- 1School of Public Health, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan-Teferi, Ethiopia
- 2Faculty of Health, School of Medicine, Deakin University, Waurn Ponds, VIC, Australia
- 3Department of Midwifery, College of Medicine and Health Sciences, Mizan-Tepi University, Mizan-Teferi, Ethiopia
- 4School of Public Health, College of Medicine and Health Sciences, Wachemo University, Hosanna, Ethiopia
- 5Department of Public Health, College of Medicine and Health Sciences, Injibara University, Injibara, Ethiopia
Background: Hepatitis B Virus (HBV) infection during pregnancy poses serious risks by raising the likelihood of chronic infection in newborns by 90% and the risk of cirrhosis and liver cancer by 25% in chronic infections. This study aimed to identify determinants of HBV infection among pregnant women in the Bench Sheko zone, Southwest Ethiopia.
Methods: An unmatched case-control study was conducted from May 15 to July 15, 2022, in selected health facilities of the Bench Sheko zone, Southwest Ethiopia. Medical charts were reviewed to collect the HBsAg status of participants, as all pregnant women attending antenatal care underwent routine screening. It involved 228 pregnant women (76 HBV-positive cases and 152 HBV-negative controls). Data were collected using structured questionnaires, and analyzed using SPSS 21. A multivariable logistic regression was performed to identify significant determinants of HBV infection, and statistical significance was declared at p-value <0.05.
Results: After controlling potential confounders, having no formal education (AOR = 4.94, 95% CI: 2.01, 8.29; P = 0.007), urban residency (AOR = 2.56, 95% CI: 1.43, 6.86; P = 0.010), history of unsafe abortion (AOR = 3.87, 95% CI: 2.17, 6.98; P < 0.001), sharing sharp materials (AOR = 8.43, 95% CI: 5.54, 10.9; P < 0.001), contact with HBV-infected persons in the family (AOR = 2.18, 95% CI: 1.72, 4.87; P < 0.001), tribal scarification (AOR = 3.23, 95% CI: 1.24, 8.91; P = 0.017), and history of unsafe tooth extraction (AOR = 4.52, 95% CI: 2.18, 9.76; P = 0.039) were identified as significant predictors of HBV infection.
Conclusion: The study identifies multiple factors contributing to HBV infection in pregnant women. Therefore, it is crucial to promote safe abortion practices and the responsible use of sharp materials, avoid high-risk contact with infected individuals within the family, raise awareness about the risks associated with tribal scarification while advocating for safer practices, and offer education on safe tooth extraction methods to reduce the risk of HBV.
Introduction
Hepatitis B infection, caused by the Hepatitis B Virus (HBV), infects the liver and can lead to cirrhosis and hepatocellular carcinoma (HCC) (1). The incubation period is 45 days to 6 months. Around 10% of children and 30%–50% of adults with acute infection experience symptoms (2, 3). HBV, primarily transmitted through blood, semen, vaginal secretions, and contaminated fluids, poses significant health risks. Major transmission routes include sexual contact, household contact, injecting drug use, and medical exposure (1–3). Possible modes of transmission are unscreened blood transfusions, needle sharing, hemodialysis, acupuncture, tattooing, and injuries from contaminated sharp instruments (1, 2). Chronic HBV infections can lead to severe complications in 20%–30% of cases, resulting in approximately 650,000 annual deaths (2).
Globally, hepatitis B infection remains a significant health concern, with approximately 1.5 million new infections each year and 820,000 related deaths reported in 2019. The global prevalence stands at 3.8% (4). In Africa, there were 990,000 new infections and 80,000 deaths in 2019, with carrier prevalence in sub-Saharan Africa ranging from 10% to 20% (4). Ethiopia, designated a high-burden country, exhibits an HBsAg prevalence of 35.8% among chronic liver disease patients (5). Recent data from 2018 indicates a pooled prevalence of 4.7% among pregnant women, with higher rates observed in specific regions such as Gambella (7.9%) and Bench Sheko Zone (5.9%) (6).
In endemic areas, the high prevalence of HBeAg in women of reproductive age correlates with increased prenatal HBV transmission (7). HBV infection during pregnancy poses significant risks to both mother and child (8), with approximately 10%–20% of HBsAg-positive pregnant women transmitting the virus to their babies. Transmission rates approach 100% for women positive for both HBsAg and HBeAg (7), leading to newborns facing an 85%–90% risk of developing chronic liver diseases (9). Tragically, about 25% of those infected as infants or young children succumb to cirrhosis and liver cancer, often without symptoms until late-stage disease (10).
