- 1Faculty of Medicine, Catholic University of Bukavu, Bukavu, Democratic Republic of the Congo
- 2Department of Obstetrics and Gynecology, Hôpital Provincial Général de Référence de Bukavu, Bukavu, Democratic Republic of the Congo
- 3Department of Internal Medicine and Pediatrics, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium
- 4Department of Diagnostic Sciences, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium
- 5Department of Laboratory Medicine, Ghent University Hospital, Ghent, Belgium
Introduction: Vaginal Candida colonization (CC) can lead to vulvovaginal candidiasis, the second most prevalent vaginal condition worldwide, and has been associated with adverse birth outcomes. However, no data on CC in the Democratic Republic of the Congo are available. We investigated the prevalence, Candida species, clinical correlates, risk factors and pregnancy outcomes in women with CC in the second trimester of pregnancy.
Material and methods: In Bukavu, the Democratic Republic of the Congo, pregnant women were recruited during antenatal care between 16 and 20 weeks of gestation from January 2017 to October 2017 and followed until delivery. Sociodemographics, sexual behavioral, hygienic and clinical characteristics, microbiological data and pregnancy outcomes were collected. Candida detection and speciation was performed with microscopy (Gram-stained smears and wet-mount) and/or quantitative PCR. Multivariate regression models were used to estimate the different associations with CC.
Results: The prevalence of CC by wet mount, microscopy of Gram-stain smears and qPCR was 27.9%, 28.1% and 38.2%, respectively. C. albicans was the most prevalent Candida species (91.0%). Previous genital infections, an intermediate vaginal microbiota, bacterial vaginosis, and the use of pit toilets were risk factors for CC. Clinically, CC was associated with itching only. Women with CC had twice the odds for preterm birth, if Candida concentration was high, the odds were four times higher.
Conclusions: In Bukavu, the Democratic Republic of the Congo, the prevalence of CC was high and associated with microbiological and modifiable risk factors. Screening and treatment for CC during antenatal care should be investigated as a possible strategy to reduce preterm birth.
Introduction
A healthy vaginal microbiota (VMB) of women of reproductive age is typically dominated by only a single Lactobacillus species, mostly L. crispatus, L. gasseri or L. jensenii (1). The role of L. iners in reproductive health is under debate (2). These single Lactobacillus species or consortia of different Lactobacillus species (3) produce lactic acid by metabolizing the amylase breakdown products of glycogen present in the vagina. This acidifies the vagina and is a crucial first-line defense protecting women against urogenital tract infections. Furthermore, a healthy VMB is associated with healthy pregnancy outcomes. Vice versa, bacterial vaginosis (BV) is a dysbiosis of this healthy VMB predominated by anaerobes such as Gardnerella and Fannyhessea and linked with adverse pregnancy outcomes such as preterm birth, low birth weight and premature ruptures of membranes (1).
The yeast Candida can colonize the VMB as a commensal. This vaginal Candida colonization (CC) is more prevalent in case of elevated endogenous estrogen levels, such as during pregnancy (4). CC can transition from a commensal state to a pathogenic state—vulvovaginal candidiasis—under certain conditions that are incompletely understood (4, 5). Clinical therapy of vulvovaginal candidiasis is difficult due to the capacity of Candida to form biofilms (6) and withstand the host immune system (7).
In most populations, vulvovaginal candidiasis and bacterial vaginosis are the leading causes of vaginal dysbiosis in women of reproductive age (8). In by far most cases, vulvovaginal candidiasis is caused by Candida albicans (9), the remainder by non-albicans Candida spp., the most common of which is Candida glabrata. The usual presenting symptoms are acute pruritus and vaginal discharge, but none is specific (9). Vaginal discharge is present inconsistently and is often neglible, not uncommon symptoms include vaginal soreness, irritation, vulvar burning, dyspareunia (9). Importantly, asymptomatic CC be present in up to 50% of women (10). Besides clinical suspicion, microscopy has been used to diagnose vulvovaginal candidiasis for decades, however, culture has remained the gold standard (11).
In the Democratic Republic of the Congo (DRC), in case of absence of laboratory capacity, the syndromic management is implemented as recommended by the WHO (12). This syndromic management is based on the identification of consistent groups of symptoms and easily recognized signs, such as the vaginal discharge syndrome, and the treatment of the majority of, or the most serious, pathogens responsible for the syndrome (12). In practice, women presenting with vaginal discharge are often treated for BV, vulvovaginal candidiasis and trichomoniasis (13). This strategy results in frequent undertreatment or overtreatment, which is frequently accompanied by recurring vaginal problems.
In observational studies, vulvovaginal candidiasis but also CC have been conflictingly associated with increased risk of spontaneous preterm birth (PTB), the birth before 37 weeks of gestation (14, 15). PTB directly accounts for 35% of the annual neonatal mortality worldwide (16). A review approximated that 14.8 million infants were born preterm in 2014 with most cases occurring in Sub-Saharan Africa and Asia (17). In DRC, the neonatal mortality rate is estimated to be 2.8% (18) and 32% of these mortalities in DRC are caused by PTB complications (19).
Despite the importance of vulvovaginal candidiasis, and the potential detrimental role of CC in pregnancy, the latter is immensely understudied, especially in sub-Saharan Africa and DRC. There are no data on prevalence, Candida species distribution, clinical presentation, risk factors and the role of Candida in birth outcomes. Therefore, we aimed to address these major gaps in a population of pregnant women from Bukavu, DRC.
Material and methods
Study design and population
The current study was part of the AVEONS study, described previously (20). AVEONS was a prospective observational study and included women between 16 and 20 weeks of gestational age [visit 1 (V1)], between 36 and 38 weeks (V2) and at delivery. The main aim of AVEONS was to investigate the role of vaginal infections in adverse pregnancy outcomes in Bukavu, DRC. Newborns were followed from delivery until day seven of life. This manuscript only considers CC at V1 and pregnancy outcomes.
Recruitment
Between January and October 2017, pregnant women were recruited when seeking antenatal care at the Provincial Referral Hospital of Bukavu (PRHB). Church announcements, radio and TV spots, community leader's meetings and posters were used to promote the study. Pregnant women who were interested in participating were informed individually and requested to sign an informed consent form. Participants were reimbursed for their transportation costs.
Inclusion and exclusion criteria
Pregnant women were considered for inclusion if (i) they were between 16 and 20 weeks of gestation, (ii) they accepted to be followed by a referral hospital team and were willing to deliver at PRHB and (iii) they accepted to be contacted by phone or other means. Women were not considered eligible in case (i) they planned to move out of Bukavu during their pregnancy, (ii) of twin pregnancies, (iii) of a fetus with a visible malformation at ultrasound examination, (iv) they had genital bleeding and/or (v) of use of antibiotics during the 2 weeks before recruitment.
Routine antenatal care and delivery procedures
A general physical and gynecological examination was performed. Urine (to diagnose urinary tract infections or bacteriuria) and five mL of total blood (to test for HIV, malaria and hemoglobin) was collected. Also, a gynecological examination, including a speculum examination with a sterile non-moistened speculum, was performed. During this examination, both the vaginal mucosa and cervix were inspected for the presence of sores and tumors. A vaginal infection was diagnosed according to the syndromic-based guidelines for the management of pregnancy issued by the Ministry of Public Health of DRC (21), which are based on WHO recommendations (22). This syndromic-based diagnosis was made in case the participant suffered from abnormal vaginal discharge (thick curdy), itching, redness, abnormal foul smell and/or burning sensation after intercourse. In case of vaginal infection, women were treated empirically with a combination of clotrimazole (200 mg) and clindamycin (100 mg). This combination is an efficient, safe and well-tolerated combination for treating mixed vaginitis caused by bacteria and fungi, also in pregnancy (23). Due to a lack of diagnostic tools in DRC, the syndromic method is employed to manage vulvovaginal diseases and a broad-spectrum combination medication capable of treating fungal, bacterial, and mixed infections is needed.
