Abstract
Background:
Patients with genetic cancer susceptibility are presented with complex management options involving difficult decisions, for example about genetic testing, treatment, screening and risk-reducing surgery/medications. This review sought to explore the experience of patients using decision support resources in this context, and the impact on decision-making outcomes.
Methods:
Systematic review of quantitative, qualitative and mixed-methods studies involving adults with or without cancer who used a decision support resource pre- or post-genetic test for any cancer susceptibility. To gather a broad view of existing resources and gaps for development, digital or paper-based patient resources were included and not limited to decision aids. Narrative synthesis was used to summarise patient impact and experience.
Results:
Thirty-six publications describing 27 resources were included. Heterogeneity of resources and outcome measurements highlighted the multiple modes of resource delivery and personal tailoring acceptable to and valued by patients. Impact on cognitive, emotional, and behavioural outcomes was mixed, but mainly positive. Findings suggested clear potential for quality patient-facing resources to be acceptable and useful.
Conclusions:
Decision support resources about genetic cancer susceptibility are likely useful to support decision-making, but should be co-designed with patients according to evidence-based frameworks. More research is needed to study impact and outcomes, particularly in terms of longer term follow-up to identify whether patients follow through on decisions and whether any increased distress is transient. Innovative, streamlined resources are needed to scale up delivery of genetic cancer susceptibility testing for patients with cancer in mainstream oncology clinics. Tailored patient-facing decision aids should also be made available to patients identified as carriers of a pathogenic gene variant that increases future cancer risks, to complement traditional genetic counselling.
Systematic Review Registration:
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020220460, identifier: CRD42020220460.
1. Introduction
Patients who have cancer susceptibility gene testing and are found to carry a pathogenic variant that causes increased future cancer risks (“carriers”) are presented with choices about primary prevention (e.g., risk-reducing surgery, chemoprevention, changes in diet), screening (e.g., earlier, more frequent mammogram/colonoscopy) and treatment for cancer or premalignant conditions (1, 2). They are also encouraged to communicate with at-risk relatives so they can be offered cascade predictive testing (3). Genetic testing has traditionally been supported through genetic counselling: the “process of helping people understand and adapt to the medical, psychological and familial implications of genetic contributions to disease” (4). Genetic counselling promotes personalised, values-based decision-making made possible through formation of a therapeutic alliance during the consultation (5). Increased importance of genomic test results to guide cancer treatment (6–10) and calls for population screening to identity carriers and offer targeted treatment, prevention and surveillance options to high risk groups (11–13) have created increased pressure on already stretched genetics and oncology services. Knowledge can be increased through genetic counselling (14), but people may not accept that risks apply to them (15, 16) or events will happen to them personally, for example due to framing (17) and anchoring-and-adjustment biases (18). Therefore, communicating risks in a personally meaningful way is crucial to quality decision-making.
Shared decision-making between healthcare providers and patients is recommended (19, 20), particularly where choices are personal and complex, as is typical regarding cancer susceptibility genes. Patient decision support resources have been employed in many areas of medicine, including genetics, to promote shared decision-making, streamline clinical consultations and improve decision quality. Patient decision aids (ptDA) are a type of decision support resource that encourage patients to become engaged in difficult decisions by considering not only information about the options, pros and cons but also how personal values influence their decision (21, 22). This is often achieved through the inclusion of a values-based exercise, for example a sliding scale or worksheet for patients to record the importance of personal values relevant to the particular decision. PtDA are useful when there is no clearly preferred option, or when feelings and choices may differ according to individual values. A Cochrane review of 105 studies including 31,043 patients using ptDA before/during clinic compared to usual care revealed increased knowledge and confidence about decisions aligned with personal values, without harmful effects (23). However, clinicians must be mindful that the design and effectiveness of ptDAs is variable, with many not meeting the International Patient Decision Aids Standards (IPDAS) (24) or lacking a theoretical framework (25) to inform content delivery. Also, whilst web-based education may be highly acceptable (26), patients will often seek online sources of information themselves (27), value individual choice, and may not view information if asked to do so at home rather than in clinic (28).
There are two contexts in which patient resources could be relevant to support decisions regarding genetic cancer susceptibility:
- i)
pre-genetic testing (29): to support patients making a decision about whether to have a genetic test, either with or without a diagnosis of cancer (30).
- ii)
post-genetic testing: to complement shared decision-making with a healthcare professional for patients identified to have a genetic cancer susceptibility, and their relatives (31, 32).
At a time of increasing demand for genetic testing and limited in-person resources to support patients, there is a need to better understand the impact and experience of PtDA for genetic cancer susceptibility in both contexts.
This systematic review aimed to identify patient resources to support decision-making pre- testing for any genetic cancer susceptibility, or regarding cancer management options for carriers. The net was cast widely to find any existing resources, including brief educational materials or interventions as well as ptDA, to explore their potential for supporting patients. A secondary goal was to highlight gaps to guide future work co-designing a PtDA with patients.
The extent to which existing decision support resources meet patients' needs and preferences was explored in terms of:
- i)
impact on outcomes, e.g. cognitive, emotional, or behavioural.
- ii)
patient experience.
Recommendations for clinical practice and future research were proposed.
2. Methods
The Centre for Reviews and Dissemination's guidance for reviews in health care (33) and the Preferred Reporting Items for Systematic Reviews (PRISMA) statement (34) guided methods and reporting for this systematic review. The protocol was published on PROSPERO: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID = 220460.
2.1. Co- design
Stakeholders were consulted from the earliest planning phases and throughout this review, includingpatient engagement in design and data synthesis. Patients have been included from the Cancer Research UK-funded CanGene-CanVar programme. An International Lynch Decision Aid Stakeholder Panel has been established to provide advice and guidance. Individuals have been invited based on expertise in clinical care, academic work, public policy, patient charities, peer support groups and public bodies.
2.2. Literature searching
The following databases (and host platforms) were searched and re-run prior to final analysis (from database inception to 02/07/2021, English language only): MEDLINE (EBSCOhost), PsycINFO (EBSCOhost), Embase (OVID), CINAHL (EBSCOhost), Web of Science Core Collection, and the Cochrane Library (Cochrane Database of Systematic Reviews (CDSR); Cochrane Central Register of Controlled Trials (CENTRAL)). The search strategy combined key word and subject terms targeting three concepts: cancer genetics, decision-making, and written resources (Supplementary Material Table S1).
In addition, other relevant studies were identified by examining bibliographies of included publications and using forward citation searching in Web of Science Core Collection. Grey literature was searched to identify unpublished resources (Supplementary Material Table S2).
2.3. Study selection
Inclusion and exclusion criteria are detailed in Table 1. In brief, studies were included if they involved adults (with or without cancer) who used a decision support resource pre- or post- testing for any cancer susceptibility genes. These could be delivered digitally or paper-based, and included one or more of the following: information, education, visual presentation of cancer risk and personalised resources, including ptDA. Quantitative, qualitative and mixed methods studies were included.
Table 1
| Population |
1. People with a cancer diagnosis deciding about:
|
| Intervention | Written or pre-recorded patient-facing resources, including information, education, risk presentation, and decision support. Digital (e.g. web-based, email, smartphone, text messaging, non-live webinars) or paper-based.
Exclusion:
|
| Comparator | Control group if the study has one, but not necessary |
| Outcomes | Quantitative or qualitative evaluations of acceptability and impact of decision support resource, including cognitive outcomes (e.g. knowledge, intention to use genetic testing, perceived risk), emotional outcomes (e.g. satisfaction, decisional conflict, emotional burden, anxiety) and behaviour change following test results. Studies describing resource development process only included if impact or experience of patient captured in some way.
Exclusion:
|
| Study design | Any |
Study inclusion and exclusion criteria.
All search results were exported to EndNote X9 software for de-duplication. Two reviewers (KK, KM) independently screened 20% of titles and abstracts (sampled in alphabetical order) as a pilot to test whether the inclusion/exclusion criteria were appropriate and to assess whether their application was accurately applied by both reviewers. Rayyan, a web application for collaboration on systematic reviews (35) was used. After each batch of 100 references in the 20% pilot, reviewers' decisions were unblinded and compared. There were 29 disagreements out of 500 and these were all resolved through discussion. Informed by the pilot, the eligibility criteria were adjusted following discussion with the wider research team (DE, CF, CG, LT). The remaining 80% of titles and abstracts were screened by the lead author (KK). Both reviewers completed full text screening. Where discrepancies arose, discussion took place (involving the wider research team where necessary) until agreement was reached.
2.4. Data extraction and critical appraisal
Data from eligible publications were extracted into an Excel database with fields guided by the TIDieR (template for intervention description and replication) checklist (36). Both reviewers performed independent data extraction for 10% of the included studies. Disagreements were resolved through discussion. Subsequently, KK extracted data from the remaining studies, checked by the second reviewer.
Critical appraisal was performed by KK and KM (Supplementary Material Table S3). Depending upon the type of study, this was guided by National Institute for Health and Care Excellence (NICE) checklists for quantitative intervention studies and qualitative studies (37) or the mixed-methods appraisal tool (38). Aspects of study design and reporting were appraised. Each study was awarded an overall study quality grading for internal validity and external validity and subject to an overall assessment grading of how well the study was conducted, as far as could be ascertained from the paper. Appraisal of study criteria in Supplementary Material Table S3 is presented as yes, no, or N/A (not applicable or not able to ascertain from the paper).
2.5. Data synthesis
The review aims necessitated inclusion of a wide range of studies, mostly of quantitative but also mixed methods and qualitative design. Meta-analysis was not possible due to the heterogeneity of methodologies, populations and outcome measures. Narrative synthesis, often used for systematic reviews of healthcare interventions (39), was selected as the most appropriate method to synthesise findings without diluting the individual value contributed by different study designs (40, 41). Tabulated data were examined to describe patterns and summarise estimates of effect direction and size (39, 42). Studies were grouped into clusters and subclusters based on pre- or post-genetic test setting and patient outcomes (Figure 1) to facilitate post-hoc subgroup analysis (40). Qualitative data were subjected to thematic analysis to interpret primary themes and concepts, and representative patient narratives were chosen to be presented in their original form to highlight these themes (41).
Figure 1

Studies included in the systematic review were grouped into clusters based on the context of the patient decision support resource (pre- and post-genetic testing) and subclusters relating to the impact on outcomes and patient-reported experiences.
3. Results
Sixty-four publications regarding 46 patient decision support resources were found to be eligible from the searches (more than one publication was included regarding some of these). Figure 2 shows the flow of study selection using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (34). Most studies were from United States (n = 26), followed by Australia (n = 12) and Netherlands (n = 12). A pragmatic decision was taken to focus data synthesis on studies published from 2011 onwards, and the protocol was amended accordingly. Older studies were based on outdated guidelines and less likely to be relevant to our study aim of identifying resources that could be used or easily adapted for current practice. As shown in Figure 3, 36 publications were retained, describing 27 resources divided into two clusters (pre-and post-genetic test) and four subclusters (impact on cognitive, emotional or behavioural outcomes and patient-reported preferences/experience for each cluster) as illustrated in Figure 1.
