- 1Department of Midwifery, College of Health and Medical Sciences, Dilla University, Dilla, Ethiopia
- 2School of Nursing and Midwifery, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia
- 3School of Public Health, College of Health and Medical Science, Haramaya University, Harar, Ethiopia
Background: Anemia in the postpartum period remains a considerable public health problem in developing countries, particularly in sub-Saharan Africa. It is the most common indirect cause of maternal morbidity and mortality. It is also a major health problem in women of reproductive age, affecting their quality of life, occupational capacity, lactation, and immunological function. Immediate postpartum anemia has a significant impact on women's quality of life, although its predictors have received little attention in Ethiopia, notably in Harari Regional State. Therefore, this study aimed to determine its magnitude and contributing factors in Eastern Ethiopia.
Methods: A facility-based cross-sectional study was conducted from June 1st to August 30th, 2021, among 484 postpartum women admitted to two public hospitals in Harari Regional State, Eastern Ethiopia. Data were collected using a pre-tested, structured interviewer-administered questionnaire. About 2 mL of blood samples were collected and analyzed using the cell-Dyne 1,800 machine. The collected data were entered into Epi-Data version 4.6 and analyzed using SPSS version 25. A multivariable logistic regression analysis was conducted to estimate the effect of independent variables on immediate postpartum anemia. An adjusted odds ratio (AOR) with a 95% confidence interval (CI) was computed to report the presence of the association. Statistical significance was declared at a p-value of < 0.05.
Results: The overall magnitude of immediate postpartum anemia was 28.1% [95% CI (23.7, 32.1)]. Lack of formal education [AOR: 3.92; 95% CI: (1.85, 8.33)], having antenatal care < 4 visits [AOR: 3.18; 95% CI: (1.53, 6.61)], a history of cesarean delivery [AOR: 3.40; 95% CI: (1.89, 6.10)], a history of maternal blood loss [AOR: 4.78; 95% CI: (2.22, 10.30)], pre-delivery Hgb level < 11 g/dl [AOR:5.46; 95% CI: (3.09,9.67)], and having no iron-folate supplementation [AOR:3.27; 95% CI: (1.31, 8.15)] were factors statistically associated with immediate postpartum anemia.
Conclusions: In this study, nearly one-third of mothers admitted for postpartum care developed anemia within 48 h of giving birth. Women's educational level, frequency of antenatal care, mode of delivery, a history of maternal blood loss, pre-delivery hemoglobin level, and iron-folate supplementation status were identified as immediate postpartum anemia risk factors. Therefore, promoting the benefits of adequate antenatal care and iron-folate supplementation during pregnancy is crucial to avoiding the risks of postpartum anemia.
Introduction
Anemia is a condition characterized by a reduction in the number and/or size of red blood cells below cut-off values, thereby impairing the blood's ability to transport oxygen to meet physiologic needs (1). Even though postpartum anemia (PPA) lacks a consensual definition, it can be inferred from the definitions provided by various scholars, depending on the duration of the postpartum period. It can be defined as Hgb < 10 g/dl, Hgb < 11g/dl, and Hgb < 12g/dl cut-off values within the first 48 h of delivery, at 1 week and 6 weeks of postpartum duration, respectively (2–5).
Although hopeful progress has been made in lowering maternal mortality in many countries, there is still evidence of a continuous increment in the rate of indirect causes of maternal mortality (6). Globally, indirect causes of maternal mortality are attributable to 35% of all causes of maternal deaths, with anemia accounting for 7% of maternal mortality due to indirect causes and 2.3% of all causes (7, 8). Moreover, severe postpartum anemia increases the risk of maternal mortality by 3 folds during the postpartum period (9).
In 2019, anemia caused 1.74 billion cases and 58.6 million years of disability worldwide (10). More specifically, PPA affects 50–80% of postpartum women in developing countries and less than 30% in developed countries (7, 11). Approximately 36.5% of lactating women were particularly vulnerable to PPA in East African countries (12). The prevalence of PPA during lactation in Ethiopia also ranges from 11.6% in Addis Ababa to 58.7% in the Somali Region (13).
Furthermore, researchers have shown that postpartum anemia (PPA) has been found to be one of the most common causes of morbidity in the early postpartum period. In addition, it is also a major health problem in women of reproductive age, affecting their quality of life, occupational capacity, lactation, and immunological function (11, 14, 15). Additionally, PPA has been strongly associated with postpartum depression and emotional instability, negatively affecting mother-infant bonding (16, 17). This implies alterations during the child's psycho-neurological development, which can negatively affect infant development (18).
Studies have revealed that socio-demographic factors, obstetric-related factors, and nutritional and maternal health status are associated with PPA. For instance, various research reports have found that the age of the mother (19, 20), area of residence (21), level of education (21, 22), history of maternal blood loss (23, 24), pre-delivery Hgb level (23, 25), history of episiotomy during childbirth (26), mode of delivery (27), number of antenatal care visits, maternal parity (13), maternal nutritional status (24), history of iron and folic acid supplementation (28), and household food insecurity status (29) are significantly associated with PPA. Moreover, chronic medical illnesses such as malaria and HIV (28) are also associated with an increased risk of PPA.
In Ethiopia, although few studies have been conducted in the last decade, almost all previous studies focused on assessing lactating women after they were discharged from the facility (29–31), which may delay the testing of PPA, resulting in logistical challenges and late initiation of the treatment, finally resulting in short- and long-term complications of anemia (15, 32). Moreover, although the maternity ward is found to be a favorable place for the diagnosis of anemia, approximately 64% of women in Ethiopia received postpartum care late (33). Therefore, this study aimed to assess the magnitude of immediate postpartum anemia and its contributing factors among postpartum women admitted to maternity wards within 48 h of delivery in public hospitals in Harari Regional State, Eastern Ethiopia.
Methods and materials
Study setting and design
A facility-based cross-sectional study was conducted from June 1st to August 30th, 2021, in two public hospitals in Harari Regional State, Eastern Ethiopia. The Harari region is one of the 11 regions in Ethiopia, which are found in Eastern Ethiopia. Harar is the capital city of the Harari Regional State, located 526 km east of the capital city, Addis Ababa. According to the Ethiopian census projection for 2019/2020, the region has an estimated population of 263,656, including 60,667 reproductive-age women (34). There are two public hospitals, one private hospital, one police hospital, eight health centers, 54 private clinics, and 24 health posts in the region.
The study was conducted at Hiwot Fana Comprehensive Specialized Hospital (HFCSH) and Jugal Regional Hospital (JRH). HFCSH is the tertiary health care level (a referral hospital hosted by Haramaya University), and Jugal Regional Hospital (JRH) is the only regional hospital in Harari Regional State. Both hospitals currently provide comprehensive care for more than five million people in their catchment area. Hiwot Fana Comprehensive Specialized Hospital (HFCSH) and Jugal Regional Hospital (JRH) have 60 and 23 beds in the maternity ward, respectively. According to Health Management and Information System (HMIS), the estimated annual maternal delivery services of HFCSH was 4,680 (35), and JRH was 3,204 (36).
Population, eligibility criteria, and sampling procedure
In this study, all postpartum women admitted to the maternity wards of two public hospitals (HFCSH and JRH) were enrolled within the first 48 h of delivery. However, women who delivered by cesarean hysterectomy or laparotomy after uterine rupture and multiple pregnancies were excluded to reduce the overestimation of immediate postpartum anemia. Moreover, all postpartum women with the following preconditions (had known anemia before conception, transfused blood in the intrapartum and postpartum period, and were critically ill during the data collection period) were excluded from the study.
In this study, the sample size was calculated by using statistical software Epi-info version 7.2.4.0 (the USA, 2018) by considering the following assumptions: A proportion of immediate postpartum anemia (p = 24.2%) taken from a study conducted at Mekele, Ethiopia (21), a 4% tolerable margin of error (d = 0.04), a 95% confidence level (Zα/2 = 1.96), and 10% contingency for non-response rate. The final sample size for this study was 484.
