- 1Department of Obstetrics and Gynecology, University of California, Davis, Sacramento, CA, United States
- 2Academic Unit of Obstetrics and Gynecology, DINOGMI, IRCCS-Azienda Ospedaliera Universitaria San Martino di Genova, Genova, Italy
- 3Collegium Medicum, University of Zielona Gora, Zielona Gora, Poland
- 4Department of Obstetrics and Gynecology, Bern University Hospital, Bern, Switzerland
- 5Gynécologie—Obstétrique et Médecine de la Reproduction—Maternité, Hospital Tenon, Paris, France
- 6Reprovision Clinical Consultancy, Oss, Netherlands
- 7Division of Obstetrics and Gynecology, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy
Introduction: The evolution of contraception has been crucial for public health and reproductive well-being. Over the past 60 years, combined oral contraceptives (COCs) have remained an important part of the contraceptive landscape worldwide; continued development has worked toward maintaining efficacy and improving safety.
Methods: Seven global experts convened to discuss the clinical relevance of the oestrogen in COCs, focusing on the impact of the new oestrogen, oestetrol (E4). Participants then commented through an online forum on the summary content and other participants' feedback. We prepared this report to describe the experts' views, their follow-up from the open forum and the evidence supporting their views.
Results: Ethinylestradiol (EE) and oestradiol (E2) affect receptors similarly whereas E4 has differential effects, especially in the liver and breast. Adequate oestrogen doses in COCs ensure regular bleeding and user acceptability. EE and E4 have longer half-lives than E2; accordingly, COCs with EE and E4 offer more predictable bleeding than those with E2. Oestrogen type and progestin influence VTE risk; E2 poses a lower risk than EE; although promising, E4/DRSP VTE risk is lacking population-based data. COCs alleviate menstrual symptoms, impact mental health, cognition, libido, skin, and bone health.
Conclusion: Oestrogens play an important role in the contraceptive efficacy, bleeding patterns, and overall tolerability/safety of COCs. Recent studies exploring E4 combined with DRSP show promising results compared to traditional formulations, but more definitive conclusions await further research.
1 Introduction
The development of safe and effective contraception is one of the most significant public health achievements of the 20th century (1). The availability and effective use of modern contraceptives to allow individuals to space or prevent pregnancies results in improved birth outcomes, decreased maternal morbidity and mortality, and improved well-being and population health (2–4). Combined oral contraceptives (COCs) have been available for more than 60 years; many advances have been made during this time, resulting in fewer adverse events while maintaining contraceptive efficacy (5).
In this publication the perspectives of an expert panel on the clinical relevance of the oestrogen component in combined hormonal contraceptives, with a particular emphasis on estetrol (E4), the newest oestrogen introduced for contraception, are presented with the aim of providing better support for oral contraceptive choices.
2 Methods
To address the significance of the oestrogen component of combined hormonal contraceptives, including the impact of a new oral contraceptive containing E4, seven international experts (the authors of this paper) were invited to participate in an interactive virtual forum discussion on April 8, 2022. A moderator (MDC) guided a two-hour discussion through open-ended questions covering the following topics:
• The role of oestrogens in combined oral contraceptives.
• Key features of oestrogens in combined oral contraceptives, in relation to contraceptive efficacy and safety.
• The impact of oestrogenicity of COCs on safety.
• The non-contraceptive benefits of COCs, e.g., menstruation symptoms, mood and well-being, sexual life, body weight, skin care, and migraine.
• The effects of oestrogens in COCs used by adolescents and young adults.
The online session was audio-recorded and transcribed, and a summary prepared and posted in an online forum available to the experts. During the 3 weeks following the live session, the experts were asked to comment through the online forum on the summary content and other participants' feedback. In this report, we describe the experts' common views related to the questions, their follow-up from the open forum and the evidence supporting their views.
This publication is intended for healthcare professionals seeking up to date clinical information on the relevance of the estrogenic component in a COC. The main outcomes of the panel discussion related to each question and corroborating references are presented with a summary of the panel members' main points summarized in boxes at the beginning of each section.
3 What is the role of oestrogens contained in combined oral contraceptives?
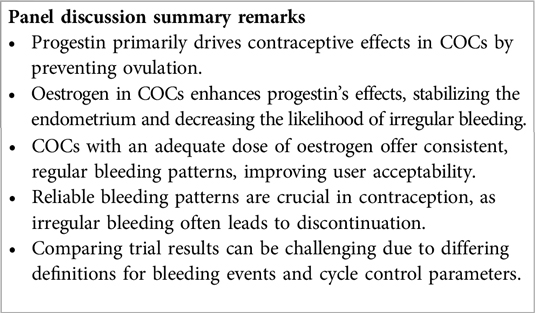
In a COC, the progestin component is primarily responsible for the contraceptive effect, mainly through ovulation inhibition (6, 7). The oestrogen component amplifies the progestational component's effectiveness, enhancing its ability to inhibit gonadotropin secretion and exert antifertility effects within the reproductive tract. The oestrogen also supports endometrial stability, preventing irregular shedding and undesired breakthrough bleeding, resulting in a consistent bleeding pattern (8–11).
The oestrogen-progestin equilibrium required for a regular bleeding pattern is disrupted by using an excessively low dose of ethinylestradiol (EE), as is present in the COC EE 10 µg/norethindrone acetate 1 mg. Substituting EE with 17β-estradiol (E2) or E2 valerate can also result in a less predictable bleeding pattern, likely related to the shorter half-life of E2 in comparison to the progestin (12). In contrast, replacing EE with estetrol (E4) 15 mg in combination with drospirenone (DRSP) 3 mg results most commonly in a consistent and predictable bleeding pattern (11). When estrogen is removed, as with the progestin-only pill (POP) containing DRSP 4 mg in a 24/4 regimen, high rates of unscheduled bleeding and a lack of scheduled bleeding occur as compared to combination products (11).
From a practical, user-orientated perspective, the greatest non-contraceptive advantage of combined oestrogen-progestin pills over progestin-only pills is their ability to produce a consistent, regular bleeding pattern (13). Therefore, a good reason for adding an oestrogen in a contraceptive is to enhance cycle control, users' acceptability, and product continuation (14–17). Nonetheless, comparing results across clinical trials can be challenging due to the varied definitions employed for reporting bleeding events or describing cycle control parameters (18).