Studies in Sub-Saharan countries investigating determinants of hepatitis B virus (HBV) among pregnant women have yielded inconsistent findings. Research in Uganda found no significant relationship between HBsAg positivity and factors like scarification, number of sexual partners, blood transfusion, polygamy, surgical procedures, or marital status (11). However, other studies highlighted scarification, blood transfusion, concurrent HIV infection, multiple sexual partners, and tribal scar as significant factors associated with HBV infection (12–15).
The high prevalence of HBV among pregnant women in Ethiopia represents a significant public health concern (16). Despite government efforts to screen for viral hepatitis in pregnant women, there's limited focus on identifying the root causes of HBV infection within the health system (6, 17). Given the potential transmission from mother to child, understanding the main factors contributing to its spread among pregnant women is crucial. Thus, this study aims to identify significant determinants of HBV infection in pregnant women.
Methods
Study design, area, and period
A health institution-based case-control study was conducted in Bench Sheko Zone, southwest Ethiopia from May 15 to July 15, 2022. The zone is located 585 km from Addis Ababa. The zone's population was 653,986, with 330,459 females and 323,527 males. Among them, 152,378 are women of childbearing age, with an estimated 22,627 pregnancies. Healthcare services are provided by 1 teaching hospital, 1 primary hospital, 26 health centers, and 130 health posts. The data collection took place at health facilities in North and Sheko districts, as well as at the Mizan-Aman town administration.
Populations
The source population comprised pregnant women attending Antenatal care (ANC) follow-ups at selected health facilities during the data collection period. The study population included pregnant women meeting the inclusion criteria during the same period. Cases were pregnant women diagnosed with HBV infection (positive HBsAg test) attending ANC at the selected health institution. Controls were pregnant women without HBV infection (negative HBsAg test) attending ANC at the same institution.
Inclusion Criteria
✓ Pregnant women attending antenatal care (ANC) follow-ups at selected health facilities during the data collection period.
✓ Pregnant women who underwent HBV testing and met the study criteria.
Exclusion Criteria
✓ Pregnant women who did not provide written informed consent.
Sample size determination
The sample size was calculated using Open Epi version 3.01 software by considering the following assumptions. The expected proportion of multiple sexual partners among HBV-infected cases and controls was 68.9% and 47.5% respectively (18), 95% CI, 80% power, and a case-to-control ratio of 1:2. The sample size was 207. Thus, after considering the 10% non-response rate, the total study participants were 228 (76 cases and 152 controls).
Sampling procedure
North and Sheko districts, along with the Mizan-Aman town administration, were chosen at random from a total of six districts and one town administration within the Bench Sheko zone. All health centers and hospitals providing ANC in these areas were included. The sample size was allocated to each institution based on average monthly client flow. Pregnant women's serologic status was checked from ANC registration books, categorizing them into case and control groups. For each case, the next two controls were consecutively interviewed until reaching the required sample size.
Study variables
The dependent variable was HBV infection. The independent variables included socio-demographic factors (age, residence, education, marital status, and occupation), reproductive health history (unsafe abortion, pregnancies, delivery location and method, pregnancy complications), medical/surgical history (hospitalization, surgery, unsafe tooth extraction, blood transfusion, STI/HIV history), behavioral factors (multiple sexual partners, household HBV contact, sharing items), and cultural/cosmetic practices (tribal scarification, ear piercing, tattoo, traditional removal of the uvula/tonsil, and circumcision/genital mutilation) (Supplementary Table S1).
Data collection tools and procedures
Data collection involved face-to-face interviews using a structured and pre-tested questionnaire adapted from relevant literature and tailored to the local context. The questionnaire, initially in English, was translated to Amharic and back to English for consistency. It covered socio-demographic, reproductive, medical, surgical, behavioral, cultural, and cosmetic aspects. Medical charts of the participants were reviewed to collect only the HBsAg status, as routine screening was conducted for all pregnant women attending antenatal care. A pretest was conducted on 5% of pregnant women (4 cases and 8 controls) at a health institution out of the actual study area. Training that lasted one day was provided to data collectors and supervisors about data collection procedures and quality assurance.
