- 1Department of Public Health, Wachemo University, Hossana, Ethiopia
- 2Department of Orthopedic Surgery, Wachemo University, Hossana, Ethiopia
Background: Anemia is a severe public health problem affecting 54% of pregnant women in SSA Yet, only a limited number of studies have provided a partial assessment of the pooled prevalence and related determinants of the severity levels of anemia in pregnant women in SSA. Therefore, this study provides the most recent estimates of anemia severity levels and related determinants.
Methods: The most recent Demographic Health Survey (DHS) dataset of 21 Sub-Saharan African countries which were collected between 2015 and 2022 were used. A total of 14,098 pregnant women were included. Multilevel ordinal logistic regression was used.
Results: The pooled prevalence of anemia was 51.26%. Pregnant women who were in the old age groups, and who have attended secondary and higher education were less likely to be at higher levels of anemia. Those women who have given birth to >1 children in the last 5 years, pregnant women in second and third trimester and living in poorest households had greater odds of being at higher levels of anemia.
Conclusion: In Sub-Saharan Africa, anemia is a severe public health concern for pregnant mothers. When developing and implementing strategies for the prevention and control of anemia, it is imperative to take into account the individual and community circumstances. Programs for the prevention and control of anemia should incorporate the economic and educational empowerment of women.
1 Introduction
Anemia is a condition in which the amount and size of red blood cells, or the hemoglobin (Hb) concentration, falls below a set threshold, hence decreasing the blood's capacity to deliver oxygen throughout the body (1). A pregnant woman is considered anemic when her Hb level is less than 11 g/dl (1). Based on Hb level, anemia is categorized as mild, moderate, or severe (2).
About half (50%) of anemia in women is due to iron deficiency. Folate and Vitamin B12—deficiency, parasitic infections, malaria, chronic diseases, loss of appetite, and low dietary diversity are among other causes of anemia in pregnancy (3, 4).
Because of increased blood volume, increased body need for iron to produce additional blood for the growing fetus, poor absorption and inadequate dietary intake make pregnant women more prone to anemia (5, 6).
Anemia increases the risk of preterm birth, maternal death, low birth weight, miscarriages, and stillbirths during pregnancy. It is an indication of poor health and inadequate nutrition (7, 8). It has contributed to over 25% of maternal deaths, newborn mortality, fetal impairment, and infant death (9, 10). Anemia caused 50 million years of healthy life lost due to disability in 2019 (11).
Anemia impairs the economic productivity and development of communities and countries (7). Each individual with anemia bears an economic burden ranging from $US29, 511 to $US7, 092 (12).
Since 2000, the prevalence of anemia in pregnant women has decreased only slightly. In 2022, around 50% of pregnant women worldwide suffered from anemia. The highest prevalence of anemia is observed in low- and lower-middle income countries. Sub-Saharan Africa faces a significant public health challenge with over half (57.0%) of the pregnant women affected by anemia (13, 14). Age, education, reproductive and obstetric factors, and maternal health service usage and residency have been found to be associated with anemia (15).
As a matter of public health concern, governmental and non-governmental organizations are keeping a close eye on anemia since it affects billions of people worldwide and puts them at risk for a variety of social, psychological, and economic difficulties (16). The World Health Assembly adopted a goal in 2012 to reduce the prevalence of anemia in pregnant women by 50% by the year 2025 (17). Furthermore, the Sustainable Development Goal states that by 2030, the worldwide maternal mortality ratio should be fewer than 70 per 100,000 live births. To achieve these objectives at the regional level, reliable and effective data and monitoring systems would be necessary. It is essential to gather enough knowledge about the individual and community-level factors leading to anemia in order to design timely interventions in the prevention and treatment of anemia. Yet, only a limited number of studies have provided a partial assessment of the pooled prevalence and related factors of the severity levels of anemia in pregnant women in SSA. Therefore, using the most recent data available from the DHS of the 21 SSA nations, this study provides the most recent estimates of anemia severity levels and related determinants, which will be beneficial to public health authorities.
The health, well-being, and capacity for economic growth and community development of future generations will all benefit from addressing the variables that contribute to anemia in SSA pregnant women. This will also help to ensure that mothers and newborns have healthier pregnancy outcomes. In order to create prompt interventions for the prevention and treatment of anemia, it is imperative that sufficient evidence regarding the individual and community-level factors contributing to anemia be generated (7).
