- 1Camborne School of Mines, Department of Earth and Environmental Sciences, University of Exeter, Penryn, United Kingdom
- 2Environment and Sustainability Institute, University of Exeter, Penryn, United Kingdom
- 3Department of Geology, School of Mines and Mineral Sciences, The Copperbelt University, Kitwe, Zambia
Mine wastes can pose environmental and human health risks, especially when they contain high concentrations of potentially toxic metal(loid)s. In this study, the geochemistry (bulk and sequential extraction analysis, paste pH) and mineralogy (X-ray diffraction, scanning electron microscopy, electron microprobe analysis) of Co in mine wastes from Cobalt (Canada) and Cornwall (UK) were characterised to assess potential Co and other metal(loid) mobility in the aquatic environment. Cobalt concentrations in Nipissing high- and low-grade tailings at Cobalt were high (up to 5,630 mg kg−1 and 1,230 mg kg−1, respectively), and were several orders of magnitude higher than those at Poldice, Wheal Unity, and Dolcoath in Cornwall (average 40 mg kg−1, 76 mg kg−1, and 59 mg kg−1, respectively). Community Bureau of Reference (BCR)-sequential extraction analysis suggested that Co was equally mobile within the samples from Cobalt and Cornwall, with averages of 46% extracted in the exchangeable fraction. Erythrite was the most important secondary Co-bearing mineral that occurred widely in the Nipissing tailings. Other Co-bearing secondary minerals included arseniosiderite, scorodite, and Fe oxyhydroxides. Primary Co-bearing minerals identified included cobaltite and safflorite-skutterudite, and Co was also taken up in primary arsenopyrite, loellingite, pyrite and chalcopyrite. At the sites in Cornwall, however, Co-bearing primary and secondary minerals were not identified. Instead, Co was observed as a trace component in primary arsenopyrite, pyrite, and chalcopyrite and in secondary scorodite and Fe-Mn oxyhydroxides. Despite these mineralogical and other geological and processing differences, Co showed consistently high potential for mobilization from the wastes. In addition, risk assessment codes for Co fell in the medium to very high risk category in the aquatic and non-aquatic environments. This classification suggests that the mine waste-hosted Co is likely to affect humans via the food chain. Further research is required to determine if Co shows similar behaviour in mine wastes globally.
1 Introduction
Waste generated by mining and mineral processing activities is expected to continue to grow into the future, owing to increasing global demand for mineral resources, as increasingly lower-grade and larger-scale deposits are being mined (Hudson-Edwards and Dold, 2015; Vriens et al., 2020). The consumption of technology metals such as Co has expanded considerably due to their growing demand, and use in digital technologies and batteries for electric vehicles (Gifford, 2020). Cobalt has been recognised by the European Commission and many governments as a “Critical Raw Material” (European Commission, 2020; Natural Resources Canada, 2022; Us Department Of the Interior, 2022). Despite this impending upscaling in Co mining in our near future that will result in increases in the production of Co-bearing mine wastes, key knowledge gaps remain regarding the potential environmental impact of such activity.
To bridge these gaps detailed geochemical and mineralogical characterisation of mine wastes, including changes in their composition as a function of varying environmental conditions, is required, (Dold, 2010; Jamieson, 2011; Hudson-Edwards et al., 1999; Beane et al., 2016; Jamieson et al., 2015). The characterisation involves determination of the total concentration, chemical speciation, solubility and mineralogy of ecotoxic metal(loid)s (Jamieson et al., 2015). Total metal(loid) concentrations are the first reference indicator for comparing environmental contamination levels with legislative limits and guidelines (Iavazzo et al., 2012; Mees et al., 2013). Sequential extractions, involving exposing the waste to a series of leaching reagents with different abilities to dissolve solid phases, give information on the chemical speciation of the metal(loid)s (Collins and Kinsela, 2010; Tessier et al., 1979; Rauret et al., 1999). These geochemical data can be combined with information on the mineralogical types, compositions, interrelationships and solubilities to develop an understanding of the nature of mineral-water interactions within mine waste. The latter is important because potential leaching of metal(loid)s into the aqueous phase is a key contributor to the environmental impact of mine waste (Jamieson et al., 2015). Geochemical-mineralogical datasets can also to be used to develop risk assessments, guide appropriate mine planning, and optimise the design of remediation measures at legacy mine sites (Dold, 2003; Hudson-Edwards et al., 2011; Filippi et al., 2015; Jamieson et al., 2015).
Detailed characterisation of Co-bearing mine wastes has been relatively limited. To fill this knowledge gap, the aim of this study was to characterise the geochemistry and mineralogy of Co at representative legacy mining sites in Cobalt, Canada, and Cornwall, UK. The results will provide data and insights into the concentrations, storage and potential mobility of Co in mine wastes in other affected sites globally.
2 Materials and methods
2.1 Site descriptions
In Cobalt, Canada, mining activities commenced 1903 and ended in 1989. Economic mineralization at this site has been classified by Kissin (1992) as a five-element (Ag-Ni-Co-Cu-Bi) vein deposit. In this mineralisation type, Ag is the principal commodity of interest whereas Co and Ni are by-products. Mineralization was hosted within silica-carbonate and quartz veins associated with tholeiitic intrusions in Archean rocks (Petruk, 1971). According to Dumaresq (2009), most of the by-product Co (with grades ranging from 0.5% Co for high grade and 0.02% Co for low grade) mined before 1950 ended up in the tailings owing to its little or no known uses.
At the Cobalt site, sampling was conducted on the Nipissing Hill Tailings (47.39111, −79.68028) (Figure 1). Mine tailings were deposited on this site from 1913 to 1932 and are divided into high- and low-grade tailings (Anderson, 1993). They were deposited in depressions covering areas of approximately 2,500 m2 and 60,000 m2, respectively, and formed layers that were locally up to about 3.6 m thick.
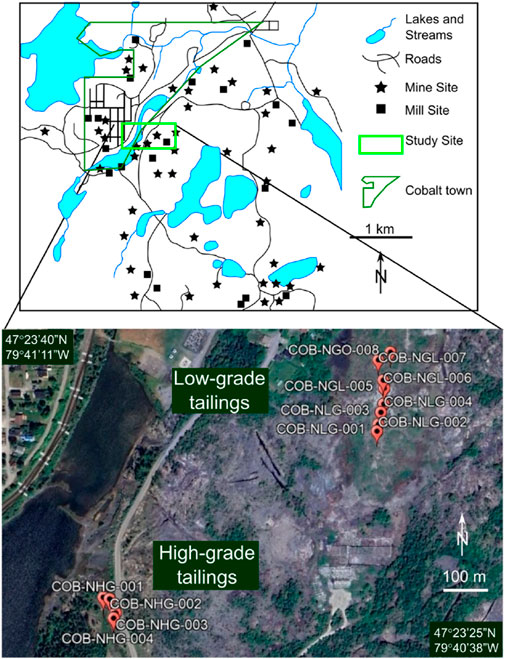
Figure 1. Mine and Mill sites of the Cobalt Mining Camp, Canada [after Dumaresq (1993)], showing the study area which is enlarged in the Google Earth satellite image showing low-grade and high-grade sampling points (base map from Google Earth).
The tailings were fine grained, with laminations and stratification resulting from changes in the material deposited and variations in processing methods and deposition periods. They were typically light grey to brown, and host localized iron oxide staining within the upper 50 cm of tailings with localised erythrite blooms. According to Dumaresq (1993), the dam structures containing the high-grade and low-grade tailings both ruptured (dates are not documented), forcing several tonnes of the tailings to be washed into nearby water bodies such as Cobalt Lake and Mill Creek.
In Cornwall, a ca. 1,000 years Sn-Cu mining period (12th century to 1998) has been recorded (LeBoutillier, 1996). In this region, granites, dykes, and their Devonian–Carboniferous host rocks are cut by extensional fault systems containing mineralised veins with polymetallic W-Sn-Cu-As-Pb-Zn magmatic-hydrothermal mineralization (Pirrie and Shail, 2018).
In Cornwall, samples were collected in June 2019 from three mine waste stockpiles at three legacy mine sites (Figure 2): Poldice Mine (50.24231, −5.16582), Wheal Unity (50.24278, −5.17556) and Dolcoath Mine (50.21750, −5.28056). According to Dines (1956), these mines historically produced Co as a by-product, yet the quantities were not well documented. The material at the Poldice mine site typically comprised fine grained (<100 µm) tailings that were observed to be stratified into layers, representing different episodes of historic processing. The material at Wheal Unity and Dolcoath mine typically comprised heterogeneous mine-affected soils with particle size distributions typically less than 2 mm diameter.
