- 1Department of Endocrinology, National University Health Systems, Singapore, Singapore
- 2Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore
- 3Department of Digestion, Metabolism and Reproduction, Imperial College London, London, United Kingdom
Gestational diabetes (GDM), defined as glucose intolerance during pregnancy, affects one in six pregnancies globally and significantly increases a woman’s lifetime risk of type 2 diabetes mellitus (T2DM). Being a relatively young group, women with GDM are also at higher risk of developing diabetes related complications (e.g., cardiovascular disease, non-alcoholic fatty liver disease) later in life. Children of women with GDM are also likely to develop GDM and this perpetuates a cycle of diabetes, escalating our current pandemic of metabolic disease. The global prevalence of GDM has now risen by more than 30% over the last two decades, making it an emerging public health concern. Antepartum management of maternal glucose is unable to fully mitigate the associated lifetime cardiometabolic risk. Thus, efforts may need to focus on improving care for women with GDM during the postpartum period where prevention or therapeutic strategies could be implemented to attenuate progression of GDM to DM and its associated vascular complications. However, strategies to provide care for women in the postpartum period often showed disappointing results. This has led to a missed opportunity to halt the progression of impaired glucose tolerance/impaired fasting glucose to DM in women with GDM. In this review, we examined the challenges in the management of women with GDM after delivery and considered how each of these challenges are defined and could present as a gap in translating evidence to clinical care. We highlighted challenges related to postpartum surveillance, postpartum glucose testing strategies, postpartum risk factor modification, and problems encountered in engagement of patients/providers to implement interventions strategies in women with GDM after delivery. We reasoned that a multisystem approach is needed to address these challenges and to retard progression to DM and cardiovascular disease (CVD) in women with GDM pregnancies. This is very much needed to pave way for an improved, precise, culturally sensitive and wholistic care for women with GDM.
1 Introduction
Gestational diabetes (GDM), defined as glucose intolerance during pregnancy, has risen in prevalence by more than 30% across all population groups over the last two decades, giving rise to an emerging public health burden (1). Globally, GDM is known to affect one in six pregnancies, with higher prevalence in Middle East and North Africa (30.2%) and in South-east Asia (23.7%) (1) (Figure 1).
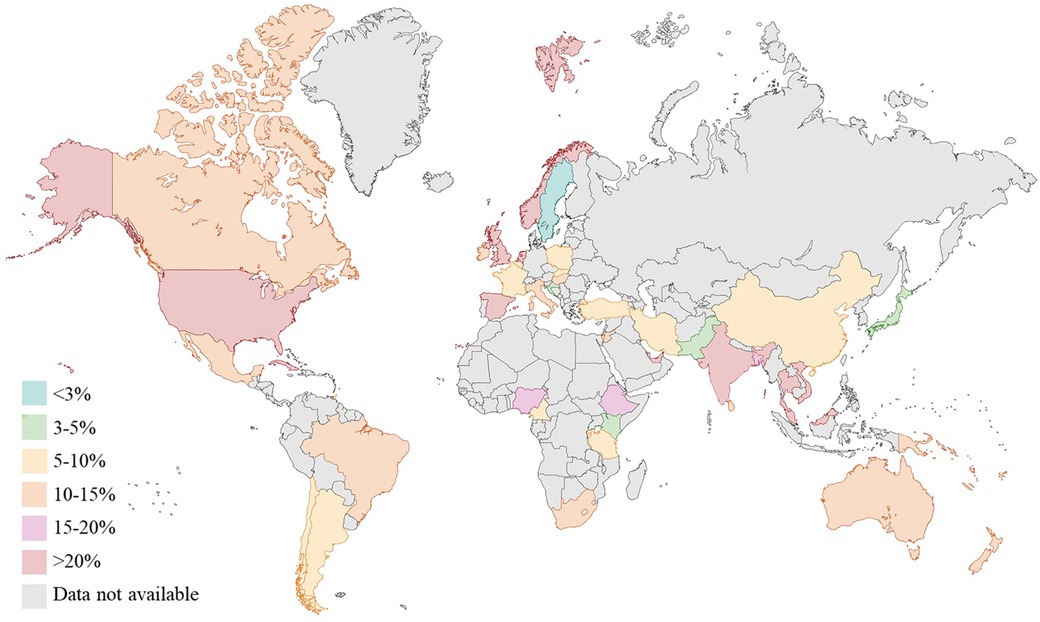
Figure 1. Prevalence (%) of gestational diabetes (GDM) worldwide (data from international diabetes federation atlas 2021). Created with Biorender.
Compared to women without GDM, women with GDM have a ten-fold increased risk of developing type 2 diabetes (T2DM) after the index pregnancy (2). In women with GDM, the linear risk of progression to diabetes is 9.6% per year after delivery, with the risk being higher in the first 5 years after delivery (2, 3). Ethnicity modifies diabetes risk in different ethnic groups. Women of South Asians and Black ethnicity are associated with an increased absolute risk of T2DM compared to White (4). However, the relative incremental risk of progression from GDM to T2DM could actually be higher in White ethnic groups compared to women of Chinese and South Asian ethnicity (White: adjusted HR13.6; 95% CI 13.2,14.0), Chinese: adjusted HR9.2; 95% CI 8.1, 10.3; South Asian women: adjusted HR9.6; 95% CI 8.8, 10.5) (5). Additionally, women with GDM, despite being a relatively young cohort, have a two-fold increased risk of cardiovascular disease (CVD) (6) and non-alcoholic fatty liver disease (NAFLD) (7) after delivery. Children from women with GDM are more likely to be macrosomic at birth and have a greater propensity to develop obesity and T2DM later in life (8). Female offsprings are also likely to experience GDM in their own pregnancies resulting in a vicious intergenerational cycle of GDM (9).
Given that T2DM, CVD and NAFLD are significant sequels to GDM, close monitoring of postpartum GDM is essential to prevent the development of T2DM. This is because detection of dysglycaemia early in the trajectory of cardiometabolic disease could enable implementation of risk-modifying intervention that reduce the growing prevalence of diabetes (Figure 2) but also mitigate associated cardiometabolic complications. However, an optimal cost-effective program to identify, monitor and manage women with GDM with elevated cardiometabolic risk post-delivery is currently lacking. In this review, we aim to summarize the key challenges in managing the metabolic sequalae in women with GDM during the postpartum period.
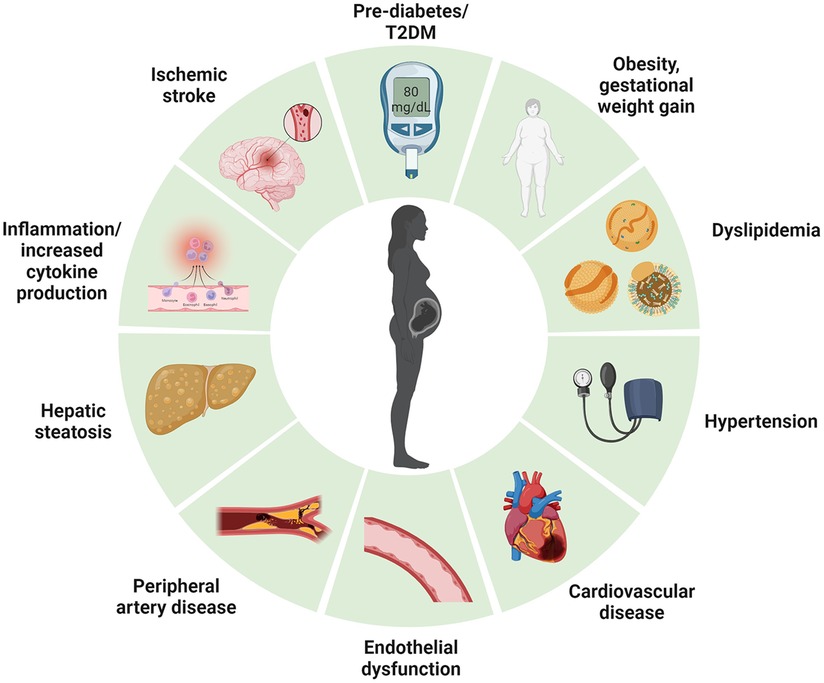
Figure 2. Long term cardiometabolic consequences of women with gestational diabetes mellitus. Created with Biorender.
2 Current challenges in postpartum management of women with GDM
2.1 Challenges in postpartum testing
2.1.1 Is OGTT sufficient in stratifying glycaemic status postpartum?
The World Health Organisation (WHO) recommends a 75-grams oral glucose tolerance test (OGTT) as the screening test to reclassify glycaemic status in women with GDM after delivery (10). The OGTT involves a fasting glucose and a 2 h post-glucose load measurement and uses non-pregnancy criteria to identify women with impaired fasting glucose (IFG), impaired glucose tolerance (IGT), diabetes mellitus (DM), or normoglycaemia in the first 6 months after delivery (10). The IGT represents an intermediate state between normal and overt diabetes and individuals with IGT typically convert to T2DM at a rate of ∼5%–10% per year (11, 12). However, the risk of dysglycaemia could extend into women with normal glucose tolerance (NGT); 17.1% of women with GDM with NGT at 3 months postpartum developed prediabetes/diabetes within a year after delivery (13). Women with NGT who progressed to prediabetes/diabetes have higher fasting, 1 h and 2 h glucose level and tend to have a delayed peak blood glucose level at 60 min (16.1% of the progressors peak at 60 min on an OGTT compared to 6.5% of the progressors who peak at 30 min) (13). Conceivably, the defects in insulin secretion are likely to be a continuous process that begins long before the onset of overt diabetes. A ∼40%–50% loss in β-cell function is expected in women who had NGT with a 2 h OGTT of 6.6 mmol/L to 7.8 mmol/L (120–140 mg/dl) (14). Ravi Retnakaran et al. observed that women with mild glucose intolerance during pregnancy that do not meet criteria for diagnosis of GDM had β-cell dysfunction at 3–12 months postpartum (13, 15, 16), suggesting a progressive loss of β-cell function beyond pregnancy. Loss of β-cell function is likely to be independent of changes in adiposity or insulin sensitivity (16), highlighting a key pathophysiologic process that drives dysglycaemia (13, 17, 18) in women with GDM after delivery.