An ultrasound examination was performed to assess the viability of the fetus and to measure the cervical length. Women followed antenatal care as usual and were offered a single dose of 500 mg mebendazole (against intestinal worm infections) and a single dose of sulfadiazine-pyrimethamine (500 mg) against malaria. At delivery, pediatricians evaluated the newborns and assessed their anthropometric features. The WHO criteria were used to suspect an early newborn infection. Nurses and a senior assistant monitored labor and collected the relevant data, such as vital signs, vaginal delivery mode, obstetrical criteria of delivery, and symptoms of vulvovaginal infection.
Study specific procedures
Data on sociodemographics and reproductive health history, sexual behavior, vaginal practices and complaints were obtained by questionnaires. The vaginal pH was determined by indicator pH papers (Hilindicator® pH paper). Three vaginal swabs and one cervicovaginal lavage (CVL) were taken. The first swab was rolled on three glass slides, one for direct wet mount microscopy and two for future Gram staining, the second swab was used for culturing and the third for molecular testing. CVLs were taken as previously described (24). Briefly, using a pipette, 10 ml of normal saline was flushed over the cervix and the lateral vaginal walls, aspirated as much as possible from the posterior fornix and collected in a vacutube. CVLs where then put in a cooled box (4 °C) and immediately transported to the laboratory, where they were frozen at −20 °C until shipment to the Laboratory Bacteriology Research (LBR) (Ghent University, Ghent, Belgium).
Laboratory tests as part of the routine antenatal care
The serum was used to test for HIV (Alere DetermineTM HIV-1/2, Abbott, Diegem, Belgium), malaria (AG P.f/pan, Bioline®, Abbott, Diegem, Belgium) and hemoglobin (HemoCue Hb201+, HemoCue, Ängelholm, Sweden). The urine was tested for the presence of white blood cells and nitrite (indicating urinary tract infection or bacteriuria) by Multistix® dipsticks (Siemens, Groot-Bijgaarden, Belgium). The wet mounts were examined for the presence of clue cells (25), Candida and Trichomonas vaginalis.
Study specific laboratory procedures
Microscopic detection of Candida and BV
The two vaginal smears were fixated, shipped to the LBR, Gram-stained using an automated Poly Stainer and scored by two independent readers according to the Nugent scoring system (26) to categorize the vaginal microbiota as healthy, intermediate microbiota or BV. In case of discrepancy in categorization, slides were reassessed by the two reviewers and discussed. In case no consensus was obtained, a third reader assessed the slide as a tie breaker. The presence of Candida (cells and/or hyphae) upon microscopic examination of Gram-stained smears was used as the case definition of CC.
Molecular detection and speciation of Candida
DNA was extracted from CVLs using the RNeasy PowerMicrobiome Kit (Qiagen, Antwerp, Belgium) according to manufacturer's instructions with minor modifications. To detect and speciate Candida in the CVL DNA extracts, fungal DNA was amplified in a qPCR assay using universal fungal primers targeting the internal transcribed spacer-2 region followed by high resolution melting of the amplicons and analysis of the melting curves for speciation as described previously (27). Briefly, a total reaction volume of 10 µl was prepared containing the primers ITS4 (5'-TCCTCCGCTTATTGATATGC-3') and ITS86 (5'-GTGAATCATCGAATCTTTGAAC-3'), both at a final concentration of 0.5 μM, 1× LightCycler 480 HRM master mix (Roche) and two μl of DNA extract or control DNA. Amplification was carried out on a LightCycler 480 (Roche) by pre-incubating the reaction mixture for 10 min at 95 °C, followed by 45 cycles of 20 s at 95 °C, 30 s at 55 °C and 30 s at 72 °C. Hereafter, a high resolution melting curve was generated by first melting all DNA at 95 °C for 5 s, followed by renaturating DNA for 1 min at 60 °C, and gradually increasing the temperature from 60 °C to 97 °C at a ramp rate of 0.02 °C /s. Results were analyzed with the LightCycler 480 Software, version 1.5 (Roche), as described previously (27). As positive controls in each run, DNA from Candida dubliniensis, C. famata, C. glabrata, C. guillermondi, C. inconspicua, C. kefyr, C. krusei, C. lipolytica, C. lusitania, C. metapsilosis, C. nivariensis, C. norvegensis, C. metapsilosis, C. ortopsilosis, C. parapsilosis, C. tropicalis and Saccharomyces cerevisiae was used. In each run, ten negative template controls were used, and each sample was analyzed in duplicate. High resolution melting curves peaks of positive samples were compared with those of reference standards for identification of the yeast.
Data analysis
A case of CC was defined based on the microscopic observation of Candida cells and/or hyphae on Gram-stained vaginal smears. PTB was defined as delivery before 37 completed weeks of gestation and after 28 completed weeks of gestation, low birth weight as birth weight <2,500 g (28–30).
For a comparison of the diagnostic performance of different assays, in case of absence of a true gold standard assay, a composite reference standard (CRS) can be used as the gold standard (31). For Candida detection, there is no gold standard, hence, a CRS for Candida colonization was used to assess sensitivity and specificity of the different Candida diagnostic methods. A case of Candida colonization using a CRS was defined if from the three diagnostic methods (wet mount microscopy, Gram-stain microscopy and qPCR) at least two of the assays were positive. This comparison of diagnostic performance of three methods was based on a subset of 330 samples. To investigate to which extent the sensitivity of Gram-stain and wet mount-based microscopy were function of the Candida concentration, we (i) stratified the qPCR positive samples into three more or less equal groups, i.e., low (<9.0E + 03 Candida cells/ml), moderate (9.0E + 03 Candida cells/ml—2.0E + 05 Candida cells/ml) and high concentration (>2.0E + 05 Candida cells/ml) and (ii) assessed the Gram-stain and wet mount positive rate in each of the concentration categories. Fleiss Cohen's kappa test was used to determine the agreement between Candida assays (32).
The Chi-squared test was used to compare CC with categorical independent variables. A binary logistic regression was performed to assess the associations between CC and different sociodemographic, sexual behavioral, hygienic, clinical, and anthropometric characteristics and laboratory findings (independent variables). These associations were expressed as crude odds ratios and 95% confidence interval (CI). Then, a multiple logistic regression model (using a combination of the stepwise forward or backward method) was applied to select CC risk factors and calculate adjusted odds ratios and 95% CI. This model was based on both probability-to-enter of 0.05 and probability-to-remove of 0.10.
Another multiple logistic regression model stratified by vaginal microbiota status was employed to estimate the association of CC with clinical symptoms. The goodness-of-fit of the final models was tested using the Hosmer-Lemeshow test.
A modified Poisson regression model with robust standard error was built to further investigate the extent to which CC was as an independent risk factor for adverse pregnancy outcomes [preterm birth (PTB), low birth weight, and premature ruptures of membranes (PROM)]. We corrected for a priori defined (possible) confounding factors influencing pregnancy outcome suggested by other studies (33–36) (vaginal microbiota, adjusted hemoglobin rate, cervix length, parity, body mass index, maternal age, education level and poverty).
