371/journal.pntd.0006166
11. Mekete K, Ower A, Dunn J, Sime H, Tadesse G, Abate E, et al. The geshiyaro project: a study protocol for developing a scalable model of interventions for moving towards the interruption of the transmission of soil-transmitted helminths and schistosome infections in the wolaita zone of Ethiopia. Parasit Vectors. (2019) 12:1–12. doi: 10.1186/s13071-019-3757-4
13. Schünemann HJ, Fretheim A, Oxman AD. Improving the use of research evidence in guideline development: 1. Guidelines for Guidelines. Health Res Policy Syst. (2006) 4:1–6.
14. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J. (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
15. Rosenbaum SE, Moberg J, Glenton C, Schünemann HJ, Lewin S, Akl E, et al. Developing evidence to decision frameworks and an interactive evidence to decision tool for making and using decisions and recommendations in health care. Glob Chall. (2018) 2(9):1700081. doi: 10.1002/gch2.201700081
16. Meneses-Echavez JF, Bidonde J, Yepes-Nuñez JJ, Poklepovic Pericic T, Puljak L, Bala MM, et al. Evidence to decision frameworks enabled structured and explicit development of healthcare recommendations. J Clin Epidemiol. (2022) 150:51–62. doi: 10.1016/j.jclinepi.2022.06.004
17. Dolley S, Hartman D, Norman T, Hudson I. DAC Target Policy Profile (TPoP). (2021). Available at: https://dac-trials.tghn.org/resources/target-policy-profile-overview/tpop-doi-landing-page/. (Accessed June 21, 2023).
18. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. (2018) 18:1–7. doi: 10.1186/s12874-018-0611-x
19. WHO. Guideline: Preventive chemotherapy to control soil-transmitted helminth infections in at-risk population groups. World Health Organization (2017).
20. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. (2002) 12(6):855–66. doi: 10.1177/104973230201200611
21. Lewinski AA, Crowley MJ, Miller C, Bosworth HB, Jackson GL, Steinhauser K, et al. Applied rapid qualitative analysis to develop a contextually appropriate intervention and increase the likelihood of uptake. Med Care. (2021) 59(6 Suppl 3):S242. doi: 10.1097/MLR.0000000000001553
22. Nasa P, Jain R, Juneja D. Delphi methodology in healthcare research: how to decide its appropriateness. World J Methodol. (2021) 11(4):116. doi: 10.5662/wjm.v11.i4.116
23. Lange T, Kopkow C, Lützner J, Günther KP, Gravius S, Scharf HP, et al. Comparison of different rating scales for the use in Delphi studies: different scales lead to different consensus and show different test-retest reliability. BMC Med Res Methodol. (2020) 20(1):1–11. doi: 10.1186/s12874-020-0912-8
24. Campbell SJ, Nery SV, Doi SA, Gray DJ, Soares Magalhães RJ, et al. Complexities and perplexities: a critical appraisal of the evidence for soil-transmitted helminth infection-related morbidity. PLoS Negl Trop Dis. (2016) 10(5):e0004566. doi: 10.1371/journal.pntd.0004566
25. Pilotte N, Manuel M, Walson JL, Ajjampur SSR. Community-wide mass drug administration for soil-transmitted helminths–risk of drug resistance and mitigation strategies. Front Trop Dis. (2022) 3:897155. doi: 10.3389/fitd.2022.897155
26. Joseph SA, Montresor A, Casapía M, Pezo L, Gyorkos TW. Adverse events from a randomized, multi-arm, placebo-controlled trial of mebendazole in children 12–24 Months of age. Am J Trop Med Hyg. (2016) 95(1):83.27139441
27. WHO. WHO Target Product Profiles. Target Product Profiles 2023. Available at: https://www.who.int/observatories/global-observatory-on-health-research-and-development/analyses-and-syntheses/target-product-profile/who-target-product-profiles. (Accessed June 16, 2023).
Keywords: soil-transmitted helminths, neglected tropical diseases, guidelines, Delphi, policy implementation, participatory methods
Citation: Means AR, List K, Roll A, Gwayi-Chore M-C, Dolley S, Schünemann HJ, Norman TC and Walson JL (2024) Participatory development of a target policy profile to support soil-transmitted helminth elimination. Front. Health Serv. 3:1310694. doi: 10.3389/frhs.2023.1310694
Received: 9 October 2023; Accepted: 21 December 2023;
Published: 19 January 2024.
Edited by:
Michael Trisolini, Northeastern University, United StatesReviewed by:
Maria Victoria Periago, National Scientific and Technical Research Council (CONICET), Argentina© 2024 Means, List, Roll, Gwayi-Chore, Dolley, Schünemann, Norman and Walson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arianna Rubin Means YWVydWJpbkB1dy5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.