- 1Department of Infectious Diseases, Washington University School of Medicine, Washington University in St. Louis, St. Louis, MO, United States
- 2Department of Psychiatry and Human Behavior, Warren Alpert Medical School, Brown University, Providence, RI, United States
- 3Center for Healthcare Organization and Implementation Research, VA Bedford Healthcare System, Bedford, MA, United States
- 4College of Public Health, Temple University, Philadelphia, PA, United States
- 5Implementation Science Center for Cancer Control and Prevention Research Center, Brown School, Washington University in St. Louis, St. Louis, MO, United States
- 6School of Social Work, Brigham Young University, Provo, UT, United States
- 7School of Nursing and Public Health, University of KwaZulu-Natal, Durban, South Africa
- 8Bernard Becker Medical Library, Washington University in St. Louis School of Medicine, St. Louis, MO, United States
- 9Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, United States
- 10Shanti K. Khinduka Distinguished Professor Emerita, Brown School, Washington University in St. Louis, St. Louis, MO, United States
Recent articles have highlighted the importance of incorporating implementation science concepts into pandemic-related research. However, limited research has been documented to date regarding implementation outcomes that may be unique to COVID-19 vaccinations and how to utilize implementation strategies to address vaccine program-related implementation challenges. To address these gaps, we formed a global COVID-19 implementation workgroup of implementation scientists who met weekly for over a year to review the available literature and learn about ongoing research during the pandemic. We developed a hierarchy to prioritize the applicability of “lessons learned” from the vaccination-related implementation literature. We identified applications of existing implementation outcomes as well as identified additional implementation outcomes. We also mapped implementation strategies to those outcomes. Our efforts provide rationale for the utility of using implementation outcomes in pandemic-related research. Furthermore, we identified three additional implementation outcomes: availability, health equity, and scale-up. Results include a list of COVID-19 relevant implementation strategies mapped to the implementation outcomes.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic vividly exemplifies an implementation crisis: life-saving remedies exist, but their adoption and spread have lagged world-wide. Many longed for vaccines and heralded their development. However, many medical and public health experts were surprised at the skepticism, hesitancy, and outright resistance to available vaccines, even as the pandemic led to sky-rocketing rates of mortality and morbidity and compromised even the most resource-rich health systems. Despite recommendations from the World Health Organization, we are still far from achieving the proposed goal of 70% vaccination coverage globally (1). Though there is some debate as to whether 70% vaccination is still sufficient due to the emergence of new variants (2), the challenges with vaccine program implementation remain.
Implementation science is well-suited to tackle crises like the COVID-19 pandemic. But what specifically does it offer? This Perspective article addresses one part of this question by demonstrating the importance of conceptualizing vaccine roll-out through the lens of two implementation science concepts: implementation outcomes and implementation strategies. These concepts help clarify some of the most pressing questions about the implementation challenges surrounding vaccination programs: What needs to be achieved, and how do we get there? This article is not meant to be an exhaustive review of current research; rather, it is intended to provide the reader with guidance on how we might more clearly conceptualize the implementation outcomes—both anticipated and actual (3)—that are most relevant for increasing the uptake of COVID-19 vaccines. Additionally, we will provide examples of implementation strategies, developed to ensure successful implementation outcomes, to better aid researchers and practitioners in evaluating the implementation of COVID-19 vaccines.
Applying an Implementation Science Lens to the Covid-19 Pandemic
Implementation science is the “study of methods to promote the systematic uptake of research findings and other evidence-based practices into routine practice” (4). This field seeks to provide guidance in cases where evidence-based interventions exist but are poorly implemented or, in some instances, not implemented at all. In the case of the COVID-19 pandemic, an intervention—a vaccine—exists, but it has been underutilized for myriad reasons (e.g., issues surrounding supply and distribution, mistrust, misinformation, vaccine hesitancy). Implementation science provides an opportunity to apply existing methods to study these challenges and improve the uptake of the COVID-19 vaccine.
Implementation science is inherently pragmatic and involves real-world, diverse populations, data collection that is meaningful and actionable, and a focus on the application of an evidence-based practice (e.g., vaccination) in local contexts (5). Thus, ensuring the successful uptake of COVID-19 vaccines globally requires a pragmatic, low burden assessment of stakeholders' perceptions of implementation outcomes related to vaccination (e.g., acceptability, cost) (6), as well as specific and operationalized implementation strategies to address barriers to vaccine uptake (7).
Previous articles have underscored the importance of incorporating implementation science concepts into pandemic-related research (8–10). For example, it is vital to engage stakeholders from project inception and consider context during implementation, as factors such as available resources, policy support, health system and population characteristics can impact the uptake of an intervention (9). Additionally, implementation science theories and methods can help inform the equitable development, implementation, and evaluation of interventions to address health disparities and promote health equity (11–14). However, there has been a limited research focus to date regarding implementation outcomes that may be unique to COVID-19 vaccinations and how to utilize implementation strategies to address vaccine program-related implementation challenges. To address these gaps, we formed a global COVID-19 implementation workgroup of implementation scientists who met weekly for over a year, to review the available literature and learn about ongoing research during the pandemic. Our efforts resulted in a list of implementation outcomes that can be used to evaluate the implementation of vaccine programs globally. Likewise, we have compiled a list of implementation strategies, which can be mapped onto the aforementioned outcomes to address common challenges related to vaccine program implementation.