A systematic sampling technique was used to select a total of 484 study participants. Initially, the only two public hospitals in Harari Regional State were selected purposely as they provided delivery services in the region. According to the previous information obtained from the Health Management and Information System (HMIS) report (6-month report), 2,380 and 1,560 women were admitted to the maternity wards of HFCSH and JGH, respectively, for postnatal care services, including those who gave birth at home or other health facilities. The data collection period was adjusted for 2 months to get the required sample size. Therefore, the total number of the 6-month report was divided into three to get 2 months' report. Thus, the total number of 2-month postpartum women at both hospitals became 1,314. Proportionally, we allocated 794 and 520 women to HFCSH and JRH, respectively. Then, the total sample size (n = 484) was proportionally allocated to 292 and 192 women from HFCSH and GRH, respectively. We also calculated the Kth interval for both hospitals (Kth = 1314/484 ≈ 3). Therefore, every third eligible postpartum woman was selected and interviewed until the required samples were obtained (Figure 1).
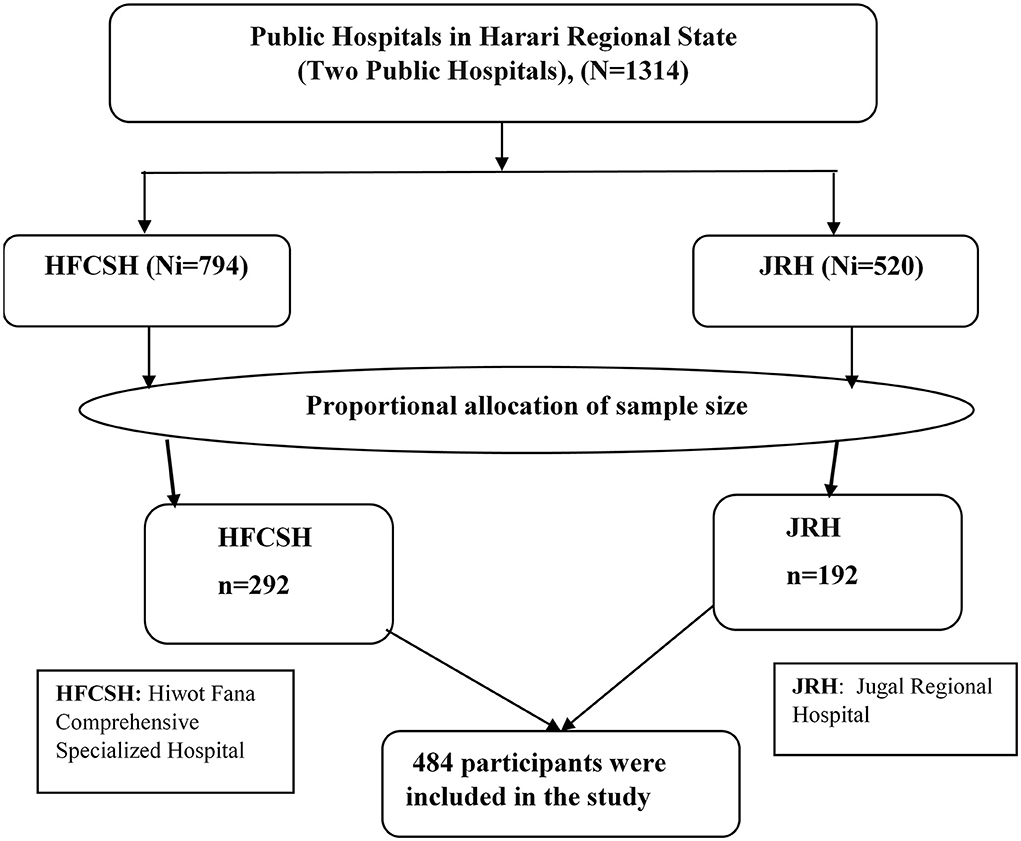
Figure 1. Schematic presentation of sampling procedure to select immediate postpartum women admitted to the maternity ward in public hospitals in Harari Regional State, Eastern Ethiopia, 2021.
Data collection tools and procedures
The data were collected using a pre-tested structured and interviewer-administered questionnaire adopted and customized from different kinds of literature (13, 21, 24, 37). Reviews of maternal charts were also undertaken using validated checklists. The data were collected over-period of 2 months by six trained data collectors (four new graduates of bachelor of science (BSc) degree midwives and two bachelor of sciences (BSc) degrees in medical laboratory scientists). In addition, two masters of midwives were recruited for the supervision of data collectors and the data collection process.
In this study, the questionnaire for maternal information contains four main parts: socio-demographic factors, obstetric-related factors, nutritional-related factors, and comorbid disease-related factors. Food insecurity status was measured using the Household Food Insecurity Access Scale (HFIAS), which was recommended by food and nutrition technical assistance (FANTA) to stratify individuals as food secure or food insecure (38). It is valid and reliable in Ethiopia as measured by Cronbach's alpha value of 0.85 for both rural and urban samples (39). Mid-Upper Arm Circumference (MUAC) was measured using tape measures on non-dominant hands, and the result was interpreted to the UNICEF and WHO recommendation cut-off point.
A combination of data collection methods was used. The data from mothers were collected using a pre-tested structured and face-to-face interviewer-administered questionnaire with reviews of maternal charts for clarity of diagnosis and intervention. The data collection tool for maternal (40) was prepared first in English, translated into a local language (Afan Oromo and Amharic), and then re-translated back to English by the experts.
Initially, all medical records/charts of mothers were screened for the presence of any diagnosed medical and/or obstetrical complications, pre-delivery Hgb level, newborn weight, and other clinical characteristics. The laboratory technician collected approximately 2 mL of blood from each study participant, especially after 8 h of delivery. Hgb concentration was determined using the automated blood analyzer Cell-Dyne1800 (Abbott Laboratories Diagnostic Division, USA) by the laboratory technician. Hemoglobin levels were collected and attached to the participants' folders before they were discharged from the hospitals. Finally, anemic mothers were managed based on their Hgb levels according to national guidelines with IFA or blood transfusion and counseled on iron-rich diet intake after being informed by the focal person of the unit (ward).
Variables and measurements
Outcome variable
An outcome variable was immediate postpartum anemia. If a woman's Hgb level was <10 g/dl, it was considered to be anemic (yes), and if the Hgb level was 10 g/dl or above, it was considered non-anemic (no) (24). We recorded this dependent variable during the analysis stage as the binary outcome of 0 and 1. Thus, if the woman's Hgb level was <10 g/dl, it was recorded as 1; if the Hgb level was ≥10 g/dl, it was recorded as 0.
Independent variables
In this study, the explanatory variables include socio-demographic-related characteristics (maternal age, place of residence, education level, occupation, and monthly income of the family), obstetrics or reproductive-related factors (parity, ANC visits, time of ANC contacts, mode of delivery, birth weight of the newborn, episiotomy, history of maternal blood loss, pre-delivery Hgb level, birth interval, intrapartum and postpartum complications, medical conditions or chronic disease-related factors (malaria, HIV positive, medical diseases, intestinal infestation), and nutritional related factors (frequency of meals and levels of MUAC measurement).
Measurements
In this study, adherence to iron supplementation is said to be good if the mother took iron supplementation for ≥3 months but poor if taken for less than 3 months during the most recent pregnancy) (41, 42), and food insecurity is a lack of consistent access to a sufficient amount of healthy, nutritious, and culturally appropriate food for every person in a household due to a lack of money and other resources to live an active, healthy life. It is measured by the Household Food Insecurity Access Scale (HFIAS) based on nine occurrence questions developed by NATA and validated in Ethiopia (43). It is classified as food secure if the women scored two or fewer affirmative (yes) answers and food insecure if they scored more than two affirmative (yes) answers.