4 What are key features of oestrogens contained in combined oral contraceptives in relation to contraceptive efficacy and safety?
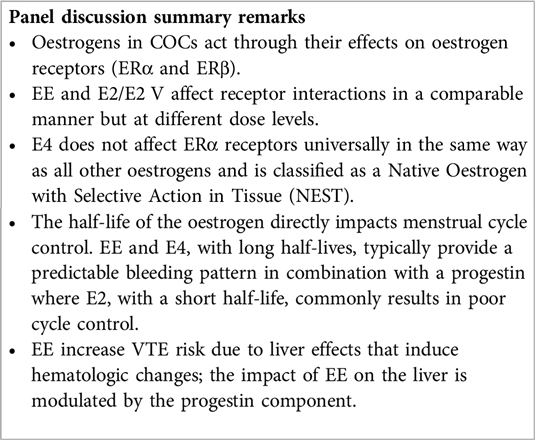
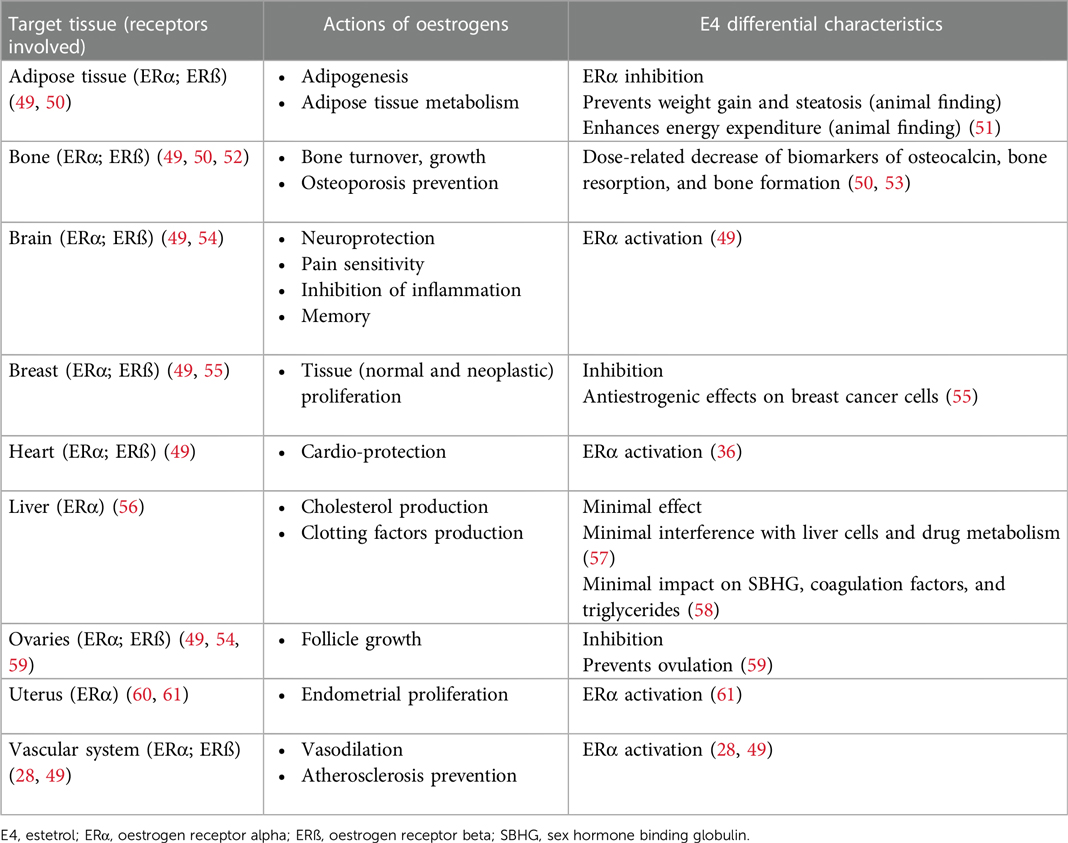
The physiological functions of oestrogenic products are modulated by the oestrogen receptors (ER) subtypes alpha (ERα) and beta (ERβ) present in different tissues such as the breast, brain, liver, skin and epithelial and fibromuscular tissues (19). The oestrogens used in COCs all have different properties (20) (see Box 1). These properties relate to the way in which oestrogens exert their effects at their receptors which is more important than their relative amount in the pill (36).
E4 activates the nuclear ERα, but it antagonizes/agonizes (mixed) the membrane ERα, in contrast to other oestrogens (20, 28). Because of this differential mechanism of action, it is classified as a Native Oestrogen with Selective Action in Tissue (NEST). The selective tissue activities of E4 are the consequence of its unique pharmacological profile (42), displaying distinct effects on different tissues which implies a limited impact on the production of liver proteins, and on haemostasis, endocrine and metabolic parameters, rendering a better safety profile compared with other oral oestrogens (28, 36, 39, 40, 42). E4 is a terminal oestrogen that does not convert into other oestrogens and has a long half-life of about 28 h (20, 36, 43). EE also has a prolonged half-life of up to 30 h (20, 44, 45) and therefore both EE and E4, with a progestin, typically offer a predictable bleeding pattern (43, 46). However, EE has been linked to an increased risk of venous thromboembolism (VTE); its dosage and the type of progestin in an EE based COC play crucial roles in modifying this risk (47). Additionally, EE significantly affects the cytochrome P450 system influencing the metabolism of other drugs (23) (see Box 2). Oral E2, with a short half-life of up to 20 h (20), requires micronization to extend its half-life, yet still it tends to result in poor cycle control (32, 45). E2 and E4 in COCs may have a lesser impact on stimulating coagulant proteins compared to EE (20) (Box 1).
