- 1Department of Maternal Intensive Care Unit, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital, Beijing, China
- 2Department of Gynecology, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Beijing Maternal and Child Health Care Hospital, Beijing, China
Background: Maternal hypertensive disorders (MHD) are leading causes of maternal morbidity and mortality worldwide, particularly among reproductive-age women of advanced maternal age (AMA), representing a significant global public health challenge.
Objective: This study aimed to analyze the global trends, inequalities, and disparities in the burden of MHD among reproductive-age AMA women from 1990 to 2021.
Methods: We conducted a population-based study using data from the Global Burden of Disease (GBD) 2021 study, covering 204 countries and territories. The study included women aged 35–49 years with hypertensive disorders during pregnancy. We assessed age-standardized incidence rate (ASIR) and age-standardized death rate (ASDR) of MHD among reproductive-age AMA women. Temporal trends were evaluated using joinpoint regression analysis, while health inequalities were measured using the concentration index and the slope index of inequality (SII).
Results: Between 1990 and 2021, the global ASIR of MHD decreased from 568.10 (95% UI: 412.06–738.55) to 491.49 (95% UI: 368.78–619.84) per 100,000 population (AAPC: −0.46%, 95% CI: −0.54% to −0.38%), and ASDR declined from 2.57 (95% UI: 2.23–2.97) to 1.44 (95% UI: 1.19–1.76) per 100,000 population (AAPC: −1.83%, 95% CI: −1.99% to −1.67%). Substantial disparities persisted across socio-demographic index (SDI) regions, with high and high-middle SDI regions showing increasing incidence trends (AAPC: 2.36% and 1.45%, respectively). The slope index of inequality (SII) for ASIR improved from −3,052.73 (95% CI: −3,329.55 to −2,775.91) to −1,209.36 (95% CI: −1,393.12 to −1,025.61) per 100,000 women, while the SII for ASDR decreased from −11.29 (95% CI: −12.38 to −10.20) to −3.66 (95% CI: −4.13 to −3.20) deaths per 100,000 women. The concentration index for ASIR showed slight improvement (from −0.46 to −0.34), while ASDR inequality marginally worsened (from −0.62 to −0.66).
Conclusion: Despite overall declines in MHD burden, significant disparities persist, particularly in low SDI regions. These findings highlight the need for targeted public health interventions to reduce inequalities, improve healthcare access, and enhance maternal outcomes for reproductive-age AMA women globally.
Introduction
Maternal hypertensive disorders (MHD) are leading causes of maternal and perinatal morbidity and mortality worldwide (1, 2).These disorders, including gestational hypertension, preeclampsia, and eclampsia, have significant implications for both maternal and neonatal health (3–7). An increasing trend of delayed childbearing has led to a notable rise in pregnancies among women of advanced maternal age (AMA), typically defined as age ≥35 years (8).This shift in maternal demographics has impacted the epidemiology of MHD, necessitating a deeper understanding of their burden and trends among reproductive-age AMA women. Previous clinical studies and meta-analyses have demonstrated that AMA is associated with an increased risk of hypertensive disorders during pregnancy. A comprehensive systematic review and meta-analysis by Lean et al. observed that advanced maternal age was associated with an increased risk of hypertensive disorders of pregnancy and other adverse pregnancy outcomes (9). This risk increases progressively with maternal age, with women aged ≥40 years showing even higher risk (10). Recent epidemiological data from the United States shows substantial variations in the prevalence of maternal hypertensive disorders across different age groups, with consistently higher rates among women aged ≥35 years (11). This pattern has also been observed in large-scale population studies in other regions, such as China, where AMA was identified as an independent risk factor for hypertensive disorders during pregnancy (12). This demographic shift, combined with the potentially higher risk profile of AMA pregnancies, makes understanding the global burden of hypertensive disorders in reproductive-age AMA women particularly important for public health planning and healthcare resource allocation.
These hypertensive disorders can lead to severe maternal complications such as stroke, organ failure, and maternal death, as well as adverse neonatal outcomes including preterm birth and low birth weight (10, 13–15). Despite advancements in maternal healthcare and the management of hypertensive disorders, comprehensive global and regional data on the burden and trends of MHD among reproductive-age AMA women remain limited. Existing clinical guidelines often lack specific recommendations for reproductive-age AMA women, and there is a scarcity of population-based data on the inequalities and trends of these disorders across different regions and socioeconomic groups (9, 16). As the number of pregnancies at advanced maternal age continues to rise globally, understanding the burden, trends, and disparities of MHD among this population is increasingly urgent.
Addressing these knowledge gaps is crucial for informing public health interventions and updating clinical guidelines to better manage and mitigate the risks associated with hypertensive disorders in reproductive-age AMA pregnancies. Significant disparities in maternal outcomes related to socioeconomic factors, healthcare access, and regional inequalities need to be systematically explored to develop targeted health policies aimed at reducing preventable maternal morbidity and mortality. Given the unique challenges faced by reproductive-age AMA women, tailored interventions are essential to improve maternal outcomes and reduce health inequities.
The present study aims to examine the global, regional, and national burden, temporal trends, and inequalities in MHD among reproductive-age women of advanced maternal age from 1990 to 2021. We also aim to explore the impact of social determinants, regional differences, and healthcare development on the prevalence and outcomes of MHD in this population. By providing comprehensive and up-to-date data, this study seeks to fill critical gaps in knowledge, ultimately contributing to the improvement of maternal health outcomes and the reduction of disparities for reproductive-age AMA women worldwide.
Methods
Data sources and study population
This study utilized data from the Global Burden of Disease (GBD) 2021 study, which provides comprehensive health metrics for 204 countries and territories from 1990 to 2021 (17). We focused on data specific to reproductive-age women of advanced maternal age (35–49 years) with hypertensive disorders in pregnancy. MHD were defined according to the International Classification of Diseases codes: 642–642.9 in ICD-9 and O10–O16.9 in ICD-10, encompassing chronic hypertension, gestational hypertension, preeclampsia, and eclampsia (18). We categorized each country and territory into one of five Socio-demographic Index (SDI) quintiles based on their SDI values (19). The SDI is a composite measure reflecting development status, incorporating income per capita, average educational attainment, and total fertility rate. This classification facilitated the analysis of trends in MHD incidence and mortality across varying levels of socioeconomic development.
