- 1Department of Anesthesia, Wolaita Sodo University, Sodo, Ethiopia
- 2School of Medicine, Arsi University, Asella, Ethiopia
- 3Department of Anesthesia, Hawassa University, Hawassa, Ethiopia
Background: Prolapse is one of the sub-types of pelvic floor dysfunction (PFD) which occurs due to abnormal fall of the pelvic organs from their normal anatomic positions. Although the cause of prolapse is multifactorial, it primarily occurs due to pregnancy and vaginal delivery. Hence, the present study aimed to identify risk factors of prolapse among women who undergo gynecological surgery.
Materials and methods: Facility-based-unmatched case–control design was employed. Cases were all gynecological women who were diagnosed with pelvic organ prolapse (POP) at Asella teaching referral hospital (ATRH) while controls were all charts of gynecological women who were diagnosed with other gynecological problems rather than POP at ATRH. For each case, two controls were selected using a simple random sampling technique. The data were entered into Epidata version 4.3.1 and finally exported to SPSS version 25 for further analysis. Then variables that had an association in the bivariate model (p < 0.25) were entered and analyzed by a multivariable conditional logistic regression model to identify the independent effect of different factors. Statistical significance was declared at p < 0.05.
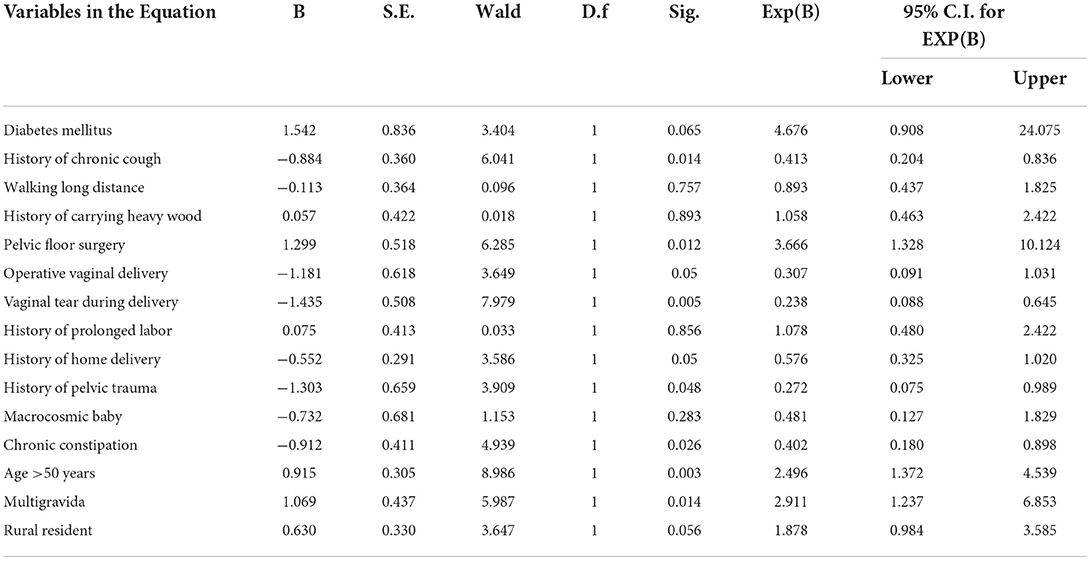
Results: A total of 147 cases and 293 controls were included in this study. Women who had a history of chronic cough, previous pelvic floor surgery, constipation, and vaginal tear during delivery, history of pelvic trauma, age of the women, rural resident, and maternal gravidity were strongly associated with prolapse at p-value of < 0.05. Multigravida [adjusted odds ratio (AOR) 2.987 (95% CI 1.237–6.853), p = 0.014], age >50 years [AOR: 2.496 (95% CI 1.372–4.539), p = 0.003], women with a history of pelvic floor surgery [AOR: 0.3.666 (95% CI 1.328–10.124), p = 0.012], women who had diabetes mellitus [AOR: 4.676 (95% CI 0.908–24.075), p = 0.065], and resided in rural areas [AOR = 1.878; (95% CI: 0.984–3.585), I2 = 47.5%, p = 0.056] were the independent predictors were of prolapse.
Conclusions: In this study, women with diabetes mellitus, previous pelvic floor surgery, rural residents, being multigravida, and age >40 were independent predictors of prolapse. Therefore, delivering health education by focusing on the identified risk factors was strongly recommended.