5 How can oestrogenicity of COCs impact safety?
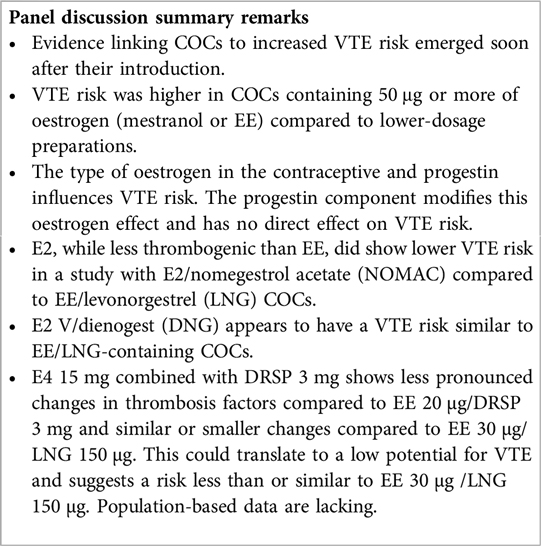
Evidence suggesting that oral contraceptives were associated with an increased VTE risk appeared rapidly after they were marketed (62). VTE occurred more frequently with COCs containing more than 50 μg of oestrogen, either mestranol or EE, compared to preparations containing a lower dosage (62). However, oestrogens differ in their effects on thrombotic markers irrespective of the progestin, and newer progestins may affect the impact of oestrogens on haemostatic markers differently than older progestins (62). For instance, the combination of E4 with DRSP resulted in less pronounced alterations in thrombosis factors compared to EE 20 µg/DRSP 3 mg, and comparable or smaller effects compared to EE 30 µg/LNG 150 µg (63). Also, E2 has less of an effect than EE on thrombosis parameters (13, 20). For instance, VTE in E2 COC users has been estimated at 20 cases in 61,600 woman-years (3.2/10,000 users per year) and in EE/LNG COC users at 28 cases in 62,807 woman-years (4.5/10,000 users per year) (64).
Thrombotic marker data are encouraging but are not sufficient to fully assess VTE risk, which requires more research, in particular large scale population based studies (63). Until now, limited epidemiological evidence suggests that E2 V/dienogest (DNG) may have an estimated VTE risk similar to LNG containing COCs (65), or lower vs. EE/LNG (66) while a population-based phase 4 study of E2/nomegestrol acetate (NOMAC) revealed a notably reduced risk of VTE in comparison to EE/LNG COCs [HR: 0.31 (95% CI, 0.13–0.75)] (67). Across the full E4/DRSP clinical program (pooled phase 2 and 3 trials) one VTE case was reported and the estimated annual VTE incidence was relatively low (3.66/10,000 woman-years). This relatively low risk was also reported in a recent modelling study that used the Activated Protein C Resistance as a predictor of VTE risk (68).
Overall, COCs containing natural oestrogens may be preferable over those with synthetic oestrogens due to their lower liver impact. Currently, the clinical and real-world data regarding E2 suggest a VTE profile that may be safer than EE. This promising finding raises expectations that a similar trend may be observed with the non-synthetic oestrogen E4 when combined with DRSP. Nevertheless, definitive conclusions regarding the VTE risk await the completion of post-marketing surveillance studies (40, 63, 69).
6 What are the non-contraceptive benefits of COCs?
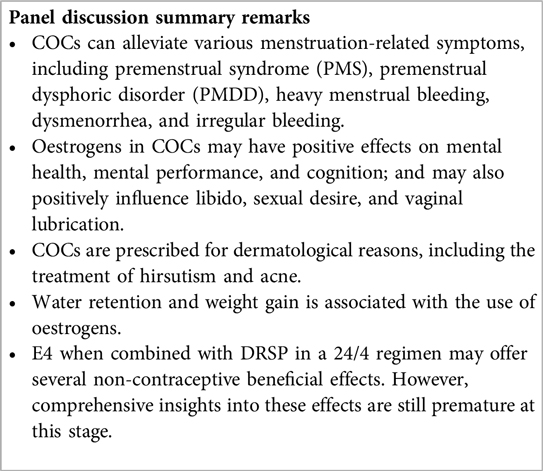
Oestrogens play a vital role in the non-contraceptive benefits of COCs. Using the right oestrogen-progestin combination can alleviate symptoms and signs in various clinical scenarios, as acknowledged by the expert panel. Examples provided include menstrual-related depressive symptoms, heavy menstrual bleeding (HMB), painful periods, and irregular bleeding (70–74). Certain COCs are approved for treating these conditions (75). For example, E2 V/DNG is indicated for HMB treatment (76) and EE 20 µg/DRSP 3 mg for PMDD treatment (77).
Changes in hormone levels, whether natural or due to contraceptives, can significantly impact emotions (78). COCs with E2 may improve mental health and cognition (79–81), potentially easing depressive symptoms in PMDD (82, 83). COCs can influence libido and vaginal health (84, 85). COCs are also used to treat dermatologic conditions like hirsutism and acne due to their antiandrogenic effects (73, 74, 86–89).
Although trials suggest no direct evidence of COCs causing weight gain, patients still commonly associate their use with this outcome (90). COCs containing DRSP, however, may help prevent weight gain (91). Additionally, COCs could affect migraine frequency, especially menstruation-related migraines, with some formulations showing potential for providing relief (92, 93).
The evidence regarding the non-contraceptive effects of E4 on women's health is still at an early stage. Theoretically, due to its extended half-life and limited impact on the liver, E4 might effectively alleviate physical symptoms associated with PMS (94), such as breast tenderness, headaches, and water retention. Pre-clinical studies suggest that E4 may support vaginal health by promoting epithelial proliferation and lubrication (95, 96). Combining E4 with DRSP also seems to have minimal impact on weight gain (97, 98). There is speculation that contraceptives with natural oestrogens like E4 might be preferred by women experiencing menstrual migraines due to potentially lower cardiovascular risks compared to those containing synthetic oestrogens (92). However, further clinical data is necessary for a more comprehensive understanding of the effects of E4 on users' health, especially those effects that will require follow-up of long-term use. It is noteworthy that in combination with DRSP, E4 has demonstrated high user acceptability and satisfaction, with users expressing a willingness to continue with the contraceptive and reporting a sense of well-being (98).
7 What are the bone effects of oestrogens in COCs in adolescents and young adults?
Oestrogens are key regulators of bone turnover in both females and males and play a major role in longitudinal and width growth throughout puberty as well as in the regulation of bone turnover (52). Oestrogen deficiency in adolescence might increase the likelihood of osteoporosis, particularly in individuals with high baseline bone turnover (52, 99–101). Limited data exist regarding the effects of COCs containing E4 on bone tissue. E4, when compared to EE, may have a lesser impact on SHBG and androgens, with a potentially positive effect on bone development in teens (36). Initial findings suggest that E4 might be more effective in preventing bone loss and bone demineralization than other EE (36, 43, 102), but further research is necessary to confirm its efficacy.