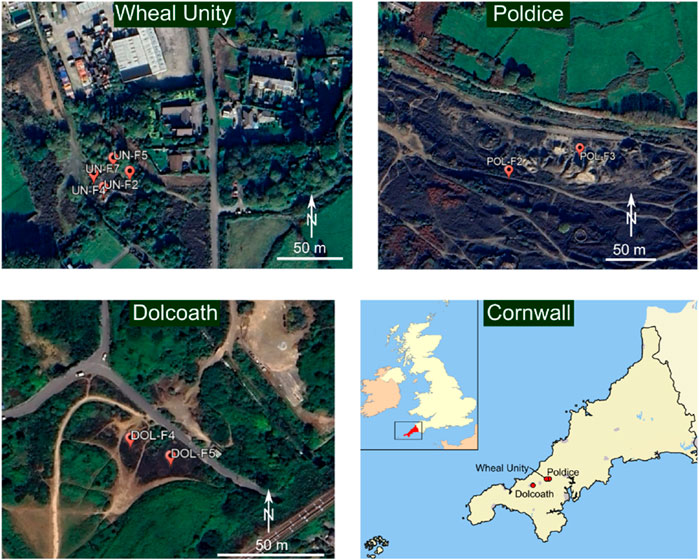
Figure 2. Dolcoath, Wheal Unity and Poldice sampling sites in Cornwall. Dolcoath mine site showing the tailings sampling sites. Poldice and Wheal Unity sites showing the mine-affected soils sampling sites. Base maps for the sampling sites were taken from Google Earth.
2.2 Sample collection and preparation
At Cobalt, 12 near surface tailings samples (8 low-grade and four high-grade) were collected and in Cornwall, 17 tailings and mine-affected soils were collected. At each sampling point, several sub-samples of c. 100 g were collected with a steel trowel composited, and 400 g of this material was placed in a labelled sealable plastic sample bag. Prior to sampling, the top 10 cm layer of the material was removed to avoid collecting from the A-O horizons which are influenced by plant and biological activity (Fawcett et al., 2015). During sampling, latex gloves were worn and all sampling equipment was cleaned with ethanol (Reagent Grade) to avoid any cross contamination. All samples were dried in an oven at 50°C for 12 h and then split using a “Jones Type” riffle splitter to obtain two 25–40 g representative samples, one of which was then ground using a TEMA disc mill (TEMA SIEBTECHNIK Ltd) to produce a fine powder of grain size <50 µm (for analyses which require a milled sample) and the other retained (for analyses which require an “as-received” sample).
<50 µm sub-samples (from the TEMA mill) were taken for geochemical analysis. The samples were digested using four acids [hydrofluoric (HF), hydrochloric (HCl), nitric (HNO3) and perchloric (HClO4)], and total metal(loid) concentrations were determined with ICP-MS (Agilent 7,700).
Four high-grade and two low-grade tailings samples from Cobalt, Canada, and six waste samples from Cornwall, UK (uncrushed and unmilled) were analysed by X-ray Diffraction and Scanning Electron Microscopy (SEM).
2.3 Geochemical analysis
2.3.1 Total acid digestion followed by inductively-coupled plasma mass spectrometry (ICP-MS)
Bulk metal concentrations were determined by total acid digestion via the four acid digestion method (hydrofluoric (HF), hydrochloric (HCl), nitric (HNO3) and perchloric (HClO4) following the methods of Garbe-Schönberg (1993) and Okeme et al. (2021), using <50 µm 100 mg mass sub-samples (generated from milling) followed by inductively-coupled plasma mass spectrometry (ICP-MS) (Agilent 7,700). Reagent blanks, sample duplicates, and certified reference materials (CRM 73305 ‘rock; blended’ and CRM RTS-3a) were analysed. Concentrations of blanks were very low, and precisions (based on analyses of the duplicates; Supplementary Table 1) were mostly <10%, except for samples with very low concentrations. Recoveries, based on comparison of the certified values of the CRMs and the analytical values obtained, were between 88% and 112% (Supplementary Table 2). The reagents used for sample preparation and extraction were analytical grade (AR) or trace analytical grade (TAG) supplied by Fisher Scientific, UK and Sigma-Aldrich.
2.3.2 Paste pH
Paste pH is a simple static test for assessing the presence of soluble acid salts and reactive minerals (Lapakko, 2002, European Commission (EC), 2020), or the presence of stored acidity that is readily available in mine waste samples (Weber et al., 2006). Paste pH of the mine waste samples was determined following the ASTM D4972-13 procedure in 0.01 M calcium chloride (American Society For Testing And Materials, 2013). Briefly, approximately 20 g of the mine waste sample was weighed into a labelled 50 mL centrifuge tube and added to it approximately 20 mL of 0.01 M CaCl2. The mixture was shaken vigorously and left to stand for 24 h before reading the pH on a potentiometer that had been calibrated with pH four and pH seven buffer solutions.
2.3.3 Sequential extraction
Sequential extractions can be used to give indications of the amounts of metal(loid)s in various chemical forms which could be released (mobilised) in solution under various environmental conditions (Tessier et al., 1979), notably pH and Eh (Anju and Banerjee, 2010). Tessier et al. (1979) introduced the first comprehensive sequential extraction scheme that subsequent procedures used as a basis. To standardise the methodology throughout Europe, the Community Bureau of Reference (BCR) developed a three-stage, sequential extraction protocol (Ure et al., 1993). Although there are limitations to the method (Martin et al., 1987) it has proved reproducible and has produced good recoveries with respect to acid dissolution for soils (Davidson et al., 1998; Rauret et al., 1999) and Co- and As-bearing mine wastes (Kříbek et al., 2011).
The BCR sequential extraction procedure was applied to 0.5 g of selected dried mine waste samples (<50 μm) to determine the chemical speciation of Co and related metal(loid)s. The full procedure is described elsewhere (Sipos et al., 2016). Briefly, four steps were followed: 1) the exchangeable fraction was extracted by 0.11 M acetic acid (CH3COOH) at pH 5; 2) the reducible fraction was extracted using 0.5 M hydroxylamine hydrochloride (H3NO·HCl) at pH 2 (adjusted by nitric acid); 3) the oxidisable fraction was extracted in two steps: In step one, the sample was oxidised with 5 mL of 8.8 M hydrogen peroxide (H2O2) and was then placed in a water bath and allowed to evaporate until near dryness. This whole step was repeated. In step two, the moist residue was extracted with 1 M ammonium acetate (CH3CO2NH4) at pH 2 (adjusted with HNO3). All extractions were carried out at solid:solution ratio of 1:40 with continuous shaking for 16 h at 22°C. The residue from step 3 was digested using the four-acid digestion method specified in Section 2.3.1.
The extracts were diluted with clean HNO3 and analysed with the Agilent 7700 ICP-MS. Total metal(loid) concentrations were calculated as the sum of the concentrations in the BCR extraction steps and in the residual digestion. To evaluate the precision of the analysis, samples were all prepared in triplicate. As with the bulk geochemical analysis, the reagents used for the sequential extraction procedures were analytical grade (AR) or trace analytical grade (TAG) supplied by Fisher Scientific, UK and Sigma-Aldrich.
2.3.4 Risk assessment code (RAC)
To evaluate the potential toxicity of Co and related metal(loid)s in the mine waste samples, the risk assessment code (RAC) method was used. The RAC was first introduced by Perin et al. (1985) for evaluating the mobility and bioavailability of metal(loid)s in sediments based on the exchangeable fraction (F1) content of the sequential extraction results. According to RAC guidelines, metal(loid)s with values less than or equal to 1% extracted have no risk to the environment. Percentages ranging from 1% to 10% are considered to be low risk, 11%–30% as medium risk and 31%–50% as high risk. Percentages greater than 50% are considered as very high risk because the dissolved metal(loid)s are thought to easily enter the food chain (Perin et al., 1985).
2.4 Mineralogical analysis
2.4.1 X-ray diffraction (XRD)
X-ray Diffraction (XRD) analysis was used to determine the bulk mineralogy of the mine waste samples. The samples were prepared by pressing the sample powder into an XRD sample holder using a glass plate. Analyses were conducted using a Siemens D5000 X-ray diffractometer set up in Bragg-Brentano configuration using monochromatic Kα radiation. A 1.5 kW Cu anode filament was operated at 40 kV and 30 mA. Data were collected using a scintillation point detector with a scan range between 2° and 70° 2θ, a step size of 0.02° 2θ and a scan time of 1 s per step and the XRD spectra were interpreted using EVA v.18.0.0.0. software.
2.4.2 Scanning electron microscopy (SEM) and electron microprobe analysis (EPMA)
For mineral identification, about 1 g of selected samples was embedded in a mixture of carbon powder, epoxy resin and hardener to obtain 30 mm polished sample mounts. These were then carbon coated to about a 25 nm thickness. Scanning electron microscope (SEM-EDS; TESCAN VEGA3) equipped with a secondary- and backscattered electron (BSE) detector and an X-ray energy dispersive detector (EDS) was used to examine the mineralogy of Co and other metal(loid)s. The instrument used an accelerating voltage of 20 keV, a beam current of 17 nA and a working distance of 15–20 mm. For imaging, software: VegaTC x 64 (v. 4.2.26.0) was used while chemical analysis was aided by the Oxford EDS system (XMax 80 mm EDS), Aztec software (version 3.3 SP1).
