- 1Department of Pharmacy, School of Health Sciences, University of Zambia, Lusaka, Zambia
- 2School of Public Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
- 3Department of Health Behavior, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 4School of Public Health, University of Zambia, Lusaka, Zambia
- 5Department of Obstetrics and Gynaecology, School of Medicine, The University of Zambia, Lusaka, Zambia
- 6Health Sciences Library, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
- 7Department of Obstetrics and Gynecology, University of North Carolina at Chapel Hill, Chapel Hill, NC, United States
Background: Hypertensive disorders of pregnancy can lead to persistent hypertension (pHTN) in the months and even years following delivery. However, its prevalence in low- and middle-income countries (LMICs) is not well characterized.
Objective: To synthesize available evidence on the pHTN prevalence following a pregnancy complicated by hypertensive disorders of pregnancy in LMICs.
Search strategy: PubMed, CINAHL Plus, Global Health (EBSCOhost), and Scopus from inception through a search date of July 12, 2022, and updated on January 2, 2024.
Selection criteria: Cross-sectional studies and cohort studies reporting pHTN prevalence were eligible.
Data collection and analysis: We conducted a narrative synthesis of data and categorized reported prevalence time points into several broader categories. We used the Newcastle-Ottawa checklist to assess the risk of bias. The protocol is registered in PROSPERO (CRD42022345739).
Results: We reviewed 1,584 abstracts and identified 22 studies that reported pHTN between 2000 and 2023 from 14 LMICs. The overall prevalence of pHTN ranged between 6.9% and 62.2%, with the highest prevalence noted within African studies and the lowest in South American studies. Estimates at different follow-up periods postpartum were 6.9%–42.9% at six weeks, 34.0%–62.2% at three months, 14.8%–62.2% at six months, 12.7%–61.2% at 12 months, and 7.5%–31.8% at more than 12 months. The quality score of the selected studies ranged from 50% to 100%.
Conclusions: The extant literature reports a high prevalence of pHTN in LMICs following a pregnancy complicated by hypertensive disorders. To reduce long-term complications of pHTN, programs should emphasize early screening and linkages to long-term care for at-risk women.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=345739, PROSPERO (CRD42022345739)
Background
Hypertension (HTN) is a metabolic condition characterized by elevated blood pressure (1). Nearly 1.3 billion adults globally have HTN, with less than half (42%) diagnosed and treated (2). A non-communicable disease, HTN is currently one of the main causes of death among men and women globally (3). Low- and middle-income countries (LMICs) bear a disproportionate burden due to unplanned rapid urbanization, population ageing and globalization of unhealthy lifestyles (2). Between 1990 and 2013, HTN-related deaths increased by 49%—to 10.4 million—a trend that is projected to continue (4). Hypertension can lead to cardiovascular diseases, including strokes and cardiac arrest, which are leading causes of premature deaths (5). Some risk factors for cardiovascular diseases are specific to women, and one of them is hypertensive disorders of pregnancy (HDP) (6).
Hypertensive disorders of pregnancy refer to gestational hypertension, preeclampsia or eclampsia (7–9). Preeclampsia and eclampsia are the most significant causes of maternal and perinatal morbidity and mortality after haemorrhage (10). Similarly, adverse events in neonates largely depend on the type and severity of the hypertensive disorder (11). Risk factors for developing HDP include but are not limited to a family history of hypertension, obesity, smoking, previous history of HDP, extremes of maternal age, alcohol use, smoking, and left ventricular hypertrophy (12).
Hypertensive disorders affect 5% to 10% of pregnant women globally and are increasing with the rise in cardiometabolic diseases in women of childbearing age (13). Studies have reported delayed recovery and persistent HDP symptoms that have been linked to the development of persistent hypertension (pHTN) long after giving birth (14, 15). HDP predisposes women to early cardiovascular diseases (3), which is why early diagnosis and follow-up of chronic hypertension are important in clinical management. While there have been several individual studies that reported pHTN, there are existing gaps. A recent systematic review and meta-analysis only assessed the severity of HDP and risk of cardiovascular diseases in different years after index pregnancy (16). A similar review focused on recurrent preeclampsia but did not assess gestational hypertension and eclampsia (17). However, we are unaware of systematic reviews on pHTN following a pregnancy complicated by gestational hypertension, preeclampsia and eclampsia in LMICs. Understanding the prevalence of pHTN by timing postpartum can inform in developing screening guidelines and promote linkage to HTN care among women of childbearing age. Through this systematic review, we sought to address this scientific gap.
Methods
This review used the Preferred Reporting Items of Systematic Reviews and Meta-Analysis 2020 checklist (18). The protocol is registered in the International Prospective Register of Systematic Reviews PROSPERO (CRD42022345739).
Eligibility criteria
This review included cross-sectional studies, retrospective and prospective cohort studies reporting pHTN burden after deliveries following a pregnancy complicated by HDP. Case-control studies, in which participants were selected based on the diagnosis of pHTN, were excluded to minimize bias. Only studies conducted in LMICs as defined by the World Bank (http://data.worldbank.org/about/country-and-lending-groups) were considered because this is where two-thirds of the global HTN cases occur (2), and monitoring of cardiovascular diseases is limited in these settings. We documented the criteria used within these selected studies to define gestational hypertension, preeclampsia and eclampsia. If the definition of HDP was not explicitly stated, we assumed that the definition used was derived from a widely used classification (7, 8, 19).
Search strategy
We searched the following electronic bibliographic databases through the last search date of July 12, 2022, and updated on January 2, 2024: (PubMed, Scopus, Global Health (EBSCOhost), and CINAHL Plus with Full Text (EBSCOhost)) using a comprehensive search strategy (Supplementary Appendix S1). The search strategy was adapted for use across the different databases. No time restrictions were imposed; however, the searches were restricted to studies published in English as the research team members were only fluent in English. Furthermore, included articles and reference lists were screened for extra inclusion material.
Data selection and abstraction
Search results from databases were downloaded to EndNote (Clarivate, Philadelphia, PA, USA), where duplicates were removed. Primary screening and data extraction was done using Covidence (Vertias Health Innovation, Melbourne, Australia), an internet-based systematic review study screening program. Two reviewers (MM and MKL) independently screened titles, abstracts, and full text for inclusion. Any discrepancies between the two reviewer’s summary reports were resolved through consensus. Data abstraction was conducted using a standardized data extraction form designed to capture variables of interest. Potentially relevant studies were retrieved in full, and a calibration exercise was conducted to assure clarity and non-duplication amongst reviewers. The following data were extracted from each study: country, study design, author, study period, sample sizes, publication year, follow-up period, source population, study setting/data source, the timing of HTN measurement, outcome measures (including definitions criteria), and effect measure estimates. Two reviewers (MM and MKL) conducted data abstraction, and any discrepancies were addressed through consensus. For any missing or unclear information, we contacted the corresponding authors at least three times if no response was received on the first attempt.
