- 1The George Institute for Global Health, Faculty of Medicine, University of New South Wales, Sydney, NSW, Australia
- 2Stroke Division, The George Institute for Global Health, Beijing, China
- 3Sydney School of Public Health, Sydney Medical School, The University of Sydney, Sydney, NSW, Australia
- 4Neurology Department, Royal Prince Alfred Hospital, Sydney Health Partners, Sydney, NSW, Australia
- 5The George Institute for Global Health, School of Public Health, Imperial College London, London, United Kingdom
Background: Studies of sex differences in the use and outcomes of endovascular treatment (EVT) for acute ischemic stroke report inconsistent results
Methods: We systematically searched PubMed and Embase databases for studies examining sex-specific utilization of EVT for acute ischemic stroke published before 31 December 2021. Estimates were compared by study type: randomized clinical trials (RCTs) and non-RCTs (hospital-based, registry-based or administrative data). Random effects odds ratios (ORs) were generated to quantify sex differences in EVT use. To estimate sex differences in functional outcome on the modified Rankin scale after EVT, the female:male ratio of ORs and 95% confidence intervals (CIs) were obtained from ordinal or binary analysis.
Results: 6,396 studies were identified through database searching, of which 594 qualified for a full review. A total of 51 studies (36 non-RCT and 15 RCTs) reporting on sex-specific utilization of EVT were included, and of those 10 estimated the sex differences of EVT on functional outcomes. EVT use was similar in women and men both in non-RCTs (OR: 1.03, 95% CI: 0.96–1.11) and RCTs (1.02, 95% CI: 0.89–1.16), with consistent results across years of publication and regions of study, except that in Europe EVT treatment was higher in women than men (1.15, 95% CI: 1.13–1.16). No sex differences were found in the functional outcome by either ordinal and binary analyses (ORs 0.95, 95% CI: 0.68–1.32] and 0.90, 95% CI: 0.65–1.25, respectively).
Conclusions: No sex differences in EVT utilization or on functional outcomes were evident after acute ischemic stroke from large-vessel occlusion. Further research may be required to examine sex differences in long-term outcomes, social domains, and quality of life.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=226100, identifier: CRD42021226100.
Introduction
Stroke is the second leading cause of death and the third leading cause of disability, consequently causing considerable suffering and economic and social burden worldwide (1). Evidence suggests that there are sex differences in the association between major risk factors and the incidence of stroke. Hypertension, smoking and atrial fibrillation are more strongly associated with increased risk of stroke in women compared to men (2). Sex also impacts stroke outcomes. Women have worse functional recovery and quality of life after stroke compared to men due to poorer health at the time of stroke onset, advanced age, and greater severity compared to men (3, 4). From a large population-based study conducted in Australia, women were more likely to arrive at hospital by ambulance, but less likely to receive stroke care management prior to hospital admission than men (5). However, the data on the aspects of accessibility and quality of clinical treatment that may contribute to worse outcomes in women are still scarce (4), there is a need to understand potential sex differences in treatment outcomes (6).
Endovascular treatment (EVT) is a guideline-recommended therapy for acute ischemic stroke (AIS) due to large-vessel occlusion (LVO) (7–11). Compared to the standard medical management with intravenous thrombolysis, in which intravenous (IV) recombinant tissue plasminogen activator (rtPA) is used within the first 4.5 h of symptom onset, EVT can be applied in a long time window (≥6 h) (12), to increase the odds of disability-free survival, and improved quality of life, life expectancy, and cost of treatment (13–16). A recent meta-analysis of studies examining sex-specific rates for IV-rtPA found that women were 13% less likely to receive treatment than men (17). Despite the rising utilization of EVT for AIS, it is unclear whether sex differences exist in this patient group and of any impact on outcomes in real-world populations (6, 18, 19). Furthermore, an individual patient data meta-analysis of randomized controlled trials (RCT) from the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration indicates that sex does not modify the treatment effect of EVT, showing women and men benefit equally (14, 20). However, post hoc analysis of the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) indicated that women had higher 90-day mortality and adverse events, compared to men (21). Whilst much of the analysis examining sex difference in EVT outcomes has relied on RCT data, real-world data from registries and surveys are more representative of the current clinical practice (19). Limited systematic reviews have been undertaken to summarize sex-specific utilization of EVT in routine practice. The aim of this systematic review was to examine sex differences in the utilization of EVT and clinical outcomes in patients with AIS from LVO.
Methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (22), and the protocol was registered in international database (PROSPERO) of prospectively registered systematic reviews in health and social care (ID: CRD42021226100).
Eligibility criteria and search
We searched PubMed/Medline and EMBASE for relevant articles published until December 2021. We used a combination of the following MeSH terms and keywords and their synonyms: stroke, brain ischemia, ischemic stroke, therapy, fibrinolytic agents, adverse effects, thrombectomy, mortality, thrombectomy trends, tissue plasminogen activator and female, male, sex characteristics, sex factors and sex difference. Details of the search strategy used in PubMed are given in Supplementary Appendix S1. The reference lists of any systematic or narrative reviews identified in the search and included studies were also screened for additional potentially relevant studies. We limited the search to studies undertaken in human adults.
Studies were included if they reported on adult women and men who received EVT for AIS, where EVT was defined as the intra-arterial use of a microcatheter, stent or other device for mechanical thrombectomy, with or without the use of a chemical thrombolytic agent (intra-arterial thrombolysis), and had a comparison group who received best medical management according to national and international guidelines, which may include intravenous thrombolysis. We also included studies which estimated sex differences in outcomes of EVT [such as functional outcome at discharge or after 90 days, mortality, symptomatic intracerebral hemorrhage (sICH), quality of life etc.]. Studies were excluded if they only included single-sex populations, did not focus on the treatment of EVT but explored the predictors, time onset, blood pressure indexes or procedure, were general discussions, contained duplicate data to another paper, were protocols or abstracts with no detailed data, case reports, or included fewer than 50 participants.