In general, COCs are popular among adolescents for birth control, but poor adherence can result in contraceptive failure (103). Choosing the most suitable COCs among adolescents should include counselling on the non-contraceptive benefits of COC. Discussions about improvements in HMB and dysmenorrhea, cycle regularity, possibly acne, hirsutism, and premenstrual symptoms, which are all common amongst adolescents, are important (104). Additionally, information about the COC protective effect against endometrial and ovarian cancer can also be provided (104).
8 Conclusions
Oestrogens play an important role in the contraceptive efficacy, bleeding patterns, and overall tolerability/safety of COCs. Beyond contraception, oestrogens in COCs also can alleviate menstrual symptoms, have a positive impact on mood, and potentially affect bone health, and other aspects of well-being. All these properties, alongside the risk of VTE are crucial in tailoring contraceptive choices. For adolescents, discussions around adherence and bone health should also shape COC choices. Recent studies exploring E4 combined with DRSP show promising results compared to traditional formulations. However, population-based outcomes and long-term effects will only come with further research. Continuous research promises deeper insights into the nuanced effects of E4 in COCs, enabling more informed and tailored contraceptive decisions.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Author contributions
MC: Conceptualization, Writing – original draft, Writing – review & editing. AC: Writing – review & editing. RS: Writing – review & editing. PS: Writing – review & editing. NC: Writing – review & editing. TK: Writing – review & editing. TS: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The authors declare the study received funding from Gedeon Richter Plc., Hungary. The funder had the following involvement in the study: support of forum, research of evidence, and publication.
Acknowledgments
The authors thank Silvia Paz Ruiz MD MMedSci (Terminal 4 Communications) for providing medical writing support in accordance with the Good Publication Practice (GPP 2022) guidelines (https://www.ismpp.org/gpp-2022).
Conflict of interest
MC has received speaking honoraria from Gedeon Richter, Mayne and OLIC, served on Advisory Boards for Gedeon Richter and Mayne, has stock options with Femasys, and has consulted for Estetra SRL, and Medicines360. The Department of Obstetrics and Gynecology, University of California, Davis, receives contraceptive research funding for MDC from Chemo Research SL, Evofem, Medicines360, Merck, Sebela, and Sumitomo Pharma. AC has received speaking honoraria and served on Advisory Boards for Gedeon Richter, Bayer, Organon, Exeltis, and Italfarmaco. RS has received speaking honoraria from Gedeon Richter and Novo Nordisk Pharma, has consulted for Theramex Poland and Gedeon Richter, and participates in phase 4 clinical trial nr HUXX-NIS-E4DRSP-1/2020. PS has received speaking honoraria from Gedeon Richter, Max Zeller & Soehne, Jenapharm, Drossapharm, EFFIK, Labatec, Besins Healthcare, Astellas, Theramex, Schaer Pharma, Bayer and Exeltis. PS served on Advisory Boards for Gedeon Richter, Theramex and Astellas. NC-B has served on Advisory Boards for for BESINS, Exeltis, and Gedeon Richter. NC-B participates on clinical trial for Bayer and Organon. TK has received consulting fees from Hervana, TEVA, Osteopharma, Starodub, Eagle Rock, Merck, Luye, QPharma, Disphar, PantaRhei, Gedeon Richter, Mithra, IPM, Myovant and Organon. TS has received consulting fees from Abbott, Astellas, Gedeon Richter, Mitsubishi Tanabe, Sojournix, Estetra, Mithra, Actavis, Medtronic, Shionogi, Applied Medical and speakers’ honoraria from Shionogi, Gedeon Richter, Intuitive Surgical, Applied Medical and Theramex.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Centers for Disease Control and Prevention. Achievements in public health, 1900–1999: family planning. JAMA. (2000) 283(3):326–31. doi: 10.1001/jama.283.3.326
2. Ahmed S, Li Q, Liu L, Tsui AO. Maternal deaths averted by contraceptive use: an analysis of 172 countries. Lancet. (2012) 380(9837):111–25. doi: 10.1016/S0140-6736(12)60478-4
3. Maguire K, Westhoff C. The state of hormonal contraception today: established and emerging noncontraceptive health benefits. Am J Obstet Gynecol. (2011) 205(4 Suppl.):S4. doi: 10.1016/j.ajog.2011.06.056
4. Center on the Economics of Reproductive Health. The Economic Effects of Contraceptives Access: A Review of the Evidence. (2019). Available online at: www.iwpr.org
5. Szarewski A, Mansour D, Shulman LP. 50 Years of “the pill”: celebrating a golden anniversary. J Fam Plan Reprod Heal Care. (2010) 36(4):231–8. doi: 10.1783/147118910793048665
6. Chappell CA, Rohan LC, Moncla BJ, Wang L, Meyn LA, Bunge K, et al. The effects of reproductive hormones on the physical properties of cervicovaginal fluid. Am J Obstet Gynecol. (2014) 211(3):1–14. doi: 10.1016/j.ajog.2014.03.041
7. Cooper DB, Patel P, Mahdy H. Oral Contraceptive Pills. [Updated 2022 Nov 24] StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing (2024). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK430882/
8. Seidman L, Kroll R, Howard B, Ricciotti N, Hsieh J, Weiss H. Ovulatory effects of three oral contraceptive regimens: a randomized, open-label, descriptive trial. Contraception. (2015) 91(6):495–502. doi: 10.1016/j.contraception.2015.03.001
9. Yu K, Huang ZY, Xu XL, Li J, Fu XW, Deng SL. Estrogen receptor function: impact on the human endometrium. Front Endocrinol (Lausanne). (2022) 13:827724. doi: 10.3389/fendo.2022.827724