Introduction
Prolapse occurs when abnormal descent of the pelvic organs occurs from their normal anatomic positions. It is primarily a common gynecological condition that is considered as a medical and social problem, deeply rooted with poor health services and socio-cultural beliefs affecting women in childbearing age and post-menopausal age (1, 2). Patients generally present with several complaints, such as bladder, bowel, and pelvic symptoms; however, with the exception of vaginal bulging, none is specific to prolapse. Women with symptoms suggestive of prolapse should undergo pelvic examination and medical history checkups. However, many patients with pelvic organ prolapse are asymptomatic and do not need treatment (3). In a general population, only 12% of women aged between 45 and 85 years women are symptomatic though over two-thirds of these women have anatomical evidence of pelvic organ prolapse (POP) (4, 5). However, women with symptomatic pelvic floor dysfunction PFD suffer from physical and emotional distress which has a great negative impact on women's social, physical, and psychological wellbeing (6, 7).
Therapeutic options for POP include surgery and conservative treatments. Although surgical management of POP is currently adopted, non-surgical treatments such as pessaries, pelvic floor muscle training, or both can be useful in symptomatic improvement (8, 9) as well as weight loss in case of obesity. Nevertheless, most of these treatments are not helpful for women with severe prolapse; therefore, surgical therapy is more appropriate in these cases. The surgical management, depending on the type of POP, includes apical suspension (sacral colpopexy and sacrospinous ligament fixation), anterior and posterior (colporrhaphy, perineorrhaphy, and obliterative procedures) vaginal prolapse repair (8).
Surgical repair is the first choice of treatment in case of severe POP (stage III–IV, according to the International Continence Society POP-Q classification (10). Surgery usually includes hysterectomy, performed through different approaches (vaginal, laparoscopic/robotic, and abdominal) (11). The two most accepted surgical techniques for primary VPP are laparoscopic sacrocolpopexy (LSC) and sacrospinous fixation (SF) (12). The second recurrence of vaginal vault prolapse (VVP) is defined as prolapse of the vaginal vault or upper vagina after two previous reconstructive surgeries. The recurrence of VVP occurs when the top of the vagina descends below a point that is 2 cm less than the total vaginal length above the plane of the hymen (13).
Although the exact prevalence of pelvic organ prolapse is unknown, the lifetime risk of requiring at least one operation to correct prolapse has been roughly estimated at 11% (14). Prolapse procedures are known to have a high reoperation rate, with a lifetime risk for surgery of 10–20% (15). Although several approaches are available for the management of POP, the best strategy in case of recurrence after vaginal vault prolapse still remains debated (16, 17). However, it is assumed that the success rate of POP surgery would increase by combining surgery with PFMT (18). Recent systematic reviews have concluded that PFMT reduces POP symptoms and severity stage (17). PFMT has been shown to increase pelvic floor muscle (PFM) strength and endurance, reduce the levator hiatus area, lift the bladder and rectal ampulla, increase PFM volume, and reduce PFM length (18).
The cause of prolapse is primarily related with pregnancy and vaginal delivery, which lead to direct pelvic floor muscle and connective tissue injury. These defects may be due to stretching and tearing of the endopelvic fascia, levator muscles, and perineal body during childbirth (19). The combinations of anatomical, physiological, genetic, lifestyle, and reproductive factors that interact throughout a woman's lifespan also contribute to PFD. Hysterectomy, pelvic surgery, and conditions associated with sustained episodes of increased intra-abdominal pressure, such as obesity, chronic cough, constipation, and repeated heavy lifting, also contribute to prolapse (20).
The prolapse affects severely affects women's quality of life in several ways. Women with POP can feel different prolapse symptoms like “something coming down” and other urinary, bowel, and sexual symptoms. It has socioeconomic and health consequences, affecting overall health and sexual function (21). These women frequently report disorders of sexual desire, arousal, orgasm, and pain and these problems can decrease the quality of life and affect the relationship between partners (22). The disease impairs healthcare seeking behavior of women due to a series of socio-cultural myths, lack of familial support, treatment cost, women's reluctance and wrong perception of the prolapse as a malignancy (23).
A previous study conducted in northern Ethiopia showed that sphincter damage, family history of POP, being uneducated, having ≥ 4 vaginal deliveries, carrying heavy objects, maternal gravidity, and BMI < 18.5 kg/m2 as determinants of POP (24). However, this study identified additional factors like chronic cough, previous pelvic floor surgery, constipation, vaginal tear during delivery, history of trauma, age of the women, rural resident as associated and, women with diabetes mellitus, previous pelvic floor surgery, rural resident, being multigravida and age >40 years as the independent predictors of POP. Besides, there may be different in sociodemographic, socioeconomic, and lifestyles differences between south-eastern Ethiopia and northern Ethiopia. Hence, this study aimed to identify risk factors of pelvic organ prolapse among gynecological patients who underwent surgery at Asella Teaching and Referral Hospital in order to segment interventional on identified risk factors.