Statistical analysis
Data coding and entry were performed using Epi-Data version 3.1 and then analyzed using SPSS version 21. Descriptive statistics, including frequencies and percentages, were performed, and findings were presented through tables. Each potential independent factor related to HBV infection underwent bivariate logistic regression analysis. To prevent overfitting, only variables showing a p-value <0.25 in the bivariate analysis were included in the multivariable logistic regression analysis. Those with a p-value <0.05 in the multivariable analysis were deemed determinants of HBV. The model's independent variables showed an acceptable variance inflation factor (VIF) indicating low multicollinearity (VIF <2), and the model fit well, as indicated by the Hosmer-Lemeshow goodness of fit test (p = 0.566).
Results
Socio-demographic characteristics
A total of 212 pregnant women participated in the study, including 71 cases and 141 controls, with response rates of 93.4% and 92.8%, respectively. The mean age was 27.6 ± 6.4 years, with cases slightly older (28.1 ± 6.9) than controls (27.3 ± 6.2). Among cases, 16 (22.5%) had no formal education, compared to 14 (9.9%) of controls. Sixty-one (85.9%) were married, while 107 (75.8%) of controls were married. Thirty-one (43.6%) of cases were housewives, compared to 73 (51.7%) of controls (Table 1).

Table 1. Socio-demographic characteristics of study participants in Bench Sheko zone, Southwest Ethiopia.
Reproductive health history
Among cases, 38 (53.5%) were experiencing their first pregnancy, compared to 48 (34%) of controls. Thirteen (39.3%) of cases and 27 (23.6%) of controls gave their recent birth at home. Spontaneous vaginal delivery was more common among controls (81, 87%) than cases (23, 69.6%). Pregnancy-related complications affected 15 (21.2%) of cases and 25 (17.7%) of controls. Additionally, 15 (10.7%) of controls and 16 (22.5%) of cases had a history of unsafe abortion. Controls were more likely to have heard about HBV infection (102, 72.3%) compared to cases (41, 57.7%) (Table 2).
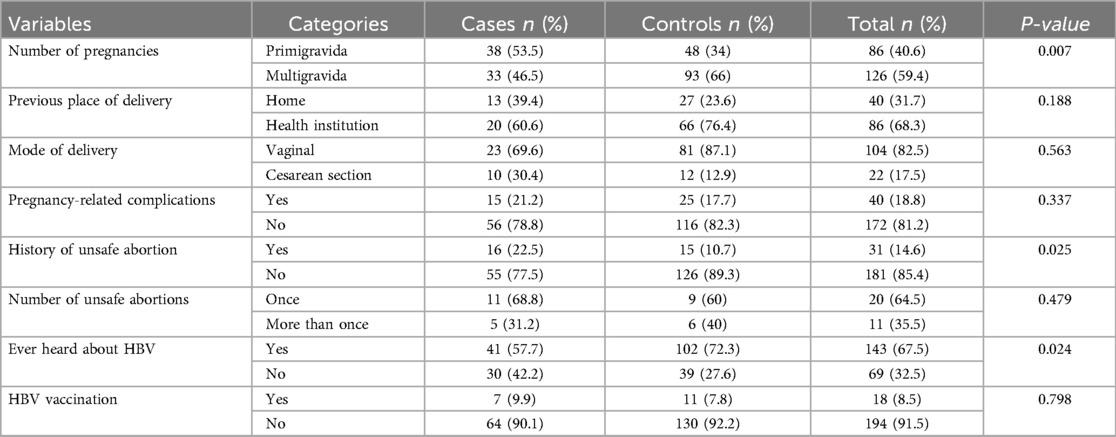
Table 2. Reproductive health-related factors of study participants in Bench Sheko zone, Southwest Ethiopia.
Behavioral and cultural-related characteristics
Participants with a history of sharing sharp materials were higher among cases (51, 71.8%) compared to controls (26, 18.4%). Cases had a higher proportion of history of contact with HBV-infected persons (12, 16.9%) compared to controls (11, 7.8%). Cases had a higher proportion of tribal scarification (25, 35.2%) compared to controls (25, 17.7%). Similarly, cases had a lower proportion of removal of the uvula (3, 4.2%) compared to controls (24, 17%) (Table 3).