Most guidelines have recommended repeating OGTT in 1-year after delivery to re-stratify diabetes risk (19–21). Longitudinal studies consistently reported increased CVD and T2DM risk in women with NGT (6, 13) after delivery, thus a single 2 h OGTT measurement at 6–12 weeks postpartum may not have the sensitivity to identify women who are at high-risk for metabolic disease (22). Furthermore, OGTT is cumbersome, requires overnight fasting and additional staffing.
Abnormal glucose challenge test following an antepartum OGTT has been shown to predict pre-diabetes at 3 months postpartum with an AuROC of 0.754 in women with GDM compared to women with NGT during an antepartum OGTT (15). The glucose excursion during antepartum OGTT is a far more predictive metabolic marker compared to other metabolic measures such as the insulinogenic index or the homeostatic model assessment of insulin resistance (HOMA-IR) (15). Indeed, the number of abnormal OGTT values on a three-point OGTT test during pregnancy predicts the risk of developing T2DM at 5 years after the index pregnancy in a dose-response manner (23). A high fasting glucose during OGTT in pregnancy is strongly associated with development of T2DM in women with GDM compared to a high 2 h post-glucose load level (23). If glucose excursion values during pregnancy could provide insight into the future maternal risk of prediabetes (15), it would be reasonable to utilize it as a means to identify women at high-risk of glycemic and cardiometabolic deterioration in the postpartum period. This might be far more feasible especially when women rarely return for a postpartum OGTT test (24, 25) (described in sections below).
2.1.2 Using 1-hour-post glucose level to predict diabetes and complications?
The 1 h plasma glucose level ≥8.6 nmol/L (155 mg/dl) during an OGTT may identify individuals with NGT at high risk of progressing to T2DM and CVD (26–28). A cohort study of 1945 non-diabetic men and women followed over 24 years showed that individuals with a 1 h prandial glucose of ≥8.6 mmol/L and a 2 h post-glucose level of <7.8 mmol/L had a 4.35-odds (95% CI 2.50−7.73) and a 1.87-odds (95% CI 1.09−3.26) of developing diabetes and prediabetes respectively (29). Elevated 1 h post glucose level of 8.6 mmol/L was also associated with an adverse cardiovascular risk profile characterised by higher blood pressure, elevated low-density lipoprotein, triglycerides and increased inflammatory markers and carotid intima thickness (30–32). In addition to macrovascular complications, 1 h plasma glucose of ≥8.6 mmol/L also predicted progression to microvascular complications, such as diabetic retinopathy and peripheral vascular complications, in individuals with NGT and IGT during 39 years follow-up (33). Compared to the 2 h post-glucose level, the 1 h post-glucose level of ≥8.6 mmol/L offered greater sensitivity in identifying a high-risk NGT group at an earlier time point before β-cell decline (22, 29, 33) in multiethnic groups (34–37) and predicted future diabetes better than fasting plasma glucose (FPG), 2 h plasma glucose, and HbA1c (AuROC of for 1 h plasma glucose of 0.84; AuROC for FPG 0.75; AuROC of 2 h plasma glucose is 0.79 and AuROC of HbA1c is 0.73) (27, 28, 38, 39).
The utility of 1 h post glucose value was endorsed by International Diabetes Federation (IDF) (40). In a recent position statement, individuals with 1 h post-glucose value of ≥8.6 mol/L were categorized as intermediate hyperglycaemia and should be commenced on lifestyle prevention program (40). People with 1 h post glucose level of ≥11.6 mmol/L were classified as T2DM and should have a repeat OGTT to confirm diagnosis (40). Overall, the accrued data suggested better stratification of risk of future T2DM, diabetes-related complications, and NAFLD with the 1 h post-glucose level of 8.6 nmol/L (40, 41). This would be of great relevance to women with GDM who are likely to have an underlying mild β-cell defect, which may not become apparent until years after pregnancy (20). The shortened OGTT procedure (from 2 h to 1 h) is also more cost-effective and clinically appealing to women with GDM who found the 2 h OGTT procedure to be time-consuming (40).
2.1.3 Accuracy of other measures to assess glycaemic status in early postpartum period
Fasting plasma glucose (FPG) and HbA1c have been suggested as alternative screening tests to determine if a woman’s glucose status had returned to normal after delivery. FPG was correlated to HbA1c (r = 0.39) and the 2 h post-glucose value (r = 0.34) (42) but using FPG alone (at ≥6.1 mmol/L) resulted in missed diagnosis of impaired glucose tolerance (IGT) in 54% of women with GDM after delivery (43). In another study, 38.3% of women classified as glucose intolerance using OGTT test were reclassified as normal with a FPG (44). A postpartum FPG alone, whilst useful, may not be sensitive enough to ascertain glucose tolerance in high-risk multi-ethnic population (43), and is likely to lead to missed cases of diabetes and IGT.
Unlike FPG, HbA1c is relatively easy to perform but it could be affected by age, race, haematological factors or iron deficiency (45–48). HbA1c is not reliable in the first 1 year postpartum, due to blood loss during labour and persistence of high red cell turnover state (49). A HbA1c cut-off of 6.5% would misclassify 75% of the women with GDM who were previously categorized as abnormal glucose regulation by an OGTT test in the postpartum period (44). HbA1c is also weakly correlated with glycaemic parameters such as insulin sensitivity (r = −0.25, p = 0.010) or glucose disposition index (r = −0.26, p = 0.007) in women with GDM during early post-partum (3–6 months) (50). Using a lower HbA1c cutoff of ≥6% (42 mmol/mol) would increase the number of false negative that does not sufficiently identify IFG or IGT in postpartum GDM women (Specificity: 83.9%, 95% CI 73.2–92.9; Sensitivity: 23.8%, 95% CI 9.5–42.9) (50). Further lowering of the HbA1c cut-off to 5.7% would reduce its specificity (50). Notably, HbA1c 5.7−6.4% was a less precise predictor of glucose abnormalities in at risk individuals or in women with GDM in early postpartum period (42, 50) but could inform progression of glucose intolerance if assessed longitudinally and periodically during postpartum period (50). FPG could be used in combination with HbA1c in the prediction of diabetes during the postpartum period (51). A study from India showed that a FPG of ≥6.1 mmol/L or HBA1c ≥ 6.0% avoided OGTT in 80.9% of the women, without missing any cases of diabetes compared to missing 2.4% cases of diabetes when either FPG ≥5.6 mmol/L or HbA1c ≥ 5.7% were used alone (51).
2.1.4 Lack of consensus in the guidelines on postpartum follow-up
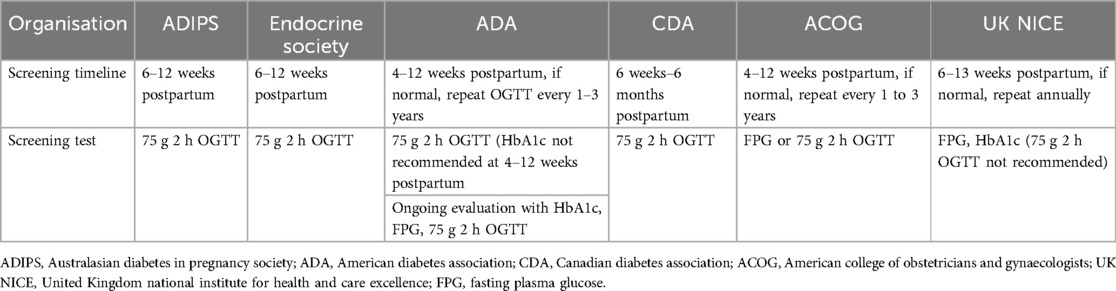
Guidelines differ in terms of timing and the type of screening test for postpartum glycaemic status in women with GDM (Table 1).
The Australasian Diabetes in Pregnancy Society (52) and Endocrine Society (53) recommend screening for type 2 diabetes in women with previous GDM at least 6–12 weeks postpartum with a 75-g oral glucose tolerance test (OGTT), using non pregnancy criteria. The American Diabetes Association recommends screening for T2DM with an OGTT at an earlier time frame (4–12 weeks after delivery) to enable discussion of result at the 6-week postpartum obstetrical assessment (49, 54), whereas the Canadian Diabetes Association (CDA) suggests the same test over a longer period of assessment (from 6 weeks to 6 months) (19). The American Congress of Obstetrician and Gynaecologist indicates screening with either the OGTT or testing with fasting plasma glucose (FPG) at 6–12 weeks postpartum (21). On the other hand, the National Institute of Health and care Excellence (NICE) excludes a routine OGTT and suggests testing with a FPG or HbA1c at 6–13 weeks postpartum if FPG is not done earlier at discharge (55). The substantial variation in clinical recommendations throughout the world has made it challenging to understand the trajectory of cardiovascular and metabolic risk of women with GDM after pregnancy.
2.2 Challenges in adherence to postpartum testing
2.2.1 Adherence to post-partum OGTT (patient and provider’s perspective)
Despite the clinical relevance of OGTT in classifying postpartum dysglycaemia, uptake of postpartum OGTT has been universally low globally, ranging from 31%–49% in most studies (56–58). This is much lower compared to postnatal cervical screening (94%) and antenatal GDM screening (98%) (59).