To determine the elemental composition of various mineral grains on a micro scale, quantitative analysis was carried out using a JOEL JXA-8200 equipped with one energy (EDS) and four wavelength (WDS) dispersive spectrometers. Wavelength dispersive X-ray spectrometry (WDS) analyses were performed at an accelerating voltage of 15 kV, a beam current of 20 nA and a probe diameter of 5 μm; the same conditions have been used by Hiller et al. (2016) and Sracek et al. (2010a). BSE images of the sample surface were used to select suitable crystal grains and point analysis was performed avoiding grain boundaries, and inclusions. Prior to the analysis, the equipment was calibrated. To account for the matrix effects on the samples, the ZAF correction routine was used (Carpenter and Vicenzi, 2017).
3 Results
3.1 Geochemistry
3.1.1 Paste pH
Paste pH values for Cobalt and Cornwall are given in Tables 1, 2, respectively. The Nipissing (Cobalt) tailings had paste pH values ranging between 7.50 and 7.84, suggesting that they have relatively low acid-generating capacity (Dumaresq, 1993). At the Cornwall sites, the paste pH values were lower and ranged between 3.72 and 5.77. These low values may be due to the presence of sulfide phases such as pyrite (detected by XRD and SEM) that have high acid-generating potential.
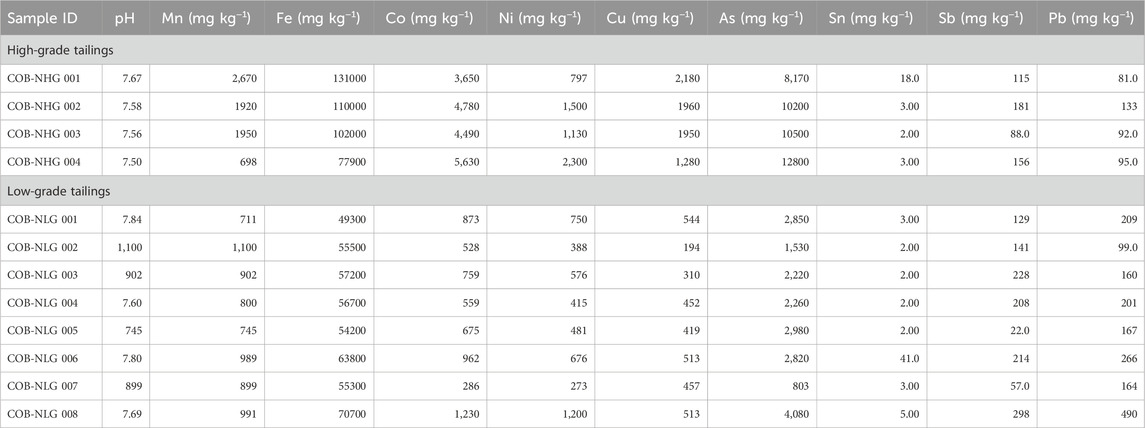
Table 1. Paste pH values and metal(loid) concentrations of high- and low-grade tailings from Cobalt, Canada.
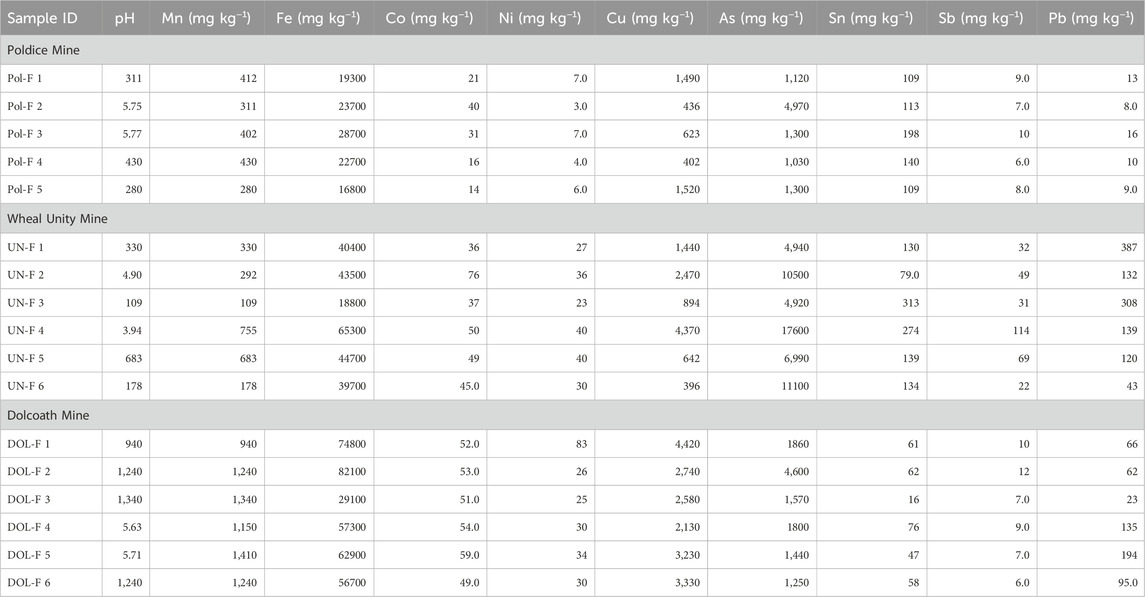
Table 2. Paste pH values and metal(loid) concentrations of mine wastes from Cornwall, United Kingdom.
3.1.2 Metal(loid) concentrations
Total concentrations of metal(loid)s in the tailings and mine-affected soils for Cobalt and Cornwall, respectively, are given in Tables 1, 2, respectively. To further understand the geochemistry of Co and associated metal(loid)s in the mine waste, a correlation analysis was conducted with these data using Pearson’s correlation coefficients (Cobalt: Supplementary Table 1; Cornwall; Supplementary Table 2).
The Nipissing tailings contain relatively high concentrations of Co, between two and three orders of magnitude higher in the high-grade (3,650–5,630 mg kg−1) than in the low-grade tailings (286–1,230 mg kg−1; Table 1). The concentrations of Ni (793–2,300 and 273–1,200 mg kg−1, respectively) followed a similar trend. Concentrations of As were also higher in the high-grade tailings (8,170–12800 mg kg−1) compared to those in the low grade tailings (803–4,080 mg kg−1) by four and three orders of magnitude, respectively. These results were expected because at Cobalt, Ag and Co were the primary commodities that were extracted (Sprague and Vermaire, 2018). Moreover, As was mineralogically associated with several Ag and Co ores from Cobalt and was principally present as Co-Ni arsenides, arsenates, and sulfarsenides (Boyle, 1968). As such, As was not only naturally elevated in the host rock but was also enriched from milling processes and subsequent oxidation of the mine waste (Dumaresq, 1993; Kwong et al., 2007). Percival et al. (2007) also recorded high concentrations of Co (5,400 mg kg−1) and Ni (up to 2,400 mg kg−1) in the Nipissing tailings.
Correlation analysis of Co in the high-grade and low-grade tailings revealed significant positive linear correlations with Fe (0.64, low-grade tailings only), As (0.98, 0.92) and Ni (0.97, 0.95) (Supplementary Table 1). These relationships suggest a common source of the metal(loid)s with a strong mineralogical association (Percival et al., 2007). These relationships were expected because in the Cobalt mining district, Co grades were associated with Co-Fe-Ni, Ni-Co-As and Co-As mineral assemblages (Petruk, 1971; Potter, 2009).
For the samples taken from Cornwall, the concentrations of Co did not vary significantly across the three sites. The mine waste at Dolcoath mine had the highest concentration of Co (average 53 mg kg−1), whereas tailings at Poldice mine had the lowest Co (average 25 mg kg−1). Cobalt had a positive linear correlation with As (0.81) and Fe (0.64) at Poldice mine (Supplementary Table 2). This correlation can be attributed to Co incorporation in minerals such as arsenopyrite (FeAsS) (Meyer et al., 2019). Cobalt also had a positive and weak correlation with Ni (0.58) at the Wheal Unity mine site (Supplementary Table 2), which may reflect Ni incorporation in pyrite and iron oxyhydroxides (Lottermoser et al., 2011). These potential associations are explored in Section 3.3. No significant correlations were calculated between Co and the other metals for the Dolcoath mine wastes.
3.1.3 Sequential extractions
In the BCR-sequential extraction scheme used in this study, the mobility and hence possible bioavailability of metal(loid)s decreased in the order of extracted fractions from exchangeable to residual. The concentrations and extracted percentages of Co and other metal(loid)s are summarized in Supplementary Tables 3, 4 for Cobalt and Cornwall, respectively, and the sequential extraction concentrations normalized to total concentrations are shown in Figure 3.
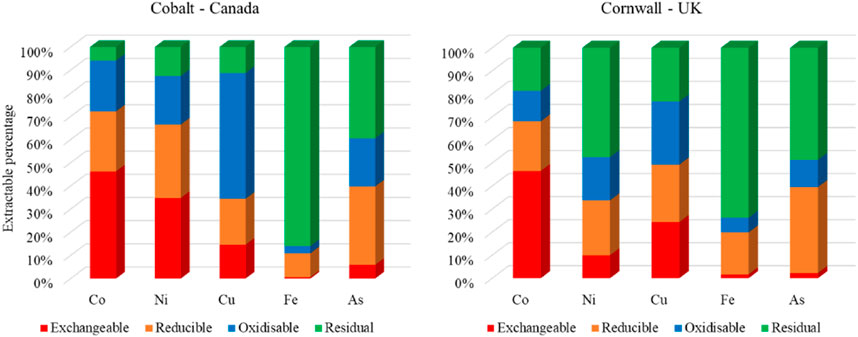
Figure 3. BCR sequential extraction results for Co, Ni, Cu, Fe and As for Cobalt and Cornwall, normalised to total concentrations of these metal(loid)s.