All forms (laboratory forms, questionnaires, case reports forms) were scanned and stored digitally as a singular file. All data were entered by double data entry using the CSPRO software. The comparison data tool of CSPRO was used to compare the two data files (from the double data entry) for discrepancies. Discrepancies were resolved by consulting the original data in the scanned documents. Analyses were performed in STATA 14 (Stata Corp, College Station, US), p-values below 0.05 were considered as statistically significant.
Ethics statement
This study was ethically approved by the Internal Review Board of the Catholic University of Bukavu (UCB/CIE/NC/016/2016), by the Ministry of Public health (062/CD/DPS/SK/2017) and by the Ethical Committee of Ghent University Hospital (PA2014/003). An informed consent form was signed by each pregnant woman who accepted to participate in the study.
Results
Sociodemographic description of the study population
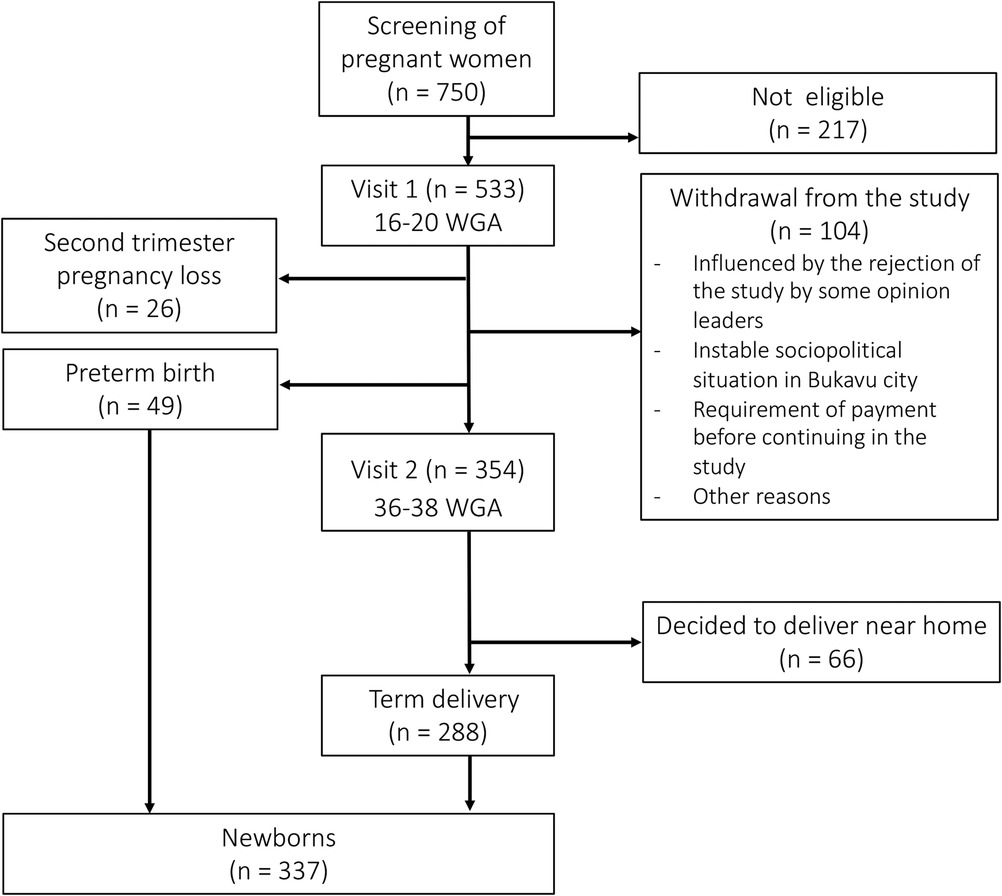
The number of pregnant women and neonates withheld at each visit is shown in Figure 1.
A total of 750 pregnant women were screened of which 533 were found eligible and were enrolled in the cohort (V1). A total of 104 women (19.5%) who started in the study withdrew from the study before V2. A total of 26 (4.9%) women had a second trimester pregnancy loss. Of all newborns in the study, 288 (85.5%) were born at term and 49 (14.5%) were born preterm. Of the 354 women who completed V2, 66 (12.4%) decided to not deliver at PRHB but near their home.
The sociodemographic characteristics of the study population are outlined in Supplementary Information S1.
Near the half of pregnant women (48.4%) were symptomatic, i.e., suffered from an abnormal vaginal discharge, vaginal itching, burning sensation after sexual intercourse, and/or a foul smell from the vagina.
Prevalence of vaginal Candida colonization and speciation
Table 1 documents the prevalence assessed in both symptomatic and asymptomatic pregnant women according to the different diagnostic tests. The overall prevalence of CC by means of microscopy of Gram-stained smears was 28.1%, in symptomatic and asymptomatic women, this was 48.1% and 8.3%, respectively. Using wet mount microscopy, the overall CC prevalence was 27.9%, in symptomatic and asymptomatic women, the prevalence was 55.6% and 1.2%, respectively. The overall CC prevalence assessed by qPCR (38.2%) was significantly higher compared to the microscopic methods, and similar in symptomatic and asymptomatic women (38.9% and 37.5%, respectively).

Table 1. Prevalence (with 95% confidence interval) of vaginal Candida colonization among symptomatic and asymptomatic pregnant women assessed by three diagnostic methods.
Among all women who carried Candida as assessed by means of qPCR, Candida albicans was the most prevalent species (91.0%) (Supplementary Information S2). The other yeast species were C. famata (1.6%), C. glabrata (0.8%), C. dubliensis (2.5%), C. inconspicua (0.8%), C. kefyr (0.8%), C. krusei (1.6%), C. tropicalis (0.8%) and Sacharomyces cerevisiae (3.2%).
Diagnostic performance of microscopic and molecular tests
The diagnostic performance parameters are shown in Table 2. The highest sensitivity was found for qPCR (performed on CVLs) (99.0%), followed by microscopic examination of Gram-stained vaginal smears (82.9%) and wet mount preparations (76.2%). The specificity of the three assays was within the same range (90.5%–97.7%).
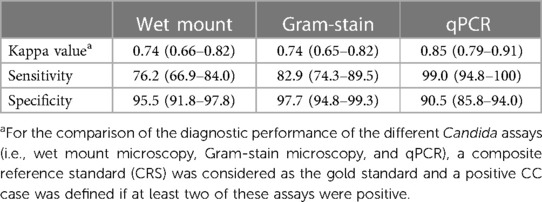
Table 2. Diagnostic performance (with 95% confidence interval) of the different tests for Candida vaginal colonization (wet mount, gram-stain, qPCR) based on 330 women.
In comparison with the CRS, a good agreement was observed with both the wet mount and Gram-stain-based microscopy (κ = 0.74 (0.66–0.82) and 0.74 (0.65–0.82), respectively) and an almost perfect agreement with qPCR [κ = 0.85 (0.79–0.91)].
The positivity rate of the two microscopic techniques as function of the Candida concentration (as assessed by means of qPCR) is shown in Figure 2. The positivity rate of microscopic techniques increased with increasing concentration, with the highest microscopic detection rate for Gram stain microscopy (80%) in the high concentration group (>2E05 Candida cells/ml).