The GBD 2021 study employs rigorous methodologies for data collection and processing, as described in detail elsewhere (20). The study synthesizes a large and growing number of data input sources including surveys, censuses, vital statistics, and other health-related data sources. These data sources and detailed methodological information are publicly accessible through the Global Health Data Exchange (GHDx) Sources Tool (http://ghdx.healthdata.org/gbd-2021/sources). This interactive tool allows users to access specific methodological details for different GBD components, causes, risks, and locations.
Disease burden indicators
We extracted age-standardized incidence and mortality rates as the primary metrics for assessing the burden of MHD. To calculate these rates for the 35–49 age group, we employed the direct standardization method using the GBD 2021 standard population as the reference (21). Age-specific incidence and mortality rates were obtained from our study population and weighted by the corresponding proportion of the GBD 2021 standard population for each age group (22). This approach ensured comparability across different populations by accounting for variations in age distribution. The final age-standardized rates were calculated by multiplying the age-specific rates by the standard population weights and summing the results across the relevant age groups.
Statistical analysis
We conducted a descriptive analysis of the age-standardized incidence and mortality rates across regions and countries. To assess temporal trends from 1990 to 2021, we performed joinpoint regression analysis to calculate the average annual percentage change (AAPC) and 95% confidence intervals (CIs) for each metric (23). Trends were considered significant if the 95% CI of the AAPC did not include zero. Trends were classified as increasing (both CI limits positive), decreasing (both CI limits negative), or stable (CI includes zero).
Health inequality analysis
To assess inequalities in the distribution of MHD across countries, we employed two complementary measures: the concentration index and the slope index of inequality (SII).
The concentration index was calculated using the Lorenz curve approach to evaluate the unequal distribution of incidence and mortality rates across countries ranked by their gross domestic product (GDP) per capita (24). The concentration index ranges from −1 to 1, with negative values indicating a concentration of the health outcome among poorer countries and positive values indicating a concentration among wealthier countries. A value of 0 represents perfect equality.
For the SII, we modeled the age-standardized mortality rates against a relative social position scale based on GDP per capita using weighted least squares regression to account for heteroskedasticity (25). The SII represents the absolute difference in the health outcome between the hypothetical countries at the bottom and top of the socioeconomic spectrum. Both inequality measures were calculated for each year from 1990 to 2021 to assess changes in health inequalities over time.
Software and reporting guidelines
All statistical analyses were performed using the Health Equity Assessment Toolkit from the World Health Organization (WHO), R version 4.4.1, and Stata version 16.0. We adhered to the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) (26).
Results
Global trends in ASIR and ASDR
The primary outcome measures were the age-standardized incidence rate (ASIR) and age-standardized death rate (ASDR). ASIR represents the number of new cases of maternal hypertensive disorders per 100,000 population, adjusted for differences in age structure across populations and time periods. ASDR represents the number of deaths from maternal hypertensive disorders per 100,000 population, similarly age-standardized to allow for valid comparisons across different populations and time periods. From 1990 to 2021, the global ASIR of MHD decreased from 568.10 (95% UI: 412.06–738.55) to 491.49 (95% UI: 368.78–619.84) per 100,000 population, with an average annual percentage change (AAPC) of −0.46% (95% CI: −0.54% to −0.38%) (Figure 1A, Table 1). During the same period, the ASDR declined from 2.57 (95% UI: 2.23–2.97) to 1.44 (95% UI: 1.19–1.76) per 100,000 population, with an AAPC of −1.83% (95% CI: −1.99% to −1.67%) (Figure 1B, Table 1).
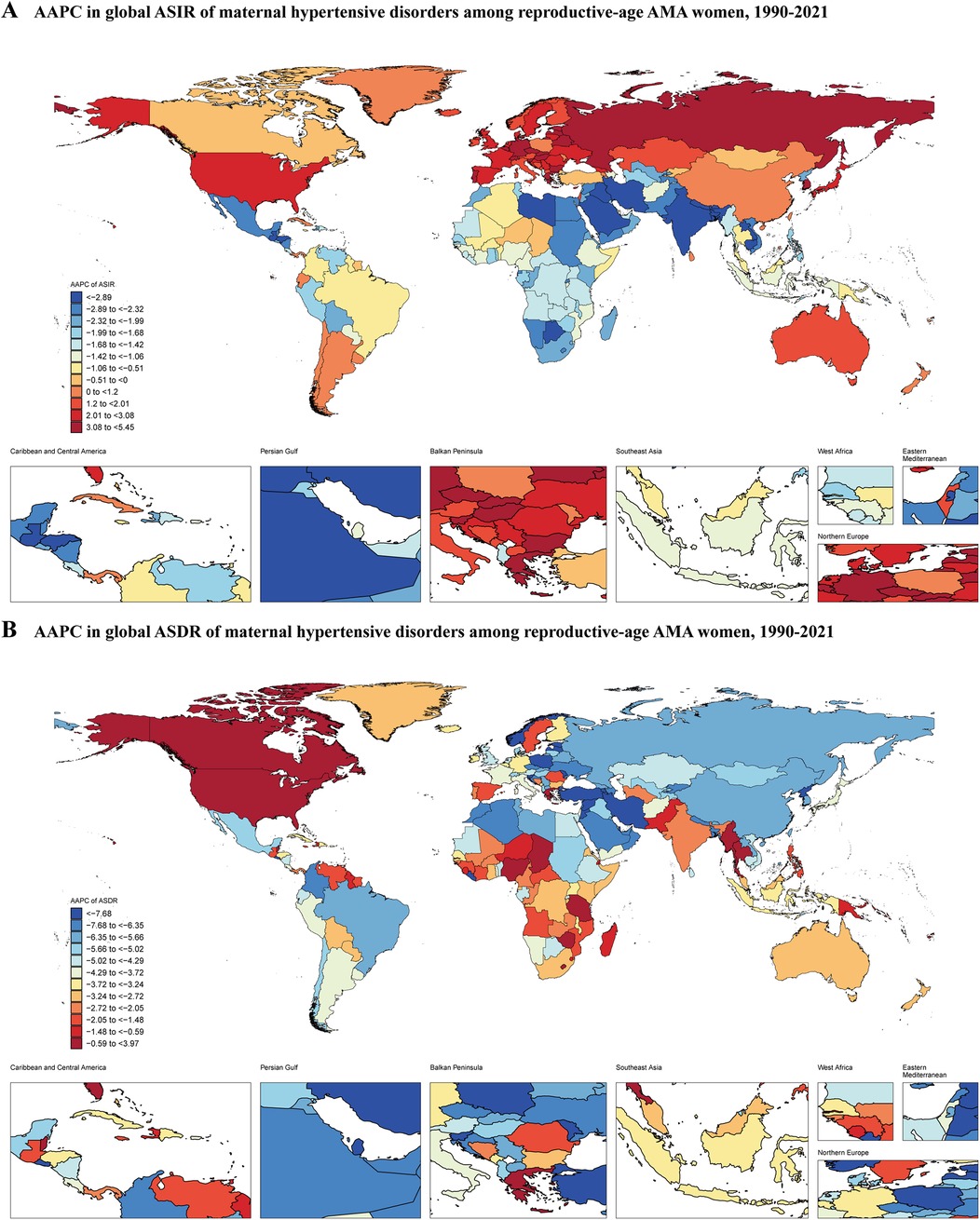
Figure 1. Average annual percentage change in global ASIR and ASDR of maternal hypertensive disorders among reproductive-age AMA women, 1990–2021. (A) ASIR; (B) ASDR; ASIR, age-standardized incidence rate; ASDR, age-standardized death rate; AMA, advanced maternal age.
