- 1Department of Biomedical and Clinical Technology, Faculty of Health Sciences, Durban University of Technology, KwaZulu-Natal, South Africa
- 2Faculty of Medicine, University of Oslo, Oslo, Norway
- 3Discipline of Public Health Medicine, University of KwaZulu-Natal, Durban, South Africa
- 4Department of Pathology, Oslo University Hospital, Oslo, Norway
- 5Norwegian Centre for Imported and Tropical Diseases, Department of Infectious Diseases, Oslo University Hospital, Oslo, Norway
Background: Globally, Africa has the highest HIV, cervical cancer and schistosomiasis prevalence. Female Genital Schistosomiasis (FGS) is hypothesized to be associated with HIV and cervical atypia. Young women aged 15 and above, constituting almost 3 million of the South African population, have limited health care access and are at risk for this triad of diseases. Urinary HPV DNA analysis is a non-invasive sampling method that can assist in evaluating risk among this population. This study compared the analysis of HPV DNA in urine and cervico-vaginal lavage (CVL) samples to cytology Pap smear, Schistosoma microscopy and HIV results.
Methods: In this cross-sectional study, 235 young women aged 16 years and older from rural high schools in KwaZulu-Natal participated. HPV DNA analysis was done in urine and CVL samples. Pap smears were analysed for squamous cell atypia and urine microscopy was used for the identification of Schistosoma ova.
Results: Urinary schistosomiasis was reported in 49 (20.9%) and HIV detected in 49 (20.4%). Urinary and CVL HPV DNA was found in 147 (62.6%) and 177 (75.3%) respectively. Any atypia was detected cytologically among 173 (73.6%). The following associations were found using the Pearson Chi-Square and a Likelihood Ratio test: (a) between HIV positive status and urinary HPV DNA positive cases on both the urine (X2 = 5.007; p-value = 0.025) and (X2 = 4.264; p-value = 0.039) and between HIV positive status and CVL HPV DNA tests respectively (X2 = 5.165; p-value = 0.023) and (X2 = 4.321; p-value = 0.015), and (b) among urine HPV DNA and the CVL HPV DNA tests, where (X2 = 52.966; p-value = 0.001) and (X2 = 50.716; p-value = 0.001). Urine HPV DNA showed a sensitivity of 75.7% and specificity of 77.6% relative to the CVL HPV DNA. There was no statistical association between urinary schistosomiasis and HPV or with any atypia.
Conclusion: Urine has the potential of being optimized as an alternative and possibly more acceptable sample for HPV detection among young adolescent populations at risk in comparison to CVL samples. An integrated targeted intervention incorporating Schistosoma in addition to HPV and HIV testing needs consideration among young women in this age group from endemic areas.
1 Background
Globally, Africa has the highest prevalence of HIV, cervical cancer and schistosomiasis (1–3). Female genital schistosomiasis (FGS) is hypothesized to be associated with both HIV and HPV (4–6). Human papillomavirus (HPV) is a well-established causal agent for anogential cancers (vulva, vagina, cervix, and anus) among women, with most invasive cancers being associated with high risk (HR) HPV types 16 and 18 (7). It has been reported that women aged 15 and older who are at greatest risk for acquiring HPV make up 21.9 million of the South African population (8). Risks for HPV and other sexually transmitted infections (STIs) increase at the age of sexual debut. Unsafe sex practice was reported to be the leading risk factor requiring further public health intervention (9). It is also established that HIV positive women have an increased risk for cervical cancer (10). While HPV is known to regress in some women, the rate of regression among those who are also at risk for HIV pose additional challenges (11). Strategies to reduce cervical cancer include screening programmes, however in low to middle income countries like South Africa where the burden is high coupled with HIV, the cervical screening policy, has only recently been amended to accommodate HIV positive women (12). Another strategy that has been adopted is the HPV vaccination programme which has been rolled out in primary schools across South Africa in 2014, targeting girls aged 9–12 years, however many young women older than the vaccination age (typically the participants included in this study), but lower than the cervical screening age particularly with the same profile as the study participants remain at risk.
In Schistosoma endemic areas, it has been suggested that schistosomiasis could be a risk factor for acquisition and maintenance of HPV, thus being a co-factor for the development of cervical cancer, a major health burden in developing countries (5). The highest disease burden of HIV, HPV and FGS is borne by adolescent girls and women in sub-Saharan Africa (SSA), who are affected in their reproductive years (13). Mortality of women from HIV and cervical cancer in turn has detrimental impacts on the lives of their children and families (14).
In schistosomiasis endemic areas, urine samples are collected for microscopy. These samples commonly also contain cervical epithelial cells (which may be HPV DNA infected) as “contaminants” from the genital tract due to the close proximity of the urethra to the genital tract (15). It is in these cells originating from the genital tract that the HPV DNA can be detected in urine samples. In light of the challenges with conventional cervical cancer screening and the ethical implications of attaining genital samples from young women, HPV DNA analysis of urine samples may be a more socially acceptable, non-invasive diagnostic tool for monitoring and evaluating the HPV risk as well as to monitor the follow up of young women post HPV vaccination (16). The aim of the present study was to investigate HPV DNA analysis using polymerase chain reaction (PCR) in more easily obtainable urine samples from young women aged 16 years and above, to assess the risk of cervical cancer in schistosomiasis endemic areas.
2 Methods
2.1 Study population
This research project is nested in a large clinical study of FGS on young women, aged 16 years old and above, attending high schools in the Ugu, King Cetshwayo and Ilembe Districts, KwaZulu-Natal. Within the province of KwaZulu-Natal, Ugu District is situated along the coast south of Durban and has a population of 754.000 people, 38% are below the age of 14 years and 52% are female (17). King Cetshwayo and Ilembe Districts are situated on the north coast of KwaZulu-Natal. The King Cetshwayo District hosts a population of approximately 982,726 people. Females make up 52.6% of the population and 49.8% of the households are headed by women (17). The Ilembe District has a population of 678,048 people and comprises 52% of women (17). The areas included are endemic for both schistosomiasis and HIV. A subsample of 235 young women aged 16–23 years from the larger sample was selected for HPV DNA analysis in urine and cervico-vaginal lavage. This convenience sample of young women was selected because they were part of a cohort that were followed up.
2.2 Sample collection and storage
The urine and genital samples were collected from young sexually active women. Consenting participants underwent a semi-structured face-to-face interview in the local language isiZulu and urine samples for microscopy were collected and processed locally, prior to gynaecological examination. Cervico-vaginal lavages were collected by spraying 10 ml saline on the vaginal wall and cervix twice, and then drawn back into the syringe. Thereafter, 1 ml of each sample was then dispensed into labelled cryotubes. Pap smears were collected by scraping a wooden spatula in the cervix and the fornices, the material then smeared onto a slide which was then spray-fixed using a commercial cytological fixative for preservation and further analysis using cytology. The detailed procedures for data and sample collection and other analyses, for urine and genital samples have been described previously (18–20). HPV DNA analysis was conducted on collected urine and CVL samples. The collected samples for DNA analysis in this study were stored at −80 °C as per the storage protocols for future DNA analysis (21).