10. Speroff L. The formulation of oral contraceptives: does the amount of estrogen make any clinical difference? Johns Hopkins Med J. (1982) 150(5):170–6.7043035
11. Archer DF, Mansour D, Foidart JM. Bleeding patterns of oral contraceptives with a cyclic dosing regimen: an overview. J Clin Med. (2022) 11(4634):1–15. doi: 10.3390/jcm11154634
12. Mansour D, Verhoeven C, Sommer W, Weisberg E, Taneepanichskul S, Melis GB, et al. Efficacy and tolerability of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol in a 24/4 regimen, in comparison to an oral contraceptive containing ethinylestradiol and drospirenone in a 21/7 regimen. Eur J Contracept Reprod Heal Care. (2011) 16(6):430–43. doi: 10.3109/13625187.2011.614029
13. Teal S, Edelman A. Contraception selection, effectiveness, and adverse effects: a review. JAMA. (2021) 326(24):2507–18. doi: 10.1001/jama.2021.21392
14. Britton LE, Alspaugh A, Greene MZ, McLemore MR. An evidence-based update on contraception. Am J Nurs. (2020) 120(2):22–33. doi: 10.1097/01.NAJ.0000654304.29632.a7
15. Regidor PA, Colli E, Palacios S. Overall and bleeding-related discontinuation rates of a new oral contraceptive containing 4 mg drospirenone only in a 24/4 regimen and comparison to 0.075 mg desogestrel. Gynecol Endocrinol. (2021) 37(12):1121–7. doi: 10.1080/09513590.2021.1963432
16. Nappi RE, Fiala C, Chabbert-Buffet N, Häusler G, Jamin C, Lete I, et al. Women’s preferences for menstrual bleeding frequency: results of the inconvenience due to women’s monthly bleeding (ISY) survey. Eur J Contracept Reprod Heal Care. (2016) 21(3):242–50. doi: 10.3109/13625187.2016.1154144
17. Polis CB, Hussain R, Berry A. There might be blood: a scoping review on women’s responses to contraceptive-induced menstrual bleeding changes. Reprod Health. (2018) 15(1):1–17. doi: 10.1186/s12978-017-0439-6
18. Creinin MD, Vieira CS, Westhoff CL, Mansour DJA. Recommendations for standardization of bleeding data analyses in contraceptive studies. Contraception. (2022) 112:14–22. doi: 10.1016/j.contraception.2022.05.011
19. Paterni I, Granchi C, Katzenellenbogen J, Minutolo F. Estrogen receptors alpha and beta subtype-selective ligands and clinical potential. Steroids. (2014) 0:13–29. doi: 10.1016/j.steroids.2014.06.012
20. Stanczyk FZ, Winer SA, Foidart JM, Archer DF. Comparison of estrogenic components used for hormonal contraception. Contraception. (2024) 130:110310. doi: 10.1016/j.contraception.2023.110310
21. National Center for Biotechnology Information. PubChem Compound Summary for CID 5991, Ethinylestradiol. Compound summary. (2022). Available online at: https://pubchem.ncbi.nlm.nih.gov/compound/Ethinylestradiol (cited Aug 6, 2022)
22. Mukherjee TK, Reynolds PR, Hoidal JR. Differential effect of estrogen receptor alpha and beta agonists on the receptor for advanced glycation end product expression in human microvascular endothelial cells. Biochim Biophys Acta. (2005) 1745(3):300–9. doi: 10.1016/j.bbamcr.2005.03.012
23. DrugBank. Ethinylestradiol. DrugBank Online. (2022). Available online at: https://go.drugbank.com/drugs/DB00977 (cited Aug 24, 2022).
24. Fruzzetti F, Cagnacci A. Venous thrombosis and hormonal contraception: what’s new with estradiol-based hormonal contraceptives? Open Access J Contracept. (2018) 9:75–9. doi: 10.2147/OAJC.S179673
25. Sitruk-Ware R, Nath A. Metabolic effects of contraceptive steroids. Rev Endocr Metab Disord. (2011) 12(2):63–75. doi: 10.1007/s11154-011-9182-4
26. US Food and Drug Administration. Yasmin. Highlights of Prescribing Information. (2012). p. 1–28. Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021098s019lbl.pdf (cited Sep 5, 2022)
27. Gialeraki A, Valsami S, Pittaras T, Panayiotakopoulos G, Politou M. Oral contraceptives and HRT risk of thrombosis. Clin Appl Thromb. (2018) 24(2):217–25. doi: 10.1177/1076029616683802
28. Foidart JM, Gaspard U, Pequeux C, Jost M, Gordenne V, Tskitishvili E, et al. Unique vascular benefits of estetrol, a native fetal estrogen with specific actions in tissues (NEST). In: Brinton R, Genazzani A, Simoncini T, Stevenson J, editors. Sex Steroids’ Effects on Brain, Heart and Vessels ISGE Series. Cham: Springer (2019). p. 169–95. Available online at: https://www.researchgate.net/publication/333465497_Unique_Vascular_Benefits_of_Estetrol_a_Native_Fetal_Estrogen_with_Specific_Actions_in_Tissues_NEST
29. Abou-Ismail M, Sridhar D, Nayak L. Estrogen and thrombosis: a bench to bedside review. Thromb Res. (2020) 192:40–51. doi: 10.1016/j.thromres.2020.05.008
30. Tang Z, Liu ZH, Wang H, Dang Z, Liu Y. A review of 17α-ethynylestradiol (EE2) in surface water across 32 countries: sources, concentrations, and potential estrogenic effects. J Environ Manage. (2021) 292:112804. doi: 10.1016/j.jenvman.2021.112804
31. Adeel M, Song X, Wang Y, Francis D, Yang Y. Environmental impact of estrogens on human, animal and plant life: a critical review. Environ Int. (2017) 99:107–19. doi: 10.1016/j.envint.2016.12.010
32. DrugBank. Estradiol. DrugBank Online. (2022). Available online at: https://go.drugbank.com/drugs/DB00783 (cited Aug 24, 2022).
33. Arnal JF, Fontaine C, Billon-Galés A, Favre J, Laurell H, Lenfant F, et al. Estrogen receptors and endothelium. Arterioscler Thromb Vasc Biol. (2010) 30(8):1506–12. doi: 10.1161/ATVBAHA.109.191221
34. Burke A. Nomegestrol acetate-17b-estradiol for oral contraception. Patient Prefer Adherence. (2013) 7:607–19. doi: 10.2147/PPA.S39371
35. National Center for Biotechnology Information. PubChem Compound Summary for CID 13791, Estradiol Valerate. Compound Summary. (2022). Available online at: https://pubchem.ncbi.nlm.nih.gov/compound/Estradiol-valerate (cited Aug 6, 2022).