Methodological approach
A PRISMA 2020 guideline for a systematic review and flow chart summarised the review’s search and selection of studies (20). We described the included articles and provided a summary of their characteristics. In addition, we reported estimates of the magnitude of pHTN with additional details about definitions and timing of measurements following a pregnancy complicated by HDP.
For pHTN, we assessed the proportion of women with a recent pregnancy complicated by HDP who remained hypertensive at the time of assessment. This review relied on the definitions of HTN used within individual studies. Estimates were reported with 95% confidence intervals (CIs). The estimates were reported at return visits and study endpoints for cohort studies. In addition, the total number of women with pHTN reported during a postpartum period was used for cross-sectional studies. We found that most studies did not clearly define all components of HDP, and assessment time points varied. Given this considerable heterogeneity, meta-analysis was deemed not feasible, and we proceeded with a narrative review alone (21).
Methodological quality
Two independent reviewers (MM and MKL) used the Newcastle-Ottawa checklist to assess individual studies’ quality and risk of bias (22). Disagreement between two reviewers was resolved through consensus. Three domains were assessed: selection of participants, comparability of cohorts and outcome assessment. For the presence of a rating, a ‘yes’ was given one point; otherwise, a zero was assigned. The item scores were summed to obtain total scores. The percentage scores were calculated by dividing the article’s score by the maximum score possible and multiplying by 100. The total score ranged from 0 to 100%, with scores less than 65%, less than 76% and between 76% and 100% considered high, moderate and low-risk studies, respectively. We used the assessment of risk of bias to inform a sensitivity analysis that considered only studies with low overall risk of bias.
Results
Search results
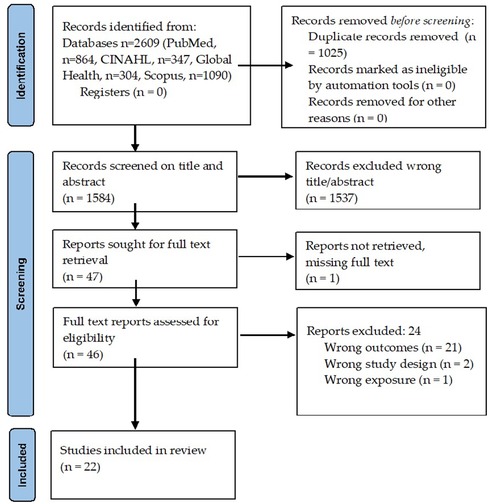
Overall, 2,609 articles were identified, with 1,025 duplicates removed (Figure 1). Full texts were obtained for 47 papers, of which 25 were excluded (Supplementary Table S1) upon further screening. The reasons for excluding studies were not being able to retrieve report (n = 1) (23), while the rest did not report pHTN as the primary outcome (n = 20) (24–43), wrong design (n = 2) (44, 45), oral presentation (n = 1) (46), or wrong exposure (n = 1) (47). After retrieval and full-text review, 22 published reports (Supplementary Table S1) were selected for this systematic review (14–16, 48–65).
Characteristics of included studies
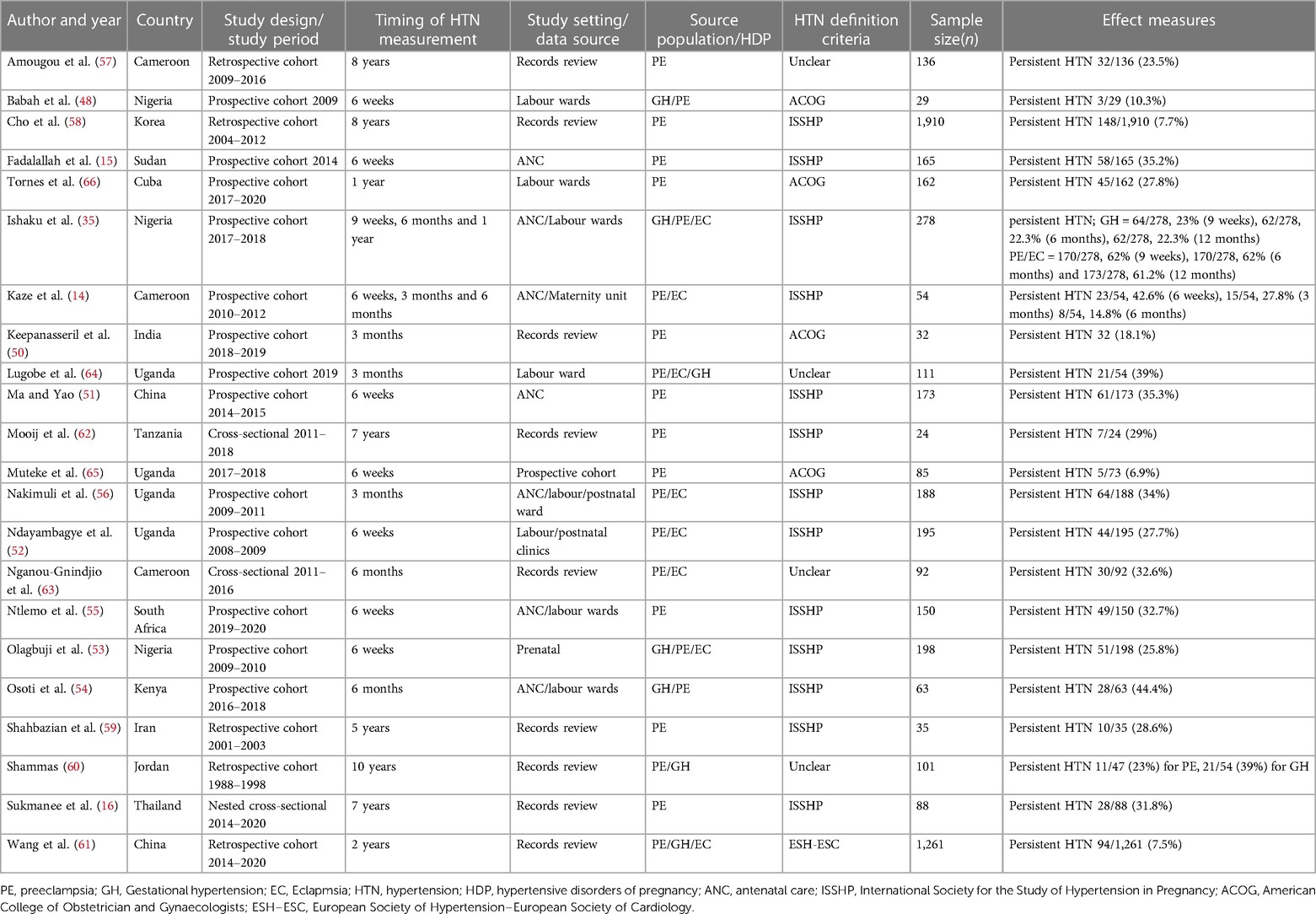
Characteristics of the studies are summarised in Table 1. Information regarding the prevalence of pHTN reported between 2000 and 2023 was obtained from 14 LMICs, with Uganda accounting for the largest proportion (18.2%), followed by Nigeria (13.6%) and Cameroon (13.6%). Fourteen studies were prospective cohorts (14, 15, 48–56, 64, 65), five were retrospective cohorts (57–61), and three were cross-sectional studies (16, 62, 63).