Given this novel disease and the unprecedented times, evidence directly related to COVID-19 is still developing. As a result, our recommendation for implementation strategies have been informed by COVID-19 evidence, as well as previous public health efforts, which we believe are transferable or applicable to this pandemic. To aid in this conceptualization of evidence, our team constructed the following hierarchy by which to prioritize recommendations and “lessons learned.” We drew from existing evidence hierarchies [e.g., GRADE (15) and AGREE II (16)] and used a consensus-based approach among the authors similar to that espoused for developing guideline recommendations [e.g., the DECIDE framework (17–19)]. The implementation science base of evidence to guide strategy selection for COVID-19 mitigation ranges from scant to very strong.
1. Direct evidence from COVID-19 vaccination: scant and emergent, especially early in the pandemic.
2. Evidence from other preventative strategies used during COVID-19 (e.g., masking, social distancing): emergent.
3. Evidence from vaccinations of non-COVID-19 diseases that are respiratory, novel, or heterogeneous in severity, or during a pandemic (e.g., influenza): solid to strong.
4. Evidence from vaccination for other infections (e.g., measles, polio): strong.
5. Evidence from other health conditions: very strong.
Implementation Outcomes
Implementation outcomes provide a means to evaluate the implementation success of interventions, treatments, policies, and protocols and are distinct from other, traditionally measured outcomes, such as service system and clinical outcomes (6). In the case of COVID-19 prevention and treatment, service system outcomes include the timeliness and efficiency of the health system, and perceived equity and patient-centeredness of treatments, while clinical outcomes reflect population and patient health and safety, as well as satisfaction with treatment options, including vaccines.
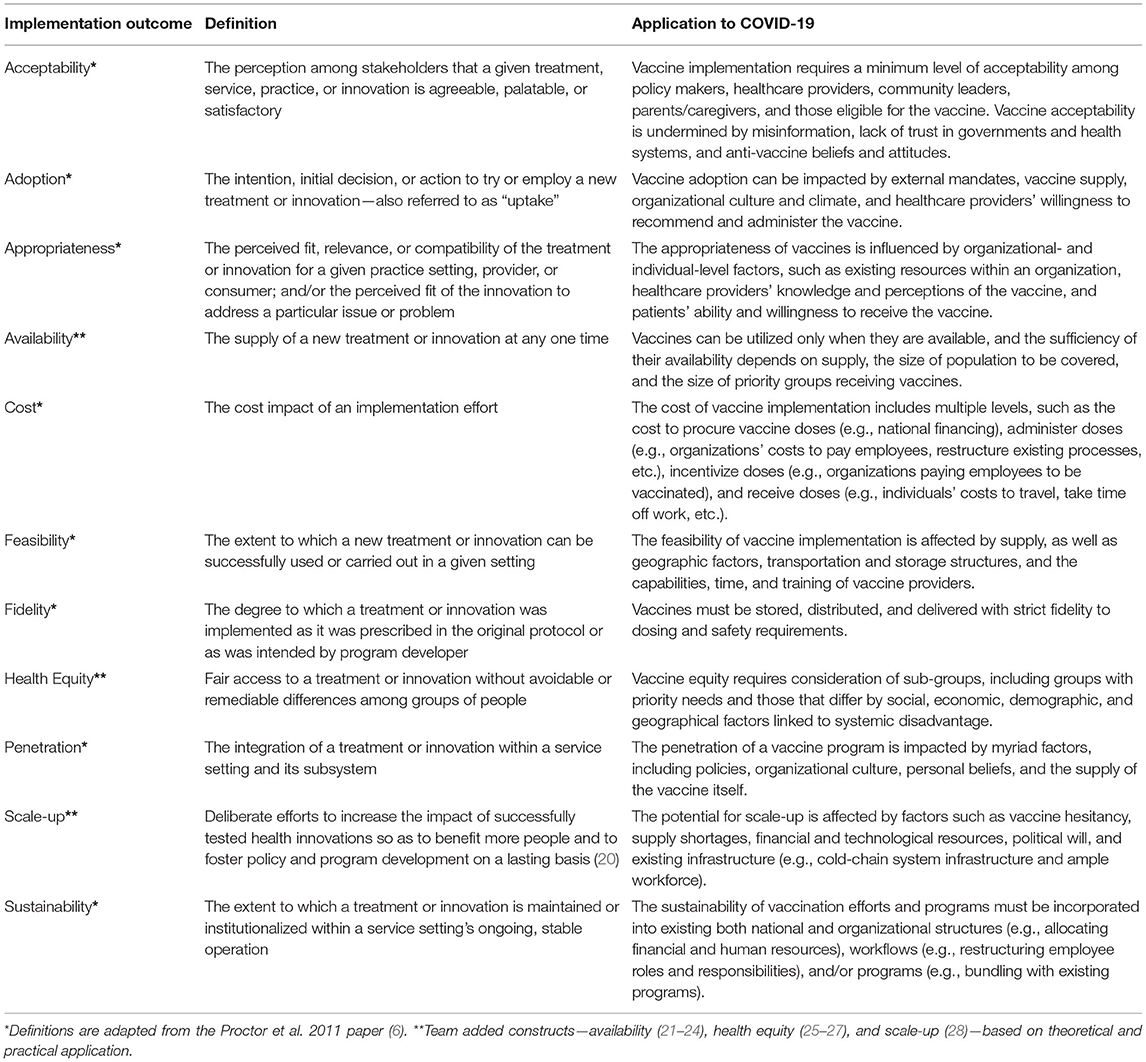
In 2011, Proctor and colleagues developed the Implementation Outcomes Framework as a way to conceptualize and measure eight distinct implementation outcomes—acceptability, adoption, appropriateness, cost, feasibility, fidelity, penetration, and sustainability (6). This framework has since been widely cited and applied within the implementation science community. However, based on our review of the literature, there are additional outcomes that could—and should—be considered within the context of the COVID-19 pandemic to provide a more comprehensive evaluation of implemenation of vaccine programs. As a result, we have operationalized the eight well-known implementation outcomes based on their relevance to the COVID-19 pandemic and have proposed three additional outcomes (availability, health equity, and scale-up) for consideration (Table 1).