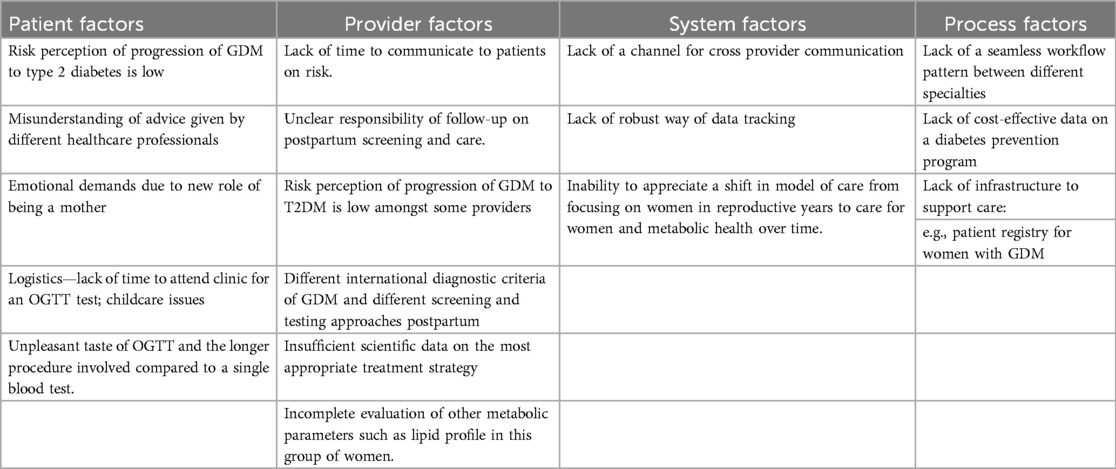
Both patients and providers have highlighted several barriers to postpartum OGTT. Bennett et al. conducted a semi-structured interviews in women with GDM and identified several themes of barriers to postpartum OGTT testing, which include: (1) emotional stress of prioritizing newborn’s needs before a woman’s postpartum care needs, the challenging adjustment to the new role as a mother and fear of receiving a diagnosis of diabetes, (2) lack of communication from providers resulting in underappreciation of the condition and a perceived sense of lack of continuity of care due to change of healthcare providers (60–62). Interestingly, the barriers reported were largely congruent across different ethnic groups (61, 63). Hewage SS et al. conducted an exploratory study in Singapore and found that despite universal GDM education, 37% of the women with GDM did not feel that postpartum OGTT was very important (61). The time-consuming nature of the OGTT test, the unpleasant taste of the glucose drink, inadequate education on postnatal care and lack of communication from relevant healthcare providers were highlighted as common barriers to postpartum OGTT amongst women with GDM in Singapore (61). Similarly, women with GDM of Hispanic, African American and White ethnic group would not adopt behaviour change before a subsequent pregnancy because they did not view prevention of GDM in future pregnancy as a priority (63). Although GDM was often seen as an important “wake-up” call for action, healthy behaviour change after pregnancy was typically not sustained (61, 63) could also influence motivation for sustained behaviour change. In Singapore, cultural practices such as confinement diet [diet consisting of red date tea (high sugar content) and herbal soups] for 14–40 days after delivery resulted in women consuming more refined carbohydrates and indulging in cravings after confinement period (64). Thus, addressing the perceived beliefs regarding continuation of health behaviours after childbirth is crucial in a successful postpartum program (65) (Table 2).
From the healthcare providers’ perspective, challenges in postpartum OGTT include lack of familiarity of screening protocols, attitudinal barriers such as having patients underestimating the severity of T2DM and perceiving the postpartum OGTT as unnecessary or costly (66). Even more worryingly, a study reported 49% of the incomplete OGTT was attributed to providers not requesting the test (67) (Table 2).
2.2.2 Uncertainty between primary and secondary care for postpartum screening
A challenge in the management of postpartum GDM is the lack of clear directions as to who should bear the responsibility of postpartum care for women. In some countries, the primary care providers (68) are expected to follow up women with GDM with a postpartum OGTT, whereas in other countries, internists are involved in the postpartum care for women with GDM (69). In practice, the type of tests to be used in assessment of glycaemic status after childbirth, frequency and duration of follow up deviated from national guidelines (70). Most specialists (73%) recommended long-term postpartum follow up but only 39% of primary care providers recalled women with GDM for diabetes screening (70).
Fragmentation of health services is a major barrier to postpartum screening (68, 70, 71). Hewage SS et al. pointed out that women were more likely to comply to T2DM preventive measures if recommended by healthcare providers (61). However, including a postpartum specialist clinician visit did not always result in higher rates of postpartum OGTT completion (56), particularly if women with GDM were not motivated to return for postpartum screening. Of the 81.1% of women who had postpartum clinician visit, 52% did not have a postpartum OGTT despite being arranged for them prior to presentation to a postpartum clinic (56). This suggests that the way the message was framed and delivered could influence a women’s decision to adhere to postpartum healthy behaviours (61).
In some countries, establishing a registry of women with previous GDM was expected to improve uptake of postpartum OGTT (72) but real-life data on the effectiveness of the GDM registry is not yet known. In Australia, the gestational diabetes registry had facilitated the process of sending automatic reminders for women with GDM to attend pre-booked postpartum OGTT screening, leading to a 9% increase in postpartum OGTT testing (73). Using a registry to recall women with GDM into primary care for postpartum screening was also shown to be effective, suggesting a potential utility of incorporating GDM register into family practice (74) (Table 2).
2.2.3 Interventions to improve OGTT uptake may not be translatable in clinical practice
Various measures have been undertaken to overcome the barriers to postpartum OGTT testing. These include patient reminders in the form of postal (75), email or phone messages (76), verbal and written antepartum counselling, flexible appointment times, advanced order sets for glucose monitoring at 35 weeks pregnancy visit, educational modules to increase awareness amongst women regarding metabolic risk (77). Whilst all these measures show reasonable improvement in the uptake of postpartum OGTT in clinical studies, changes in postpartum OGTT screening rates in clinical practice outside the context of clinical studies were minimal (24). This suggests a gap in the translation of research to healthcare practice. Involvement of other healthcare professionals, such as nurses or case managers, seems to improve postpartum OGTT adherence (25, 78). As seen in the Women in India with Gestational Diabetes Strategies (WINGS) project in India, it is possible to obtain a 95.8% (203/212) postpartum follow-up rate through sustained efforts by trained healthcare professionals to contact women (79). Aside from periodic reminders, strategies such as offering postpartum screening to women with GDM during child immunization visits and integrating GDM screening with national public health programs have also been suggested (80). An electronic self-administered capillary OGTT device was reported to have good user-applicability by untrained individuals in community and could be tested as a screening tool in women with GDM in future (81).
Mobile applications such as smartphones and mobile apps are utilized as practical tools to motivate women to return for their postpartum follow-up (82). Early studies on mobile application-based interventions showed promising results, but long-term effectiveness of mobile applications in postpartum GDM management is unclear (83). Much work is still needed to determine the effectiveness of mobile applications in engaging a broad audience with various levels of literacy and digital experience (83).
2.2.4 Is postpartum OGTT enough to evaluate other metabolic risks?
Dyslipidemia is a physiological response in pregnancy driven by secretion of steroid hormone (e.g., progesterone), increased hepatic synthesis of triglycerides, and reduced lipoprotein lipase activity in adipose tissue (84). The characteristic finding at the 12th week of gestation is an elevated maternal triglyceride (TG) level and a mild increase in low-density lipoproteins (LDLs) and high-density lipoproteins (HDLs) (84). Altered lipid levels at 3 months postpartum (85) rarely normalise within a year after delivery (86–88). Of note, one in six women with abnormal glucose tolerance had an abnormal lipid profile postpartum, and one in four women with NGT had dyslipidemia (89). Another study reported 43% of women with GDM who had normoglycaemia at 6 months postpartum had dyslipidaemia (90). Dyslipidemia during and after pregnancy (88) aggravated endothelial dysfunction and promoted premature atherosclerosis (91), leading to increased CVD events per 10,000 person-years in women with GDM compared to those without (5.8 vs. 2.5, p < 0.0001) (88). CVD events could occur in a subset of women with GDM who did not develop intercurrent T2DM (3.2 vs. 2.2, p < 0.0001) (92). In these women, mediation analysis showed that HDL, triglycerides and LDL cholesterol (without glycaemia) contributed to elevated CVD risk at 40.8% 12.1% and 9.9%, respectively (92).
CVD monitoring and modification of CVD risk are thus critically needed in women with GDM after pregnancy. However, surveillance protocols for CVD have been mostly focused on individuals aged 40–80 years with T2DM and not on younger women with GDM (93). Females of reproductive age are less likely to be offered statin, and even if offered, they are less likely to comply (94). Therefore, future research should consider intervention strategies to reduce progression of atherosclerotic disease in women with GDM, beyond preserving the β-cell function.
2.3 Challenges in implementing postpartum interventions
2.3.1 Decision on the most appropriate postpartum intervention
Currently, the most appropriate lifestyle intervention to prevent diabetes during postpartum period is not known. The Diabetes Prevention Program (DPP) and the Finnish Diabetes Prevention Study (FDPS) have shown that lifestyle interventions were effective in reducing risk of T2DM by ∼58% in women with a history of GDM (95, 96) and in at risk non-pregnant individuals (97). However, other lifestyle intervention trials during pregnancy did not show changes in fasting glucose or insulin sensitivity (98, 99). Women enrolled in the Tianjin Gestational Diabetes Mellitus Prevention Program, had significant weight loss and reduction in plasma insulin levels in the lifestyle intervention arm compared to the control group during the first year (100) but it is unclear if these effects were sustained (101). A systematic review on lifestyle intervention conducted in at-risk population in lower-middle income countries (LMIC) showed a possible reduction in T2DM incidence by 25% but the type of lifestyle intervention was heterogenous (102).
Various factors could impact on the success of a diabetes prevention program. Besides the type of intervention (physical activity or dietary changes or both), the level of intensity of contact between the healthcare worker and women, the mode of contact and whether the trial design included patients with prior education or elements of behavioural therapy such as goal setting, stimulus control and motivational interview could influence outcomes. Participants in the DPP received 16-sessions (6 months) of intensive curriculum on behavioral change (103) to reach a 58% reduction in diabetes risk (95). In the Mothers After Gestational Diabetes in Australia Diabetes Program, a 12-months intervention consisting of program handbook, face-to-face and telephone follow up calls ensured participants achieve their health goals (104). Latino women with GDM received an 8-weeks culturally appropriate education classes and monthly support sessions over a 6-months period to sustain health behaviour change (105). In South Asian population (India, Sri Lanka and Bangladesh), a 12-months lifestyle intervention trial on diet and physical activity did not yield any change in glycaemic status at 14 months in women with GDM (106). The South Asian ethnic group is likely to have a different trajectory for developing dysglycaemia during the postpartum period. Thus, a cultural and country specific approach is clearly needed to implement diabetes prevention care after delivery (106).