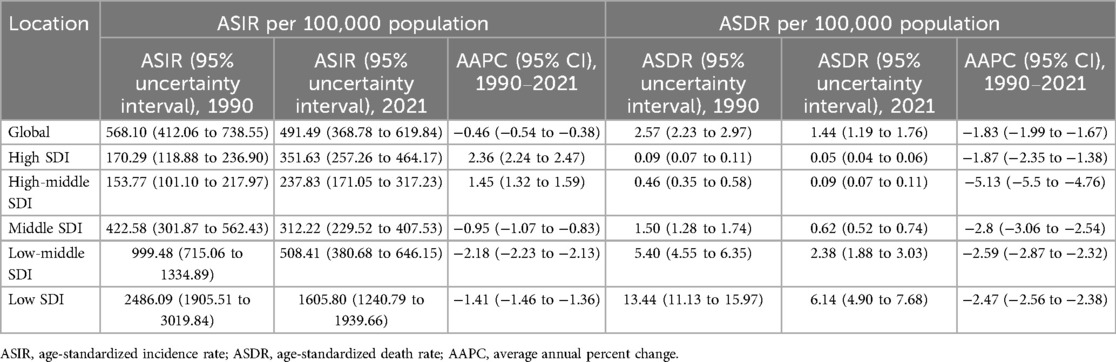
Table 1. ASIR and ASDR of maternal hypertensive disorders among women of advanced maternal age in 1990 and 2021, and average annual percent change (1990–2021) at global and socio-demographic index regional levels.
Disparities across socio-demographic index regions
Significant disparities in disease burden were observed across Socio-demographic Index (SDI) regions. For ASIR, the low SDI region consistently showed the highest burden, though decreasing from 2,486.09 (95% UI: 1,905.51–3,019.84) per 100,000 population in 1990 to 1,605.80 (95% UI: 1,240.79–1,939.66) per 100,000 population in 2021, with an annual average percent change (AAPC) of −1.41% (95% CI: −1.46 to −1.36) (Table 1). In contrast, both high SDI and high-middle SDI regions showed increasing trends, with their ASIRs rising from 170.29 to 351.63 (AAPC: 2.36%, 95% CI: 2.24 to 2.47) and from 153.77 to 237.83 (AAPC: 1.45%, 95% CI: 1.32 to 1.59), respectively (Table 1). The middle SDI and low-middle SDI regions experienced decreasing trends, with AAPCs of −0.95% (95% CI: −1.07 to −0.83) and −2.18% (95% CI: −2.23 to −2.13), respectively (Table 1).
For ASDR, all SDI regions showed declining trends, but with varying magnitudes. The low SDI region maintained the highest ASDR, despite decreasing from 13.44 (95% UI: 11.13–15.97) per 100,000 population in 1990 to 6.14 (95% UI: 4.90–7.68) per 100,000 population in 2021 (AAPC: −2.47%, 95% CI: −2.56 to −2.38) (Table 1). The high-middle SDI region showed the most substantial decline (AAPC: −5.13%, 95% CI: −5.50 to −4.76), while the high SDI region maintained the lowest ASDR throughout the period, decreasing from 0.09 to 0.05 (AAPC: −1.87%, 95% CI: −2.35 to −1.38) (Table 1).
Regional variations
At the Global Burden of Disease (GBD) regional level, Western Sub-Saharan Africa had the highest ASIR in 2021 at 2,234.25 (95% UI: 1,780.30–2,613.28) per 100,000 population, while East Asia had the lowest at 103.89 (95% UI: 69.45–150.93) (Supplementary Table S1). In 2021, the highest ASDR was observed in Central Sub-Saharan Africa at 8.86 (95% UI: 5.65–13.14) per 100,000 population, with Central Europe showing the lowest at 0.01 (95% UI: 0.01–0.02) (Supplementary Table S1). From 1990 to 2021, Eastern Europe experienced the fastest increase in ASIR with an AAPC of 3.17% (95% CI: 2.93% to 3.40%), while South Asia saw the most rapid decrease with an AAPC of −3.21% (95% CI: −3.40% to −3.03%) (Supplementary Table S1). East Asia demonstrated the most rapid decrease in ASDR with an AAPC of −6.35% (95% CI: −7.01% to −5.68%). High-income North America was the only region among the 21 GBD regions that showed an increase in ASDR, with an AAPC of 1.3% (95% CI: 0.49% to 2.12%) (Supplementary Table S1).