2 Methods
2.1 Data source and sampling procedure
The most recent Demographic Health Survey (DHS) dataset, which were gathered in 21 Sub-Saharan African nations between 2015 and 2022, were used in this investigation. Enhancing the collection, processing, and exchange of population, health, and nutrition data while expediting their application to planning, policy-making, and program administration is the main objective of the nationally representative DHS survey. The Inner City Fund (ICF) International gave us permission to use the datasets. The DHS website (http://dhsprogram.com) was then used to download each individual data set. The DHS report for each nation contains information about the DHS.
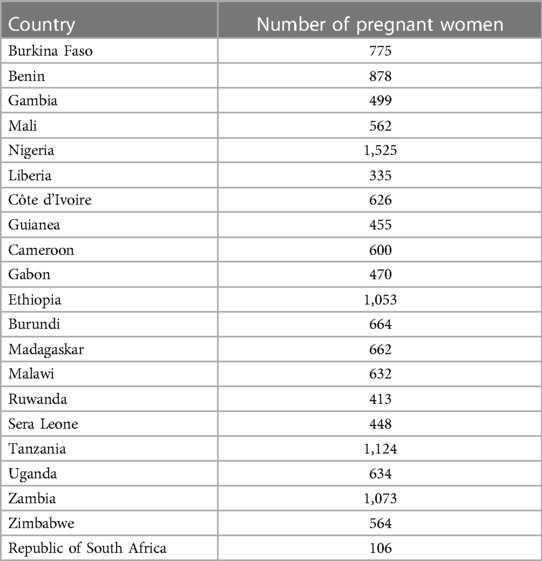
The datasets from 21 countries have been appended together in order to estimate the pooled prevalence and identify factors that are related to the severity levels of anemia among pregnant women in Sub-Saharan Africa. A two-stage stratified sampling approach was used select women during DHS data collection. Firstly, Enumeration Areas (EAs) were chosen with a probability proportional to EA size. Next, using an equal probability systematic selection, a fixed number of 20–28 households per EA were chosen. Next, utilizing the HemoCue fast testing technology, hemoglobin levels were measured in willingly consenting women living in the designated homes. Next, depending on the hemoglobin level, the anemic status was established. Since the fetus's iron requirements and the mother's increased blood volume during pregnancy lower blood hemoglobin levels, pregnancy-related Hb levels were modified. Altitude and smoking were also taken into consideration. A total of 14,098 pregnant women from 21 SSA countries were included in this study (18) (Table 1).
2.2 Dependent variable
Depending on the Hb level, anemia is classified as none, mild, moderate, or severe and is an ordinal dependent variable.
2.3 Independent variables
Owing to the DHS dataset’s hierarchical structure, the independent variables were divided into categories at the individual and community levels.
Individual-level variables include age, marital status, level of education, exposure to the media, use of tobacco products, number of births within the previous 5 years, the number of household members, wealth index, access to a toilet, distance to health facility, and type of cooking fuel.
Community-level variables include place of residence and region of residence.
2.4 Statistical analysis
STATA version 14 was used for data cleaning and analysis. Means, medians, and proportions were used to summarize the data. To present the data, tables and graphs were employed. Because of the hierarchical nature of the DHS data, multilevel (two-level) ordinal logistic regression was used to identify factors related to anemia severity levels.
Four different models were fitted.
The first model, known as the null model, had no independent variables and represented the variation in anemia among pregnant women that was explained by cluster differences in the absence of the explanatory variables. The second model involved individual-level variables, the third involved community-level variables, and the fourth involved a model that combined significant variables from the second and third models.
A sample weight was used in consideration of the survey's complex sampling design. The adjusted odds ratio was estimated with a 95% confidence interval. Variables in each model with a p-value <0.05 were taken into account for the full model. The likelihood ratio test was used to test the proportional odds assumption, and the result showed that the assumption was satisfied (p-value = 0.87). Akaike information criteria (AIC) was used to compare the models, and the model that had the lowest AIC was deemed to be the best fit.