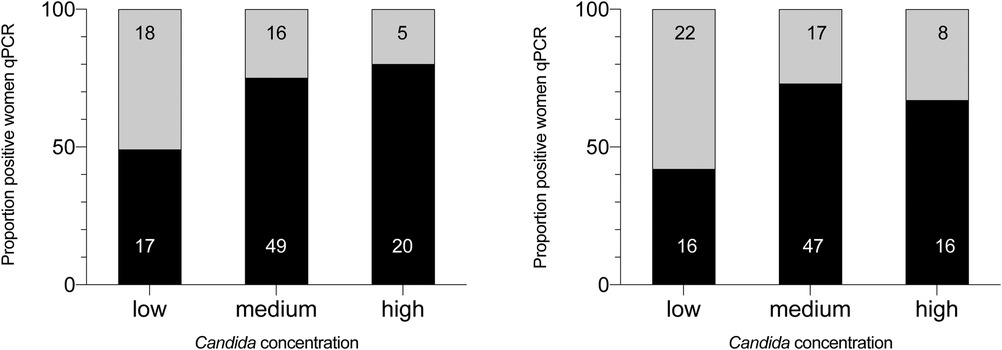
Figure 2. Positivity rate of vaginal Candida colonization as assessed by microscopic techniques according to Candida concentration measured by qPCR. The different classes of Candida concentration (assessed by means of qPCR) are categorized into low, moderate and high. The black part of each column represents the proportion of samples found positive by Gram-stain or wet mount in the different categories, the grey part the samples found negative. The number in the columns are absolute numbers, the numbers on the Y-axis are percentages. GS, microscopic examination of Gram-stained smears; WM, microscopic examination of wet mounts.
Independent risk factors for vaginal Candida colonization
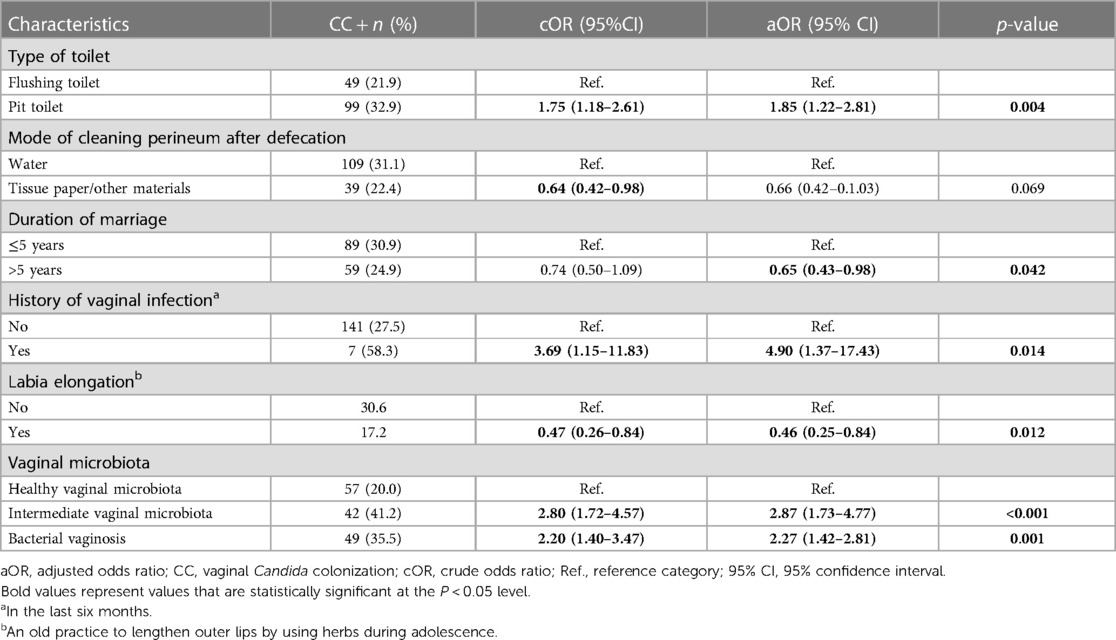
The univariate and multivariate associations are shown in Supplementary Information S1 and Table 3, respectively. CC was significantly and independently associated with the use of a pit toilet compared to a flush toilet (aOR: 1.85; 95% CI: 1.22–2.81), a history of vaginal infection in the six previous months (aOR: 4.90; 95% CI: 1.37–17.43), the practice of labia elongation (aOR: 0.46; 95% CI: 0.25–0.84), the presence of an intermediate vaginal microbiota (aOR: 2.87; 95% CI: 1.73–4.77) and BV (aOR: 2.27; 95% CI: 1.42–2.81).
Symptomatology of vaginal Candida colonization
The univariate associations of clinical signs and symptoms with CC are shown in Supplementary Information S1. The multivariate model stratified by the VMB status is shown in Table 4. Vaginal itching was statistically significantly associated with CC across strata (aOR: 1.98, 95% CI: 1.02–3.83; aOR: 3.26, 95% CI: 1.30–8.17; aOR: 3.40, 95% CI: 1.50–7.70 for healthy VMB, intermediate VMB, or bacterial vaginosis, respectively).
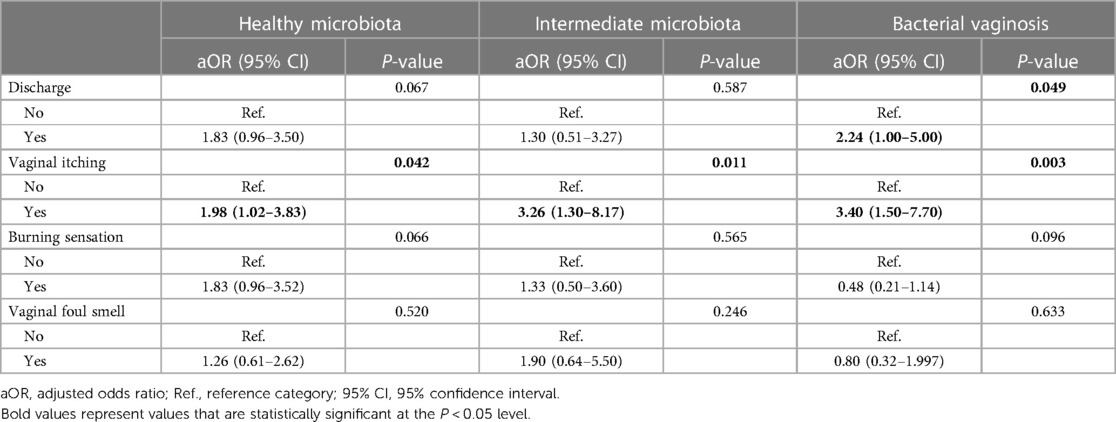
Table 4. Clinical symptoms associated with vaginal Candida colonization stratified by the vaginal microbiota status.
The univariate and multivariate associations of Candida with adverse pregnancy outcomes
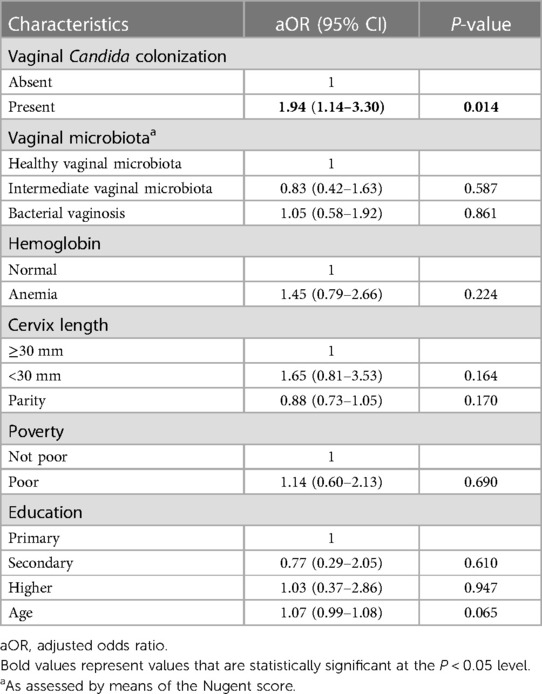
The univariate associations between CC and delivery characteristics and neonatal outcomes are shown in Supplementary Information S3. Statistically significant associations were found between CC and PTB (OR: 1.59; 95% CI: 1.06–2.39), non-cephalic presentation (compared to cephalic presentation) (OR: 5.05; 95% CI: 1.44–17.72), neonatal fever (OR: 2.84; 95% CI: 1.56–5.15) and an elevated neonatal temperature (>37.2 °C, compared to 36.6 °C-37.2 °C) (OR: 2.09; 95% CI: 1.23–3.55). In the multivariate model (Table 5), CC remained significantly associated only with PTB (aOR: 1.94; 95% CI: 1.14–3.30, p = 0.014).