Materials and methods
This retrospective unmatched case–control study was conducted using a simple random sampling technique from February to March 2021 at Asella Teaching Referral Hospital (ATRH), South East Ethiopia. Age at the first delivery was used to calculate the final sample size as it gave the maximum sample size, 440 [147 cases and 293 controls] with the following assumptions (24): 91.9% proportion of exposed control, 64.9% proportion of case (24), 95% confidence interval (CI), 80% power, 6.1 odds ratio, 2:1 controls to cases ratio. The case definition of this study was charts of women who reported to have at least one of the pelvic floor disorders (utero-vaginal prolapse, rectocele, cystocele, vault prolapse, and delivered myoma) with stages two and above (23). On the other hand, all charts of women who were diagnosed gynecological problems other than pelvic floor disorders were controls. In this study charts of women with both pelvic floor disorder and other gynecological problems, charts of women with stage one pelvic floor disorder and charts with at least three incomplete identified risk factors were excluded. Before selecting cases and controls, all 2 years charts of women with all gynecological problems were identified. After that, a simple random sampling technique was used to separately select both cases and controls from its respective group. Four nurse degree holders' and two masters of Science degree holders were recruited as data collectors and supervisors, respectively. The training was given for the data collectors and supervisors for 2 days. The aim of the training was to make understanding on the objective of the study, data collection tool, data quality assurance, and data collection procedures. Data were entered into Epidata version 4.3.1 and then exported to Stata v14.0 (Statacorp, College Station, Texas, USA) software for analyses. Then variables that had an association in the bivariate model (p < 0.25) were transported and analyzed by a multivariable logistic regression model to identify the independent effect of different factors. A stepwise approach will be performed to select variables for inclusion in modeling. Statistical significance will be declared at p < 0.05. To check co-linearity between risk factors, tolerance and variance inflation factor (VIF) were used (25). Adjusted odds ratio (AOR) with a 95% CI was used to measure the strength of association. Calibration of the model was determined by a non-significant Hosmer–Lemeshow goodness of fit test (26).
Result
Sociodemographic characteristics of the patient
A total of 440 women were included in this case–control study to determine risk factors of pelvic organ prolapse. The majority of the patients with pelvic organ prolapse were found in the age >50 years and the lowest percentage of women were found between the age group of 18–24 years. The mean age of respondents was 35.3386 ± SD (10.77292), (minimum 18 and maximum 65). In addition, the majority of women were from rural areas 343 (77.95%). The regarding the residence of women, about 94.55% of all patients were from rural areas while the remaining 22% were from urban. Majorities of the patients 94.5% of all women were married (Table 1).
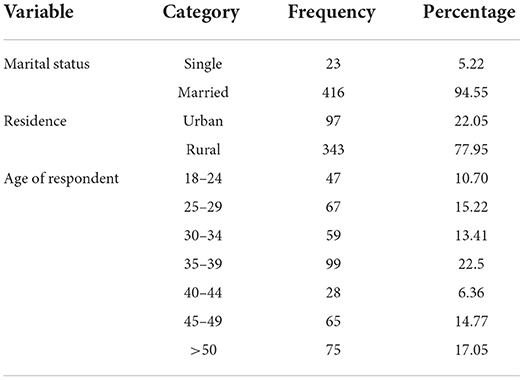
Table 1. Sociodemographic of patients with pelvic organ prolapse at Asella Teaching Referral Hospital (ATRH), 2021.
Bivariate analysis on sociodemographic, medical, and other risk factors of POP
Among all sociodemographic and other determinants, intra-abdominal mass and marital status were not associated with POP on bivariable analysis at p value of <0.25; hence excluded from the multivariable analysis. On the other hand, sociodemographic and other factors which were associated on bivariable analysis were history of chronic cough, constipation, history of walking long distance, history of carrying heavy wood, history of trauma, age of the women, and resident of women (Table 2).