National-level findings
At the national level, South Sudan had the highest ASIR in 2021 (3,404.51; 95% UI: 2,744.38–3,945.33), while Canada had the lowest (48.80; 95% UI: 32.15–75.37) (Supplementary Table S2, Supplementary Figure S1B). The Central African Republic had the highest ASDR (14.41; 95% UI: 7.74–24.58), whereas Slovenia had the lowest (0.002; 95% UI: 0.001–0.003) (Supplementary Table S2, SupplementaryFigure S1D). Between 1990 and 2021, Nepal showed the most substantial annual decline in ASIR (AAPC: −5.88%; 95% CI: −6.04% to −5.72%), while Czechia demonstrated the most substantial annual increase (AAPC: 5.45%; 95% CI: 5.12% to 5.79%) (Supplementary Table S2). Jordan exhibited the most substantial decrease in ASDR (AAPC: −11.34%; 95% CI: −11.72% to −10.96%), whereas Guam demonstrated the highest increase in ASDR (AAPC: 3.97%; 95% CI: 2.65% to 5.31%) over the study period (Supplementary Table S2).
Correlation with socio-demographic development
A significant negative correlation was observed between MHD and socio-demographic development for both ASIR and ASDR across countries throughout the study period (1990–2021). For ASIR, the strength of the negative correlation between ASIR and SDI has gradually decreased over time, from ρ = −0.85 in 1990 to ρ = −0.65 in 2021 (P < 0.001) (Figures 2A,B, E), indicating that while countries with lower SDI generally exhibited higher incidence rates, this relationship has become relatively less pronounced over the past three decades. The correlation between ASDR and SDI has remained consistently strong throughout the study period (ranging from ρ = −0.89 in 1990 to ρ = −0.87 in 2021, P < 0.001) (Figures 2C,D and E), suggesting that ASDR decreased more consistently with increasing socio-demographic development.The temporal trend analysis further revealed that the correlation coefficient for ASIR steadily weakened over time, while that for ASDR remained relatively stable throughout the study period (Figure 2E), highlighting the divergent patterns in the relationship between socio-demographic development and disease incidence vs. mortality.
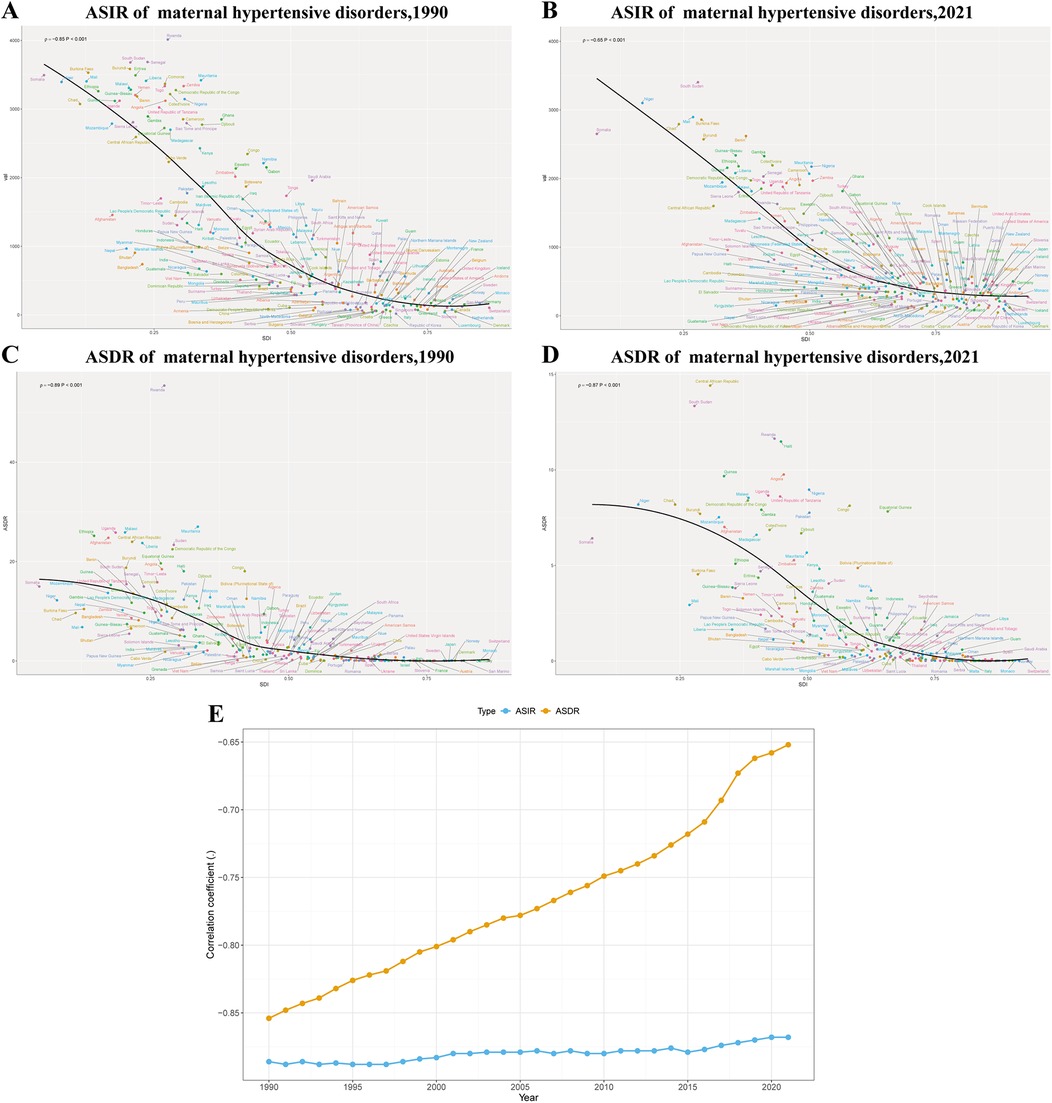
Figure 2. Correlation between SDI and ASIR and ASDR of maternal hypertensive disorders among reproductive-age AMA women at the national level in 1990 and 2021 and trends of correlation, 1990–2021. (A) The association between SDI and the ASIR, 1990; (B) The association between SDI and the ASIR, 2021; (C) The association between SDI and the ASDR,1990; (D) The association between SDI and the ASDR,2021. (E) Temporal trends in correlation coefficients between SDI and disease burden (ASIR and ASDR) from 1990 to 2021. SDI, socio-demographic Index; ASIR, age-standardized incidence rate; ASDR, age-standardized death rate; AMA, advanced maternal age.