Data quality control
Structured and pre-tested tools were used. The final version of the questionnaire for maternal information was translated from the English language into the local language by experts. The training was given to data collectors and supervisors. A pre-test was conducted on 5% of the sample size at another public hospital (40) with similar characteristics to the study population. Any ambiguity, confusion, and difficult words were removed and verified based on pretested experience. A continuum of close supervision was ensured on each data collection day. Two independent data clerks did double data entry.
Data processing and analysis
The collected data were coded, cleaned, and entered into Epi-Data version 4.6 and then exported to SPSS version 25 (IBM SPSS Statistics, 2016) for further analysis. Descriptive statistics were carried out using frequency tables, proportions, and summary measures. Bivariable and multivariable logistic regression analyses with a 95% Confidence Interval (CI) were used to identify factors independently associated with immediate PPA. From the bi-variable analysis, variables with a significant level of p-value < 0.25 were considered for the multivariable analysis model to control for potential confounders. During multivariable analysis, the model fitness was checked by the Hosmer-Lemeshow model fitness test, and the result was insignificant (p = 0.828). Moreover, multi-collinearity was also checked using the variance inflation factor (all VIF values were < 2.8) and standard error < 2. Finally, an adjusted odds ratio (AOR) with a 95% CI was used to indicate the relationship between the independent variables and the immediate postpartum anemia. The statistical significance was declared at a p-value less than 0.05.
Ethical considerations
The study protocol was approved by the Institutional Health Research Ethics Review Committee (IHRERC) of the College of Health and Medical Sciences, Haramaya University (IHRERC/091/2021). All participants were informed about the procedure and purpose of the study, and informed, written, and signed voluntary consent was obtained before participation. Additionally, informed, written, and signed voluntary assent was taken from the parent, husband, or guardian using normal disclosure processes for participants less than 18 years of age. Other additional data needed were extracted from medical records; confidential information about the identity of individual patients was not collected. The standard safety measures for the prevention and transmission control measures of COVID-19 were strictly followed and carried out during the data collection period as per the WHO 2021 standard.
Results
Socio-demographic characteristics of the study participants
A total of 477 selected postnatal women were enrolled in this study, yielding a response rate of 98.6%. The mean age of the study participants was 25.33 (SD± 5.24) years. Nearly one-third of the study participants, 134 (28.1%), had no formal education. Slightly more than half (51.8%) of the participants' income was less than 2,500 ETB, with a median income of 2,500 ETB and IQ ± 2500 ETB (Table 1).
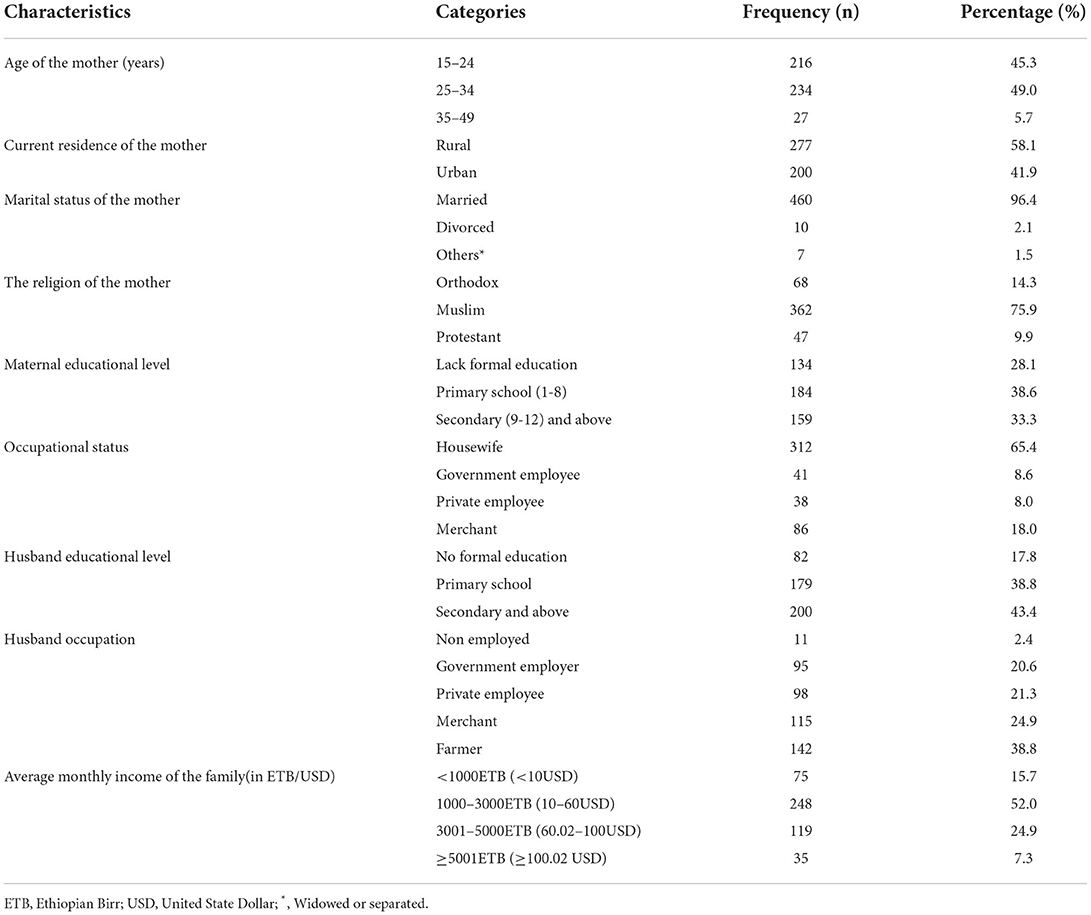
Table 1. Socio-demographic characteristics of postpartum women admitted to the maternity ward in public hospitals in Harari Regional State, Eastern Ethiopia, 2021 (n = 477).
Obstetric-related characteristics of the study participants
The majority of the participants, 252 (52.8%), were multiparous, and 126 (65.8%) had short inter-pregnancy intervals (<2 years). More than half (56.7%) of the participants had <4 ANC visits, and about one-eighth (11.9%) of the participants had a history of maternal blood loss in recent pregnancy (Table 2).