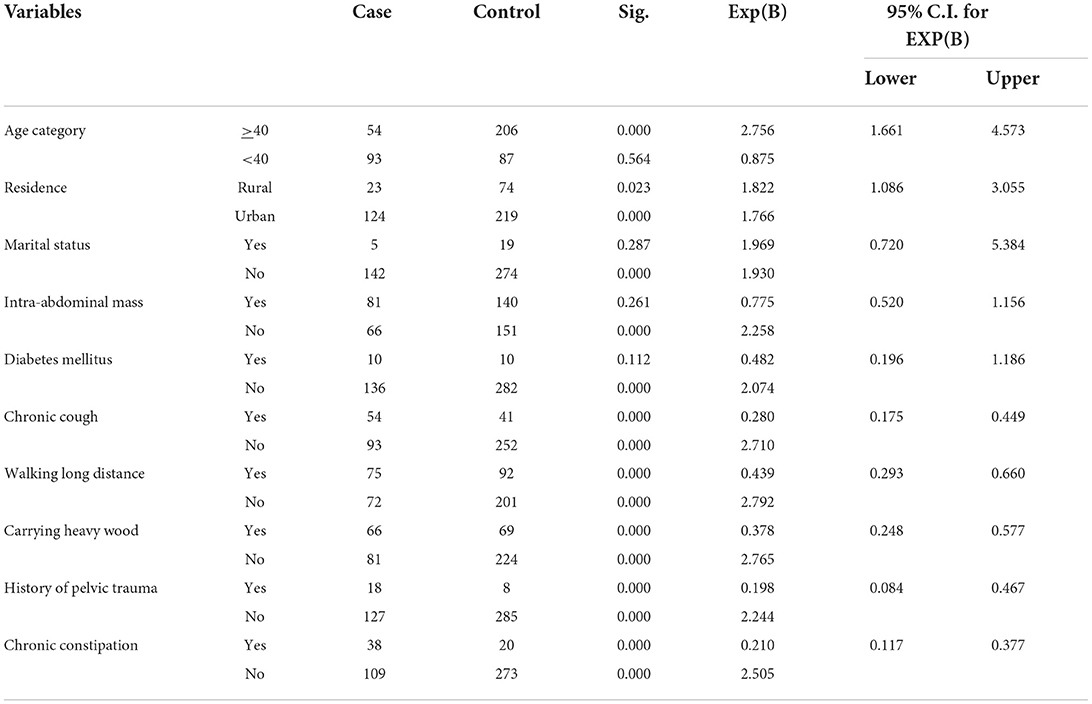
Table 2. Bivariable analyses of sociodemographic, medical, and other risk factors of POP at ATRH, 2021.
Obstetrics and surgery-related risk factors
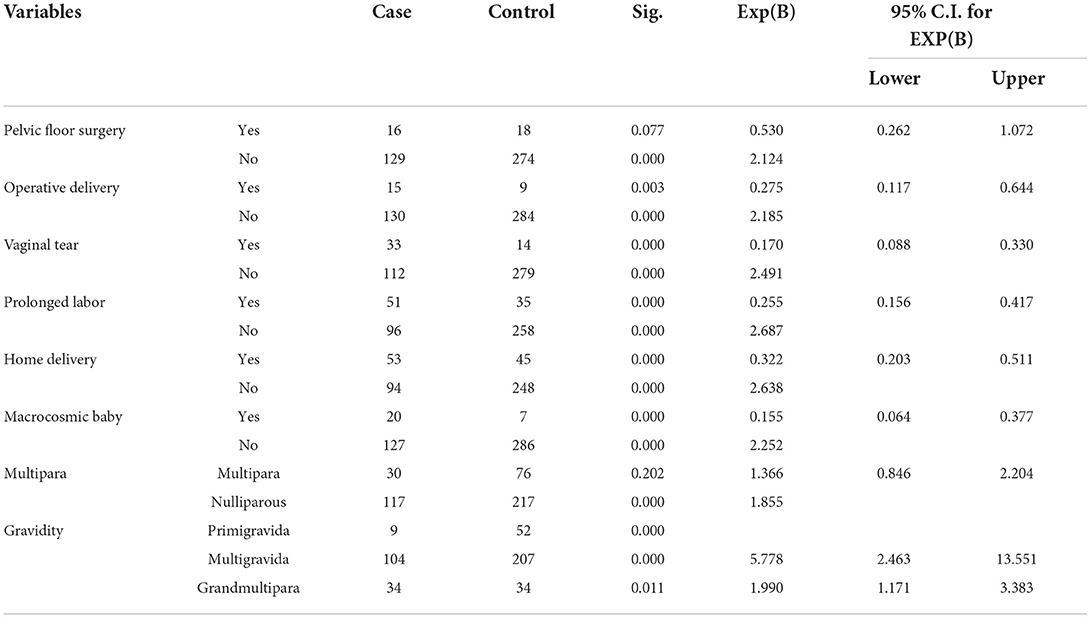
All obstetrics- and gynecological-related determinants of POP were associated on bivariable analysis at p value of <0.25; hence transported to multivariable analysis. These factors include previous pelvic floor surgery, vaginal tear during delivery, operative vaginal delivery, birth weight, history of prolonged labor, history, home delivery, maternal parity, and gravidity (Table 3).