Trends across SDI regions
From 1990 to 2021, trends in ASIR varied across different SDI regions (Figure 3A). High SDI regions showed a consistent increase (AAPC: 2.36%). The trend accelerated from 1990 to 2014, peaking with an annual percent change (APC) of 3.35% during 2011–2014, before slightly decelerating to 2.90% from 2014 to 2021. High-middle SDI regions experienced overall growth (AAPC: 1.45%) but with fluctuations. After an initial decline (APC: −5.07%) from 1990 to 1994, the trend reversed, reaching a 5.66% annual increase from 2006 to 2015, before stabilizing and slightly declining in recent years. Middle SDI regions showed a slight overall decrease (AAPC: −0.95%), characterized by periods of decline from 1990 to 2001, followed by an increase (APC: 1.41%) from 2006 to 2015, and then declining again. Low-middle SDI regions demonstrated a consistent decrease (AAPC: −2.18%), with the most rapid decline (APC: −3.10%) between 1996 and 2004. Low SDI regions also showed an overall decrease (AAPC: −1.41%), with varying rates over time and the most rapid decrease (APC: −2.26%) observed from 2011 to 2014.
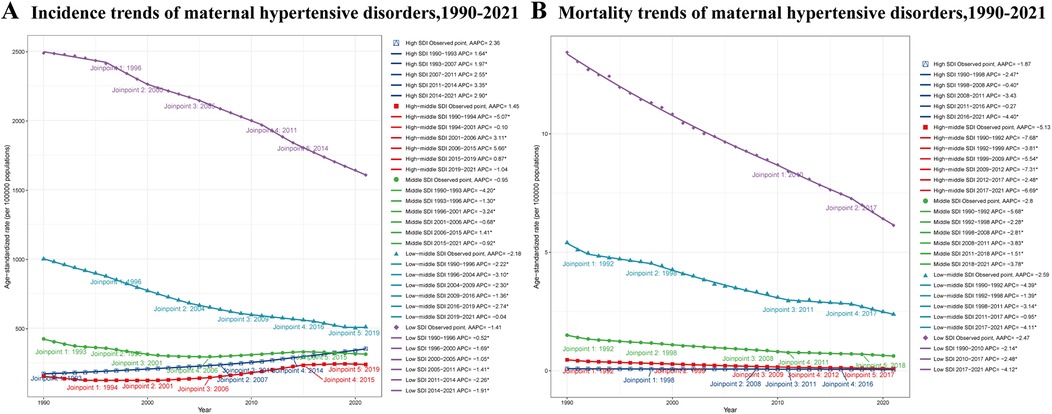
Figure 3. ASIR and ASDR trends of maternal hypertensive disorders among reproductive-age AMA women across different SDI regions, 1990–2021. (A) ASIR trends of maternal hypertensive disorders (B) ASDR trends of maternal hypertensive disorders. ASIR, age-standardized incidence rate; ASDR, age-standardized death rate; AMA, advanced maternal age; SDI, socio-demographic Index.
The ASDR exhibited a decreasing trend across all SDI regions from 1990 to 2021 (Figure 3B). High SDI regions experienced the smallest decline (AAPC: −1.87%), with periods of slower decrease (APC: −0.40% from 1998 to 2008) and more rapid decline (APC: −4.40% from 2016 to 2021). High-middle SDI regions showed the most substantial decrease (AAPC: −5.13%), with rapid declines (APC: −7.68%) from 1990 to 1992 and (APC: −6.69%) from 2017 to 2021. Middle SDI regions demonstrated a consistent decline (AAPC: −2.80%), with the most rapid decrease (APC: −5.68%) observed from 1990 to 1992. Low-middle SDI regions showed a steady decrease (AAPC: −2.59%), with rapid declines from 1990 to 1992 (APC: −4.39%) and from 2017 to 2021 (APC: −4.11%). Low SDI regions experienced a consistent decline (AAPC: −2.47%), with the rate of decrease accelerating in recent years (APC: −4.12% from 2017 to 2021).
Health inequalities among reproductive-age AMA women
Significant inequalities in the burden of MHD among reproductive-age women of advanced maternal age were observed across different SDI levels from 1990 to 2021. The ASIR showed a consistent decrease, with notable disparities between countries at different SDI levels. In 1990, the SII for ASIR was −3,052.73 (95% CI: −3,329.55 to −2,775.91) per 100,000 women, indicating substantial absolute inequality between countries with the lowest and highest SDI (Figure 4A). By 2021, the SII had reduced to −1,209.36 (95% CI: −1,393.12 to −1,025.61) per 100,000 women, representing a 60.4% reduction (Figure 4A).