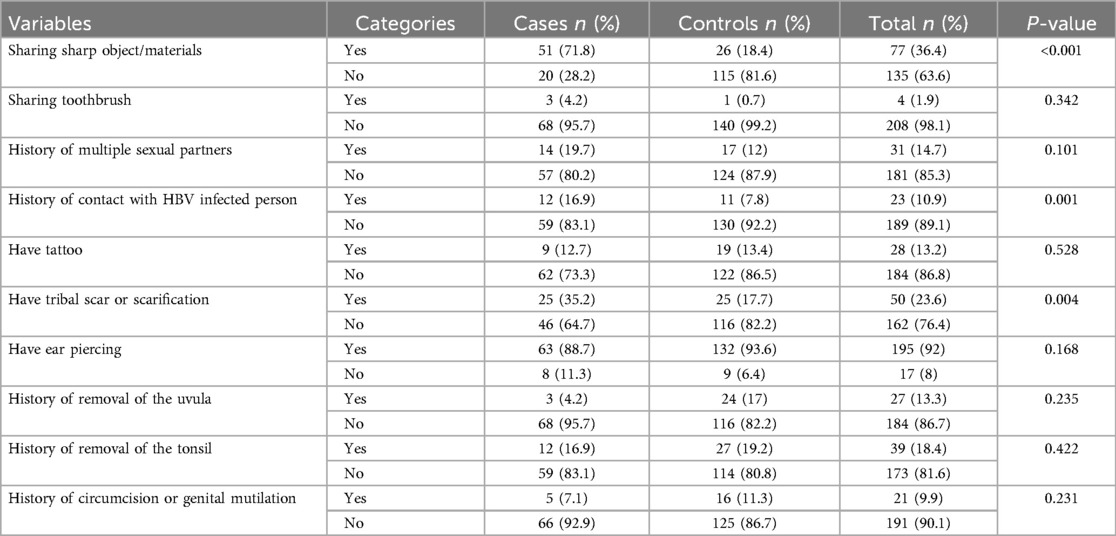
Table 3. Behavioral and cultural-related factors of study participants in Bench Sheko zone, Southwest Ethiopia.
Medical and surgical history
Cases had a higher history of surgery (12, 16.9%) compared to controls (15, 10.6%). Unsafe tooth extraction was more common among cases (16, 22.5%) than controls (8, 5.6%). Sexually transmitted diseases were reported more frequently among cases (15, 21%) compared to controls (15, 10.6%). Additionally, the HIV/AIDS positivity rate was higher among cases (8, 11.2%) than controls (6, 4.3%) (Table 4).
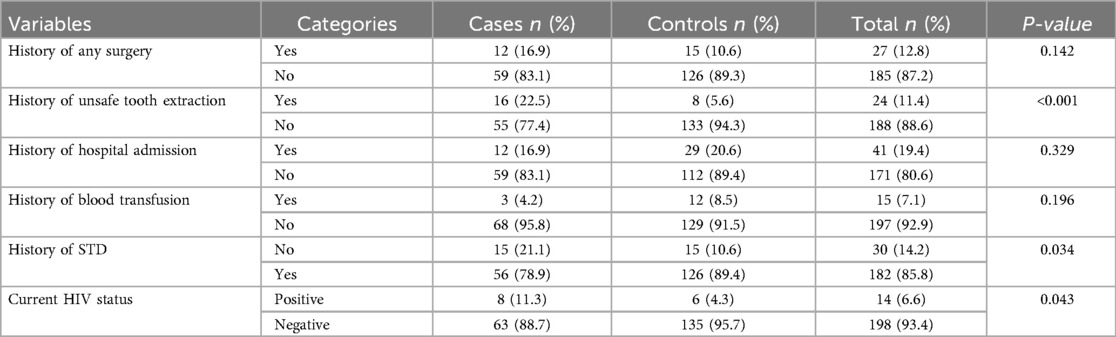
Table 4. Medical and surgical history of study participants in bench sheko zone, southwest Ethiopia.