For the definitions of HTN, 13 studies used the International Society for the Study of Hypertension in Pregnancy recommendations (8), four used the American College of Obstetricians and Gynecologists (7), one used the European Society of Hypertension–European Society of Cardiology, and four did not mention criteria used (Table 1).
We noted considerable variability from selected studies concerning HTN definition, study design, study setting/data source and follow-up period post-delivery. Preeclampsia as a component of HDP was reported in all 22 studies. However, only seven studies reported gestational hypertension, and eight studies reported eclampsia (Table 1).
Persistent hypertension
All 22 studies reported pHTN post-delivery of pregnancy complicated by HDP, ranging between 6.9% and 62.2% (Table 1). There was high heterogeneity with the timing of HTN measurement reported as an interval from delivery until the development of HTN at the time of assessment. We categorized these time points into several broader categories: six weeks, three months, six months, 12 months and more than 12 months to examine the prevalence of pHTN. The ranges reported were 6.9%–42.9% at six weeks, 34.0%–62.2% at three months, 14.8%–62.2% at six months, 12.7%–61.2% at 12 months, and 7.5%–31.8% at more than 12 months. The prevalences are shown in Figure 2A.
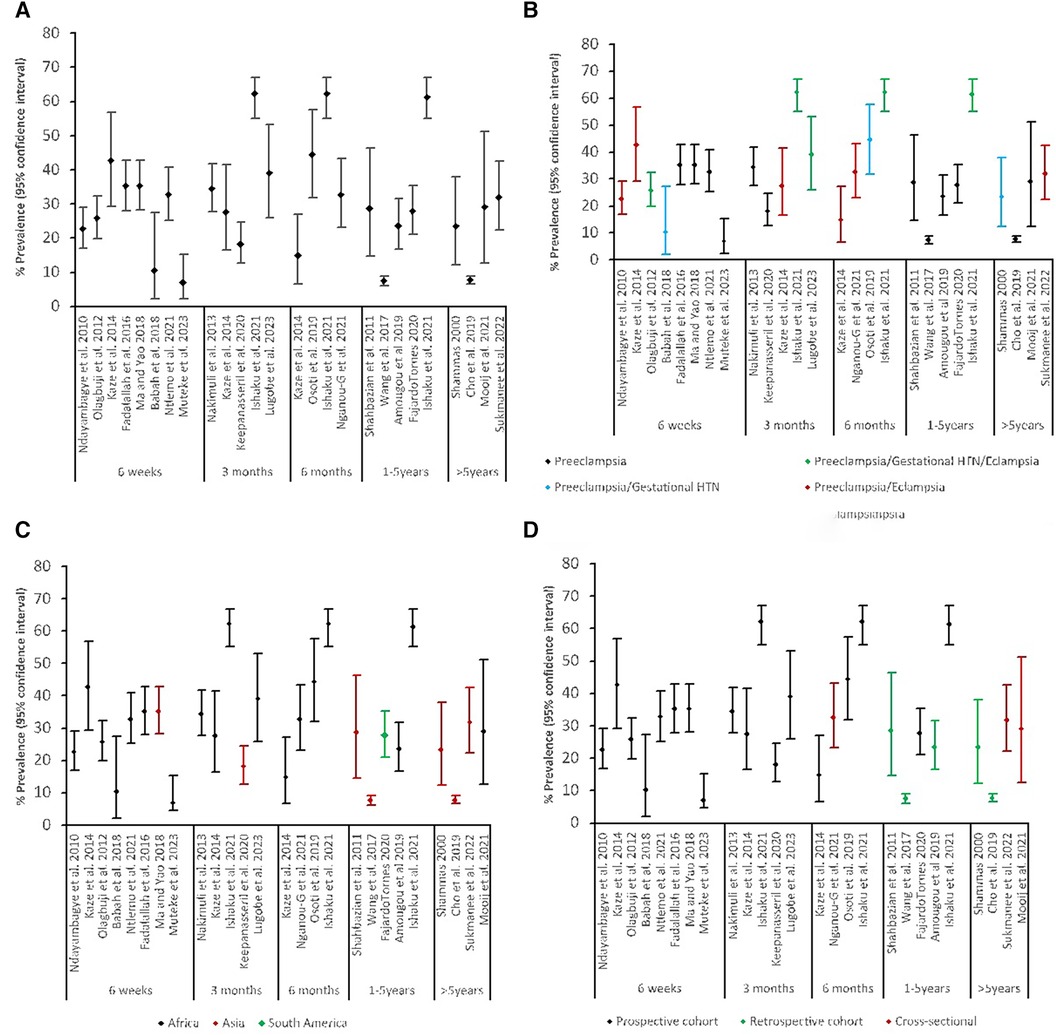
Figure 2. Prevalence of persistent HTN: (A) by timing of measurement, (B) by timing of measurement and source population, (C) by timing of measurement and region, (D) by timing of measurement and study design.
The prevalence varied considerably by region of LMICs (Figure 2C). The prevalence of pHTN in the African region was highest at any given time, followed by South America, and Asia was the least. Figure 2B shows the types of HDP considered within the study populations. Studies that reported all three hypertensive disorders had higher rates of pHTN at any time, followed by combined preeclampsia and eclampsia. When stratified by the study design (Figure 2D), prospective cohort studies reported higher prevalence rates (10.3%–62.2%) than retrospective (7.2%–31.6%) and cross-sectional studies (23.2%–42.2%), regardless of the source population.
Quality appraisal
Twenty studies were included in quality appraisals (Table 2). Two studies were excluded from the quality appraisal because they reported pHTN as part of the composite outcome. The risk of bias was low in 17 studies, moderate in one study and high in four studies. Most studies were considered to be at high risk of bias because they did not have a non-exposed group. Overall, the quality score of the selected studies ranged from 50% to 100%.
