- 1Key Laboratory of Environmental Medicine and Engineering of Ministry of Education, and Department of Nutrition and Food Hygiene, School of Public Health, Southeast University, Nanjing, China
- 2Clinical Medical Research Center for Plateau Gastroenterological Disease of Xizang Autonomous Region, and School of Medicine, Xizang Minzu University, Xianyang, China
Objectives: Anemia in pregnancy has been a topic of interest for researchers due to its potential impact on various adverse pregnancy outcomes. This study aims to explore the relationship between anemia and adverse pregnancy outcomes such as preterm birth, low birth weight, and maternal mortality.
Methods: We conducted both a systematic review and a meta-analysis on the associations between anemia during pregnancy and adverse pregnancy outcomes. We searched Chinese databases (CNKI, Wanfang, CBM, VIP) and English ones (Cochrane Library, PubMed, Embase, Web of Science). Two researcher-authors independently assessed study quality with the Newcastle-Ottawa Scale. After extracting data, we analyzed heterogeneity and used a random-effects model for higher heterogeneity and a fixed-effects model for low heterogeneity in the meta-analysis while also systematically synthesizing and narratively describing findings in the systematic review.
Results: A total of 31 cohort studies were included. Meta-analysis showed that the risk of postpartum hemorrhage [RR [95% CI], 2.76 [1.63, 4.66]], premature rupture of membranes (PROM) [1.94 (1.26, 3.00)], preterm delivery [1.51 (1.33, 1.72)], low birth weight (LBW) [1.40 (1.19, 1.63)], cesarean section[1.33 (1.02, 1.74)], gestational hypertension[1.28 (1.14, 1.44)] and neonatal asphyxia[1.21 (1.07, 1.37)] was higher in the group of anemia in pregnancy than in the control group.
Conclusion: Maternal anemia is associated with an increased risk of seven adverse pregnancy outcomes: postpartum hemorrhage, PROM, preterm delivery, LBW, cesarean section, gestational hypertension and neonatal asphyxia. Appropriate nutritional supplementation and screening for anemia before and during pregnancy are recommended to improve maternal health and manage adverse pregnancy outcomes.
1 Introduction
Anemia during pregnancy is a significant global health concern that affects a substantial number of women worldwide, with a prevalence rate of 30% among women of childbearing age (1). It is defined as a condition where hemoglobin levels are below average, resulting in a decreased oxygen-carrying capacity of red blood cells in tissues (2). During pregnancy, as the fetus grows, pregnant women experience metabolic changes, hormonal level fluctuations and an increase in blood volume, and the physiological demands for iron, folate, and other nutrients increase significantly to support the growth and development of the fetus, as well as to meet the expanded blood volume requirements of the mother (3–6). This heightened need often places pregnant women at a higher risk of developing anemia if these nutrients are inadequately supplied through diet or if there are underlying health conditions that interfere with nutrient absorption or utilization (7, 8). In developing countries, where access to proper nutrition and prenatal care may be limited, the rates tend to be even higher (9). This not only impacts the health and well-being of the mother but also has far-reaching implications for the pregnancy itself and the health of the newborn. Severe anemia during pregnancy can have both short-term and long-term effects on the mother, fetus and newborn, such as leading to the occurrence of adverse pregnancy outcomes like gestational hypertension, miscarriage, preterm birth and low birth weight infants (10, 11).
Despite the well-documented associations between anemia during pregnancy and these adverse outcomes, there remains some variability in the reported findings across different studies. This could be attributed to differences in the diagnostic criteria used for anemia, variations in study populations, and disparities in the methods employed to measure and assess adverse pregnancy outcomes. Furthermore, the complex interplay of multiple factors such as socioeconomic status, access to healthcare, and coexisting medical conditions makes it challenging to fully understand the precise mechanisms through which anemia leads to these adverse events. Given these uncertainties and the significant impact on both maternal and neonatal health, this study conducts a systematic review and meta-analysis of prospective and retrospective cohort studies published domestically and internationally to explore the relationship and impact degree between anemia during pregnancy and various adverse pregnancy outcomes.
2 Methods
2.1 Literature search
Computer searches were conducted in Chinese databases and English databases: CNKI (China National Knowledge Infrastructure), Wanfang, VIP (Chongqing VIP Information Co., Ltd.), CBM (China Biology Medicine disc), Cochrane Library, PubMed, Embase and Web of Science. A search strategy combining both MeSH terms and free-text terms was used. The complete search strings used in English databases is (“Anemia” OR “Anaemia” OR “Iron Deficiency Anemia”) AND (“Pregnancy” OR “Pregnant Women”) AND (“PROM” OR “SGA” OR “Pre-eclampsia” OR “Fetal Distress” OR “Preterm Birth” OR “Premature Delivery” OR “Neonatal Asphyxia” OR “Low Birth Weight” OR “Postpartum Hemorrhage” OR “Cesarean Section” OR “Gestational Hypertension” OR “Adverse Pregnancy Outcomes”). The search strategy used in different databases is different, but the basic keywords and search logic are the same.
2.2 Inclusion criteria
(1) Cohort study literature on anemia and pregnancy outcomes published from the establishment of the database until December 2023; (2) The researchers excluded multiple pregnancies from the sample at the time of design; (3) Studies must include outcomes for pregnant mothers in both the anemia group and the control group, as well as birth outcomes for newborns; (4) Outcome variables include gestational hypertension, gestational diabetes mellitus (GDM), puerperal infection, cesarean section, premature rupture of membranes (PROM), preterm birth, postpartum hemorrhage, congenital malformations, fetal distress, neonatal asphyxia, low birth weight (LBW).
2.3 Exclusion criteria
(1) Research on specific types of anemia, such as thalassemia or aplastic anemia; (2) Studies without a control group or with weak comparability (studies not adhering to the diagnostic criteria or using other standards for diagnosis); (3) Duplicate reports or studies of poor quality.
2.4 Diagnostic criteria
According to the World Health Organization (WHO) “Hemoglobin Concentration for the Diagnosis and Severity Assessment of anemia” released in 2011, pregnant women with hemoglobin concentrations less than 11 g/dl can be diagnosed as anemia (12). The Indian Council of Medical Research (ICMR) established the following criteria in 1989: Very severe anemia: hemoglobin level < 4 g/dl; Severe anemia: 4 g/dl ≤ hemoglobin level < 6.9 g/dl; Moderate anemia: 7 g/dl ≤ hemoglobin level < 9.9 g/dl; Mild anemia: 10 g/dl ≤ hemoglobin level < 10.9 g/dl (13). The diagnostic criteria for anemia are based on either WHO standards or ICMR standards, depending on the content of the studies.
2.5 Literature screening and data extraction
To avoid bias in literature screening, two researchers involved in the study independently conducted a preliminary screening of the literature by reading abstracts based on the search strategy and inclusion/exclusion criteria. Anemia in pregnancy is not a rare disease, and when the sample size is small, it does not achieve the ideal statistical test efficacy (14). For these reasons, we excluded studies with small sample sizes (n < 85), incomplete information or data, unclear statistical methods, or duplicate reports due to poor quality. After completing the screening independently, they compared their selections. For any literature with disagreement on inclusion, consensus was reached through discussion.