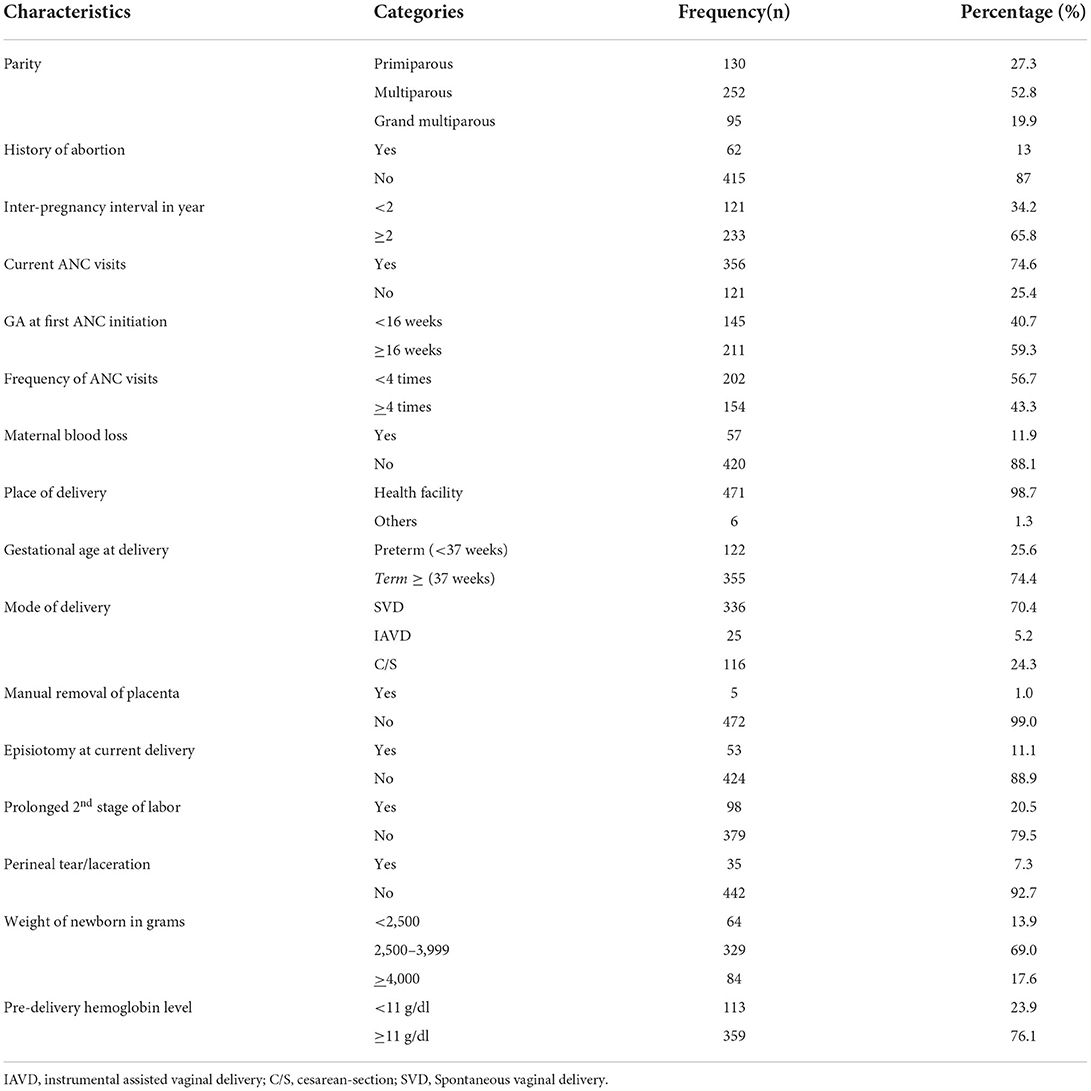
Table 2. Obstetrics-related factors of postpartum women admitted to the maternity ward in the public hospitals in Harari regional state, Eastern Ethiopia, 2021.
Comorbid disorders-related characteristics
Among all study participants, 132 (27.7%) had clinical or diagnostic tests that confirmed medical and/or obstetric complications during recent pregnancy. Pregnancy-induced hypertension was the most reported condition (14.8%), whereas a history of malaria was the most minor medical disease reported in this study (0.6%) (Figure 2).
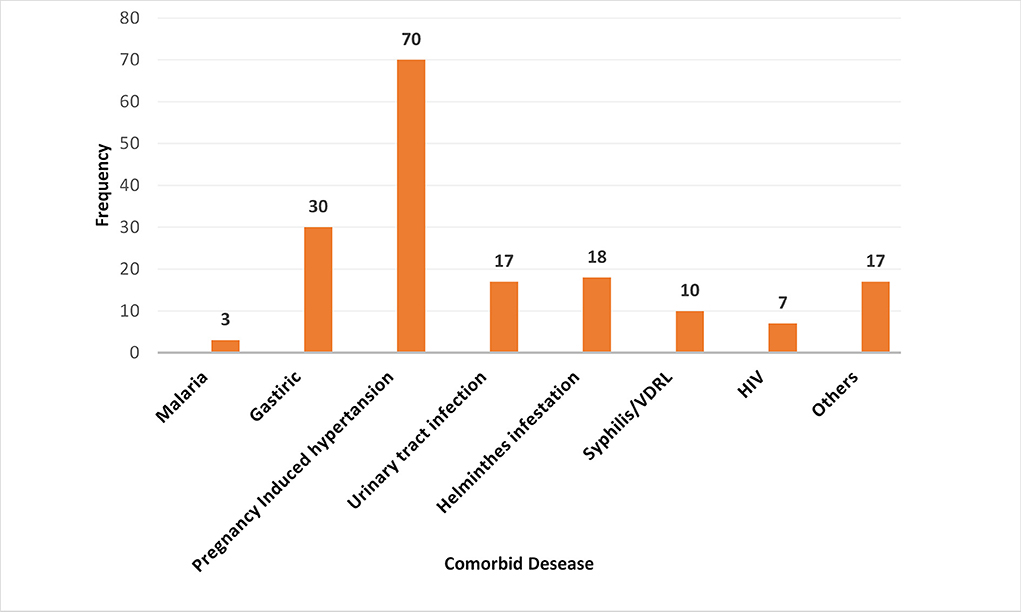
Figure 2. Comorbid disorders during pregnancy among postpartum women admitted to the maternity ward in public hospitals in Harari Regional State, Eastern Ethiopia, 2021. Other: Diabetes Mellitus, Tuberculosis, Pre-conception anemia, Heart failure, chronic hypertension.
Dietary diversity and micronutrient-related characteristics
In this study, nearly three-fourths (73.8%) of the study participants started iron-folate during the most recent pregnancy, and more than half (57.4%) of them had poor adherence to iron-folate. The majority, 397 (83.2%), of the study participants' mid-upper arm circumference was 23 cm or above (Table 3).
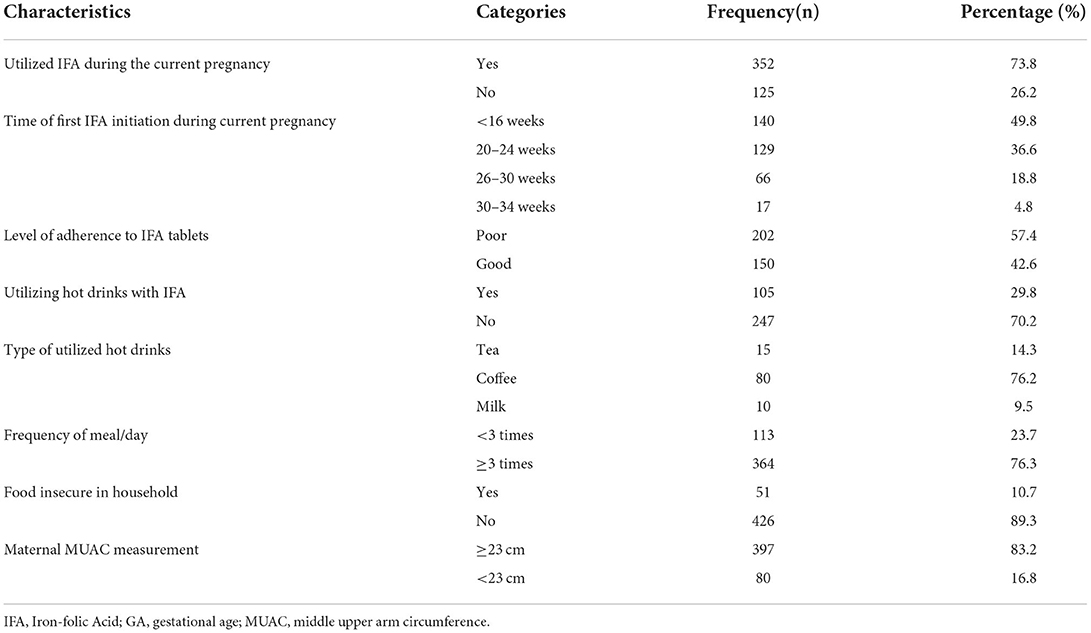
Table 3. Dietary and micronutrient-related factors of postpartum women admitted to the maternity ward in public hospitals in Harari Regional State, Eastern Ethiopia, 2021.