2.3 Laboratory analyses
2.3.1 HPV-DNA
Samples were prepared using the Abbot m2000 RealTime (Abbot) system for DNA extraction, and the Real Time High-Risk HPV test for detection. This assay is able to detect and type 16 and 18 as high-risk DNA types, in addition the following genotypes were detectable as high-risk HPV but not typed: 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68. For both urine and cervico-vaginal lavage, 500 µl of sample was processed using the Abbot mSample PreparationDNA System. Once DNA was isolated, samples were then ready for the amplification master mix and thereafter the Abbott RealTime HR-HPV assay protocol was followed (22). In the present study, the PCR analysis was conducted at a private accredited diagnostic laboratory in South Africa using their calibrated routine protocol for HPV DNA analysis.
2.3.2 Urine microscopy
Urine samples were processed in the field site laboratory by a laboratory technician. Urine samples for microscopy were preserved with 1 ml of 2% tincture of merthiolate in 5% formalin solution (23). The samples were spun for 10 min at 4,000 rates per min (rpm) and the sediment examined microscopically, magnification with objective 10, by trained field workers. The samples underwent quality control by an independent microscopist on 10% of randomly chosen samples.
2.3.3 Cytology
The Pap smears were stained and evaluated by a trained cytologist and reported in accordance with the Bethesda system (24). This system uses the following categories for atypia which could be reported as either atypical squamous cells of undetermined significance (ASCUS), low grade squamous intraepithelial lesion (LSIL) and high grade squamous intraepithelial lesion (HSIL). Each of these categories includes HPV. ASCUS is an entity which is used when the distinction between reactive changes or LSIL is difficult to determine cytologically (25). For the analysis in the present study, the category of “any atypia” was used to describe the collective sum of the ASCUS, LSIL and HSIL cases.
2.4 Data analysis
The aim of this study was to investigate whether the urinary HPV DNA could be an indicator of gynaecological infection in young rural women in Schistosoma and HIV endemic South Africa. As such, a questionnaire was used as the primary source of data collection in this study as well as the results from laboratory analysis. Laboratory analysis included the urine and CVL HPV DNA testing, urine microscopy, cytology and other STI results. Following the data collection, the researchers cleaned, organized, and then analysed the data using SPSS software (version 28.0). To analyse the collated data and demonstrate the findings of this study, the researchers used both descriptive and inferential statistical matrices.
Descriptive statistical techniques were used to measure the central tendencies and dispersion among variables. On the other hand, to demonstrate the relationship among variables and assess the degree of association, probability, and nature of relationship among variables in the dataset, inferential statistical techniques were used. All calculated inferential tests were based on the traditional significance level of p = <0.05.
3 Results
3.1 Respondents' profile and schistosomiasis risk factors
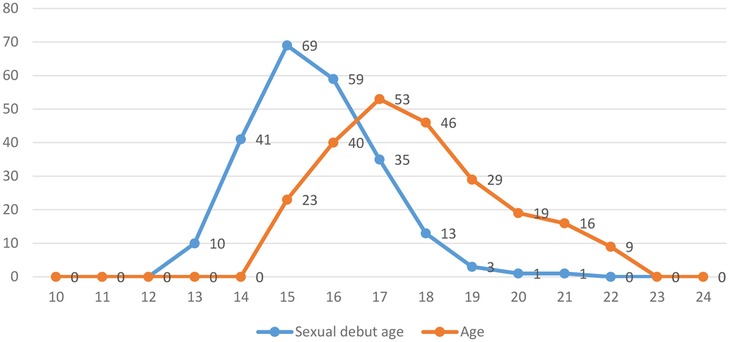
The results in Figure 1 and Table 1 show that most of respondents are either 18 or more years old (n = 173; 73.2%), with a mean (M) age of 18.78 years and a standard deviation (SD) of 1.85 years. Likewise, the results in Table 1 and Figure 1 show that most of the young women began their sexual debut before the age of 18 (n = 179; 76.2%), where the M = 16.34 years and SD = 2.30 years. The results in Figure 1 further indicates that the minimum and maximum sexual age debut was 14 years and 22 years, respectively. The majority of the study participants indicated that they had only one sexual partner during the last month (n = 143; 60.9%).
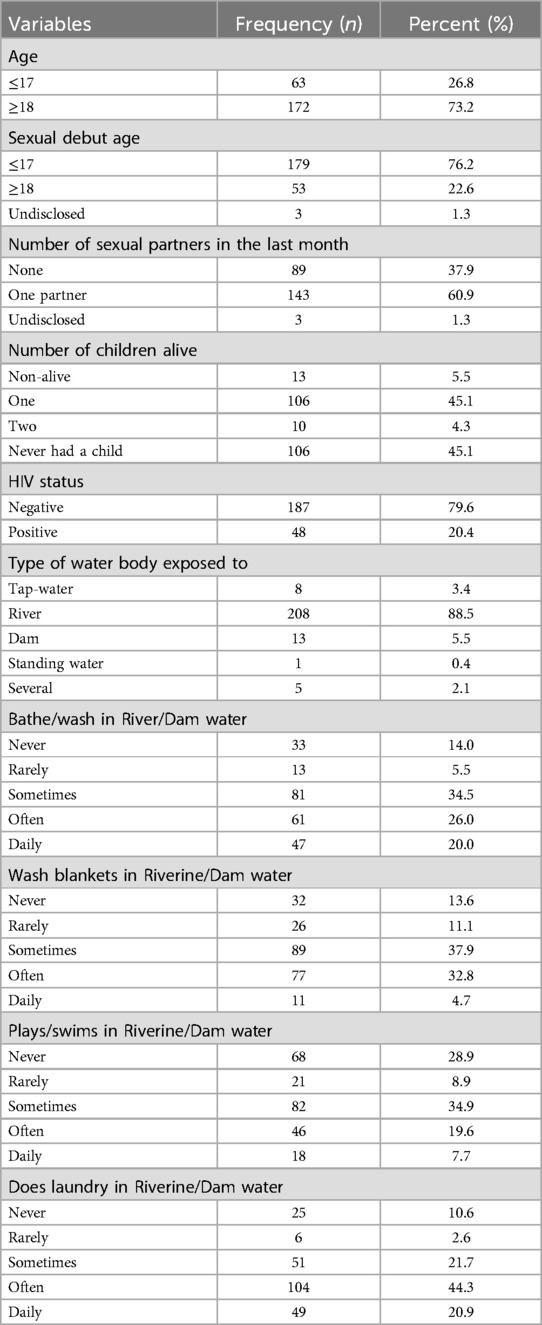
Table 1. Shows demographic profile and the exposure to risk factors for schistosomiasis and sexually transmitted infections (n = 235).