Study selection and data extraction
Authors MO, SS, and XL scrutinized titles and abstracts, and excluded clearly irrelevant references independently. They independently reviewed abstracts of potential relevance to identify studies for review in full text. Two reviewers extracted data independently from the included studies. Where possible, the following data at study baseline were extracted from each report: year, first author and country of study, study size, number of women and men, age (years), the average length of follow-up (years), sex-stratified number of patients who received EVT or other management, study design, such as RCT or observational studies, such as national registries, hospital based or administrative data-based studies to make comparison with the study on use of thrombolysis (17). Hospital settings could include individual community-based or stroke-specialist centers or hospital networks. Administrative data included studies that used hospital billing data, such as hospital discharge data or Medicare. Any sex-stratified data on the following outcomes were also included: functional outcome defined by modified Rankin scale (mRS) at 90 days, mortality, sICH, and length of hospital stay measured using any measure. Any discrepancies in study selection or data extraction were resolved by mutual consent amongst the 3 authors.
Quality assessment
For those studies examining sex differences in utilization of EVT, a modified quality assessment tool was developed by adapting items from Newcastle-Ottawa Scale (NOS) (23) and a recent systematic review of sex differences in intravenous thrombolysis treatment (17). Eligible studies were assigned a score of 0, 1, or 2 on the following four criteria: (1) the representativeness of the overall study population, (2) the proportion and impact of exclusions applied to the initial patient cohort, (3) adjustment of confounding variables, (4) the method by which the outcome (EVT or not) was ascertained. The overall study quality score will thus be in the range 0–8, where higher scores represent better study quality. A detailed description of the adapted quality assessment tool is provided in Supplementary Appendix S2. For studies which reported data on outcomes, the NOS scale was used for non-RCTs whilst the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) tool was used for RCTs to determine the quality of evidence.
Statistical analysis
Crude odds ratios (OR) for EVT, for women vs. men, were pooled across identified studies using random effects inverse-variance weighted meta-analysis. The adjusted OR was only reported in a single study. Subgroup analyses for observational studies were conducted by study design (hospital-based, registries or administrative), time of study publication (before 2015 or later), and geographic region (North America, South America, Europe, Asia or multiple regions). Similarly, subgroup analyses were also conducted for RCTs by publication year and geographic region.For those studies with multiple-adjusted sex-specific ORs for functional outcomes, EVT vs. not, were used to obtain women:men ratios (taking the maximum adjustment set) of ORs (RORs) (24). These were then pooled using the same method of meta-analysis as above. We used the Q and I2 statistics to assess heterogeneity, and contour-enhanced funnel plots were used to test for publication bias with 1%, 5% and 10% significance contours, and the regression-based Egger test was used to examine funnel-plot asymmetry for publication bias. Two-sided p values of less than or equal to 0.05 were deemed statistically significant. Meta-analyses used the meta forestplot command in Stata version 17 (25).
Results
Characteristics of included studies
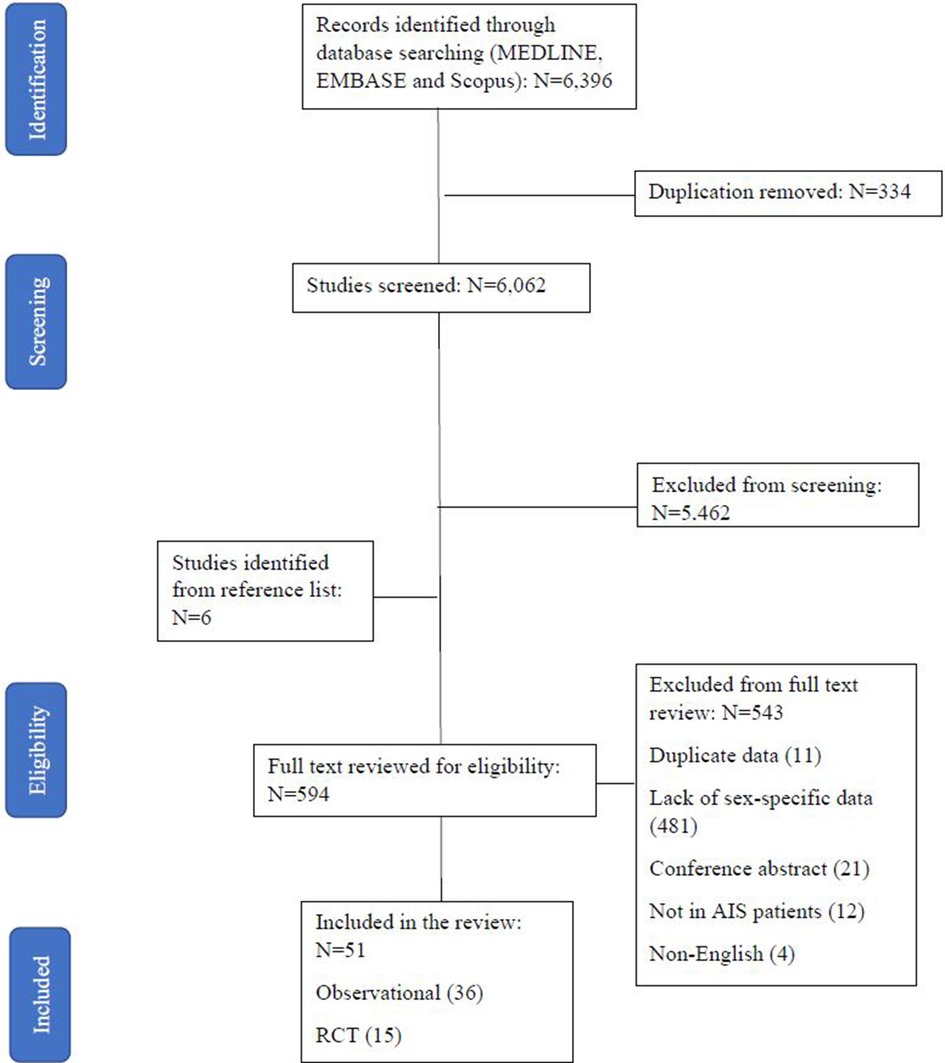
Screening identified 6,396 studies, of which 594 were reviewed in full text (Figure 1). A total of 51 studies (26–72) (Supplementary Table S1) including 4,316,668 AIS patients (49.1% female) reported utilization of EVT stratified by sex in AIS patients with LVO. Nine of the studies (10, 11, 22, 29, 30, 36, 43, 57, 62) reported sex differences in EVT outcomes of mRS score at 90 days and two reported sex difference in mortality and safety outcome. Of all the included studies on EVT use, there were 36 observational studies (percentage of EVT use: 1.7%–78.0%) and 15 RCTs (33.3%–66.2%). The quality assessment score ranged from 2 to 6, with most of the studies scoring low to moderate quality scores (63% with a score of 3 to 4) (Supplementary Tables S2, S3).