Evaluations of COVID-19 mitigation efforts could have been—and still have potential to be—more precise and robust with a greater focus on implementation outcomes. These outcomes can play three important roles in relation to COVID-19 control. First, they allow researchers and implementers to focus efforts on assessing the baseline or starting point, thereby quantifying the gap between desired and achieved outcomes. Vaccines need to “have an efficacy of at least 70% to prevent an epidemic and of at least 80% to largely extinguish an epidemic without any other measures (e.g., social distancing)” (29). Within the context of COVID-19 vaccination, where many countries included other mitigation measures in their efforts to control the pandemic, such as mask wearing and social distancing, this could include monitoring the vaccination status of a population. In addition to quantifying gaps in implementation, incorporating implementation outcomes into vaccine program planning and evaluation also provides a source of accountability for implementers at the national-, state-, or local-levels. The additional implementation outcomes of availability and health equity are essential for increasing vaccine uptake. Vaccines can be utilized only when they are available, and the sufficiency of their availability depends on supply, the size of population to be covered, and the size of priority groups receiving vaccines. Similarly, vaccine equity requires consideration of sub-groups, including groups with priority needs for vaccination and those that differ by social, economic, demographic, and geographical factors linked to systemic disadvantage.
Second, implementation outcomes provide a direction for implementation efforts. For instance, factors such as the feasibility and sustainability of public health programs may be prioritized when implementation outcomes are incorporated into the planning and evaluation process. These outcomes can also highlight potential barriers or facilitators that may arise during implementation and help researchers identify implementation strategies to address potential challenges. As we have seen over the past 2 years, myriad challenges can arise during vaccine development, distribution, and implementation. For example, factors such as unfamiliar technology or a lack of staff buy-in may decrease the feasibility of implementing a COVID-19 vaccine program. The additional implementation outcome of scale-up we have defined illustrates how vaccine scale-up is affected by factors such as vaccine hesitancy, supply shortages, financial and technological resources, and existing infrastructure (e.g., cold-chain system infrastructure and ample workforce). Scale-up differs from the original penetration implementation outcome, which refers to the degree to which an intervention has infiltrated a service system. Scale-up, on the hand, is broader, encompassing multiple service systems thereby requiring different strategies at higher socioecological levels. Additionally, the deluge of information disseminated through social media has increasingly delivered misinformation impacting acceptability of COVID-19 vaccines globally. As a result, specific strategies can be selected based on the implementation context. Challenges with technology could be addressed through the provision of technical assistance, while buy-in could be encouraged by identifying champions within the organization. Finally, vaccine-related misinformation could be counteracted through a targeted public health campaign emphasizing the transparency of the vaccine development process, and using jargon-free messaging that takes into account socioeconomic and cultural factors, as well as personal and media sources needed for communicating with specific populations (30).
Third, implementation outcomes can increase the precision of evaluation efforts surrounding public health programs. Too often, public health systems' data collection is limited to clinical outcomes (e.g., number of COVID-19 cases, hospitalizations, and mortality). However, measuring intermediate outcomes, such as acceptabilty, adoption and fidelity, paints a more complete picture of program implementation and illuminates the challenges to current implementation efforts. For example, several global surveys conducted throughout the pandemic have assessed the degrees of acceptability of COVID-19 vaccines over time (31, 32). Important findings from these surveys have revealed that those who trust their governments and their messaging (31) and receive information and guidance from healthcare providers (32) reported that they will be more likely to become vaccinated against COVID-19. In another project, COVID-19 vaccine acceptability among patients and employees of a large integrated healthcare system in the United States was assessed through surveys (33) and interviews (34). Specific, tailored communication strategies were then created to overcome identified barriers to receiving a vaccine and were disseminated widely across the healthcare system, for use in one-to-one conversations between trusted providers and patients, and among employees (34). The additional information regarding, for example, program acceptability can enable implementers to better evaluate program efforts and adapt practices to promote the uptake of COVID-19 vaccines.