36. Fruzzetti F, Fidecicchi T, Montt Guevara MM, Simoncini T. Estetrol: A New Choice for Contraception. J Clin Med. (2021) 10(23):5625. doi: 10.3390/jcm10235625
37. National Center for Biotechnology Information. PubChem Compound Summary for CID 27125, Estetrol. Compound summary. (2022). Available online at: https://pubchem.ncbi.nlm.nih.gov/compound/Estetrol (cited Aug 6, 2022)
38. European Medicines Agency-Committee for Medicinal Products for Human Use. Drovelis-Summary of Product Characteristics. Drovelis-Assessment Report. (2021). Available online at: https://www.ema.europa.eu/en/documents/assessment-report/drovelis-epar-public-assessment-report_en.pdf (cited March 18, 2024).
39. Coelingh Bennink HJT, Foidart J. Estetrol, a fetal steroid for the treatment of adults. J Reprod Med Endocrinol. (2015) 12(4):399–403. Available online at: http://www.kup.at/kup/pdf/9658.pdf
40. Mawet M, Gaspard U, Foidart JM. Estetrol as estrogen in a combined oral contraceptive, from the first in-human study to the contraceptive efficacy. Eur Gynecol Obstet. (2021) 3(1):13–21. doi: 10.53260/EGO.213012
41. Křepelka P. Estetrol and the possibilities of its clinical use. Ces Gynekol. (2021) 86(3):217–21. doi: 10.48095/cccg2021217
42. Gérard C, Foidart JM. Estetrol: from preclinical to clinical pharmacology and advances in the understanding of the molecular mechanism of action. Drugs R D. (2023) 23(2):77–92. doi: 10.1007/s40268-023-00419-5
43. Gérard C, Arnal JF, Jost M, Douxfils J, Lenfant F, Fontaine C, et al. Profile of estetrol, a promising native estrogen for oral contraception and the relief of climacteric symptoms of menopause. Expert Rev Clin Pharmacol. (2022) 15(2):121–37. doi: 10.1080/17512433.2022.2054413
44. Kanarkowski R, Tornatore KM, D'Ambrosio R, Gardner MJ, Jusko WJ. Pharmacokinetics of single and multiple doses of ethinyl estradiol and levonorgestrel in relation to smoking. Clin Pharmacol Ther. (1988) 43(1):23–31. doi: 10.1038/clpt.1988.7
45. Stanczyk FZ, Archer DF, Bhavnani BR. Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception. (2013) 87(6):706–27. doi: 10.1016/j.contraception.2012.12.011
46. Lee A, Syed YY. Estetrol/drospirenone: a review in oral contraception. Drugs. (2022) 82(10):1117–25. doi: 10.1007/s40265-022-01738-8
47. Heikinheimo O, Toffol E, Partonen T, But A, Latvala A, Haukka J. Systemic hormonal contraception and risk of venous thromboembolism. Acta Obstet Gynecol Scand. (2022) 101(8):846–55. doi: 10.1111/aogs.14384
48. Fruzzetti F, Trémollieres F, Bitzer J. An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest. Gynecol Endocrinol. (2012) 28(5):400. doi: 10.3109/09513590.2012.662547
49. Morselli E, Santos RS, Criollo A, Nelson MD, Palmer BF, Clegg DJ. The effects of oestrogens and their receptors on cardiometabolic health. Nat Rev Endocrinol. (2017) 13(6):352–64. doi: 10.1038/nrendo.2017.12
50. Mawet M, Maillard C, Klipping C, Zimmerman Y, Foidart JM, Coelingh Bennink HJ. Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives. Eur J Contracept Reprod Heal Care. (2015) 20(6):463–75. doi: 10.3109/13625187.2015.1068934
51. Buscato M, Davezac M, Zahreddine R, Adlanmerini M, Métivier R, Fillet M, et al. Estetrol prevents western diet–induced obesity and atheroma independently of hepatic estrogen receptor α. Am J Physiol Endocrinol Metab. (2021) 320(1):E19–29. doi: 10.1152/ajpendo.00211.2020
52. Emmanuelle NE, Marie-Cécile V, Florence T, Jean-François A, Françoise L, Coralie F, et al. Critical role of estrogens on bone homeostasis in both male and female: from physiology to medical implications. Int J Mol Sci. (2021) 22(4):1–18. doi: 10.3390/ijms22041568
53. Douxfils J, Gaspard U, Taziaux M, Jost M, Bouvy C, Lobo RA, et al. Impact of estetrol (E4) on hemostasis, metabolism and bone turnover in postmenopausal women. Climacteric. (2022) 0(0):1–9. doi: 10.1080/13697137.2022.2139599
54. Coelingh Bennink HJT, Holinka CF, Diczfalusy E. Estetrol review: profile and potential clinical applications. Climacteric. (2008) 11(Suppl. 1):47–58. doi: 10.1080/13697130802073425
55. Singer CF, Bennink HJ, Natter C, Steurer S, Rudas M, Moinfar F, et al. Antiestrogenic effects of the fetal estrogen estetrol in women with estrogen-receptor positive early breast cancer. Carcinogenesis. (2014) 35(11):2447–51. doi: 10.1093/carcin/bgu144
56. Palmisano BT, Zhu L, Stafford JM. Role of estrogens in the regulation of liver lipid metabolism. Adv Exp Med Biol. (2017) 1043(615):227–56. doi: 10.1007/978-3-319-70178-3_12
57. Visser M, Holinka CF, Coelingh Bennink HJT. First human exposure to exogenous single-dose oral estetrol in early postmenopausal women. Climacteric. (2008) 11(Suppl. 1):31–40. doi: 10.1080/13697130802056511
58. Kluft C, Zimmerman Y, Mawet M, Klipping C, Duijkers IJM, Neuteboom J, et al. Reduced hemostatic effects with drospirenone-based oral contraceptives containing estetrol vs. ethinyl estradiol. Contraception. (2017) 95(2):140–7. doi: 10.1016/j.contraception.2016.08.018
59. Duijkers IJ, Heger-Mahn D, Drouin D, Skouby S. A randomised study comparing the effect on ovarian activity of a progestogen-only pill (POP) containing desogestrel and a new POP containing drospirenone in a 24/4 regimen. Eur J Contracept Reprod Heal Care. (2015) 20(6):419–27. doi: 10.3109/13625187.2015.1044082
60. Weihua Z, Saji S, Mäkinen S, Cheng G, Jensen EV, Warner M, et al. Estrogen receptor (ER) beta, a modulator of ERalpha in the uterus. Proc Natl Acad Sci U S A. (2000) 97(11):5936–41. doi: 10.1073/pnas.97.11.5936
61. Apter D, Zimmerman Y, Beekman L, Mawet M, Maillard C, Foidart JM, et al. Bleeding pattern and cycle control with estetrol-containing combined oral contraceptives: results from a phase II, randomised, dose-finding study (FIESTA). Contraception. (2016) 94(4):366–73. doi: 10.1016/j.contraception.2016.04.015
62. Morimont L, Haguet H, Dogné JM, Gaspard U, Douxfils J. Combined oral contraceptives and venous thromboembolism: review and perspective to mitigate the risk. Front Endocrinol (Lausanne). (2021) 12:1–17. doi: 10.3389/fendo.2021.769187
63. Douxfils J, Klipping C, Duijkers I, Kinet V, Mawet M, Maillard C, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. (2020) 102(6):396–402. doi: 10.1016/j.contraception.2020.08.015
64. Grandi G, Facchinetti F, Bitzer J. Confirmation of the safety of combined oral contraceptives containing oestradiol on the risk of venous thromboembolism. Eur J Contracept Reprod Heal Care. (2022) 27(2):83–4. doi: 10.1080/13625187.2022.2029397
65. Electronic Medicines Compendium (EMC). Qlaira film-coated tablets. SmPC. (2022). Available online at: https://www.medicines.org.uk/emc/product/6536/smpc#gref (cited Nov 20, 2022).