Sex-specified EVT use
Among the 36 observational studies which reported EVT use in women compared to men, the pooled crude OR was 1.03 (95% CI: 0.96–1.11) (Figure 2), indicating no significant sex difference in the odds of receiving EVT between women and men. The one study which reported an adjusted OR of 1.20, (95% CI: 0.92–1.56) for EVT use in women compared to men, adjusted for age, onset-to-door time and severity score (66). There was substantial heterogeneity among these observational studies, where the Q statistic was highly statistically significant (p < 0.01) and I2 was 95.0% (Figure 2). Figure 3 summarized the subgroup analysis of pooled estimates by region, publication year and study design. The findings of the pre-specified subgroup analysis by study design of observational studies were consistent with the main result: there was no significant difference of EVT use in women compared to men (registry OR: 1.04, 95% CI: 0.92–1.18; administrative data OR: 1.09, 95% CI: 0.95–1.25; hospital-based studies OR: 0.97, 95% CI: 0.92–1.04; test of group differences p = 0.27; Figure 3A, Supplementary Figure S1). Similar results were found in the subgroup analyses by publication year (Supplementary Figure S2). However, significant differerences were identified across region (p < 0.01); the studies conducted in Europe showed a higher odds of EVT use in women compared to men (OR: 1.15, 95% CI: 1.13–1.16; Supplementary Figure S3).
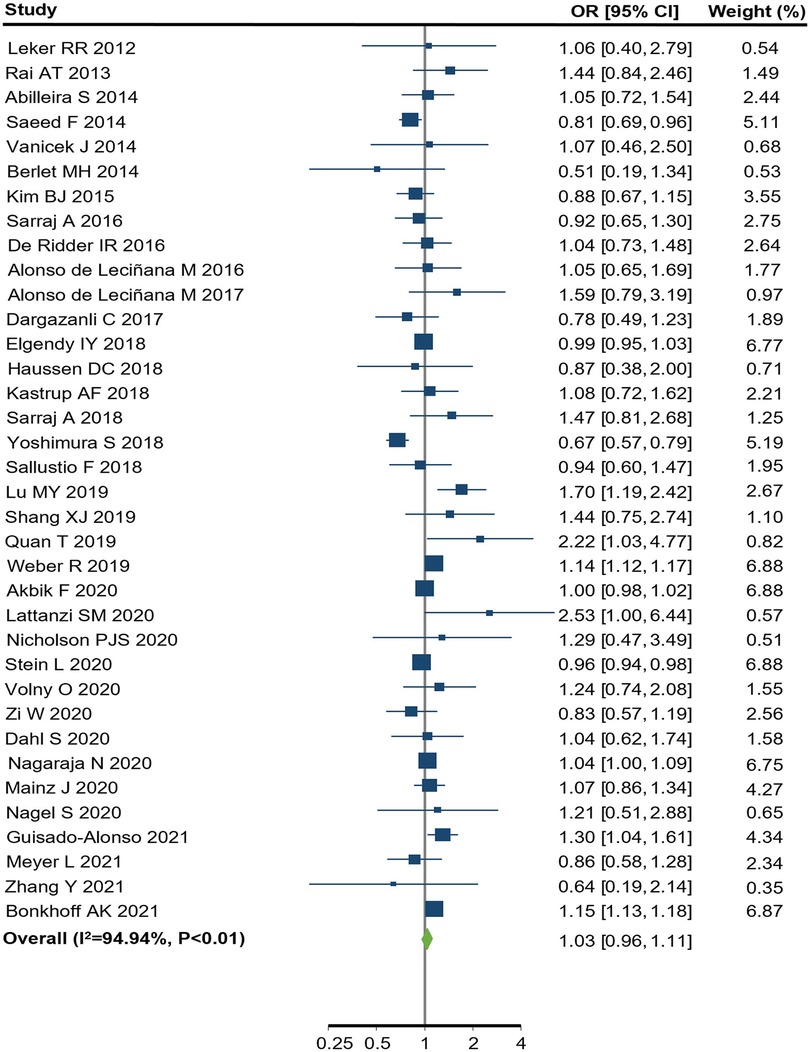
Figure 2. endovascular treatment use for women compared to men in observational studies. CI, confidence interval; OR, odds ratio. p value from Q statistics.
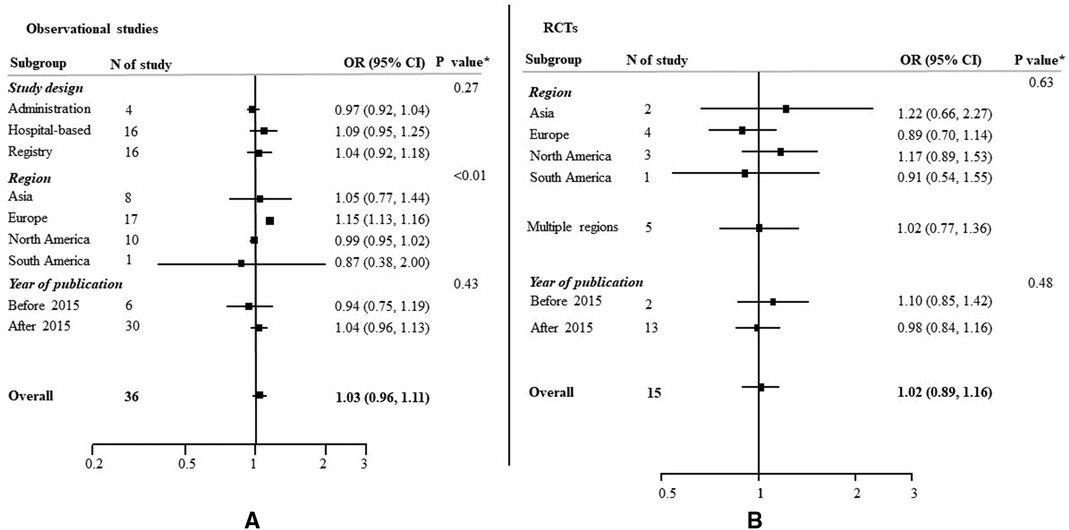
Figure 3. Odds ratios (OR) of EVT use for women compared to men, with 95% confidence intervals (CIs), by study type. CI, confidence interval; OR, odds ration; RCT, randomized controlled trial. *p value from Qb statistics.