Figure 2
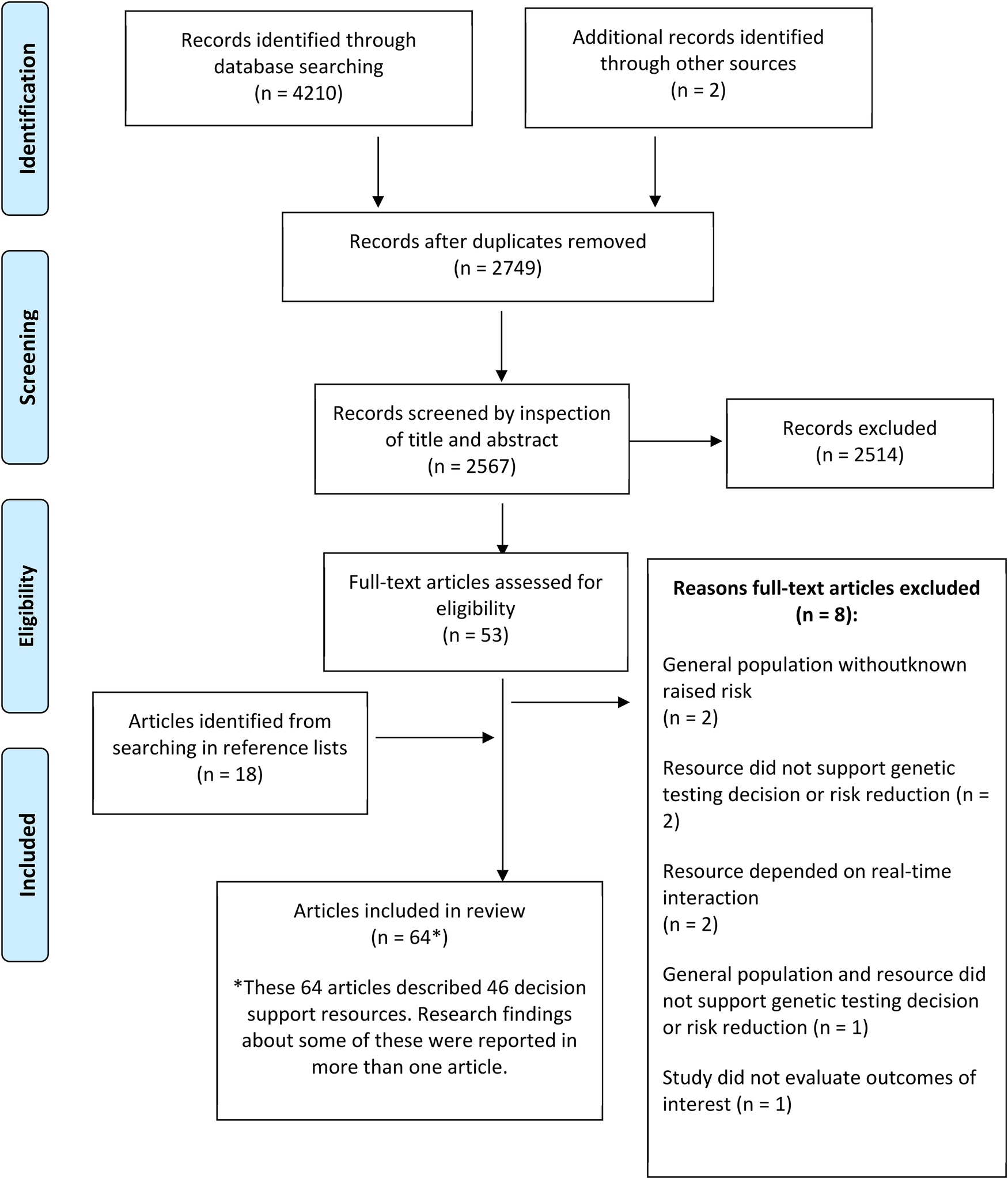
Flowchart showing the flow of study selection for the systematic review.
Figure 3
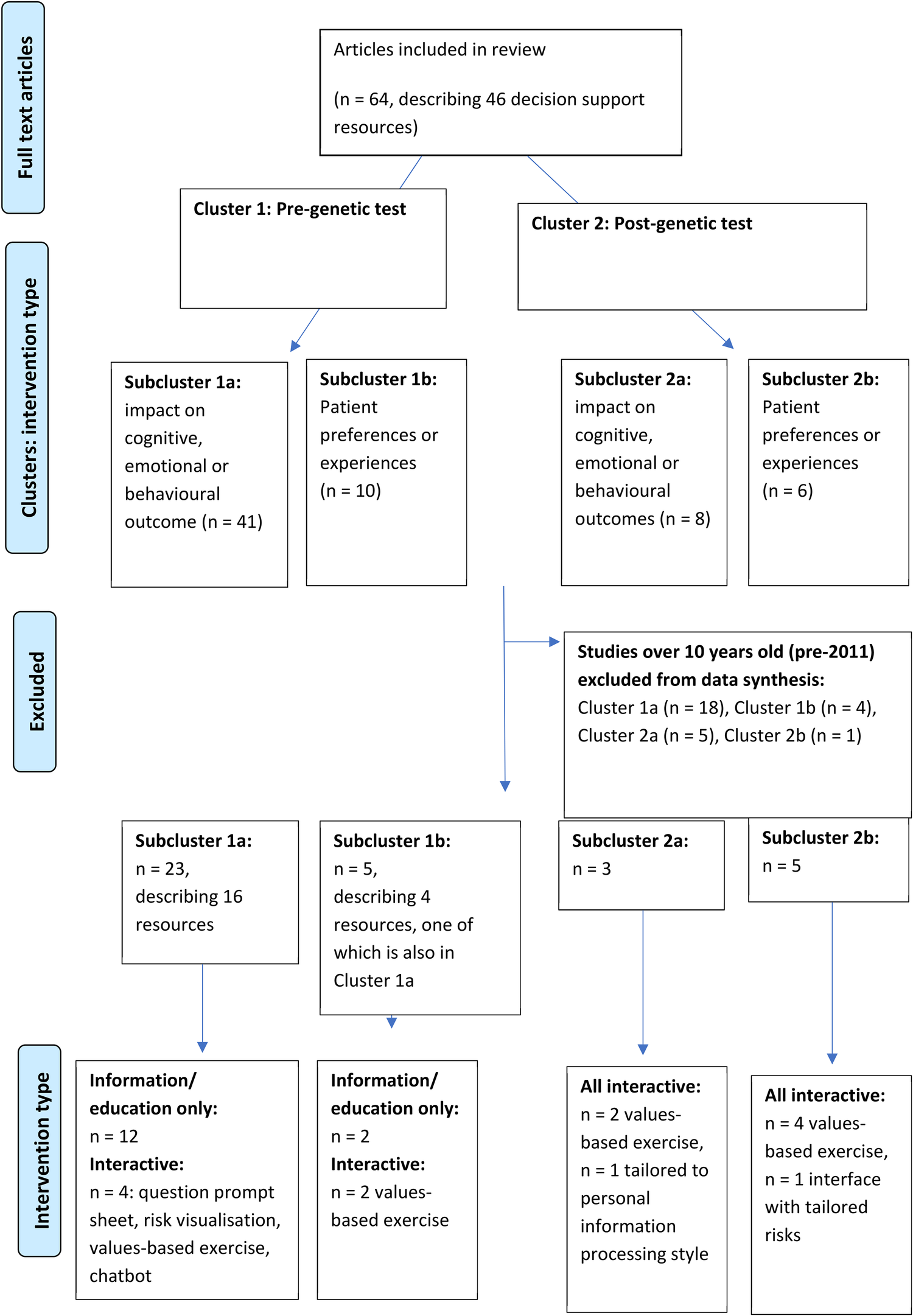
Studies included in data synthesis, grouped into clusters and subclusters.
3.1. Critical appraisal of studies
Most (n = 17) studies contained strong certainty of evidence/low risk of bias, 13 contained medium certainty of evidence/moderate risk of bias and three contained weak certainty of evidence/high risk of bias (Supplementary Appendix S3). Study design varied, from publications describing development of resources with some preliminary patient evaluation in a hypothetical decision-making setting (32, 43–50), to larger randomised controlled trials in target populations (51–56). Follow-up was often short (less than two months), with only a few studies recording outcomes as long as 12 months after resource use (53, 55, 57–59). Qualitative study designs resulted in some rich findings (60–64) but some lacked rigorous analysis methods leading to more shallow data (45, 60). Overall, external validity was limited by lack of patient diversity.
3.2. Target population
Cluster 1 resources supported pre-genetic test decisions for patients diagnosed with breast (28, 57, 60, 61, 64, 65, 66, 67), ovarian (68), breast/ovarian (69) and colorectal cancer (70, 71) as well as patients (mostly) unaffected by cancer with a family history (48, 49, 53, 54, 72, 73) or of Ashkenazi Jewish heritage (51, 62) (see Table 1). All Cluster 2 resources targeted BRCA1 or BRCA2 carriers to support decisions about risk management post-genetic test. These were designed for carriers unaffected by cancer (45, 55), with personal history of breast cancer (63), separate versions tailored to breast cancer history (32) or not specified (47, 46, 52, 59).
3.2.1. Setting for delivery
Cluster 1 resources were designed to replace face-to-face genetic counselling pre-test (28, 48, 57, 62, 67, 68, 69) or to supplement genetic counselling (44, 49, 51, 53, 54, 60, 64, 66, 70, 72, 73), which may have been delivered in the mainstream setting by oncology professionals (52, 65, 71). Cluster 2 resources were exclusively to supplement standard of care genetic counselling post-test for carriers.
3.3. Characteristics of decision support resources
3.3.1. Conceptual/theoretical framework
In most publications, a conceptual/theoretical framework to inform design was not specified. However, six resources were based on underlying theory (43, 49, 53, 54, 59, 64, 66, 71, 74) (see Table 2). These included theories related to information tailoring (43, 53, 74), such as the elaboration likelihood model of communication persuasion, which examines how presenting personalised messages can encourage more thoughtful decision-making (75, 76). The health belief model (77) suggests behaviour change is maximised if resources address threat severity and personal benefits and the transtheoretical model of health behaviour change (78) describes stages of change that patients move through before taking action. These two behaviour change theories informed content in psychoeducational resources and measured intention to pursue genetic counselling and testing (66). Another theory informed information presentation (64, 66): the fuzzy-trace model postulates decision-making is influenced by a quick, intuitive “getting the gist” which is personal and values-based, and can be more important than memory of information learned verbatim (76).