Risk factors of pelvic organ prolapse
In this study, women who had a history of chronic cough, previous pelvic floor surgery, constipation, vaginal tear during delivery, history of trauma, age of the women, rural resident, and maternal gravidity were strongly associated with pelvic organ prolapse at p value of <0.05. The result of multivariable analysis also showed women with diabetes mellitus, previous pelvic floor surgery, rural residents, being multigravida, and age >40 years independent predictors of factors of pelvic organ prolapse. Women who had diabetes mellitus had about five times more likely to have pelvic organ prolapse than patients who had no diabetes mellitus [(AOR: 4.676 (95% CI 0.908–24.075), p = 0.065].
The odds of having pelvic organ prolapse were two times more prevalent among women who resided in rural areas than those who were living in urban areas [(AOR = 1.878; 95% CI: 0.984–3.585), I2 = 47.5%, p = 0.056].
Women who had a history of walking long distance were 89.3% more likely to develop POP than those who were not [AOR: 0.893 (95% CI 0.437–1.825), p = 757]. Additionally, women who had a history of carrying heavy wood were about 105.8 more likely to develop POP than who were not [AOR: 1.058 (95% CI 0.463–2.422), p = 0.893].
Being multigravida women had about three times more likely to develop POP when compared to single gestation [AOR 2.91 (95% CI (1.237–6.853)], p = 0.014). The odds of having pelvic organ prolapse were about 2.5 and 3.7 times among women aged >50 years and women with a history of pelvic floor surgery [AOR: 2.496 (95% CI 1.372–4.539), p = 0.003] and [AOR: 0.3.666 (95% CI 1.328–10.124), p = 0.012], respectively (Table 4).
Discussion
Pelvic organ prolapse is downward descent of female pelvic organs, such as the bladder, uterus or post-hysterectomy vaginal cuff, and the small or large bowel, resulting in protrusion of the vagina, uterus, or both. The most valid symptom of POP is the sensation of a bulge in the vagina (27). It is a major female health problem that causes considerable physical and emotional distress, bothers quality of life and influence a large financial burden (15). The effect of disorder is not only limited to the physical health, sexual lives, ability to work, and earn a livelihood of the individual women, but also it affects their families, caregivers, and society at large (22, 24). Hence, women want to preserve their physique and capacity for sexual function well beyond menopause (1).
This study showed that previous pelvic floor surgery was independent predictors of POP which is in line with other studies (28, 29). Another population-based study has shown that at least 30% of women treated surgically for pelvic organ prolapse, urinary incontinence, or both will require subsequent surgery for a recurrence of these conditions (30, 31). It was also founded by another study that the first vaginal delivery and forceps delivery are risk factors of POP (32). In contrast to the current study, operative vaginal delivery and vaginal tear during birth were not associated with POP (4, 33). On the other hand, elective cesarean delivery was protective when compared with spontaneous or operative vaginal delivery (19).
The present study found that multiparty was one of the independent predictors of POP which agrees with other similar studies (34–36). It was also revealed by other studies that pregnancy and childbirth are considered as risk factors for POP (32, 33). This might be due to the fact that repeated pregnancy and birth damages sphincter muscles and ligaments, which sometimes never fully regain its strength and elasticity. However, another study found that operative vaginal delivery other than forceps delivery, age at last delivery, and gravidity were not significantly associated with POP (35).
Like other several studies, we found that the risk of POP increases with age (33, 37–43). Similarly, another study conducted in Jimma, southwest Ethiopia revealed that women aged ≥ 40 years were about three times more likely to have had POP compared to its counterpart (38). The findings of the review article showed that odds of having pelvic organ prolapse were about seven times more likely among women having more than 40 years old than in the younger population (23). The increase in prevalence of POP as age increases might be due the weakening sphincter muscles and surrounding tissues as the age increases (44, 45).
According to the findings of this study, rural resident was independent predictors of POP which agrees with another study (38). A systematic review and meta-analysis done on the burden of pelvic organ prolapse in Ethiopia showed that the odds of having pelvic organ prolapse were 3.29 times more prevalent among women who resided in rural areas than those who were living in urban areas (23). This might be due to the fact that rural women had been assisting in farmland, marketing, wood and water fetching, child rearing and carrying the baby on the back even during pregnancy which has detrimental effects for the loss of genitourinary supporting structures.
The finding of the current study also revealed that chronic cough and chronic constipation were strongly associated with POP which agrees with another study (46). In contrast, other studies found that chronic cough and chronic constipation were associated with prolapse (33, 41, 47). These might be due to the fact that conditions such as chronic cough, constipation, and obesity may predispose some women to disruption, stretching, or dysfunction of the levator anti complex, connective-tissue attachments of the vagina, or both, resulting in prolapse (46).