Implementation Strategies
Implementation strategies are defined as “methods or techniques used to enhance the adoption, implementation, and sustainability of a clinical program or practice” (7). Implementation scientists have emphasized the importance of identifying and compiling evidence-based strategies (35), selecting strategies based on implementation context and barriers (36), and specifying the use of implementation strategies in research (7). Building on this body of work, our team set out to map implementation strategies onto specific implementation outcomes and challenges to provide guidance for researchers and practitioners to increase the uptake of vaccines and aid in the scale-up and spread of successful vaccination programs.
After identifying and conceptualizing critical implementation outcomes to assess to determine the effectiveness of COVID-19 vaccine implementation globally (Table 1), our research group considered some of the most common challenges encountered during vaccine program implementation (e.g., mistrust, misinformation, vaccine hesitancy, supply issues). We then compiled a range of implementation strategies to determine which of these may be most useful in overcoming common implementation challenges and increasing uptake of COVID-19 vaccines. We present the implementation outcomes, implementation challenges, and the implementation strategies our team identified, and details on these strategies (e.g., specified actions or tools) identified in the literature to improve implementation success (Table 2).
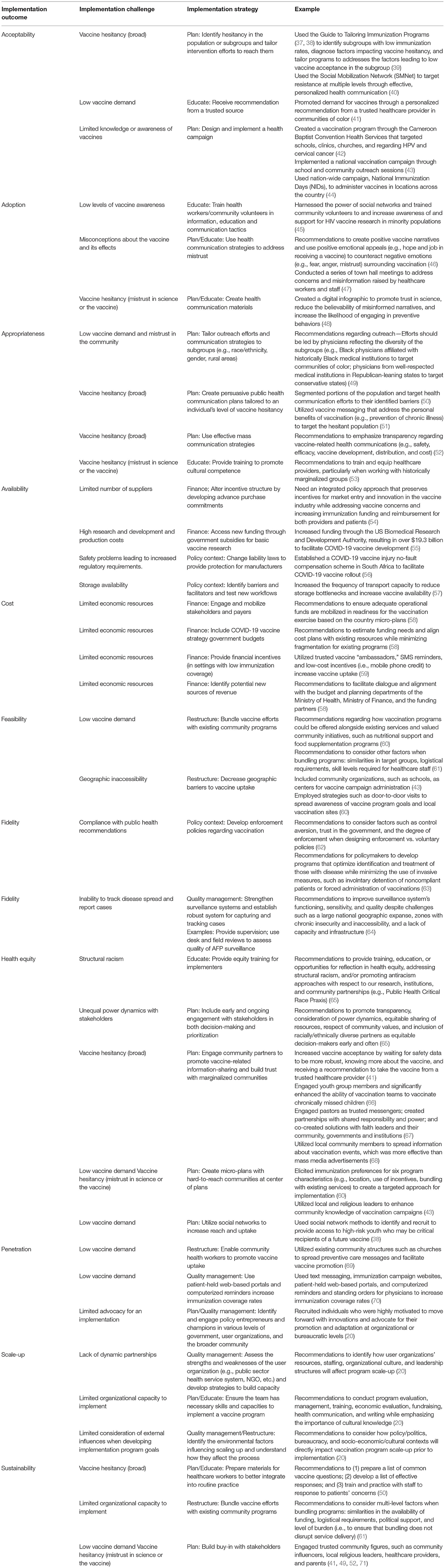
Table 2. Implementation outcomes, challenges, and strategies applicable to the COVID-19 vaccine program implementation.
In many cases, we found that several implementation strategies were needed to address these complex, multi-level implementation challenges (5), especially prevalent in vaccination programs. For example, Rajkumari and colleagues identified two barriers related to the uptake of a measles-rubella vaccine in India—limited knowledge of the vaccine and geographic inaccessibility—which impacted the vaccine program's acceptability and feasibility, respectively (43). As a result, the team selected implementation strategies to address these barriers, which included creating a national health campaign and administering vaccines through community organizations, such as schools (43). Momplaisir et al., explored attitudes and beliefs related to COVID-19 vaccinations within Black communities in the United States (41). Though participants reported low demand and high levels of vaccine hesitancy, they also identified that recommendations from a healthcare provider might increase their trust in the vaccine's safety and efficacy (41). These findings illustrate the importance of engaging community partners and promoting trust, particularly in historically underserved communities, to increase the acceptability and equitable distribution of the COVID-19 vaccine.
Discussion
The COVID-19 pandemic is an evolving situation, and implementation science needs to respond accordingly. Many of our theories and study designs have an implicit long period of time that's required to assess implementation (72). However, this situation requires rapid assessments and evaluations, as well as the measurement of implementation outcomes that may have been previously overlooked (73).
Our team's activity provided evidence that the original Proctor implementation outcomes are still essential for examining the success of COVID-19 vaccination programs globally; yet three outcomes—those of availability, health equity and scale-up—are welcome additions to the implementation outcomes framework. Addressing implementation challenges related to availability, for example, allows governments and policymakers to focus on the earlier, or pre-implementation, factors that support widespread vaccine scale-up, such as increasing vaccine suppliers and providing incentives and additional funding structures to address high research and development costs. In certain contexts, policies may also need to change regarding manufacturing liability, which is essential for increasing the availability and eventual scale-up of vaccines. Vaccine availability is also impacted by storage issues and can be mitigated by additional infrastructure support, such as increasing transportation, which is needed for future scale-up.