Since there are several water sources that serves as risk factors for schistosomiasis among rural women, the results in this study revealed that most of the respondents (n = 208; 88.5%) had come in contact with water from the river and about (n = 13; 5.5%) indicated that they had come in contact with water from dams. The results for the frequencies of their contact and activities (such as laundry, bathing, swimming or playing) with these water bodies are detailed in Table 1.
3.2 Detection of HPV DNA, cytology and urinary schistosomiasis
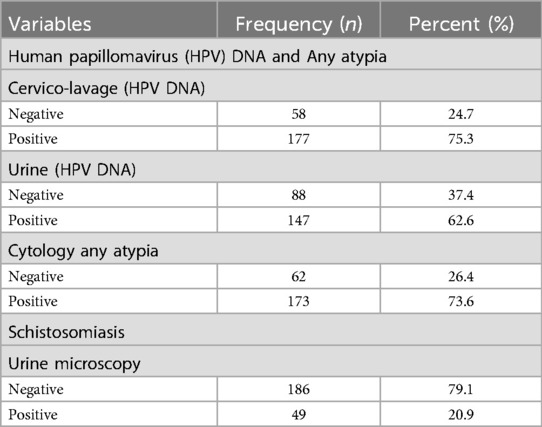
The Real Time High-Risk HPV test was able to identify and type HPV-16 and HPV-18 as well as the following range of other high-risk DNA genotypes comprising the following: HPV-31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68. All the respondents in this study were tested for HPV DNA using CVL and urine, for any atypia using cytology Pap smears and for schistosomiasis using microscopy as shown in Table 2. The results from the PCR analysis revealed that (n = 177; 75.3%) of the participants tested HPV DNA positive in CVL, and (n = 147; 62.6%) of the sample tested positive HPV DNA in urine, while (n = 173; 73.6%) were tested positive for any atypia using cytology samples.
Of the 75.3% HPV DNA cervico-vaginal lavage positive participants, 51.5% of them were positive for other high-risk HPV, 12.5% were positive for HPV 16 and other high-risk-HPV, 4.7% were positive for HPV 18 and other high-risk HPV, 3.0% were positive for HPV 16, 2.1% were positive for HPV 18, and 1.3% were positive for HPV 16, HPV 18 and other high-risk HPV types. Of the 62.6% of the urine HPV PCR positive participants, 43.0% of them were positive for other high-risk HPV, 9.8% tested positive for HPV 16 and other high-risk HPV, 4.3% were positive for HPV 18 and other high-risk HPV, 3.0% were positive for HPV 18, 2.1% were positive for HPV 16, and 0.4% were positive for HPV 16, HPV 18 and other high-risk HPV.
Furthermore, all the respondents in this study were tested for schistosomiasis using urine microscopy as shown in Table 2. Here, the urine microscopy diagnostic results revealed that (n = 49; 20.9%) of the participants tested positive for schistosomiasis.
3.3 Urinary HPV DNA detection in comparison with the respondents' profile, sexual behaviour and sexual health
The results of the urine PCR test identified 147 cases (62.6%) as positive for HPV (Table 2). Of the 147 urine HPV DNA positive cases, 48 (76.2%) were ≤17 years old, 117 (65.4%) had their sexual debut at ≤17 years old, 34 (77.3%) were tested HIV positive, 60 (67.4%) had no sexual partner at the time of data collection, and 85 (59.4%) of them had one sexual partner at the time of the data collection (see Table 3).
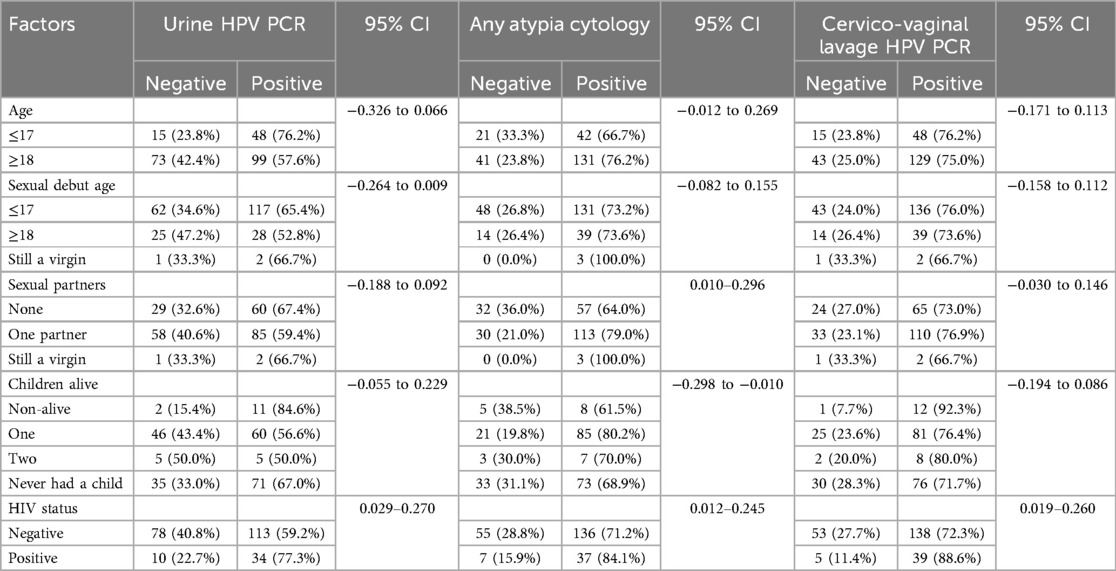
Table 3. Shows the cytology and polymerase chain reaction (PCR) test results for HPV and any atypia relative to respondents’ profile, sexual behaviour and sexual health (n = 235).
In addition, the cytology results for any atypia identified 173 cases (73.6%) as positive for either ASCUS, LSIL or HSIL. These positive results were obtained in cytology samples from 173 suspected HPV participants. Of these 173 positive cases, 131 (76.2%) were ≥18 years old, 131 (73.2%) had their sexual debut at ≤17 years old, 37 (84.1%) were tested positive for HIV, 113 (79.0%) of them had one sexual partner at the time of data collection, and 57 (64.0%) had no sexual partner when the data was collected.
On the other hand, the results of the PCR examination using the CVL identified 177 (75.3%) as positive cases for HPV from the sample (Table 2). Of the 177 positive cases, 129 (75.0%) were ≥18 years old, 136 (76.0%) had their sexual debut at ≤17 years old, 39 (88.6%) were tested positive for HIV, 110 (76.9%) had one sexual partner at the time of data collection, and 65 (73.0%) had no sexual partner at the time of data collection (see Table 3).