Table 2
| Authors (year), country, time of recruitment | Study type | Description of resource/intervention | Theoretical underpinning/framework | Population | Setting | Intervention: number of subjects | Comparator | Control: number of subjects | Main outcomes | Main findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1a. Deciding whether to have a genetic test: intervention impact on cognitive, emotional or behavioural outcome (n = 22 studies, describing 15 interventions) | ||||||||||
| Albada et al. (2011), Netherlands, 02/2008–04/2010 | Process evaluation (quantitative) | E-info geneca: patient-facing website about breast cancer genetic counselling tailored on age, cancer history, extensiveness of information, with question prompt sheet (QPS) | Elaboration likelihood model | Consecutive female counsellees aged >18 years, no familial pathogenic variant | At home, prior to pre-test genetic counselling | 101, of which 85 (84.2%) completed evaluation | NA | NA | factors influencing use, evaluation of website | All counselees used website, mean time 21 min (range 39s-1 h36 m), with those affected by breast cancer spending longer. Most selected extensive detail, but 8/85 (9.6%) then found information too much and 12 (14.4%) upsetting. Counselees highly satisfied with website. Those with greater information needs found most helpful. Less than half viewed genetic counselling information. Forty-two (41.7%) formulated questions to discuss at appointment. |
| Albada et al. (2012) | RCT | 101 | brief leaflet before pre-test genetic counselling | 89 | genetic counselling expectations, knowledge, information needs | Use of tailored website prior to genetic counselling resulted in more realistic expectations (p = 0.03) and reduced requirement for education, freeing up time for Genetic Counsellor to focus on personalised risk communication and psychosocial factors. | ||||
| Albada et al. (2015) | RCT | 86 | 76 | genetic counselling satisfaction, knowledge, anxiety, risk perception, surveillance adherence | One-year follow-up revealed higher satisfaction (p = 0.02) and more perceived personal control (p = 0.02) in intervention group. However, overestimation of cancer risk persisted and no significant improvement in knowledge, anxiety or adherence to recommendations. | |||||
| Arrick et al. (2019), USA, dates not specified | RCT | Visual aids with calculated vs self-perceived 10-year cancer risk: 1) spinner pie chart game, 2) computer-generated random dot arrays | not stated | consecutive counselees | During clinic, to supplement pre-test genetic counselling | n/66 | pre-test genetic counselling with 10-year cancer risk calculation | n/66 | accuracy of risk perception | More than 85% of counsellees overestimated cancer risk by at least two-fold before genetic counselling. Enhanced counselling significantly improved risk perception accuracy, sustained at two-week and six-month follow-up for both spinner game “out of 1,000” and random dot array. |
| Cragun et al. (2020), USA, 11/2018–03/2020 | Process evaluation (quantitative) | Educational tool: twelve-minute narrated, animated slides about multigene panel testing | Not stated | Consecutive counsellees aged ≥18 years | Via tablet at hospital before pre-test genetic counselling | 305 | NA | NA | knowledge, empowerment, attitudinal values about genetic testing, evaluation of health literacy relationship with outcomes | Intervention significantly increased knowledge, based on unvalidated questionnaire. Genetic testing decision empowerment increased (29% to 74%, p < 0.001), but was lower before-and after-tool with lower health literacy. Values about genetic counselling did not change. Triage of counsellees to identify lower health literacy and/or sustained decisional conflict could identify need for traditional pre-test genetic counselling. |
| Dekker et al. (2014), Netherlands, CRC < 70 01/-07/2009, all CRC 06/2010–01/2011 |
RCT | Novel strategy (NS): patient/clinician website about familial CRC risk, with risk calculators and decision support intervention (DSI) for high risk patients, patient brochure | Not stated | Patients newly diagnosed with CRC, no known pathogenic variant | At home, prior to usual care | 140 | usual care | 252 | cancer prevention measures (genetic counselling for high risk, colonoscopy in first degree relatives for moderate risk) | Website used by 94/140 (67%). Genetic counselling uptake lower (15% vs 33%, p = 0.003) in high risk after NS. No significant difference in cancer prevention measures in moderate/low risk, but familial risk discussed more frequently. Although web- and paper-based tools appreciated as supplement to improve knowledge, communication and shared decision-making, CRC patients preferred doctor's advice when considering cancer prevention. |
| Gornick et al. (2018), USA, 02/2014–05/2016 | RCT (secondary analysis of data collected in RCT) | iCan-Decide: web-based interactive decision tool with values-based exercise and testimonials tailored to age, ethnicity and timing of breast surgical consult | IPDAS | Patients newly diagnosed early stage breast cancer aged 21–84 years enrolled at surgical practice | At home or on tablet at hospital; prior to or after surgical consultation | 245 | static (non-tailored) website | 251 | knowledge, uptake of genetic testing | Knowledge regarding probability of BRCA1 or BRCA2 pathogenic variant low but significantly increased (35.8% vs 24.4%, p < 0.006) after intervention. However, knowledge also related to educational status, younger age and ethnicity, suggesting potential for more impact in subgroups. Website complements but should not replace professional advice and need to address HCP knowledge gaps. |
| Hall et al. (2011), USA, 06/2005–12/2008 | RCT | CD-ROM about MSI testing: graphics and pictures with professional narration, based on structural equation modelling | Theoretical model of association between knowledge, attitudes and decisional conflict | Patients diagnosed CRC offered MSI testing, did not meet Amsterdam criteria | At hospital, after education session | 120 | brief description of MSI and IHC by health educator | 119 | knowledge, self-efficacy, preparedness, decisional conflict | Decision aid not tailored, did not address barriers. Knowledge regarding MSI increased in intervention group (p < 0.05) along with preparedness for decision-making (p < 0.05). Decisional conflict mediated through multiple pathways including knowledge-independent and attitude. |
| Heald et al. (2020), USA, 05/2019–03/2020 | Process evaluation (quantitative) | Chatbot-deployed education and consent for 55 pan-cancer gene panel | Not stated | Patients presenting for colonoscopy aged ≥18 years | At home, via electronic medical record patient portal | 487 | NA | NA | feasibility, uptake of genetic testing | Chat initiated by 487/4,254 (11.4%). Genetic testing ordered for 161 (chat) + 8 (via Genetic Counsellor). No Lynch Syndrome identified. Twelve (9.3%) had pathogenic variant identified, four with known personal/family history of hereditary syndrome. Chatbot was used as a genetic counselling extender, evaluating three times as many patients compared to traditional counselling time in previous study. |
| Hoberg-Vetti et al. (2016), Norway, 09/2012–04/2015 | Cohort analytic (two groups pre- + post-) | DNA-BONus study: written information on hereditary breast and ovarian cancer, testing BRCA1 and BRCA2 Norweigian founder pathogenic variants | Not stated | Patients newly diagnosed breast/ovarian cancer recruited from surgical/gynaecological clinics | In oncology clinic, in place of pre-test genetic counselling | 1,015 (breast cancer n = 405, ovarian cancer n = 83) | NA | NA | uptake of genetic testing, anxiety, depression | Genetic testing accepted by 68.0% with ovarian and 45.4% with breast cancer. Older patients may have declined study. Baseline anxiety/depression similar to newly diagnosed patients with cancer in general. Anxiety significantly decreased by six-months (p < 0.001), during which time genetic testing was completed. No change in depression. Only 20 patients called Genetic Counsellor with questions. |
| Hoberg-Vetti et al. (2019) | Cohort analytic (two groups pre- + post-) | 309 (breast cancer n = 259, ovarian cancer n = 50) | NA | NA | cancer-related distress, perceived social support, decisional conflict | Participants psychological substudy (309/1,015, 40.0%) significantly younger. Severe stress response decreased from baseline to six-months’ post-test for intrusion (32.1 to 14.0%) and avoidance (23.6 to 16.0%). Factors for increased cancer-related distress included younger age, ovarian cancer, less perceived social support, higher decisional conflict. | ||||
| Kasting et al. (2019), USA, 03/2015–09/2015 | RCT | Psychoeducational intervention (PEI): booklet, 12-minute DVD about hereditary breast cancer featuring patients, oncologists, genetic counsellor | Health Belief Model, Transtheoretical Model of Behaviour | Patients post-surgery for breast cancer, ‘high risk’ due to diagnosis aged ≤50 years or family history | At home, prior to pre-test genetic counselling | 53 | one-page factsheet: information about hereditary cancer and appointment logistics | 56 | feasibility, acceptability, preliminary efficacy, cancer worry and distress | Based on self-report 88% viewed video. Intervention group greater intention to pursue genetic counselling after four-months (28.0 vs 7.7%, p = 0.027), although only 3% had attended. PEI no effect on perceived barriers or cancer worry, both factors for behaviour change. Control group greater decrease in perceived cancer risk (p = 0.064). |
| Manchanda et al. (2016), UK, 02/2009–07/2010 | RCT | GCaPPS study: DVD presentation to small groups (2–5) about testing for BRCA1 and BRCA2 Ashkenazi Jewish founder pathogenic variants | Not stated | Ashkenazi Jewish aged >18 years, no known familial pathogenic variant, recruited from community | during clinic, followed by individual pre-test genetic counselling | 409 | pre-test genetic counselling | 527 | knowledge, genetic testing uptake, perceived cancer risk, counselling time/satisfaction | DVD non-inferior with respect to knowledge, counselling satisfaction and perceived risk, and decreased appointment time by mean 20.5 min (p < 0.005), saving £14 per person. DVD well received: 98% satisfied with length/information, 87–95% not significantly worried, 89% proceeded with genetic testing. |
| McCuaig et al. (2019), Canada, 05/2015–03/2018 | RCT | Prevent Ovarian Cancer Program: 20-minute, voice-recorded presentation about 52-gene panel testing for hereditary ovarian cancer | Tiered and binned approach to informed consent for multigene panel testing (Bradbury et al. 2015) | Females aged ≥18 years, FDR died ovarian cancer, no known familial pathogenic variant, recruited from community | At home, prior to brief, telephone pre-test genetic counselling | 237 | pre-test genetic counselling (option via video if not local) | 116 | knowledge, genetic testing uptake, perceived cancer risk, distress, anxiety, depression, decisional conflict, counselling time/satisfaction | Intervention non-inferior for knowledge, distress, anxiety, depression, decisional conflict but not perceived cancer risk. There was a 26-minute time savings (p < 0.001). Nearly all (>90%) participants in both groups pursued genetic testing, 86%–90% wanted all gene results. Both groups highly satisfied. |
| Meiser et al. (2012), Australia, dates not specified | Process evaluation (qualitative) | One-page educational pamphlet with information about treatment-focused BRCA1 and BRCA2 genetic testing (TFGT) | Not stated | Females aged ≥18 years with breast cancer diagnosed aged ≤50 years | At home, retrospective (previously tested) or hypothetical (recently diagnosed) | 17 | NA | NA | acceptability, preferred timing and impact of pamphlet | Semi-structured interviews that informed pamphlet development suggested patients preferred to be told about TFGT at or around time of diagnosis in face-to-face consultation with HCP. Sixteen out of 17 patients satisfied with information provided, 15 self-reported improved understanding, and only four were worried by reading the pamphlet. |
| Quinn et al. (2017), Australia, 07/2010–10/2012 | RCT | see Meiser et al. 2012 | Females aged 18–49 years with breast cancer, no prior genetic testing, high risk | At home, in place of pre-test genetic counselling with brief Genetic Counsellor telephone intake | 65 | traditional pre-test genetic counselling | 70 | decisional conflict, cost effectiveness | All 135 patients consented to testing, 20 (14.8%) had a pathogenic variant and 18 (13.3%) had a variant of uncertain significance. Pamphlet saved AUD$84 per patient and not inferior with respect to decisional regret at 12-months’ follow-up for genetic testing or risk-reducing surgeries. | |