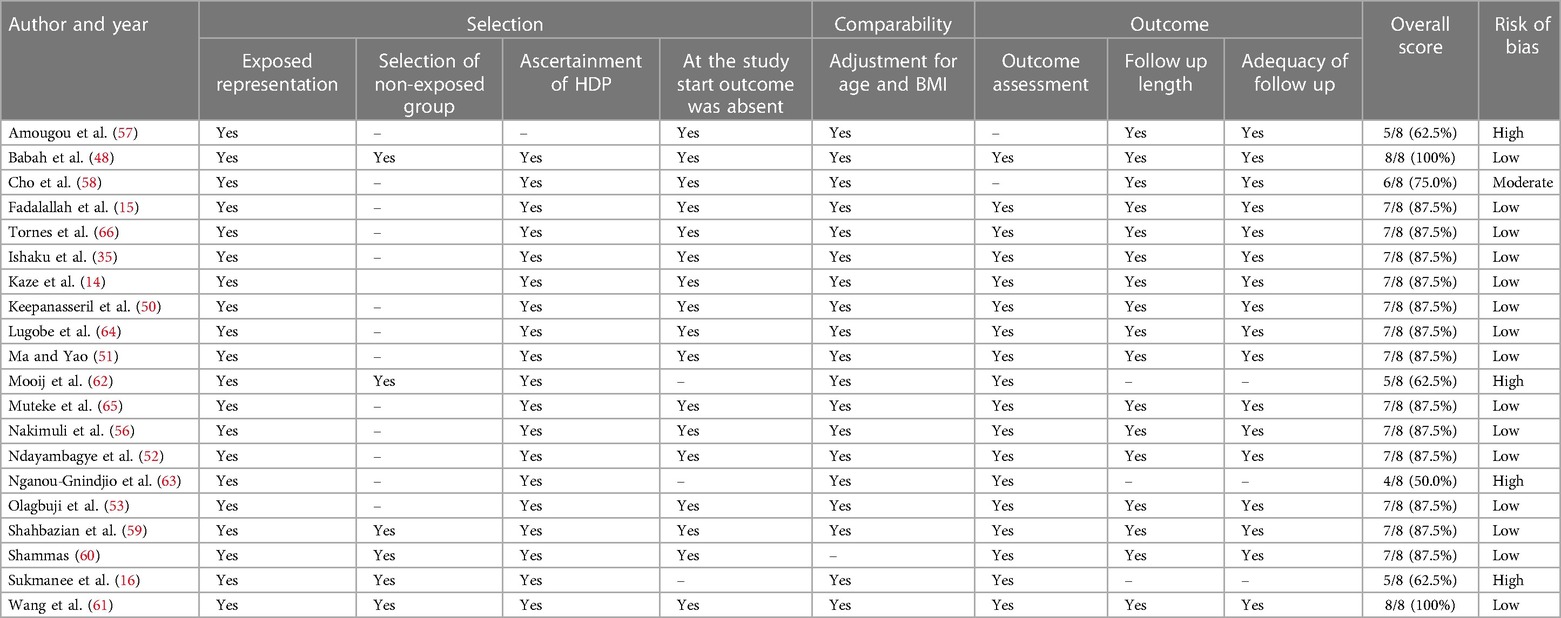
Table 2. Risk of bias assessment of included studies with persistent hypertension as a primary outcome (n = 20).
Sensitivity analysis
In sensitivity analysis, including only studies with a low risk of methodological bias, the overall prevalence of pHTN ranged from 6.9%–62.2%. The prevalence was less varied 7.5%–23.5% at more than 12 months.
Discussion
This systematic review aimed to examine the prevalence of pHTN among women with prior HDP in LMICs. We found that the prevalence of pHTN post-delivery varied greatly among women diagnosed with HDP in this region. The prevalence appeared to differ by region, type of HDP, timing of HTN measurement postpartum, and study design—but remained high throughout.
Among the included studies, there was a high variation in the prevalence of pHTN. Large proportions of women with HDP had high blood pressure six weeks after delivery, with the longest follow-up period being ten years. This was consistent with prior systematic reviews that included only preeclampsia as a source population (17, 67). In this systematic review, studies that included all HDP in the source population reported the highest prevalence rates of pHTN after pregnancy, followed by those with combined preeclampsia and eclampsia, and the least was preeclampsia. The mechanism explaining the relation between pHTN and HDP is considered multifactorial and challenging to study if thresholds are different and there is no standard approach. Studies have shown gestational hypertension and preeclampsia to be independent risk factors after adjusting for known cardiovascular risk markers like obesity and advanced age (68). A meta-analysis by Heida and colleagues (69) found that the risk of pHTN after delivery among women with gestational hypertension was comparable to women with preeclampsia. Other mechanisms that play a role in gestational hypertension or preeclampsia are of an inflammatory nature (70). Gestational hypertension and preeclampsia have been linked to elevated inflammatory biomarkers during pregnancy (71, 72), providing biological plausibility for persistent changes after pregnancy. Therefore, inflammatory biomarkers may provide insight into the development of pHTN after complicated pregnancies. Furthermore, at the time of diagnosis, pregnant women with gestational hypertension and preeclampsia have profound systemic inflammation and disruption of the endothelium (73). This suggests that pregnancy could be an early stress test providing an opportunity to identify women at risk of HTN early in life (37).
Among the studies in this review, the prevalence of pHTN was high. The prevalence was highest in African countries like Nigeria (49) and Kenya (54) and the lowest in South America (Cuba) (66) and Asia (China) (51), regardless of the timing of measurement. In high-income countries, the rates are lower. For instance, in the United Kingdom, they reported 13% (74), in the USA, 58.6% (75) and 3% in Australia (76). It is plausible that the variability in sociodemographic factors, maternal risk factors, and differences in postpartum care service utilization across the LMICs might explain the observed results. For instance, in sub-Saharan Africa, women often present late and with advanced disease states due to poor health-seeking behaviour, contributing to high co-morbidity and mortality (77, 78). Other plausible explanations for the observed estimates may be attributable to genetic differences between African populations and other regions (79), but the quality of care social and structural determinants of health have also been proposed as contributing factors (80).
Persistent hypertension prevalence rates depended on the timing of its measurement. Although HDP typically resolves in the window following delivery, its effects remain in the postpartum period due to the delay for most body systems to return to normal (81). It is possible that women at different time points do not have persistent long-term hypertension but are still resolving their HDP. However, this is less likely to be a problem over the course of follow-up, especially at the 12- and 24-month time points. The timing of hypertension screening is key to developing clinical guidelines for postpartum care for women with prior HDP. These findings suggest that, in LMICs, services to screen and treat HTN may be needed for women with HDP. In middle and high-income countries, several guidelines have been developed regarding pHTN post-delivery abeit inconsistent on the intervention thresholds. Some guidelines recommend initiating follow-up at 6–8 weeks post-delivery (82, 83), whereas others advise starting 6–12 months post-delivery (7, 84, 85). There is no consensus approach in many national programs, and the result is a non-standardized approach that may be difficult to implement. The observed regional and between-country disparities highlight the need, following delivery, to monitor women with HDP to ensure their well-being and prevent complications (8).