Similar results were found in the 15 included RCTs, with no evidence of substantial heterogeneity (OR: 1.02, 95% CI: 0.89–1.16; I2 = 0.0%, p = 0.68; Figure 3B; Supplementary Figure S4). No heterogeneity was identified across the subgroup analysis by publication year (Supplementary Figure S5) and region (Supplementary Figure S6).
Sex differences in clinical outcomes of EVT
Only five (4 RCTs and 1 Registry-based study) out of the 52 studies reported sex-specific estimates of clinical outcomes on favourable shift of mRS (Figure 4A). There was no significant difference found on favourable shift of mRS for women relative to men (ROR: 0.95, 95% CI: 0.68–1.32) and no heterogeneity identified across the studies (I2 = 0.0%, p = 0.73). Of another five studies (3 RCTs and 2 Registry-based studies), the women to men ROR for reported functional outcome, by mRS score 0–2 vs. 3–6, was 0.90 (95% CI: 0.65–1.25), with no heterogeneity identified (I2 = 0.0%, p = 0.67, Figure 4B). Only two studies reported sex differences in death or safety outcomes, such as sICH. There was a higher percentage of in-hospital mortality in women (16.32% vs. 12.60%) and sICH (9.2% vs. 6.7%) compared to men (Supplementary Table S4).
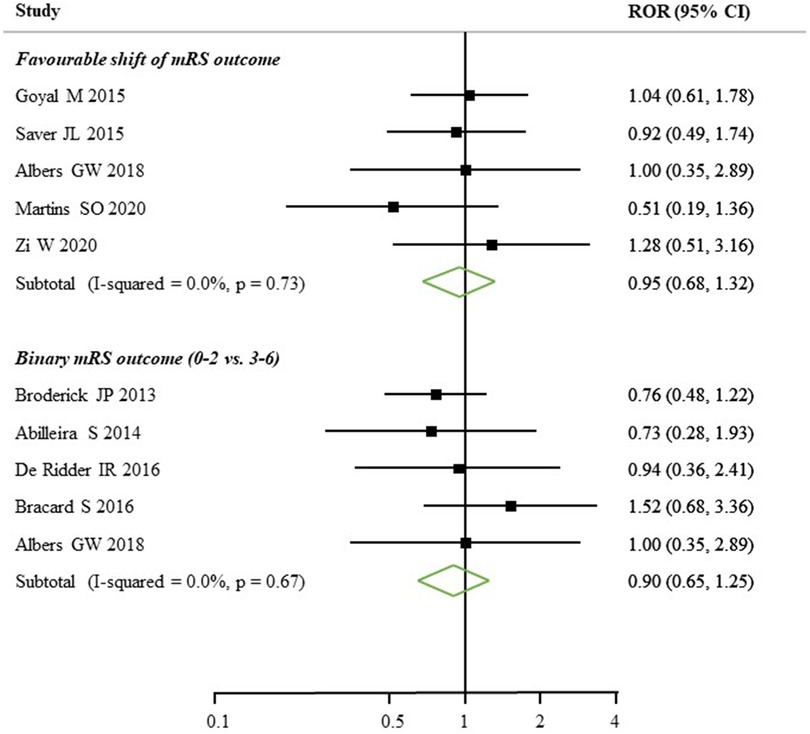
Figure 4. Women to men ratios of sex-specific odds ratios, with 95% confidence intervals (CIs), of functional outcomes after EVT treatment. mRS, modified Rankin scale; ROR, relative ratio of odds ratios.
Publication bias
The funnel plots were almost symmetrical, with no significant publication bias observed through the Egger regression-based test in the results that focused on utilization of EVT (Supplementary Figure S7) and EVT outcomes (Supplementary Figure S8).
Discussion
This review provides a comprehensive analysis of the current evidence on sex differences in the utilization and outcomes of EVT. Among 4,316,668 AIS patients, in 51 studies, we found no significant difference in the use of EVT among women compared to men, either in observational studies or RCTs. Furthermore, there was no sex difference in the functional outcomes of EVT according to the mRS score assessed at 90 days.
A recent meta-analysis showed that women were less likely than men to receive intravenous thrombolysis (17). Similarly, a study that examined data available from 1997 to 2006 showed that women were also less likely to be given several revascularisation interventions after stroke (73). However, our review demonstrated that sex did not modify the utilization of EVT, which might be related to an increased uptake of reperfusion treatment over time. This is consistent with recent evidence highlighting an increase in the utilization of EVT in both sexes when data were restricted to the period, 2006 to 2014 (74). Our subgroup analysis also supports this result, which showed that before 2014, the utilization of EVT was significantly lower in women. However, in subgroup analysis by region, there was significant differences between the groups. Women were more likely to receive EVT compared to men in studies conducted in Europe. This might be explained by the greater weight given by a European registry on EVT, which reflects the high capacity of EVT treatment in this region as 40/44 European countries were reported providing endovascular procedures to patients with AIS (75). A study conducted in UK has shown that women had a higher frequency of anterior circulation LVO AIS than men (76), and which were more likely to be eligible to undertake EVT according to clinical guideline recommendations. Women experience delayed arrival to hospital and door-to-scan times due to differences in acute stroke presentation compared to men (77, 78). Therefore, they were ineligible for thrombolysis and more likely to receive EVT with a prolonged time window. Though the pooled estimates shows no sex differences on EVT use from Asia, there was significant differences of EVT utilisation across the studies within the region group. This may be due to accessibility of EVT, adequacy of specialist available to perform procedures and availability of stroke/neuro-interventional units differed across countries in Asia (79).