Determinants of HBV infection
After adjusting for confounding factors, the study identified several significant determinants of HBV infection: pregnant women without formal education had 4.9 times higher odds of HBV infection compared to those with diplomas or higher education (AOR = 4.94, 95% CI: 2.01, 8.29; P = 0.007). Urban residents were 2.6 times more likely to have HBV than rural residents (AOR = 2.56, 95% CI: 1.43, 6.86; P = 0.010). A history of unsafe abortion increased the odds of HBV infection by 3.9 times (AOR = 3.87, 95% CI: 2.17, 6.98; P < 0.001). Sharing sharp materials like syringes and needles increased the odds by 8.4 times (AOR = 8.43, 95% CI: 5.54, 10.9; P < 0.001). Contact with HBV-infected persons in the family raised the odds by 2.2 times (AOR = 2.18, 95% CI: 1.72, 4.87; P < 0.001). Tribal scarification increased the odds by 3.2 times (AOR = 3.23, 95% CI: 1.24, 8.91; P = 0.017). The history of unsafe tooth extraction increased the odds by 4.5 times (AOR = 4.52, 95% CI: 2.18, 9.76; P = 0.039) (Table 5).
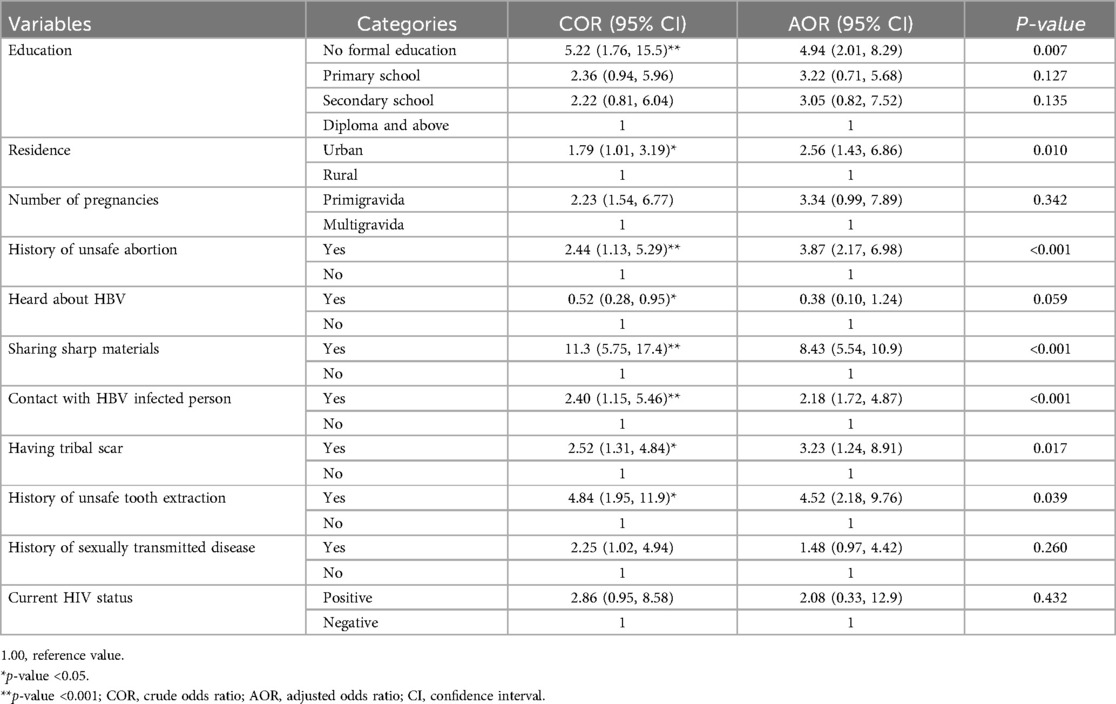
Table 5. Determinants of HBV infection among study participants in Bench Sheko zone, Southwest Ethiopia.
Discussion
In Ethiopia, despite screening efforts, the health system lacks focus on identifying the root causes of HBV infection (6, 17). The study aimed to identify potential determinants of HBV infection among pregnant women. The study identified key risk factors for HBV infection, such as education level, residence, unsafe abortion history, sharing sharp materials, contact with hepatitis patients, tribal scars, and unsafe tooth extraction. Understanding these factors helps target preventive measures and interventions more effectively.
In line with the findings of previous studies, there was a positive association between level of education and HBV infection (14, 19). Low literacy levels may contribute to HBV transmission due to limited health literacy, barriers to healthcare access, socioeconomic challenges, risky behaviors, and stigma. Enhancing education, improving healthcare access, and addressing social determinants are crucial for reducing HBV infection among illiterate populations.