Strengths and limitations
The strength of this analysis was that, to the best of our knowledge, regional estimates of pHTN in LMICs have not been previously reported. The results from this systematic review challenge the status quo in most LMICs where women are mostly discharged and never followed up beyond six weeks after giving birth. Despite the strengths of this systematic review, these findings should be interpreted in the context of some limitations. The present systematic review excluded all sources written in languages other than English. In addition, there are several limitations to this combination of study designs, including the use of a checklist for quality appraisal and different guidelines, which can account for a large part of the variations in reported pHTN rates in the reviewed studies. Furthermore, the small number of studies from some regions of LMICs, such as South America, may have affected the true estimates for this region.
Conclusion
The present systematic review provided a narrative synthesis of available evidence regarding pHTN in LMICs following a pregnancy complicated by HDP. The prevalence appeared heterogeneous across the region due in part to the varied study timing and source populations.
Implications
Rates of pHTN appeared high across multiple settings and populations, suggesting that standard approaches to HTN screening, care, and treatment may be needed for women with HDP. Future research should focus on the design of such services, weighing both their costs and long-term benefits.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
MM: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing, Methodology. AH: Conceptualization, Resources, Supervision, Writing – review & editing. WM: Conceptualization, Resources, Supervision, Writing – review & editing. ML: Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. JC: Methodology, Software, Writing – review & editing. BC: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Research funding and trainee support for MM was provided by the UNC-UNZA-Wits Partnership for HIV and Women’s Reproductive Health, which is funded by the Fogarty International Center (D43 TW010558). Additional investigator salary was supported by the National Institute of Allergy and Infectious Diseases (K24 AI120796). The contents of this article are the authors’ responsibility and do not necessarily reflect the views of the funders. The funders had no role in the study design/conduct, decision to publish, or manuscript preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgwh.2024.1315763/full#supplementary-material
References
1. Zhou B, Perel P, Mensah GA, Ezzati M. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension. Nat Rev Cardiol. (2021) 18(11):785–802. doi: 10.1038/s41569-021-00559-8
2. Zhou B, Carrillo-Larco RM, Danaei G, Riley LM, Paciorek CJ, Stevens GA, et al. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398(10304):957–80. doi: 10.1016/S0140-6736(21)01330-1
3. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics—2017 update: a report from the American heart association. Circulation. (2017) 135(10):146–603. doi: 10.1161/CIR.0000000000000485
4. Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, et al. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the lancet commission on hypertension. Lancet. (2016) 388(10060):2665–712. doi: 10.1016/S0140-6736(16)31134-5
5. Yuyun MF, Sliwa K, Kengne AP, Mocumbi AO, Bukhman G. Cardiovascular diseases in sub-saharan Africa compared to high-income countries: an epidemiological perspective. Glob Heart. (2020) 15(1):15. doi: 10.5334/gh.403
6. Hoffman RM, Newhouse C, Chu B, Stringer JS, Currier JS. Non-communicable diseases in pregnant and postpartum women living with HIV: implications for health throughout the life course. Curr HIV/AIDS Rep. (2021) 18(1):73–86. doi: 10.1007/s11904-020-00539-6
7. Practice ACoO. ACOG Practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American college of obstetricians and gynecologists. Int J Gynecol Obstet. (2002) 77(1):67–75. doi: 10.1016/S0020-7292(02)80002-9
8. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: iSSHP classification, diagnosis, and management recommendations for international practice. Hypertension. (2018) 72(1):24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803
9. Tranquilli AL, Brown MA, Zeeman GG, Dekker G, Sibai BM. The definition of severe and early-onset preeclampsia. Statements from the international society for the study of hypertension in pregnancy (ISSHP). Pregnancy Hypertens. (2013) 3(1):44–7. doi: 10.1016/j.preghy.2012.11.001
10. Zhang WH, Alexander S, Bouvier-Colle MH, Macfarlane A, Group MB. Incidence of severe preeclampsia, postpartum haemorrhage and sepsis as a surrogate marker for severe maternal morbidity in a European population-based study: the MOMS-B survey. BJOG Int J Obstet Gynaecol. (2005) 112(1):89–96. doi: 10.1111/j.1471-0528.2004.00303.x
11. Bridwell M, Handzel E, Hynes M, Jean-Louis R, Fitter D, Hogue C, et al. Hypertensive disorders in pregnancy and maternal and neonatal outcomes in Haiti: the importance of surveillance and data collection. BMC Pregnancy Childbirth. (2019) 19(1):1–11. doi: 10.1186/s12884-019-2361-0
12. Shey Wiysonge C, Ngu Blackett K, Mbuagbaw J. Risk factors and complications of hypertension in Yaounde, Cameroon: cardiovascular topics. Cardiovasc J S Afr. (2004) 15(5):215–9.15483733
13. Benschop L, Duvekot JJ, van Lennep JER. Future risk of cardiovascular disease risk factors and events in women after a hypertensive disorder of pregnancy. Heart. (2019) 105(16):1273–8. doi: 10.1136/heartjnl-2018-313453
14. Kaze FF, Njukeng FA, Kengne AP, Ashuntantang G, Mbu R, Halle MP, et al. Postpartum trend in blood pressure levels, renal function and proteinuria in women with severe preeclampsia and eclampsia in Sub-Saharan Africa: a 6-months cohort study. BMC Pregnancy Childbirth. (2014) 14(1):1–7. doi: 10.1186/1471-2393-14-1
15. Fadalallah ZM, Elhassan EM, Rayis DA, Abdullahi H, Adam I. Prospective cohort study of persistent hypertension following preeclampsia at medani hospital, Sudan. Int J Gynaecol Obstet. (2016) 134(1):66–8. doi: 10.1016/j.ijgo.2015.11.014
16. Sukmanee J, Liabsuetrakul T. Risk of future cardiovascular diseases in different years postpartum after hypertensive disorders of pregnancy: a systematic review and meta-analysis. Medicine (Baltimore). (2022) 101(30):29646. doi: 10.1097/MD.0000000000029646
17. Brouwers L, van der Meiden-van Roest AJ, Savelkoul C, Vogelvang TE, Lely AT, Franx A, et al. Recurrence of preeclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta-analysis. BJOG Int J Obstet Gynaecol. (2018) 125(13):1642–54. doi: 10.1111/1471-0528.15394
18. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6(7):1000097. doi: 10.1371/journal.pmed.1000097
19. Sinkey RG, Battarbee AN, Bello NA, Ives CW, Oparil S, Tita AT. Prevention, diagnosis, and management of hypertensive disorders of pregnancy: a comparison of international guidelines. Curr Hypertens Rep. (2020) 22:1–0. doi: 10.1007/s11906-020-01082-w
20. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372(88):105906. doi: 10.1186/s13643-021-01626-4
21. Cumpston MS, McKenzie JE, Welch VA, Brennan SE. Strengthening systematic reviews in public health: guidance in the cochrane handbook for systematic reviews of interventions. J Public Health (Bangkok). (2022) 44(4):588–92. doi: 10.1093/pubmed/fdac036
22. Peterson J, Welch V, Losos M, Tugwell PJ. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. vol. 2 (iss. 1). Ottawa: Ottawa Hospital Research Institute (2011). p. 1–2.