2.6 Quality assessment
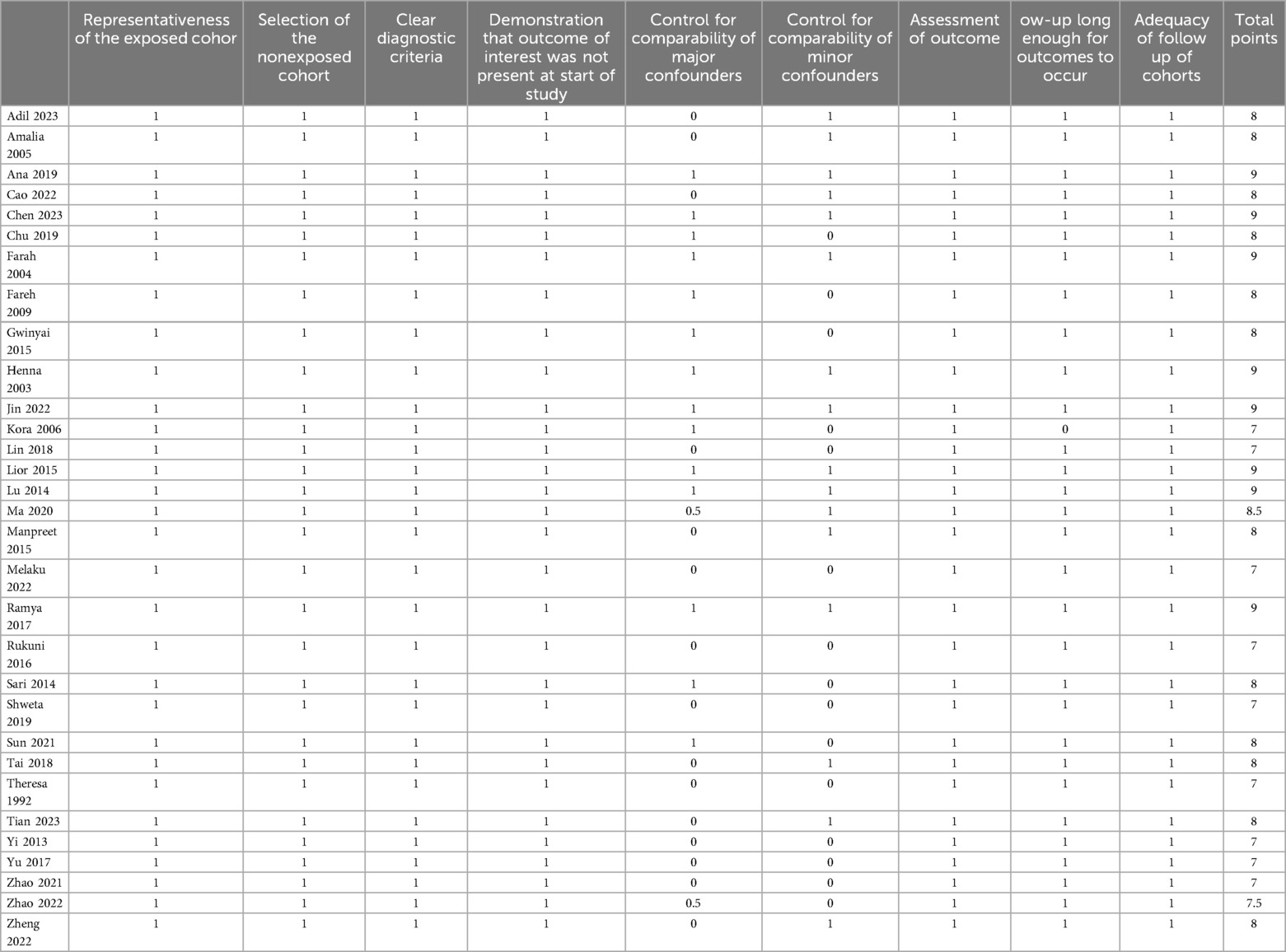
Two evaluators with relevant expertise independently assessed the quality of the included studies using the Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomized studies (15). For any literature where there was disagreement in the assessment, consensus was reached through discussion. The NOS scale has a total score of 9 points, with studies scoring seven or above considered higher quality and eligible for inclusion in the systematic review and meta-analysis.
2.7 Statistical analysis
Meta-analysis was conducted using RevMan 5.0. The first step involved testing for heterogeneity among the included studies, which refers to the consistency or trend of the results across the studies, indicated by the I2 value. The I2 values are 25%, 50%, and 75%, respectively, representing low, medium, and high degrees of heterogeneity. The relative risk (RR) was used to compare the combined effect. If I2 < 50%, a fixed-effect model was employed for analysis; otherwise, a random-effect model was used. Meta-regression was utilized for sensitivity analysis and subgroup analysis to identify potential sources of heterogeneity. Forest plots were used to display the RR values and 95% CI of factors from the included studies, and funnel plots were employed to understand potential publication bias.
3 Results
3.1 Literature search and study selection
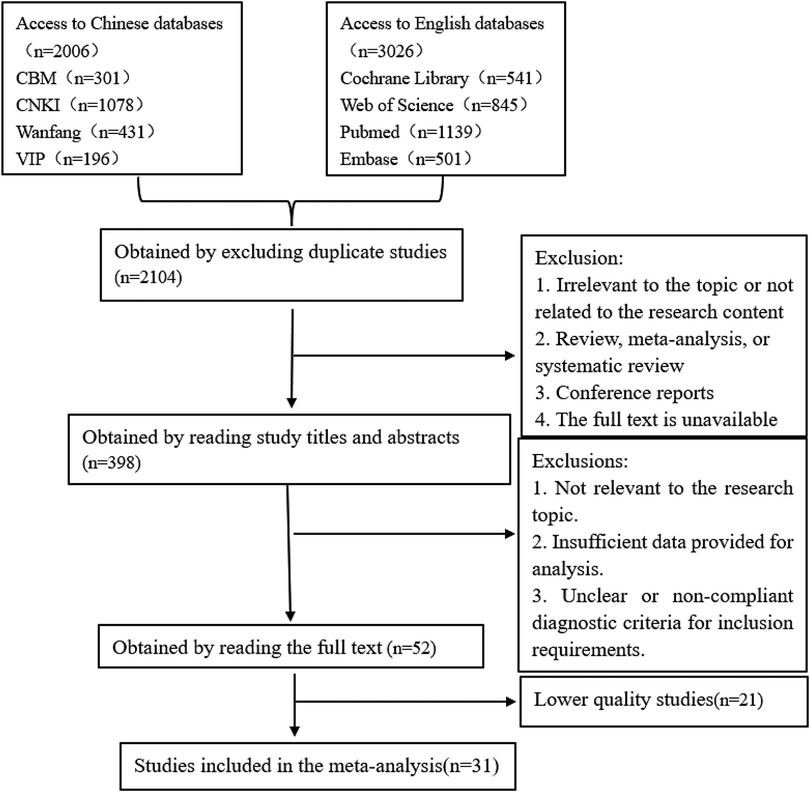
The systematic review identified 3,032 articles. Following the screening process outlined in Figure 1, 31 studies were ultimately included, of which 9 were prospective cohort studies, and 22 were retrospective cohort studies.