In this study, diabetes mellitus was identified as among independent predictors of POP. Similarly, another study found that diabetes mellitus was significantly associated with primary POP (4, 41). Other studies also revealed that obesity (BMI ≥ 25 kg/m2) could increase the risk of POP (48, 49). The findings of systematic review and meta-analysis revealed a contrary finding that being underweight (BMI, 18.5 kg/m2) increases the risk of POP by a threefold (48, 50). However, we did not collect BMI of the patient.
Limitations of this study
The main limitations of the present study included failure to assess some important variables like age at first delivery, age at last delivery, and BMI of women as our data were secondary source. However, we tried to assess all other documented factors and important characteristics.
Conclusions
In conclusion, women who had a history of chronic cough, previous pelvic floor surgery, constipation, vaginal tear during delivery, history of trauma, age of the women, rural residents, and being gravida women were strongly associated with prolapse. And the independent predictors of POP were women with diabetes mellitus, previous pelvic floor surgery, rural residents, being multigravida, and age >40 years. Therefore, delivering health education by focusing on the identified risk factors was strongly recommended. Further, multicenter cohort studies with a higher sample size should be conducted to further investigate the risk factors responsible for occurrences of POP.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
MO and TW were involved in the conception, study design, execution, acquisition of data, analysis and interpretation of data, took part in drafting the article, and revising it critically for important intellectual content. NGK and NAK were involved in study design, execution, acquisition of data, analysis, interpretation, drafted, and final manuscript writing. All authors reviewed and agreed on all versions of the manuscript before submission, agreed to submit to the current journal, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Acknowledgments
We would like to thank Arsi University for providing support to conduct this project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ATRH, Asella Teaching Referral Hospital; BMI, body mass index; UVP, utero vaginal prolapse.
References
1. Okonkwo J, Obiechina N, Obionu C. Incidence of pelvic organ prolapse in Nigerian women. J Natl Med Assoc. (2003) 95:132.
2. Dietz H. The aetiology of prolapse. Int Urogynecol J. (2008) 19:1323–9. doi: 10.1007/s00192-008-0695-7
3. Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. (2007) 369:1027–38. doi: 10.1016/S0140-6736(07)60462-0
4. Slieker-ten Hove M, Pool-Goudzwaard A, Eijkemans M, Steegers-Theunissen R, Burger C, Vierhout M. Pelvic floor muscle function in a general population of women with and without pelvic organ prolapse. Int Urogynecol J. (2010) 21:311–9. doi: 10.1007/s00192-009-1037-0
5. Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am. (1998) 25:723–46. doi: 10.1016/S0889-8545(05)70039-5
6. Abdel-Fattah M, Familusi A, Fielding S, Ford J, Bhattacharya S. Primary and repeat surgical treatment for female pelvic organ prolapse and incontinence in parous women in the UK: a register linkage study. BMJ Open. (2011) 1:e000206. doi: 10.1136/bmjopen-2011-000206
7. Subak LL, Waetjen LE, Van Den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. (2001) 98:646–51. doi: 10.1097/00006250-200110000-00021
8. Laganà AS, La Rosa VL, Rapisarda AMC, Vitale SG. Pelvic organ prolapse: the impact on quality of life and psychological well-being. J Psychosom Obstet Gynecol. (2018) 39:164–6. doi: 10.1080/0167482X.2017.1294155
10. Haylen BT, De Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodynamics. (2010) 29:4–20. doi: 10.1002/nau.20798
11. Serati M, Laganà AS, Casarin J, Gisone B, Cantaluppi S, Ghezzi F. Laparoscopic duplication of the uterosacral ligaments following hysterectomy for stage III–IV apical pelvic organ prolapse. Updates Surg. (2020) 72:199–204. doi: 10.1007/s13304-019-00690-9
12. Coolen ALW, van IJsselmuiden MN, van Oudheusden AM, Veen J, van Eijndhoven HW, Mol BWJ, et al. Laparoscopic sacrocolpopexy versus vaginal sacrospinous fixation for vaginal vault prolapse, a randomized controlled trial: SALTO-2 trial, study protocol. BMC women's health. (2017) 17:1–8. doi: 10.1186/s12905-017-0402-2
13. Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, Van Kerrebroeck P, Victor A, Wein AJ: The standardization of terminology of lower urinary tract function: report from the standardization sub-committee of International Continence Society. In: Textbook of Female Urology and Urogynecology, 3rd Edn. CRC Press. (2010) 1098–108. doi: 10.3109/9781439807217-110
14. Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol. (2002) 186:1160–6. doi: 10.1067/mob.2002.123819
15. Friedman T, Eslick GD, Dietz HP. Risk factors for prolapse recurrence: systematic review and meta-analysis. Int Urogynecol J. (2018) 29:13–21. doi: 10.1007/s00192-017-3475-4
16. Funk MJ, Edenfield AL, Pate V, Visco AG, Weidner AC, Wu JM. Trends in use of surgical mesh for pelvic organ prolapse. Am J Obstet Gynecol. (2013) 208:79 e71–79. e77. doi: 10.1016/j.ajog.2012.11.008
17. Li C, Gong Y, Wang B. The efficacy of pelvic floor muscle training for pelvic organ prolapse: a systematic review and meta-analysis. Int Urogynecol J. (2016) 27:981–92. doi: 10.1007/s00192-015-2846-y
18. Brækken IH, Majida M, Engh ME, Bø K. Are pelvic floor muscle thickness and size of levator hiatus associated with pelvic floor muscle strength, endurance and vaginal resting pressure in women with pelvic organ prolapse stages I–III? A cross sectional 3D ultrasound study. Neurourol Urodynamics. (2014) 33:115–20. doi: 10.1002/nau.22384
19. Handa VL, Blomquist JL, Knoepp LR, Hoskey KA, McDermott KC, Muñoz A. Pelvic floor disorders 5-10 years after vaginal or cesarean childbirth. Obstet Gynecol. (2011) 118:777. doi: 10.1097/AOG.0b013e3182267f2f
20. DeLancey JO, Low LK, Miller JM, Patel DA, Tumbarello JA. Graphic integration of causal factors of pelvic floor disorders: an integrated life span model. Am J Obstet Gynecol. (2008) 199:610 e611–610, e615. doi: 10.1016/j.ajog.2008.04.001
21. Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in US Women: 2010 to 2050. Obstet Gynecol. (2009) 114:1278–83. doi: 10.1097/AOG.0b013e3181c2ce96
22. Vitale SG, Caruso S, Rapisarda AMC, Valenti G, Rossetti D, Cianci S, et al. Biocompatible porcine dermis graft to treat severe cystocele: impact on quality of life and sexuality. Arch Gynecol Obstet. (2016) 293:125–31. doi: 10.1007/s00404-015-3820-0
23. Gedefaw G, Demis A. Burden of pelvic organ prolapse in Ethiopia: a systematic review and meta-analysis. BMC Womens Health. (2020) 20:1–9. doi: 10.1186/s12905-020-01039-w
24. Asresie A, Admassu E, Setegn T. Determinants of pelvic organ prolapse among gynecologic patients in Bahir Dar, North West Ethiopia: a case–control study. Int J Womens Health. (2016) 8:713. doi: 10.2147/IJWH.S122459
25. Fagerland MW, Hosmer DW. A goodness-of-fit test for the proportional odds regression model. Stat Med. (2013) 32:2235–49. doi: 10.1002/sim.5645
26. Fernández D, Liu I, Arnold R, Nguyen T, Spiess M. Model-based goodness-of-fit tests for the ordered stereotype model. Stat Methods Med Res. (2020) 29:1527–41. doi: 10.1177/0962280219864708
27. Jelovsek JE, Barber MD. Women seeking treatment for advanced pelvic organ prolapse have decreased body image and quality of life. Am J Obstet Gynecol. (2006) 194:1455–61. doi: 10.1016/j.ajog.2006.01.060
28. Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. BJOG. (1997) 104:579–85. doi: 10.1111/j.1471-0528.1997.tb11536.x
29. Lowenstein L, Kenton K, Pierce K, FitzGerald MP, Mueller ER, Brubaker L: Patients' pelvic goals change after initial urogynecologic consultation. Am J Obstet Gynecol. (2007) 197:640. e641–640. e643. doi: 10.1016/j.ajog.2007.08.021
30. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. (1997) 89:501–6. doi: 10.1016/S0029-7844(97)00058-6
31. Symmonds RE, Williams TJ, Lee RA, Webb MJ. Posthysterectomy enterocele and vaginal vault prolapse. Am J Obstet Gynecol. (1981) 140:852–9. doi: 10.1016/0002-9378(81)90074-0
32. Vergeldt TF, Weemhoff M, IntHout J, Kluivers KB. Risk factors for pelvic organ prolapse and its recurrence: a systematic review. Int Urogynecol J. (2015) 26:1559–73. doi: 10.1007/s00192-015-2695-8
33. Swift S, Woodman P, O'Boyle A, Kahn M, Valley M, Bland D, et al. Pelvic Organ Support Study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. (2005) 192:795–806. doi: 10.1016/j.ajog.2004.10.602
34. Whitcomb EL, Rortveit G, Brown JS, Creasman JM, Thom DH, Van Den Eeden SK, et al. Racial differences in pelvic organ prolapse. Obstet Gynecol. (2009) 114:1271. doi: 10.1097/AOG.0b013e3181bf9cc8
35. Glazener C, Elders A, MacArthur C, Lancashire R, Herbison P, Hagen S, et al. Childbirth and prolapse: long-term associations with the symptoms and objective measurement of pelvic organ prolapse. BJOG. (2013) 120:161–8. doi: 10.1111/1471-0528.12075
36. Yeniel AÖ, Ergenoglu AM, Askar N, Itil IM, Meseri R. How do delivery mode and parity affect pelvic organ prolapse? Acta Obstet Gynecol Scand. (2013) 92:847–51. doi: 10.1111/aogs.12129