| Nilsson et al. (2018), Sweden, 02/2015–08/2016 | Process evaluation (quantitative) | BRCAsearch study: short letter about BRCA2 and BRCA2 testing with invitation to contact Genetic Counsellor if required | Not stated | Patients with newly diagnosed breast cancer taking part in Swedish Cancerome Analysis Network biobank study | Oncology clinic, in place of pre-test genetic counselling | 818 | NA | NA | uptake of genetic testing, number of patients contacting genetic counsellor | Of 818 patients given the letter, 542 (66.2%) consented to genetic testing. Eleven pathogenic variant carriers were identified (2.0% prevalence), six of whom fulfilled Swedish testing criteria. Only 11 (2.0%) contacted Genetic Counsellor with questions, and 19 (3.5%) had questions regarding administrative processes. |
| Nilsson et al. (2019) | Process evaluation (quantitative) | 805 | NA | NA | patient-, tumour- and treatment-related predictors of genetic testing uptake | Analysis from medical records in 539/805 (67%) who consented to genetic testing. Consenting patients younger (mean 61.9 vs 67.1 years, p < 0.001), more educated (p = 0.003) and more likely to have family history (p = 0.02). Patients with psychiatric disorders less likely to have testing (p = 0.006). | ||||
| Nilsson et al. (2019a) | Process evaluation (quantitative) | 448 | NA | NA | one-year post-results satisfaction | Satisfaction 96.0%; only 11.1% preferred more oral information, more likely with comorbidities (p = 0.02), born outside Sweden (p = 0.01), lower education (p = 0.06). Twenty-nine contacted genetic counsellor and had pre-test counselling. All 11 pathogenic variant carriers satisfied with testing decision, 10/11 with information; all attended post-test counselling and had risk-reducing BSO. | ||||
| Sie et al. (2014), Netherlands, 08/2011–02/2012 | Cohort analytic (two groups pre- + post-) | DNA-direct study: triage call by doctor followed by written and digital information (website, educational movie) about BRCA and BRCA2 testing. | Not stated | Females (previously) diagnosed breast cancer, referred to Genetics, no known familial pathogenic variant | At home, in place of pre-test genetic counselling | 95 | DNA intake: pre-test genetic counselling | 66 | choice of DNA-direct vs. DNA-intake, satisfaction with genetic counselling, distress | More patients (59%) chose streamlined DNA-direct (p = 0.03), received results quicker (70 vs 103 days, p = 0.002), and 89% would choose again. DNA-direct more informed, lower decisional conflict, and lower distress baseline and two-week follow-up (p = 0.001). Identification of pathogenic carriers 8% in both groups. Satisfaction with genetic counselling equal. Most DNA-direct did not view video, only read letter, and none contacted doctor with clinical questions. |
| Sie et al. (2016) | Cohort analytic (two groups pre- + post-) | 59 | 49 | one-year post-results satisfaction, distress, Genetic Counsellor experience | Both self-selected groups satisfied with choice of procedure, with no regret at one-year. DNA-direct lower distress, although both groups below clinical thresholds and difference smaller at one-year. Genetic Counsellors who performed post-test counselling believed most (76%) DNA-direct patients made informed choice for genetic testing and felt 85% were suited for streamlined model. | |||||
| Tea et al. (2018), Austria, 02/2015–02/2016 | Process evaluation (quantitative) | New Genetic Counselling Tool (NGCT): visual aid about BRCA1 and BRCA2 testing using lay language, with pictures, diagrams and tables | Not stated | Patients aged >18 years referred for genetic counselling | During clinic, to supplement pre-test genetic counselling | 34 | pre-test genetic counselling (without visual aid) | 36 | knowledge | More NGCT (44% vs 17%, p = 0.012) correctly answered all seven knowledge questions correctly on unvalidated post-counselling questionnaire. Visual aid was used in genetic counselling sessions, and effect on counselling style and language not measured in this study. |
| Watson et al. (2016), USA, 7/2014–12/2014 (standard), 3/2015–8/2015 (video) | Process evaluation (quantitative) | Seven-minute educational video explaining choice between BRCA1 and BRCA2 testing with reflex to multigene panel |
Not stated | Patients with ovarian, fallopian tube or primary peritoneal cancer | During oncology clinic, on a tablet, in place of pre-test genetic counselling | 295 | pre-test genetic counselling | 267 | uptake of genetic testing | Fifty-five per cent (162/295) of patients who viewed video consented to testing on same day, a significant increase (p ≤ 0.001) compared to previous referral method. Detection of pathogenic variants similar in both groups (8.1 vs 7.9%). |
Characteristics and main findings of studies.
Whilst not all ptDA were informed by theory, some (32, 45, 49, 55, 59, 63, 64) followed recommended guidelines such as the Ottawa Decision-Support Framework (ODSF) (79) which includes a values-based exercise to improve decision quality and process. Similarly, the multiattribute value and utility models (80, 81) guided inclusion of pros and cons of cancer risk management choices that patients rated according to personal importance (59), and the informed choice model (82, 83) was used to theorise that increased knowledge would improve decision-making quality and genetic testing uptake (49).
3.3.2. Format and content delivery mode varied widely
For studies aimed at replacing the need for in-person counselling pre-test, a brief information letter was used (57, 69, 67), or letter and digital options including a website (49), video (28) or chatbot (48). Educational presentations to supplement genetic counselling ranged in length from seven-minute video (68), 12 minute DVD (66) or animated slides (73), 15 minute DVD delivered to small groups (51), 20 minute voice-recorded presentation (54) and CD-ROM about microsatellite instability testing in colorectal cancers that took on average 24 min to view (71).
Paper-based resources were either brief (one-page) and focused on treatment-related implications of genetic testing for people with breast cancer (60) or longer (15-page) including a worksheet to record personal weighing up of options (32, 47, 55). Others made resources available online with printable content (45, 64). Visual aids were created to improve genetic cancer risk communication using pictures, diagrams and tables (44) or by comparing an interactive spinner-game format to random dot icon arrays (72). Digital decision support resources were mostly interactive (62, 63, 64, 52, 59, 70) and some also included computer-tailoring for personal characteristics to present more relevant information (46, 49, 53, 65).
3.4. Impact of decision support resources
A summary of the main findings is presented in Table 2, with narrative synthesis below.
3.4.1. Knowledge, understanding and expectations
Improvement in genetic cancer susceptibility knowledge or realistic expectations was statistically significant for many (44, 51, 53, 54, 65, 71) but not all resources (70). However, measurement was often by self-report, using unvalidated questionnaires and/or with lack of pre- and post-counselling measures or control group. In one study, patients stated viewing an educational video increased knowledge, however qualitative data gathered via semi-structured interviews suggested gaps remained, for example many thought genetic counselling was the same as genetic testing (61).
Accuracy of cancer risk perception significantly improved by adding personalised visual aids to genetic counselling (72), with effects sustained up to six-months. However, recorded presentations before a shortened session had mixed results when compared to traditional counselling in randomised trials. An educational video followed by abbreviated genetic counselling was non-inferior to traditional counselling for increased knowledge, satisfaction and risk perception for community-recruited participants offered Ashkenazi Jewish founder BRCA1 and BRCA2 gene testing (51). Patients with a relative who died of ovarian cancer were offered 52-gene panel testing using a digital presentation viewed at home followed by short telephone genetic counselling, which was non-inferior to traditional counselling for knowledge, satisfaction and psychological factors but not for ovarian cancer risk perception (54).
3.4.2. Distress, anxiety and cancer worry
Mental well-being outcomes such as distress, anxiety, cancer worry and depression tended to be below clinically relevant thresholds at baseline and follow-up, indicating no significant evidence of psychological harm from pre-test resources (51, 53, 54, 66, 67, 69, 84). For people newly diagnosed with cancer, levels were transiently increased as expected at this challenging stage of life;genetic testing using decision support resources did not increase symptoms (28, 58, 66, 67, 69). However, several studies excluded patients with psychological conditions (28, 57, 58, 60, 85, 86), so it is not known how resources might have influenced mental well-being in these groups.
Some post-test resources showed time-dependent results, with cancer-related distress higher in the PtDA group compared to usual care at one month, but lower from one- to six-months, possibly indicative of a deliberative decision-making process (59) or declining over time but similar in ptDA and usual care groups (55). Distress was also shown to vary by topic, with lower levels regarding chemoprevention compared to risk-reducing breast and ovarian surgery decisions (55).
3.4.3. Genetic testing uptake
Written information instead of pre-test counselling led to genetic testing uptake in 405/1015 (45.4%) (84) and 542/818 (66.2%) (85) patients with breast and 83/1,015 (68.0%) with ovarian cancer (84), although there was no comparator group and older people were less likely to take part. An educational video followed by brief counselling in community-recruited patients led to high testing uptake, with 92% of video vs. 96% of the traditional counselling group electing testing, and video delivered a significant time saving (19.4 vs. 45.8 min, p < 0.001) (54). In a similar study, 89% across DVD and traditional counselling groups had testing, with the DVD saving 20.5 min of counselling time (51). However, there was lower uptake of testing amongst patients with ovarian cancer shown a video in their oncology clinic instead of referral for genetic counselling (162/295, 55%) (68). Despite interventions increasing knowledge and interest in testing, patients particularly valued their doctor's advice and did not always follow through on scheduling a genetic counselling appointment (65, 66). Some resources were used to manage expectations about being offered testing (48, 56), a successful approach for people at lower risk who do not meet current eligibility guideline criteria.
3.4.4. Decisional conflict
Decisional conflict describes level of uncertainty about making a choice. The decisional conflict scale measures contributing factors such as support, information and personal values (87). Results from this review suggest patients with higher decisional conflict may need additional decision support and genetic counselling (57, 73, 65).
An educational resource to shorten pre-test genetic counselling was non-inferior to standard care with respect to decisional conflict in patients with a family history of ovarian cancer (video (54),) and patients diagnosed with breast cancer before age 50 years (pamphlet (67),). Baseline levels of decisional conflict were moderate in patients referred for genetic risk assessment, and showed significant decrease at two-months after using an interactive, web-based ptDA about breast reconstruction after risk-reducing mastectomies, compared to standard of care counselling (52). One study in 239 high risk patients with colorectal cancer (71) showed that a ptDA impacted decisional conflict by increasing knowledge and preparedness to make a decision. Decisional conflict was also influenced by knowledge-independent factors such as attitudes about testing and learning about hereditary cancer risk which, along with other barriers, are often not addressed in ptDA.
BRCA1 and BRCA2 carriers with no history of cancer had low baseline decisional conflict about breast risk management options in intervention and control groups, which declined with time up to 12-months and was not significantly influenced by a paper-based ptDA used at home after post-test genetic counselling (55).