The prevalence of PTB further stratified by Candida concentration is shown in Supplementary Information S4. There was an increasing prevalence of PTB, going from 11.2% (no Candida group), 16.0% (women with low Candida concentration), 19.4% (moderate concentration) and 31.3% (high concentration). Only high concentrations of Candida were found to be statistically significantly associated with PTB (OR, 3.60; 95% CI, 1.09–11.90; p = 0.035).
Discussion
We aimed to investigate the prevalence, species, risk factor and clinical correlates of CC, as well as the association with pregnancy outcomes. To be best of our knowledge, no studies on Candida in pregnancy have been performed in DRC. The prevalence of CC was high, and associated with other genital tract infections, hygiene, and preterm birth.
The prevalence of vaginal Candida colonization in pregnant women from Bukavu was high
We found a prevalence of 28.1% of CC in second trimester of pregnancy, as assessed by microscopy of Gram-stained smears; wet mount gave very similar results. Not surprisingly, in our study, the prevalence of CC as assessed by qPCR was much higher (38%). Interestingly, there was a clear difference in prevalence between symptomatic (48% and 56% for Gram-stain and wet mount, respectively) and asymptomatic women (8% and 1% for Gram-stain and wet mount, respectively) for the microscopic methods only. Such as difference was not seen for the qPCR method (symptomatic and asymptomatic women 39% and 38%, respectively). This might be due to the higher sensitivity of qPCR. The concentration of Candida is likely higher when the patient is symptomatic and it is also possible that qPCR is detecting DNA from dead/lysed Candida cells, hence, not visible by microscopy.
An overview of studies from other countries in sub-Saharan Africa reporting CC rates in pregnant women is given in Supplementary Information S5. In Sub-Saharan Africa in pregnancy, prevalence ranges from 14% (Burkina Faso) to 57% (South Africa) in the normal pregnant population. Differences in prevalence between all studies may be explained by different populations and the use of different diagnostic tests, which have different sensitivities, as has been shown in current study and by others (37–39). Also, the different mean gestational age between populations can impact Candida prevalence (36), although some studies from sub-Saharan Africa found no statistically significant association with gestational age (10, 40, 41).
We found that C. albicans—the most virulent Candida species colonizing the vagina (42–44)—was the most prevalent yeast (91.0%). Other studies from sub-Saharan Africa documented C. albicans as the most yeast species in pregnant women, ranging from 40.4% to 90.7% (Supplementary Information S3). The non-albicans species (C. glabrata, C. dubliensis, C. krusei, and C. tropicalis) in our study all have been isolated previously from cases of vaginitis, with C. glabrata being considered the most prevalent cause of non-albicans vaginitis. Other species found in our study, C. famata, C. inconspicua, C. kefyr and S. cerevisiae, are less common to (very) rare causes of vaginitis (45, 46).
Previous vaginal infections and concurrent VM disturbances were independent risk factors for vaginal Candida colonization
Women with a history of vaginal infection in the last six months had a nearly five-time higher odds of Candida colonization. Although we have no specific information on the type of these previous vaginal infections, not unlikely, these were (i) (recurrent) vulvovaginal candidiasis, which has a global prevalence of 9% in the age group 25–34 years (47), (ii) and/or a previous STI, a risk factor for vulvovaginal candidiasis (48), (iii) and/or (recurrent) intermediate VM/BV, which were also risk factors for CC in our study. Indeed, women with an intermediate VMB or BV had a two- to three-fold higher odds for CC. The protective role of (probiotic) lactobacilli against Candida albicans is controversial (49) and clinical studies have shown conflicting findings on the association between the VM and vaginal Candida (50, 51). It has been demonstrated different Lactobacillus species have different probiotic effects against Candida in vitro (52).
Improved sanitation might reduce the prevalence of vaginal Candida colonization
Women using a pit toilet had a higher risk for CC compared to women using a flushing toilet. Commonly, pit toilets lack (sufficient) running water and/or soap for a proper cleaning of hands and/or perineum. As the gut has been documented as a reservoir for vaginal Candida (53), poor cleansing could enhance the spread Candida from perineum to the vagina and everywhere hand-touched in the toilet. Pit toilets have also been associated with other urogenital infections such as trichomoniasis (54, 55). Our findings further stress the need of improving sanitation facilities—also in urban areas such as in our study—to reduce urogenital infections, especially among pregnant women.
We further found that pregnant women who practiced labia elongation had a lower risk of CC. In several central African countries, lengthening of the labia is done as an esthetic improvement, to increase sexual pleasure, and/or to be accepted by the tribe (56).
Vaginal Candida colonization was independently associated with preterm birth
Prevention of PTB remains one of the most important challenges in maternity and neonatology care (16, 17). Although the etiology of PTB is multifactorial, both local and systemic infections are a major cause (57).
Here, we found that CC during the second trimester of pregnancy was independently associated with PTB, with women who carried Candida having a two-fold higher odds for PTB. Importantly, these odds were nearly four-fold in women colonized with high concentrations of Candida, defined in our study as >2E05 Candida cells/ml by means of qPCR.
A high concentration of Candida might increase the possibility of an ascending infection leading to PTB. In some cases, vaginal pathogen concentrations are indeed important in reproductively health. For example, Goodfellow and colleagues showed that the vaginal concentration of L. iners was associated with early PTB recurrence (58). Also, in Group B Streptococcus (GBS) neonatal sepsis, GBS is transmitted from the maternal vagina to the neonate during birth or after breaking of the fetal membranes. It is known that there is a direct relationship between the maternal vaginal GBS concentration, the risk of vertical transmission and the likelihood of serious disease in neonates (59).
Future studies investigating the role of the VMB in pregnancy should consider absolute concentrations. This is currently a shortcoming of the widely used 16S rRNA gene metagenomic next-generation sequencing studies that report in relative abundances. Himschoot et al. (2024, under revision) showed that across samples, the same relative abundances of vaginal key species can represent a large range (several logs) of absolute concentrations.
Two meta-analyses (one considering only asymptomatic CC (14) and one considering both symptomatic and asymptomatic CC (15)) reported no association between (asymptomatic) vaginal Candida and PTB. Several factors might explain why our findings are contrasting.
First, only two studies (from Kenya) in these meta-analyses were performed in sub-Saharan Africa. Both PTB and CC, common among black women, are influenced by maternal ethnicity (60, 61). Also, the VMB differs between African and Caucasian populations (62). Furthermore, there is an important difference in the management (including treatment) of vaginal Candida between high-income countries and low-and-middle income countries (see further), and treatment differences between studies are present. Second, we tested for CC in the second trimester of pregnancy, but the timepoint(s) of testing varied in other studies. Candida later in pregnancy might have less impact on PTB. Third, diagnostic methods differ between studies.