The magnitude of immediate postpartum anemia
The overall magnitude of immediate postpartum anemia was 28.1% [95% CI (23.7–32.1%)]. The postpartum hemoglobin concentration of the participants ranged from 3 g/dl to 14.8 g/dl, with median values of ± 10.5 g/dl (Figure 3).
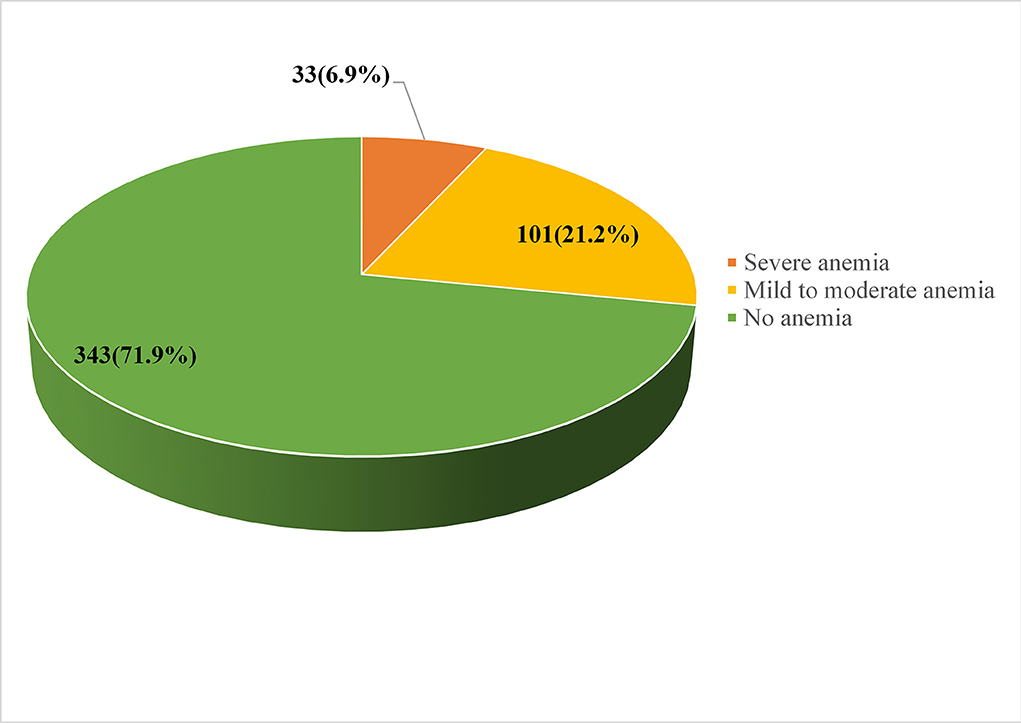
Figure 3. Magnitude of immediate postpartum anemia among women admitted to the maternity ward in public hospitals in Harari Regional State, Eastern Ethiopia, 2021.
Factors associated with immediate postpartum anemia
In bivariable analysis, mothers' age, place of residence, educational level, maternal parity, inter-pregnancy interval, frequency of ANC follow-up, mode of delivery, gestational age at delivery, a history of maternal blood loss, pre-delivery Hgb level, iron-folate supplementation, and MUAC level of the mothers were independently associated with immediate postpartum anemia among postpartum mothers. However, in the final model of multivariable logistic regression analysis, the educational level of the mother, frequency of ANC follow-up, mode of delivery, a history of maternal blood loss, pre-delivery Hgb level, and IFA supplementation during recent pregnancy were factors that remained statistically associated with immediate postpartum anemia.
Accordingly, the likelihood of immediate PPA was four times higher among women with no formal education than among those with at least secondary education or above [AOR: 3.92; 95% CI (1.85, 8.33)]. Likewise, women who had fewer than four ANC visits were three times more likely to be anemic than those who had at least ≥ 4 ANC visits during their current pregnancy [AOR: 3.18; 95% CI (1.53, 6.61)]. Similarly, the odds of immediate PPA were three times higher among women who gave birth through cesarean section (C/S) than among those who delivered vaginally [AOR: 3.40; 95% CI (1.89, 6.10)]. Moreover, the likelihood of immediate PPA was nearly five times higher among women with a history of blood loss than their counterparts [AOR: 4.78; 95% CI (2.22, 10.30)]. Likewise, women with a pre-delivery Hgb level of <11 g/dl were 5.5 times more likely to be anemic than those whose Hgb levels were ≥ 11 gm/dl [AOR: 5.46; 95% CI (3.09, 9.67)]. Furthermore, women with no history of iron-folate supplementation during their current pregnancy were three times more likely [AOR: 3.27; 95% CI: (1.31, 8.15)] to be anemic than those with a history of iron-folate supplementation during their most recent pregnancy (Table 4).
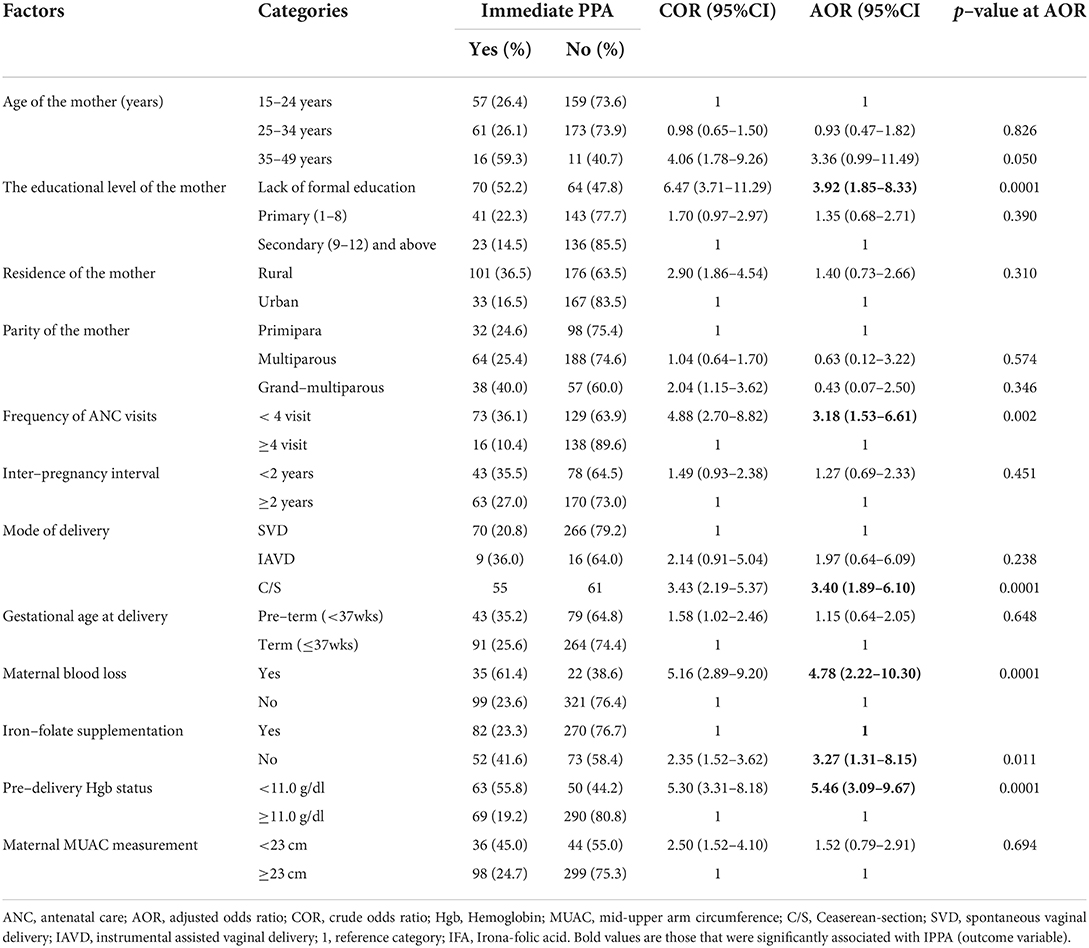
Table 4. Bi-variable and multivariable analysis of factors associated with immediate postpartum anemia among postpartum women admitted to the maternity ward in public hospitals in Harari Regional State, Eastern Ethiopia, 2021 (N = 477).