Cost-effectiveness is an important factor to consider in the implementation of prevention programs for women with GDM. Unfortunately, few studies studied the cost-effectiveness of T2DM prevention in women with GDM. Werbrouck et al. concluded that an OGTT every three years could potentially lead to the lowest cost per T2DM case detected (107) but the modelling studies done were 14–30 years ago (1993–2010) and did not include incremental analysis or a comparator population of “no screening/prevention” (107). No further randomized controlled trials on the cost-effectiveness of lifestyle intervention programs has since been conducted (108), representing a clear research gap in women’s health.
Metformin and Troglitazone were studied as potential agents to reduce the risk of diabetes in women with previous GDM. Compared to the placebo, women with previous GDM (n = 350) benefited from metformin and intensive lifestyle modification, with both these interventions achieving a ∼50% and ∼53% risk reduction of diabetes, respectively (95). The effect of metformin or lifestyle intervention also persisted for 15 years in DPP study (109). Likewise, in the Troglitazone in Prevention of Diabetes (TIRPOD) study, treatment with Troglitazone (400 mg per day) in 133 women with GDM of Hispanic origin for 30 months resulted in more than 50% reduction in the incidence rate of T2DM (12.1% in Troglitazone vs. 5.4% in placebo group, P = 0.03) (110). Two-thirds of the women receiving Troglitazone had improved insulin sensitivity and a greater mean decrease in fasting glucose (110) and protection against diabetes for 8 months after stopping therapy (110). Due to concerns about hepatotoxicity, troglitazone was discontinued. Dipeptidyl-peptidase IV (DPPIV) inhibitors and sodium-glucose co-transporter 2 (SGLT2) inhibitors were studied in small number of patients with previous GDM. A proof-of-concept study in forty women with prior GDM showed that a 16-weeks treatment with metformin and sitagliptin significantly increased first-phase insulin secretion from 720.3 ± 299.0 to 995.5 ± 370.3 pmol/L (P = 0.02) but no significant change was observed with sitagliptin or metformin alone (111). In another study, women with previous GDM lost 4.9% of their original weight after 24 months of dapagliflozin-metformin combination compared to metformin (1.4% weight loss) or dapagliflozin alone (3.2%) (111). Women with prior GDM randomized to 84-weeks of metformin 2000 mg and liraglutide 1.8 mg subcutaneously per day had improved postpartum insulin sensitivity and reduced body weight compared to women receiving metformin alone (112). More studies are clearly needed to establish the optimal early postpartum treatment for this high-risk young cohort.
2.3.2 Implementation of care in high-income (HIC) and low middle-income (LMIC) countries
Challenges faced in implementing postpartum GDM care are contextual and highly dependent on the societal/cultural barriers and health system resources available for maternal care in each country. Postpartum care for women with GDM in high income countries (HIC) is at present, suboptimal (66). On an individual level, the barriers identified in HIC include fear of diagnosis of diabetes, inadequate information on postpartum care, difficulties in adhering to a healthy lifestyle long term (60, 113–117). From a health system perspective (60), challenges perceived are lack of concern on postpartum health by policy makers (67), lack of agreed quality and accountability measures between providers and patients on a global/local level (66, 118). Most countries by default, would refer women with GDM to primary care as a standard practice but quality of postpartum care in each practice varies (20, 21, 119). In Finland, a universal healthcare system exists to provide a series of intervention from primary care to preventive care and through to treatment for women with GDM (120). However, even in Finland, return rate for postpartum OGTT testing ranges from 30.9%–85.2%, with higher rates of return in areas that offer lifestyle intervention (121). In the United States, continuous care to pregnant women with or without GDM during the postpartum period depends on whether the women were enrolled in health systems that offer prevention programs (122). In Australia, postpartum care depends on whether the woman is followed up in a public or private sector (123). Those receiving postpartum OGTT test in a public sector are likely to have fragmented care due to inadequate staffing, difficulty in establishing a continuity of care after delivery (123) while those in private sectors are more likely to be enrolled in a long-term follow up programme (123).
The data on postpartum care for women with GDM in LMIC are limited, compared to HIC (66, 118, 124). Some of the challenges identified in LMIC are similar to those seen in HIC (e.g., fear/anxiety about the perceived diagnosis of overt diabetes) (125, 126). However, the more pertinent issues are associated with social and cultural issues and differences in health systems between countries (60, 118, 124, 127). Shortage of trained healthcare professionals (118), issues with transportation to health centres (128) or lack of financial means to see a healthcare professional and poor understanding on implication of GDM on long term metabolic health (125, 129) are highlighted as barriers to postpartum follow up (127). The lack of robust follow-up systems (124), guidelines or glucose equipment for postpartum care (118) pose substantial barriers to screening and counselling. Healthcare professionals in LMIC such as India or Turkey often do not recommend women with GDM to have postpartum testing according to latest evidence (130, 131). It is therefore not surprising that fewer than one in ten people with diabetes in LMIC receive the standard level of care as detailed in international guidelines (132). In LMIC, inadequate collaboration between different specialists impairs the process of coordinating care for women (124). Women often have to consult different services and specialists and the delays experienced in receiving care increases the risk of drop-outs (124).
Society and cultural factors influence the provision of care. In Southeast China (133) or Vietnam (134), GDM is perceived by women or family members as an insignificant condition that disappears after delivery and this greatly influence their care-seeking behaviour (134). Husbands’ approvals are sometimes needed before a woman seek for medical care (124). Illiteracy and the cultural expectation for woman to deliver at home results in missed opportunities to educate women and family (118). In Tonga, physical activity as a preventive measure is perceived as a “foreign” concept, resulting in a reluctance to engage in physical activity measures after delivery (135). Although society and cultural issues emerge as a prevailing factor in shaping care in LMIC, factors such as low perceived importance of postpartum GDM care by policy makers (66, 67, 118, 127), absence of financing strategies and disorganized care processes remain a common issue globally (129, 133, 134). Despite these issues, delivery of postpartum care is still possible if innovative, country-specific and culturally appropriate methods are carried out (see below) (58, 79, 136, 137).
Medical specialisation has continued to expand in LMIC but the type and number of specialists available to deliver care in a particular field may not necessarily translate to improved service availability (138). Factors like inadequate incentivisation and career advancement opportunities for specialists in public sector often lead to migration of specialists from public to private sector, which influence delivery of equitable public health services (138). Thus, country-specific policies should be in place to determine the level of health systems that require specialists’ involvement (138). Public health services data in Iran and China showed that community health workers could play a beneficial role in coaching, hypertension and diabetes prevention (139, 140). In Nepal and India, early preliminary studies suggest that mobile or tablet-based electronic decision support systems led by health workers could support patient education and improve screening and management of GDM (141, 142). A good example of success is the Women in India with GDM Strategy Project (WINGS) in Southern India (136) which showed improved GDM complications rate (79), postpartum follow up and a reversal of trend of declining physical activity associated with pregnancy with low-cost intervention n (137). Innovative measures used include having trained health workers educate on nutrition through cooking demonstrations (130, 136) or via a diet and nutrition “snakes and ladders” game (136), providing women with GDM a nutrition booklet (136) and a pedometer to increase daily step count (137), and contacting women to remind them to return for postpartum follow up (79).
3 Conclusion
Management of women with GDM has conventionally been focused on lowering the glycaemic excursion during pregnancy with the overarching aim of reducing pregnancy complications and fetal macrosomia. However, evidence suggests life-long metabolic sequalae of GDM impacts on a woman’s overall health (6, 8), and with this, the larger social construct. Despite this, care for women with GDM in the postpartum period is suboptimal. A seamless transition from obstetric care to primary care with an emphasis metabolic and cardiovascular health in women with GDM is currently non-existent. Thus, it is critical to recognize GDM as a double-edge sword, which presents as a risk to mother and child during antenatal period but also an opportunity to modify the progression to overt T2DM and CVD (143). This needs to occur in tandem with efforts from clinicians, policy makers and professional bodies. Whilst novel and emerging anti-diabetic medications could offer promise, this risk is unlikely to be fully mitigated if efforts are not made to engage, educate and empower these “high-risk” women. A system level change is required to facilitate transfer of medical information between healthcare professionals and community, and this should occur in parallel with social support programs that promote lifestyle intervention to promote a global shift in healthcare beliefs and practice. Women with previous GDM are in the most productive years of their lives, not limiting to economy contribution and family building. Evidently, an orchestrated program of care amongst different specialists and various domains is urgently needed to improve women’s health. There is clearly much work to be done before we could bridge evidence into clinical practice but overcoming the obstacles ahead is a necessary step to realise a future of diminished diabetes risk in women with GDM and their future generations.