3 Results
3.1 Characteristics of the participants
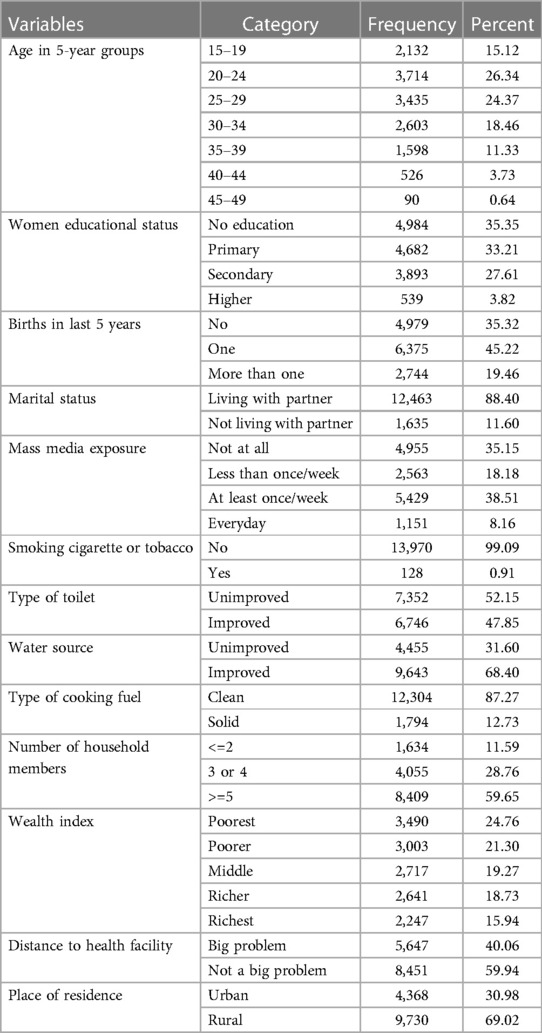
More than quarter (26.34%) of the pregnant women were in the age group of 20–24 years. The mean age of the pregnant women was 26.76 years (SD = 6.81years). More than one-third of the pregnant women had not attended formal education (35.35%) and 35.15 had no mass media exposure. Nearly one-fourth (24.76%) were living in poorest households and 35.32% had no birth in the last five years. More than half (52.15%) and 31.6% of the pregnant women had to access to improved toilet facility and improved water source. Majority of the pregnant women (87.27%) were living in households using clean cooking fuel and 69.02% were rural residents (Table 2).
3.2 Prevalence of anemia in pregnant women in SSA
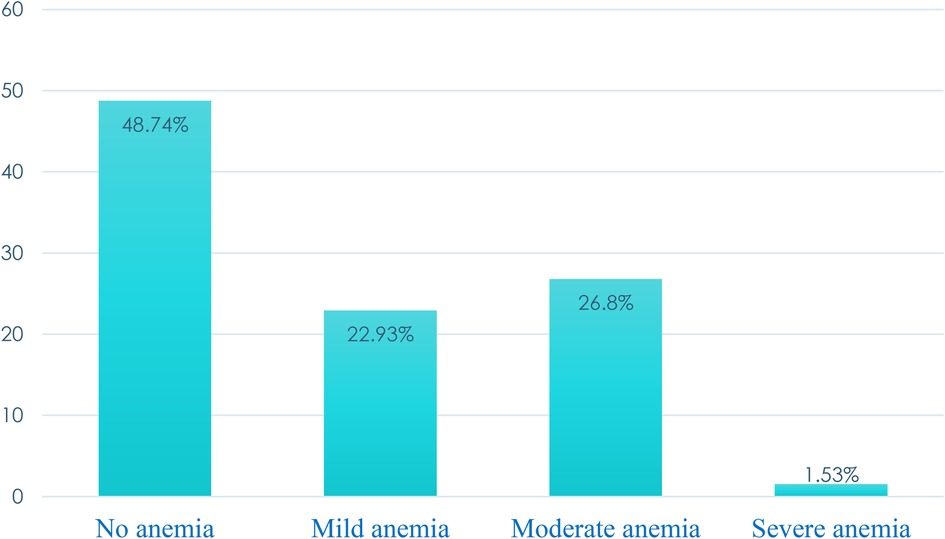
The pooled prevalence of anemia was 51.26% with a median hemoglobin level of 10.9 g/dl (IQR: 9.8, 12 g/dl). More than one-fourth (26.8%) and one in ten (1.53%) were moderately and severely anemic, respectively (Figure 1).