These results further suggested that there is a significant statistical relationship between HPV and the respondents’ profile, sexual behaviour and sexual health. As determined by a Pearson Chi-Square test and a Likelihood Ratio test, the results suggested that there is a strong link between respondents' age category and urine HPV DNA, with (X2 = 6.834; p-value = 0.009) and (X2 = 6.062; p-value = 0.014), respectively. Thus, these findings support the descriptive results of this study, and suggest that participants of lower ages (≤17 years old) are associated with positive cases of HPV based on urine PCR. This is an indication that younger respondents are more likely to be tested positive for HPV DNA in urine. Similarly, an association was found between respondents' number of sexual partners and HPV. The results suggested that there is a strong statistical significance between respondents with one active sexual partner and positive cases of any atypia cytology and urine HPV DNA diagnostics, where (X2 = 7.424; p-value = 0.024) and (X2 = 8.027; p-value = 0.018) as determined by a Pearson Chi-Square test and a Likelihood Ratio test, respectively. As such, these findings support the descriptive results of this study, and suggest that participants with active sexual partners are associated with positive cases of HPV DNA and any atypia as diagnosed by cytology. In a similar fashion, the results revealed that there is a statistical association between respondents' HIV positive status and HPV positive cases based on both the urine and the CVL PCR tests. The results from the Pearson Chi-Square test and the Likelihood Ratio test suggested that there is a strong link between respondents' HIV positive status and HPV DNA diagnostics using urine, with (X2 = 5.007; p-value = 0.025) and (X2 = 4.264; p-value = 0.039), respectively. Similarly, the findings from the Pearson Chi-Square test and the Likelihood Ratio test suggested that there is a statistical relationship between respondents' HIV positive status and PCR HPV diagnostics based on the CVL DNA diagnosis, with (X2 = 5.165; p-value = 0.023) and (X2 = 4.321; p-value = 0.015), respectively. Thus, these findings support the descriptive results of this study, and propose that participants with positive HIV status are associated with positive cases of HPV.
No statistical association was found between respondents' age, sexual age debut, number of sexual partners, number of children alive, and HPV positive cases based on the CVL DNA diagnosis. Likewise, no association was found between respondents' sexual age debut, number of children alive and HPV based on urine PCR and cytology.
3.3.1 Sensitivity and specificity of urine HPV PCR in comparison with cervico-vaginal lavage HPV PCR
The findings in Table 4 shows the sensitivity and specificity of urine HPV DNA test of the sample in relation to the HPV DNA diagnoses based on the CVL. The results of the examination using the urine, showed a sensitivity of 75.7% and specificity of 77.6% relative to the CVL HPV DNA test.
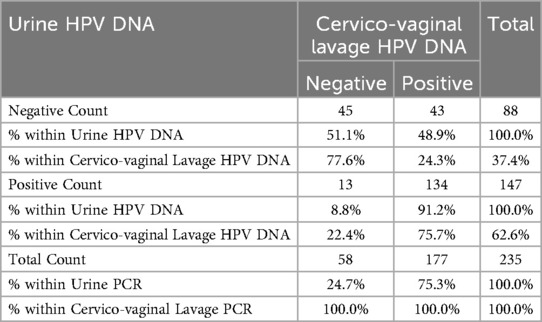
Table 4. Sensitivity and specificity of HPV urine PCR test in comparison with the HPV cervico-vaginal lavage PCR test (n = 235).
Using the CVL HPV DNA test as a gold standard, Table 4 also shows the positive predictive value (i.e., the probability that a positive test means that they have the disease) and the negative predictive value (i.e., the probability that a negative test means that they really do not have the disease). These results revealed that using the CVL HPV DNA test, the positive predictive value of the participants that really have the disease was 91.2%; while the negative predictive value of the participants that do not really have the disease was 51.1%.
The results in Table 4 further shows the prevalence of HPV (i.e., the proportion of the participants in the population that have the disease) in the study population was 75.3%. Therefore, the CVL HPV DNA test had a very good measure of agreement (k = 0.127; p-value = 0.050) relative to the urine HPV DNA test.
Table 5 shows the findings for the sensitivity and specificity of HPV based on any atypia as detected by cytology in relation to the CVL HPV DNA. The results from the any atypia showed a sensitivity of 76.8% and specificity of 36.2% relative to the HPV DNA test using CVL. Using the CVL HPV DNA test as a gold standard, Table 5 equally demonstrates the positive predictive value (i.e., the probability that a positive test means that they have the disease) and the negative predictive value (i.e., the probability that a negative test means that they really do not have the disease). The findings revealed that by using the CVL HPV DNA test, the positive predictive value of the participants that really have the disease was 78.6%; while the negative predictive value of the participants that do not really have the disease was 33.9%. The results in Table 5 further indicates the prevalence of HPV based on any atypia cytology test (i.e., the proportion of the participants in the population that have the disease). These results suggested that the prevalence of HPV in the population was 75.3%. Therefore, the CVL PCR test had a very good measure of agreement (k = 0.127; p-value = 0.050) relative to any atypia as seen in cytology.
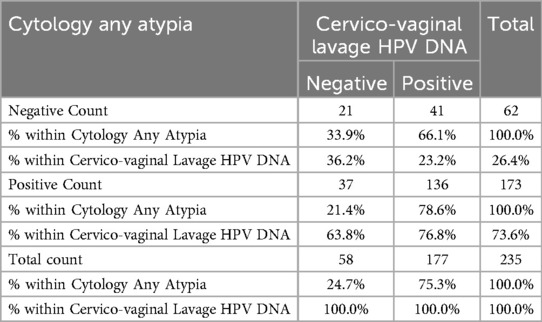
Table 5. Sensitivity and specificity of HPV based on any atypia cytology test in comparison with the HPV DNA test (n = 235).
In addition, the findings of this study further reveal that there is a significant statistical relationship between the two HPV DNA diagnostic test results. As determined by a Pearson Chi-Square test and a Likelihood Ratio test, the results suggested that there is a strong association between urine HPV DNA and the CVL HPV DNA, where (X2 = 52.966; p-value = 0.001) and (X2 = 50.716; p-value = 0.001), respectively. This is an indication that urine HPV DNA test can be used in placed of the CVL PCR test for diagnosing HPV among women. Similarly, an association was found between any atypia as seen in cytology and the CVL DNA test results, where (X2 = 3.826; p-value = 0.050) as determined by a Pearson Chi-Square test. As such, these findings indicate that any atypia diagnosed by cytology can be use in placed of the CVL DNA test for diagnosing HPV among women. On the contrary, no statistical association was found between urine HPV PCR and urinary Schistosomiasis as detected by microscopy.