Author contributions
PE: Conceptualization, Supervision, Writing – original draft, Writing – review & editing. AT: Writing – review & editing. TY: Writing – review & editing. CK: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
PCE is funded by NUS ExxonMobil and NMRC (National Medical Research Council) New Investigator Grant. The views expressed are those of the authors and not necessarily those of the abovementioned funders.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res Clin Pract. (2022) 183:109050. doi: 10.1016/j.diabres.2021.109050
2. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. Br Med J. (2020) 369:m1361. doi: 10.1136/bmj.m1361
3. Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes. Diabetes Care. (2002) 25:1862–8. doi: 10.2337/diacare.25.10.1862
4. Vounzoulaki E, Miksza JK, Zaccardi F, Tan BK, Davies MJ, Khunti K, et al. Association of ethnicity and socioeconomic status with health outcomes in women with gestational diabetes: clinical practice research datalink cohort study. Diabetes Metab Syndr. (2024) 18:103010. doi: 10.1016/j.dsx.2024.103010
5. Mukerji G, Chiu M, Shah BR. Impact of gestational diabetes on the risk of diabetes following pregnancy among Chinese and South Asian women. Diabetologia. (2012) 55:2148–53. doi: 10.1007/s00125-012-2549-6
6. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. (2019) 62:905–14. doi: 10.1007/s00125-019-4840-2
7. Kubihal S, Gupta Y, Shalimar Kandasamy D, Goyal A, Kalaivani M, et al. Prevalence of non-alcoholic fatty liver disease and factors associated with it in Indian women with a history of gestational diabetes mellitus. J Diabetes Investig. (2021) 12:877–85. doi: 10.1111/jdi.13411
8. Carr DB, Utzschneider KM, Hull RL, Tong J, Wallace TM, Kodama K, et al. Gestational diabetes mellitus increases the risk of cardiovascular disease in women with a family history of type 2 diabetes. Diabetes Care. (2006) 29:2078–83. doi: 10.2337/dc05-2482
9. Lee SC, Pu YB, Chow CC, Yeung VT, Ko GT, So WY, et al. Diabetes in Hong Kong Chinese: evidence for familial clustering and parental effects. Diabetes Care. (2000) 23:1365–8. doi: 10.2337/diacare.23.9.1365
10. López Stewart G. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: a world health organization guideline. Diabetes Res Clin Pract (2014) 103:341–63. doi: 10.1016/j.diabres.2013.10.012
11. Unwin N, Shaw J, Zimmet P, Alberti KGMM. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. (2002) 19:708–23. doi: 10.1046/j.1464-5491.2002.00835.x
12. Gerstein HC, Santaguida P, Raina P, Morrison KM, Balion C, Hunt D, et al. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Res Clin Pract. (2007) 78:305–12. doi: 10.1016/j.diabres.2007.05.004
13. Retnakaran R, Qi Y, Connelly PW, Sermer M, Hanley AJ, Zinman B. Risk of early progression to pre-diabetes or diabetes in women with recent gestational dysglycemia but normal glucose tolerance at 3-months postpartum. Clin Endocrinol (Oxf). (2010) 73:476–483. doi: 10.1111/j.1365-2265.2010.03834.x
14. Gastaldelli A, Ferrannini E, Miyazaki Y, Matsuda M, DeFronzo RA. Beta-cell dysfunction and glucose intolerance: results from the San Antonio metabolism (SAM) study. Diabetologia. (2004) 47:31–9. doi: 10.1007/s00125-003-1263-9
15. Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJG, Zinman B. An abnormal screening glucose challenge test in pregnancy predicts postpartum metabolic dysfunction, even when the antepartum oral glucose tolerance test is normal. Clin Endocrinol (Oxf). (2009) 71:208–14. doi: 10.1111/j.1365-2265.2008.03460.x
16. Retnakaran R, Qi Y, Sermer M, Connelly PW, Hanley AJG, Zinman B. β-Cell function declines within the first year postpartum in women with recent glucose intolerance in pregnancy. Diabetes Care. (2010) 33:1798–804. doi: 10.2337/dc10-0351
17. Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. Can Med Assoc J. (2009) 181:371–6. doi: 10.1503/cmaj.090569
18. Retnakaran R, Ye C, Kramer CK, Hanley AJ, Connelly PW, Sermer M, et al. Deteriorating beta cell function is the dominant determinant of progression from normal glucose tolerance to prediabetes/diabetes in young women following pregnancy. Diabetologia. (2023) 66:2154–63. doi: 10.1007/s00125-023-05994-5
19. Cheng AYY. Canadian diabetes association 2013 clinical practice guidelines for prevention and management of diabetes in Canada. Can J Diabetes. (2013) 37:S1–3. doi: 10.1016/j.jcjd.2013.01.009
20. College of Obstetrictians and Gynaecologist Singapore. Guidelines for the Management of Gestational Diabetes Mellitus. Available online at: https://www.ams.edu.sg/view-pdf.aspx?file=media%5c4163_fi_430.pdf&ofile=COGS+GDM+Guidelines+2018.pdf (Accessed August 1, 2024).
21. American College of Obstetrics and Gynecology. Practice bulletin no. 137: gestational diabetes mellitus. Obstet Gynecol. (2013) 122:406–16. doi: 10.1097/01.AOG.0000433006.09219.f1
22. Bergman M, Jagannathan R, Buysschaert M, Pareek M, Olsen MH, Nilsson PM, et al. Lessons learned from the 1 h post-load glucose level during OGTT: current screening recommendations for dysglycaemia should be revised. Diabetes Metabolism Res. (2018) 34:e2992. doi: 10.1002/dmrr.2992
23. Hiersch L, Shah BR, Berger H, Geary M, McDonald SD, Murray-Davis B, et al. Oral glucose tolerance test results in pregnancy can be used to individualize the risk of future maternal type 2 diabetes mellitus in women with gestational diabetes mellitus. Diabetes Care. (2021) 44:1860–7. doi: 10.2337/dc21-0659
24. Pastore I, Chiefari E, Vero R, Brunetti A. Postpartum glucose intolerance: an updated overview. Endocrine. (2018) 59:481–94. doi: 10.1007/s12020-017-1388-0
25. Hunt KJ, Conway DL. Who returns for postpartum glucose screening following gestational diabetes mellitus? Am J Obstet Gynecol. (2008) 198:404.e1–e6. doi: 10.1016/j.ajog.2007.09.015
26. Abdul-Ghani MA, Abdul-Ghani T, Ali N, DeFronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. (2008) 31:1650–5. doi: 10.2337/dc08-0225
27. Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care. (2007) 30:1544–8. doi: 10.2337/dc06-1331
28. Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes. Diabetes Care. (2009) 32:281–6. doi: 10.2337/dc08-1264
29. Bergman M, Chetrit A, Roth J, Jagannathan R, Sevick M, Dankner R. One-hour post-load plasma glucose level during the OGTT predicts dysglycemia. Diabetes Res Clin Pract. (2016) 120:221–8. doi: 10.1016/j.diabres.2016.08.013
30. Bianchi C, Miccoli R, Trombetta M, Giorgino F, Frontoni S, Faloia E, et al. Elevated 1 h postload plasma glucose levels identify subjects with normal glucose tolerance but impaired β-cell function, insulin resistance, and worse cardiovascular risk profile: the GENFIEV study. J Clin Endocrinol Metab. (2013) 98:2100–5. doi: 10.1210/jc.2012-3971
31. Succurro E, Marini MA, Arturi F, Grembiale A, Lugarà M, Andreozzi F, et al. Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis. (2009) 207:245–9. doi: 10.1016/j.atherosclerosis.2009.04.006
32. Andreozzi F, Mannino GC, Perticone M, Perticone F, Sesti G. Elevated 1 h post-load plasma glucose levels in subjects with normal glucose tolerance are associated with a pro-atherogenic lipid profile. Atherosclerosis. (2017) 256:15–20. doi: 10.1016/j.atherosclerosis.2016.11.020
33. Pareek M, Bhatt DL, Nielsen ML, Jagannathan R, Eriksson K-F, Nilsson PM, et al. Enhanced predictive capability of a 1 h oral glucose tolerance test: a prospective population-based cohort study. Diabetes Care. (2018) 41:171–7. doi: 10.2337/dc17-1351
34. Oh TJ, Lim S, Kim KM, Moon JH, Choi SH, Cho YM, et al. One-hour postload plasma glucose concentration in people with normal glucose homeostasis predicts future diabetes mellitus: a 12-year community-based cohort study. Clin Endocrinol (Oxf). (2017) 86:513–9. doi: 10.1111/cen.13280
35. Oka R, Aizawa T, Miyamoto S, Yoneda T, Yamagishi M. One-hour plasma glucose as a predictor of the development of type 2 diabetes in Japanese adults. Diabet Med. (2016) 33:1399–405. doi: 10.1111/dme.12994
36. Paddock E, Looker HC, Piaggi P, Knowler WC, Krakoff J, Chang DC. One-hour plasma glucose compared with two-hour plasma glucose in relation to diabetic retinopathy in American Indians. Dia Care. (2018) 41:1212–7. doi: 10.2337/dc17-1900
37. Sai Prasanna N, Amutha A, Pramodkumar TA, Anjana RM, Venkatesan U, Priya M, et al. The 1 h post glucose value best predicts future dysglycemia among normal glucose tolerance subjects. J Diabetes Complicat. (2017) 31:1592–6. doi: 10.1016/j.jdiacomp.2017.07.017
38. Abdul-Ghani MA, Abdul-Ghani T, Müller G, Bergmann A, Fischer S, Bornstein S, et al. Role of glycated hemoglobin in the prediction of future risk of T2DM. J Clin Endocrinol Metab. (2011) 96:2596–600. doi: 10.1210/jc.2010-1698
39. Jagannathan R, Sevick MA, Fink D, Dankner R, Chetrit A, Roth J, et al. The 1 h post-load glucose level is more effective than HbA1c for screening dysglycemia. Acta Diabetol. (2016) 53:543–50. doi: 10.1007/s00592-015-0829-6
40. Bergman M, Manco M, Satman I, Chan J, Schmidt MI, Sesti G, et al. International diabetes federation position statement on the 1 h post-load plasma glucose for the diagnosis of intermediate hyperglycaemia and type 2 diabetes. Diabetes Res Clin Pract. (2024) 209:111589. doi: 10.1016/j.diabres.2024.111589
41. Succurro E, Fraticelli F, Franzago M, Fiorentino TV, Andreozzi F, Vitacolonna E, et al. Hyperglycemia at 1 h-OGTT in pregnancy: a reliable predictor of metabolic outcomes? Front Endocrinol. (2021) 12:612829. doi: 10.3389/fendo.2021.612829
42. Lorenzo C, Wagenknecht LE, Hanley AJG, Rewers MJ, Karter AJ, Haffner SM. A1c between 5.7 and 6.4% as a marker for identifying pre-diabetes, insulin sensitivity and secretion, and cardiovascular risk factors. Diabetes Care. (2010) 33:2104–9. doi: 10.2337/dc10-0679