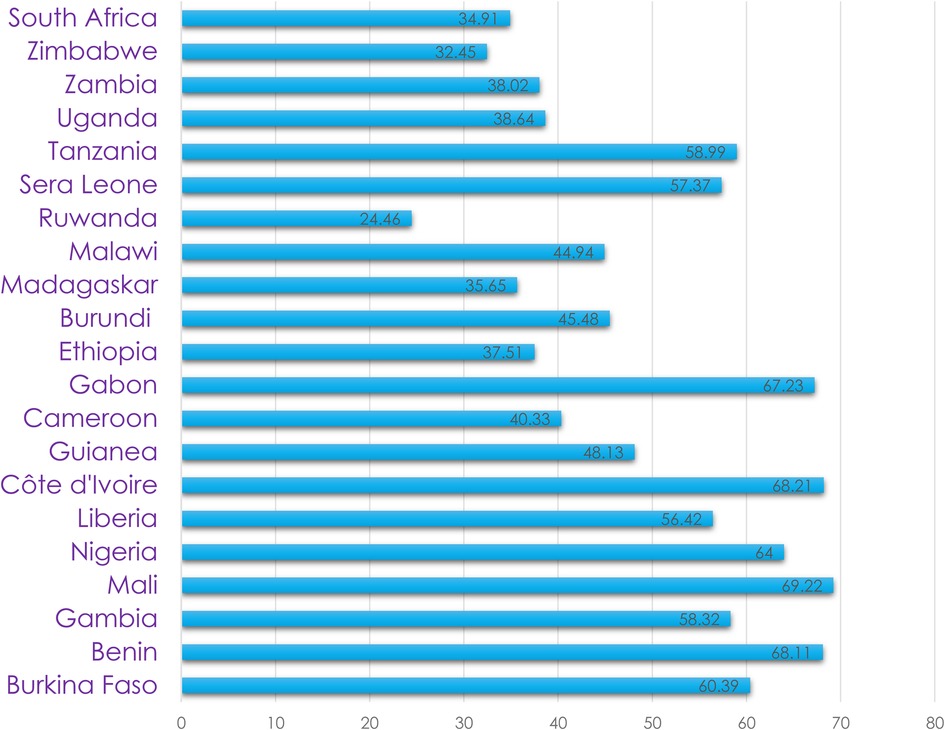
The prevalence of anemia was the highest in Mali (69.21%) followed by Cote D'Ivoire (68.21%) (Figure 2).
3.3 Predictors of severity levels of anemia among pregnant women in SSA
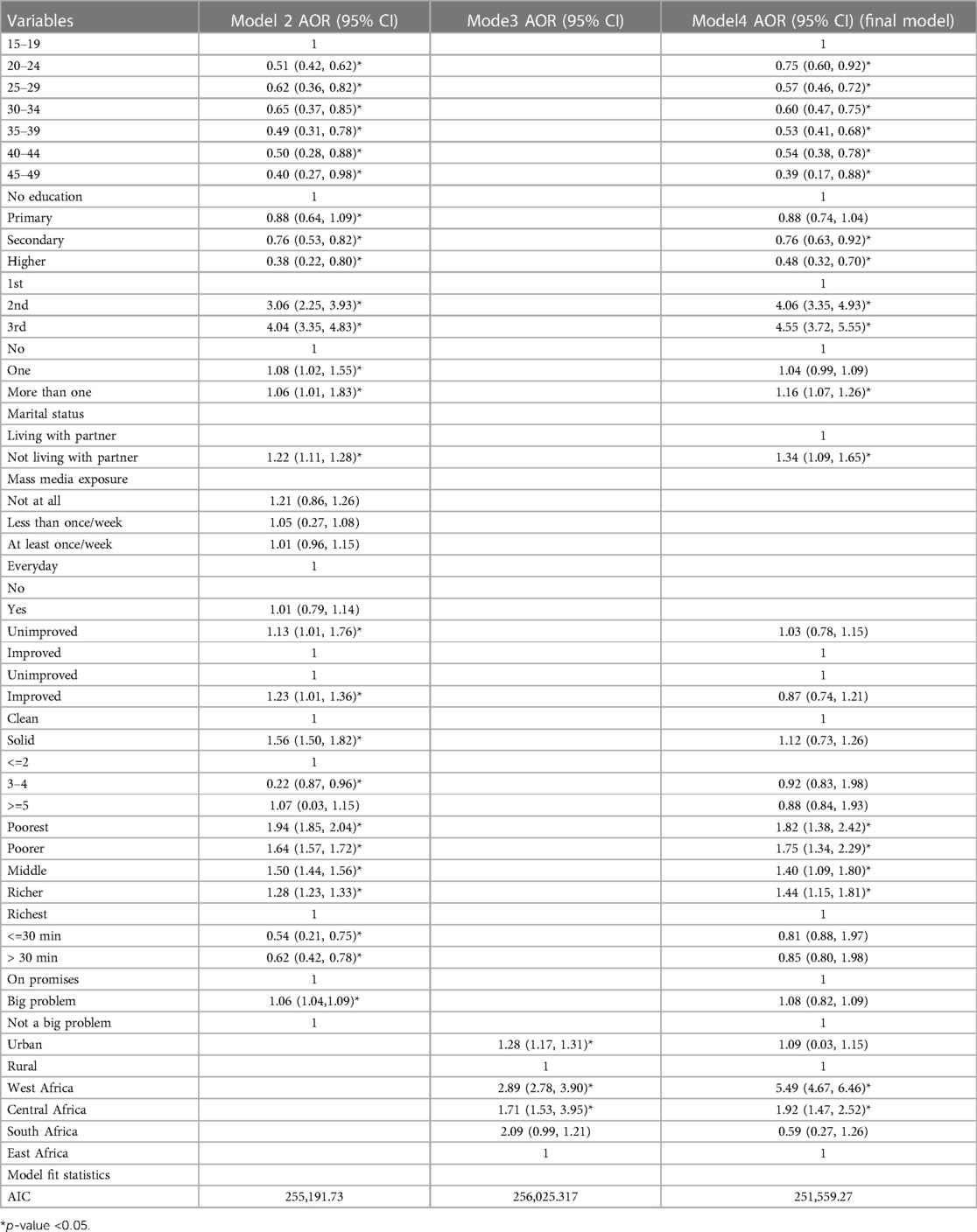
Age, education, marital status, number of births in the last 5 years, pregnancy trimester, and wealth index, and region of residence were significantly associated with anemia.
Pregnant women who were in the age groups of 20–24, 25–29, 30–34, 35–39, 40–44 and 45–49 were 25% (AOR = 0.75), 43% (AOR = 0.57), 40% (AOR = 0.60), 47% (AOR = 0.57), 46% (AOR = 0.54), and 61% (AOR = 0.39) less likely to be at higher levels of anemia as compared to those in the age group of 15–19 years, respectively.
Pregnant women who have attended secondary and higher education had 24% (AOR = 0.76) and 52% (AOR = 0.48) lesser odds of being at higher levels of anemia, respectively, as compared to those who have not attended education. Those women who have given birth to more than one births in the last five years before survey had 16% greater odds of being at higher levels of anemia compared to women who gave no birth. The odds of being at higher levels of anemia were 4.06(AOR = 4.06) and (AOR = 4.55) times greater for pregnant women in second and third trimester, respectively as compared to those in first trimester.
Pregnant women not living with their partner had 34% (AOR = 1.34) greater odds of being at higher levels of anemia as compared with those living with their partner. The odds of higher levels of anemia were 1.82(AOR = 1.82), 1.75(AOR = 1.75), 1.40(AOR = 1.40), and 1.44(AOR = 1.44) times greater for pregnant women living in poorest, poor, middle and rich households, respectively, as compared to those living in richest households (Table 3).
4 Discussion