66. Dinger J, Möhner S, Heinemann K. Combined oral contraceptives containing dienogest and estradiol valerate may carry a lower risk of venous and arterial thromboembolism compared to conventional preparations: results from the extended INAS-SCORE study. Front Womens Health. (2020) 5(1):1–8. doi: 10.15761/FWH.1000178
67. Reed S, Koro C, DiBello J, Becker K, Bauerfeind A, Franke C, et al. Prospective controlled cohort study on the safety of a monophasic oral contraceptive containing nomegestrol acetate (2.5 mg) and 17β-oestradiol (1.5 mg) (PRO-E2 study): risk of venous and arterial thromboembolism. Eur J Contracept Reprod Heal Care. (2021) 26(6):439–46. doi: 10.1080/13625187.2021.1987410
68. Douxfils J, Bouvy C, Morimont L. Evaluation of activated protein C resistance using thrombin generation test. Methods Mol Biol. (2023) 2663:211–24. doi: 10.1007/978-1-0716-3175-1_12
69. Haverinen AH, Luiro KM, Szanto T, Kangasniemi MH, Hiltunen L, Sainio S, et al. Combined oral contraceptives containing estradiol valerate vs ethinylestradiol on coagulation: a randomized clinical trial. Acta Obstet Gynecol Scand. (2022) 101(10):1102–11. doi: 10.1111/aogs.14428
70. Lete I, Lapuente O. Contraceptive options for women with premenstrual dysphoric disorder: current insights and a narrative review. Open Access J Contracept. (2016) 7:117–25. doi: 10.2147/OAJC.S97013
71. Freeman EW, Halbreich U, Grubb GS, Rapkin AJ, Skouby SO, Smith L, et al. An overview of four studies of a continuous oral contraceptive (levonorgestrel 90 mcg/ethinyl estradiol 20 mcg) on premenstrual dysphoric disorder and premenstrual syndrome. Contraception. (2012) 85:437–45. doi: 10.1016/j.contraception.2011.09.010
72. Marr J, Niknian M, Shulman LP, Lynen R. Premenstrual dysphoric disorder symptom cluster improvement by cycle with the combined oral contraceptive ethinylestradiol 20 mcg plus drospirenone 3 mg administered in a 24/4 regimen. Contraception. (2011) 84(1):81–6. doi: 10.1016/j.contraception.2010.10.010
73. ESHRE Capri Workshop Group. Noncontraceptive health benefits of combined oral contraception. Hum Reprod Update. (2005) 11(5):513–25. doi: 10.1093/humupd/dmi019
74. Carey MS, Allen RH. Non-contraceptive uses and benefits of combined oral contraception. Obstet Gynaecol. (2012) 14(4):223–8. doi: 10.1111/j.1744-4667.2012.00126.x
75. de Wit AE, de Vries YA, de Boer MK, Scheper C, Fokkema A, Janssen CAH, et al. Efficacy of combined oral contraceptives for depressive symptoms and overall symptomatology in premenstrual syndrome: pairwise and network meta-analysis of randomized trials. Am J Obstet Gynecol. (2021) 225(6):624–33. doi: 10.1016/j.ajog.2021.06.090
76. Chaplin S, Briggs P. Qlaira: first COC licensed for heavy menstrual bleeding. Prescriber. (2011) 22(22):15–8. doi: 10.1002/psb.837
77. U.S. Food and Drug Administration. Yaz (drospirenone/ethinyl estradiol) for Oral Contraception and Premenstrual Dysphoric Disorder. Drug Approval Package. (2006). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021873_yaz_toc.cfm (cited Jun 20, 2022).