43. McClean S, Farrar D, Kelly CA, Tuffnell DJ, Whitelaw DC. The importance of postpartum glucose tolerance testing after pregnancies complicated by gestational diabetes. Diabetic Med. (2010) 27:650–4. doi: 10.1111/j.1464-5491.2010.03001.x
44. Picón MJ, Murri M, Muñoz A, Fernández-García JC, Gomez-Huelgas R, Tinahones FJ. Hemoglobin A1c versus oral glucose tolerance test in postpartum diabetes screening. Diabetes Care. (2012) 35:1648–53. doi: 10.2337/dc11-2111
45. Hardikar PS, Joshi SM, Bhat DS, Raut DA, Katre PA, Lubree HG, et al. Spuriously high prevalence of prediabetes diagnosed by HbA1c in young Indians partly explained by hematological factors and iron deficiency anemia. Diabetes Care. (2012) 35:797–802. doi: 10.2337/dc11-1321
46. Lapolla A, Mosca A, Fedele D. The general use of glycated haemoglobin for the diagnosis of diabetes and other categories of glucose intolerance: still a long way to go. Nutr Metab Cardiovasc Dis. (2011) 21:467–75. doi: 10.1016/j.numecd.2011.02.006
47. Herman WH, Ma Y, Uwaifo G, Haffner S, Kahn SE, Horton ES, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the diabetes prevention program. Diabetes Care. (2007) 30:2453–7. doi: 10.2337/dc06-2003
48. Kim C, Bullard KM, Herman WH, Beckles GL. Association between iron deficiency and A1C levels among adults without diabetes in the national health and nutrition examination survey, 1999–2006. Diabetes Care. (2010) 33:780–5. doi: 10.2337/dc09-0836
49. American Diabetes Association. 14. Management of diabetes in pregnancy: standards of medical care in diabetes—2020. Diabetes Care. (2020) 43:S183–92. doi: 10.2337/dc20-S014
50. Göbl CS, Bozkurt L, Yarragudi R, Tura A, Pacini G, Kautzky-Willer A. Is early postpartum HbA1c an appropriate risk predictor after pregnancy with gestational diabetes mellitus? Acta Diabetol. (2014) 51:715–22. doi: 10.1007/s00592-014-0574-2
51. Goyal A, Gupta Y, Kubihal S, Kalaivani M, Bhatla N, Tandon N. Utility of screening fasting plasma glucose and glycated hemoglobin to circumvent the need for oral glucose tolerance test in women with prior gestational diabetes. Adv Ther. (2021) 38:1342–51. doi: 10.1007/s12325-020-01618-1
52. Rudland VL, Price SAL, Hughes R, Barrett HL, Lagstrom J, Porter C, et al. ADIPS 2020 guideline for pre-existing diabetes and pregnancy. Aust NZ J Obst Gynaeco. (2020) 60:E18–52. doi: 10.1111/ajo.13265
53. Blumer I, Hadar E, Hadden DR, Jovanovič L, Mestman JH, Murad MH, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. (2013) 98:4227–49. doi: 10.1210/jc.2013-2465
54. American Diabetes Association. Management of diabetes in pregnancy. Diabetes Care. (2021) 44:S200–10. doi: 10.2337/dc21-S014
55. National Institute of Clinical Excellence (NICE) guidelines. Committee Opinion, Diabetes in Pregnancy: Management of Diabetes and Its Complications from Preconception to the Postnatal Period. Clinical Guideline NG3 Article Online (2017). http://www.nice.org.uk/guidance/ng3/resources/diabetes-in-pregnancy-management-of-diabetes-and-itscomplications-from-preconceptionto-the-postnatal-period-51038446021 (Accessed November 14, 2023).
56. Paul JC, Fitzpatrick JJ. Postpartum glucose screening among women with gestational diabetes. Appl Nurs Res. (2020) 56:151341. doi: 10.1016/j.apnr.2020.151341
57. de Gennaro G, Bianchi C, Aragona M, Battini L, Baronti W, Brocchi A, et al. Postpartum screening for type 2 diabetes mellitus in women with gestational diabetes: is it really performed? Diabetes Res Clin Pract. (2020) 166:108309. doi: 10.1016/j.diabres.2020.108309
58. Goyal A, Gupta Y, Kalaivani M, Sankar MJ, Kachhawa G, Bhatla N, et al. Long term (>1 year) postpartum glucose tolerance status among Indian women with history of gestational diabetes mellitus (GDM) diagnosed by IADPSG criteria. Diabetes Res Clin Pract. (2018) 142:154–61. doi: 10.1016/j.diabres.2018.05.027
59. Smirnakis KV, Chasan-Taber L, Wolf M, Markenson G, Ecker JL, Thadhani R. Postpartum diabetes screening in women with a history of gestational diabetes. Obstet Gynecol. (2005) 106:1297–303. doi: 10.1097/01.AOG.0000189081.46925.90
60. Bennett WL, Ennen CS, Carrese JA, Hill-Briggs F, Levine DM, Nicholson WK, et al. Barriers to and facilitators of postpartum follow-up care in women with recent gestational diabetes mellitus: a qualitative study. J Women’s Health. (2011) 20:239–45. doi: 10.1089/jwh.2010.2233
61. Hewage SS, Aw S, Chi C, Yoong J. Factors associated with intended postpartum OGTT uptake and willingness to receive preventive behavior support to reduce type 2 diabetes risk among women with gestational diabetes in Singapore: an exploratory study. Nutr Metab Insights. (2021) 14:117863882110168. doi: 10.1177/11786388211016827
62. Dennison RA, Fox RA, Ward RJ, Griffin SJ, Usher-Smith JA. Women’s views on screening for type 2 diabetes after gestational diabetes: a systematic review, qualitative synthesis and recommendations for increasing uptake. Diabet Med. (2020) 37:29–43. doi: 10.1111/dme.14081
63. Tang JW, Foster KE, Pumarino J, Ackermann RT, Peaceman AM, Cameron KA. Perspectives on prevention of type 2 diabetes after gestational diabetes: a qualitative study of hispanic, African-American and white women. Matern Child Health J. (2015) 19:1526–34. doi: 10.1007/s10995-014-1657-y
64. Teh K, Quek IP, Tang WE. Postpartum dietary and physical activity-related beliefs and behaviors among women with recent gestational diabetes mellitus: a qualitative study from Singapore. BMC Pregnancy Childbirth. (2021) 21:612. doi: 10.1186/s12884-021-04089-6
65. Neven ACH, Lake AJ, Williams A, O’Reilly SL, Hendrieckx C, Morrison M, et al. Barriers to and enablers of postpartum health behaviours among women from diverse cultural backgrounds with prior gestational diabetes: a systematic review and qualitative synthesis applying the theoretical domains framework. Diabet Med. (2022) 39:e14945. doi: 10.1111/dme.14945
66. Nielsen KK, Kapur A, Damm P, de Courten M, Bygbjerg IC. From screening to postpartum follow-up—the determinants and barriers for gestational diabetes mellitus (GDM) services, a systematic review. BMC Pregnancy Childbirth. (2014) 14:41. doi: 10.1186/1471-2393-14-41
67. Battarbee A, Yee L. Barriers to postpartum follow-up and glucose tolerance testing in women with gestational diabetes mellitus. Amer J Perinatol. (2018) 35:354–60. doi: 10.1055/s-0037-1607284
68. Stuebe A, Ecker J, Bates D, Zera C, Bentley-Lewis R, Seely E. Barriers to follow-up for women with a history of gestational diabetes. Amer J Perinatol. (2010) 27:705–10. doi: 10.1055/s-0030-1253102
69. Shah B, Lipscombe L, Feig D, Lowe J. Missed opportunities for type 2 diabetes testing following gestational diabetes: a population-based cohort study: type 2 diabetes testing after GDM. BJOG: An Int J Obstet Gynaecol. (2011) 118:1484–90. doi: 10.1111/j.1471-0528.2011.03083.x
70. Pierce M, Modder J, Mortagy I, Springett A, Hughes H, Baldeweg S. Missed opportunities for diabetes prevention: post-pregnancy follow-up of women with gestational diabetes mellitus in England. Br J Gen Pract. (2011) 61:e611–9. doi: 10.3399/bjgp11X601316
71. Devsam BU, Bogossian FE, Peacock AS. An interpretive review of women’s experiences of gestational diabetes mellitus: proposing a framework to enhance midwifery assessment. Women Birth. (2013) 26:e69–76. doi: 10.1016/j.wombi.2012.12.003
72. Atalag K, Zivaljevic A, Eagleton C, Pickering K. A standards-based approach to development of clinical registries—initial lessons learnt from the gestational diabetes registry. In Conference Paper: 13th Annual Health Informatics New Zealand (HINZ) at: Auckland. (2014). p. 1–7. doi: 10.13140/2.1.2861.7283
73. Boyle DIR, Versace VL, Dunbar JA, Scheil W, Janus E, Oats JJN, et al. Results of the first recorded evaluation of a national gestational diabetes mellitus register: challenges in screening, registration, and follow-up for diabetes risk. PLoS One. (2018) 13:e0200832. doi: 10.1371/journal.pone.0200832
74. O’Reilly SL, May CR, Ford D, Dunbar JA. Implementing primary care diabetes prevention for women with previous gestational diabetes: a mixed-methods study. Fam Pract. (2022) 39:1080–6. doi: 10.1093/fampra/cmac022
75. Clark HD, Graham ID, Karovitch A, Keely EJ. Do postal reminders increase postpartum screening of diabetes mellitus in women with gestational diabetes mellitus? A randomized controlled trial. Am J Obstet Gynecol. (2009) 200:634.e1–e7. doi: 10.1016/j.ajog.2009.01.003
76. Sanderson H, Loveman E, Colquitt J, Royle P, Waugh N, Tan B. Improving uptake of postnatal checking of blood glucose in women who had gestational diabetes mellitus in universal healthcare settings: a systematic review. JCM. (2018) 8:4. doi: 10.3390/jcm8010004
77. Huang J, Forde R, Parsons J, Zhao X, Wang J, Liu Y, et al. Interventions to increase the uptake of postpartum diabetes screening among women with previous gestational diabetes: a systematic review and Bayesian network meta-analysis. Am J Obstet Gynecol MFM. (2023) 5:101137. doi: 10.1016/j.ajogmf.2023.101137
78. Vesco KK, Dietz PM, Bulkley J, Bruce FC, Callaghan WM, England L, et al. A system-based intervention to improve postpartum diabetes screening among women with gestational diabetes. Am J Obstet Gynecol. (2012) 207:283.e1–e6. doi: 10.1016/j.ajog.2012.08.017
79. Bhavadharini B, Anjana RM, Mahalakshmi MM, Maheswari K, Kayal A, Unnikrishnan R, et al. Glucose tolerance status of Asian Indian women with gestational diabetes at 6 weeks to 1 year postpartum (WINGS-7). Diabetes Res Clin Pract. (2016) 117:22–7. doi: 10.1016/j.diabres.2016.04.050
80. Tandon N, Gupta Y, Kalra S. Postpartum screening after gestational diabetes mellitus: aiming for universal coverage. Indian J Endocrinol Metab. (2015) 19:1–4. doi: 10.4103/2230-8210.144634
81. Bethel MA, Price HC, Sourij H, White S, Coleman RL, Ring A, et al. Evaluation of a self-administered oral glucose tolerance test. Diabetes Care. (2013) 36:1483–8. doi: 10.2337/dc12-0643