3.2 Study quality and characteristics
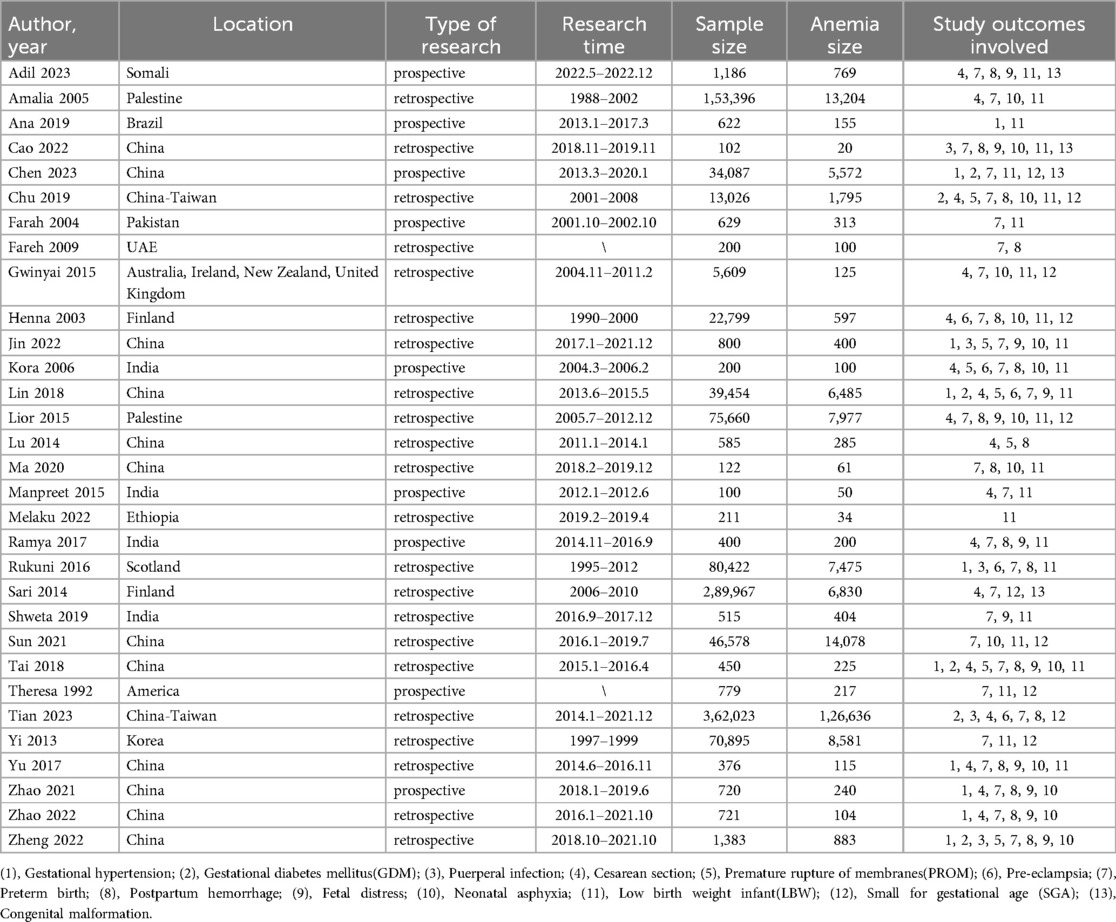
Quality assessment of the included studies based on the NOS (Newcastle-Ottawa Scale) showed scores ≥7 for all 31 selected articles (Table 1) (16–46). The primary studies was conducted in Asia, the Americas, Europe, Oceania, and Africa. Due to the search results of the Chinese datebases, 14 papers from China and 4 from Africa have the highest participation. Other sources include the United States, Ireland, the United Kingdom, Korea, Pakistan, Somalia, the United Arab Emirates, and Palestine. The publication years of the included studies ranged from 1992 to 2023. A total of 1,204,017 pregnant women were included, with 204,030 cases in the anemia group. The basic characteristics of each study are presented in Table 2.
3.3 Meta-analysis results
3.3.1 Meta-analysis of the relationship between anemia and adverse pregnancy outcomes
The pooled meta-analysis results for all possible adverse pregnancy outcomes are presented in Table 3. A total of 10 studies (18, 20, 26, 28, 35, 39, 43–46) investigated the relationship between anemia during pregnancy and pregnancy hypertension. The analysis showed that anemia during pregnancy may increase the risk of hypertension-induced pregnancy. Significant heterogeneity was found among the studies (I2 = 86%, P < 0.0001). The meta-analysis revealed that the rate of pregnancy-induced hypertension was higher in the anemia group compared to the control group, with a pooled RR of 1.33 (95% CI 1.02, 1.74). The forest and funnel plots are shown in Supplementary Figures S1 and S2.
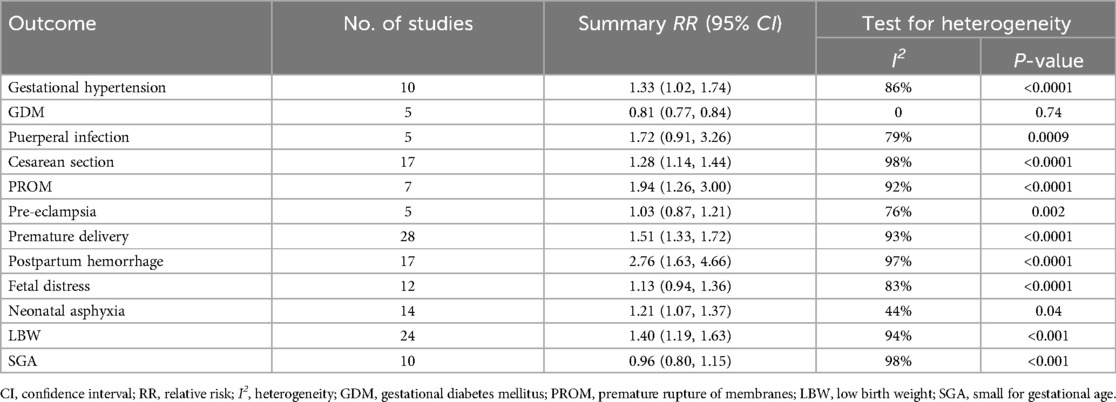
Table 3. Meta-analysis of the association between anemia during pregnancy and the risk of adverse maternal outcomes.
Seventeen studies (16, 17, 21, 24, 25, 27–30, 32, 34, 36, 39, 42–45) examined the association between anemia during pregnancy and mode of delivery. Anemia during pregnancy may increase the probability of cesarean section. Significant heterogeneity was observed among the studies (I2 = 98%, P < 0.0001), indicating that pregnant women with anemia had a higher likelihood of undergoing cesarean section compared to the control group, with a pooled RR of 1.28 (95% CI 1.14, 1.44). The forest and funnel plots are shown in Supplementary Figures S3 and S4.
Seven studies (21, 26–28, 30, 39, 46) investigated the relationship between anemia during pregnancy and PROM. Anemia during pregnancy will increase the risk of PROM. Significant heterogeneity was present among the studies (I2 = 92%, P < 0.0001), and pregnant women with anemia had a significantly higher probability of experiencing PROM compared to the control group, with a pooled RR of 1.94 (95% CI 1.26, 3.00). The forest and funnel plots are shown in Supplementary Figures S5 and S6.