Our findings were consistent to a previously published meta-analyses restricted to RCTs which found that sex does not modify functional outcomes after EVT; concluding that the treatment should be considered equally for women and men (21). However, their results were based exclusively on RCT data, and did not allow an assessment of differences in real-world circumstances. As RCTs are designed with particular inclusion and exclusion criteria for the purposes of efficiently assessing outcomes for a particular outcome, they may not be fully representative of a diverse range of real-world populations (80). Although there is a recent published study of multi-national registries which has shown no sex differences on the functional outcomes after EVT conducted in the late time window (6–24 h), the observational studies on the sex disparities on EVT treatment are still scarce (81). It is, therefore, important to include any administrative or registry based data for such analysis to determine if there is any significant modification emerging in the association of sex and outcomes after EVT in clinical practice.
Implications for clinical practice and future research
From our review, we can conclude that in real-world data, such as registry-based, hospital-based or administrative data, there are no sex differences in the utilization and outcomes of EVT in AIS. Our findings reflect pooled estimates of a previously published meta-analysis on RCTs (21), that is, of no sex differences in EVT outcomes, and therefore both women and men should be considered for EVT where possible.
However, future research in this area should consider reporting data by sex to determine any potential sex differences in outcomes after EVT. Only five studies provided sex stratified data on functional outcomes after EVT. The majority of the RCTs (6/10) did not report functional outcomes by sex, and even fewer real-world studies reported sex-specified outcomes. This will be crucial in determining if real world outcomes are different compared to RCTs. According to a recent statement from the American Heart Association/American Stroke Association, evidence is still lacking regarding sex differences in long-term outcomes after EVT (6). Further evaluation of additional outcome measures, such as long-term disability, health-related quality of life, and post-stroke depression and other social domains, is essential to have a thorough understanding of sex differences in EVT outcomes (6).
Strengths and limitations
This study had several strengths. We utilized a broad search strategy to identify all relevant studies, and duplicate screening and extraction was conducted to reduce reviewer bias. We also conducted subgroup analysis by publication year and region to explore any potential heterogeneity. We also included hospital-based, administrative and registry data to reduce selection bias that can be induced by only including RCTs. However, there were also some limitations. Our analysis was based on crude estimates of EVT utilization, since limited studies reported adjusted estimates, and most of the studies were identified as having low to moderate level quality based on the quality assessment. In addition, the esitmates of sex differences in EVT utilization in RCTs were due to random chance.
Conclusion
There were no sex differences in the utilization and functional outcomes of EVT for patients with AIS. However, further research is needed using real-world data to identify long-term sex-stratified outcomes after EVT.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
XW and MO contributed to the concept and rationale for the study. MO, SS, XL and LS conducted screening, full text review and data extraction. MO and SS wrote the first draft of the manuscript with input from MW, CC, CA, KH and XW. All authors contributed to the article and approved the submitted version.
Funding
There was no specific funding for this systematic review. XW is supported by the National Heart Foundation Postdoctoral Fellowship (102117); New South Wales investigator development grant, and National Health and Medical Research Council of Australia (NHMRC) investigator grant (APP1195237). CC acknowledges the support of the National Heart Foundation of Australia (Postdoctoral fellowship 102741). MW is supported by NHMRC grants APP1149987 and APP1174120. CSA holds an NHMRC Senior Investigator Fellowship.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgwh.2022.1032592/full#supplementary-material.
References
1. Johnson W, Onuma O, Owolabi M, Sachdev S. Stroke: a global response is needed. Bull W H O. (2016) 94:634–634A. doi: 10.2471/BLT.16.181636
2. Peters SAE, Carcel C, Millett ERC, Woodward M. Sex differences in the association between major risk factors and the risk of stroke in the UK biobank cohort study. Neurology. (2020) 95:e2715–26. doi: 10.1212/WNL.0000000000010982
3. Rexrode KM, Madsen TE, Yu AYX, Carcel C, Lichtman JH, Miller EC. The impact of sex and gender on stroke. Circ Res. (2022) 130:512–28. doi: 10.1161/CIRCRESAHA.121.319915
4. Carcel C, Wang X, Sandset EC, Delcourt C, Arima H, Lindley R, et al. Sex differences in treatment and outcome after stroke: pooled analysis including 19,000 participants. Neurology. (2019) 93:e2170–80. doi: 10.1212/WNL.0000000000008615
5. Wang X, Carcel C, Hsu B, Shajahan S, Miller M, Peters S, et al. Differences in the pre-hospital management of women and men with stroke by emergency medical services in New South Wales. Med J Aust. (2022) 217:143–8. doi: 10.5694/mja2.51652
6. Ospel JM, Schaafsma JD, Leslie-Mazwi TM, Amin-Hanjani S, Asdaghi N, Gordon-Perue GL, et al. Toward a better understanding of sex- and gender-related differences in endovascular stroke treatment: a scientific statement from the American heart association/American stroke association. Stroke. (2022) 53:e396–e406. doi: 10.1161/STR.0000000000000411