37. Lukman Y. Utero-vaginal prolapse: a rural disability of the young. East Afr Med J. (1995) 72:2–9.
38. Akmel M, Segni H. Pelvic organ prolapse in Jimma University specialized hospital, Southwest Ethiopia. Ethiop. J. Health Sci. (2012) 22:85–92.
39. Swift S, Pound T, Dias J. Case–control study of etiologic factors in the development of severe pelvic organ prolapse. Int Urogynecol J. (2001) 12:187–92. doi: 10.1007/s001920170062
40. Megabiaw B, Adefris M, Rortveit G, Degu G, Muleta M, Blystad A, et al. Pelvic floor disorders among women in Dabat district, northwest Ethiopia: a pilot study. Int Urogynecol J. (2013) 24:1135–43. doi: 10.1007/s00192-012-1981-y
41. Kudish BI, Iglesia CB, Gutman RE, Sokol AI, Rodgers AK, Gass M, et al. Risk factors for prolapse development in white, black, and Hispanic women. Female Pelvic Med Reconstr Surg. (2011) 17:80. doi: 10.1097/SPV.0b013e31820e5d06
42. Whiteside JL, Weber AM, Meyn LA, Walters MD. Risk factors for prolapse recurrence after vaginal repair. Am J Obstet Gynecol. (2004) 191:1533–8. doi: 10.1016/j.ajog.2004.06.109
43. Diez-Itza I, Aizpitarte I, Becerro A. Risk factors for the recurrence of pelvic organ prolapse after vaginal surgery: a review at 5 years after surgery. Int Urogynecol J. (2007) 18:1317–24. doi: 10.1007/s00192-007-0321-0
44. Cattani L, Decoene J, Page AS, Weeg N, Deprest J, Dietz HP: Pregnancy, labour and delivery as risk factors for pelvic organ prolapse: a systematic review. Int Urogynecol J. (2021) 7:1623–31. doi: 10.1007/s00192-021-04724-y
45. Thapa S, Angdembe M, Chauhan D, Joshi R. Determinants of pelvic organ prolapse among the women of the western part of N epal: a case–control study. J Obstet Gynaecol Res. (2014) 40:515–20. doi: 10.1111/jog.12168
46. Dwyer P, Lee E, Hay D. Obesity and urinary incontinence in women. BJOG. (1988) 95:91–6. doi: 10.1111/j.1471-0528.1988.tb06486.x
47. Nygaard I, Bradley C, Brandt D. Pelvic organ prolapse in older women: prevalence and risk factors. Obstet Gynecol. (2004) 104:489–97. doi: 10.1097/01.AOG.0000136100.10818.d8
48. Lien YS, Chen GD, Ng SC. Prevalence of and risk factors for pelvic organ prolapse and lower urinary tract symptoms among women in rural Nepal. Int J Gynecol Obstet. (2012) 119:185–8. doi: 10.1016/j.ijgo.2012.05.031
Keywords: pelvic organ prolapse, pelvic floor dysfunction, maternal health, risk factors, Asella
Citation: Obsa MS, Worji TA, Kedir NA and Kute NG (2022) Risk factors of pelvic organ prolapse at Asella Teaching and Referral Hospital: Unmatched case control study. Front. Glob. Womens Health 3:833823. doi: 10.3389/fgwh.2022.833823
Received: 12 December 2021; Accepted: 18 August 2022;
Published: 13 September 2022.
Edited by:
Alex Siu-Wing Chan, Hong Kong Polytechnic University, Hong Kong SAR, ChinaReviewed by:
Antonio Simone Laganà, University of Palermo, ItalyBedru Jemal, Dilla University, Ethiopia
Copyright © 2022 Obsa, Worji, Kedir and Kute. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammed Suleiman Obsa, bXN1bGVpbWFuNDNAZ21haWwuY29t
 Mohammed Suleiman Obsa
Mohammed Suleiman Obsa Tahir A. Worji
Tahir A. Worji Nemo A. Kedir2
Nemo A. Kedir2