23. Mirza Muhammad Ilyas B, Talat M, Shabnum Tahir S, Aftab A, Omer S. Renal outcome in patients with preeclampsia and eclampsia at presentation, three and six months postpartum. Med Forum Mon. (2011) 22(9):8–10.
24. Vigil-De Gracia P, Ruiz E, López JC, Alveo de Jaramillo I, Vega-Maleck JC, Pinzón J. Management of severe hypertension in the postpartum period with intravenous hydralazine or labetalol: a randomized clinical trial. Hypertens Pregnancy. (2007) 26(2):163–71. doi: 10.1080/10641950701204430
25. Guida JP, Parpinelli MA, Surita FG, Costa ML. The impact of proteinuria on maternal and perinatal outcomes among women with preeclampsia. Int J Gynaecol Obstet. (2018) 143(1):101–7. doi: 10.1002/ijgo.12487
26. Farrokh-Eslamlou H, Aghlmand S, Oshnouei S. Persistence of hemorrhage and hypertensive disorders of pregnancy (HDP) as the main causes of maternal mortality: emergence of medical errors in Iranian healthcare system. Iran J Public Health. (2014) 43(10):1395.26060702
27. Choi SK, Shin JC, Park YG, Park IY, Kwon JY, Ko HS, et al. The efficacy of peripartum transthoracic echocardiography in women with preeclampsia. Pregnancy Hypertens. (2017) 10:187–91. doi: 10.1016/j.preghy.2017.05.002
28. Rezk M, Abo-Elnasr M, Al Halaby A, Zahran A, Badr H. Maternal and fetal outcome in women with gestational hypertension in comparison to gestational proteinuria: a 3-year observational study. Hypertens Pregnancy. (2016) 35(2):181–8. doi: 10.3109/10641955.2015.1130832
29. Berhan Y, Endeshaw G. Clinical and biomarkers difference in prepartum and postpartum eclampsia. Ethiop J Health Sci. (2015) 25(3):257–66. doi: 10.4314/ejhs.v25i3.9
30. Singh S, Ahmed EB, Egondu SC, Ikechukwu NE. Hypertensive disorders in pregnancy among pregnant women in a Nigerian Teaching Hospital. Niger Med J. (2014) 55(5):384. doi: 10.4103/0300-1652.140377
31. Machano MM, Joho AA. Prevalence and risk factors associated with severe preeclampsia among postpartum women in Zanzibar: a cross-sectional study. BMC Public Health. (2020) 20:1–10. doi: 10.1186/s12889-020-09384-z
32. Kreepala C, Luangphiphat W, Villarroel A, Kitporntheranunt M, Wattanavaekin K, Piyajarawong T. Effect of magnesium on glomerular filtration rate and recovery of hypertension in women with severe preeclampsia. Nephron. (2018) 138(1):35–41. doi: 10.1159/000481463
33. El-Qatrawi KJ. Effect of hypertension on pregnancy outcomes at UNRWA health centres in gaza governorates: a comparative study. Lancet. (2021) 398:S26. doi: 10.1016/S0140-6736(21)01512-9
34. Vasquez DN, Das Neves AV, Zakalik G, Intile DA, Cicora F, Saenz MG, et al. OS024. Characteristics and outcomes of critically ill obstetric patientswith hypertensive disease of pregnancy in Argentina: multicenter study. Pregnancy Hypertens. (2012) 2(3):188–9. doi: 10.1016/j.preghy.2012.04.025
35. Ishaku SM, Karima T, Oboirien KA, Innocent AP, Lawal O, Jamilu T, et al. Metabolic syndrome following hypertensive disorders in pregnancy in a low-resource setting: a cohort study. Pregnancy Hypertens. (2021) 25:129–35. doi: 10.1016/j.preghy.2021.05.018
36. Osoti AO, Page ST, Richardson BA, Guthrie BL, Kinuthia J, Polyak SJ, et al. Postpartum metabolic syndrome and high-sensitivity C-reactive protein after gestational hypertension and preeclampsia. Int J Gynaecol Obstet. (2020) 151(3):443–9. doi: 10.1002/ijgo.13352
37. Facca TA, Mastroianni-Kirsztajn G, Sabino AR, Passos MT, Dos Santos LF, Famá EA, et al. Pregnancy as an early stress test for cardiovascular and kidney disease diagnosis. Pregnancy Hypertens. (2018) 12:169–73. doi: 10.1016/j.preghy.2017.11.008
38. Behboudi-Gandevani S, Amiri M, Rahmati M, Amanollahi Soudmand S, Azizi F, Ramezani Tehrani F. Preeclampsia and the ten-year risk of incident chronic kidney disease. Cardiorenal Med. (2020) 10(3):188–97. doi: 10.1159/000506469
39. Louw M, Adeyemo A, Soma-Pillay P, Makin J, Pattinson RC. Cardiac diastolic function after recovery from preeclampsia. Cardiovasc J Afr. (2018) 29(1):26–31. doi: 10.5830/CVJA-2017-031
40. Jones E, Rayner B. Hypertension in pregnancy: a future risk for chronic kidney disease in South Africa. SAMJ S Afr Med J. (2019) 109(9):665–7. doi: 10.7196/SAMJ.2019.v109i9.13883
41. Ishaku SM, Olanrewaju TO, Browne JL, Klipstein-Grobusch K, Kayode GA, Franx A, et al. Prevalence and determinants of chronic kidney disease in women with hypertensive disorders in pregnancy in Nigeria: a cohort study. BMC Nephrol. (2021) 22(1):229. doi: 10.1186/s12882-021-02419-6
42. Wibhuti IR, Soesanto AM, Shahab F. Diastolic function in patients with preeclampsia during pre-and postpartum period using tissue Doppler imaging. Med J Indones. (2016) 25(2):93–7. doi: 10.13181/mji.v25i2.1410
43. Ravikumar T, Prasannakumar P. Association of hypertension and cerebral venous thrombosis among the women in the puerperal period–a prospective study. Int J Curr Res Med Sci. (2016) 2(2):472–80.