Twenty-eight studies (16, 17, 19–29, 31, 32, 34–46) explored the association between pregnancy with anemia and preterm birth. Anemia during pregnancy will increase the risk of preterm birth. Significant heterogeneity was detected among the studies (I2 = 95%, P < 0.0001), indicating that pregnant women with anemia had a significantly higher risk of preterm birth compared to the control group, with a pooled RR of 1.51 (95% CI 1.33, 1.72). The forest and funnel plots are shown in Supplementary Figures S7 and S8.
Seventeen studies (16, 19, 21, 23, 25, 27, 29–31, 34, 35, 39, 43–46) investigated the relationship between pregnancy with anemia and postpartum hemorrhage. Anemia during pregnancy will increase the risk of postpartum hemorrhage.Significant heterogeneity was found among the studies (I2 = 97%, P < 0.0001), and pregnant women with anemia had a significantly higher probability of experiencing postpartum hemorrhage compared to the control group, with a pooled RR of 2.76 (95% CI 1.63, 4.66). The forest and funnel plots are shown in Supplementary Figures S9 and S10.
Fifteen studies (17, 19, 21, 22, 24–27, 29, 31, 38, 43–46) examined the association between pregnancy with anemia and neonatal asphyxia. Anemia during pregnancy may increase the risk of neonatal asphyxia. Sensitivity analysis revealed that there was high heterogeneity before removing the study by Lior et al. (29) (I2 = 74%). Therefore, it was excluded from the analysis. There was lower heterogeneity among the studies (I2 = 44%, P = 0.04), and the incidence of neonatal asphyxia was higher in the anemia group compared to the control group, with a pooled RR of 1.21 (95% CI 1.07, 1.37). The forest and funnel plots are shown in Supplementary Figures S11 and S12.
Twenty-four studies (16–22, 24–29, 31–35, 37–40, 42, 43) investigated the relationship between pregnancy with anemia and LBW. Anemia during pregnancy may increase the risk of LBW. Significant heterogeneity was observed among the studies (I2 = 94%, P < 0.0001), and the incidence of LBW was significantly higher in the anemia group compared to the control group, with a pooled RR of 1.40 (95% CI 1.19, 1.63). The forest and funnel plots are shown in Supplementary Figures S13 and S14.
Through the method of sequentially excluding individual studies for sensitivity analysis, the results indicated that the study by Tian et al. (31) was the source of heterogeneity for GDM (I2 = 97%, P < 0.001). After excluding the study by Tian et al, the heterogeneity became 0, but the result showed a meaningless negative correlation with RR (95% CI) of 0.81 (0.77, 0.84).
The meta-analysis indicated that there was no statistically significant difference in the risk of puerperal infection, pre-eclampsia, fetal distress and SGA between the anemia group and the non-anemia group. The pooled RRs (95% CIs) were 1.72 (0.91, 3.26), 1.03 (0.87, 1.21), 2.76 (1.63, 4.66) and 0.96 (0.80, 1.15) respectively. The forest and funnel plots are shown in Supplementary Figures S15–S22.
3.3.2 Subgroup analysis
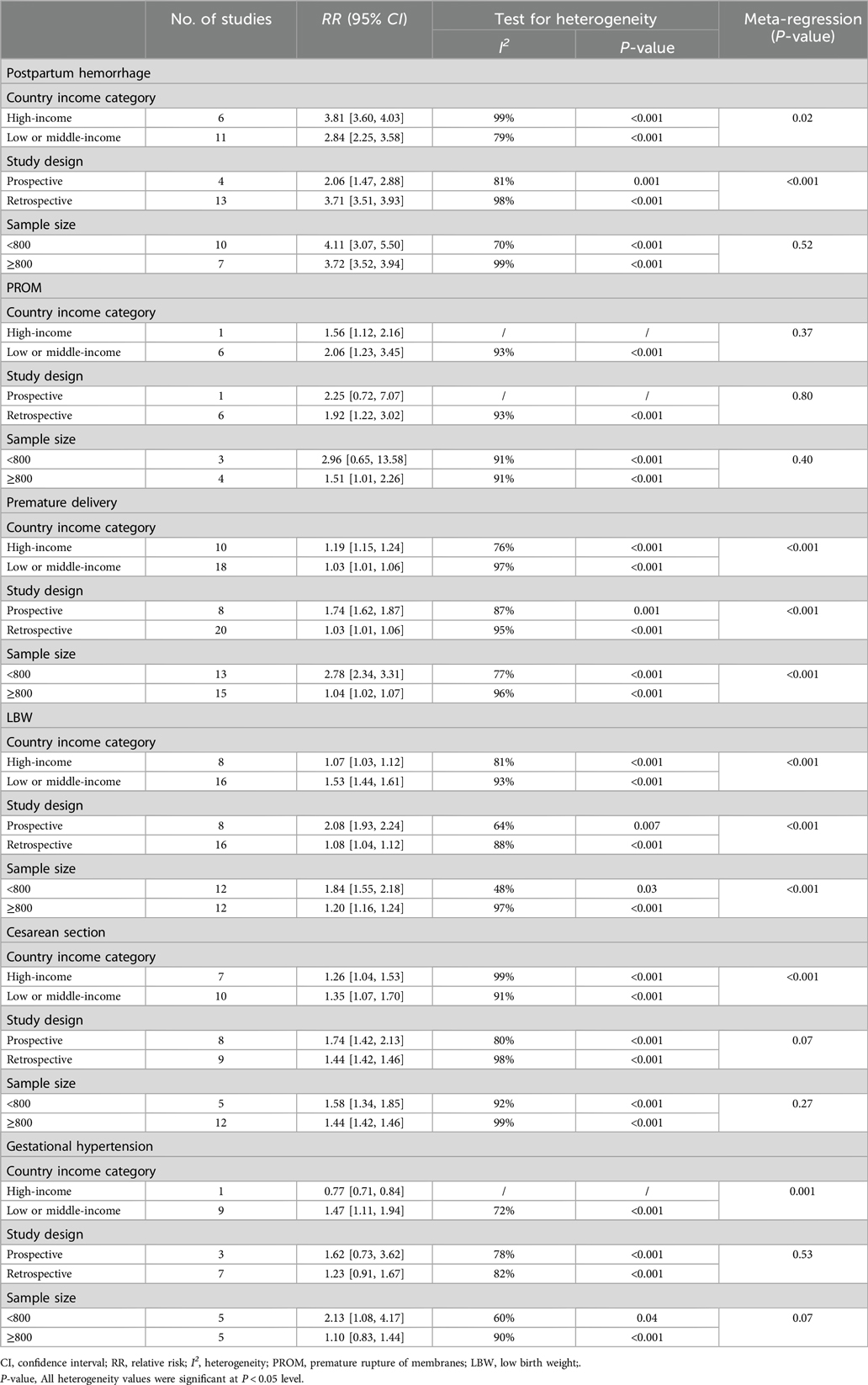
As shown in Table 4, meta-regression analyses were conducted on subgroups of pregnancy outcomes with high heterogeneity, including postpartum hemorrhage, PROM, preterm birth, LBW, cesarean section and gestational hypertension based on national income levels, study types and sample sizes. The results indicated that income levels may be a source of heterogeneity for postpartum hemorrhage (P = 0.02), premature delivery (P < 0.001) and cesarean section (P < 0.001). Study design types may be a source of heterogeneity for postpartum hemorrhage (P < 0.001) and preterm birth (P < 0.001). Sample sizes may be a source of heterogeneity for preterm birth (P < 0.001) and low birth weight (P < 0.001).