Discussion
In this study, the overall magnitude of immediate postpartum anemia was 28.1%, 95% CI: (23.7–32.1%), which is comparable with findings from similar studies reported in Mbarara, Uganda (30.0%) (44); Coastal Karnataka (26.5%) (22); Madrid, Spain (29%) (27); Jimma, Southern Ethiopia (28.7) (30); Debre Markos, Northern Ethiopia (24.3) (24) and Mekelle (24.2) (21). However, the result of this study is encouraging as the current prevalence of immediate postpartum anemia is comparatively lower than the various previous studies conducted in Madrid, Spain (49.7%) (25); Beijing, China (32.7%) (45); Tamil Nadu, India (47.3%) (19); Bursa, Turkey (45.1%) (26) and Jeddah, Saudi Arabia (59.3%) (46). The possible explanation for this variation might be attributed to using different sub-standards of Hgb concentration cut-off points among the countries. Moreover, the selection of the study participants and assessment methods might be other factors for the discrepancy. For instance, Dundar and Cakmak selected women who had undergone episiotomy (26), and Rakesh et al. took Hgb < 12 g/dl at 6 weeks as a cut-off point, in contrast to this study (Hgb < 10 g/dl within 48 h of the postpartum period) (19). Moreover, in this study, we excluded women with certain preconditions such as diagnosed pre-conception anemia, uterine rupture, laparotomy, and multiple pregnancies to minimize the overestimation of postpartum anemia. Another possible explanation might be that the government is currently increasing the number of health extension workers in the rural community and has introduced a community health insurance program, motivating community members—including pregnant women—for health service utilization.
On the contrary, the number of postpartum anemia reported in the current study is higher when compared to the previous studies conducted in different parts of the world, like in Germany (22%) (47); Mariakani sub-country hospital, Kenya (16.4%) (48), and Ghana (16%) (20). The possible justification for these discrepancies might be attributed to ANC coverage, as can be seen in the studies by Bergmann et al., Rukiya et al. (48), and Kofie et al. (20), who selected only women who benefited from ANC follow-up. Besides, 65% of participants in the study undertaken in Ghana had completed secondary education (20) compared to only 23% of participants who had completed secondary and above education level in our study. Likewise, Kofie et al. and Rukiya et al. conducted a study at 6 weeks of the postpartum period, which is far from the immediate postpartum period (20, 48). As a result, the women may have recovered from anemia over time. Other possible reasons might be study settings, geographical differences, dietary habits, health-seeking behaviors, or different lifestyles of the community.
In the final model of the multivariable analysis, the educational status of the women was independently associated with immediate PPA. Accordingly, women without formal education had a significantly higher likelihood of developing anemia than women who had attended secondary and above-secondary education. This result is in agreement with a study conducted in Northern Ethiopia (21) and Nairobi, Kenya (48). This could be because education could allow mothers to use health care services, improve nutritional status by enhancing their preference for more diversified food, and make decisions for their health and their unborn fetus. Moreover, women's higher levels of education increase the use of ANC services, allow women more access to nutritional counseling during pregnancy and get the advantages of IFA supplementation (49). In other words, uneducated women have less access to maternal health care services such as ANC follow-up during pregnancy. Hence, they might not consume iron-rich nutritious food or make inappropriate use of IFA tablets during pregnancy, which is a pillar in the preventive strategies of anemia (29).
Likewise, the frequency of antenatal care visits was found to be an independent predictor of immediate PPA. Thus, women who had fewer than four ANC visits were more likely to develop immediate PPA when compared to those who had four or more ANC visits. This finding is in line with studies conducted in Debra Markos, Northern Ethiopia (24) and Jimma, Southern Ethiopia (30), and data from Ethiopia DHS (13), in which a higher number of anemia was observed in women with fewer ANC follow-ups. The possible justification could be that women who had < 4 ANC visits might not get enough provision of nutrition and health education adequately, and even if they do, they may not get enough provision of iron and folic acid, which contribute to the reduction of anemia and are not screened early for the detection of some diseases, including anemia and other complications that predispose to anemia. On the contrary, women who had adequate ANC visits benefited from prophylactic measures like malaria infection, monthly IFA supplementation, and early treatment of intestinal parasites (33, 40).
Moreover, in this study, the mode of delivery also increased the risk of being anemic during the postpartum period. Thus, the odds of developing immediate PPA increased among women who gave birth via cesarean section compared with those who gave birth via SVD. This finding is congruent with previous studies investigated in developing countries such as Southern Ethiopia (21), Jeddah, Saudi Arabia (46), and Pakistan (50) and developed countries like Madrid, Spain (27), and Bursa, Turkey (26). The possible explanation is that women who had undergone cesarean section might be predominantly susceptible to PPH, which, in turn, decreased RBC production and increased nutrient losses from bleeding (23). Another suggestion could be uterine atony from prolonged labor, uterine tears, lacerations from obstructed labor, and retro placental clot due to placenta abruption, which causes severe bleeding by preventing uterine contraction; they are all indications for cesarean delivery (51).
Similarly, the odds of immediate PPA among postnatal women with a history of blood loss during pregnancy or delivery were five times higher than those without a history of blood loss during the most recent pregnancy. Previous various studies also support this result report from California (23), Berlin, Germany (47), Tamil Nadu, India (19), and Debra Markos, Ethiopia (24). This is due to excessive bleeding at and/or after birth, which decreases the RBC component called Hgb. Moreover, hemoglobin values dropped by ≥2 mg/dL in women with 500–1,000 mL of blood loss (52). The other explanation might be the loss of iron stores during pregnancy, and blood loss during delivery could be the complications of antepartum bleeding.
Furthermore, hemoglobin levels less than eleven at delivery were another independent factor strongly associated with PPA during the immediate postpartum period. This finding is in line with different studies conducted in developed countries such as Berlin, Germany (47), Spain (25), California (23), and Tamil Nadu, India (19). The possible causes might be a low Hgb level before delivery and decreased myometrial contractility, as well as impaired coagulation, which results from impaired transport of Hgb and oxygen to the uterus, causing tissue enzymes and cellular dysfunction that lead to uterine atony, which is the most common cause of PPH (53).
Finally, women with no iron-folate supplementation during their most recent pregnancy were at risk of developing postpartum anemia. This finding is in agreement with studies from developing countries such as Debra Markos, Ethiopia (24), Karachi, Pakistan (50), and India (19). The possible explanation could be that iron is a necessary replacement for blood loss and tissue accretion during pregnancy and labor, as high physiologic requirements and depletion of iron during pregnancy and labor occur (54). Studies have implied that consumption of at least 90 iron-containing tablet supplements during pregnancy can reduce maternal anemia by up to 70%(55). Another justification might be that most women who are iron deficient but not anemic early in pregnancy become anemic due to decreased/ineffective/erythrocyte production, resulting in immediate PPA (56).
Limitations of the study
As the women were asked about their activities in the past 10 months, recall bias was expected. However, best efforts were made to manage them through pre-testing questionnaires, training data collectors and supervisors on how to approach participants, interviewing postpartum women privately, close supervision of data collectors, and explaining the purpose of the study to the study participants well. Because the study was institutional-based, it may be difficult to generalize and apply the results to the general population. Moreover, as the study participants were from different geographical areas with different altitudes, adjusting Hgb levels based on altitudes was challenging. Additionally, this study also lacks an assessment of the dietary source of iron and the deworming status of the women during their recent pregnancy.