Health equity, a critical part of implementation science, and fair access to COVID-19 vaccines has been a continual challenge globally. Health equity is also closely related to the implementation outcomes of availability and scale-up. For example, when there is limited vaccine supply or organizational capacity for vaccine program implementation, power struggles that exist among stakeholders with varying social, economic, demographic, and geographical differences may lead to an inequitable distribution of vaccines (e.g., vaccines only being available to groups whose power aligns with the organizations distributing vaccines). Additionally, greater distrust in science and vaccines within historically underserved populations may further impede vaccine acceptance and uptake. This underscores the importance of considering the myriad external influences that lead to misinformation campaigns and distrust when assessing the potential for vaccine program scale-up.
Through engagement of a collaborative working group on global vaccine implementation, our team was able to apply a well-known implementation science framework to current and past literature, policies, and country-level knowledge about vaccines. As a result, we identified three additional implementation outcomes and compiled a list of specific implementation strategies that can be applied at multiple levels to increase vaccine uptake.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
EP, EG, MM, and OA contributed to conception of the study. MP, AE, and EP wrote the first draft of the manuscript. GH and LL wrote portions of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This work was supported in part by the National Institute of Allergy and Infectious Diseases (No. K24AI134413). EP effort was supported by the Implementation Science-Entrepreneurship Unit of the Washington University Institute of Clinical and Translational Sciences (No. UL1TR002345) from the National Center for Advancing Translational Sciences of the National Institutes of Health; the National Institute of Mental Health (No. R25MH080916); National Cancer Institute (No. P50CA244431); National Institute of Mental Health (No. 5P50MH113662); Patient-Centered Outcomes Research Institute Award (No. TRD-1511-33321).
Author Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official positions of the National Institutes of Health. Additionally, the views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge the support of the Washington University Center for Dissemination and Implementation.
References
1. World Health Organization. Strategy to Achieve Global Covid-19 Vaccination by mid-2022 (2021). Available online at: https://reliefweb.int/report/world/strategy-achieve-global-covid-19-vaccination-mid-2022 (accessed March 31, 2022).
2. Aizenman N. The goal: Vaccinate 70% of the world against COVID. In: Scientists are proposing a reboot. NPR. (2022).
3. Damschroder LJ, Reardon CM, Opra Widerquist MA, Lowery J. Conceptualizing outcomes for use with the Consolidated Framework for Implementation Research (CFIR): the CFIR outcomes addendum. J Article Implement Sci. (2022) 17:1–10. doi: 10.1186/s13012-021-01181-5
4. Bauer MS, Damschroder L, Hagedorn H, Smith J, Kilbourne AM. An introduction to implementation science for the non-specialist. BMC Psychol. (2015) 3:32–32. doi: 10.1186/s40359-015-0089-9
5. Glasgow RE. What does it mean to be pragmatic? Pragmatic methods, measures, and models to facilitate research translation. Health Educ Behav. (2013) 40:257–65. doi: 10.1177/1090198113486805
6. Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: Conceptual distinctions, measurement challenges, and research agenda. Admin Policy Mental Health Mental Health Serv Res. (2011) 38:65–76. doi: 10.1007/s10488-010-0319-7
7. Proctor EK, Powell BJ, McMillen JC. Implementation strategies: recommendations for specifying and reporting. Implement Sci. (2013) 8:139. doi: 10.1186/1748-5908-8-139
8. Means AR, Wagner AD, Kern E, Newman LP, Weiner BJ. Implementation science to respond to the COVID-19 pandemic perspective. Front Public Health. (2020) 8:462. doi: 10.3389/fpubh.2020.00462
9. Chambers DA. Considering the intersection between implementation science and COVID-19. Implement Res Pract. (2020) 1:0020764020925994. doi: 10.1177/0020764020925994
10. Wensing M, Sales A, Armstrong R, Wilson P. Implementation science in times of Covid-19. Implement Sci. (2020) 15:42. doi: 10.1186/s13012-020-01006-x
11. Jacobson TA, Smith LE, Hirschhorn LR, Huffman MD. Using implementation science to mitigate worsening health inequities in the United States during the COVID-19 pandemic. Int J Equity Health. (2020) 19:170. doi: 10.1186/s12939-020-01293-2
12. Galaviz KI, Breland JY, Sanders M, Breathett K, Cerezo A, Gil O, et al. Implementation Science to Address Health Disparities During the Coronavirus Pandemic. Health Equity. (2020) 4:463–7. doi: 10.1089/heq.2020.0044
13. Woodward EN, Singh RS, Ndebele-Ngwenya P, Melgar Castillo A, Dickson KS, Kirchner JE, et al. more practical guide to incorporating health equity domains in implementation determinant frameworks. Implement Sci Commun. (2021) 2:61. doi: 10.1186/s43058-021-00146-5
14. Shelton RC, Chambers DA, Glasgow RE. An extension of RE-AIM to enhance sustainability: addressing dynamic context and promoting health equity over time. Front Public Health. (2020) 8:134. doi: 10.3389/fpubh.2020.00134
17. Treweek S, Oxman AD, Alderson P, Bossuyt PM, Brandt L, Brozek J, et al. Developing and evaluating communication strategies to support informed decisions and practice based on evidence (DECIDE): protocol and preliminary results. Implement Sci. (2013) 8:6–6. doi: 10.1186/1748-5908-8-6
18. Guldbrandsson K, Stenström N, Winzer R. The DECIDE evidence to recommendation framework adapted to the public health field in Sweden. Health Promot Int Dec. (2016) 31:749–54. doi: 10.1093/heapro/dav060
19. Glenton C, Lewin S, Gülmezoglu AM. Expanding the evidence base for global recommendations on health systems: strengths and challenges of the OptimizeMNH guidance process. Implement Sci. (2016) 11:98. doi: 10.1186/s13012-016-0470-y
20. World Health Organization. Practical Guidance for scaling up health service innovations. (2009). Available online at: https://www.who.int/reproductivehealth/publications/strategic_approach/9789241598521/en/ (accessed March 31, 2022).