4 Discussion
Young women in South Africa are known to be at high risk for HIV. To some extent there has been advocacy around HIV over the years, yet the prevalence is still rife. Cervical cancer is also known to be a leading cancer among the South African population, despite it being a preventable disease. In a bid to curb cervical cancer, the South African Department of Health rolled out a school-based HPV vaccination programme among school girls aged 10–12 years in 2014, while this programme has been in existence for several years, there are still several challenges (26). The HPV vaccination programme only targets girls within the specified age, while this has been done in alignment with resources available and other considerations, the HPV disease burden may be continuing amongst those who have missed the vaccination age. The association between HIV and the progression to invasive cervical cancer linked to high risk HPV strains is also another co-factor (11). FGS on the other hand is a highly neglected disease, which is a challenge to diagnose and treat. The link between FGS and HIV has been established as well as the link between HIV and HPV. Convincing evidence that HPV and FGS are associated has however, been more difficult to ascertain thus far.
Based on our findings, the young women included in the study were found to have risk factors for all three of the aforementioned diseases as determined by their demographic profile, history of water contact and sexually transmitted infection status. The risk factors include reported sexual activity among majority of the participants, with the minimum and maximum reported sexual debut age being 14 and 22 years respectively. They also reported varying frequencies and duration of water contact with rivers or dams, which are risk factors for schistosomiasis, with the prevalence of 20.9% detected using microscopy. Based on molecular testing for HPV DNA, the prevalence of HPV infection among our study population was found to be 75.3% and the overall HIV prevalence was 20.4%. Presently routine HIV testing, reproductive health care and deworming programmes are limited or non-existent for young women at risk of these diseases (4).
HPV is known to have a higher prevalence among younger sexually active women in comparison to older women and is known to regress with age in some (27). The extent of regression in a population who are already at risk for HIV, schistosomiasis and other STI's is however, largely unknown (28). Our study revealed that that participants of lower ages (≤17 years old) are associated with positive cases of HPV based on urine PCR—confirming the arguments established in other studies that younger women are more likely to test positive for HPV (in our case HPV DNA in urine) (29). An association was also found in our study between respondents' number of sexual partners and HPV—indicating that there is a strong statistical significance between respondents with one active sexual partner and positive cases of any atypia cytology and urine HPV DNA diagnostics. Consequently, the findings from our study revealed that being HIV positive is strongly associated with HPV DNA diagnostics using urine. Similarly, the results of the current study found that HIV positive status and PCR HPV diagnostics based on CVL DNA diagnosis are strongly correlated. Although, the collection of gynaecological samples in young women, requires logistical infrastructure that is not easily available in the field. The aim of this study therefore was to determine if urine, which is a relatively easy sample to collect can be used as an indirect indicator for HPV DNA detection among young women also at risk for FGS.
4.1 Urine HPV PCR analysis
In these young South African women, we found that almost three quarters of the study population tested positive for HPV DNA and based on our results, urine analysis was a good predictor for HPV positivity in gynaecological samples. This has been seen in several prior studies using various types of gynaecological and urine samples either clinician collected or self-collected (30–32). In the present study, correlation of HPV DNA detection among CVL and urine samples indicated that urine could be used as an alternate sample to CVL for HPV detection since there was a good measure of agreement (k = 0.127; p-value = 0.050) relative to the urine HPV DNA test.
When investigating the link between risk factors such as age and age of onset of sexual activity and HPV, active sexual partners and HIV, associations were found with urine detected HPV and or squamous atypia. Our investigation of the risk factors however did not take into account the sexual activity of the respondent's partners, who are equally responsible for transmitting HPV, HIV and other STI's as well.
While it is known that HPV can regress, it has been found that HR HPV strains are more likely to persist and progress to invasive cervical cancer and it is also known that women with HIV are more likely to have HR HPV strains (28). In the present study the following HR strains were detected: HPV 16, 18 as well as the following range of “other high-risk” genotypes: 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68, among 75.3% and 62.6% of the women in their CVL and urine samples respectively. These strains have been identified to have varying progression rates to invasive cancer (33). A point of concern is that women in this age range, who have not qualified or missed HPV vaccination, do not qualify for cervical screening and are also at risk for HIV, may be at a higher risk for progression to invasive cancer of the cervix.
The South African cervical cancer screening programme has been in existence for 23 years, which was traditionally limited to women aged 30 and above. It was recently amended to accommodate HIV positive women so that all HIV positive women are able to be screened for cervical cancer at diagnosis and if found to have any positive atypia, they will be screened annually and if negative every three years as per the WHO guidelines (8, 12).
Younger women have limited reproductive healthcare options in South Africa, and many do not know their HIV status to be eligible for cervical screening despite their known proneness to acquiring HIV and other STIs. In addition to cervical cancer progression, young women are prone to have teenage pregnancies and are at risk for adverse pregnancy related events including HIV transmission from mother-to-child (29, 34). While HPV vaccination was rolled out in South Africa in 2014 to primary school girls aged 9–12, its impact on cervical cancer may only be seen in years to come (8, 12).
Consequently, based on the preliminary findings from the present study using urine to detect HPV DNA could be an alternative to genital sampling among women in resource limited settings—since the results of our study show that urine HPV DNA correlated well with CVL HPV DNA (35). Urine is an easily obtainable sample and our findings of 75.7% sensitivity and 77.6% specificity with the additional good measure of agreement with the CVL HPV DNA testing has potential to be optimized for HPV testing using more cost-efficient methods and applied in low resource countries amongst high risk populations. In the present study the HPV DNA analysis was performed on samples post collection in a laboratory setting, however there are newly available point of care diagnostic methods which could be used in clinics (36). In some low resource countries, where conventional screening with cytology is limited, HPV testing has been adopted as part of a screen and treat campaigns. HPV DNA testing has the potential to provide results with a faster turn-around time and has been found to have superior sensitivity to conventional cytology (37). In addition to HPV DNA testing, the WHO recommends onsite treatment and follow-up facilities (35). One of the drawbacks to caution against is over-treatment for otherwise reversible HPV infection along with the risk of HIV progression due to the adverse effects of the treatment among women with HIV (38).
4.2 HPV and schistosomiasis
While the natural progression to invasive cancer is known to take years to decades, maintaining a balance in terms of avoiding over-treatment or misdiagnosis of lesions is required. In women who are at risk for FGS it is necessary to ensure that FGS is considered a clinical differential diagnosis to cervical cancer and pre-cancer, since FGS often is misdiagnosed or underdiagnosed (39). Clinical lesions on the cervix due to HPV or cervical cancer and FGS may be difficult to distinguish clinically. While it is hypothesized that there is a possible association of FGS and cervical atypia it has been difficult to prove a strong association between these entities. In the present study there was no distinct association between the two entities. However, due to the genital tract being a common site for these two entities, we cannot ignore either and therefore need to ensure that we are able to differentiate them. Treatment, requires resources and infrastructure (20).