Candida and BV management in antenatal care
In DRC, self-reported vaginal symptoms remain the backbone of the syndromic management of genital infections, and clinical examination or microscopy are not often used. This approach has been shown to perform poorly, for example, an evaluation in Kenya showed that only 50% of women presenting with vaginal complaints received appropriate treatment (63). Currently, the WHO guidelines for the management of genital infections have included recommendations for the use of molecular assays or more simple point-of-care tests, but implementing this remains a challenge/unfeasible in most resource-limited settings. We showed that microscopic examination of wet mount preparations and/or Gram-stained smears—even with a sub-optimal sensitivity compared to qPCR—only fails to detect Candida if present at low concentrations (as assessed by qPCR), which might be clinically less relevant and was not associated with PTB in our study.
In our study, 45% of women had CC and/or BV (19% CC, 17% BV and 9% CC/BV coinfection). Often, symptoms of these conditions are similar, complicating clinical diagnosis and effective treatment (47, 64). In our study, the only symptom associated with CC across VMB categories of healthy (OR 1.98), intermediate (3.26) and BV (OR 3.40) was vaginal itching, similar to findings reported by Gloria and colleagues (OR 2.20, 95% CI 1.40–3.46) (63).
WHO recommends to screen women with previous spontaneous abortion, stillbirth and/or preterm delivery for BV and trichomoniasis (65), and our findings call for further investigation if Candida should be included. Microscopy could be considered as routine screening of women in antenatal care. Although microscopy is an affordable and rather easy to implement technique, it's use often seems to be arduous and tedious owing to the lack of a relevant practitioner's training. Development and implementation of cheap and quick point-of-care tests should be intensified as a means for the reduction of PTB in resources constrained countries. These assays should include multiple targets (Candida, BV, and Trichomonas vaginalis).
Limitations and future perspectives
Our study was limited by the drop-out of approximately one third of the study participants during follow-up due to socio-economic and political reasons. Because of this, our study might be underpowered to assess the association between CC and PTB, hence, results should be interpreted with care.
Considering we found a substantial part of pregnant women, not only from our study but also other Sub-Saharan African countries (see Supplementary Information S3), were colonized by Candida, and CC was associated with increased risk for PTB, further research should be done. A meta-analysis of two randomized controlled trials indicated that clotrimazole treatment for vaginal Candida reduced the risk for spontaneous preterm birth compared to standard of care (risk ratio 0.36, 95% CI 0.17–0.75) (60). Similar trials should investigate if a similar approach also can reduce the incidence of PTB in DRC. To guide such interventions, research on the development and implementation of cheap point-of-care tests and/or microscopy to diagnose Candida, BV and sexually transmitted infections in antenatal care in LMIC should be intensified.
Conclusion
In this first report on vaginal Candida in women from DRC, we document a high prevalence of CC in pregnancy, mainly C. albicans. CC was associated with microbiological and modifiable risk factors. Vaginal Candida, especially when present at high concentrations, was an independent risk factor for PTB.
Key message
Vaginal Candida colonization is associated with preterm birth in a population from the Democratic Republic of the Congo.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors upon request, without undue reservation.
Ethics statement
The study involving humans was approved by the Internal Review Board of the Catholic University of Bukavu (UCB/CIE/NC/016/2016), the Ministry of Public health (062/CD/DPS/SK/2017) and by the Ethical Committee of Ghent University Hospital (PA2014/003). The study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Visualization, Methodology, Validation, Writing – original draft. KD: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. IM: Writing – review & editing, Investigation. MK: Writing – review & editing, Investigation. AD: Data curation, Investigation, Methodology, Writing – review & editing. JB: Resources, Writing – review & editing. HD: Methodology, Writing – review & editing. EH: Investigation, Writing – review & editing. BK: Investigation, Writing – review & editing. BB: Writing – review & editing, Resources. AR: Writing – review & editing, Methodology. MV: Resources, Supervision, Writing – review & editing, Methodology. SC: Resources, Writing – review & editing, Conceptualization, Data curation, Funding acquisition, Project administration, Supervision. PC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was funded by the The Flemish Interuniversity Council—University Development Co-operation (VLIR-UOS) (grant number, ZIUOS2012AP024).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgwh.2024.1339821/full#supplementary-material
Abbreviations
BV, bacterial vaginosis; CI, confidence interval; cOR, crude odds ratio; CVL, cervicovaginal lavage; DRC, the Democratic Republic of the Congo; aOR, adjusted odds ratio; PTB, preterm birth; qPCR, quantitative PCR; VMB, vaginal microbiota; CC, vaginal Candida colonization.
References
1. Vaneechoutte M. The human vaginal microbial community. Res Microbiol. (2017) 168(9–10):811–25. doi: 10.1016/j.resmic.2017.08.001
2. Vaneechoutte M. Lactobacillus iners, the unusual suspect. Res Microbiol. (2017) 168(9–10):826–36. doi: 10.1016/j.resmic.2017.09.003
3. Pacha-Herrera D, Erazo-Garcia MP, Cueva DF, Orellana M, Borja-SerranoP AC, Tejera E, et al. Clustering analysis of the multi-microbial consortium by Lactobacillus species against vaginal dysbiosis among Ecuadorian women. Front Cell Infect Microbiol. (2022) 12:863208. doi: 10.3389/fcimb.2022.863208
4. David H, Solomon AP. Molecular association of Candida albicans and vulvovaginal candidiasis: focusing on a solution. Front Cell Infect Microbiol. (2023) 13:1245808. doi: 10.3389/fcimb.2023.1245808
5. Alvarez PS, Trujillo AMB, Hoyos MP, Castro GY, Lengua MD, Arechavala AI. Vaginal colonization and vulvovaginitis by Candida species in pregnant women from northern of Colombia. Archivos de Medicina (Manizales). (2018) 18:51–9. doi: 10.30554/archmed.18.1.2010.2018
6. Atiencia-Carrera MB, Cabezas-Mera FS, Vizuete K, Debut A, Tejera E, Machado A. Evaluation of the biofilm life cycle between Candida albicans and Candida tropicalis. Front Cell Infect Microbiol. (2022) 12:953168. doi: 10.3389/fcimb.2022.953168
7. Cangui-Panchi SP, Ñacato-Toapanta AL, Enríquez-Martínez LJ, Salinas-Delgado GA, Reyes J, Garzon-Chavez D, et al. Battle royale: immune response on biofilms–host-pathogen interactions. Curr Res Immunol. (2023) 4:100057. doi: 10.1016/j.crimmu.2023.100057
8. Goncalves B, Ferreira C, Alves CT, Henriques M, Azeredo J, Silva S. Vulvovaginal candidiasis: epidemiology, microbiology and risk factors. Crit Rev Microbiol. (2016) 42:905–27. doi: 10.3109/1040841X.2015.1091805
9. Sobel JD. Vulvovaginal candidosis. Lancet. (2007) 369(9577):1961–71. doi: 10.1016/S0140-6736(07)60917-9
10. Konadu DG, Owusu-Ofori A, Yidana Z, Boadu F, Iddrisu LF, Adu-Gyasi D, et al. Prevalence of vulvovaginal candidiasis, bacterial vaginosis and trichomoniasis in pregnant women attending antenatal clinic in the middle belt of Ghana. BMC Pregnancy Childbirth. (2019) 19(1):341. doi: 10.1186/s12884-019-2488-z
11. Nyirjesy P, Brookhart C, Lazenby G, Schwebke J, Sobel JD. Vulvovaginal candidiasis: a review of the evidence for the 2021 centers for disease control and prevention of sexually transmitted infections treatment guidelines. Clin Infect Dis. (2022) 74(Suppl_2):S162–8. doi: 10.1093/cid/ciab1057
12. World Health Organization. Guidelines for the Management of Symptomatic Sexually Transmitted Infections. Geneva: World Health Organization (2021).