3.5. Evaluation of decision support resources
3.5.1. Satisfaction and acceptability by patient report
Satisfaction and acceptance was high across clinical settings and patient groups; however, resource usage was often untracked, and many studies did not compare to standard care in a randomised controlled trial. Where optional usage was monitored or self-reported, this revealed 64/100 (64%) used an interactive CD-ROM (59), 94/140 (67%) used a website (70), and 53/60 (88%) viewed a video (66). A much lower percentage (487/4,254, 11.4%) of patients presenting for colonoscopy screening engaged with a chatbot to answer questions about their family history to determine eligibility for genetic testing (48). Most (95/161, 59%) patients with breast cancer chose to use streamlined pre-test information instead of genetic counselling; when presented with letter and video options, most only read the letter and none contacted the doctor with questions (28). There was no regret at 12-months about choosing streamlined testing, which identified a pathogenic BRCA variant in 8/95 (8%) of patients (58). Similarly, 96% of patients with breast cancer were satisfied at 12-months with a short letter instead of pre-test counselling and only 11/818 (2%) contacted the genetic counsellor for support (57). Only 20/1,015 (1.9%) of patients with breast or ovarian cancer who received brief written pre-test information in oncology contacted the genetic counsellor (84).
3.5.2. Experience and emotional outcomes by patient report
Two studies explored the experience of patients with breast cancer using resources to decide about genetic counselling/testing. In a telephone structured interview study to evaluate acceptability and emotional impact of a one-page pamphlet about treatment-focused genetic testing, 7/17 people thought the pamphlet sufficient for decision making, whilst 10/17 believed more information was needed, e.g., discussion with healthcare professional (HCP) or searching online (60). Four out of 17 were worried by reading the pamphlet: three were reminded of their breast cancer diagnosis and one was worried about their relatives. Think-aloud interviews reviewing a web-based ptDA revealed patients with breast cancer preferred less text, to get the “gist”, with optional, more detailed information, and wanted a “friendlier” feel to patient pictures (64). This is in contrast to study findings about another web-based ptDA tailored for personal characteristics (43), in which patients with breast cancer spent more time looking at information and selected to receive extensive detail, however when looking at the information 12/85 (14.4%) then found it upsetting.
The JeneScreen web-based programme for Ashkenazi Jewish BRCA testing was evaluated in a pre- and post-test interview study of 11 patients without cancer (62). Similar to findings from another study involving patients with breast cancer (64), some wanted less pre-test information up front and suggested a staged approach: “…But you may not get that result, so you wouldn”t need to go into as much detail about that topic…only if you need the information”. Ten out of 11 were satisfied with online consent to testing and suggested it was more convenient: “Online, everybody prefers online”; “If it required me going somewhere to meet someone, then it probably would have taken me longer to get around to doing it”. However, one patient referred to her age as the reason she would prefer in-person support: “Well, I am over 70, I prefer to do things where I am speaking to someone”.
A psychoeducational intervention (PEI) containing information about breast cancer genetic testing was explored by focus groups (paper version (50),) and semi-structured interviews (video format (61),). The paper PEI was visually attractive and culturally acceptable: “I like the cover; you have a variety of ethnic groups and ages” and appreciated as a take-home resource: “you”re only going to remember a little piece of what [your health care professional says]…but hand me books…I can flip through it and then…write down notes to ask the next time I see somebody”; “I would see it; I can hold it; I can turn the pages…it prompts me to start thinking” (50). Patients with breast cancer had emotional reactions to patient narratives in the video PEI: “It just makes you realize [sic] that other people feel or felt like that….touching to watch the stories because I can relate, I kind of teared up” (61).
Focus groups with 15 BRCA carriers with breast cancer guided development and evaluation of a web-based ptDA (63). Key decision-making motivating factors were identified, such as feeling obligated e.g., “do the right thing” to save life by having risk-reducing ovarian surgery, or HCP being strong influencers, e.g., “my surgeon was gung-ho”, describing consultations about risk-reducing mastectomies. Inclusion of a values-based exercise was appreciated: “When it is in black and white in front of me and I am able to block out everybody else, what they want, what they think I should do and I can look at it and say what is the best thing step by step for me and then get a print out—that is huge”. PtDA timing was debated, suggesting need for personalisation: “The patient will probably let you know if they are ready for it or not”. Patients preferred to use ptDAs in clinical settings: “more geared up….more serious about it if it was in an office than at home”, while clinicians (also included in focus groups) were keen for at-home use but cautioned that support was needed: “…are they really going to know how to self-interpret with what they”ve just done?”
4. Discussion
4.1. Discussion
This systematic literature review identified a range of decision support resources about genetic cancer susceptibility testing or cancer risk management for carriers. The heterogeneity of resources and study designs precluded meta-analysis. However, to meet study aims it was important that the systematic review search strategy was inclusive and broad to capture any types of resources, including digital, paper-based or educational that might benefit patients with different preferences and backgrounds. Any existing resources that could be delivered in current clinical practice or easily adapated might improve decision-making experience and outcomes, and needed to be considered.
Regarding the aims of this review:
- i)
decision support resources used to streamline cancer susceptibility genetic testing were non-inferior in terms of knowledge and decision satisfaction, however there was limited rigorous evaluation in randomised control trials compared to usual care. Decision support resources for carriers did not have a sustained effect on cancer-related distress, with transiently increased levels in one study possibly indicative of more deliberative decision-making. However, few studies included longer term follow-up beyond one to two months after resource use. Measures such as distress could change over time due to impact of the cancer diagnosis trajectory with changes in prognosis due to worsening disease. It is therefore important to make comparisons at multiple timepoints between patients with cancer who have used decision support resources to those who have not, to investigate the effect. Decisional conflict was low or moderate at baseline and with use of the intervention.
- ii)
all studies evaluating patient experience reported positive feedback on satisfaction and usefulness, however this was often by self-report, and many studies lacked a control group receiving usual care. There was a lack of patient diversity.
On balance, there was clear potential for decision support resources to be useful to help patients make more deliberative decisions in line with their personal values and situation, and may save time in clinic and healthcare resources. Taking into account the importance of varied patient preferences, there was no one best method of delivery, suggesting a flexible, multi-modal approach should be considered for future co-design of patient resources. This review highlighted several gaps in the availability of patient decision support resources to fulfil what patients in focus groups have told our research team that they want: trusted, up-to-date sources of information from experts that can also help them to educate their healthcare professionals and relatives (
88).
Our findings align with results from a systematic review of Ottawa Decision Support Framework (ODSF)-based resources in 24 randomised controlled trials which showed PtDA used across a variety of medical specialities resulted in higher quality decisions and less HCP resource, compared to usual care (89). Some of the resources included in this review used a framework such as ODSF/IPDAS, but many did not which could have impacted effectiveness. The ODSF was recently updated following a review of use across 18 countries and >50,000 patients (90) to include decisional outcomes such as proportion of patients undecided, feeling uninformed, unsupported or unsure of values. There has been limited evaluation of these outcomes in decision support for genetic cancer susceptibility, and their inclusion should be given consideration in future research.
Streamlined, cost-effective pathways and patient resources are needed for HCP to deliver genomic testing “routinely to all people with cancer”, a commitment of the NHS Genomic Medicine Service (91) to inform surgical/treatment options, future cancer risks and risk to relatives. This is particularly relevant as genetic testing is moved into “mainstream” care, with patient-facing resources presenting an opportunity to more safely scale up delivery of testing without compromising informed decisions. Written or digital educational resources can be non-inferior to genetic counselling in the pre-test setting to increase knowledge (51, 54, 92).
Setting, mode of delivery and accessibility should be given due consideration; there was limited evaluation of this for the resources identified in this review. The results presented suggest people may not use a resource if asked to view a video/website/chatbot, or look at something at home, and they will rarely contact HCP with questions. It is not known whether these people did not need support or did not realise what support and benefit genetic counselling could provide. Those with more decision support needs should be referred to genetic counselling along with being offered tailored paper- and/or web-based patient resources because there is a suggestion that those with the greatest information needs may benefit most from additional decision support (43).
Quantifying genetic cancer susceptibility (known as “penetrance”) depends on the gene variant as well as age, medical and family history (93–96). Understanding of risk conferred by variants in cancer susceptibility genes has progressed at pace due to advances in genomic sequencing technology and large consortium studies (97–99). However, uncertainty remains about the likelihood that a carrier will develop cancer, which type of cancer and at what age. This is often experienced as trading one type of uncertainty for another, first finding out the genetic test result and then diverting thoughts and energy to thinking (or worrying) about what happens next. In patient-centred healthcare, uncertainty is multidimensional, including ethical (100), as well as scientific, system-based and personal factors linked to values, understanding and context (101, 102). Communication about uncertainty in a transparent, accessible way that engenders trust is a challenge for creators of ptDA, but if done successfully could improve understanding and emotional response (103–105) and make patients feel part of a team with their HCP (106). The findings of this review suggest the importance of including visual presentations of cancer risk to improve understanding of genetic cancer susceptibility and inform personalised decision-making.
Emerging research suggests that using digital technology such as smartphone applications could be acceptable and accessible for patients with lower literacy to consent for genetic testing (107), however continued bioethics exploration is needed to optimise inclusivitys. One size does not fit all, but harnessing digital technology to personalise decision support resources could empower more patients to take an active role in their care plan and improve health outcomes, consistent with the goals of the NHS Long Term Plan (108) and Universal Personalised Care Action Plan (109). This review has highlighted the usefulness, acceptability and time-saving nature of patient decision support resources, but has not identified one best method of delivery or any resources suitable for implementation in current clinical practice.
4.2. Future work
This review provides the groundwork to inform co-design with patients and other expert stakeholders from clinical genetics, oncology, charities, ethics, academic and health care bodies of a ptDA for Lynch syndrome, a genetic predisposition to certain cancers, mainly colorectal (bowel), endometrial (womb/uterine), ovarian and gastro-intestinal. The learning will be applied to create an adaptable template ptDA for genetic predispositions to other cancers. Most ptDA have focussed on pre-test decisions rather than genetic cancer susceptibility risk management. This highlighted the need for more resources for carriers, particularly for genes other than BRCA1 and BRCA2. The ptDA we are co-designing is tailored based on personal characteristics to present risk estimates and relevant options spanning from targeted treatment of cancer to primary prevention of future cancers. This will be multi-modal to allow wider dissemination, using an interactive website with the option to print personalised paper-based versions, question checklists and summaries of values-based exercises to take to an appointment with a healthcare professional. The Person-Based Approach (110) is being used to develop and iteratively optimise content and delivery of the ptDA, with attention to accessibility such as for patients with lower health literacy.
Future research is needed in the following areas:
- •
to determine the best modes of implementation in clinical practice
- •
co-design of decision support resources with patients, including from diverse communities
4.3. Strengths and limitations
Non-peer reviewed, published resources may not have been identified. The decision to exclude pre-2011 publications resulted in loss of some relevant (but dated) evidence. Since genetics and technology are evolving so rapidly, it was decided that the review would be more relevant if it was focussed on more recent publications. However, even recent studies lacked exploration of the impact of more complex genetic testing, for example large gene panel tests or whole genome sequencing, which lead to more additional, unexpected findings and variants of uncertain significance requiring more interpretation and uncertainty about recommended management options.