Urban participants exhibited higher risk of HBV infection compared to rural participants, consistent with previous studies (20, 21). This phenomenon may be due to high urban population density, which increases transmission through close contact and shared resources, along with significant population mobility that spreads HBV among diverse groups. Additionally, higher rates of risky behaviors like unsafe injections and sharing sharp materials, coupled with socioeconomic disparities affecting access to health resources, contribute to the increased risk (21).
A history of unsafe abortion was significantly correlated with HBV infection, which aligns with studies in Ethiopia (13, 22, 23) and Nigeria (18). The increased risk of HBV transmission during abortions outside healthcare facilities is due to inadequate infection prevention measures, including the use of contaminated instruments, and the absence of medical supervision and sterile conditions, heightening the likelihood of exposure.
Participants who shared sharp materials had a greater chance of having HBV infection. This finding is consistent with other studies (15, 24). Sharing sharp materials, such as needles or razors, significantly increases the risk of contracting HBV due to direct blood-to-blood contact. This close exchange of bodily fluids provides an efficient pathway for the transmission of the virus, elevating the likelihood of infection.
Participants with a history of family contact with hepatitis patients exhibited higher odds of HBV infection compared to those without such contact, aligning with findings from studies conducted elsewhere (12, 13, 15, 18, 25, 26). The increased risk of HBV infection may be due to unprotected caregiving contact and limited awareness of transmission modes and prevention measures, leading to behaviors that facilitate virus transmission, such as sharing personal items or engaging in unprotected sexual activity.
The history of tribal scarification is associated with HBV infection in this study. This finding is supported by studies conducted elsewhere (27, 28). The increased likelihood of risk can be elucidated by the utilization of inadequately sterilized equipment throughout the procedure (28). This increased risk arises from the potential for pathogens and contaminants to persist on improperly cleaned instruments, thus increasing the probability of infections or complications for individuals undergoing the procedure.
Pregnant women with a history of unsafe tooth extraction exhibited 4.5 times higher odds of HBV infection compared to those without such a history, consistent with studies in Ethiopia (12, 29), and Saudi Arabia (30). The increased risk may arise from inadequate infection control measures during unsafe tooth extraction procedures, posing threats to both healthcare workers and patients. Additionally, traditional tooth removal methods that involve non-sterile materials could further contribute to HBV transmission.
Limitations of the study
Blood samples weren't directly collected from participants due to financial limitations, so HBV status relied on secondary data. Using only the HBsAg marker to define the clinical status of pregnant women is a limitation. Including markers like anti-HBe antibodies and HBeAg could have provided a more detailed assessment of HBsAg-positive women. Exposure variables, like STI history, were symptom-based, possibly leading to recall bias. Sensitive questions, such as those about multiple partners, might have faced social desirability bias.
Conclusion
The study identifies several determinants of HBV infection in pregnant women, including no formal education, urban residency, history of unsafe abortion, sharing sharp materials, contact with HBV-infected family members, tribal scarification, and history of unsafe tooth extraction. Effective reduction of HBV risk relies on targeted interventions, such as health education to increase awareness, ensuring safe healthcare practices, promoting HBV vaccination, and offering support to high-risk pregnant women. Addressing these factors can significantly lower HBV infection rates among pregnant women and improve maternal and child health outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Mizan-Tepi University Ethical Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
TY: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EE: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NS: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. GA: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. DG: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. AH: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. AAh: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. AAs: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Firstly, we extend our gratitude to all study participants for their valuable participation. Secondly, we would like to express our thanks to the administrative staff at MTUTH for their full collaboration during the study period.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgwh.2024.1453231/full#supplementary-material
References
1. World Health Organization. Fact Sheet: Hepatitis B in the WHO European Region. Geneva, Switzerland: World Health Organization (2022). p. 2.
2. World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva, Switzerland: World Health Organization (2015). p. 134.
3. Abdi F, Novin MG, Afrakhteh M, Khorvash F. Hepatitis B and pregnancy: an update review article. World J Obstet Gynecol. (2015) 4(1):1–8. doi: 10.5317/wjog.v4.i1.1
4. World Health Organization. Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021. Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact. Geneva: World Health Organization (2021). p. 108.