The pooled prevalence of anemia was 51.26% among pregnant women in SSA. This is higher than the global (37%) and continental (46.7%) estimates of prevalence of anemia among pregnant women. This shows that anemia is a severe public health problem among pregnant women in SSA.
Pregnant women who have attended secondary and higher education had lesser odds of being at higher levels of anemia as compared to those who have not attended education. This is inline the finding in study done in Ethiopia (19), SSA (20) and Nepal (21). This can be due to the fact that attending school often leads to increased knowledge and awareness about proper nutrition and health practices. Women who have received education are more likely to have access to information about balanced diets and the importance of consuming iron-rich foods. Additionally, attending school may also provide opportunities for women to engage in physical activities, which can contribute to better overall health. Furthermore, education can empower women to make informed decisions about their own health and seek appropriate medical care when needed (22–24).
Pregnant women who were in the old age groups were less likely to be at higher levels of anemia than adolescent pregnant women. This is in line to the finding in study done in Ethiopia (25), East Africa (26) and Uganda (27). It's worth noting that older women may have accumulated more life experiences, including a better understanding of nutrition and healthcare. Older pregnant women may be more likely to follow recommended prenatal care guidelines, including proper nutrition and iron supplementation, which can contribute to a lower risk of anemia during pregnancy (23, 28). Additionally, older women may have a higher baseline iron stores due to a longer period of exposure to dietary sources of iron throughout their lives.