The findings of this systematic review may be subject to uncertainty due to methodological limitations of the included studies, including: lack of comparison to usual care using randomised controlled trials; use of unvalidated and patient-reported outcome measures; potential bias due to missing data (included untracked usage of resources) and short-term follow-up. Samples often lacked carriers (where included, these were exclusively BRCA1 and BRCA2). Studies were commonly conducted in a single centre or one country and therefore may not be generalisable.
4.4. Conclusions
More longitudinal research is needed regarding whether people complete actions in line with the decisions they make about cancer susceptibility genetic testing, cancer treatment and prevention. Longer term studies are important to understand whether people retain knowledge and accurate risk perception and maintain low levels of distress and decisional conflict over time after using decision support resources, compared to usual care. However, there has been little exploration of how measures such as distress might change over the longer term, and how this could be related to the psychological effects of a cancer diagnosis with changing prognosis, vs. the effect from using a PtDA which may be more transient. Evaluation should drive an iterative process of development and refinement of ptDA and determine the most effective mode of delivery in oncology and genetics services to improve health outcomes. Sustainable funding to update and securely host ptDA is essential to provide personalised cancer risk estimates and options based on current evidence and clinical guidelines.
Overall, the findings of this review suggest there is clear potential for ptDA and other decision support resources to complement the usual standard of care involving shared decision-making with healthcare professionals about genetic cancer susceptibility, but further development is needed to meet needs in current clincial practice. Psychological behavioural theory and a proven framework e.g., the ODSF (79) should underpin co-design, and quality standards should be met, e.g., IPDAS (24).
4.5. Practice implications
There are two main settings in which genetic cancer susceptibility patient decision support resources should be considered. First, healthcare professionals in oncology offering genetic cancer susceptibility testing for patients with cancer, where decisions to inform personalised treatment are often needed urgently. Patient-facing resources in this context could be brief and paper-based, with the option to delve into interactive, web-based resources and/or genetic counselling referral for those requiring or desiring a higher level of decision support. Secondly, post-genetic testing, carriers will need ongoing management of their lifelong condition, and ptDA can complement genetic counselling, encouraging decisions in accordance with personal values. Relatives of carriers will similarly need genetic counselling with a healthcare professional, but could additionally benefit from ptDA to increase knowledge pre-test.
A heterogeneous group of decision support resources has been identified in this review, each designed for a specific local care pathway and patient poulation. Clinical implementation shuld involves evaluating resources in complex care pathways, dealing with the realities of funding and staffing shortages present in the healthcare setting. Further research is needed to understand what decision support resources for genetic cancer susceptibility work for whom, how, why and in what setting (111, 112), with particular attention to patient values and preferences and ensuring inclusive accessibility.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
KK: conceptualisation, methodology, formal analysis, writing- original draft preparation. KM: conceptualisation, methodology, investigation, writing- reviewing and editing. LT: conceptualisation, formal analysis, writing- reviewing and editing. JS: methodology, writing- reviewing and editing. VF: methodology, writing- reviewing and editing. LW: methodology, writing- reviewing and editing. DE: conceptualisation, writing- reviewing and editing, supervision. CG: conceptualisation, writing- reviewing and editing, supervision. CF: conceptualisation, writing- reviewing and editing, supervision. All authors contributed to the article and approved the submitted version
Funding
This work was supported by Cancer Research UK [CG1296/A27223].
Acknowledgments
We would like to thank our CanGene-CanVar Patient Reference Panel members: Caroline Dale, Sue Duncombe, Rochelle Gold, Sonia Patton, Warren Rook, Richard Stephens, Lesley Turner, Frankie Vale, Helen White, Ivan Woodward, Steve Worrall, and Julie Young, other Patient and Public Involvement contributors from the community and our International Lynch Syndrome Decision Aid Stakeholder panel: Munaza Ahmed, Lyndsy Ambler, Antonis Antoniou, Stephanie Archer, Ruth Armstrong, Elizabeth Bancroft, Kristine Barlow-Stewart, Elizabeth Barnett, Marion Bartlett, Julian Barwell, Dany Bell, Cheryl Berlin, Matilda Bradford, John Burn, Sarah Cable, Dharmisha Chauhan, Ruth Cleaver, Elizabeth Coad, Gaya Connolly, Gillian Crawford, Emma Crosbie, Victoria Cuthill, Tabib Dahir, Karina Dahl Steffensen, Eleanor Davies, Glyn Elwyn, Mary Jane Esplen, D Gareth Evans, Pia Fabricius, Andrea Forman, Kaisa Fritzell, Claire Giffney, Joana Gomes, Rebecca Hall, Helen Hanson, Menna Hawkins, Deborah Holliday, Roberta Horgan, Karen Hurley, Margaret James, Ros Jewell, Sarah John, Siobhan John, Victoria Kiesel, Anna Koziel, Anjana Kulkarni, Fiona Lalloo, Helen Liggett, Aela Limbu, Kate Lippiett, Anne Lowry, Manami Matsukawa, Tracie Miles, Shakira Milton, Pål Møller, Kevin Monahan, Laura Monje-Garcia, Alex Murray, Jennie Murray, Kai-Ren Ong, Anbu Paramasivam, Alison Pope, Sarah Pugh, Gabriel Recchia, Nicola Reents, Peter Risby, Neil Ryan, Sibel Saya, Raza Sayyed, Salma Schickh, Lucy Side, Sian Smith, Tracy Smith, Dawn Stacey, Eriko Takamine, Katrina Tatton-Brown, Helle Vendel Petersen, Robert Volk, Jennifer Wiggins, Lisa Wilde, Jennet Williams, Catherine Willis, Elizabeth Winchester, Kristi Withington, Emma Woodward.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhs.2023.1092816/full#supplementary-material.
References
1.
Excellence, N. I. f. H., & Care. Clinical Guideline [CG164] Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer | Guidance | NICE. 2013, NICE.
2.
Cairns SR Scholefield JH Steele RJ Dunlop MG Thomas HJ Evans GD et al Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. (2010) 59(5):666–89. 10.1136/gut.2009.179804
3.
Royal College of Physicians, Royal College of Pathologists and British Society for Genetic Medicin. Consent and confidentiality in genomic medicine: Guidance on the use of genetic and genomic information in the clinic. 2019, Report of the Joint Committee on Genomics in Medicine.: London.
4.
Resta R Biesecker BB Bennett RL Blum S Hahn SE Strecker MN et al A new definition of genetic counseling: national society of genetic Counselors’ task force report. J Genet Couns. (2006) 15(2):77–83. 10.1007/s10897-005-9014-3
5.
Biesecker B Austin J Caleshu C . Theories for psychotherapeutic genetic counseling: fuzzy trace theory and cognitive behavior theory. J Genet Couns. (2017) 26(2):322–30. 10.1007/s10897-016-0023-1
6.
Pilié PG Gay CM Byers LA O’Connor MJ Yap TA . PARP Inhibitors: extending benefit beyond BRCA-mutant cancers. Clin Cancer Res. (2019) 25(13):3759–71. 10.1158/1078-0432.CCR-18-0968
7.
Luchini C Bibeau F Ligtenberg MJL Singh N Nottegar A Bosse T . ESMO Recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. (2019) 30(8):1232–43. 10.1093/annonc/mdz116
8.
Schon K Tischkowitz M . Clinical implications of germline mutations in breast cancer: tP53. Breast Cancer Res Treat. (2018) 167(2):417–23. 10.1007/s10549-017-4531-y
9.
Mosele F Remon J Mateo J Westphalen CB Barlesi F Lolkema MP et al Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO precision medicine working group. Ann Oncol. (2020) 31(11):1491–505. 10.1016/j.annonc.2020.07.014
10.
Mandelker D Donoghue M Talukdar S Bandlamudi C Srinivasan P Vivek M et al Germline-focussed analysis of tumour-only sequencing: recommendations from the ESMO precision medicine working group. Ann Oncol. (2019) 30(8):1221–31. 10.1093/annonc/mdz136
11.
Turnbull C Sud A Houlston RS . Cancer genetics, precision prevention and a call to action. Nat Genet. (2018) 50(9):1212–8. 10.1038/s41588-018-0202-0
12.
Grzymski JJ Elhanan G Morales Rosado JA Smith E Schlauch KA Read R et al Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat Med. (2020) 26(8):1235–9. 10.1038/s41591-020-0982-5
13.
Manchanda R Burnell M Gaba F Desai R Wardle J Gessler S et al Randomised trial of population-based BRCA testing in ashkenazi Jews: long-term outcomes. Bjog. (2020) 127(3):364–75. 10.1111/1471-0528.15905
14.
Madlensky L Trepanier AM Cragun D Lerner B Shannon KM Zierhut H . A rapid systematic review of outcomes studies in genetic counseling. J Genet Couns. (2017) 26(3):361–78. 10.1007/s10897-017-0067-x
15.
Bayne M Fairey M Silarova B Griffin SJ Sharp SJ Klein WMP et al Effect of interventions including provision of personalised cancer risk information on accuracy of risk perception and psychological responses: a systematic review and meta-analysis. Patient Educ Couns. (2020) 103(1):83–95. 10.1016/j.pec.2019.08.010
16.
Turbitt E Roberts MC Taber JM Waters EA McNeel TS Biesecker BB et al Genetic counseling, genetic testing, and risk perceptions for breast and colorectal cancer: results from the 2015 national health interview survey. Prev Med. (2019) 123:12–9. 10.1016/j.ypmed.2019.02.027
17.
Tversky A Kahneman D . The framing of decisions and the psychology of choice. Science. (1981) 211(4481):453. 10.1126/science.7455683
18.
Senay I Kaphingst KA . Anchoring-and-Adjustment bias in communication of disease risk. Med Decis Making. (2008) 29(2):193–201. 10.1177/0272989X08327395
19.
Montgomery v Lanarkshire Health Board (2015). Available at: https://www.supremecourt.uk/cases/docs/uksc-2013-0136-judgment.pdf
20.
Excellence, N. I. f. H. a. C. Clinical Guideline [CG138] Patient experience in adult NHS services: improving the experience of care for people using adult NHS services | Guidance | NICE. (2021). NICE Available at: https://www.nice.org.uk/guidance/cg138.
21.
O’Connor AM Bennett CL Stacey D Barry M Col NF Eden KB et al Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. (2009) 3. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001431.pub2/full
22.
Elwyn G O’Connor AM Bennett C Newcombe RG Politi M Durand MA et al Assessing the quality of decision support technologies using the international patient decision aid standards instrument (IPDASi). PLoS One. (2009) 4(3):e4705. 10.1371/journal.pone.0004705
23.
Stacey D Legare F Lewis K Barry MJ Bennett CL Eden KB et al Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. (2017) 4. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001431.pub5/full
24.
O’Connor AM Bennett C Stacey D Barry MJ Col NE Eden KB et al Do patient decision aids meet effectiveness criteria of the international patient decision aid standards collaboration? A systematic review and meta-analysis. Med Decis Making. (2007) 27(5):554–74. 10.1177/0272989X07307319
25.