While there was no statistical association in the present study between urinary schistosomiasis and HPV, it must however be noted that genital schistosomiasis has been found to be associated with HIV in several studies, this cannot be ignored due to the geographical overlap in disease burden with these diseases and HIV (5, 40). FGS is also a neglected entity which is rarely diagnosed, this is confounded by limitations in health care workers' knowledge of the entity at public health facilities, limited resources, limited knowledge among women at risk, and the reduced health seeking behaviour amongst women for STIs and other genital symptoms (5, 41, 42). This is possibly why no association between schistosomiasis and squamous cell atypia was found due to the small sample size used in the present study.
Validating the use of urine samples for HPV DNA and Schistosoma DNA genotyping using point of care testing could be used as an alternative to genital sampling. Such point of care tests could be useful in providing DNA evidence of the infective agents to inform the diagnosis. Validation with rapid point of care HPV and Schistosoma DNA testing will be useful since such testing could be used to complement other diagnostic tests such as colposcopy and imaging techniques that are being developed for FGS (43).
4.3 HPV and HIV
Considering the supporting finding that there was a statistical association between respondents’ HIV positive status and HPV PCR positive cases based on both the urine and the cervico-vaginal lavage, warrants intervention. It may be argued that HPV in its natural course has the tendency to clear, but it has been found that this tendency is halved among people co-infected with HIV, likewise clearance is decreased in women with FGS (44). In turn women infected with HIV and HPV have been found to progress much quicker to invasive cancer (44). In young HIV positive women, who may not be able to get access to gynaecological examinations, urine HPV analysis may be a practical way of identifying those with HR HPV strains in order to refer them for further management. Cervical cancer is treatable when detected in the pre-invasive stages, however, sadly, for most women in low to middle income countries, it is only detected when it is invasive and the survival chances are limited. Furthermore, with the advent of HIV and other co-factors, cervical cancer is now ranked third most common in women 45 years and younger (45).
4.4 Study limitations
The urine and cervico-vaginal lavage samples were collected several years ago and were in storage at −80 °C, it is possible that the samples used in this investigative study were not optimal, however it is well documented that DNA, can be preserved for several years (21) The sample was also a convenience sample, larger studies are recommended to fully validate urine as an alternative to gynaecological samples. The laboratory testing was done at a private diagnostic laboratory, and the current assay was validated for gynaecological samples and not validated for urine PCR. However, urinary HPV DNA analyses showed an association with the cervico-vaginal lavage results and there was a good measure of agreement between the tests. The cytology results were not validated with histology. Due to this being a cross- sectional study, it was not possible to determine in those who were both positive for HIV and HPV, which disease had occurred first.
5 Conclusion
While it may be possible to raise awareness on HIV transmission, and to offer counselling and antiretroviral therapy to try to reduce HIV transmission, coupled with mass anti-schistosomal treatment to these young women, they are currently neglected when it comes to HPV or cervical cancer awareness and treatment options. This is simply because the cohort of young women like those included in this study, do not meet the criteria for HPV vaccination as per South African policy.
Using urine samples as an alternative to genital sampling may be promising, to assess the risk of HPV, though DNA analysis. It is recommended that young women especially in the 16–24 age group who are from Schistosoma endemic areas are targeted with specific public health interventions to increase their awareness of this triad of diseases for which they are at risk for. Efforts to raise awareness of HPV and schistosomiasis at community and school level are vital. Strategies to strengthen health seeking behaviour and reproductive health services specifically geared for adolescent women aged 16 and above should be committed to by the Department of Health (to upscale youth friendly reproductive health services) and the Department of Education (to upscale reproductive health literacy at high school level) respectively. Additionally, further research needs to be done to develop point of care urine HPV and Schistosoma assays, which may aid in rapid diagnosis. It will also be necessary for the anti-parasitic treatment to be provided in schools and HPV vaccination to be available to not only girls aged 9–12, but to boys as well and to consider vaccination among the slightly older age group of women who are also at high risk.
While adolescent health services do exist in South Africa, they are limited in number and also not optimally utilised by youth (46). While HIV services and family planning services may exist in adolescent health facilities, services related to cervical cancer and FGS are limited.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This project is nested in a large study entitled “Schistosomiasis in young women and girls in KwaZulu-Natal, manifestations, effect of treatment, and association with HIV”. Ethical approval was obtained from the Biomedical Ethics Research Committee of the University of KwaZulu-Natal (Ref BF029/07). Ethical approval was given by the Department of Health, Pietermaritzburg, KZN, February 3rd 2009, Reference HRKM010-08. REK Øst-Norge, the Norwegian ethics committee, gave ethical clearance Ref 469-07066a1.2007.535, September 17th 2007. In the present study, ethical clearance was received for the laboratory diagnostic aspects of this study from the Biomedical Ethics Research Committee of the University of KwaZulu-Natal (BF057/11). All data and sample collection procedures were collected in accordance with relevant ethical guidelines and regulations. Informed consent was obtained from all participants and or their legal guardians where required. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable data included in this article.
Author contributions
PP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HG: Investigation, Writing – review & editing, Writing – original draft. MT: Conceptualization, Writing – original draft, Writing – review & editing. BR: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. EK: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research received funding from the European Research Council under the Seventh Framework Programme, ERC Grant agreement no. PIRSES-GA-2010-269245, University of Copenhagen with the support from the Bill and Melinda Gates Foundation grant no. OPPGH5344, Norwegian Research Council ref. 213702/H10, the South Eastern Regional Health Authority, Norway (no. 2016055, 2011073, 2012032), National Research Foundation (NRF) SGD14052367807 and NRF FUNDING INSTRUMENT (Post-PhD Track) no. TK170519231548.
Acknowledgments
The authors would like the thank Dr IK Christiansen, Professor OH Ambur Norwegian HPV Reference Laboratory, Dept. Microbiology and Infection Control, Akershus University Hospital, Lørenskog, Norway for their introduction and guidance on the prospect of conducting HPV DNA analysis in urine samples. Recruitment, data collection and data management would not have been possible without the tremendous support from the BRIGHT Project Team South Africa, with special mentioning of Svein Gunnar Gundersen and Birgitte Jyding Vennervald as mentors on the project. We are most grateful to the study community district including the KwaZulu-Natal, Department of Health and Department of Education.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DNA, deoxy ribonuclease; FGS, female genital schistosomiasis; HPV, human papillomavirus; HR, high risk; HIV, human immunodeficiency virus; PCR, polymerise chain reaction; SIL, squamous intraepithelial lesion; LSIL, low grade squamous intraepithelial lesion; HSIL, high grade squamous intraepithelial lesion; SCA, squamous cell atypia; STIs, sexually transmitted infections; SSA, sub-saharan africa.