78. Engman J, Sundström Poromaa I, Moby L, Wikström J, Fredrikson M, Gingnell M. Hormonal cycle and contraceptive effects on amygdala and salience resting-state networks in women with previous affective side effects on the pill. Neuropsychopharmacology. (2018) 43(3):555–63. doi: 10.1038/npp.2017.157
79. Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. (2014) 66(4):602–18. doi: 10.1016/j.yhbeh.2014.08.011
80. Bustamante-Barrientos FA, Méndez-Ruette M, Ortloff A, Luz-Crawford P, Rivera FJ, Figueroa CD, et al. The impact of estrogen and estrogen-like molecules in neurogenesis and neurodegeneration: beneficial or harmful? Front Cell Neurosci. (2021) 15:1–19. doi: 10.3389/fncel.2021.636176
81. Stute P, Wienges J, Koller AS, Giese C, Wesemüller W, Janka H, et al. Cognitive health after menopause: does menopausal hormone therapy affect it? Best Pract Res Clin Endocrinol Metab. (2021) 35(6):101565. doi: 10.1016/j.beem.2021.101565
82. Yonkers KA, O’Brien PS, Eriksson E. Premenstrual syndrome. Lancet. (2008) 371(9619):1200–10. doi: 10.1016/S0140-6736(08)60527-9
83. Wright KP, Johnson JV. Evaluation of extended and continuous use oral contraceptives. Ther Clin Risk Manag. (2008) 4(5):905–11. doi: 10.2147/tcrm.s2143
84. de Castro Coelho F, Barros C. The potential of hormonal contraception to influence female sexuality. Int J Reprod Med. (2019) 2019:1–9. doi: 10.1155/2019/9701384
85. Tomczyk K, Chmaj-Wierzchowska K, Wszołek K, Wilczak M. New Possibilities for Hormonal Vaginal Treatment in Menopausal Women. J Clin Med. (2023) 12(14):4740. doi: 10.3390/jcm12144740
86. Requena C, Llombart B. Oral contraceptives in dermatology. Actas Dermo-Sifiliográficas (English Ed). (2020) 111(5):351–6. doi: 10.1016/j.adengl.2019.06.008
87. Trivedi MK, Shinkai K, Murase JE. A review of hormone-based therapies to treat adult acne vulgaris in women. Int J Womens Dermatol. (2017) 3(1):44–52. doi: 10.1016/j.ijwd.2017.02.018
88. European Medicines Agency. Dienogest/ethinylestradiol can be used for acne after certain other treatments have failed. Vol. 44. (2017). Available online at: https://www.ema.europa.eu/en/documents/press-release/dienogest/ethinylestradiol-can-be-used-acne-after-certain-other-treatments-have-failed_en.pdf (cited March 18, 2024).
89. Markovski M, Hall J, Jin M, Laubscher T, Regier L. Approach to the management of idiopathic hirsutism. Can Fam Physician. (2012) 58(2):173–7.22337742
90. Gallo MF, Lopez LM, Grimes DA, Carayon F, Schulz KF, Helmerhorst FM. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. (2014) 2014(1):CD003987. doi: 10.1002/14651858.CD003987.pub5
91. Regidor PA, Schindler AE. Antiandrogenic and antimineralocorticoid health benefits of COC containing newer progestogens: dienogest and drospirenone. Oncotarget. (2017) 8(47):83334–42. doi: 10.18632/oncotarget.19833
92. Nappi RE, Tiranini L, Sacco S, De Matteis E, De Icco R, Tassorelli C. Role of estrogens in menstrual migraine. Cells. (2022) 11:18–25. doi: 10.3390/cells11081355
93. Reddy N, Desai MN, Schoenbrunner A, Schneeberger S, Janis JE. The complex relationship between estrogen and migraines: a scoping review. Syst Rev. (2021) 10(1):1–13. doi: 10.1186/s13643-021-01618-4
94. Chen MJ, Jensen JT, Kaunitz AM, Achilles SL, Zatik J, Weyers S, et al. Tolerability and safety of the estetrol/drospirenone combined oral contraceptive: Pooled analysis of two multicenter, open-label phase 3 trials. Contraception. (2022) 116:44–50. doi: 10.1016/j.contraception.2022.10.004
95. Benoit T, Valera MC, Fontaine C, Buscato M, Lenfant F, Raymond-Letron I, et al. Estetrol, a fetal selective estrogen receptor modulator, acts on the vagina of mice through nuclear estrogen receptor α activation. Am J Pathol. (2017) 187(11):2499–507. doi: 10.1016/j.ajpath.2017.07.013
96. Gallez A, Dias Da Silva I, Wuidar V, Foidart JM, Péqueux C. Estetrol and mammary gland: friends or foes? J Mammary Gland Biol Neoplasia. (2021) 26(3):297–308. doi: 10.1007/s10911-021-09497-0
97. Ma S, Song SJ. Oral contraceptives containing drospirenone for premenstrual syndrome. Cochrane Database Syst Rev. (2023) 6(6):CD006586. doi: 10.1002/14651858.CD006586.pub5
98. Apter D, Zimmerman Y, Beekman L, Mawet M, Maillard C, Foidart JM, et al. Estetrol combined with drospirenone: an oral contraceptive with high acceptability, user satisfaction, well-being and favourable body weight control. Eur J Contracept Reprod Heal Care. (2017) 22(4):260–7. doi: 10.1080/13625187.2017.1336532
99. Hadji P, Colli E, Regidor P. Bone health in estrogen-free contraception. Osteoporos Int. (2020) 2020:2391–400. doi: 10.1007/s00198-019-05103-6
100. Herrmann M, Seibel MJ. The effects of hormonal contraceptives on bone turnover markers and bone health. Clin Endocrinol (Oxf). (2010) 72(5):571–83. doi: 10.1111/j.1365-2265.2009.03688.x
101. Hammond GL. Diverse roles for sex hormone-binding globulin in reproduction. Biol Reprod. (2011) 85(3):431–41. doi: 10.1095/biolreprod.111.092593
102. Coelingh Bennink HJ, Heegaard AM, Visser M, Holinka CF, Christiansen C. Oral bioavailability and bone-sparing effects of estetrol in an osteoporosis model. Climacteric. (2008) 11(Suppl. 1):2–14. doi: 10.1080/13697130701798692
103. Todd N, Black A. Contraception for adolescents. JCRPE J Clin Res Pediatr Endocrinol. (2020) 12(Suppl 1):28–40. doi: 10.4274/jcrpe.galenos.2019.2019.S0003
Keywords: oestrogens, oestetrol, E4, oral contraception, combined hormonal contraceptives, effectiveness, safety, non-contraceptive benefits
Citation: Creinin MD, Cagnacci A, Spaczyński RZ, Stute P, Chabbert-Buffet N, Korver T and Simoncini T (2024) Experts' view on the role of oestrogens in combined oral contraceptives: emphasis on oestetrol (E4). Front. Glob. Womens Health 5:1395863. doi: 10.3389/fgwh.2024.1395863
Received: 4 March 2024; Accepted: 26 March 2024;
Published: 9 April 2024.
Edited by:
Ronald Anguzu, Medical College of Wisconsin, United StatesReviewed by:
Yan Che, Fudan University, China© 2024 Creinin, Cagnacci, Spaczyński, Stute, Chabbert-Buffet, Korver and Simoncini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. D. Creinin bWRjcmVpbmluQHVjZGF2aXMuZWR1
 M. D. Creinin
M. D. Creinin A. Cagnacci
A. Cagnacci R. Z. Spaczyński
R. Z. Spaczyński P. Stute4
P. Stute4 T. Simoncini
T. Simoncini