Durand M-A Stiel M Boivin J Elwyn G . Where is the theory? Evaluating the theoretical frameworks described in decision support technologies. Patient Educ Couns. (2008) 71(1):125–35. 10.1016/j.pec.2007.12.004
26.
Hilgart JS Hayward JA Coles B Iredale R . Telegenetics: a systematic review of telemedicine in genetics services. Genet Med. (2012) 14(9):765–76. 10.1038/gim.2012.40
27.
Morgan D Sylvester H Lucas FL Miesfeldt S . Hereditary breast and ovarian cancer: referral source for genetic assessment and communication regarding assessment with nongenetic clinicians in the community setting. Genet Med. (2010) 12(1):25–31. 10.1097/GIM.0b013e3181c60955
28.
Sie AS van Zelst-Stams WA Spruijt L Mensenkamp AR Ligtenberg MJ Brunner HG et al More breast cancer patients prefer BRCA-mutation testing without prior face-to-face genetic counseling. Fam Cancer. (2014) 13(2):143–51. 10.1007/s10689-013-9686-z
29.
Sturm AC Schmidlen T Scheinfeldt L Hovick S McElroy JP Toland AE et al Early outcome data assessing utility of a post-test genomic counseling framework for the scalable delivery of precision health. J Pers Med. (2018) 8(3). 10.3390/jpm8030025
30.
Grimmett C Pickett K Shepherd J Welch K Recio-Saucedo A Streit E et al Systematic review of the empirical investigation of resources to support decision-making regarding BRCA1 and BRCA2 genetic testing in women with breast cancer. Patient Educ Couns. (2018) 101(5):779–88. 10.1016/j.pec.2017.11.016
31.
Krassuski L Vennedey V Stock S Kautz-Freimuth S . Effectiveness of decision aids for female BRCA1 and BRCA2 mutation carriers: a systematic review. BMC Med Inform Decis Mak. (2019) 19. 10.1186/s12911-019-0872-2. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-019-0872-2
32.
Kautz-Freimuth S Redaèlli M Rhiem K Vodermaier A Krassuski L Nicolai K et al Development of decision aids for female BRCA1 and BRCA2 mutation carriers in Germany to support preference-sensitive decision-making. BMC Med Inform Decis Mak. (2021) 21(1):180–180. 10.1186/s12911-021-01528-4
33.
Systematic Reviews: Guidance from the Centre for Reviews and Dissemination. (2009) [05/03/2021]; Available at:https://www.york.ac.uk/crd/guidance/
34.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. 10.1136/bmj.n71
35.
Rayyan . (cited 2021 01/05/2021); Available at:https://rayyan.ai
36.
Hoffmann TC Glasziou PP Boutron I Milne R Perera R Moher D et al Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ: British Medical Journal. (2014) 348:g1687. 10.1136/bmj.g1687
37.
Excellence, N. I. f. H., & Care. Process and methods [PMG4] Methods for the development of NICE public health guidance (third edition).(2012). Available at: https://www.nice.org.uk/process/pmg4/chapter/appendix-f-quality-appraisal-checklist-quantitative-intervention-studies.
38.
Hong Q Pluye P Fàbregues S Bartlett G Boardman F Cargo M et al Mixed Methods Appraisal Tool (MMAT). 2018, Canadian Intellectual Property Office, Industry Canada.
39.
Campbell M McKenzie JE Sowden A Katikireddi SV Brennan SE Ellis S et al Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. Br Med J. (2020) 368:l6890. 10.1136/bmj.l6890
40.
Popay J . Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. (2006) [12/03/2021]; Available at:https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf
41.
Mays N Pope C Popay J . Systematically reviewing qualitative and quantitative evidence to inform management and policy-making in the health field. J Health Serv Res Policy. (2005) 10(Suppl 1):6–20. 10.1258/1355819054308576
42.
Mulrow C Langhorne P Grimshaw J . Integrating heterogeneous pieces of evidence in systematic reviews. Ann Intern Med. (1997) 127(11):989–95. 10.7326/0003-4819-127-11-199712010-00008
43.
Albada A Ausems M Otten R Bensing JM van Dulmen S . Use and evaluation of an individually tailored website for counselees prior to breast cancer genetic counseling. J Cancer Educ. (2011) 26(4):670–81. 10.1007/s13187-011-0227-x
44.
Tea MKM Tan YY Staudigl C Eibl B Renz R Asseryanis E et al Improving comprehension of genetic counseling for hereditary breast and ovarian cancer clients with a visual tool. Plos One. (2018) 13(7). 10.1371/journal.pone.0200559
45.
Jabaley T Underhill-Blazey ML Berry DL . Development and testing of a decision aid for unaffected women with a BRCA1 or BRCA2 mutation. J Cancer Educ. (2020) 35(2):339–44. 10.1007/s13187-019-1470-9
46.
Schackmann EA Munoz DF Mills MA Plevritis SK Kurian AW . Feasibility evaluation of an online tool to guide decisions for BRCA1/2 mutation carriers. Fam Cancer. (2013) 12(1):65–73. 10.1007/s10689-012-9577-8
47.
Harmsen MG Steenbeek MP Hoogerbrugge N van Doorn HC Gaarenstroom KN Vos MC et al A patient decision aid for risk-reducing surgery in premenopausal BRCA1/2 mutation carriers: development process and pilot testing. Health Expect. (2018) 21(3):659–67. 10.1111/hex.12661
48.
Heald B Keel E Marquard J Burke CA Kalady MF Church JM et al Using chatbots to screen for heritable cancer syndromes in patients undergoing routine colonoscopy. J Med Genet. (2020) 58(12):807–14. 10.1136/jmedgenet-2020-107294
49.
Peshkin BN Ladd MK Isaacs C Segal H Jacobs A Taylor KL et al The genetic education for men (GEM) trial: development of web-based education for untested men in BRCA1/2-positive families. J Cancer Educ. (2021) 36(1):72–84. 10.1007/s13187-019-01599-y
50.
Vadaparampil ST Malo TL Nam KM Nelson A de la Cruz CZ Quinn GP . From observation to intervention: development of a psychoeducational intervention to increase uptake of BRCA genetic counseling among high-risk breast cancer survivors. J Cancer Educ. (2014) 29(4):709–19. 10.1007/s13187-014-0643-9
51.
Manchanda R Burnell M Loggenberg K Desai R Wardle J Sanderson SC et al Cluster-randomised non-inferiority trial comparing DVD-assisted and traditional genetic counselling in systematic population testing for BRCA1/2 mutations. J Med Genet. (2016) 53(7):472–80. 10.1136/jmedgenet-2015-103740
52.
Sherman KA Kilby CJ Shaw LK Winch C Kirk J Tucker K et al Facilitating decision-making in women undergoing genetic testing for hereditary breast cancer: bRECONDA randomized controlled trial results. Breast. (2017) 36:79–85. 10.1016/j.breast.2017.10.001
53.
Albada A van Dulmen S Spreeuwenberg P Ausems MG . Follow-up effects of a tailored pre-counseling website with question prompt in breast cancer genetic counseling. Patient Educ Couns. (2015) 98(1):69–76. 10.1016/j.pec.2014.10.005
54.
McCuaig JM Tone AA Maganti M Romagnuolo T Ricker N Shuldiner J et al Modified panel-based genetic counseling for ovarian cancer susceptibility: a randomized non-inferiority study. Gynecol Oncol. (2019) 153(1):108–15. 10.1016/j.ygyno.2018.12.027
55.
Metcalfe KA Dennis CL Poll A Armel S Demsky R Carlsson L et al Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Genet Med. (2017) 19(3):330–6. 10.1038/gim.2016.108
56.
Albada A van Dulmen S Ausems M Bensing JM . A pre-visit website with question prompt sheet for counselees facilitates communication in the first consultation for breast cancer genetic counseling: findings from a randomized controlled trial. Genet Med. (2012) 14(5):535–42. 10.1038/gim.2011.42
57.
Nilsson MP Nilsson ED Borg Å Brandberg Y Silfverberg B Loman N . High patient satisfaction with a simplified BRCA1/2 testing procedure: long-term results of a prospective study. Breast Cancer Res Treat. (2019) 173(2):313–8. 10.1007/s10549-018-5000-y
58.
Sie AS Spruijt L van Zelst-Stams WA Mensenkamp AR Ligtenberg MJ Brunner HG et al High satisfaction and low distress in breast cancer patients one year after BRCA-mutation testing without prior face-to-face genetic counseling. J Genet Couns. (2016) 25(3):504–14. 10.1007/s10897-015-9899-4
59.
Hooker GW Leventhal KG DeMarco T Peshkin BN Finch C Wahl E et al Longitudinal changes in patient distress following interactive decision aid use among BRCA1/2 carriers: a randomized trial. Med Decis Making. (2011) 31(3):412–21. 10.1177/0272989X10381283
60.
Meiser B Gleeson M Watts K Peate M Zilliacus E Barlow-Stewart K et al Getting to the point: what women newly diagnosed with breast cancer want to know about treatment-focused genetic testing. Oncol Nurs Forum. (2012) 39(2):E101–11. 10.1188/12.ONF.E101-E111
61.
Scherr CL Nam K Augusto B Kasting ML Caldwell M Lee MC et al A framework for pilot testing health risk video narratives. Health Commun. (2020) 35(7):832–41. 10.1080/10410236.2019.1598612
62.
Yuen J Cousens N Barlow-Stewart K O’Shea R Andrews L . Online BRCA1/2 screening in the Australian Jewish community: a qualitative study. J Community Genet. (2020) 11(3):291–302. 10.1007/s12687-019-00450-7
63.
Culver JO MacDonald DJ Thornton AA Sand SR Grant M Bowen DJ et al Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. (2011) 20(3):294–307. 10.1007/s10897-011-9350-4
64.
Grimmett C Brooks C Recio-Saucedo A Armstrong A Cutress RI Evans DG et al Development of breast cancer choices: a decision support tool for young women with breast cancer deciding whether to have genetic testing for BRCA1/2 mutations. Support Care Cancer. (2019) 27(1):297–309. 10.1007/s00520-018-4307-x
65.
Gornick MC Kurian AW An LC Fagerlin A Jagsi R Katz SJ et al Knowledge regarding and patterns of genetic testing in patients newly diagnosed with breast cancer participating in the iCanDecide trial. Cancer. (2018) 124(20):4000–9. 10.1002/cncr.31731
66.
Kasting ML Conley CC Hoogland AI Scherr CL Kim J Thapa R et al A randomized controlled intervention to promote readiness to genetic counseling for breast cancer survivors. Psychooncology. (2019) 28(5):980–8.
67.
Quinn VF Meiser B Kirk J Tucker KM Watts KJ Rahman B et al Streamlined genetic education is effective in preparing women newly diagnosed with breast cancer for decision making about treatment-focused genetic testing: a randomized controlled noninferiority trial. Genet Med. (2017) 19(4):448–56. 10.1038/gim.2016.130
68.