21. Haldane V, Chuah FLH, Srivastava A, Singh SR, Koh GCH, Seng CK, et al. Community participation in health services development, implementation, and evaluation: a systematic review of empowerment, health, community, and process outcomes. PLoS ONE. (2019) 5. doi: 10.1371/journal.pone.0216112
22. Jackson JA, Spilman SK, Kingery LK, Oetting TW, Taylor MJ, Pruett WM, et al. Implementation of high-flow nasal cannula therapy outside the intensive care setting. Respir Care. (2021) 66:357. doi: 10.4187/respcare.07960
23. Wang T, Lurie M, Govindasamy D, Mathews C. The Effects of school-based condom availability programs (CAPs) on condom acquisition, use and sexual behavior: a systematic review. AIDS Behav. (2018) 22:308–20. doi: 10.1007/s10461-017-1787-5
24. Tanahashi T. Health service coverage and its evaluation. Bull World Health Organ. (1978) 56:295–303.
25. Brownson RC, Kumanyika SK, Kreuter MW, Haire-Joshu D. Implementation science should give higher priority to health equity. Implement Sci. (2021) 16:28. doi: 10.1186/s13012-021-01097-0
26. Baumann AA, Cabassa LJ. Reframing implementation science to address inequities in healthcare delivery. BMC Health Serv Res. (2020) 20:190. doi: 10.1186/s12913-020-4975-3
27. Odeny B. Closing the health equity gap: a role for implementation science? PLoS Med. (2021) 18:e1003762–e1003762. doi: 10.1371/journal.pmed.1003762
28. Milat AJ, Bauman A, Redman S. Narrative review of models and success factors for scaling up public health interventions. Implement Sci. (2015) 10:113. doi: 10.1186/s13012-015-0301-6
29. Bartsch SM, O'Shea KJ, Ferguson MC, Bottazzi ME, Wedlock PT, Strych U, et al. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am J Prev Med Oct. (2020) 59:493–503. doi: 10.1016/j.amepre.2020.06.011
30. Quinn SC, Jamison AM, Freimuth V. Communicating effectively about emergency use authorization and vaccines in the COVID-19 pandemic. Am J Public Health. (2021) 111:355–8. doi: 10.2105/AJPH.2020.306036
31. Lazarus JV, Ratzan SC, Palayew A, Gostin LO, Larson HJ, Rabin K, et al. global survey of potential acceptance of a COVID-19 vaccine. Nat Med. (2021) 27:225–8. doi: 10.1038/s41591-020-1124-9
32. Solís Arce JS, Warren SS, Meriggi NF, Scacco A, McMurry N, Voors M, et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat Med. (2021) 27:1385–94. doi: 10.1101/2021.03.11.21253419
33. Jasuja GK, Meterko M, Bradshaw LD, Carbonaro R, Clayman ML, LoBrutto L, et al. Attitudes and intentions of US veterans regarding COVID-19 vaccination. JAMA Netw Open. (2021) 4:e2132548. doi: 10.1001/jamanetworkopen.2021.32548
34. Elwy AR, Clayman ML, LoBrutto L, Miano D, Ann Petrakis B, Javier S, et al. Vaccine hesitancy as an opportunity for engagement: A rapid qualitative study of patients and employees in the U.S. Veterans Affairs healthcare system. Vaccine X Dec. (2021) 9:100116. doi: 10.1016/j.jvacx.2021.100116
35. Powell BJ, Waltz TJ, Chinman MJ, Damschroder LJ, Smith JL, Matthieu MM, et al. refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project. Implement Sci. (2015) 10:21. doi: 10.1186/s13012-015-0209-1
36. Powell BJ, Beidas RS, Lewis CC, Aarons GA, McMillen JC, Proctor EK, et al. Methods to improve the selection and tailoring of implementation strategies. J Behav Health Serv Res Apr. (2017) 44:177–94. doi: 10.1007/s11414-015-9475-6
37. Dubé E, Leask J, Wolff B, Kickler B, Balaban V, Hosein E, et al. The WHO tailoring immunization programmes (TIP) approach: review of implementation to date. Vaccine. (2018) 36:1509–15. doi: 10.1016/j.vaccine.2017.12.012
38. Butler R, MacDonald NE. Diagnosing the determinants of vaccine hesitancy in specific subgroups: the Guide to Tailoring Immunization Programmes (TIP). Vaccine. (2015) 33:4176–9. doi: 10.1016/j.vaccine.2015.04.038
39. MacDonald NE, Butler R, Dubé E. Addressing barriers to vaccine acceptance: an overview. Hum Vaccin Immunother. (2018) 14:218–24. doi: 10.1080/21645515.2017.1394533
40. Siddique AR, Singh P, Trivedi G. Role of Social Mobilization (Network) in Polio Eradication in India. Indian Pediatr. (2016) 53:S50–6.