Conclusion
This study indicated that the magnitude of immediate postpartum anemia is a moderate public health problem per the WHO cut-off value for the public health significance of anemia. About one-third of mothers admitted for postpartum care developed anemia within 48 h of giving birth. In this study, women's educational status, frequency of ANC visits, mode of delivery, maternal blood loss, pre-delivery hemoglobin concentration value, and iron and folic acid supplementation during recent pregnancy were independent factors associated with immediate PPA. This finding may help concerned bodies/stakeholders improve women's health through monitoring, implementing preventive measures, and sustained efforts on the identified risk factors of immediate postpartum anemia during pregnancy, labor, and delivery by understanding the local context of anemia in the early postpartum period.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Health Research Ethics Review Committee (IHRERC) of the College of Health and Medical Sciences, Haramaya University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
We are grateful to the study participants, data collectors, supervisors, and staff of Hiwot Fana Comprehensive Specialized Hospital and Jugal Regional Hospital for their kind, invaluable collaboration during data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Ruiz de Viñaspre-Hernández R, Gea-Caballero V, Juárez-Vela R, Iruzubieta-Barragán FJ. The definition, screening, and treatment of postpartum anemia: a systematic review of guidelines. Birth. (2020) 48:14–25. doi: 10.1111/birt.12519
3. Munoz M, Peña-Rosas J, Robinson S, Milman N, Holzgreve W, Breymann C, et al. Patient blood management in obstetrics: management of anemia and hematinic deficiencies in pregnancy and postpartum period: nata consensus statement. Transf Med. (2018) 28:22–39. doi: 10.1111/tme.12443
4. Breymann C, Honegger C, Holzgreve W, Surbek D. Diagnosis and treatment of iron-deficiency anemia during pregnancy and postpartum. Arch Gynecol Obstet. (2010) 282:577–80. doi: 10.1007/s00404-010-1532-z
5. Barroso F, Allard S, Kahan BC, Connolly C, Smethurst H, Choo L, et al. Prevalence of maternal anemia and its predictors: a multi-center study. Eur J Obstetr Gynecol Reprod Biol. (2011) 159:99–105. doi: 10.1016/j.ejogrb.2011.07.041
6. Nair M, Nelson-Piercy C, Knight M. Indirect maternal deaths: the uk and global perspectives. Obstetr Med. (2017) 10:10–5. doi: 10.1177/1753495X16689444
7. WHO. Nutritional Tools for Effective Prevention and Control. Geneva: World Health Organization (2017).
8. Bailey PE, Andualem W, Brun M, Freedman L, Gbangbade S, Kante M, et al. Institutional maternal and perinatal deaths: a review of 40 low and middle-income countries. BMC Pregn Childbirth. (2017) 17:295. doi: 10.1186/s12884-017-1479-1
9. Daru J, Zamora J, Fernández-Félix BM, Vogel J, Oladapo OT, Morisaki N, et al. Risk of maternal mortality in women with severe anemia during pregnancy and postpartum: a multilevel analysis. Lancet Global Health. (2018) 6:e548–e54. doi: 10.1016/S2214-109X(18)30078-0
10. Gardner W, Kassebaum N. Global, Regional, and National Prevalence of Anemia and Its Causes in 204 Countries and Territories, 1990–2019. Curr Develop Nutr. (2020) 4:830. doi: 10.1093/cdn/nzaa053_035
11. Milman N. Postpartum anemia I: definition, prevalence, causes, and consequences. Ann Hematol. (2011) 90:1247–53. doi: 10.1007/s00277-011-1279-z
12. Tusa BS, Weldesenbet AB, Bahiru N, Enyew DB. Magnitudes of anemia and its determinant factors among lactating mother in east africa countries; using generalized mixed effect model. Front Nutr. (2021) 8:401. doi: 10.3389/fnut.2021.667466
13. Seifu B, Yilma D. Prevalence and associated factors of anemia among lactating women in ethiopia from 2010 to 2020: a systematic review and meta-analysis. BioMed Res J. (2020) 5:327–42.
14. Iyengar K. Early postpartum maternal morbidity among rural women of rajasthan, india: a community-based study. J Health Popul Nutr. (2012) 30:213. doi: 10.3329/jhpn.v30i2.11316
15. Butwick A, McDonnell N. Antepartum and postpartum anemia: a narrative review. Int J Obstet Anesth. (2021) 47:102985. doi: 10.1016/j.ijoa.2021.102985
16. Wassef A, Nguyen QD, St-André M. Anemia and depletion of iron stores as risk factors for postpartum depression: a literature review. J Psychosom Obstetr Gynecol. (2019) 40:19–28. doi: 10.1080/0167482X.2018.1427725
17. Maeda Y, Ogawa K, Morisaki N, Tachibana Y, Horikawa R, Sago H. Association between perinatal anemia and postpartum depression: A prospective cohort study of japanese women. Int J Gynecol Obstetr. (2020) 148:48–52. doi: 10.1002/ijgo.12982
18. Horie S, Nomura K, Takenoshita S, Nakagawa J, Kido M, Sugimoto M, et al. Relationship between a Level of Hemoglobin after Delivery and Exclusive Breastfeeding Initiation at a Baby-Friendly Hospital in Japan. Environ Health Prev Med. (2017) 22:1–6. doi: 10.1186/s12199-017-0650-7
19. Rakesh P, Gopichandran V, Jamkhandi D, Manjunath K, George K, Prasad J. Determinants of Postpartum Anemia among Women from a Rural Population in Southern India. Int J Women's Health. (2014) 6:395. doi: 10.2147/IJWH.S58355
20. Kofie P, Tarkang EE, Manu E, Amu H, Ayanore MA, Aku FY, et al. Prevalence and associated risk factors of anemia among women attending antenatal and postnatal clinics at a public health facility in Ghana. BMC Nutr. (2019) 5:1–9. doi: 10.1186/s40795-019-0303-x
21. Fanta GA. Prevalence and associated risk factors of immediate postpartum anemia in two teaching hospitals in mekelle. Ethiopian J. Reprod Health. (2020) 12:7.
22. Bhagwan D, Kumar A, Rao CR, Kamath A. Prevalence of Anemia among Postnatal Mothers in Coastal Karnataka. J Clin Diagnostic Res. (2016) 10:LC17. doi: 10.7860/JCDR/2016/14534.7086
23. Butwick W, Kuzniewicz M, Li SX, Escobar GJ. Patterns and predictors of severe postpartum anemia after cesarean section. Transfusion. (2017) 57:36–44. doi: 10.1111/trf.13815
24. Abebaw A, Gudayu TW, Kelkay B. The proportion of immediate postpartum anemia and associated factors among postnatal mothers in northwest ethiopia: a cross-sectional study. Anemia. (2020) 2020:8979740. doi: 10.1155/2020/8979740
25. Brichs XU, Carballeira MR, Fernández AG, Picañol EP. Anemia in pregnancy and immediate postpartum period prevalence and risk factors in pregnancy and childbirth. Med Clín. (2016) 146:429–35. doi: 10.1016/j.medcle.2016.06.050
26. Dündar B, Çakmak BD. The Prevalence and analysis of risk factors for postpartum anemia in women without prepartum anemia. Haydarpaşa Numune Train Res Hospital Med J. (2019) 59:165–70. doi: 10.14744/hnhj.2019.75436
27. Medina Garrido C, León J, Romaní Vidal A. Maternal anemia after delivery: prevalence and risk factors. J Obstet Gynaecol. (2018) 38:55–9. doi: 10.1080/01443615.2017.1328669
28. Obua RD. Prevalence and factors associated with postpartum anemia among women attending the postnatal clinic at six weeks at Apac General Hospital: Makerere University. (2019).