References
4. Engels D, Hotez PJ, Ducker C, Gyapong M, Bustinduy AL, Secor WE, et al. Integration of prevention and control measures for female genital schistosomiasis, HIV and cervical cancer. Bull World Health Organ. (2020) 98(9):615. doi: 10.2471/BLT.20.252270
5. Rafferty H, Sturt A, Phiri CR, Webb EL, Mudenda M, Mapani J, et al. Association between cervical dysplasia and female genital schistosomiasis diagnosed by genital PCR in Zambian women. BMC Infect Dis. (2021) 21(1):1–9. doi: 10.1186/s12879-021-06380-5
6. Patel P, Rose CE, Kjetland EF, Downs JA, Mbabazi PS, Sabin K, et al. Association of schistosomiasis and HIV infections: a systematic review and meta-analysis. Int J Infect Dis. (2021) 102:544–53. doi: 10.1016/j.ijid.2020.10.088
7. Bruni L, Albero G, Serrano B, Mena M, Collado JJ, Gómez D, et al. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Spain: Human Papillomavirus and Related Diseases in South Africa (2021).
8. ICO/IARC. South Africa Human Papillomavirus and Related Cancers, Fact Sheet 2021. Barcelona: HPV Information Centre (2021).
9. Bradshaw D, Pillay-Van Wyk V, Neethling I, Roomaney R, Cois A, Joubert J, et al. Second comparative risk assessment for South Africa (SACRA2) highlights need for health promotion and strengthened surveillance. S Afr Med J. (2022) 112:556–70.36458357
10. Stelzle D, Tanaka LF, Lee KK, Khalil AI, Baussano I, Shah ASV, et al. Estimates of the global burden of cervical cancer associated with HIV. Lancet Glob Health. (2021) 9(2):e161–e69. doi: 10.1016/S2214-109X(20)30459-9
11. Liu G, Sharma M, Tan N, Barnabas RV. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. Aids. (2018) 32(6):795–808. doi: 10.1097/QAD.0000000000001765
13. Bustinduy AL, Randriansolo B, Sturt AS, Kayuni SA, Leutscher PDC, Webster BL, et al. An update on female and male genital schistosomiasis and a call to integrate efforts to escalate diagnosis, treatment and awareness in endemic and non-endemic settings: the time is now. Adv Parasitol. (2022) 115:1–44. doi: 10.1016/bs.apar.2021.12.003
14. Ngcobo N, Jaca A, Iwu-Jaja CJ, Mavundza E. Reflection: burden of cervical cancer in sub-Saharan Africa and progress with HPV vaccination. Curr Opin Immunol. (2021) 71:21–6. doi: 10.1016/j.coi.2021.03.006
15. Pattyn J, Van Keer S, Téblick L, Van Damme P, Vorsters A. HPV DNA detection in urine samples of women:‘an efficacious and accurate alternative to cervical samples?’. Expert Rev Anti-Infect Ther. (2019) 17(10):755–57. doi: 10.1080/14787210.2019.1668776
16. Sayed S, Chung M, Temmerman M. Point-of-care HPV molecular diagnostics for a test-and-treat model in high-risk HIV populations. Lancet Glob Health. (2020) 8(2):e171–e72. doi: 10.1016/S2214-109X(19)30559-5
17. COGTA. Profile Analysis and District Development Model. King Cetshwayo District: Cooperative Governance and Traditional Affairs (2020).
18. Holmen SD, Kleppa E, Lillebø K, Pillay P, Van Lieshout L, Taylor M, et al. The first step toward diagnosing female genital schistosomiasis by computer image analysis. Am J Trop Med Hyg. (2015) 93(1):80. doi: 10.4269/ajtmh.15-0071
19. Pillay P, Van Lieshout L, Taylor M, Sebitloane M, Zulu SG, Kleppa E, et al. Cervical cytology as a diagnostic tool for female genital schistosomiasis: correlation to cervical atypia and schistosoma polymerase chain reaction. Cytojournal. (2016) 13:10. doi: 10.4103/1742-6413.180784
20. Nemungadi TG, Kleppa E, van Dam GJ, Corstjens PLAM, Galappathi-Arachige HN, Pillay P, et al. Female genital schistosomiasis lesions explored using circulating anodic antigen as an indicator for live schistosoma worms. Front Trop. Dis. (2022) 3:26. doi: 10.3389/fitd.2022.821463
21. Holland NT, Smith MT, Eskenazi B, Bastaki M. Biological sample collection and processing for molecular epidemiological studies. Mutat Res. (2003) 543(3):217–34. doi: 10.1016/S1383-5742(02)00090-X
22. Huang S, Tang N, Mak W-B, Erickson B, Salituro J, Li Y, et al. Principles and analytical performance of Abbott RealTime high risk HPV test. J Clin Virol. (2009) 45:S13–7. doi: 10.1016/S1386-6532(09)70003-4
23. Thomassen Morgas DE, Kvalsvig SG, Gundersen M, Kjetland EF, Kvalsvig JD, Taylor M, et al. Schistosomiasis and water-related practices in school girls in rural KwaZulu-Natal, South Africa. S Afr J Epidemiol Infect. (2010) 25(4):30–3. doi: 10.1080/10158782.2010.11441406
24. Kurman RJ. The Bethesda System for Reporting Cervical/vaginal Cytologic Diagnoses: Definitions, Criteria, and Explanatory Notes for Terminology and Specimen Adequacy. Germany: Springer Science & Business Media (2012).