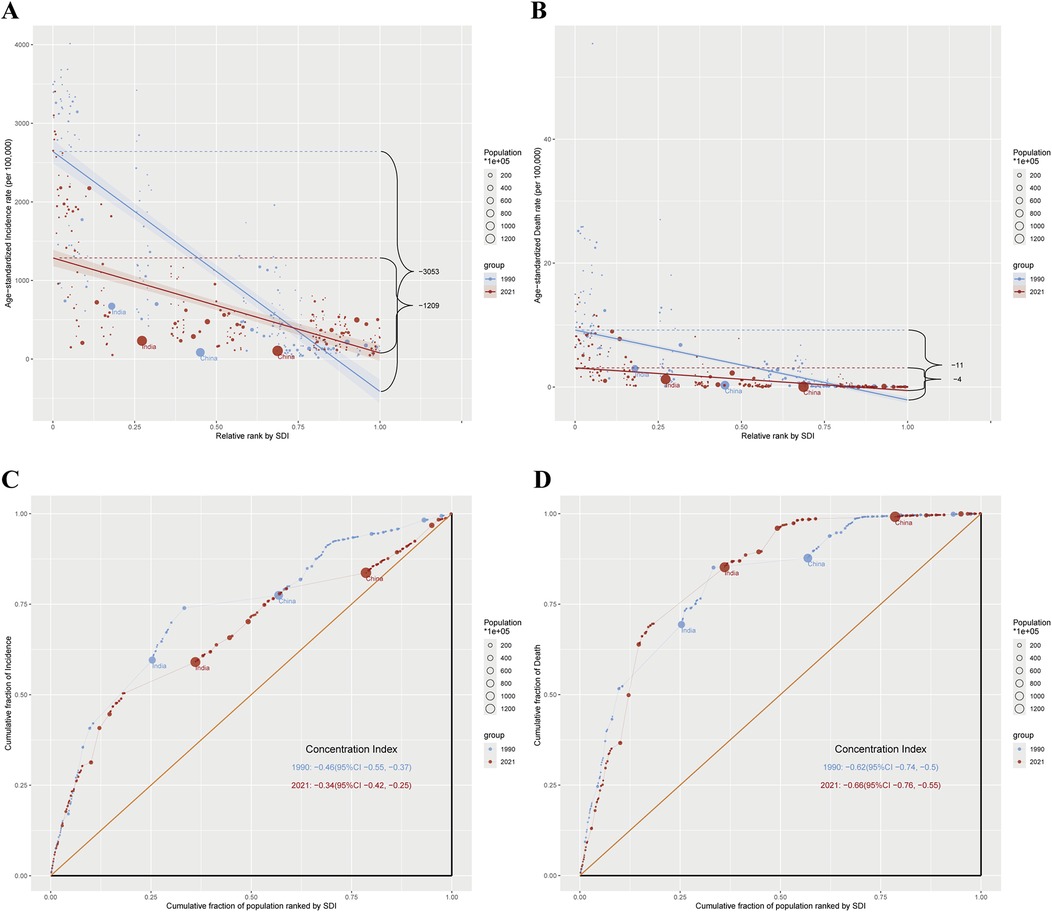
Figure 4. Absolute and relative cross-country inequality in ASIR and ASDR of maternal hypertensive disorders among reproductive-age AMA women, 1990–2021. (A) Health inequality regression curves for ASIR of maternal hypertensive disorders among reproductive-age AMA women; (B) Health inequality regression curves for ASDR of maternal hypertensive disorders among reproductive-age AMA women; (C) Concentration curves for ASIR of maternal hypertensive disorders among reproductive-age AMA women; (D) Concentration curves for ASDR of maternal hypertensive disorders among reproductive-age AMA women; ASIR, age-standardized incidence rate; ASDR, age-standardized death rate; AMA, advanced maternal age.
The SII for ASDR showed a similar trend. In 1990, the SII was −11.29 deaths per 100,000 women (95% CI: −12.38 to −10.20), indicating that countries with the lowest SDI experienced approximately 11 more deaths per 100,000 women compared to those with the highest SDI (Figure 4B). By 2021, the SII had decreased to −3.66 deaths per 100,000 women (95% CI: −4.13 to −3.20) (Figure 4B). Although inequality persisted, it decreased over time. The consistently negative SII values indicate that the burden among reproductive-age AMA women was consistently higher in countries with lower SDI levels.
The concentration index for ASIR among reproductive-age AMA women indicated a disproportionate burden among poorer populations, with slight improvement over time. The global concentration index for incidence was −0.46 (95% CI: −0.55 to −0.37) in 1990, improving to −0.34 (95% CI: −0.42 to −0.25) in 2021 (Figure 4C). For ASDR, the concentration index showed a marginal increase in inequality, from −0.62 (95% CI: −0.74 to −0.50) in 1990 to −0.66 (95% CI: −0.76 to −0.55) in 2021 (Figure 4D).
Discussion
The main objective of this study was to analyze temporal trends, disparities, and health inequalities in the burden of MHD among reproductive-age women of AMA between 1990 and 2021. Our findings revealed a global decline in both the ASIR and ASDR of MHD over the study period. Specifically, the ASIR decreased from 568.10 to 491.49 per 100,000 population, and the ASDR decreased from 2.57 to 1.44 per 100,000 population, with annual percentage changes (AAPC) of −0.46% and −1.83%, respectively. Despite this overall decline, significant disparities in incidence and mortality persisted across Socio-demographic Index (SDI) regions. Notably, low SDI regions consistently had higher ASIR and ASDR compared to other regions, while high and high-middle SDI regions demonstrated increasing trends in ASIR. Although absolute inequalities in both incidence and mortality have decreased over time, relative inequalities in mortality have persisted or even increased.
The observed overall decline in global ASIR and ASDR of MHD may be attributed to improvements in healthcare access, increased awareness of maternal health risks, and advancements in obstetric care (7, 27, 28). The significant disparities across SDI regions reflect underlying socioeconomic, healthcare, and policy differences. Low SDI regions bore the highest burden of MHD, likely due to limited access to quality maternal healthcare, inadequate healthcare infrastructure, and socioeconomic challenges. This high burden underscores the need for strengthened maternal healthcare services and policies targeting maternal health risks for reproductive-age AMA women in these regions (29). However, contrasting trends were observed across different SDI regions. The increasing ASIR trends in high and high-middle SDI regions also warrant attention. Factors such as rising maternal age, increased prevalence of obesity, and associated comorbidities like diabetes and cardiovascular disease are likely contributing to this trend (14, 30, 31). Studies from developed countries have reported an increased risk of hypertensive disorders in pregnancies among older mothers, highlighting the need for preventive strategies focused on these risk factors (32–36). These risk factors, particularly obesity and its associated comorbidities, are not limited to developed nations but are increasing globally, affecting both high and low SDI regions (37). However, the impact of these conditions on MHD trends appears to be offset by different factors across regions. Particularly in lower SDI regions, despite the rising prevalence of obesity and resource constraints, the aforementioned improvements in healthcare infrastructure, enhanced access to prenatal care services, better awareness and management of traditional risk factors, coupled with targeted maternal health programs and early identification of high-risk pregnancies, have likely contributed to their declining ASIR trends (38).