4 Discussion
Anemia is one of the common complications during pregnancy, even among pregnant women in developed countries (47). Due to the increased demand for nutrients during pregnancy, some pregnant women may experience anemia due to inadequate daily intake of nutrients or lower nutrient utilization rates (48). Chowdhury et al's (49) study indicated maternal age, BMI levels, economic status and educational level are associated with hemoglobin levels during pregnancy. Previous research has suggested that severe anemia during pregnancy, resulting in decreased hemoglobin levels and reduced oxygen-carrying capacity of the blood, can lead to various adverse pregnancy outcomes, including pregnancy-induced hypertension, miscarriage, preterm birth and LBW (50, 51).
This study found that pregnant women with anemia were at higher risk of developing pregnancy-induced hypertension, cesarean section, PROM, preterm birth, postpartum hemorrhage, neonatal asphyxia and LBW compared to non-anemic pregnant women with respective RR values of 1.33, 1.28, 1.94, 1.51, 2.76, 1.21 and 1.40. In terms of risk severity, the order from highest to lowest was postpartum hemorrhage, PROM, preterm birth, LBW, cesarean section, pregnancy-induced hypertension and neonatal asphyxia.
Our study corroborates previous research in identifying several adverse pregnancy outcomes associated with anemia. Xiong et al's (10) meta-analysis on the relationship between anemia during pregnancy and pregnancy outcomes investigated the ORs (95% CIs) of early pregnancy anemia with preterm birth, LBW, fetal growth restriction and hypertension, which were 1.32 (1.01, 1.74), 1.39 (0.70, 2.74), 1.01 (0.73, 1.38) and 0.80 (0.53, 1.20) respectively. In comparison, this study identified four adverse outcomes related to pregnancy anemia: PROM, cesarean section, postpartum hemorrhage and neonatal asphyxia. While Xiong et al' 's (10) study did not find a statistically significant association between anemia and pregnancy-induced hypertension, this study demonstrated a strong correlation between anemia during pregnancy and pregnancy-induced hypertension, with an RR value of 1.89. This difference may be attributed to the inclusion of only two relevant studies with potentially lower credibility in Xiong et al's analysis (52, 53). Our study encompassed a more comprehensive set of relevant studies, enhancing the reliability of our results. Additionally, some studies treated hypertension as a confounding factor and excluded it from their analysis, potentially influencing the results. When pregnant women have anemia, the decreased oxygen-carrying capacity of the blood leads to increased cardiovascular and peripheral vascular pressure to meet the demands for blood supply, thereby increasing the risk of hypertension (54).
The mechanisms underlying the adverse pregnancy outcomes in anemic pregnant women are multifactorial and biologically plausible. The decreased oxygen-carrying capacity of the blood due to anemia leads to compensatory physiological responses (55, 56). For instance, the increased cardiovascular and peripheral vascular pressure to meet the oxygen demands of the fetus can precipitate hypertension (57, 58). Postpartum hemorrhage is a significant cause of maternal mortality. Omotayo et al's (59) study showed that severe anemia during pregnancy increases the risk of postpartum hemorrhage, and there is no significant correlation between mild to moderate anemia and postpartum hemorrhage. The mechanism behind this may be related to iron deficiency anemia and increased secretion of nitric oxide. During pregnancy, trophoblasts and placental cells secrete nitric oxide through paracrine action, which then binds with nitric oxide synthase in the uterine muscle layer. This process plays a crucial role in maintaining the stability of the uterus. In the case of anemia, this process is intensified, leading to a decrease in the contraction function of uterine smooth muscles, thereby causing uterine muscle weakness and increasing the risk of postpartum uterine bleeding (60). Nur et al (61). also confirmed in a case-control study that anemia is a significant risk factor for postpartum hemorrhage.
Fetal hypoxia, a consequence of maternal anemia, is a key contributor to neonatal asphyxia. Maternal anemia causes a reduction in hemoglobin levels in the maternal blood, decreasing the blood's capacity to carry oxygen and affecting the fetus's ability to obtain sufficient oxygen in the uterus. When the fetus experiences hypoxia in the maternal uterus, it may result in asphyxia (62, 63). Additionally, this study demonstrates a strong association between maternal anemia during pregnancy and LBW and preterm birth with RR values of 1.40 and 1.51, respectively. Allen's (64) research suggests that placental insufficiency in anemic patients leading to hypoxia is considered one of the mechanisms causing LBW. Maternal anemia is often accompanied by inadequate nutrient intake and insufficient placental blood supply. Due to the lack of essential nutrients, the metabolic capacity of pregnant women's cells decreases, affecting fetal development and growth, leading to LBW (65). Furthermore, when the immune system function of newborns is immature, there is an increased risk of complications such as respiratory infections, indirectly raising the likelihood of neonatal asphyxia (66).
In general, maternal anemia during pregnancy impacts fetal oxygen supply and overall health, potentially leading to inadequate uterine contractions that can affect fetal growth and development. This condition may trigger preterm birth or delivery complications such as placental abruption, PROM, among others (67–69). Apart from oxidative damage, another known biological mechanism indicates that anemia and iron deficiency stimulate the synthesis of corticotropin-releasing hormone (CRH), leading to stress responses in both the mother and the fetus (70). Elevated levels of CRH are major risk factors for preterm birth, gestational hypertension, preeclampsia and PROM (64, 71). This study did not find an association between maternal anemia during pregnancy and gestational diabetes, puerperal infections, fetal distress, etc., which may be related to unpublished negative study results or differences in literature diagnostic criteria. Further, future high-quality research and relevant data are needed for supplementation and improvement.
The high heterogeneity observed in our meta-analysis, as indicated by the I2 values, warrants further exploration. Subgroup analyses based on factors such as country income levels, study design, and sample size have provided valuable insights. For example, income levels influenced the risk of postpartum hemorrhage, preterm birth, and cesarean section. In high-income countries, the risk of postpartum hemorrhage was higher, potentially due to differences in healthcare utilization and management practices. Study design also emerged as a significant source of heterogeneity, with retrospective studies often showing different results compared to prospective ones. This could be attributed to differences in data collection methods, recall bias in retrospective studies, and the ability to control for confounding factors. Sample size differences further contributed to heterogeneity, particularly in outcomes like preterm birth and LBW. Smaller studies may be more susceptible to sampling variability and less likely to capture the full spectrum of associations.
The strength of this study lies in focusing solely on the pregnancy outcomes of singleton anemic women. This is because twin or multiple pregnancies carry a higher risk of adverse perinatal outcomes. If there were a significant number of multiple pregnancies in the anemic group, it would lead to selection bias and magnify the impact of anemia on adverse pregnancy outcomes. Our study also has several limitations that should be considered when interpreting the results. On the one hand, the reliance on publicly available Chinese and English literature may have introduced selection bias, potentially overlooking relevant studies in other languages or unpublished research. The exclusion of specific types of anemia, such as aplastic anemia and thalassemia, limits the generalizability of our findings. On the other hand, while we standardized certain factors like singleton pregnancies, the lack of control for other confounding variables such as parity and BMI may have affected the accuracy of our estimates. Future studies should expand the literature search to include non-English and non-Chinese databases and grey literature sources more comprehensively. Collaborations with international research groups could also help in accessing a broader range of studies. In addition, future researchs can specifically investigate the impact of different types of anemia on pregnancy outcomes. This could involve conducting separate meta-analyses for each major type of anemia or including detailed subgroup analyses within a comprehensive study.