41. Momplaisir F, Haynes N, Nkwihoreze H, Nelson M, Werner RM, Jemmott J. Understanding drivers of coronavirus disease 2019 vaccine hesitancy among blacks. Clin Infect Dis. (2021) 73:1784–9. doi: 10.1093/cid/ciab102
42. Wamai RG, Ayissi CA, Oduwo GO, Perlman S, Welty E, Manga S, et al. Assessing the effectiveness of a community-based sensitization strategy in creating awareness about HPV, cervical cancer and HPV vaccine among parents in north west cameroon. J Community Health. (2012) 37:917–26. doi: 10.1007/s10900-012-9540-5
43. Rajkumari B, Keisam A, Haobam D, Thounaojam T, Haobam DS. Evaluation of vaccination coverage of measles-rubella campaign in Imphal East District, Manipur: a cross-sectional study. Indian J Public Health. (2020) 64:173–7. doi: 10.4103/ijph.IJPH_361_19
44. Bonu S, Rani M, Razum O. Global public health mandates in a diverse world: the polio eradication initiative and the expanded programme on immunization in sub-Saharan Africa and South Asia. Health Policy. (2004) 70:327–45. doi: 10.1016/j.healthpol.2004.04.005
45. Kelley RT, Hannans A, Kreps GL, Johnson K. The Community Liaison Program: a health education pilot program to increase minority awareness of HIV and acceptance of HIV vaccine trials. Health Educ Res Aug. (2012) 27:746–54. doi: 10.1093/her/cys013
46. Chou W-YS, Budenz A. Considering emotion in COVID-19 vaccine communication: addressing vaccine hesitancy and fostering vaccine confidence. Health Commun. (2020) 35:1718–22. doi: 10.1080/10410236.2020.1838096
47. Berry SD, Johnson KS, Myles L, Herndon L, Montoya A, Fashaw S, et al. Lessons learned from frontline skilled nursing facility staff regarding COVID-19 vaccine hesitancy. J Am Geriatr Soc. (2021) 69:1140–6. doi: 10.1111/jgs.17136
48. Agley J, Xiao Y, Thompson EE, Golzarri-Arroyo L. COVID-19 misinformation prophylaxis: protocol for a randomized trial of a brief informational intervention. JMIR Res Protoc. (2020) 9:e24383. doi: 10.2196/24383
49. SteelFisher GK, Blendon RJ, Caporello H. An uncertain public - encouraging acceptance of Covid-19 vaccines. N Engl J Med. (2021) 384:1483–7. doi: 10.1056/NEJMp2100351
50. Wood S, Schulman K. Beyond politics - promoting Covid-19 vaccination in the United States. N Engl J Med. (2021) 384:e23. doi: 10.1056/NEJMms2033790
51. Freeman D, Loe BS Yu L-M, Freeman J, Chadwick A, Vaccari C, Shanyinde M, et al. Effects of different types of written vaccination information on COVID-19 vaccine hesitancy in the UK (OCEANS-III): a single-blind, parallel-group, randomised controlled trial. Lancet Public health. (2021) 6:e416–27. doi: 10.1016/S2468-2667(21)00096-7
52. Khubchandani J, Sharma S, Price JH, Wiblishauser MJ, Sharma M, Webb FJ. COVID-19 vaccination hesitancy in the united states: a rapid national assessment. J Commun Health. (2021) 46:270–7. doi: 10.1007/s10900-020-00958-x
53. Volpp KG, Loewenstein G, Buttenheim AM. Behaviorally informed strategies for a national COVID-19 vaccine promotion program. JAMA. (2021) 325:125–6. doi: 10.1001/jama.2020.24036
54. Muzumdar JM, Cline RR. Vaccine supply, demand, and policy: a primer. J Am Pharm Assoc. (2009) 49:e87–99. doi: 10.1331/JAPhA.2009.09007
55. Frank R, Dach L, Lurie N. It was the government that produced COVID-19 vaccine success. Health Affairs May. (2021) 14:2021. doi: 10.1377/forefront.20210512.191448
56. Zuma ND. Disaster Management Act, 2002: draft amendments to regulations issued in terms of section 27(2) of the disaster management act, 2002. In: Governance DoC-O, editor. (2021).