The exchangeable fraction contains metal(loid)s which are carbonate bound, found in their ionic form, or are exchangeable (Anju and Banerjee, 2010). Metal(loid)s extracted in this stage are more susceptible to changes in pH (Tessier et al., 1979), and therefore considered to be more mobile as they are soluble and likely to be bioavailable. Metal(loid)s that fall in this category are potentially ecotoxic, especially if present in significant concentrations (Matong et al., 2016). The average exchangeable fractions of Co, Ni, Fe, Cu and As accounted for 46%, 35%, 1%, 15%, and 6% of the total metal(loid)s content in the samples at Cobalt, respectively. Analysis of the samples from Cornwall determined that Co, Ni, Fe, Cu and As accounted for 46%, 10%, 2%, 24%, and 2% of the total metal(loid)s content in the samples, respectively.
The reducible fraction is typically ascribed to those bound to iron and manganese oxyhydroxides. According to Pickering (1986), iron and manganese oxyhydroxides exist as nodules, concretions, cement between particles, and in certain cases as coatings on particles. They are also efficient scavengers for metal(loid)s and are thermodynamically unstable under anoxic conditions (Tessier et al., 1979; Anju and Banerjee, 2010). As such, the metal(loid)s extracted here are considered to be less mobile than those extracted in the exchangeable fraction. At Cobalt, As accounted for the highest extracted fraction (34%) of the reducible fraction, followed by Ni (32%), Co (26%), Cu (20%) and Fe (10%). At the Cornwall sites, the average extracted fraction proportions were As (37%), Cu (25%), Ni (24%), Co (22%), and Fe (18%) (Figure 3).
Oxidisable metal(loid)s are typically those which are bound to sulfides and organic material. They can be released into the environment under oxidising conditions (Tessier et al., 1979; Anju and Banerjee, 2010). Metal(loid)s bound to this fraction are generally less mobile than those bound in the reducible fraction. In the Cobalt tailings Co, Ni, Fe, Cu and As were present in the following average respective proportions: 22%, 21%, 3%, 54%, and 21%. At the Cornwall sites, the average proportions of metal(loid)s were Co (13%), Ni (19%), Fe (6%), Cu (27%), and As (12%).
The metal(loid)s found in the residual fraction are strongly bound to and within the crystalline structure of the minerals present, and are therefore not typically labile (Anju and Banerjee, 2010; Rauret et al., 1999) and are unlikely to be released over a reasonable time span under known natural conditions (Tessier et al., 1979). As such they are unlikely to present a risk to humans or the environment. At Cobalt the most prominent deportment within this category included: Fe (86%), As (39%), Ni (13%), Cu (11%), and Co (6%). A similar fractionation trend was observed in the samples from Cornwall where the proportions were Fe (74%), As (49%), Ni (47%), Cu (23%), and Co (19%).
3.2 Risk assessment code (RAC)
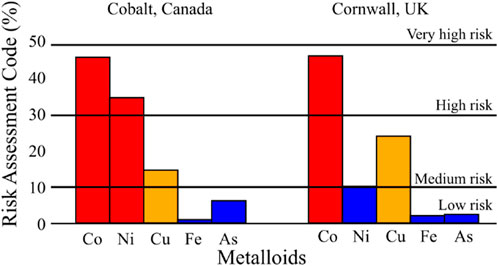
The proportion of Co in the exchangeable fraction of the Nipissing tailings varied from 28% to 58% (Figure 3; Supplementary Table 3). These proportions indicate medium, high, and very high risk in various samples (Table 3). The RAC site average for Ni was 35%. Although this places Ni in the high-risk category, some samples indicated medium risk. Thus, Co and Ni are in the medium to high-risk category in the aquatic and non-aquatic environments and likely to affect humans via the food chain. The RAC values for Cu ranged between 9.10% and 17.8% which classifies it as a medium risk metal(loid) in the Nipissing tailings. The average RAC values of As (5.9%) and Fe (0.58%) classified these metal(loid)s as low risk and no risk to the environment (Figure 4).
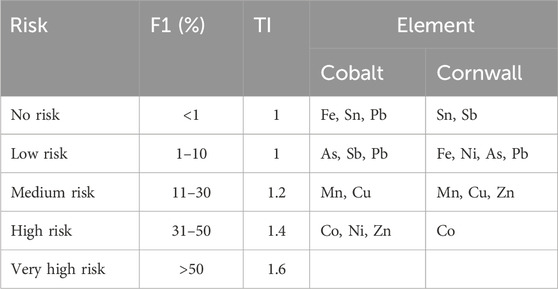
Table 3. Risk Assessment Code and toxicity index (TI) values for the mine waste samples taken from Cobalt (Canada) and Cornwall (United Kingdom).
In the Cornwall samples Co had RAC values ranging from 30% to 62% (Figure 4) which indicates high risk and very high risk in various samples across the sites, and by average (46%) as a high risk metal(loid) (Table 3). Although on average Cu (28%) was placed in the medium risk category, some samples were classified as low risk. The average RAC values for Ni were 9.9% which placed it in the low risk category. However, Ni was classified as medium risk in some samples. The site averages for Fe (1.7%) and As (2.2%) classified the metal(loid)s as low risk, and no risk to the environment in some samples.
3.3 Mineralogy
3.3.1 Bulk mineralogy
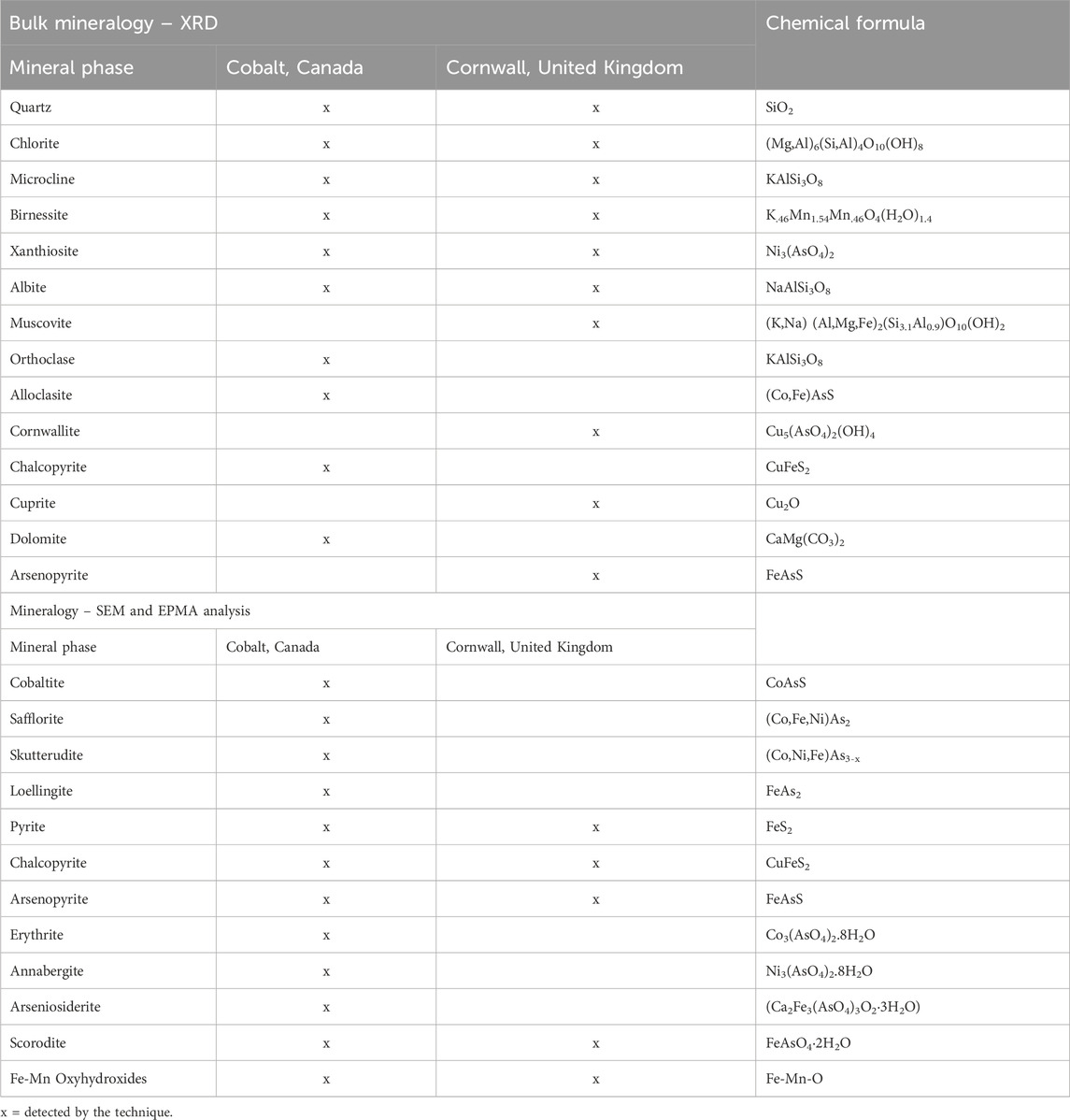
Typical X-ray diffraction patterns for Cobalt and Cornwall are displayed in Supplementary Figure 1. The mineralogy of the high- and low-grade tailings at Cobalt comprised predominantly quartz, chlorite and albite, with lesser amounts of orthoclase, microcline, and dolomite (Table 4). Ore minerals identified in the high-grade tailings include xanthiosite and alloclasite, while birnessite and chalcopyrite occurred in the low-grade tailings.