29. Liyew AM, Teshale AB. Individual and community-level factors associated with anemia among lactating mothers in ethiopia using data from ethiopian demographic and health survey, 2016; a multilevel analysis. BMC Public Health. (2020) 20:75. doi: 10.1186/s12889-020-08934-9
30. Alemayehu M. Factors associated with anemia among lactating mothers in subsistence farming households from selected districts of jimma zone, southwestern ethiopia: a community-based cross-sectional study. J Nutr Food Sci. (2017) 7:595. doi: 10.4172/2155-9600.1000595
31. Feleke BE, Feleke TE. Pregnant Mothers Are More Anemic Than Lactating Mothers, a Comparative Cross-Sectional Study, Bahir Dar, Ethiopia. BMC Hematol. (2018) 18:1–7. doi: 10.1186/s12878-018-0096-1
32. Prabhu M, Bateman BT. Postpartum Anemia: Missed Opportunities for Prevention and Recognition. Boston, MA: Wiley Online Library. (2017). doi: 10.1111/trf.13927
33. EPHI, ICF. Ethiopia Mini Demographic and Health Survey 2019: Key Indicators. Rockville, Maryland, USA: Ephi and Icf. USA: EPHI and ICF (2019).
34. CSA, International I. Ethiopia Demographic and Health Survey 2011. Ethiopia: Central Statistical Agency and ICF International Addis Ababa. (2012).
37. Liyew AM, Kebede SA, Agegnehu CD, Teshale AB, Alem AZ, Yeshaw Y, et al. Spatiotemporal patterns of anemia among lactating mothers in ethiopia using data from ethiopian demographic and health surveys (2005, 2011 and 2016). PLoS ONE. (2020) 15:e0237147. doi: 10.1371/journal.pone.0237147
38. Beloosesky R, Ross MG, Levine D. Oligohydramnios: Etiology, Diagnosis, and Management. Waltham, MA (2020).
39. Gebreyesus SH, Lunde T, Mariam DH, Woldehanna T, Lindtjørn B. Is the Adapted Household Food Insecurity Access Scale (Hfias) Developed Internationally to Measure Food Insecurity Valid in Urban and Rural Households of Ethiopia? BMC Nutrition. (2015) 1:2. doi: 10.1186/2055-0928-1-2
40. Yada TA, Dessie Y, Darghawth R, Wilfong T, Kure MA, Roba KT. Magnitude of intestinal parasitosis, malnutrition, and predictors of anemia among nonpregnant reproductive-age women attending healthcare services in olenchity general hospital, central Ethiopia. Front Trop Dis. (2021) 2:655690. doi: 10.3389/fitd.2021.655690
41. Kassa ZY, Awraris T, Daba AK, Tenaw Z. Compliance with iron-folic acid and associated factors among pregnant women through pill count in hawassa city, south Ethiopia: A community-based cross-sectional study. Reprod Health. (2019) 16:1–8. doi: 10.1186/s12978-019-0679-8
42. Ba DM, Ssentongo P, Kjerulff KH, Na M, Liu G, Gao X, et al. Adherence to Iron Supplementation in 22 Sub-Saharan African Countries and Associated Factors among Pregnant Women: A Large Population-Based Study. Curr Develop Nutr. (2019) 3:nzz120. doi: 10.1093/cdn/nzz120
43. Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (Hfias) for Measurement of Household Food Access: Indicator Guide (V. 3). Washington, DC: Food and Nutritiontechnical Assistance Project, Academy for Educational Development. (2017). doi: 10.1037/e576842013-001
44. Nawagi F. Incidence and factors associated with postpartum anemia at mbarara regional referral hospital. J Health Med Nursing. (2016) 23:2422–8419.
45. Zhao A, Zhang J, Wu W, Wang P, Zhang Y. Postpartum anemia is a neglected public health issue in china: a cross-sectional study. Asia Pac J Clin Nutr. (2019) 28:793–9. doi: 10.6133/apjcn.201912_28(4).0016
46. Mattar G, Alsahafi N, Shami B, Abulkhair S, Alhazmi N, Alsaleh R. Incidence of Postpartum Anemia among Postpartum Patients in East Jeddah Hospital. Int J Pharma BioSci. (2019) 9:39–46. doi: 10.22376/ijpbs/lpr.2019.9.2.P39-46
47. Bergmann RL, Richter R, Bergmann KE, Dudenhausen JW. Prevalence and risk factors for early postpartum anemia. Eur J Obstetr Gynecol Reprod Biol. (2010) 150:126–31. doi: 10.1016/j.ejogrb.2010.02.030
48. Rukiya A, Gachuno O, Machoki J. Determinants of postpartum anemia–a cross-sectional study. J Obstetr Gynecol Eastern Central Africa. (2015) 27:33–82.
49. CSA ICF. Central Statistical Agency (Csa)[Ethiopia] and Icf. Maryland, USA: Ethiopia demographic and health survey, Addis Ababa, Ethiopia and Calverton. (2016).
50. Rabia S, Jalil N, Feroze S, Iqbal M. Frequency and determinants of maternal anemia in the early postpartum period. Ann Abbasi Shaheed Hospital Karachi Med Dental College. (2018) 23:15–20.
51. Fawcus S, Moodley J. Postpartum Hemorrhage Associated with Cesarean Section and Cesarean Hysterectomy. Best Pract Res Clin Obstetr Gynecol. (2013) 27:233–49. doi: 10.1016/j.bpobgyn.2012.08.018
52. Yefet E, Yossef A, Suleiman A, Hatokay A, Nachum Z. Hemoglobin drop following postpartum hemorrhage. Sci Rep. (2020) 10:1–8. doi: 10.1038/s41598-020-77799-0
53. Frass KA. Postpartum Hemorrhage Is Related to the Hemoglobin Levels at Labor: Observational Study. Alexandria J Med. (2015) 51:333–7. doi: 10.1016/j.ajme.2014.12.002
54. WHO. Global Anemia Reduction in Reproductive Age Group. Available online at: https://apps.who.int/iris/rest/bitstreams/1315161/retrieve (2020).
55. Abraha I, Bonacini MI, Montedori A, Di Renzo GC, Angelozzi P, Micheli M, et al. Oral iron-based interventions for the prevention of critical outcomes in pregnancy and postnatal care: an overview and update of systematic reviews. J Evid Based Med. (2019) 12:155–66. doi: 10.1111/jebm.12344
Keywords: anemia, postpartum, associated factors, hemoglobin, Ethiopia, immediate postpartum
Citation: Abebe GT, Kure MA, Yadeta TA, Roba KT and Amante TD (2022) Immediate postpartum anemia and associated factors among women admitted to maternity ward at public hospitals in Harari Regional State, Eastern Ethiopia: A facility-based cross-sectional study. Front. Glob. Womens Health 3:916245. doi: 10.3389/fgwh.2022.916245
Received: 08 April 2022; Accepted: 19 August 2022;
Published: 20 September 2022.
Edited by:
Rahat Najam Qureshi, Aga Khan University, PakistanReviewed by:
Enoch Odame Anto, Kwame Nkrumah University of Science and Technology, GhanaSidrah Nausheen, Aga Khan University, Pakistan
Negussie Boti, Arba Minch University, Ethiopia
Copyright © 2022 Abebe, Kure, Yadeta, Roba and Amante. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed Abdurke Kure, bWFtZWVsZW1vQGdtYWlsLmNvbQ==
 Gizaw Taddesse Abebe
Gizaw Taddesse Abebe Mohammed Abdurke Kure
Mohammed Abdurke Kure Tesfaye Assebe Yadeta
Tesfaye Assebe Yadeta Kedir Teji Roba
Kedir Teji Roba Tariku Dingeta Amante
Tariku Dingeta Amante