82. Yadav P, Kant R, Kishore S, Barnwal S, Khapre M. The impact of mobile health interventions on antenatal and postnatal care utilization in low- and middle-income countries: a meta-analysis. Cureus. (2022) 14:e21256. doi: 10.7759/cureus.21256
83. Sherifali D, Nerenberg KA, Wilson S, Semeniuk K, Ali MU, Redman LM, et al. The effectiveness of eHealth technologies on weight management in pregnant and postpartum women: systematic review and meta-analysis. J Med Internet Res. (2017) 19:e337. doi: 10.2196/jmir.8006
84. Herrera E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. ENDO. (2002) 19:43–56. doi: 10.1385/ENDO:19:1:43
85. Retnakaran R, Qi Y, Connelly PW, Sermer M, Hanley AJ, Zinman B. The graded relationship between glucose tolerance status in pregnancy and postpartum levels of low-density-lipoprotein cholesterol and apolipoprotein B in young women: implications for future cardiovascular risk. J Clin Endocrinol Metab. (2010) 95:4345–53. doi: 10.1210/jc.2010-0361
86. Prados M, Flores-Le Roux JA, Benaiges D, Llauradó G, Chillarón JJ, Paya A, et al. Previous gestational diabetes increases atherogenic dyslipidemia in subsequent pregnancy and postpartum. Lipids. (2018) 53:387–92. doi: 10.1002/lipd.12040
87. Zhu Y, Zhu H, Dang Q, Yang Q, Huang D, Zhang Y, et al. Changes in serum TG levels during pregnancy and their association with postpartum hypertriglyceridemia: a population-based prospective cohort study. Lipids Health Dis. (2021) 20:119. doi: 10.1186/s12944-021-01549-y
88. Lowe LP, Perak AM, Kuang A, Lloyd-Jones DM, Sacks DA, Deerochanawong C, et al. Associations of glycemia and lipid levels in pregnancy with dyslipidemia 10–14 years later: the HAPO follow-up study. Diabetes Res Clin Pract. (2022) 185:109790. doi: 10.1016/j.diabres.2022.109790
89. Tobias DK, Stuart JJ, Li S, Chavarro J, Rimm EB, Rich-Edwards J, et al. Association of history of gestational diabetes with long-term cardiovascular disease risk in a large prospective cohort of US women. JAMA Intern Med. (2017) 177:1735. doi: 10.1001/jamainternmed.2017.2790
90. Jotic AZ, Stoiljkovic MM, Milicic TJ, Lalic KS, Lukic LZ, Macesic MV, et al. Development of ALOHa-G risk score for detecting postpartum dyslipidemia among normoglycemic women with previous gestational diabetes: observational cohort study. Diabetes Ther. (2023) 14:857–67. doi: 10.1007/s13300-023-01387-4
91. Van JAD, Luo Y, Danska JS, Dai F, Alexeeff SE, Gunderson EP, et al. Postpartum defects in inflammatory response after gestational diabetes precede progression to type 2 diabetes: a nested case-control study within the SWIFT study. Metab Clin Exp. (2023) 149:155695. doi: 10.1016/j.metabol.2023.155695
92. Retnakaran R, Shah BR. Mediating effect of vascular risk factors underlying the link between gestational diabetes and cardiovascular disease. BMC Med. (2022) 20:389. doi: 10.1186/s12916-022-02581-0
93. Authors/Task Force Members: Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, et al. European guidelines on cardiovascular disease prevention in clinical practice (version 2012): the fifth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) * developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Eur Heart J. (2012) 33:1635–701. doi: 10.1093/eurheartj/ehs092
94. Gheorghe G, Toth PP, Bungau S, Behl T, Ilie M, Pantea Stoian A, et al. Cardiovascular risk and statin therapy considerations in women. Diagnostics. (2020) 10:483. doi: 10.3390/diagnostics10070483
95. Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, et al. The diabetes prevention program research group. prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. (2008) 93:4774–9. doi: 10.1210/jc.2008-0772
96. Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. (2002) 346:393–403. doi: 10.1056/NEJMoa012512
97. Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. (2001) 344:1343–50. doi: 10.1056/NEJM200105033441801
98. Oostdam N, van Poppel M, Wouters M, Eekhoff E, Bekedam D, Kuchenbecker W, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG. (2012) 119:1098–107. doi: 10.1111/j.1471-0528.2012.03366.x
99. Jelsma JG, van Poppel MN, Galjaard S, Desoye G, Corcoy R, Devlieger R, et al. DALI: vitamin D and lifestyle intervention for gestational diabetes mellitus (GDM) prevention: an European multicentre, randomised trial—study protocol. BMC Pregnancy Childbirth. (2013) 13:142. doi: 10.1186/1471-2393-13-142
100. Hu G, Tian H, Zhang F, Liu H, Zhang C, Zhang S, et al. Tianjin gestational diabetes mellitus prevention program. Diabetes Res Clin Pract. (2012) 98:508–17. doi: 10.1016/j.diabres.2012.09.015
101. Egan AM, Simmons D. Lessons learned from lifestyle prevention trials in gestational diabetes mellitus. Diabet Med. (2019) 36:142–50. doi: 10.1111/dme.13772
102. Sagastume D, Siero I, Mertens E, Cottam J, Colizzi C, Peñalvo JL. The effectiveness of lifestyle interventions on type 2 diabetes and gestational diabetes incidence and cardiometabolic outcomes: a systematic review and meta-analysis of evidence from low- and middle-income countries. EClinicalMedicine. (2022) 53:101650. doi: 10.1016/j.eclinm.2022.101650
103. Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the diabetes prevention program outcomes study. Lancet. (2009) 374:1677–86. doi: 10.1016/S0140-6736(09)61457-4
104. Shih ST, Davis-Lameloise N, Janus ED, Wildey C, Versace VL, Hagger V, et al. Mothers after gestational diabetes in Australia diabetes prevention program (MAGDA-DPP) post-natal intervention: study protocol for a randomized controlled trial. Trials. (2013) 14:339. doi: 10.1186/1745-6215-14-339
105. Philis-Tsimikas A, Fortmann AL, Dharkar-Surber S, Euyoque JA, Ruiz M, Schultz J, et al. Dulce mothers: an intervention to reduce diabetes and cardiovascular risk in Latinas after gestational diabetes. Behav Med Pract Policy Res. (2014) 4:18–25. doi: 10.1007/s13142-014-0253-4
106. Tandon N, Gupta Y, Kapoor D, Lakshmi JK, Praveen D, Bhattacharya A, et al. Effects of a lifestyle intervention to prevent deterioration in glycemic status among South Asian women with recent gestational diabetes: a randomized clinical trial. JAMA Netw Open. (2022) 5:e220773. doi: 10.1001/jamanetworkopen.2022.0773
107. Werbrouck A, Schmidt M, Putman K, Benhalima K, Verhaeghe N, Annemans L, et al. A systematic review on costs and cost-effectiveness of screening and prevention of type 2 diabetes in women with prior gestational diabetes: exploring uncharted territory. Diabetes Res Clin Pract. (2019) 147:138–48. doi: 10.1016/j.diabres.2018.11.012
108. Hewage SS, Wu S, Neelakantan N, Yoong J. Systematic review of effectiveness and cost-effectiveness of lifestyle interventions to improve clinical diabetes outcome measures in women with a history of GDM. Clin Nutr ESPEN. (2020) 35:20–9. doi: 10.1016/j.clnesp.2019.10.011
109. Diabetes Prevention Program Research Group, Nathan DM, Knowler WC, Edelstein S, Crandall JP, et al. Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the diabetes prevention program and diabetes prevention program outcomes study. Diabetes Care. (2019) 42:601–8. doi: 10.2337/dc18-1970
110. Willi SM, Kennedy A, Wallace P, Ganaway E, Rogers NL, Garvey WT. Troglitazone antagonizes metabolic effects of glucocorticoids in humans. Diabetes. (2002) 51:2895–902. doi: 10.2337/diabetes.51.10.2895
111. Elkind-Hirsch KE, Paterson MS, Shaler D, Gutowski HC. Short-term sitagliptin-metformin therapy is more effective than metformin or placebo in prior gestational diabetic women with impaired glucose regulation. Endocr Pract. (2018) 24:361–8. doi: 10.4158/EP-2017-0251
112. Elkind-Hirsch KE, Shaler D, Harris R. Postpartum treatment with liraglutide in combination with metformin versus metformin monotherapy to improve metabolic status and reduce body weight in overweight/obese women with recent gestational diabetes: a double-blind, randomized, placebo-controlled study. J Diabetes Complicat. (2020) 34:107548. doi: 10.1016/j.jdiacomp.2020.107548
113. Razee H, van der Ploeg HP, Blignault I, Smith BJ, Bauman AE, McLean M, et al. Beliefs, barriers, social support, and environmental influences related to diabetes risk behaviours among women with a history of gestational diabetes. Health Promot J Aust. (2010) 21:130–7. doi: 10.1071/HE10130
114. Laureij LT, van der Hulst M, Lagendijk J, Been JV, Ernst-Smelt HE, Franx A, et al. Insight into the process of postpartum care utilisation and in-home support among vulnerable women in the Netherlands: an in-depth qualitative exploration. BMJ Open. (2021) 11:e046696. doi: 10.1136/bmjopen-2020-046696
115. Collier SA, Mulholland C, Williams J, Mersereau P, Turay K, Prue C. A qualitative study of perceived barriers to management of diabetes among women with a history of diabetes during pregnancy. J Women’s Health. (2011) 20:1333–9. doi: 10.1089/jwh.2010.2676
116. Mersereau P, Williams J, Collier SA, Mulholland C, Turay K, Prue C. Barriers to managing diabetes during pregnancy: the perceptions of health care practitioners. Birth. (2011) 38:142–9. doi: 10.1111/j.1523-536X.2010.00464.x
117. Taylor C, Bhavnani V, Zasada M, Ussher M, Bick D; SWAN trial team; SWAN trial team. Barriers and facilitators to uptake and retention of inner-city ethnically diverse women in a postnatal weight management intervention: a mixed-methods process evaluation within a feasibility trial in England. BMJ Open. (2020) 10:e034747. doi: 10.1136/bmjopen-2019-034747
118. Nielsen KK, de Courten M, Kapur A. Health system and societal barriers for gestational diabetes mellitus (GDM) services—lessons from world diabetes foundation supported GDM projects. BMC Int Health Hum Rights. (2012) 12:33. doi: 10.1186/1472-698X-12-33
119. National Institute for Health and care Excellence. Diabetes in Pregnancy: Management from Preconception to the Postnatal Period (2015). Available online at: https://www.nice.org.uk/guidance/ng3 (Accessed August 7, 2024).