Quartz and muscovite were abundant in the Cornwall samples, with minor birnessite, fluorite and microcline across all sample sites. These results are similar to those obtained by Tang et al. (2021). Generally, the bulk mineralogy of samples across the three sites in Cornwall was consistent, likely due to the similarities in mineralisation in the region (Dines, 1956).
3.3.2 SEM and EPMA
3.3.2.1 Mineralogy of the samples from Cobalt, Canada
Co-bearing minerals were identified as both primary (sulfarsenides, arsenides) and secondary (arsenate) minerals within the samples taken from Cobalt. The sulfarsenides cobaltite-gersdorffite, containing up to about 32 wt% Co, and arsenides safflorite-skutterudite, containing up to about 13.7 wt% Co), were observed mainly in the high-grade samples. Cobaltite occurred as euhedral to anhedral overgrowths on chlorite and other silicates such as albite (Figure 5), whereas safflorite-skutterudite occurred as fine grained (typically ∼20 µm), subhedral-anhedral phases inter-grown with sulfarsenides, with little to no secondary alteration.

Figure 5. SEM-BSE image composite illustrating the mineralogy of the Cobalt tailings. (A) euhedral-anhedral cobaltite overgrowths on chlorite and albite, (B) fine grained safflorite-skutterudite, (C) euhedral-subhedral loellingite, (D) secondary minerals erythrite and arseniosiderite.
Arsenopyrite (∼4.4 wt% Co), and loellingite (∼9.9 wt% Co) were observed in all samples from the Nipissing tailings. The former was observed as a subhedral to anhedral phase associated with chlorite phases whereas the latter occurred as a euhedral-subhedral arsenide associated with various phases of arsenopyrite, arseniosiderite, chalcopyrite and silicate minerals.
Erythrite-annabergite minerals containing about 20.8 wt% Co were the most widespread Co-bearing phases across the Nipissing site. In several samples, erythrite-annabergite occurred as elongated, euhedral, prismatic, needle-like phases of secondary rims growing on common rock forming minerals including silicates and carbonates. Across the sites, erythrite was also observed as a replacement of the primary arsenides and sulfarsenides.
Other Co-bearing minerals detected within the samples from Cobalt included scorodite (8.3 wt% Co), observed as associated with pyrite on a chlorite-albite matrix dotted with the needle-like erythrite, arseniosiderite (5.2 wt% Co), observed as massive, leaf-like aggregates with radial growth textures, and as replacement products of arsenopyrite and scorodite, and iron oxides (1.6 wt% Co), typically observed as subhedral, fine grained stains on silicate cement.
3.3.2.2 Mineralogy of the samples from Cornwall, UK
Pyrite, chalcopyrite and arsenopyrite were the most important Co-bearing primary minerals observed in the samples from the Cornwall sites (Figure 6). Pyrite (1 wt% Co) was observed typically in samples from the Wheal Unity mine as euhedral, medium to coarse grained phases with scorodite overgrowths (Figure 6A). Chalcopyrite (∼0.5 wt% Co) was observed in several samples as both euhedral and subhedral (Figure 6B), medium grained phases associated with silicate minerals and arsenopyrite. Arsenopyrite (0.01–1.05 wt% Co), was observed as euhedral and anhedral phases, and in a few samples with visible alteration to scorodite (Figure 6C).
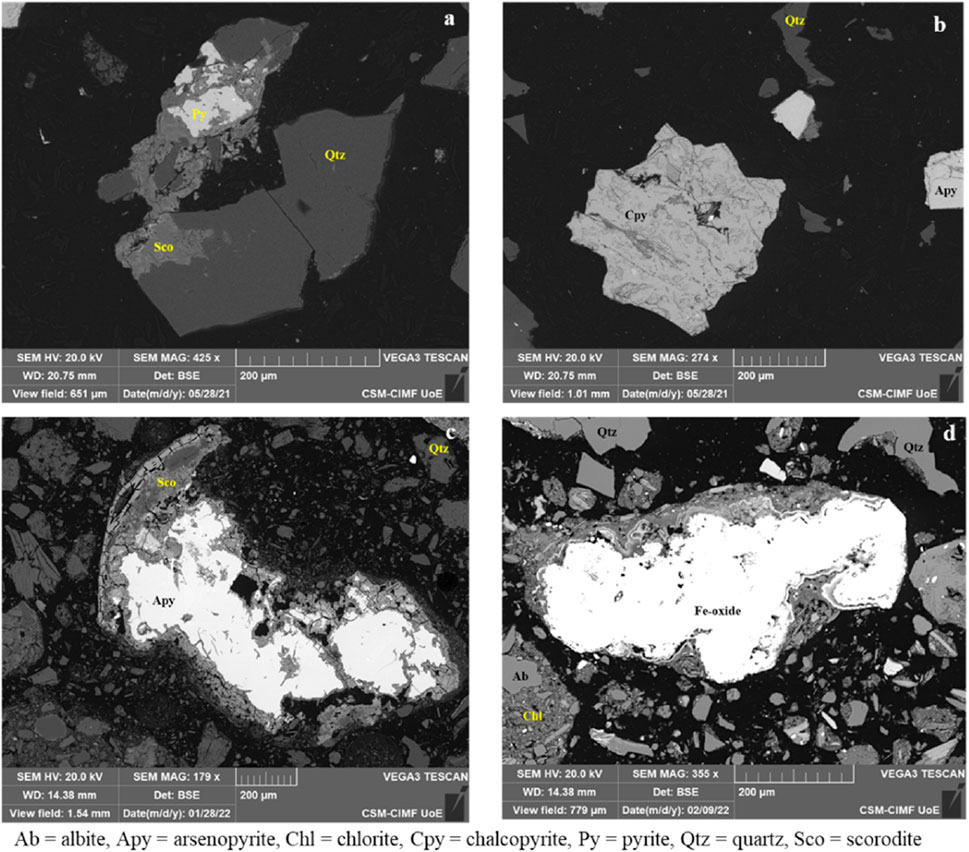
Figure 6. SEM-BSE images illustrating the mineralogy of the Cornwall mine wastes. (A) medium-coarse grained euhedral pyrite, (B) medium grained subhedral chalcopyrite, (C) arsenopyrite altering to scorodite, (D) anhedral Fe oxide phases showing visible alteration.
Scorodite and Fe-Mn oxides were observed in all Cornwall samples. Scorodite (up to ∼1.7 wt% Co) was observed as an overgrowth texture overgrowing on pyrite and chalcopyrite phases. Iron oxides (0.7–3.1 wt% Co) were observed as euhedral and anhedral phases showing visible alteration (Figure 6D), and in certain cases, pitted and overgrown on silicates. The other phase had concentric rings/outer bands around an inner core with a homogenous fabric. In some samples, Mn-oxides (0.9 wt% Co) were observed as precipitates/coatings on the silicates.
4 Discussion
4.1 Bulk Co mineralogy
Mineralogical characterization of the tailings from Cobalt, Canada showed that Co was present in primary minerals such as cobaltite, safflorite, skutterudite, loellingite, pyrite, chalcopyrite and arsenopyrite, and secondary minerals such as erythrite, annabergite, scorodite and arseniosiderite. In the mine waste samples collected in Cornwall, Co was mainly present as a trace element incorporated in both primary (pyrite, chalcopyrite, and arsenopyrite) and secondary minerals (scorodite and the iron oxyhydroxides). Trace amounts of Co (in concentrations below the c. 0.01 wt% detection limit of the SEM/EDS) could also have occurred in other minerals. The distinction in the Co mineralogy of the two sites can be attributed to differences in ore deposit geology and processing techniques. In Cobalt, Canada, the ore was derived from the five-element (Ag-Bi-Co-Ni-As) vein deposits where Ag was typically the principal commodity of interest. Throughout its history the ore was processed by a number of processing techniques including gravity concentration, flotation, cyanidation and Hg-amalgamation (Reid et al., 1922). In Cornwall, the ore was mainly derived from bulk mineable stockworks or sheeted vein systems rich in Sn, Cu, W and As (Dines, 1956; Moon, 2010). The metals were recovered by calcination which involved roasting the ore, followed by sublimation and condensation in long flues containing collection chambers (Turner, 1986; Camm et al., 2003).