5 Conclusion
Maternal anemia is associated with an increased risk of adverse pregnancy outcomes. Anemia during pregnancy poses significant risks, not only leading to poor health conditions for pregnant women, premature rupture of membranes and postpartum hemorrhage but also being detrimental to fetal development, resulting in outcomes such as low birth weight, preterm birth and neonatal asphyxia. For pregnant women, special attention should be paid to supplementing iron. Adopting excellent nutritional management measures plays an essential supportive role in preventing the occurrence of pregnancy-induced anemia and avoiding the worsening of anemia.
Author contributions
RW: Methodology, Writing – original draft. SX: Data curation, Writing – original draft. XH: Investigation, Writing – original draft. XJ: Conceptualization, Writing – review & editing. DP: Supervision, Writing – review & editing. HX: Project administration, Writing – review & editing. WL: Data curation, Writing – review & editing. LY: Investigation, Writing – review & editing. SW: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Chinese Nutrition Society (NO. CNS- HPNK2022-140).
Acknowledgments
The authors thank all the participants for their time and efforts. Thank you for the study's support from the Nutrition Research Fund of the Chinese Nutrition Society (CNS) Nutrition Science Foundation- Hyproca Maternal and Infant Nutrition Research Fund Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgwh.2025.1502585/full#supplementary-material
References
1. Stevens GA, Paciorek CJ, Flores-Urrutia MC, Borghi E, Namaste S, Wirth JP, et al. National, regional, and global estimates of anaemia by severity in women and children for 2000–19: a pooled analysis of population-representative data. Lancet Glob Health. (2022) 10(5):e627–39. doi: 10.1016/S2214-109X(22)00084-5
2. Addo OY, Yu EX, Williams AM, Young MF, Sharma AJ, Mei Z, et al. Evaluation of hemoglobin cutoff levels to define Anemia among healthy individuals. JAMA Netw Open. (2021) 4(8):e2119123. doi: 10.1001/jamanetworkopen.2021.19123
3. Jouanne M, Oddoux S, Noël A, Voisin-Chiret AS. Nutrient requirements during pregnancy and lactation. Nutrients. (2021) 13(2):692. doi: 10.3390/nu13020692
4. Kazma JM, van den Anker J, Allegaert K, Dallmann A, Ahmadzia HK. Anatomical and physiological alterations of pregnancy. J Pharmacokinet Pharmacodyn. (2020) 47(4):271–85. doi: 10.1007/s10928-020-09677-1
5. Hytten F. Blood volume changes in normal pregnancy. Clin Haematol. (1985) 14(3):601–12. doi: 10.1016/S0308-2261(21)00496-3
6. Aparicio E, Jardí C, Bedmar C, Pallejà M, Basora J, Arija V, et al. Nutrient intake during pregnancy and post-partum: ECLIPSES study. Nutrients. (2020) 12(5):1325. doi: 10.3390/nu12051325
7. Qiao Y, Di J, Yin L, et al. Prevalence and influencing factors of anemia among pregnant women across first, second and third trimesters of pregnancy in monitoring areas, from 2016 to 2020: a population-based multi-center cohort study. BMC Public Health. (2024) 24(1):1100. doi: 10.1186/s12889-024-18610-x
8. Elstrott B, Khan L, Olson S, Raghunathan V, DeLoughery T, Shatzel JJ. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur J Haematol. (2020) 104(3):153–61. doi: 10.1111/ejh.13345
9. Alem AZ, Efendi F, McKenna L, Felipe-Dimog EB, Chilot D, Tonapa SI, et al. Prevalence and factors associated with anemia in women of reproductive age across low- and middle-income countries based on national data. Sci Rep. (2023) 13(1):20335. doi: 10.1038/s41598-023-46739-z
10. Xiong X, Buekens P, Alexander S, Demianczuk N, Wollast E. Anemia during pregnancy and birth outcome: a meta-analysis. Am J Perinatol. (2000) 17(3):137–46. doi: 10.1055/s-2000-9508
11. Figueiredo ACMG, Gomes-Filho IS, Silva RB, Pereira PPS, Mata FAFD, Lyrio AO, et al. Maternal anemia and low birth weight: a systematic review and meta-analysis. Nutrients. (2018) 10(5):601. doi: 10.3390/nu10050601
12. World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva: World Health Organization (2011).
13. Givens DI, Anitha S, Giromini C. Anaemia in India and its prevalence and multifactorial aetiology: a narrative review. Nutrients. (2024) 16(11):1673. doi: 10.3390/nu16111673
14. Peterson SJ, Foley S. Clinician’s guide to understanding effect size, alpha level, power, and sample size. Nutr Clin Pract. (2021) 36(3):598–605. doi: 10.1002/ncp.10674
15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
16. Barut A, Mohamud DO. The association of maternal anaemia with adverse maternal and foetal outcomes in Somali women: a prospective study. BMC Women’s Health. (2023) 23(1):193. doi: 10.1186/s12905-023-02382-4
17. Levy A, Fraser D, Katz M, Mazor M, Sheiner E. Maternal anemia during pregnancy is an independent risk factor for low birthweight and preterm delivery. Eur J Obstet Gynecol Reprod Biol. (2005) 122(2):182–6. doi: 10.1016/j.ejogrb.2005.02.015
18. Figueiredo ACMG, Gomes-Filho IS, Batista JET, Orrico GS, Porto ECL, Cruz Pimenta RM, et al. Maternal anemia and birth weight: a prospective cohort study. PLoS One. (2019) 14(3):e0212817. doi: 10.1371/journal.pone.0212817
19. Cao LF. Analysis of influencing factors and maternal and infant pregnancy outcomes of anemia during pregnancy in Huanggang City. Chin Foreign Med Res. (2002) 1(8):72–4. doi: 10.3969/j.issn.2096-6229.2022.08.024
20. Chen Y, Zhong T, Song X, Zhang S, Sun M, Liu X, et al. Maternal anaemia during early pregnancy and the risk of neonatal outcomes: a prospective cohort study in central China. BMJ Paediatr Open. (2024) 8(1):e001931. doi: 10.1136/bmjpo-2023-001931
21. Chu FC, Shaw SW, Lo LM, Hsieh TT, Hung TH. Association between maternal anemia at admission for delivery and adverse perinatal outcomes. J Chin Med Assoc. (2020) 83(4):402–7. doi: 10.1097/JCMA.0000000000000215
22. Lone FW, Qureshi RN, Emanuel F. Maternal anaemia and its impact on perinatal outcome. Trop Med Int Health. (2004) 9(4):486–90. doi: 10.1111/j.1365-3156.2004.01222.x