Our findings indicate significant health inequalities in the burden of MHD among reproductive-age AMA women, with consistent disparities observed across countries of different SDI levels. Although absolute inequalities in both incidence and mortality have decreased over time, countries with lower SDI levels continue to bear a disproportionately higher burden. The concentration index for ASIR improved from 1990 to 2021, indicating a reduction in relative inequalities in incidence. However, for ASDR, the concentration index slightly worsened during the same period, suggesting that relative inequalities in mortality have persisted or increased. This contrasting trend between ASIR and ASDR highlights the need for continued efforts to address health disparities, particularly in mortality outcomes. To effectively address these inequalities, efforts should focus on improving healthcare access in low SDI regions, ensuring equitable distribution of resources, and addressing underlying socioeconomic determinants that contribute to health disparities.
The strengths of this study include the use of comprehensive global data from the GBD 2021 study and the analysis of long-term trends in MHD among reproductive-age AMA women. However, limitations exist, such as potential variations in data quality, underreporting in some regions, and reliance on modeled estimates, which may affect the accuracy of the findings. These limitations should be considered when interpreting the results, and future studies should aim to improve data quality and coverage.
The findings of this study have important implications for global maternal health policies. Targeted interventions are needed to address the rising burden of MHD in high and high-middle SDI regions and to reduce health inequalities in low SDI regions. Policymakers should focus on improving healthcare access, promoting preventive measures, and addressing socioeconomic determinants of health to enhance maternal outcomes among reproductive-age AMA women.
Conclusion
This study highlights the global burden, inequalities, and trends of MHD among reproductive-age AMA women from 1990 to 2021. While progress has been made in reducing the overall burden of MHD, significant disparities persist, particularly in low SDI regions. Continued efforts are essential to address these inequalities and improve maternal health outcomes for reproductive-age AMA women worldwide.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
This study used publicly available, de-identified data from the GBD 2021 study. As such, it was exempt from ethics review as per the guidelines of the University of Washington Institutional Review Board. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
XZ: Data curation, Software, Visualization, Writing – original draft, Writing – review & editing. WK: Conceptualization, Data curation, Supervision, Writing – review & editing. YJ: Software, Visualization, Writing – review & editing. FS: Software, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors express their gratitude to the Institute for Health Metrics and Evaluation (IHME) for providing access to the Global Burden of Disease 2021 study data. We also thank all individuals and organizations involved in data collection and curation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgwh.2025.1513909/full#supplementary-material
References
1. American College of Obstetricians and Gynecologists. Gestational hypertension and preeclampsia. ACOG practice bulletin No. 222. Obstet Gynecol. (2020) 135:135–260. doi: 10.1097/AOG.0000000000003891
2. Syoum FH, Abreha GF, Teklemichael DM, Chekole MK. Fetomaternal outcomes and associated factors among mothers with hypertensive disorders of pregnancy in suhul hospital, Northwest Tigray, Ethiopia. J Pregnancy. (2022) 2022:6917009. 36406161
3. Lugobe HM, Muhindo R, Kayondo M, Wilkinson I, Agaba DC, McEniery C, et al. Risks of adverse perinatal and maternal outcomes among women with hypertensive disorders of pregnancy in southwestern Uganda. PLoS One. (2020) 15(10):e0241207. doi: 10.1371/journal.pone.0241207
4. Wu P, Green M, Myers JE. Hypertensive disorders of pregnancy. Br Med J. (2023) 381:e071653. doi: 10.1136/bmj-2022-071653v
5. Ngwenya S. Severe preeclampsia and eclampsia: incidence, complications, and perinatal outcomes at a low-resource setting, mpilo central hospital, Bulawayo, Zimbabwe. Int J Womens Health. (2017) 9:353–7. doi: 10.2147/IJWH.S131934
6. Xavier IM, Simões ACZ, Oliveira R, Barros YE, Sarmento ACA, Medeiros KS, et al. Maternal-fetal outcomes of women with hypertensive disorders of pregnancy. Rev Assoc Med Bras (1992). (2023) 69(6):e20230060. 37283361
7. Huang C, Li J, Qin G, Liew Z, Hu J, László KD, et al. Maternal hypertensive disorder of pregnancy and offspring early-onset cardiovascular disease in childhood, adolescence, and young adulthood: a national population-based cohort study. PLoS Med. (2021) 18(9):e1003805. doi: 10.1371/journal.pmed.1003805
8. Office for National Statistics (ONS). Statistical bulletin, Birth characteristics in England and Wales. (2022). Available online at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2022 (accessed January 15, 2025).
9. Lean SC, Derricott H, Jones RL, Heazell AEP. Advanced maternal age and adverse pregnancy outcomes: a systematic review and meta-analysis. PLoS One. (2017) 12(10):e0186287. doi: 10.1371/journal.pone.0186287
10. Frick AP. Advanced maternal age and adverse pregnancy outcomes. Best Pract Res Clin Obstet Gynaecol. (2021) 70:92–100. 32741623
11. Vidit M, Shreyash P, Aangi JS, Marina B, Rosario C, Rhea S. Interstate and age group stratified variability in the incidence, prevalence and mortality of maternal hypertensive disorders in the United States: a 1990–2021 analysis using the global burden of disease database. Circulation. (2024) 150(Suppl_1):4145765. doi: 10.1161/circ.150.suppl_1.4145765
12. Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China: prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS One. (2014) 9(6):e100180. doi: 10.1371/journal.pone.0100180
13. Correa-De-Araujo R, Yoon SSS. Clinical outcomes in high-risk pregnancies due to advanced maternal age. J Womens Health. (2021) 30(2):160–7. doi: 10.1089/jwh.2020.8860
14. Bapayeva G, Terzic S, Dotlic J, Togyzbayeva K, Bugibaeva U, Mustafinova M, et al. The influence of advanced age and obesity on pregnancy course and outcome in patients with diabetes mellitus. Prz Menopauzalny. (2022) 21(3):170–9. doi: 10.5114/pm.2022.116351
15. Wang Y, Arvizu M, Rich-Edwards JW, Wang L, Rosner B, Stuart JJ, et al. Hypertensive disorders of pregnancy and subsequent risk of premature mortality. J Am Coll Cardiol. (2021) 77(10):1302–12. doi: 10.1016/j.jacc.2021.01.018
16. Kenny LC, Lavender T, Mcnamee R, O'Neill SM, Mills T, Khashan AS. Advanced maternal age and adverse pregnancy outcome: evidence from a large contemporary cohort. PLoS One. (2013) 8(2):e56583. doi: 10.1371/journal.pone.0056583
17. Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021 (GBD 2021). Seattle, United States: Institute for Health Metrics and Evaluation (IHME) (2024).