4.2 Bulk geochemistry
When comparing the three sites from Cornwall, Dolcoath mine site had the highest concentration of Co, followed by Wheal Unity and then Poldice mine. The Dolcoath concentration (53 mg kg−1) exceeds the soil quality guideline set by the Ontario Ministry of the Environment (Ontario Ministry of the Environment, 2011), the Canadian Council of Ministers of the Environment (CCME, 2010) and the United States Environmental Protection Agency (USEPA, 2011) for residential purposes. At the Poldice mine site, the concentration of Co is lower than the CCME (2010) soil quality guideline. When comparing the Cobalt sites to those in Cornwall, the concentration of Co at the former is 900-fold and 13-fold higher than the latter in the high-grade and low-grade Nipissing tailings, respectively. This concentration also exceeds the soil quality guidelines shown in Table 5, suggesting that Co is likely to pose greater environmental concerns at Cobalt than at the Cornwall sites. Looking from a global point of view, Co concentrations from the three Cornwall sites are 2-3 orders of magnitude lower than the other global sites shown in Table 5. However, the Co content in the tailings from Cobalt (especially the high-grade) is many orders of magnitude higher than at the other sites.
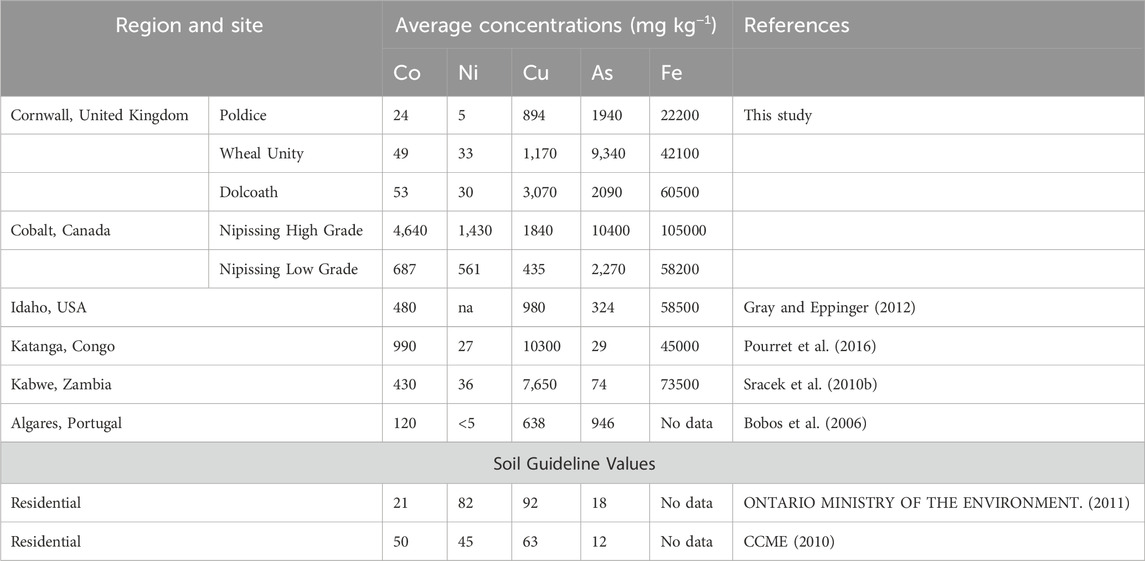
Table 5. Average Co, Ni, Ni, As, and Fe mine waste concentrations reported in this study compared to those in other areas, and to soil guideline values. na: not analysed.
4.3 Geochemical controls on Co mineralogy
Primary Co (Ni-Fe-As) minerals can undergo oxidation and dissolution to liberate Co, and associated metal(loid)s, into the near-surface environment (Ziwa et al., 2020). Conversely, the precipitation of secondary Co-bearing phases removes Co and the associated metal(loid)s from solution. Understanding these dissolution-precipitation reactions, the Co phases present, and the geochemical conditions under which they remain stable is key to sustainable mine waste management.
Primary Co-bearing phases were either absent or only detected in low concentrations in all mine waste samples analysed. This absence suggests that the Co present as secondary minerals, with some evidence for this in the form of Co-Ni arsenate phases, including erythrite-annabergite (often forming precipitation rims on silicates and carbonates), particularly in the Cobalt samples. The dominance of secondary minerals provides clear evidence of the dissolution, mobilization, precipitation and retardation of Co, Ni and As within the mine waste.
In low pH conditions, such as those recorded at Cornwall (pH < 5), Co-bearing sulfarsenides and arsenides such as cobaltite and safflorite will likely undergo oxidative dissolution, and release Co2+ and As oxyanions (AsO4−) into aquatic environments. The presence of carbonates can cause an increase in the pH of environmental waters to near neutral (such as pH ∼ 7.6 at Cobalt), which could result in dissolved Co interacting with the soluble As oxyanions to form secondary arsenate phases, including erythrite (Boyle and Dass, 1971). Following this, cobaltite is likely to be susceptible to oxidation, either by oxygen (Equation 1), Fe(III) (Equation 2) and nitrate (Equation 3) (Nordstrom and Southam, 1997; Ehrlich et al., 2015). The generation of Fe(III) is associated with microbial activity, and Fe(III) can oxidise sulfide minerals such as cobaltite much faster than oxygen (Nordstrom and Southam, 1997).
The Co2+ and AsO43- generated by the cobaltite oxidation can react with water and proton to produce erythrite (Equation 3) (Boyle and Dass, 1971).
Under prolonged oxidation, the arsenate-bearing erythrite and annabergite are suggested to be unstable (Boyle and Dass, 1971), and if such oxidative dissolution occurs then Co and related metal(loid)s would be further remobilised. SEM data show that the erythrite-annabergite phase was predominantly euhedral, and with no obvious dissolution textures. This euhedral, prismatic, needle-like crystallinity suggests that this phase was stable within the near surface oxidizing environment, particularly within the high-grade tailings damp at the time of sampling.
Other Co-hosting phases, including arsenopyrite, scorodite and arseniosiderite, have been identified. Scorodite is the most common arsenate formed from the weathering of As-bearing sulfide ores such as arsenopyrite (Palache et al., 1951) whereas arseniosiderite occurs as secondary alteration products related to the oxidation of arsenopyrite in mine wastes (Paktunc et al., 2015). These arsenate minerals are stable in the pH ranges exhibited by the Cobalt samples (Supplementary Table 1), suggesting that the Co they contain may also be relatively immobile unless redox conditions change.
4.4 Co speciation and environmental implications
Sequential extraction data show that Co is associated with the most labile “exchangeable” phase in samples taken from both Cobalt and Cornwall. This “exchangeable” association is likely due to its enrichment in the secondary mineral erythrite, as well as its incorporation into arseniosiderite, scorodite, and possibly carbonate phases. This similarity provides some evidence, despite the differences in origins (both geological and mineral processing techniques to produce the mine waste materials), that Co may have a relatively predictable behaviour in mine waste. Further study is required to explore this subject in more detail.
From an aquatic environmental standpoint, slight change in the pH could trigger the release of Co and other such bound metal(loid)s into solution thereby rendering them mobile and bioavailable. For example, if the pH conditions of the mine wastes became acidic (Figure 7), the Co bound with erythrite would likely solubilise and become mobile in the environment. At the Cobalt site, the paste pH (7.5–7.84) of the Nipissing tailings falls within the stability field of erythrite which would limit Co mobility as it is locked in the mineral crystal lattice. However, at the three sites in Cornwall, the pH range of 3.72–5.77 suggests that erythrite will undergo dissolution and release Co, As (and Ni) into the environment.

Figure 7. Eh-pH diagram for the Co-As-H2O system at 25°C (α∑As = 10−3, α∑Co = 10−3) highlighting the stability field (purple) for erythrite. Modified from Charykova and Krivovichev (2020), with permission of Springer Nature. Vertical blue and green strips: measured paste pH range for the Cobalt and Cornwall sites, respectively.
Co was also measured as strongly associated with the ‘reducible’ fraction within both the samples taken from Cobalt and Cornwall, which indicates an association with Fe-Mn oxyhydroxide phases–possibly by adsorption onto the mineral surfaces. XRD and SEM analyses of this study have confirmed the presence of birnessite and possibly goethite in samples from both sites. These phases are also excellent scavengers for metal(loid)s and are thermodynamically unstable and may release the metal(loid)s under reducing conditions (Tessier et al., 1979; Pickering, 1986; Anju and Banerjee, 2010). A smaller proportion of Co was also recorded as associated with the ‘oxidizable’ phase, which suggest some binding with sulfide phases such as pyrite and chalcopyrite. Under oxidising conditions, the Co within such phases may be liberated into the environment. There was also some association of Co with the ‘residual’ phase, which is considered largely immobile in the environment. Therefore, it is likely that only a low concentration of Co is locked in the crystal structure of primary rock forming silicate minerals such as chlorite.
5 Conclusion
There is currently a lack of robust geochemical and mineralogical data for Co in mining-affected environments. These data are useful to support both environmental protection from existing mine wastes but also to inform the development of future responsible mining approaches and regulations. This study has therefore been established to characterise the geochemistry and mineralogy of Co at two representative locations of contrasting geology and mineral processing history: Cobalt, Canada, and Cornwall, United Kingdom.