44. Amaral LM, Cunningham MW Jr, Cornelius DC, LaMarca B. Preeclampsia: long-term consequences for vascular health. Vasc Health Risk Manag. (2015) 15(11):403–15. doi: 10.2147/VHRM.S64798
45. Livergood MC, Tallmadge M, Mahlum L, Hauck J, Palatnik A. 658 Factors associated with persistent hypertension at 1-year postpartum in individuals with gestational hypertension or preeclampsia. Am J Obstet Gynecol. (2021) 224(2):S413–S4. doi: 10.1016/j.ajog.2020.12.682
46. Ueno T, Yoshimoto L, Shimizu K, Yamada T, Takeda T, Tano S, et al. The changes of maternal cardiac function due to preeclampsia. J Perinat Med. (2017) 2:45.
47. Gainder S, Saha SC, Dhaliwal L, Bagga R. OS001. Pregnancy outcome in subsequent pregnancies after eclampsia. Pregnancy Hypertens. (2012) 2(3):175. doi: 10.1016/j.preghy.2012.04.003
48. Babah OA, Olaleye O, Afolabi BB. Postpartum sequelae of the hypertensive diseases of pregnancy: a pilot study. Niger Med J. (2018) 59(1):1. doi: 10.4103/nmj.NMJ_101_18
49. Ishaku SM, Jamilu T, Innocent AP, Gbenga KA, Lamaran D, Lawal O, et al. Persistent hypertension up to one year postpartum among women with hypertensive disorders in pregnancy in a low-resource setting: a prospective cohort study. Glob Heart. (2021) 16(1):62. doi: 10.5334/gh.854
50. Keepanasseril A, Thilaganathan B, Velmurugan B, Kar SS, Maurya DK, Pillai AA. Influence of maternal and perinatal characteristics on risk of postpartum chronic hypertension after preeclampsia. Int J Gynaecol Obstet. (2020) 151(1):128–33. doi: 10.1002/ijgo.13281
51. Ma J, Yao H. Persistent hypertension post-preeclampsia: a tertiary care centre-based study in China. Clin Exp Obstet Gynecol. (2018) 45(5):741–4. doi: 10.12891/ceog4094.2018
52. Ndayambagye EB, Nakalembe M, Kaye DK. Factors associated with persistent hypertension after puerperium among women with preeclampsia/eclampsia in Mulago Hospital, Uganda. BMC Pregnancy Childbirth. (2010) 10(1):1–7. doi: 10.1186/1471-2393-10-12
53. Olagbuji B, Ezeanochie M, Ande A, Okonkwo C. Prevalence and risk factors for persistent hypertension after the puerperium in pregnancies complicated with hypertensive disorders. J Obstet Gynaecol. (2012) 32(6):529–32. doi: 10.3109/01443615.2012.689891
54. Osoti AO, Page ST, Richardson BA, Guthrie BL, Kinuthia J, Polyak SJ, et al. Postpartum metabolic syndrome after gestational hypertension and preeclampsia, a prospective cohort study. Pregnancy Hypertens. (2019) 18:35–41. doi: 10.1016/j.preghy.2019.08.088
55. Ntlemo P, Cronje T, Soma-Pillay P. Metabolic syndrome at 6 weeks aeftr delivery in a cohort of pre-eclamptic and normotensive women. S Afr Med J. (2021) 111(4):350–4. doi: 10.7196/SAMJ.2021.v111i4.15349
56. Nakimuli A, Elliott AM, Kaleebu P, Moffett A, Mirembe F. Hypertension persisting after preeclampsia: a prospective cohort study at Mulago Hospital, Uganda. PloS One. (2013) 8(12):85273. doi: 10.1371/journal.pone.0085273
57. Amougou SN, Mbita S, Danwe D, Tebeu P-M. Factor associated with progression to chronic arterial hypertension in women with preeclampsia in Yaoundé, Cameroon. Pan Afr Med J. (2019) 33:200. doi: 10.11604/pamj.2019.33.200.16857
58. Cho GJ, Kim HY, Park JH, Ahn KH, Hong SC, Kim HJ, et al. Prepregnancy factors are associated with development of hypertension later in life in women with preeclampsia. J Women’s Health. (2019) 28(7):984–9. doi: 10.1089/jwh.2018.7165
59. Shahbazian N, Shahbazian H, Ehsanpour A, Aref A, Gharibzadeh S. Hypertension and microal buminuria 5 years after pregnancies complicated by preeclampsia. Iran J Kidney Dis. (2011) 5(5):324–7.21876309
60. Shammas AG, Maayah JF. Hypertension and its relation to renal function 10 years after pregnancy complicated by preeclampsia and pregnancy induced hypertension. Saudi Med J. (2000) 21(2):190–2.11533780
61. Wang L, Leng J, Liu H, Zhang S, Wang J, Li W, et al. Association between hypertensive disorders of pregnancy and the risk of postpartum hypertension: a cohort study in women with gestational diabetes. J Hum Hypertens. (2017) 31(11):725–30. doi: 10.1038/jhh.2017.46
62. Mooij R, Kapanga RR, Mwampagatwa IH, Mgalega GC, van Dillen J, Stekelenburg J, et al. Beyond severe acute maternal morbidity: a mixed-methods study on the long-term consequences of (severe pre-) eclampsia in rural Tanzania. Trop Med Int Health. (2021) 26(1):33–44. doi: 10.1111/tmi.13507
63. Nganou-Gnindjio CN, Kenmogne D, Essama DB, Nkeck JR, Yanwou N, Foumane P. Persistent hypertension after preeclampsia in a group of cameroonians: result of a cross-sectional study and perspectives to reduce its burden in limited income countries. J Clin Hypertens. (2021) 23(6):1246–51. doi: 10.1111/jch.14260
64. Lugobe HM, Kayondo M, Mceniery CM, Catov JM, Wilkinson IB, Wylie BJ, et al. Persistent hypertension at 3 months postpartum among women with hypertensive disorders of pregnancy at a tertiary hospital in Southwestern Uganda. AJOG Global Rep. (2023) 3(1):100163. doi: 10.1016/j.xagr.2023.100163
65. Muteke K, Musaba MW, Mukunya D, Beyeza J, Wandabwa JN, Kiondo P. Postpartum resolution of hypertension, proteinuria and acute kidney injury among women with preeclampsia and severe features at Mulago National Referral Hospital, Uganda: a cohort study. Afr Health Sci. (2023) 23(3):27–36. doi: 10.4314/ahs.v23i3.6
66. Tornes YF, Mèndez DN, Aliaga AA, Ayebare DS, Ssebuufu R, Byonanuwe S. Predictors of postpartum persisting hypertension among women with preeclampsia admitted at Carlos Manuel de Cèspedes Teaching Hospital, Cuba. Int J Women’s Health. (2020) 12:765. doi: 10.2147/IJWH.S263718