18. IHME | GHDx. Global Burden of Disease Study 2021 (GBD 2021) Causes of Death and Nonfatal Causes Mapped to ICD Codes. Available from: Available online at: https://ghdx.healthdata.org/record/ihme-data/gbd-2021-cause-icd-code-mappings (accessed October 1, 2024).
19. IHME | GHDx. Global Burden of Disease Study 2021 (GBD 2021) Socio-Demographic Index (SDI) 1950–2021. Available online at: https://ghdx.healthdata.org/record/global-burden-disease-study-2021-gbd-2021-socio-demographic-index-sdi-1950%E2%80%932021 (accessed October 1, 2024).
20. GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403(10440):2133–61. doi: 10.1016/S0140-6736(24)00757-8
21. Bray F, Parkin DM. Evaluation of data quality in the cancer registry: principles and methods. Part I: comparability, validity and timeliness. Eur J Cancer. (2009) 45(5):747–55. doi: 10.1016/j.ejca.2008.11.032
22. GBD 2021 Demographics Collaborators. Global age-sex-specific mortality, life expectancy, and population estimates in 204 countries and territories and 811 subnational locations, 1950–2021, and the impact of the COVID-19 pandemic: a comprehensive demographic analysis for the global burden of disease study 2021. Lancet. (2024) 403(10440):1989–2056. doi: 10.1016/S0140-6736(24)00476-8
23. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. (2000) 19(3):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3%3C335::aid-sim336%3E3.0.co;2-z
24. O'Donnell O, Van Doorslaer E, Wagstaff A, Lindelow M. Analyzing Health Equity Using Household Survey Data: A Guide to Techniques and Their Implementation. Washington, DC: The World Bank (2007).
25. Ordunez P, Martinez R, Soliz P, Giraldo G, Mujica OJ, Nordet P. Rheumatic heart disease burden, trends, and inequalities in the Americas, 1990–2017: a population-based study. Lancet Glob Health. (2019) 7(10):e1388–97. 31537369
26. Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet. (2016) 388(10062):e19–23. doi: 10.1016/S0140-6736(16)30388-9
27. Peng R, Tong Y, Yang M, Wang J, Yang L, Zhu J, et al. Global burden and inequality of maternal and neonatal disorders: based on data from the 2019 global burden of disease study. QJM. (2024) 117(1):24–37. doi: 10.1093/qjmed/hcad220
28. Ananth CV, Brandt JS, Hill J, Graham HL, Grover S, Schuster M, et al. Historical and recent changes in maternal mortality due to hypertensive disorders in the United States, 1979 to 2018. Hypertension. (2021) 78(5):1414–22. doi: 10.1161/HYPERTENSIONAHA.121.17661
29. Nyongesa P, Ekhaguere OA, Marete I, Tenge C, Kemoi M, Bann CM, et al. Maternal age extremes and adverse pregnancy outcomes in low-resourced settings. Front Glob Women’s Health. (2023) 4:1201037.
30. Li J, Yan J, Ma L, Huang Y, Zhu M, Jiang W. Effect of gestational diabetes mellitus on pregnancy outcomes among younger and older women and its additive interaction with advanced maternal age. Front Endocrinol. (2023) 14:1158969. doi: 10.3389/fendo.2023.1158969
31. Lumsden RH, Pagidipati N. Management of cardiovascular risk factors during pregnancy. Heart. (2022) 108(18):1438–44. doi: 10.1136/heartjnl-2021-319606
32. Aljahdali EA, Alsinani NS. Pregnancy outcomes at advanced maternal age in a tertiary hospital, Jeddah, Saudi Arabia. Saudi Med J. (2022) 43(5):491–9. doi: 10.15537/smj.2022.43.5.20220023
33. Attali E, Yogev Y. The impact of advanced maternal age on pregnancy outcome. Best Pract Res Clin Obstet Gynaecol. (2021) 70:2–9. doi: 10.1016/j.bpobgyn.2020.06.006
34. Ford ND, Cox S, Ko JY, Ouyang L, Romero L, Colarusso T, et al. Hypertensive disorders in pregnancy and mortality at delivery hospitalization—United States, 2017–2019. MMWR Morb Mortal Wkly Rep. (2022) 71(17):585–91. doi: 10.15585/mmwr.mm7117a1
35. Cameron NA, Petito L, Shah N, Perak AM, Catov JM, Bello NA, et al. Abstract 042: trends in hypertensive disorders of pregnancy among nulliparous individuals with singleton live Births in the United States: an age-period-cohort analysis between 1995 and 2019. Circulation. (2022) 145(1):A42. doi: 10.1161/circ.145.suppl_1.042
36. Lopian M, Kashani-Ligumsky L, Many A. A balancing act: navigating hypertensive disorders of pregnancy at very advanced maternal age, from preconception to postpartum. J Clin Med. (2023) 12(14):4701. doi: 10.3390/jcm12144701
37. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet. (2024) 403(10431):1027–50. doi: 10.1016/S0140-6736(23)02750-2
Keywords: maternal hypertensive disorders, global burden, advanced maternal age, health inequality, joinpoint regression
Citation: Zhao X, Kong W, Jiang Y and Sui F (2025) Global burden, trends and inequalities of maternal hypertensive disorders among reproductive-age women of advanced maternal age, 1990–2021: a population-based study. Front. Glob. Women's Health 6:1513909. doi: 10.3389/fgwh.2025.1513909
Received: 19 October 2024; Accepted: 19 February 2025;
Published: 6 March 2025.
Edited by:
Judite Blanc, University of Miami, United StatesReviewed by:
Jarrod Zamparini, University of the Witwatersrand, South AfricaAlicia Chung, New York University, United States
Copyright: © 2025 Zhao, Kong, Jiang and Sui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weimin Kong, a3dtMTk2N0BjY211LmVkdS5jbg==
 Xuanyu Zhao
Xuanyu Zhao Weimin Kong
Weimin Kong Yan Jiang1
Yan Jiang1