The BCR sequential extraction procedure used in this study has proven a useful technique for indirectly assessing the potential mobility and bioavailability of Co and associated metal(loid)s in the mine wastes studied. Results from the mine wastes taken from Cobalt show that up to 58% of Co was extracted in the exchangeable fraction, corresponding to the ‘mobile’ fraction. The order of deportment was exchangeable > reducible > oxidisable > residual. Similarly, up to approximately 46% of Co was recorded as associated with the exchangeable fraction for the samples taken from Cornwall. The order of deportment was exchangeable > reducible ≥ residual > oxidisable.
Mineralogical analysis indicates that erythrite is the most important secondary Co-bearing mineral, which was recorded as widespread in the Nipissing tailings. Other secondary minerals in which Co was found included arseniosiderite, scorodite, and Fe oxyhydroxides. Primary Co-bearing minerals identified included cobaltite and safflorite-skutterudite. Cobalt was also incorporated in other primary minerals including arsenopyrite, loellingite, pyrite and chalcopyrite. No Co-bearing primary and secondary minerals were recorded in any of the Cornwall samples. Cobalt was instead incorporated in other primary (arsenopyrite, pyrite, and chalcopyrite) and secondary minerals (scorodite, and Fe-Mn oxyhydroxides). Overall the results demonstrate, that despite, their contrasting geology, mineral processing history and potential differences in Co mineralogical deportment between the two sampling locations, Co exhibited a relatively consistently high potential to leach into the aquatic environment. As a result, Co may have a relatively predictable behaviour across different locations worldwide. Further study is required to further investigate this phenomenon using different Co-bearing geological materials and processing wastes.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
GZ: Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing–original draft, Funding acquisition. RC: Methodology, Project administration, Supervision, Validation, Writing–review and editing, Investigation, Resources. KH-E: Methodology, Project administration, Supervision, Validation, Writing–review and editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The presented work was funded by the UK Department for International Development through PhD funding to GZ through the Commonwealth Scholarship Commission of the United Kingdom (CSC Reference Number ZMCS-2018-855).
Acknowledgments
We acknowledge Gavyn Rollinson, Malcolm Spence, Sharon Uren and Jens Andersen for technical support and Heather Jamieson for guidance and assistance during the sampling at Cobalt, Ontario. We thank Agnico-Eagle for permission to sample the tailings at Cobalt. We are grateful for the comments of the two reviewers as these greatly improved the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgeoc.2025.1543695/full#supplementary-material
References
AMERICAN SOCIETY FOR TESTING AND MATERIALS (2013). Standard test method for pH of soils. West Conshohocken, PA, USA.
Anju, M., and Banerjee, D. K. (2010). Comparison of two sequential extraction procedures for heavy metal partitioning in mine tailings. Chemosphere 78, 1393–1402. doi:10.1016/j.chemosphere.2009.12.064
Beane, S. J., Comber, S. D., Rieuwerts, J., and Long, P. (2016). Abandoned metal mines and their impact on receiving waters: a case study from Southwest England. Chemosphere 153, 294–306. doi:10.1016/j.chemosphere.2016.03.022
Bobos, I., Durães, N., and Noronha, F. (2006). Mineralogy and geochemistry of mill tailings impoundments from Algares (Aljustrel), Portugal: implications for acid sulfate mine waters formation. J. Geochem. Explor. 88, 1–5. doi:10.1016/j.gexplo.2005.08.004
Boyle, R. W. (1968). The geochemistry of silver and its deposits, with notes on geochemical prospecting for the element. Bull. Geol. Surv. Can. 160. doi:10.4095/101471
Boyle, R. W., and Dass, A. S. (1971). The geochemistry of the supergene processes in the native silver veins of the Cobalt-South Lorrain area, Ontario. Can. Mineralogist 11, 358–390.
Camm, G. S., Butcher, A. R., Pirrie, D., Hughes, P. K., and Glass, H. J. (2003). Secondary mineral phases associated with a historic arsenic calciner identified using automated scanning electron microscopy; a pilot study from Cornwall, UK. Miner. Eng. 16, 1269–1277. doi:10.1016/j.mineng.2003.07.005
Carpenter, P. K., and Vicenzi, E. P. (2017). Use of mineral reference standards in EPMA: instrumental calibration, standards comparison, and quality control. Microsc. Microanal. 23, 496–497. doi:10.1017/s1431927617003166
CCME (2010). Canadian environmental quality guidelines summary table. Available at: http://st-ts.ccme.ca.
Charykova, M. V., and Krivovichev, V. G. (2020). “Mineral systems and thermodynamic stability of arsenic minerals in the environment,” in Processes and phenomena on the boundary between biogenic and abiogenic nature. Editors O. Frank-Kamenetskaya, O. Vlasov, D. Panova, and S. Lessovaia Cham: Springer. doi:10.1007/978-3-030-21614-6_15
Collins, R. N., and Kinsela, A. S. (2010). The aqueous phase speciation and chemistry of cobalt in terrestrial environments. Chemosphere 79, 763–771. doi:10.1016/j.chemosphere.2010.03.003
Davidson, C. M., Duncan, A. L., Littlejohn, D., Ure, A. M., and Garden, L. M. (1998). A critical evaluation of the three-stage BCR sequential extraction procedure to assess the potential mobility and toxicity of heavy metals in industrially-contaminated land. Anal. Chim. Acta 363, 45–55. doi:10.1016/s0003-2670(98)00057-9
Dines, H. G. (1956). The metalliferous mining region of south-west england. London: Economic Memoir of the Geological Survey of Great Britain.
Dold, B. (2003). Speciation of the most soluble phases in a sequential extraction procedure adapted for geochemical studies of copper sulfide mine waste. J. Geochem. Explor. 80, 55–68. doi:10.1016/s0375-6742(03)00182-1
Dold, B. (2010). Basic concepts in environmental geochemistry of sulfidic mine-waste management. Waste Manag. 24, 173–198. doi:10.5772/8458
Dumaresq, C. G. (1993). The occurrence of arsenic and heavy metal contamination from natural and anthropogenic sources in the cobalt area of Ontario. Ottawa, Ontario: Carelton University. MSc thesis.
Dumaresq, C. G. (2009). The rise and fall of a mining camp. Cobalt mining legacy. Available at: https://www.cobaltmininglegacy.ca/mininghistory.php (Accessed November 11, 2022).
Ehrlich, H. L., Newman, D. K., and Kappler, A. E. (2015). Ehrlich's geomicrobiology. 6th Edition. Boca Raton: CRC Press.
EUROPEAN COMMISSION (EC) (2020). Communication from the commission to the European parliament, the Council, the European economic and social committee and the committee of the regions: critical Raw materials resilience: charting a path towards greater security and sustainability.
Fawcett, S. E., Jamieson, H. E., Nordstrom, D. K., and Mccleskey, R. B. (2015). Arsenic and antimony geochemistry of mine wastes, associated waters and sediments at the Giant Mine, Yellowknife, Northwest Territories, Canada. Appl. Geochem. 62, 3–17. doi:10.1016/j.apgeochem.2014.12.012
Filippi, M., Drahota, P., Machovič, V., Böhmová, V., and Mihaljevič, M. (2015). Arsenic mineralogy and mobility in the arsenic-rich historical mine waste dump. Sci. Total Environ. 536, 713–728. doi:10.1016/j.scitotenv.2015.07.113
Garbe-Schönberg, C. D. (1993). Simultaneous determination of thirty-seven trace elements in twenty-eight international rock standards by ICP-MS. Geostand. Newsl. 17, 81–97. doi:10.1111/j.1751-908x.1993.tb00122.x
Gifford, S. (2020). Lithium, cobalt and nickel: the gold rush of the 21st century. Available at: https://www.faraday.ac.uk/wp-content/uploads/2022/09/Faraday_Insights_6_Updated_Sept2022_FINAL.pdf.