5. Ayele AG, Gebre-Selassie S. Prevalence and risk factors of hepatitis B and hepatitis C virus infections among patients with chronic liver diseases in public hospitals in Addis Ababa, Ethiopia. Int Sch Res Notices. (2013) 2013:563821. doi: 10.1155/2013/563821
6. Kebede KM, Abateneh DD, Belay AS. Hepatitis B virus infection among pregnant women in Ethiopia: a systematic review and meta-analysis of prevalence studies. BMC Infect Dis. (2018) 18(1):322. doi: 10.1186/s12879-018-3234-2
7. Pennap GR, Osanga ET, Ubam A. Seroprevalence of hepatitis B surface antigen among pregnant women attending antenatal clinic in federal medical centre keffi, Nigeria. Res J Med Sci. (2011) 5(2):80–2. doi: 10.3923/rjmsci.2011.80.82
8. Hwang EW, Cheung R. Global epidemiology of hepatitis B virus (HBV) infection. N Am J Med. (2011) 4(1):7–13. doi: 10.7156/v4i1p007
9. Apuzzio J, Block JM, Cullison S, Cohen C, Leong SL, London WT, et al. Chronic hepatitis B in pregnancy A workshop consensus statement on screening, evaluation, and management, part 1. Obstetrics Report. (2012) 37:22–34.
10. Sinha S, Kumar M. Pregnancy and chronic hepatitis B virus infection. Hepatol Res. (2010) 40(1):31–48. doi: 10.1111/j.1872-034X.2009.00597.x
11. Bayo P, Ochola E, Oleo C, Mwaka AD. High prevalence of hepatitis B virus infection among pregnant women attending antenatal care: a cross-sectional study in two hospitals in Northern Uganda. BMJ Open. (2014) 4(11):e005889. doi: 10.1136/bmjopen-2014-005889
12. Molla S, Munshea A, Nibret E. Seroprevalence of hepatitis B surface antigen and anti HCV antibody and its associated risk factors among pregnant women attending maternity ward of Felege Hiwot referral hospital, Northwest Ethiopia: a cross-sectional study. Virol J. (2015) 12(1):204. doi: 10.1186/s12985-015-0437-7
13. Tanga AT, Teshome MA, Hiko D, Fikru C, Jilo GK. Sero-prevalence of hepatitis B virus and associated factors among pregnant women in Gambella hospital, South Western Ethiopia: facility based cross-sectional study. BMC Infect Dis. (2019) 19(1):602. doi: 10.1186/s12879-019-4220-z
14. Metaferia Y, Dessie W, Ali I, Amsalu A. Seroprevalence and associated risk factors of hepatitis B virus among pregnant women in southern Ethiopia: a hospital-based cross-sectional study. Epidemiol Health. (2016) 38:e2016027. doi: 10.4178/epih.e2016027
15. Asaye Z, Aferu T, Asefa A, Feyissa D, Regasa T, Kebede O, et al. Prevalence of hepatitis B virus among pregnant women on antenatal care follow-up at mizan-tepi university teaching hospital and Mizan health center, Southwest Ethiopia. Int J Gen Med. (2021) 14:195–200. doi: 10.2147/IJGM.S292070
16. Alemu AA, Zeleke LB, Aynalem BY, Kassa GM. Hepatitis B virus infection and its determinants among pregnant women in Ethiopia: a systematic review and meta-analysis. Infect Dis Obstet Gynecol. (2020) 2020:9418475. doi: 10.1155/2020/9418475
17. Belyhun Y, Maier M, Mulu A, Diro E, Liebert UG. Hepatitis viruses in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. (2016) 16(1):761. doi: 10.1186/s12879-016-2090-1
18. Rabiu KA, Akinola OI, Adewunmi AA, Omololu OM, Ojo TO. Risk factors for hepatitis B virus infection among pregnant women in Lagos, Nigeria. Acta Obstet Gynecol Scand. (2010) 89(8):1024–8. doi: 10.3109/00016349.2010.482580
19. Fessehaye N, Berhane A, Ahmed H, Mohamed S, Tecle F, Gikunju J, et al. Prevalence of hepatitis B virus infection and associated seromarkers among pregnant women in Eritrea. J Hum Virol Retrovirol. (2018) 6(1):00191.