120. Teperi J, Porter M, Vuorenkoski L, Baron JF. The Finnish Health Care System: A Value-Based Perspective. Sitra Reports (2009). https://www.hbs.edu/ris/Publication%20Files/Finnish_Health_Care_System_SITRA2009_78584c8b-10c4-4206-9f9a-441bf8be1a2c.pdf (Accessed August 8, 2024).
121. Korpi-Hyövälti E, Laaksonen DE, Schwab U, Heinonen S, Niskanen L. How can we increase postpartum glucose screening in women at high risk for gestational diabetes mellitus? Int J Endocrinol. (2012) 2012:519267. doi: 10.1155/2012/519267
122. Ferrara A, Hedderson MM, Brown SD, Albright CL, Ehrlich SF, Tsai A-L, et al. The comparative effectiveness of diabetes prevention strategies to reduce postpartum weight retention in women with gestational diabetes mellitus: the gestational diabetes’ effects on moms (GEM) cluster randomized controlled trial. Diabetes Care. (2016) 39:65–74. doi: 10.2337/dc15-1254
123. Rayner J-A, McLachlan HL, Peters L, Forster DA. Care providers’ views and experiences of postnatal care in private hospitals in Victoria, Australia. Midwifery. (2013) 29:622–7. doi: 10.1016/j.midw.2012.05.006
124. Utz B, De Brouwere V. Why screen if we cannot follow-up and manage?” challenges for gestational diabetes screening and management in low and lower-middle income countries: results of a cross-sectional survey. BMC Pregnancy Childbirth. (2016) 16:341. doi: 10.1186/s12884-016-1143-1
125. Tewari A, Praveen D, Madhira P, Josyula LK, Joshi R, Kokku SB, et al. Feasibility of a lifestyle intervention program for prevention of diabetes among women with prior gestational diabetes mellitus (LIVING study) in South Asia: a formative research study. Front Glob Womens Health. (2020) 1:587607. doi: 10.3389/fgwh.2020.587607
126. Muhwava LS, Murphy K, Zarowsky C, Levitt N. Perspectives on the psychological and emotional burden of having gestational diabetes amongst low-income women in Cape Town, South Africa. BMC Women’s Health. (2020) 20:231. doi: 10.1186/s12905-020-01093-4
127. Kragelund Nielsen K, Damm P, Bygbjerg I, Kapur A. Barriers and facilitators for implementing programmes and services to address hyperglycaemia in pregnancy in low and middle income countries: a systematic review. Diabetes Res Clin Pract. (2018) 145:102–18. doi: 10.1016/j.diabres.2018.04.009
128. Oza-Frank R, Conrey E, Bouchard J, Shellhaas C, Weber MB. Healthcare experiences of low-income women with prior gestational diabetes. Matern Child Health J. (2018) 22:1059–66. doi: 10.1007/s10995-018-2489-y
129. Ge L, Wikby K, Rask M. ‘Is gestational diabetes a severe illness?’ exploring beliefs and self-care behaviour among women with gestational diabetes living in a rural area of the South East of China. Aust J Rural Health. (2016) 24:378–84. doi: 10.1111/ajr.12292
130. Mahalakshmi M, Bhavadharini B, Maheswari K, Anjana R, Jebarani S, Ninov L, et al. Current practices in the diagnosis and management of gestational diabetes mellitus in India (WINGS-5). Indian J Endocr Metab. (2016) 20:364. doi: 10.4103/2230-8210.180001
131. Aluş Tokat M, Sancı M, Girgeç S, Kulhan NG, Özcan ÇY. Postpartum education and lifestyle changes for preventing type 2 diabetes in Turkish women with previous gestational diabetes: a retrospective study. Int J of Nursing Practice. (2016) 22:427–35. doi: 10.1111/ijn.12452
132. Flood D, Seiglie JA, Dunn M, Tschida S, Theilmann M, Marcus ME, et al. The state of diabetes treatment coverage in 55 low-income and middle-income countries: a cross-sectional study of nationally representative, individual-level data in 680 102 adults. Lancet Health Longev. (2021) 2:e340–51. doi: 10.1016/S2666-7568(21)00089-1
133. Ge L, Albin B, Hadziabdic E, Hjelm K, Rask M. Beliefs about health and illness and health-related behavior among urban women with gestational diabetes mellitus in the South East of China. J Transcult Nurs. (2016) 27:593–602. doi: 10.1177/1043659615594677
134. Gammeltoft TM, Nguyen TA, Dung TK, Thi Dang N-A, Phuong Nguyen TM, Nguyen VT, et al. The pioneers of Vietnam’s epidemiological transition: an ethnographic study of pregnant women’s experiences of gestational diabetes. Glob Health Action. (2024) 17:2341521. doi: 10.1080/16549716.2024.2341521
135. Doran F, Davis K. Gestational diabetes mellitus in Tonga: insights from healthcare professionals and women who experienced gestational diabetes mellitus. N Z Med J. (2010) 123:59–67. PMID: 21326400.21326400
136. Kayal A, Mohan V, Malanda B, Anjana R, Bhavadharini B, Mahalakshmi M, et al. Women in India with gestational diabetes mellitus strategy (WINGS): methodology and development of model of care for gestational diabetes mellitus (WINGS 4). Indian J Endocr Metab. (2016) 20:707. doi: 10.4103/2230-8210.189230
137. Anjana RM, Sudha V, Lakshmipriya N, Anitha C, Unnikrishnan R, Bhavadharini B, et al. Physical activity patterns and gestational diabetes outcomes—the wings project. Diabetes Res Clin Pract. (2016) 116:253–62. doi: 10.1016/j.diabres.2016.04.041
138. Sriram V, Bennett S. Strengthening medical specialisation policy in low-income and middle-income countries. BMJ Glob Health. (2020) 5:e002053. doi: 10.1136/bmjgh-2019-002053
139. Farzadfar F, Murray CJ, Gakidou E, Bossert T, Namdaritabar H, Alikhani S, et al. Effectiveness of diabetes and hypertension management by rural primary health-care workers (behvarz workers) in Iran: a nationally representative observational study. Lancet. (2012) 379:47–54. doi: 10.1016/S0140-6736(11)61349-4
140. Liang X, Chen J, Liu Y, He C, Li T. The effect of hypertension and diabetes management in Southwest China: a before- and after-intervention study. PLoS One. (2014) 9:e91801. doi: 10.1371/journal.pone.0091801
141. Shanmugavel A, Shakya PR, Shrestha A, Nepal J, Shrestha A, Daneault J-F, et al. Designing and developing a mobile app for management and treatment of gestational diabetes in Nepal: user-centered design study. JMIR Form Res. (2024) 8:e50823. doi: 10.2196/50823
142. Mohan S, Chaudhry M, McCarthy O, Jarhyan P, Calvert C, Jindal D, et al. A cluster randomized controlled trial of an electronic decision-support system to enhance antenatal care services in pregnancy at primary healthcare level in Telangana, India: trial protocol. BMC Pregnancy Childbirth. (2023) 23:72. doi: 10.1186/s12884-022-05249-y
Keywords: gestational diabetes, postpartum, cardiovascular disease, impaired glucose tolerance, oral glucose tolerance test (OGTT)
Citation: Eng PC, Teo AED, Yew TW and Khoo CM (2024) Implementing care for women with gestational diabetes after delivery—the challenges ahead. Front. Glob. Womens Health 5:1391213. doi: 10.3389/fgwh.2024.1391213
Received: 25 February 2024; Accepted: 31 July 2024;
Published: 16 August 2024.
Edited by:
Ilaria Fantasia, Di Venere and Sarcone Hospitals, ItalyReviewed by:
Lorrein Muhwava-Mbabala, Foundation for Innovative New Diagnostics, SwitzerlandAlpesh Goyal, All India Institute of Medical Sciences, India
Copyright: © 2024 Eng, Teo, Yew and Khoo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pei Chia Eng, cC5lbmdAbnVzLmVkdS5zZw==
†ORCID:
Pei Chia Eng
orcid.org/0000-0002-4172-1344
Ada Ee Der Teo
orcid.org/0000-0002-8832-0409
Tong Wei Yew
orcid.org/0000-0002-7349-3841
Chin Meng Khoo
orcid.org/0000-0003-1601-2391
 Pei Chia Eng
Pei Chia Eng Ada Ee Der Teo
Ada Ee Der Teo Tong Wei Yew1,2,†
Tong Wei Yew1,2,†