23. Fareh OI, Rizk DE, Thomas L, Berg B. Obstetric impact of anaemia in pregnant women in United Arab Emirates. J Obstet Gynaecol. (2005) 25(5):440–4. doi: 10.1080/01443610500160451
24. Masukume G, Khashan AS, Kenny LC, Baker PN, Nelson G, SCOPE Consortium. Risk factors and birth outcomes of anaemia in early pregnancy in a nulliparous cohort. PLoS One. (2015) 10(4):e0122729. doi: 10.1371/journal.pone.0122729
25. Hämäläinen H, Hakkarainen K, Heinonen S. Anaemia in the first but not in the second or third trimester is a risk factor for low birth weight. Clin Nutr. (2003) 22(3):271–5. doi: 10.1016/S0261-5614(02)00209-1
26. Jin D. The effect of iron deficiency anemia during pregnancy on pregnancy outcome and its clinical prevention and treatment. Dietary Health Care. (2022) 10:5–8. https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjMxMjI2EhpRS0JKQkQyMDIyMjAyMjA0MjIwMDAxMDg0MRoIeHd2d3ZseHo%3D
27. Visalakshi K. D. A Study of Impact of Anaemia on Pregnancy. (2006). Available online at: https://www.proquest.com/dissertations-theses/study-impact-anaemia-on-pregnancy/docview/2866084451/se-2
28. Lin L, Wang C, Su R, Feng H, Yang H. Prevalence of late pregnancy anemia, related factors, and pregnancy outcomes in different cities of China. Chin J Family Planning Gynecol. (2018) 10(08):37–40+49. https://kns.cnki.net/kcms2/article/abstract?v=x0sJsLzXXbhZW4boSi208jk8fLK6G-MCFbaVbHPnWOvLWr_TZeerljb3UG8_Pngyn3jXLXohXb0FXNN2D-J0JD25UOCOlq0QW6oTIGIvi2eG82gyBzf2XqLCpLnrBeuDbzwrq6TcrgNkt3sQEgPfLyI8ievACurRAwoHV7h79LnZRC7IKqVcEEhhuzXL3TZxyyrbKw0RZO8=&uniplatform=NZKPT&language=CHS
29. Drukker L, Hants Y, Farkash R, Ruchlemer R, Samueloff A, Grisaru-Granovsky S. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for cesarean section and adverse maternal and neonatal outcomes. Transfusion. (2015) 55(12):2799–806. doi: 10.1111/trf.13252
30. Lu LF. Analysis of pregnancy outcome with anemia. Jilin Med. (2014) 25:5632–3 (in Chinese). doi: 10.3969/j. https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjMxMjI2EhBqaWxpbnl4MjAxNDI1MDQ1Ggh1bHhsdGU5bw%3D%3D
31. Ma J. Correlation analysis of pregnancy complicated with anemia and adverse pregnancy outcomes. Front Med. (2020) 10(25):116–7. https://d.wanfangdata.com.cn/periodical/Ch9QZXJpb2RpY2FsQ0hJTmV3UzIwMjUwMTE2MTYzNjE0Eg55aXlxeTIwMjAyNTA3MBoIb2tzMjZzaWk=
32. Kaur M, Chauhan A, Manzar MD, Rajput MM. Maternal anaemia and neonatal outcome: a prospective study on urban pregnant women. J Clin Diagn Res. (2015) 9(12):QC04–QC8. doi: 10.7860/JCDR/2015/14924.6985
33. Engidaw MT, Eyayu T, Tiruneh T. The effect of maternal anaemia on low birth weight among newborns in Northwest Ethiopia. Sci Rep. (2022) 12(1):15280. doi: 10.1038/s41598-022-19726-z
34. Ramya G. Maternal and Fetal Outcome in Women with Severe and Very Severe Anaemia in Labour—A Prospective Study. (2017). Available online at: https://www.proquest.com/dissertations-theses/maternal-fetal-outcome-women-with-severe-very/docview/2866083749/se-2
35. Rukuni R, Bhattacharya S, Murphy MF, Roberts D, Stanworth SJ, Knight M. Maternal and neonatal outcomes of antenatal anemia in a Scottish population: a retrospective cohort study. Acta Obstet Gynecol Scand. (2017) 95(5):555–64. doi: 10.1111/aogs.12862
36. Räisänen S, Kancherla V, Gissler M, Kramer MR, Heinonen S. Adverse perinatal outcomes associated with moderate or severe maternal anaemia based on parity in Finland during 2006–10. Paediatr Perinat Epidemiol. (2014) 28(5):372–80. doi: 10.1111/ppe.12134
37. Kumari S, Garg N, Kumar A, Guru PKI, Ansari S, Anwar S, et al. Maternal and severe anaemia in delivering women is associated with risk of preterm and low birth weight: a cross sectional study from Jharkhand, India. One Health. (2019) 8:100098. doi: 10.1016/j.onehlt.2019.100098
38. Sun CF, Liu H, Hao YH, Hu HT, Zhou ZY, Zou KX, et al. Association between gestational anemia in different trimesters and neonatal outcomes: a retrospective longitudinal cohort study. World J Pediatr. (2021) 17(2):197–204. doi: 10.1007/s12519-021-00411-6
39. Tai W, Ma J. The influence of pregnancy anemia on pregnancy outcomes. J Logistics Support Coll CAPF (Med Edit). (2018) 09:765–8. doi: 10.16548/j.2095-3720.2018.09.011
40. Scholl TO, Hediger ML, Fischer RL, Shearer JW. Anemia vs iron deficiency: increased risk of preterm delivery in a prospective study. Am J Clin Nutr. (1992) 55(5):985–8. doi: 10.1093/ajcn/55.5.985
41. Tian ML, Ma GJ, Du LY, Xiao YG, Zhang YK, Tang ZJ. Prevalence and adverse perinatal outcomes of anaemia in the third trimester of pregnancy in Hebei Province, China. Int Health. (2024) 16(1):91–6. doi: 10.1093/inthealth/ihad028
42. Yi SW, Han YJ, Ohrr H. Anemia before pregnancy and risk of preterm birth, low birth weight and small-for-gestational-age birth in Korean women. Eur J Clin Nutr. (2013) 67(4):337–42. doi: 10.1038/ejcn.2013.12
43. Yu C, Zhao X, Kang Y. Risk factors for the onset of anemia during pregnancy and its impact on pregnancy outcomes. Maternal Child Health Care China. (2017) 23:5827–30. https://kns.cnki.net/kcms2/article/abstract?v=x0sJsLzXXbgPAKsGI0JgIZYPI0S_K23d5g2DkzIoUOGYRgfGHb9H-HM8UpV-R7AucP1K2ynPYIcQrpT2lgk0WfSM35cqAfKvV9VSFgKQBePbTQvaQj8i4ta17UdbRBEOB9JtNnJ6_cNbmNvt7CcwLD0tgAq2iXUb0TPUb_CqMaGNA5EeqCYvAwzE1IiHUK5ZIsYUiXobS18=&uniplatform=NZKPT&language=CHS
44. Zhao N. Risk factors for the onset of iron-deficiency anemia during pregnancy and its impact on pregnancy outcomes. Maternal Child Health Care China. (2021) 05:1139–41. doi: 10.19829/j.zgfybj.issn.1001-4411.2021.05.052
45. Zhao D, Zhang C, Ma J, Li J, Li Z, Huo C. Risk factors for iron deficiency and iron deficiency anemia in pregnant women from plateau region and their impact on pregnancy outcome. Am J Transl Res. (2022) 14(6):4146–53.35836856
46. Zheng S. Retrospective analysis of clinical data of 883 cases of late-stage iron-deficiency anemia in pregnant women. (master’s thesis). Jilin University (2022). doi: 10.27162/d.cnki.gjlin.2022.005392