7. Fransen PS, Beumer D, Berkhemer OA, van den Berg LA, Lingsma H, van der Lugt A, et al. Mr clean, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in The Netherlands: study protocol for a randomized controlled trial. Trials. (2014) 15:343. doi: 10.1186/1745-6215-15-343
8. Molina CA, Chamorro A, Rovira À, de Miquel A, Serena J, Roman LS, et al. Revascat: a randomized trial of revascularization with solitaire fr device vs. Best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int J Stroke. (2015) 10:619–26. doi: 10.1111/ijs.12157
9. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
10. Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-pa vs. T-pa alone in stroke. N Engl J Med. (2015) 372:2285–95. doi: 10.1056/NEJMoa1415061
11. Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
12. Schellinger PD, Demaerschalk BM. Endovascular stroke therapy in the late time window. Stroke. (2018) 49:2559–61. doi: 10.1161/STROKEAHA.118.021003
13. McCarthy DJ, Diaz A, Sheinberg DL, Snelling B, Luther EM, Chen SH, et al. Long-term outcomes of mechanical thrombectomy for stroke: a meta-analysis. Sci World J. (2019) 2019:7403104. doi: 10.1155/2019/7403104
14. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
15. Campbell BCV, Mitchell PJ, Churilov L, Keshtkaran M, Hong K-S, Kleinig TJ, et al. Endovascular thrombectomy for ischemic stroke increases disability-free survival, quality of life, and life expectancy and reduces cost. Front Neurol. (2017) 8:657. doi: 10.3389/fneur.2017.00657
16. Hussein HM, Saleem MA, Qureshi AI. Statewide trends in utilization and outcomes of endovascular treatment of acute ischemic stroke: analysis of Minnesota hospital association data (2014 and 2015). J Stroke Cerebrovasc Dis. (2018) 27:677–81. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.056
17. Strong B, Lisabeth LD, Reeves M. Sex differences in iv thrombolysis treatment for acute ischemic stroke: a systematic review and meta-analysis. Neurology. (2020) 95:e11–22. doi: 10.1212/WNL.0000000000009733
18. Bushnell C, Howard VJ, Lisabeth L, Caso V, Gall S, Kleindorfer D, et al. Sex differences in the evaluation and treatment of acute ischaemic stroke. Lancet Neurol. (2018) 17:641–50. doi: 10.1016/S1474-4422(18)30201-1
19. Madsen TE, DeCroce-Movson E, Hemendinger M, McTaggart RA, Yaghi S, Cutting S, et al. Sex differences in 90-day outcomes after mechanical thrombectomy for acute ischemic stroke. J Neurointerv Surg. (2019) 11:221–5. doi: 10.1136/neurintsurg-2018-014050
20. Chalos V, de Ridder IR, Lingsma HF, Brown S, van Oostenbrugge RJ, Goyal M, et al. Does sex modify the effect of endovascular treatment for ischemic stroke? Stroke. (2019) 50:2413–9. doi: 10.1161/STROKEAHA.118.023743
21. de Ridder IR, Fransen PSS, Beumer D, Berkhemer OA, van den Berg LA, Wermer MJ, et al. Is intra-arterial treatment for acute ischemic stroke less effective in women than in men. Interv Neurol. (2016) 5:174–8. doi: 10.1159/000447331
22. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. Prisma 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Br Med J. (2021) 372:n160. doi: 10.1136/bmj.n160
23. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. Newcastle-Ottawa quality assessment scale cohort studies. 2004
24. Woodward M. Rationale and tutorial for analysing and reporting sex differences in cardiovascular associations. Heart. (2019) 105:1701–8. doi: 10.1136/heartjnl-2019-315299
26. Leker RR, Eichel R, Gomori JM, Ramirez de Noriega F, Ben-Hur T, Cohen JE. Stent-based thrombectomy versus intravenous tissue plasminogen activator in patients with acute middle cerebral artery occlusion. Stroke. (2012) 43:3389–91. doi: 10.1161/STROKEAHA.112.673665
27. Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. (2013) 368:904–13. doi: 10.1056/NEJMoa1213701
28. Rai AT, Carpenter JS, Raghuram K, Roberts TD, Rodgers D, Hobbs GR. Endovascular therapy yields significantly superior outcomes for large vessel occlusions compared with intravenous thrombolysis: is it time to randomize? J Neurointerv Surg. (2013) 5:430–4. doi: 10.1136/neurintsurg-2012-010429
29. Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-pa versus t-pa alone for stroke. N Engl J Med. (2013) 368:893–903. doi: 10.1056/NEJMoa1214300
30. Abilleira S, Cardona P, Ribó M, Millán M, Obach V, Roquer J, et al. Outcomes of a contemporary cohort of 536 consecutive patients with acute ischemic stroke treated with endovascular therapy. Stroke. (2014) 45:1046–52. doi: 10.1161/STROKEAHA.113.003489
31. Saeed F, Adil MM, Piracha BH, Qureshi AI. Outcomes of endovascular versus intravenous thrombolytic treatment for acute ischemic stroke in dialysis patients. Int J Artif Organs. (2014) 37:727–33. doi: 10.5301/ijao.5000349
32. Vanicek J, Bulik M, Brichta J, Jancalek R. Utility of a rescue endovascular therapy for the treatment of major strokes refractory to full-dose intravenous thrombolysis. Br J Radiol. (2014) 87:20130545. doi: 10.1259/bjr.20130545
33. Berlet MHMD, Stambo GWMD, Kelley MRN, Van Epps KMD, Woeste TMD, Steffen DRTCV. Does modern ischemic stroke therapy in a large community-based dedicated stroke center improve clinical outcomes? A two-year retrospective study. J Stroke Cerebrovasc Dis. (2014) 23:869–78. doi: 10.1016/j.jstrokecerebrovasdis.2013.07.016
34. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 h after symptom onset in ischemic stroke. N Engl J Med. (2015) 372:2296–306. doi: 10.1056/NEJMoa1503780
35. Kim BJ, Han MK, Park TH, Park SS, Lee KB, Lee BC, et al. Trends in the effectiveness of endovascular recanalization for acute stroke: is a change taking place? J Stroke Cerebrovasc Dis. (2015) 24:866–73. doi: 10.1016/j.jstrokecerebrovasdis.2014.12.003
36. Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (thrace): a randomised controlled trial. Lancet Neurol. (2016) 15:1138–47. doi: 10.1016/S1474-4422(16)30177-6
37. Sarraj A, Sangha N, Hussain MS, Wisco D, Vora N, Elijovich L, et al. Endovascular therapy for acute ischemic stroke with occlusion of the middle cerebral artery m2 segment. JAMA Neurol. (2016) 73:1291–6. doi: 10.1001/jamaneurol.2016.2773
38. Alonso de Leciñana M, Fuentes B, Ximénez-Carrillo Á, Vivancos J, Masjuan J, Gil-Nuñez A, et al. A collaborative system for endovascular treatment of acute ischaemic stroke: the Madrid stroke network experience. Eur J Neurol. (2016) 23:297–303. doi: 10.1111/ene.12749
39. Muir KW, Ford GA, Messow C-M, Ford I, Murray A, Clifton A, et al. Endovascular therapy for acute ischaemic stroke: the pragmatic ischaemic stroke thrombectomy evaluation (piste) randomised, controlled trial. J Neurol Neurosurg Psychiatry. (2017) 88:38–44. doi: 10.1136/jnnp-2016-314117
40. Alonso de Leciñana M, Kawiorski MM, Ximénez-Carrillo Á, Cruz-Culebras A, García-Pastor A, Martínez-Sánchez P, et al. Mechanical thrombectomy for basilar artery thrombosis: a comparison of outcomes with anterior circulation occlusions. J Neurointerv Surg. (2017) 9:1173–8. doi: 10.1136/neurintsurg-2016-012797
41. Dargazanli C, Arquizan C, Gory B, Consoli A, Labreuche J, Redjem H, et al. Mechanical thrombectomy for minor and mild stroke patients harboring large vessel occlusion in the anterior circulation: a multicenter cohort study. Stroke. (2017) 48:3274–81. doi: 10.1161/STROKEAHA.117.018113
42. Khoury NN, Darsaut TE, Ghostine J, Deschaintre Y, Daneault N, Durocher A, et al. Endovascular thrombectomy and medical therapy versus medical therapy alone in acute stroke: a randomized care trial. J Neuroradiol. (2017) 44:198–202. doi: 10.1016/j.neurad.2017.01.126
43. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 h with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
44. Elgendy IY, Omer MA, Kennedy KF, Mansoor H, Mahmoud AN, Mojadidi MK, et al. 30-day Readmissions after endovascular thrombectomy for acute ischemic stroke. JACC Cardiovasc Interv. (2018) 11:2414–24. doi: 10.1016/j.jcin.2018.09.006
45. Haussen DC, Lima FO, Bouslama M, Grossberg JA, Silva GS, Lev MH, et al. Thrombectomy versus medical management for large vessel occlusion strokes with minimal symptoms: an analysis from stopstroke and gestor cohorts. J Neurointerv Surg. (2018) 10:325–9. doi: 10.1136/neurintsurg-2017-013243
46. Kastrup A, Brunner F, Hildebrandt H, Roth C, Winterhalter M, Papanagiotou P. Endovascular therapy versus thrombolysis in patients with large vessel occlusions within the anterior circulation aged ≥80 years. J Neurointerv Surg. (2018) 10:1053–6. doi: 10.1136/neurintsurg-2017-013732
47. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 h after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
48. Sarraj A, Hassan A, Savitz SI, Grotta JC, Cai C, Parsha KN, et al. Endovascular thrombectomy for mild strokes: how low should we go? Stroke. (2018) 49:2398–405. doi: 10.1161/STROKEAHA.118.022114
49. Yoshimura S, Sakai N, Uchida K, Yamagami H, Ezura M, Okada Y, et al. Endovascular therapy in ischemic stroke with acute large-vessel occlusion: recovery by endovascular salvage for cerebral ultra-acute embolism Japan registry 2. J Am Heart Assoc. (2018) 7(9):e008796. doi: 10.1161/JAHA.118.008796
50. Sallustio F, Koch G, Alemseged F, Konda D, Fabiano S, Pampana E, et al. Effect of mechanical thrombectomy alone or in combination with intravenous thrombolysis for acute ischemic stroke. J Neurol. (2018) 265:2875–80. doi: 10.1007/s00415-018-9073-7
51. Zhao QS, Li W, Li D, Liu T, Wang JH, Gao Y, et al. Clinical treatment efficiency of mechanical thrombectomy combined with rhpro-UK thrombolysis for acute moderate/severe cerebral infarction. Eur Rev Med Pharmacol Sci. (2018) 22:5740–6. doi: 10.26355/eurrev_201809_15842
52. Lu MY, Chen CH, Yeh SJ, Tsai LK, Lee CW, Tang SC, et al. Comparison between in-hospital stroke and community-onset stroke treated with endovascular thrombectomy. PLoS One. (2019) 14:e0214883. doi: 10.1371/journal.pone.0214883
53. Shang XJ, Shi ZH, He CF, Zhang S, Bai YJ, Guo YT, et al. Efficacy and safety of endovascular thrombectomy in mild ischemic stroke: results from a retrospective study and meta-analysis of previous trials. BMC Neurol. (2019) 19:150. doi: 10.1186/s12883-019-1372-9
54. Quan T, Hou H, Xue W, Yu G, Ma H, Sun J, et al. Endovascular treatment of acute intracranial vertebrobasilar artery occlusion: a multicenter retrospective observational study. Neuroradiology. (2019) 61:1477–84. doi: 10.1007/s00234-019-02282-1
55. Weber R, Krogias C, Eyding J, Bartig D, Meves SH, Katsanos AH, et al. Age and sex differences in ischemic stroke treatment in a nationwide analysis of 1.11 million hospitalized cases. Stroke. (2019) 50:3494–502. doi: 10.1161/STROKEAHA.119.026723
56. Akbik F, Xu H, Xian Y, Shah S, Smith EE, Bhatt DL, et al. Trends in reperfusion therapy for in-hospital ischemic stroke in the endovascular therapy era. JAMA Neurol. (2020) 77:1486–95. doi: 10.1001/jamaneurol.2020.3362
57. Martins SO, Mont’Alverne F, Rebello LC, Abud DG, Silva GS, Lima FO, et al. Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med. (2020) 382:2316–26. doi: 10.1056/NEJMoa2000120
58. Lattanzi S, Coccia M, Pulcini A, Cagnetti C, Galli FL, Villani L, et al. Endovascular treatment and cognitive outcome after anterior circulation ischemic stroke. Sci Rep. (2020) 10:18524. doi: 10.1038/s41598-020-75609-1
59. Nicholson P, Byun JS, Lu H, Hilditch CA, Brinjikji W, Agid R, et al. Endovascular treatment versus best medical therapy in acute ischemic stroke patients with mild symptoms. World Neurosurg. (2020) 144:e837–41. doi: 10.1016/j.wneu.2020.09.080