Watson CH Ulm M Blackburn P Smiley L Reed M Covington R et al Video-assisted genetic counseling in patients with ovarian, fallopian and peritoneal carcinoma. Gynecol Oncol. (2016) 143(1):109–12. 10.1016/j.ygyno.2016.07.094
69.
Høberg-Vetti H Eide GE Siglen E Listøl W Haavind MT Hoogerbrugge N et al Cancer-related distress in unselected women with newly diagnosed breast or ovarian cancer undergoing BRCA1/2 testing without pretest genetic counseling. Acta Oncol. (2019) 58(2):175–81. 10.1080/0284186X.2018.1502466
70.
Dekker N Hermens RP de Wilt JH van Zelst-Stams WA Hoogerbrugge N . RISCO Study Group. Improving recognition and referral of patients with an increased familial risk of colorectal cancer: results from a randomized controlled trial. Colorectal Dis. (2015) 17(6):499–510. 10.1111/codi.12880
71.
Hall MJ Manne SL Winkel G Chung DS Weinberg DS Meropol NJ . Effects of a decision support intervention on decisional conflict associated with microsatellite instability testing. Cancer Epidemiol Biomark Prev. (2011) 20(2):249–54. 10.1158/1055-9965.EPI-10-0685
72.
Arrick BA Bloch KJ Colello LS Woloshin S Schwartz LM . Visual representations of risk enhance long-term retention of risk information: a randomized trial. Med Decis Making. (2019) 39(2):100–7. 10.1177/0272989X18819493
73.
Cragun D Weidner A Tezak A Zuniga B Wiesner GL Pal T . A web-based tool to automate portions of pretest genetic counseling for inherited cancer. J Natl Compr Canc Netw. (2020) 18(7):841–7. 10.6004/jnccn.2020.7546
74.
Albada A van Dulmen S Lindhout D Bensing JM Ausems M . A pre-visit tailored website enhances counselees’ realistic expectations and knowledge and fulfils information needs for breast cancer genetic counselling. Fam Cancer. (2012) 11(1):85–95. 10.1007/s10689-011-9479-1
75.
Petty RE Cacioppo JT . The elaboration likelihood model of persuasion. In: BerkowitzL, editors. Advances in experimental social psychology. New York: Academic Press (1986). p. 123–205.
76.
Reyna VF . A theory of medical decision making and health: fuzzy trace theory. Med Decis Making. (2008) 28(6):850–65. 10.1177/0272989X08327066
77.
Janz NK Becker MH . The health belief model: a decade later. Health Educ Q. (1984) 11(1):1–47. 10.1177/109019818401100101
78.
Prochaska JO Velicer WF . The transtheoretical model of health behavior change. Am J Health Promot. (1997) 12(1):38–48. 10.4278/0890-1171-12.1.38
79.
Ottawa Decision Support Framework. Available at: https://decisionaid.ohri.ca/odsf.html
80.
Keeney RL Raiffa H . Decisions with multiple objectives: Preferences and value trade-offs. Cambridge: Cambridge University Press (1993).
81.
Edwards W Newman WEJR Newman JR Snapper K . Multiattribute evaluation. New York: SAGE Publications (1982).
82.
Michie S Dormandy E Marteau TM . The multi-dimensional measure of informed choice: a validation study. Patient Educ Couns. (2002) 48(1):87–91. 10.1016/S0738-3991(02)00089-7
83.
Michie S Dormandy E Marteau TM . Informed choice: understanding knowledge in the context of screening uptake. Patient Educ Couns. (2003) 50(3):247–53. 10.1016/S0738-3991(03)00044-2
84.
Høberg-Vetti H Bjorvatn C Fiane BE Aas T Woie K Espelid H et al BRCA1/2 Testing in newly diagnosed breast and ovarian cancer patients without prior genetic counselling: the DNA-BONus study. Eur J Hum Genet. (2016) 24(6):881–8. 10.1038/ejhg.2015.196
85.
Nilsson MP Törngren T Henriksson K Kristoffersson U Kvist A Silfverberg B et al BRCAsearch: written pre-test information and BRCA1/2 germline mutation testing in unselected patients with newly diagnosed breast cancer. Breast Cancer Res Treat. (2018) 168(1):117–26. 10.1007/s10549-017-4584-y
86.
Nilsson MP Nilsson ED Silfverberg B Borg Å Loman N . Written pretest information and germline BRCA1/2 pathogenic variant testing in unselected breast cancer patients: predictors of testing uptake. Genet Med. (2019) 21(1):89–96. 10.1038/s41436-018-0021-9
87.
O’Connor AM . Validation of a decisional conflict scale. Med Decis Making. (1995) 15(1):25–30. 10.1177/0272989X9501500105
88.
Morton K Kohut K Turner L Smith S Crosbie E. J Ryan N et al Person-based co-design of a decision aid template for people with a genetic predisposition to cancer. Front in Dig Health. (2022) 4. 10.3389/fdgth.2022.1039701
89.
Hoefel L Lewis KB O’Connor A Stacey D . 20th Anniversary update of the Ottawa decision support framework: part 2 subanalysis of a systematic review of patient decision aids. Med Decis Making. (2020) 40(4):522–39. 10.1177/0272989X20924645
90.
Stacey D Légaré F Boland L Lewis KB Loiselle M-C Hoefel L et al 20th Anniversary Ottawa decision support framework: part 3 overview of systematic reviews and updated framework. Med Decis Making. (2020) 40(3):379–98. 10.1177/0272989X20911870
91.
England NHS. NHS Genomic Medicine Service. 2021 27/07/2021]; Available at:https://www.england.nhs.uk/genomics/nhs-genomic-med-service/
92.
Biesecker BB Lewis KL Umstead KL Johnston JJ Turbitt E Fishier KP et al Web platform vs in-person genetic counselor for return of carrier results from exome sequencing A randomized clinical trial. JAMA Intern Med. (2018) 178(3):338–46. 10.1001/jamainternmed.2017.8049
93.
Shin SJ Dodd-Eaton EB Peng G Bojadzieva J Chen J Amos CI et al Penetrance of different cancer types in families with li-fraumeni syndrome: a validation study using multicenter cohorts. Cancer Res. (2020) 80(2):354–60. 10.1158/0008-5472.CAN-19-0728
94.
Tung N Domchek SM Stadler Z Nathanson KL Couch F Garber JE et al Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. (2016) 13(9):581–8. 10.1038/nrclinonc.2016.90
95.
Valle L Vilar E Tavtigian SV Stoffel EM . Genetic predisposition to colorectal cancer: syndromes, genes, classification of genetic variants and implications for precision medicine. J Pathol. (2019) 247(5):574–88. 10.1002/path.5229
96.
Batalini F Peacock EG Stobie L Robertson A Garber J Weitzel JN et al Li-Fraumeni syndrome: not a straightforward diagnosis anymore-the interpretation of pathogenic variants of low allele frequency and the differences between germline PVs, mosaicism, and clonal hematopoiesis. Breast Cancer Res. (2019) 21(1):107. 10.1186/s13058-019-1193-1
97.
Dominguez-Valentin M Sampson JR Seppälä TT Ten Broeke SW Plazzer JP Nakken S et al Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the prospective lynch syndrome database. Genet Med. (2020) 22(1):15–25. 10.1038/s41436-019-0596-9
98.
Kuchenbaecker KB Hopper JL Barnes DR Phillips KA Mooij TM Roos-Blom MJ et al Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. Jama. (2017) 317(23):2402–16. 10.1001/jama.2017.7112
99.
Mai PL Best AF Peters JA DeCastro RM Khincha PP Loud JT et al Risks of first and subsequent cancers among TP53 mutation carriers in the national cancer institute li-fraumeni syndrome cohort. Cancer. (2016) 122(23):3673–81. 10.1002/cncr.30248
100.
Cribb A . Managing ethical uncertainty: implicit normativity and the sociology of ethics. Sociol Health Illn. (2020) 42(S1):21–34. 10.1111/1467-9566.13010
101.
Han PKJ Klein WMP Arora NK . Varieties of uncertainty in health care: a conceptual taxonomy. Med Decis Making. (2011) 31(6):828–38. 10.1177/0272989X10393976
102.
Han PKJ Umstead KL Bernhardt BA Green RC Joffe S Koenig B et al A taxonomy of medical uncertainties in clinical genome sequencing. Genet Med. (2017) 19(8):918–25. 10.1038/gim.2016.212
103.
Newson AJ Leonard SJ Hall A Gaff CL . Known unknowns: building an ethics of uncertainty into genomic medicine. BMC Med Genomics. (2016) 9(1):57–57. 10.1186/s12920-016-0219-0
104.
Stivers T Timmermans S . Negotiating the diagnostic uncertainty of genomic test results. Soc Psychol Q. (2016) 79(3):199–221. 10.1177/0190272516658770
105.
Brashers DE . Communication and uncertainty management. Journal of Communication. (2001) 51(3):477–97. 10.1111/j.1460-2466.2001.tb02892.x
106.
Clarke AJ Wallgren-Pettersson C . Ethics in genetic counselling. J Community Genet. (2019) 10(1):3–33. 10.1007/s12687-018-0371-7
107.
Kraft SA Porter KM Duenas DM Guerra C Joseph G Lee SS-J et al Participant reactions to a literacy-focused, web-based informed consent approach for a genomic implementation study. AJOB Empir Bioeth. (2021) 12(1):1–11. 10.1080/23294515.2020.1823907
108.
England N . NHS Long term plan. (2019) :24–6. https://www.longtermplan.nhs.uk/
109.
England NHS. Universal Personalised Care: Implementing the Comprehensive Model.(2019). Available at: https://www.england.nhs.uk/publication/universal-personalised-care-implementing-the-comprehensive-model/
110.
Yardley L Morrison L Bradbury K Muller I . The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res. (2015) 17(1):e30. 10.2196/jmir.4055
111.
Pawson R . Evidence-based policy: the promise of `realist synthesis’. Evaluation. (2002) 8(3):340–58. 10.1177/135638902401462448
112.
Pawson R Greenhalgh T Harvey G Walshe K . Realist review—a new method of systematic review designed for complex policy interventions. J Health Serv Res Policy. (2005) 10(1_suppl):21–34. 10.1258/1355819054308530
Summary
Keywords
cancer genetics, genetic counselling, patient decision aid, shared decision-making, decision support
Citation
Kohut K, Morton K, Turner L, Shepherd J, Fenerty V, Woods L, Grimmett C, Eccles DM and Foster C (2023) Patient decision support resources inform decisions about cancer susceptibility genetic testing and risk management: a systematic review of patient impact and experience. Front. Health Serv. 3:1092816. doi: 10.3389/frhs.2023.1092816
Received
18 November 2022
Accepted
26 April 2023
Published
31 May 2023
Volume
3 - 2023
Edited by
Nisha Shah, University of Oxford, United Kingdom
Reviewed by
Leonieke Kranenburg, Erasmus Medical Center, Netherlands Erin Turbitt, University of Technology Sydney, Australia
Updates
Copyright
© 2023 Kohut, Morton, Turner, Shepherd, Fenerty, Woods, Grimmett, Eccles and Foster.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Kelly Kohut k.e.kohut@soton.ac.uk
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.