Pregnant women not living with their partner had greater odds of being at higher levels of anemia as compared with those living with their partner. This finding is supported by study conducted in East Africa (26). Living with a partner may provide increased social and emotional support. Emotional well-being and reduced stress can positively impact overall health, including nutritional status. Pregnant women living with their partners may have better financial stability and access to resources. A stable financial situation can contribute to a more nutritious diet, including foods rich in iron and other essential nutrients. Women living with their partners may have better access to healthcare resources, including prenatal care, which is crucial for monitoring and managing health during pregnancy. Regular prenatal check-ups can help identify and address anemia early on. Shared living arrangements may influence dietary habits. Pregnant women living with their partners may be more likely to have access to a variety of foods, including those that are iron-rich, leading to a healthier and more balanced diet. The stress and emotional strain associated with being pregnant and not living with a partner might contribute to lifestyle factors that can impact health. Stress can affect appetite, dietary choices, and overall well-being, potentially contributing to anemia (29, 30).
The odds of being at higher levels of anemia is greater for pregnant women who have given birth to more than one births in the last five years before survey compared to women who gave no birth. This is in line to the finding in studies done in Ethiopia (19) and Bangladesh (31). This could be because pregnancies that happen too soon after one another may leave the body unable to recuperate completely, which could result in the body losing vital nutrients like iron. The body requires time to heal and restore the nutrients that are depleted during pregnancy and delivery. Given that iron is an essential component of red blood cells, this could lead to anemia (32).
Also, frequent pregnancies can lead to increased blood loss during childbirth. Women who have shorter intervals between pregnancies may not have enough time for their bodies to fully restore their blood volume and iron stores before the next pregnancy. This can further contribute to the development of anemia (33).
The odds of being at higher levels of anemia were greater for pregnant women in second and third trimester as compared to those in first trimester. This is in agreement to finding in studies done in Bangladesh (31) and SSA (20). This is due to the fact that the mother's body needs more iron in the latter half of her pregnancy in order to produce more red blood cells and sustain the fetus's rapid growth in the second and third trimesters. Additionally, the increased risk of anemia in the second and third trimesters may be explained by the larger maternal plasma volume increments in relation to red cell mass.
The likelihood of having worse anemia was higher for women in the poorest, middle-class, rich, and poor households than for those in the wealthiest homes. These results were also seen in studies conducted in Mali (34), East Africa (35) and SSA (36). This could be due to the fact that women from lower-income households are less able to pay for preventive and curative medical care as well as diverse, iron-rich foods. One consequence of not being able to pay for medical treatment for their illnesses could be anemia (37, 38). Additionally, women who reside in lower-income households are more vulnerable to anemia-causing infectious illnesses and malnutrition (39, 40).
Lack of information on certain proximal causes of anemia, such as dietary variety, HIV, malaria, and parasite infection, may have had an impact on the reported result. Because of the cross-sectional form of the study, determining the temporal association is much more difficult.
5 Conclusion
In Sub-Saharan Africa, anemia is a severe public health concern for pregnant mothers. The percentages of those who were moderately and severely anemic were almost one-fourth (26.8%) and 1.53%, respectively. Factors associated with anemia change on an individual and community levels. When developing and implementing strategies for the prevention and control of anemia, it is imperative to take into account the individual and community circumstances.
Anemia was substantially correlated with age, education, marital status, number of births within the previous five years, pregnancy trimester, wealth index, and region of residence.
It is important to address these factors and provide support to women in order to prevent and manage anemia in this population. Adolescent females, as well as those who were not enrolled in school should receive extra consideration when developing programs to prevent and control anemia. Programs for the prevention and control of anemia should incorporate the economic and educational empowerment of women.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: http://dhsprogram.com.
Ethics statement
Inner City Fund (ICF) International authorized access to the DHS data upon registration and an explanation of the study's objectives. The DHS data collection was approved ethically by the ORC Macro Inc. Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
LT: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Writing – original draft, Writing – review & editing. AA: Data curation, Writing – review & editing. HT: Software, Writing – review & editing. AH: Methodology, Writing – review & editing. DA: Software, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We express our gratitude to Inner City Fund (ICF) International for providing access to the datasets.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva: World Health Organization (2011).
3. Clark SF. Iron deficiency anemia. Nutr Clin Pract. (2008) 23(2):128–41. doi: 10.1177/0884533608314536
4. World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life. Geneva: World Health Organization (2002).
5. Talaulikar VS, Arulkumaran S. Folic acid in obstetric practice: a review. Obstet Gynecol Surv. (2011) 66(4):240–7. doi: 10.1097/OGX.0b013e318223614c
6. Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. (2006) 83(5):993–1016. doi: 10.1093/ajcn/83.5.993
7. World Health Organization. Global Nutrition Targets 2025: Anaemia Policy Brief. Geneva: World Health Organization (2014).