25. Solomon D, Schiffman M, Tarone R. Comparison of three management strategies for patients with atypical squamous cells of undetermi baseline results from a randomized trial. J Natl Cancer Inst. (2001) 93(4):293–99. doi: 10.1093/jnci/93.4.293
26. Amponsah-Dacosta E, Blose N, Nkwinika VV, Chepkurui V. Human papillomavirus vaccination in South Africa: programmatic challenges and opportunities for integration with other adolescent health services? Front Public Health. (2022) 10:799984. doi: 10.3389/fpubh.2022.799984
27. Schiffman M, Wentzensen N, Wacholder S, Kinney W, Gage JC, Castle PE, et al. Human papillomavirus testing in the prevention of cervical cancer. J Natl Cancer Inst. (2011) 103(5):368–83. doi: 10.1093/jnci/djq562
28. Taku O, Businge CB, Mdaka ML, Phohlo K, Basera W, Garcia-Jordon M, et al. Human papillomavirus prevalence and risk factors among HIV-negative and HIV-positive women residing in rural Eastern Cape, South Africa. Int J Infect Dis. (2020) 95:176–82. doi: 10.1016/j.ijid.2020.02.051
29. Mbulawa ZZ, Van Schalkwyk C, Hu N-C, Meiring TL, Barnabas S, Dabee S, et al. High human papillomavirus (HPV) prevalence in South African adolescents and young women encourages expanded HPV vaccination campaigns. PLoS One. (2018) 13(1):e0190166. doi: 10.1371/journal.pone.0190166
30. Arbyn M, Peeters E, Benoy I, Van den Broek D, Bojers J, De Sutter P, et al. VALHUDES: a protocol for validation of human papillomavirus assays and collection devices for HPV testing on self-samples and urine samples. J Clin Virol. (2018) 107:52–6. doi: 10.1016/j.jcv.2018.08.006
31. Shin HY, Lee B, Hwang S-H, Lee DO, Sung NY, Park JY, et al. Evaluation of satisfaction with three different cervical cancer screening modalities: clinician-collected pap test vs. HPV test by self-sampling vs. HPV test by urine sampling. J Gynecol Oncol. (2019) 30(5):e76. doi: 10.3802/jgo.2019.30.e76
32. Nutthachote P, Oranratanaphan S, Termrungruanglert W, Triratanachat S, Chaiwongkot A, Baedyananda F, et al. Comparison of detection rate of high risk HPV infection between self-collected HPV testing and clinician-collected HPV testing in cervical cancer screening. Taiwan J Obstet Gynecol. (2019) 58(4):477–81. doi: 10.1016/j.tjog.2019.05.008
33. Demarco M, Hyun N, Carter-Pokras O, Raine-Bennett TR, Cheungh L, Chen X, et al. A study of type-specific HPV natural history and implications for contemporary cervical cancer screening programs. EClinicalMedicine. (2020) 22:00293. doi: 10.1016/j.eclinm.2020.100293
34. Ahinkorah BO, Kang M, Perry L, Brooks F, Hayen A. Prevalence of first adolescent pregnancy and its associated factors in sub-Saharan Africa: a multi-country analysis. PLoS One. (2021) 16(2):e0246308. doi: 10.1371/journal.pone.0246308
35. Kunckler M, Schumacher F, Kenfack B, Cararino R, Viviano M, Tincho E, et al. Cervical cancer screening in a low-resource setting: a pilot study on an HPV-based screen-and-treat approach. Cancer Med. (2017) 6(7):1752–61. doi: 10.1002/cam4.1089
36. Kelly H, Mayaud P, Segondy M, Pai NP, Peeling RW. A systematic review and meta-analysis of studies evaluating the performance of point-of-care tests for human papillomavirus screening. Sex Transm Infect. (2017) 93(S4):S36–45. doi: 10.1136/sextrans-2016-053070
37. Hu L, Bell D, Antani S, Xue Z, Yu K, Horning NP, et al. An observational study of deep learning and automated evaluation of cervical images for cancer screening. J Nat Cancer Inst. (2019) 111(9):923–32. doi: 10.1093/jnci/djy225
38. Dreyer G. Clinical implications of the interaction between HPV and HIV infections. Best Pract Res Clin Obstet Gynaecol. (2018) 47:95–106. doi: 10.1016/j.bpobgyn.2017.08.011
39. O’Brien DP, Ford N, Djirmay AG, Calmy A, Vitoria M, Jensen TO, et al. Female genital schistosomiasis and HIV: research urgently needed to improve understanding of the health impacts of this important coinfection. JAIDS J Acquir Immune Defic Syndr. (2019) 80(5):489–93. doi: 10.1097/QAI.0000000000001957
40. Kleppa E, Klinge KF, Galaphaththi-Arachchige HN, Holmen SD, Lillebo K, Onsrud M, et al. Schistosoma haematobium infection and CD4+ T-cell levels: a cross-sectional study of young South African women. PLoS One. (2015) 10(3):e0119326. doi: 10.1371/journal.pone.0119326
41. Comins CA, Rucinski KB, Baral S, Abebe SA, Mulu A, Schwartz SR. Vulnerability profiles and prevalence of HIV and other sexually transmitted infections among adolescent girls and young women in Ethiopia: a latent class analysis. PLoS One. (2020) 15(5):e0232598. doi: 10.1371/journal.pone.0232598
42. Hopkins KL, Jaffer M, Hlongwane KE, Otwembe K, Dietrich J, Cheyip M, et al. Assessing national cervical cancer screening guidelines: results from an HIV testing clinic also screening for cervical cancer and HPV in Soweto, South Africa. PLoS One. (2021) 16(7):e0255124. doi: 10.1371/journal.pone.0255124
43. Søfteland S, Sebitloane MH, Taylor M, Roald BB, Holmen S, Galaphaththi-Arachchige HN, et al. A systematic review of handheld tools in lieu of colposcopy for cervical neoplasia and female genital schistosomiasis. Int J Gynaecol Obstet. (2021) 153(2):190–99. doi: 10.1002/ijgo.13538
44. Looker KJ, Rönn MM, Brock PM, Brisson M, Drolet M, Mayoud P, et al. Evidence of synergistic relationships between HIV and human papillomavirus (HPV): systematic reviews and meta-analyses of longitudinal studies of HPV acquisition and clearance by HIV status, and of HIV acquisition by HPV status. J Int AIDS Soc. (2018) 21(6):e25110. doi: 10.1002/jia2.25110
45. Arbyn M, Weiderpass E, Bruni L, de Sanjose S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. (2020) 8(2):e191–203. doi: 10.1016/S2214-109X(19)30482-6
Keywords: urine, human papillomavirus, DNA, cervico-vaginal lavage, female genital schistosomiasis
Citation: Pillay P, Galappaththi-Arachchige HN, Taylor M, Roald B and Kjetland EF (2025) Urinary human papillomavirus DNA as an indicator of gynaecological infection in young women in Schistosoma and HIV endemic South Africa. Front. Glob. Womens Health 5:1436064. doi: 10.3389/fgwh.2024.1436064
Received: 21 May 2024; Accepted: 20 December 2024;
Published: 23 January 2025.
Edited by:
Rebecca F. Grais, Réseau International des Instituts Pasteur, FranceReviewed by:
Mehmet Sarier, University of Istinye, TürkiyeLi-Ye Yang, People's Hospital of Yangjiang, China
Copyright: © 2025 Pillay, Galappaththi-Arachchige, Taylor, Roald and Kjetland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: P. Pillay, cGlsbGF5cEBkdXQuYWMuemE=
†ORCID:
P. Pillay
orcid.org/0000-0001-9414-3384
 P. Pillay
P. Pillay H. N. Galappaththi-Arachchige2
H. N. Galappaththi-Arachchige2 M. Taylor
M. Taylor E. F. Kjetland
E. F. Kjetland