20. Stawinska-Witoszynska B, Klos J, Moryson W, Wieckowska B. Trends in the incidence of acute hepatitis B in the Polish population and their determinants. Medicina (Kaunas). (2021) 57(8):738. doi: 10.3390/medicina57080738
21. Makuwa M, Mintsa-Ndong A, Souquière S, Nkoghé D, Leroy EM, Kazanji M. Prevalence and molecular diversity of hepatitis B virus and hepatitis delta virus in urban and rural populations in Northern Gabon in Central Africa. J Clin Microbiol. (2009) 47(7):2265–8. doi: 10.1128/JCM.02012-08
22. Yohanes T, Zerdo Z, Chufamo N. Seroprevalence and predictors of hepatitis B virus infection among pregnant women attending routine antenatal care in Arba Minch hospital, South Ethiopia. Hepat Res Treat. (2016) 2016:9290163. doi: 10.1155/2016/9290163
23. Umare A, Seyoum B, Gobena T, Mariyam TH. Hepatitis B virus infections and associated factors among pregnant women attending antenatal care clinic at Deder hospital, Eastern Ethiopia. PLoS One. (2016) 11(11):e0166936. doi: 10.1371/journal.pone.0166936
24. Assefa A, Shiferaw D, Bishaw Z, Kiros T. Seroprevalence of hepatitis B virus (HBV) and hepatitis C virus (HCV) among blood donors from Bahir Dar, Ethiopia. Adv Public Health. (2020) 2020:5073171. doi: 10.1155/2022/5073171
25. Tadiwos MB, Kanno GG, Areba AS, Kabthymer RH, Abate ZG, Aregu MB. Sero-Prevalence of hepatitis B virus infection and associated factors among pregnant women attending antenatal care services in Gedeo zone, Southern Ethiopia. J Prim Care Community Health. (2021) 12:2150132721993628. doi: 10.1177/2150132721993628
26. Mezgebo TA, Niguse S, Kahsay AG, Hailekiros H, Berhe N, Dejene TA. Hepatitis B virus infection and associated risk factors among pregnant women attending antenatal care in health facilities of Tigray, Northern Ethiopia. J Med Virol. (2018) 90(3):503–9. doi: 10.1002/jmv.24987
27. Gatheru Z, Murila F, Mbuthia J, Okoth F, Kanyingi F, Mugo F, et al. Factors associated with hepatitis B surface antigen seroprevalence amongst pregnant women in Kenya. Open J Obstet Gynecol. (2018) 8:456–67. doi: 10.4236/ojog.2018.85052
28. Kafeero HM, Ndagire D, Ocama P, Kudamba A, Walusansa A, Sendagire H. Prevalence and predictors of hepatitis B virus (HBV) infection in east Africa: evidence from a systematic review and meta-analysis of epidemiological studies published from 2005 to 2020. Arch Public Health. (2021) 79(1):167. doi: 10.1186/s13690-021-00686-1
29. Bancha B, Kinfe AA, Chanko KP, Workie SB, Tadese T. Prevalence of hepatitis B viruses and associated factors among pregnant women attending antenatal clinics in public hospitals of Wolaita zone, South Ethiopia. PLoS One. (2020) 15(5):e0232653. doi: 10.1371/journal.pone.0232653
Keywords: hepatitis B virus, viral infection, pregnant women, sero-prevalence, Southwest Ethiopia
Citation: Yosef T, Eyasu E, Shifera N, Abebe GF, Girma D, Habte A, Ahmed AF and Asefa A (2024) Determinants of hepatitis B virus infection among pregnant women in Bench Sheko zone, Southwest Ethiopia: a case-control study. Front. Glob. Womens Health 5:1453231. doi: 10.3389/fgwh.2024.1453231
Received: 22 June 2024; Accepted: 30 September 2024;
Published: 14 October 2024.
Edited by:
Stephen Kennedy, University of Oxford, United KingdomReviewed by:
Cyrille Bisseye, Université des Sciences et Techniques de Masuku, GabonElisabeth Zeuko'o Menkem, University of Buea, Cameroon
Copyright: © 2024 Yosef, Eyasu, Shifera, Abebe, Girma, Habte, Ahmed and Asefa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tewodros Yosef, dGV3b2Ryb3N5b3NlZjQ3QG10dS5lZHUuZXQ=
†ORCID:
Tewodros Yosef
orcid.org/0000-0002-3173-6753
 Tewodros Yosef
Tewodros Yosef Ephrem Eyasu1
Ephrem Eyasu1 Nigusie Shifera
Nigusie Shifera Gossa Fetene Abebe
Gossa Fetene Abebe Desalegn Girma
Desalegn Girma Aklilu Habte
Aklilu Habte Adane Asefa
Adane Asefa