8. Rasmussen KM. Is there a causal relationship between iron deficiency or iron-deficiency anemia and weight at birth, length of gestation and perinatal mortality? J Nutr. (2001) 131(2):590S–603S. doi: 10.1093/jn/131.2.590S
9. Balarajan Y, Ramakrishnan U, Özaltin E, Shankar AH, Subramanian S. Anaemia in low-income and middle-income countries. Lancet. (2011) 378(9809):2123–35. doi: 10.1016/S0140-6736(10)62304-5
10. Banhidy F, Acs N, Puho EH, Czeizel AE. Iron deficiency anemia: pregnancy outcomes with or without iron supplementation. Nutrition. (2011) 27(1):65–72. doi: 10.1016/j.nut.2009.12.005
12. Ershler WB, Chen K, Reyes EB, Dubois R. Economic burden of patients with anemia in selected diseases. Value Health. (2005) 8(6):629–38. doi: 10.1111/j.1524-4733.2005.00058.x
13. Benson AE, Shatzel JJ, Ryan KS, Hedges MA, Martens K, Aslan JE, et al. The incidence, complications, and treatment of iron deficiency in pregnancy. Eur J Haematol. (2022) 109(6):633–42. doi: 10.1111/ejh.13870
14. World Health Organization. GHO| by Category| Anaemia in Pregnant Women-Estimates by Country. Geneva: World Health Organization (2021).
15. Gautam S, Min H, Kim H, Jeong H-S. Determining factors for the prevalence of anemia in women of reproductive age in Nepal: evidence from recent national survey data. PLos One. (2019) 14(6):e0218288. doi: 10.1371/journal.pone.0218288
17. World Health Organization. Comprehensive Implementation Plan on Maternal, Infant and Young Child Nutrition. Geneva: World Health Organization (2014).
18. ICF. Demography Health Surveys Standard Recode Manual for dhs7. Reston, VA: The Demographic and Health Surveys Program (2018).
19. Geta TG, Gebremedhin S. Prevalence and predictors of anemia among pregnant women in Ethiopia. Syst Rev Meta-Analysis. (2022) 17(7):e0267005. doi: 10.1371/journal.pone.0267005
20. Nyarko SH, Boateng EN, Dickson KS, Adzrago D, Addo IY, Acquah E, et al. Geospatial disparities and predictors of anaemia among pregnant women in Sub-Saharan Africa. BMC Pregnancy Childbirth. (2023) 23(1):743. doi: 10.1186/s12884-023-06008-3
21. Yadav UK, Ghimire P, Amatya A, Lamichhane A. Factors associated with anemia among pregnant women of underprivileged ethnic groups attending antenatal care at provincial level hospital of province 2, Nepal. Anemia. (2021) 2021:7–8. doi: 10.1155/2021/8847472
22. Mukhamedzhanov E, Tsitsurin V, Zhakiyanova Z, Akhmetova B, Tarjibayeva S. The effect of nutrition education on nutritional behavior, academic and sports achievement and attitudes. Int J Educ Math Sci Technol. (2023) 11(2):358–74. doi: 10.46328/ijemst.3133
23. Ba DM, Ssentongo P, Kjerulff KH, Na M, Liu G, Gao X, et al. Adherence to iron supplementation in 22 Sub-Saharan African countries and associated factors among pregnant women: a large population-based study. Curr Dev Nutr. (2019) 3(12):nzz120. doi: 10.1093/cdn/nzz120
24. Raghupathi V, Raghupathi W. The influence of education on health: an empirical assessment of OECD countries for the period 1995–2015. Arch Public Health. (2020) 78(1):20. doi: 10.1186/s13690-020-00402-5
25. Tiruneh FN, Asres DT, Tenagashaw MW, Assaye H. Decision-making autonomy of women and other factors of anemia among married women in Ethiopia: a multilevel analysis of a countrywide survey. BMC Public Health. (2021) 21(1):1497. doi: 10.1186/s12889-021-11538-6
26. Liyew AM, Tesema GA, Alamneh TS, Worku MG, Teshale AB, Alem AZ, et al. Prevalence and determinants of anemia among pregnant women in East Africa; A multi-level analysis of recent demographic and health surveys. PLoS One. (2021) 16(4):e0250560. doi: 10.1371/journal.pone.0250560
27. Nankinga O, Aguta D. Determinants of anemia among women in Uganda: further analysis of the Uganda demographic and health surveys. BMC Public Health. (2019) 19(1):1–9. doi: 10.1186/s12889-019-8114-1