13. Zemouri C, Wi TE, Kiarie J, Seuc A, Mogasale V, Latif A, et al. The performance of the vaginal discharge syndromic management in treating vaginal and cervical infection: a systematic review and meta-analysis. PLoS One. (2016) 11(10):e0163365. doi: 10.1371/journal.pone.0163365
14. Gigi RMS, Buitrago-Garcia D, Taghavi K, Dunaiski C-M, van de Wijgert JHHM, Peters RPH, et al. Vulvovaginal yeast infections during pregnancy and perinatal outcomes: systematic review and meta-analysis. BMC Womens Health. (2023) 23(1):116. doi: 10.1186/s12905-023-02258-7
15. Schuster HJ, de Jonghe BA, Limpens J, Budding AE, Painter RC. Asymptomatic vaginal Candida colonization and adverse pregnancy outcomes including preterm birth: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. (2020) 2:100163. doi: 10.1016/j.ajogmf.2020.100163
16. Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Every newborn: progress, priorities, and potential beyond survival. Lancet. (2014) 384:189–205. doi: 10.1016/S0140-6736(14)60496-7
17. Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. (2019) 7:e37–46. doi: 10.1016/S2214-109X(18)30451-0
18. United Nations Inter-agency Group for Child Mortality Estimation (UN IGME). Levels & Trends in Child Mortality: Report 2019, Estimates Developed by the United Nations Inter-agency Group for Child Mortality Estimation.
19. World Health Organization. Maternal and Child Epidemiology Estimation Group (MCEE), Child Causes of Death, by Country and by Region, 2000–2016. Geneva: World Health Organization (2018). Available online at: int/healthinfo/global_burden_disease/childcod_methods_2000_2016.pdf2018
20. Mulinganya G, De Vulder A, Bisimwa G, Boelens J, Claeys G, De Keyser K, et al. Prevalence, risk factors and adverse pregnancy outcomes of second trimester bacterial vaginosis among pregnant women in Bukavu, Democratic Republic of the Congo. PLoS One. (2021) 16(10):e0257939. doi: 10.1371/journal.pone.0257939
21. Ministry of Public Health, Democratic Republic of the Congo (Ministère de la Santé Publique, République Démocratique du Congo). Guide national de Prise en Charge des Infections Sexuellement Transmissibles Selon l'approche Syndromique (2006). Available online at: https://www.medbox.org/document/guide-national-de-prise-en-charge-des-infections-sexuellement-transmissibles-selon-lapproche-syndromique (Accessed October 01, 2023).
22. World Health Organization. Guide Pour la Prise en Charge des Infections Sexuellement Transmissibles. Geneva: Organisation mondiale de la Santé (2006).
23. Maladkar M, Patil S, Sood S. A clinical experience to assess safety and efficacy of fixed dose combination of clindamycin and clotrimazole in the treatment of patients with mixed bacterial and fungal vaginosis. J Clin Gynecol Obstet. (2015) 4:217–25. doi: 10.14740/jcgo331w
24. Kyongo JK, Crucitti T, Menten J, Hardy L, Cools P, Michiels J, et al. Cross-sectional analysis of selected genital tract immunological markers and molecular vaginal Microbiota in Sub-Saharan African women, with relevance to HIV risk and prevention. Clin Vaccine Immunol. (2015) 22(5):526–38. doi: 10.1128/CVI.00762-14
25. Modak T, Arora P, Agnes C, Ray R, Goswami S, Ghosh P, et al. Diagnosis of bacterial vaginosis in cases of abnormal vaginal discharge: comparison of clinical and microbiological criteria. J Infect Dev Ctries. (2011) 5:353–60. doi: 10.3855/jidc.1153
26. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. (1991) 29:297–301. doi: 10.1128/jcm.29.2.297-301.1991
27. Duyvejonck H, Cools P, Decruyenaere J, Roelens K, Noens L, Vermeulen S, et al. Validation of high resolution melting analysis (HRM) of the amplified ITS2 region for the detection and identification of yeasts from clinical samples: comparison with culture and MALDI-TOF based identification. PLoS One. (2015) 10:e0132149. doi: 10.1371/journal.pone.0132149
28. World Health Organisation. Recommended definitions, terminology and format for statistical tables related to the perinatal period and use of a new certificate for cause of perinatal deaths. Acta Obstet Gynecol Scand. (1977) 56:247–53. doi: 10.3109/00016347709162009
29. Cutland CL, Lackritz EM, Mallett-Moore T, Bardají A, Chandrasekaran R, Lahariya C, et al. Low birth weight: case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. (2017) 35:6492–500. doi: 10.1016/j.vaccine.2017.01.049
30. Quinn J-A, Munoz FM, Gonik B, Frau L, Cutland C, Mallett-Moore T, et al. Preterm birth: case definition & guidelines for data collection, analysis, and presentation of immunisation safety data. Vaccine. (2016) 34:6047–56. doi: 10.1016/j.vaccine.2016.03.045
31. Naaktgeboren CA, Bertens LC, van Smeden M, de Groot JA, Moons KG, Reitsma JB. Value of composite reference standards in diagnostic research. Br Med J. (2013) 347:f5605. doi: 10.1136/bmj.f5605
32. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. (1977) 33(1):159–74. doi: 10.2307/2529310
33. Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. (2008) 371:75–84. doi: 10.1016/S0140-6736(08)60074-4
34. Payne MS, Bayatibojakhi S. Exploring preterm birth as a polymicrobial disease: an overview of the uterine microbiome. Front Immunol. (2014) 5:595. doi: 10.3389/fimmu.2014.00595
35. Vogel JP, Chawanpaiboon S, Moller A-B, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. (2018) 52:3–12. doi: 10.1016/j.bpobgyn.2018.04.003
36. Holzer I, Farr A, Kiss H, Hagmann M, Petricevic L. The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch Gynecol Obstet. (2017) 295:891–5. doi: 10.1007/s00404-017-4331-y
37. Sobel JD, Akins RA. The role of PCR in the diagnosis of Candida vulvovaginitis—a new gold standard? Curr Infect Dis Rep. (2015) 17(6):488. doi: 10.1007/s11908-015-0488-3
38. Mårdh P-A, Novikova N, Witkin SS, Korneeva I, Rodriques AR. Detection of candida by polymerase chain reaction vs. microscopy and culture in women diagnosed as recurrent vulvovaginal cases. Int J STD AIDS. (2003) 14:753–6. doi: 10.1258/09564620360719796
39. Bergman JJ, Berg AO, Schneeweiss R, Heidrich F. Clinical comparison of microscopic and culture techniques in the diagnosis of Candida vaginitis. J. Fam. Pract. (1984) 18:549–52. PMID: 6368737.6368737
40. Sangaré I, Sirima C, Bamba S, Zida A, Cissé M, Bazié W, et al. Prevalence of vulvovaginal candidiasis in pregnancy at three health centers in Burkina Faso. J Mycol Med. (2018) 28:186–92. doi: 10.1016/j.mycmed.2017.08.006