67. Alonso-Ventura V, Li Y, Pasupuleti V, Roman YM, Hernandez AV, Pérez-López FR. Effects of preeclampsia and eclampsia on maternal metabolic and biochemical outcomes in later life: a systematic review and meta-analysis. Metab Clin Exp. (2020) 102:154012. doi: 10.1016/j.metabol.2019.154012
68. Männistö T, Mendola P, Vääräsmäki M, Järvelin MR, Hartikainen AL, Pouta A, et al. Elevated blood pressure in pregnancy and subsequent chronic disease risk. Circulation. (2013) 127(6):681–90. doi: 10.1161/CIRCULATIONAHA.112.128751
69. Heida KY, Franx A, Van Rijn BB, Eijkemans MJ, Boer JM, Verschuren MW, et al. Earlier age of onset of chronic hypertension and type 2 diabetes mellitus after a hypertensive disorder of pregnancy or gestational diabetes mellitus. Hypertension. (2015) 66(6):1116–22. doi: 10.1161/HYPERTENSIONAHA.115.06005
70. Mtali YS, Lyimo MA, Luzzatto L, Massawe SN. Hypertensive disorders of pregnancy are associated with an inflammatory state: evidence from hematological findings and cytokine levels. BMC Pregnancy Childbirth. (2019) 19(1):1–9. doi: 10.1186/s12884-019-2383-7
71. Wang Y, Li B, Zhao Y. Inflammation in preeclampsia: genetic biomarkers, mechanisms, and therapeutic strategies. Front Immunol. (2022) 8(13):883404. doi: 10.3389/fimmu.2022.883404
72. Redman C, Sargent I. Preeclampsia, the placenta and the maternal systemic inflammatory response—a review. Placenta. (2003) 24:S21–S7. doi: 10.1053/plac.2002.0930
73. Tanz LJ, Stuart JJ, Missmer SA, Rimm EB, Sumner JA, Vadnais MA, et al. Cardiovascular biomarkers in the years following pregnancies complicated by hypertensive disorders or delivered preterm. Pregnancy Hypertens. (2018) 13:14–21. doi: 10.1016/j.preghy.2018.04.015
74. Girsberger M, Muff C, Hösli I, Dickenmann MJ. Short term sequelae of preeclampsia: a single center cohort study. BMC Pregnancy Childbirth. (2018) 18(1):1–7. doi: 10.1186/s12884-018-1796-z
75. Ackerman-Banks CM, Grechukhina O, Spatz E, Lundsberg L, Chou J, Smith G, et al. Seizing the window of opportunity within 1 year postpartum: early cardiovascular screening. J Am Heart Assoc. (2022) 11(8):024443. doi: 10.1161/JAHA.121.024443
76. Brown MA, Roberts L, Hoffman A, Henry A, Mangos G, O'Sullivan A, et al. Recognizing cardiovascular risk after preeclampsia: the P4 study. J Am Heart Assoc. (2020) 9(22):e018604. doi: 10.1161/JAHA.120.018604
77. Maisa A, Lawal AM, Islam T, Nwankwo C, Oluyide B, Fotso A, et al. Exploring factors influencing patient mortality and loss to follow-up in two paediatric hospital wards in Zamfara, North-West Nigeria, 2016–2018. Plos One. (2021) 16(12):0262073. doi: 10.1371/journal.pone.0262073
78. Adedokun ST, Yaya S. Factors influencing mothers’ health care seeking behaviour for their children: evidence from 31 countries in Sub-Saharan Africa. BMC Health Serv Res. (2020) 20(1):1–9. doi: 10.1186/s12913-020-05683-8
79. Anand S, Bradshaw C, Prabhakaran D. Prevention and management of CVD in LMICs: why do ethnicity, culture, and context matter? BMC Med. (2020) 18:1–5. doi: 10.1186/s12916-019-1480-9
80. Gouda HN, Charlson F, Sorsdahl K, Ahmadzada S, Ferrari AJ, Erskine H, et al. Burden of non-communicable diseases in Sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. (2019) 7(10):1375–87. doi: 10.1016/S2214-109X(19)30374-2
81. Pridjian G, Puschett JB. Preeclampsia. Part 1: clinical and pathophysiologic considerations. Obstet Gynecol Surv. (2002) 57(9):598–618. doi: 10.1097/00006254-200209000-00023
82. Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 update: a guideline from the American Heart Association. Circulation. (2011) 123(11):1243–62. doi: 10.1161/CIR.0b013e31820faaf8
83. Visintin C, Mugglestone MA, Almerie MQ, Nherera LM, James D, Walkinshaw S. Management of hypertensive disorders during pregnancy: summary of NICE guidance. Br Med J. (2010) 341:c2207. doi: 10.1136/bmj.c2207
84. Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, Blomström-Lundqvist C, Cifkova R, De Bonis M, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy: the task force for the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39(34):3165–241. doi: 10.1093/eurheartj/ehy340
85. Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL, et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45(5):1545–88. doi: 10.1161/01.str.0000442009.06663.48
Keywords: hypertensive disorders, persistent hypertension, low- and middle-income countries, prevalence, pregnancy
Citation: Mukosha M, Hatcher A, Mutale W, Lubeya MK, Conklin JL and Chi BH (2024) Prevalence of persistent hypertension following pregnancy complicated by hypertensive disorders in low-and middle-income countries: a systematic review. Front. Glob. Womens Health 5:1315763. doi: 10.3389/fgwh.2024.1315763
Received: 11 October 2023; Accepted: 22 February 2024;
Published: 1 March 2024.
Edited by:
Tabassum Firoz, Yale New Haven Health System, United StatesReviewed by:
Maggie Woo Kinshella, University of British Columbia, CanadaYixiao Wang, Southeast University, China
© 2024 Mukosha, Hatcher, Mutale, Lubeya, Conklin and Chi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moses Mukosha bXVrb3NoYW1vc2VzQHlhaG9vLmNvbQ==
 Moses Mukosha
Moses Mukosha Abigail Hatcher2,3
Abigail Hatcher2,3 Jamie L. Conklin
Jamie L. Conklin Benjamin H. Chi
Benjamin H. Chi