60. Stein L, Tuhrim S, Fifi J, Mocco J, Dhamoon M. National trends in endovascular therapy for acute ischemic stroke: utilization and outcomes. J Neurointerv Surg. (2020) 12:356–62. doi: 10.1136/neurintsurg-2019-015019
61. Volny O, Zerna C, Tomek A, Bar M, Rocek M, Padr R, et al. Thrombectomy vs medical management in low nihss acute anterior circulation stroke. Neurology. (2020) 95:e3364–72. doi: 10.1212/WNL.0000000000010955
62. Zi W, Qiu Z, Wu D, Li F, Liu H, Liu W, et al. Assessment of endovascular treatment for acute basilar artery occlusion via a nationwide prospective registry. JAMA Neurol. (2020) 77:561–73. doi: 10.1001/jamaneurol.2020.0156
63. Dahl S, Hjalmarsson C, Andersson B. Sex differences in risk factors, treatment, and prognosis in acute stroke. Women's Health. (2020) 16:1745506520952039. doi: 10.1177/1745506520952039
64. Nagaraja N, Olasoji EB, Patel UK. Sex and racial disparity in utilization and outcomes of t-pa and thrombectomy in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2020) 29:104954. doi: 10.1016/j.jstrokecerebrovasdis.2020.104954
65. Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (best): an open-label, randomised controlled trial. Lancet Neurol. (2020) 19:115–22. doi: 10.1016/S1474-4422(19)30395-3
66. Mainz J, Andersen G, Valentin JB, Gude MF, Johnsen SP. Disentangling sex differences in use of reperfusion therapy in patients with acute ischemic stroke. Stroke. (2020) 51:2332–8. doi: 10.1161/STROKEAHA.119.028589
67. Nagel S, Pfaff J, Herweh C, Schieber S, Mundiyanapurath S, Schönenberger S, et al. Distal arterial occlusions in patients with mild strokes - is endovascular therapy superior to thrombolysis alone? J Stroke Cerebrovasc Dis. (2020) 29:104868. doi: 10.1016/j.jstrokecerebrovasdis.2020.104868
68. Guisado-Alonso D, Martinez-Domeno A, Prats-Sanchez L, Delgado-Mederos R, Camps-Renom P, Abilleira S, et al. Reasons for not performing mechanical thrombectomy a population-based study of stroke codes. Stroke. (2021) 52:2746–53. doi: 10.1161/STROKEAHA.120.032648
69. Meyer L, Bechstein M, Bester M, Hanning U, Brekenfeld C, Flottmann F, et al. Thrombectomy in extensive stroke may not be beneficial and is associated with increased risk for hemorrhage. Stroke. (2021) 52:3109–17. doi: 10.1161/STROKEAHA.120.033101
70. Zhang Y, He Y, Chen S, Zhao W, Chen Y, Liu Y, et al. Safety and efficacy of intravascular therapy in patients with progressive stroke caused by intracranial large vascular occlusion exceeding the time window of 24 h. Neurol Res. (2021) 43:1031–9. doi: 10.1080/01616412.2021.1948768
71. Langezaal LCM, van der Hoeven E, Mont’Alverne FJA, de Carvalho JJF, Lima FO, Dippel DWJ, et al. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med. (2021) 384:1910–20. doi: 10.1056/NEJMoa2030297
72. Bonkhoff AK, Karch A, Weber R, Wellmann J, Berger K. Female stroke sex differences in acute treatment and early outcomes of acute ischemic stroke. Stroke. (2021) 52:406–15. doi: 10.1161/STROKEAHA.120.032850
73. Towfighi A, Markovic D, Ovbiagele B. Sex differences in revascularization interventions after acute ischemic stroke. J Stroke Cerebrovasc Dis. (2013) 22:e347–53. doi: 10.1016/j.jstrokecerebrovasdis.2013.03.018
74. Lekoubou A, Bishu KG, Ovbiagele B. Stroke thrombectomy utilization rates by sex: what were things like before 2015? J Stroke Cerebrovasc Dis. (2020) 29:104587. doi: 10.1016/j.jstrokecerebrovasdis.2019.104587
75. Aguiar de Sousa D, von Martial R, Abilleira S, Gattringer T, Kobayashi A, Gallofré M, et al. Access to and delivery of acute ischaemic stroke treatments: a survey of national scientific societies and stroke experts in 44 European countries. Eur Stroke J. (2019) 4:13–28. doi: 10.1177/2396987318786023
76. Niewada M, Kobayashi A, Sandercock PAG, Kamiński B, Członkowska A. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. (2005) 24:123–8. doi: 10.1159/000082999
77. Bushnell CD, Chaturvedi S, Gage KR, Herson PS, Hurn PD, Jiménez MC, et al. Sex differences in stroke: challenges and opportunities. J Cereb Blood Flow Metab. (2018) 38:2179–91. doi: 10.1177/0271678X18793324
78. Reeves MJP, Bushnell CDMD, Howard GD, Gargano JWMS, Duncan PWP, Lynch GMD, et al. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. (2008) 7:915–26. doi: 10.1016/S1474-4422(08)70193-5
79. Toyoda K, Koga M, Hayakawa M, Yamagami H. Acute reperfusion therapy and stroke care in Asia after successful endovascular trials. Stroke. (2015) 46:1474–81. doi: 10.1161/STROKEAHA.115.008781
80. Camm AJ, Fox KAA. Strengths and weaknesses of “real-world” studies involving non-vitamin k antagonist oral anticoagulants. Open Heart. (2018) 5:e000788. doi: 10.1136/openhrt-2018-000788
Keywords: sex difference, endovascular treatment, ischemic stroke, female, treatment
Citation: Ouyang M, Shajahan S, Liu X, Sun L, Carcel C, Harris K, Anderson CS, Woodward M and Wang X (2023) Sex differences in the utilization and outcomes of endovascular treatment after acute ischemic stroke: A systematic review and meta-analysis. Front. Glob. Womens Health 3:1032592. doi: 10.3389/fgwh.2022.1032592
Received: 31 August 2022; Accepted: 22 December 2022;
Published: 18 January 2023.
Edited by:
Ilaria Campesi, University of Sassari, ItalyReviewed by:
Giuseppe Seghieri, Regional Health Agency of Tuscany, ItalyDeep Pujara, University Hospitals Cleveland Medical Center, United States
© 2023 Ouyang, Shajahan, Liu, Sun, Carcel, Harris, Anderson, Woodward and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Wang eHdhbmdAZ2VvcmdlaW5zdGl0dXRlLm9yZy5hdQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Sex and Gender Differences in Disease, a section of the journal Frontiers in Global Women's Health
 Menglu Ouyang
Menglu Ouyang Sultana Shajahan
Sultana Shajahan Xiaoying Liu
Xiaoying Liu Lingli Sun
Lingli Sun Cheryl Carcel
Cheryl Carcel Katie Harris
Katie Harris Craig S. Anderson
Craig S. Anderson Mark Woodward
Mark Woodward Xia Wang
Xia Wang