41. Mulu W, Yimer M, Zenebe Y, Abera B. Common causes of vaginal infections and antibiotic susceptibility of aerobic bacterial isolates in women of reproductive age attending at felegehiwot referral hospital, Ethiopia: a cross sectional study. BMC Womens Health. (2015) 15:42. doi: 10.1186/s12905-015-0197-y
42. Mushi MF, Bader O, Bii C, Groß U, Mshana SE. Virulence and susceptibility patterns of clinical Candida spp. Isolates from a tertiary hospital, Tanzania. Med Mycol. (2019) 57:566–72. doi: 10.1093/mmy/myy107
43. Aguin TJ, Sobel JD. Vulvovaginal candidiasis in pregnancy. Curr Infect Dis Rep. (2015) 17:462. doi: 10.1007/s11908-015-0462-0
44. Kennedy MA, Sobel JD. Vulvovaginal candidiasis caused by non-albicans Candida species: new insights. Curr Infect Dis Rep. (2010) 12:465–70. doi: 10.1007/s11908-010-0137-9
45. Papaemmanouil V, Georgogiannis N, Plega M, Lalaki J, Lydakis D, Dimitriou M, et al. Prevalence and susceptibility of Saccharomyces cerevisiae causing vaginitis in Greek women. Anaerobe. (2011) 17:298–9. doi: 10.1016/j.anaerobe.2011.04.008
46. Sobel J, Vazquez J, Lynch M, Meriwether C, Zervos M. Vaginitis due to Saccharomyces cerevisiae: epidemiology, clinical aspects, and therapy. Clin Infect Dis. (1993) 16:93–9. doi: 10.1093/clinids/16.1.93
47. Denning DW, Kneale M, Sobel JD, Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis. (2018) 18:e339–e47. doi: 10.1016/S1473-3099(18)30103-8
48. Arfiputri DS, Hidayati AN, Handayani S, Ervianti E. Risk factors of vulvovaginal candidiasis in dermato-venereology outpatients clinic of soetomo general hospital, Surabaya, Indonesia. Afr J Infect Dis. (2018) 12:90–4. doi: 10.21010/ajid.v12i1S.13
49. Tachedjian G, Aldunate M, Bradshaw CS, Cone RA. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. (2017) 168:782–92. doi: 10.1016/j.resmic.2017.04.001
50. McClelland RS, Richardson BA, Hassan WM, Graham SM, Kiarie J, Baeten JM, et al. Prospective study of vaginal bacterial flora and other risk factors for vulvovaginal candidiasis. J Infect Dis. (2009) 199:1883–90. doi: 10.1086/599213
51. Jespers V, Crucitti T, Menten J, Verhelst R, Mwaura M, Mandaliya K, et al. Prevalence and correlates of bacterial vaginosis in different sub-populations of women in Sub-Saharan Africa: a cross-sectional study. PLoS One. (2014) 9(10):e109670. doi: 10.1371/journal.pone.0109670
52. Rodríguez-Arias RJ, Guachi-Álvarez BO, Montalvo-Vivero DE, Machado A. Lactobacilli displacement and Candida albicans inhibition on initial adhesion assays: a probiotic analysis. BMC Res Notes. (2022) 15:1–7. doi: 10.1186/s13104-022-06114-z
53. Lin XL, Li Z, Zuo XL. Study on the relationship between vaginal and intestinal Candida in patients with vulvovaginal candidiasis. Zhonghua Fu Chan Ke Za Zhi. (2011) 46:496–500. Chinese. PMID: 22041440.22041440
54. Masha SC, Wahome E, Vaneechoutte M, Cools P, Crucitti T, Sanders EJ. High prevalence of curable sexually transmitted infections among pregnant women in a rural county hospital in Kilifi, Kenya. PLoS One. (2017) 12:e0175166. doi: 10.1371/journal.pone.0175166
55. Crucitti T, Jespers V, Mulenga C, Khondowe S, Vandepitte J, Buvé A. Non-sexual transmission of trichomonas vaginalis in adolescent girls attending school in Ndola, Zambia. PLoS One. (2011) 6(1):e16310. doi: 10.1371/journal.pone.0016310
56. Koster M, Price LL. Rwandan female genital modification: elongation of the labia minora and the use of local botanical species. Cult Health Sex. (2008) 10:191–204. doi: 10.1080/13691050701775076
57. Lamont RF. Advances in the prevention of infection-related preterm birth. Front Immunol. (2015) 6:566. doi: 10.3389/fimmu.2015.00566
58. Goodfellow L, Verwijs MC, Care A, Sharp A, Ivandic J, Poljak B, et al. Vaginal bacterial load in the second trimester is associated with early preterm birth recurrence: a nested case-control study. BJOG. (2021) 128(13):2061–72. doi: 10.1111/1471-0528.16816
59. Cools P, Melin P. Group B Streptococcus and perinatal mortality. Res Microbiol. (2017) 168(9-10):793–801. doi: 10.1016/j.resmic.2017.04.002
60. Roberts CL, Algert CS, Rickard KL, Morris JM. Treatment of vaginal candidiasis for the prevention of preterm birth: a systematic review and meta-analysis. Syst Rev. (2015) 4:31. doi: 10.1186/s13643-015-0018-2
61. Schaaf JM, Liem SM, Mol BWJ, Abu-Hanna A, Ravelli AC. Ethnic and racial disparities in the risk of preterm birth: a systematic review and meta-analysis. Am J Perinatol. (2013) 30(6):433–50. doi: 10.1055/s-0032-1326988
62. Jespers V, van de Wijgert J, Cools P, Verhelst R, Verstraelen H, Delany-Moretlwe S, et al. The significance of Lactobacillus crispatus and L. vaginalis for vaginal health and the negative effect of recent sex: a cross-sectional descriptive study across groups of African women. BMC Infect Dis. (2015) 15:115. doi: 10.1186/s12879-015-0825-z
63. Omosa-Manyonyi GS, de Kam M, Tostmann A, Masido MA, Nyagah N, Obimbo MM, et al. Evaluation and optimization of the syndromic management of female genital tract infections in Nairobi, Kenya. BMC Infect Dis. (2023) 23(1):547. doi: 10.1186/s12879-023-08442-2
64. Rathod SD, Klausner JD, Krupp K, Reingold AL, Madhivanan P. Epidemiologic features of vulvovaginal candidiasis among reproductive-age women in India. Infect Dis Obstet Gynecol. (2012) 2012:859071. doi: 10.1155/2012/859071
Keywords: candida, pregnancy, Democratic Republic of the Congo, newborn, preterm birth, qPCR, microscopy, vaginal microbiome
Citation: Mulinganya MG, De Keyser K, Mongane IJ, Kampara MF, De Vulder A, Boelens J, Duyvejonck H, Hendwa E, Kujirakwinja BY, Bisimwa BG, Rodriguez A, Vaneechoutte M, Callens S and Cools P (2024) Second trimester vaginal Candida colonization among pregnant women attending antenatal care in Bukavu, Democratic Republic of the Congo: prevalence, clinical correlates, risk factors and pregnancy outcomes. Front. Glob. Womens Health 5:1339821. doi: 10.3389/fgwh.2024.1339821
Received: 16 November 2023; Accepted: 29 April 2024;
Published: 23 May 2024.
Edited by:
Rebecca F. Grais, Epicentre, FranceReviewed by:
António Machado, Universidad San Francisco de Quito, EcuadorStephen E. Mshana, Catholic University of Health and Allied Sciences (CUHAS), Tanzania
© 2024 Mulinganya, De Keyser, Mongane, Kampara, De Vulder, Boelens, Duyvejonck, Hendwa, Kujirakwinja, Bisimwa, Rodriguez, Vaneechoutte, Callens and Cools. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Piet Cools, cGlldC5jb29sc0BVR2VudC5iZQ==
 Mulumeoderhwa Guy Mulinganya
Mulumeoderhwa Guy Mulinganya Karen De Keyser4
Karen De Keyser4 Antonio Rodriguez
Antonio Rodriguez Mario Vaneechoutte
Mario Vaneechoutte Piet Cools
Piet Cools