57. Haidari LA, Connor DL, Wateska AR, Brown ST, Mueller LE, Norman BA, et al. Augmenting transport versus increasing cold storage to improve vaccine supply chains. PLoS ONE. (2013) 8:e64303. doi: 10.1371/journal.pone.0064303
58. World Health Organization. Guidance on developing a national deployment and vaccination plan for COVID-19 vaccines: Interim guidance. (2021). Available online at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Vaccine-deployment-2021.1-eng
59. Banerjee A, Chandrasekhar AG, Dalpath S, Duflo E, Floretta J, Jackson MO, et al. Selecting the most effective nudge: evidence from a large-scale experiment on immunization. In: NBER Working Papers 28726. (2021). doi: 10.3386/w28726
60. Ozawa S, Zhou M, Wonodi C, Chen H-H, Bridges JFP. Parents' preferences for interventions to improve childhood immunization uptake in northern Nigeria. Vaccine. (2018) 36:2833–41. doi: 10.1016/j.vaccine.2018.03.073
61. World Health Organization. Working together: an integration resource guide for immunization services throughout the life course. Licence: CC BY-NCSA 3.0 IGO. (2018). Available online at: https://apps.who.int/iris/handle/10665/276546 (accessed March 31, 2022).
62. Schmelz K. Enforcement may crowd out voluntary support for COVID-19 policies, especially where trust in government is weak and in a liberal society. In: Proceedings of the National Academy of Sciences of the United States of America. (2021) 118. doi: 10.1073/pnas.2016385118
63. Bayer R, Dupuis L. Tuberculosis, public health, and civil liberties. Annu Rev Public Health. (1995) 16:307–26. doi: 10.1146/annurev.pu.16.050195.001515
64. Alleman MM, Meyer SA, Mulumba A, Nyembwe M, Riziki Y, Mbule A, et al. Improved acute flaccid paralysis surveillance performance in the Democratic Republic of the Congo, 2010-2012. J Infect Dis. (2014) 210:S50–61. doi: 10.1093/infdis/jit670
65. Shelton RC, Adsul P, Oh A, Moise N, Griffith DM. Application of an antiracism lens in the field of implementation science (IS): Recommendations for reframing implementation research with a focus on justice and racial equity. Implement Res Pract. (2021) 2:26334895211049482. doi: 10.1177/26334895211049482
66. Musa A, Mkanda P, Manneh F, Korir C, Warigon C, Gali E, et al. Youth group engagement in noncompliant communities during supplemental immunization activities in kaduna, Nigeria, in 2014. J Infect Dis. (2016) 213:S91–5. doi: 10.1093/infdis/jiv510
67. Privor-Dumm L, King T. Community-based strategies to engage pastors can help address vaccine hesitancy and health disparities in black communities. J Health Commun. (2020) 25:827–30. doi: 10.1080/10810730.2021.1873463
68. Lahariya C, Khandekar J, Ray TK, Meenakshi, Pradhan SK. Role of an area specific approach to increase community participation in pulse polio program in a locality of south Delhi. J Commun Dis. (2007) 39:245–8.
69. Wesevich A, Chipungu J, Mwale M, Bosomprah S, Chilengi R. Health promotion through existing community structures: a case of churches' roles in promoting rotavirus vaccination in rural zambia. J Prim Care Commun Health. (2016) 7:81–7. doi: 10.1177/2150131915622379
70. Odone A, Ferrari A, Spagnoli F, Visciarelli S, Shefer A, Pasquarella C, et al. Effectiveness of interventions that apply new media to improve vaccine uptake and vaccine coverage. Hum Vaccin Immunother. (2015) 11:72–82. doi: 10.4161/hv.34313
71. Ratzan S, Schneider EC, Hatch H, Cacchione J. Missing the point—how primary care can overcome Covid-19 vaccine “Hesitancy”. New Engl J Med. (2021) 384:e100. doi: 10.1056/NEJMp2106137
72. Khan S, Chambers D, Neta G. Revisiting time to translation: implementation of evidence-based practices (EBPs) in cancer control. Cancer Causes Control Mar. (2021) 32:221–30. doi: 10.1007/s10552-020-01376-z
Keywords: implementation science, COVID-19, vaccine, implementation outcomes, implementation strategies
Citation: Pilar M, Elwy AR, Lushniak L, Huang G, McLoughlin GM, Hooley C, Nadesan-Reddy N, Sandler B, Moshabela M, Alonge O, Geng E and Proctor E (2022) A Perspective on Implementation Outcomes and Strategies to Promote the Uptake of COVID-19 Vaccines. Front. Health Serv. 2:897227. doi: 10.3389/frhs.2022.897227
Received: 15 March 2022; Accepted: 22 April 2022;
Published: 20 May 2022.
Edited by:
Nick Sevdalis, King's College London, United KingdomReviewed by:
Denise F. Lillvis, University at Buffalo, United StatesCopyright © 2022 Pilar, Elwy, Lushniak, Huang, McLoughlin, Hooley, Nadesan-Reddy, Sandler, Moshabela, Alonge, Geng and Proctor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meagan Pilar, bWVhZ2FuLnBpbGFyQHd1c3RsLmVkdQ==
 Meagan Pilar
Meagan Pilar A. Rani Elwy2,3
A. Rani Elwy2,3 Larissa Lushniak
Larissa Lushniak Gabriella M. McLoughlin
Gabriella M. McLoughlin Nisha Nadesan-Reddy
Nisha Nadesan-Reddy Brittney Sandler
Brittney Sandler Mosa Moshabela
Mosa Moshabela Elvin Geng
Elvin Geng Enola Proctor
Enola Proctor