28. Delie AM, Gezie LD, Gebeyehu AA, Tarekegn GE, Muche AA. Trend of adherence to iron supplementation during pregnancy among Ethiopian women based on Ethiopian demographic and health surveys: a multivariable decomposition analysis. Front Nutr. (2022) 9:955819. doi: 10.3389/fnut.2022.955819
29. Atif M, Farooq M, Shafiq M, Ayub G, Ilyas M. The impact of partner’s behaviour on pregnancy related outcomes and safe child-birth in Pakistan. BMC Pregnancy Childbirth. (2023) 23(1):516. doi: 10.1186/s12884-023-05814-z
30. Saranrittichai S. The effects of health literacy development and social support program on behaviors and hematocrit levels among pregnant women with iron deficiency anemia. JNSH. (2022) 44:6–8. https://he01.tci-thaijo.org/index.php/nah/article/view/250652
31. Ahmed S, Al Mamun MA, Mahmud N, Farzana N, Sathi MSA, Biswas BK, et al. Prevalence and associated factors of Anemia among pregnant women receiving antenatal care (ANC) at fatima hospital in Jashore, Bangladesh: a cross-sectional study. Food Nutr Sci. (2019) 10(9):1056–71. doi: 10.4236/fns.2019.109076
32. Lilungulu A, Matovelo D, Kihunrwa A, Gumodoka B. Spectrum of maternal and perinatal outcomes among parturient women with preceding short inter-pregnancy interval at Bugando Medical Centre, Tanzania. Matern Health Neonatol Perinatol. (2015) 1(1):1–7. doi: 10.1186/s40748-014-0002-1
33. Saral N, Ulas SC. The effect of short pregnancy interval on perinatal outcomes in Turkey: a retrospective study. Pak J Med Sci. (2019) 35(5):1243. doi: 10.12669/pjms.35.5.837
34. Armah-Ansah EK. Determinants of anemia among women of childbearing age: analysis of the 2018 Mali demographic and health survey. Arch Public Health. (2023) 81(1):10. doi: 10.1186/s13690-023-01023-4
35. Teshale AB, Tesema GA, Worku MG, Yeshaw Y, Tessema ZT. Anemia and its associated factors among women of reproductive age in Eastern Africa: a multilevel mixed-effects generalized linear model. PLos One. (2020) 15(9):e0238957. doi: 10.1371/journal.pone.0238957
36. Worku MG, Alamneh TS, Teshale AB, Yeshaw Y, Alem AZ, Ayalew HG, et al. Multilevel analysis of determinants of anemia among young women (15-24) in Sub-Sahara Africa. PLos One. (2022) 17(5):e0268129. doi: 10.1371/journal.pone.0268129
37. Owais A, Merritt C, Lee C, Bhutta ZA. Anemia among women of reproductive age: an overview of global burden, trends, determinants, and drivers of progress in low- and middle-income countries. Nutrients. (2021) 13(8):6–7. doi: 10.3390/nu13082745
38. Mankelkl G, Kinfe B. Sociodemographic factors associated with anemia among reproductive age women in Mali; evidenced by Mali malaria indicator survey 2021: spatial and multilevel mixed effect model analysis. BMC Women’s Health. (2023) 23(1):291. doi: 10.1186/s12905-023-02351-x
39. Ord J, Monks A. Food poverty and youth work–A community response. Crit Soc Policy. (2022) 42(1):64–84. doi: 10.1177/0261018321996534
Keywords: severity levels, anemia, pregnant women, multilevel analysis, Sub-Saharan Africa
Citation: Tirore LL, Areba AS, Tamrat H, Habte A and Abame DE (2024) Determinants of severity levels of anemia among pregnant women in Sub-Saharan Africa: multilevel analysis. Front. Glob. Womens Health 5:1367426. doi: 10.3389/fgwh.2024.1367426
Received: 18 January 2024; Accepted: 11 March 2024;
Published: 9 April 2024.
Edited by:
Mojisola Olanike Kehinde, Landmark University, NigeriaReviewed by:
Tilaye Gebru, Haramaya University, EthiopiaOlutosin Ademola Otekunrin, Federal University of Agriculture, Nigeria
© 2024 Tirore, Areba, Tamrat, Habte and Abame. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lire Lemma Tirore bGVtbWFsaXJlQGdtYWlsLmNvbQ==
 Lire Lemma Tirore
Lire Lemma Tirore Abriham Shiferaw Areba
Abriham Shiferaw Areba Habtamu Tamrat2
Habtamu Tamrat2 Desta Erkalo Abame
Desta Erkalo Abame