47. Camaschella C. Iron-deficiency anemia. N Engl J Med. (2015) 372(19):1832–43. doi: 10.1056/NEJMra1401038
48. James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. (2021) 138(4):663–74. doi: 10.1097/AOG.0000000000004559
49. Chowdhury HA, Ahmed KR, Jebunessa F, Akter J, Hossain S, Shahjahan M. Factors associated with maternal anaemia among pregnant women in Dhaka city. BMC Women’s Health. (2015) 15:77. doi: 10.1186/s12905-015-0234-x
50. Haider BA, Olofin I, Wang M, Spiegelman D, Ezzati M, Fawzi WW, Nutrition Impact Model Study Group (anaemia). Anaemia, prenatal iron use, and risk of adverse pregnancy outcomes: systematic review and meta-analysis. BMJ (Clin Res Ed.). (2013) 346:f3443. doi: 10.1136/bmj.f3443
51. McClure EM, Goldenberg RL, Dent AE, Meshnick SR. A systematic review of the impact of malaria prevention in pregnancy on low birth weight and maternal anemia. Int J Gynaecol Obstet. (2013) 121(2):103–9. doi: 10.1016/j.ijgo.2012.12.014
52. Knottnerus JA, Delgado L, Knipschild PG, Essed GG, Smits F. Maternal haemoglobin and pregnancy outcome. Lancet. (1986) 2(8501):282. doi: 10.1016/S0140-6736(86)92096-9
53. Lieberman E, Ryan KJ, Monson RR, Schoenbaum SC. Risk factors accounting for racial differences in the rate of premature birth. N Engl J Med. (1987) 317(12):743–8. doi: 10.1056/NEJM198709173171206
54. Milman N, Jønsson L, Dyre P, Pedersen PL, Larsen LG. Ferrous bisglycinate 25 mg iron is as effective as ferrous sulfate 50 mg iron in the prophylaxis of iron deficiency and anemia during pregnancy in a randomized trial. J Perinat Med. (2014) 42(2):197–206. doi: 10.1515/jpm-2013-0153
55. Hare GM. Tolerance of anemia: understanding the adaptive physiological mechanisms which promote survival. Transfus Apher Sci. (2014) 50(1):10–2. doi: 10.1016/j.transci.2013.12.005
56. Gillies ID, White YS, White JM. Compensatory mechanisms for the severe anaemia caused by haemoglobin hammersmith. Eur J Clin Invest. (1976) 6(3):213–20. doi: 10.1111/j.1365-2362.1976.tb00513.x
57. Johnson A, Vaithilingan S, Avudaiappan SL. The interplay of hypertension and anemia on pregnancy outcomes. Cureus. (2023) 15(10):e46390. doi: 10.7759/cureus.46390
58. Mulatie Z, Aynalem M, Getawa S. Hematological profiles of newborns of mothers with hypertensive disorders of pregnancy delivered at the university of Gondar comprehensive specialized hospital: a comparative cross-sectional study. BMC Pediatr. (2024) 24(1):17. doi: 10.1186/s12887-023-04491-3
59. Omotayo MO, Abioye AI, Kuyebi M, Eke AC. Prenatal anemia and postpartum hemorrhage risk: a systematic review and meta-analysis. J Obstet Gynaecol Res. (2021) 47(8):2565–76. doi: 10.1111/jog.14834
60. Al-Hijji J, Andolf E, Laurini R, Batra S. Nitric oxide synthase activity in human trophoblast, term placenta and pregnant myometrium. Reprod Biol Endocrinol. (2003) 1:51. doi: 10.1186/1477-7827-1-51
61. Nur R, Sarina HS, Patui NS, Radhiah S, Suwendro NI, Ariani , Patel A, Saleem S, Ali SA, et al. Postpartum hemorrhage in maternal mothers at anutapura public hospital in palu, 2017. Gac Sanit. (2021) 35(2):S148–51. doi: 10.1016/j.gaceta.2021.10.014
62. Parks S, Hoffman MK, Goudar SS, Helill SE, Assefa B, Abdu M, et al. Maternal anaemia and maternal, fetal, and neonatal outcomes in a prospective cohort study in India and Pakistan. BJOG. (2019) 126(6):737–43. doi: 10.1111/1471-0528.15585
63. Ahmed R, Mosa H, Sultan M, et al. Prevalence and risk factors associated with birth asphyxia among neonates delivered in Ethiopia: a systematic review and meta-analysis. PLoS One. (2021) 16(8):e0255488. doi: 10.1371/journal.pone.0255488
64. Allen LH. Biological mechanisms that might underlie iron’s effects on fetal growth and preterm birth. J Nutr. (2001) 131(2S-2):581S–9. doi: 10.1093/jn/131.2.581S
65. Sharma AJ, Ford ND, Bulkley JE, Jenkins LM, Vesco KK, Williams AM. Use of the electronic health record to assess prevalence of Anemia and iron deficiency in pregnancy. J Nutr. (2021) 151(11):3588–95. doi: 10.1093/jn/nxab254
66. Qin J. Analysis of Complications and Respiratory System Treatment in Extremely Low Birth Weight Infants. (master’s thesis). Soochow University (2022).
67. Kadyrov M, Kosanke G, Kingdom J, Kaufmann P. Increased fetoplacental angiogenesis during first trimester in anaemic women. Lancet. (1998) 352(9142):1747–9. doi: 10.1016/S0140-6736(98)02069-8
68. Zhang Q, Ananth CV, Li Z, Smulian JC. Maternal anaemia and preterm birth: a prospective cohort study. Int J Epidemiol. (2009) 38(5):1380–9. doi: 10.1093/ije/dyp243
69. Lao TT, Hui SYA, Wong LL, Sahota DS. Iron deficiency anaemia associated with increased placenta praevia and placental abruption: a retrospective case-control study. Eur J Clin Nutr. (2022) 76(8):1172–7. doi: 10.1038/s41430-022-01086-6
70. Bakacak M, Avci F, Ercan O, Köstü B, Serin S, Kiran G, et al. The effect of maternal hemoglobin concentration on fetal birth weight according to trimesters. J Matern Fetal Neonatal Med. (2015) 28(17):2106–10. doi: 10.3109/14767058.2014.979149
Keywords: anemia, anemia in pregnancy, pregnancy outcome, cohort study, maternal health
Citation: Wang R, Xu S, Hao X, Jin X, Pan D, Xia H, Liao W, Yang L and Wang S (2025) Anemia during pregnancy and adverse pregnancy outcomes: a systematic review and meta-analysis of cohort studies. Front. Glob. Womens Health 6:1502585. doi: 10.3389/fgwh.2025.1502585
Received: 27 September 2024; Accepted: 10 January 2025;
Published: 31 January 2025.
Edited by:
Muhabaw Shumye Mihret, University of Gondar, EthiopiaReviewed by:
Suriya Kumareswaran, National University of Malaysia, MalaysiaPriya Chandran, Government Medical College, India
Copyright: © 2025 Wang, Xu, Hao, Jin, Pan, Xia, Liao, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaokang Wang, c2hhb2thbmd3YW5nQHNldS5lZHUuY24=
 Rui Wang
Rui Wang Shan Xu1
Shan Xu1 Xingyi Jin
Xingyi Jin Hui Xia
Hui Xia Wang Liao
Wang Liao Ligang Yang
Ligang Yang Shaokang Wang
Shaokang Wang