Gray, J. E., and Eppinger, R. G. (2012). Distribution of Cu, Co, As, and Fe in mine waste, sediment, soil, and water in and around mineral deposits and mines of the Idaho Cobalt Belt, USA. Appl. Geochem. 27, 1053–1062. doi:10.1016/j.apgeochem.2012.02.001
Hiller, E., Tóth, R., Kučerová, G., Jurkovič, Ľ., Šottník, P., Lalinská-Voleková, B., et al. (2016). Geochemistry of mine tailings from processing of siderite–Cu ores and mobility of selected metals and metalloids evaluated by a pot leaching experiment at the Slovinky impoundment, Eastern Slovakia. Mine Water Environ. 35, 447–461. doi:10.1007/s10230-016-0388-2
Hudson-Edwards, K. A., and Dold, B. (2015). Mine waste characterization, management and remediation. Minerals 5, 82–85. doi:10.3390/min5010082
Hudson-Edwards, K. A., Jamieson, H. E., and Lottermoser, B. G. (2011). Mine wastes: past, present, future. Elements 7, 375–380. doi:10.2113/gselements.7.6.375
Hudson-Edwards, K. A., Schell, C., and Macklin, M. G. (1999). Mineralogy and geochemistry of alluvium contaminated by metal mining in the Rio Tinto area, southwest Spain. Appl. Geochem. 14, 1015–1030. doi:10.1016/s0883-2927(99)00008-6
Iavazzo, P., Adamo, P., Boni, M., Hillier, S., and Zampella, M. (2012). Mineralogy and chemical forms of lead and zinc in abandoned mine wastes and soils: an example from Morocco. J. Geochem. Explor. 113, 56–67. doi:10.1016/j.gexplo.2011.06.001
Jamieson, H. E. (2011). Geochemistry and mineralogy of solid mine waste: essential knowledge for predicting environmental impact. Elements 7, 381–386. doi:10.2113/gselements.7.6.381
Jamieson, H. E., Walker, S. R., and Parsons, M. B. (2015). Mineralogical characterization of mine waste. Appl. Geochem. 57, 85–105. doi:10.1016/j.apgeochem.2014.12.014
Kříbek, B., Mihaljevič, M., Sracek, O., Knésl, I., Ettler, V., and Nyambe, I. (2011). The extent of arsenic and of metal uptake by aboveground tissues of Pteris vittata and Cyperus involucratus growing in copper-and cobalt-rich tailings of the Zambian Copperbelt. Archives Environ. Contam. Toxicol. 61, 228–242. doi:10.1007/s00244-010-9604-4
Kwong, Y. T. J., Beauchemin, S., Hossain, M. F., and Gould, W. D. (2007). Transformation and mobilization of arsenic in the historic Cobalt mining camp, Ontario, Canada. J. Geochem. Explor. 92, 133–150. doi:10.1016/j.gexplo.2006.08.002
Lapakko, K. (2002). Metal mine rock and waste characterization tools: an overview. Min. Minerals Sustain. Dev. 67, 1–30. Available at: https://www.iied.org/g00559
Lottermoser, B. G., Glass, H. J., and Page, C. N. (2011). Sustainable natural remediation of abandoned tailings by metal-excluding heather (Calluna vulgaris) and gorse (Ulex europaeus), Carnon Valley, Cornwall, UK. Ecol. Eng. 37, 1249–1253. doi:10.1016/j.ecoleng.2011.03.002
Martin, J., Nirel, P., and Thomas, A. (1987). Sequential extraction techniques: promises and problems. Mar. Chem. 22, 313–341. doi:10.1016/0304-4203(87)90017-x
Matong, J. M., Nyaba, L., and Nomngongo, P. N. (2016). Fractionation of trace elements in agricultural soils using ultrasound assisted sequential extraction prior to inductively coupled plasma mass spectrometric determination. Chemosphere 154, 249–257. doi:10.1016/j.chemosphere.2016.03.123
Mees, F., Masalehdani, M. N. N., DE Putter, T., D’Hollander, C., VAN Biezen, E., Mujinya, B. B., et al. (2013). Concentrations and forms of heavy metals around two ore processing sites in Katanga, Democratic Republic of Congo. J. Afr. Earth Sci. 77, 22–30. doi:10.1016/j.jafrearsci.2012.09.008
Meyer, N., Borg, G., and Kamradt, A. (2019). Mineralogical and geochemical aspects of vein-type ores from the carnon river mining district, Cornwall. Glasgow.
Moon, C. J. (2010). Geochemical exploration in Cornwall and devon: a review. Geochem. Explor. Environ. Anal. 10, 331–351. doi:10.1144/1467-7873/09-239
Nordstrom, D. K., and Southam, G. (1997). Geomicrobiology of sulfide mineral oxidation. Rev. mineralogy 35, 361–390. doi:10.1515/9781501509247-013
Okeme, I. C., Martin, P. G., Jones, C., Crane, R. A., Ojonimi, T. I., Ignatyev, K., et al. (2021). An advanced analytical assessment of rare earth element concentration, distribution, speciation, crystallography and solid-state chemistry in fly ash. Spectrochim. Acta Part B At. Spectrosc. 177, 105950. doi:10.1016/j.sab.2020.105950
ONTARIO MINISTRY OF THE ENVIRONMENT (2011). Soil, ground water and sediment Standards for use under Part XV. Toronto, Ontario: I of the Environmental Protection Act.
Paktunc, D., Majzlan, J., Huang, A., Thibault, Y., Johnson, M. B., and White, M. A. (2015). Synthesis, characterization, and thermodynamics of arsenates forming in the Ca-Fe (III)-As (V)-NO3 system: implications for the stability of Ca-Fe arsenates. Am. Mineralogist 100, 1803–1820. doi:10.2138/am-2015-5199
Palache, C., Berman, H., and Frondel, C. (1951). The system of mineralogy (7th ed.) vol. 2 - halides, nitrates, borates, carbonates, sulfates, phosphates, arsenates, tungstates. Molybdates, New York: John Wiley and Sons.
Percival, J. B., Kwong, Y. J., Dumaresq, C. G., and Michel, F. A. (2007). “Distribution of As, Ni and Co in tailings and surface waters in the cobalt area, Ontario,” in Mining and the environment IV conference. Sudbury (Canada).
Perin, G., Craboledda, L., Lucchese, M., Cirillo, R., Dotta, L., Zanette, M. L., et al. (1985). Heavy metal speciation in the sediments of northern Adriatic Sea. A new approach for environmental toxicity determination. Heavy Metals Environ. 2, 454–456.
Petruk, W. (1971). Mineralogical characteristics of the deposits and textures of the ore minerals. Can. Mineralogist 11, 108–139.
Pickering, W. F. (1986). Metal ion speciation—soils and sediments (a review). Ore Geol. Rev. 1, 83–146. doi:10.1016/0169-1368(86)90006-5
Pirrie, D., and Shail, R. K. (2018). Mud and metal; the impact of historical mining on the estuaries of SW England, UK. Geol. Today 34, 215–223. doi:10.1111/gto.12249
Potter, E. G. (2009). Genesis of polymetallic mineralization and the metallogeny of the paleoproterozoic cobalt embayment, northern Ontario. Ottawa, Ontario: Carleton University. PhD thesis.
Pourret, O., Lange, B., Bonhoure, J., Colinet, G., Decrée, S., Mahy, G., et al. (2016). Assessment of soil metal distribution and environmental impact of mining in Katanga (Democratic Republic of Congo). Appl. Geochem. 64, 43–55. doi:10.1016/j.apgeochem.2015.07.012
Rauret, G., López-Sánchez, J. F., Sahuquillo, A., Rubio, R., Davidson, C., Ure, A., et al. (1999). Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1, 57–61. doi:10.1039/a807854h
Reid, F. D., Denny, J. J., and Hutchison, R. H. (1922). Milling and metallurgical practice in treatment of silver ores at Cobalt.
Sipos, P., Choi, C., and May, Z. (2016). Combination of single and sequential chemical extractions to study the mobility and host phases of potentially toxic elements in airborne particulate matter. Geochemistry 76, 481–489. doi:10.1016/j.chemer.2016.08.005
Sprague, D. D., and Vermaire, J. C. (2018). Legacy arsenic pollution of lakes near Cobalt, Ontario, Canada: arsenic in lake water and sediment remains elevated nearly a century after mining activity has ceased. Water, Air, and Soil Pollut. 229, 87–16. doi:10.1007/s11270-018-3741-1
Sracek, O., Mihaljevič, M., Kříbek, B., Majer, V., and Veselovský, F. (2010a). Geochemistry and mineralogy of Cu and Co in mine tailings at the Copperbelt, Zambia. J. Afr. Earth Sci. 57, 14–30. doi:10.1016/j.jafrearsci.2009.07.008
Sracek, O., Veselovský, F., Kříbek, B., Malec, J., and Jehlička, J. (2010b). Geochemistry, mineralogy and environmental impact of precipitated efflorescent salts at the Kabwe Cu–Co chemical leaching plant in Zambia. Appl. Geochem. 25, 1815–1824. doi:10.1016/j.apgeochem.2010.09.008
Tang, J., Oelkers, E., Declercq, J., and Bowell, R. (2021). Effects of pH on arsenic mineralogy and stability in Poldice Valley, Cornwall, United Kingdom. Geochemistry 81, 125798. doi:10.1016/j.chemer.2021.125798
Tessier, A., Campbell, P. G., and Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 51, 844–851. doi:10.1021/ac50043a017
Ure, A., Quevauviller, P., Muntau, H., and Griepink, B. (1993). Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of the European communities. Int. J. Environ. Anal. Chem. 51, 135–151. doi:10.1080/03067319308027619
US DEPARTMENT OF THE INTERIOR (2022). Final list of critical minerals. Fed. Regist. 87, 10381–10382.
USEPA (2011). Cleanup regulations and standards. Available at: http://www.epa.gov/cleanup/regs.htm.
Vriens, B., Plante, B., Seigneur, N., and Jamieson, H. (2020). Mine waste rock: insights for sustainable hydrogeochemical management. Minerals 10, 728. doi:10.3390/min10090728
Weber, P. A., Hughes, J. B., Conner, L. B., Lindsay, P., and Smart, R. (2006). Short-term acid rock drainage characteristics determined by paste pH and kinetic NAG testing: cypress, prospect. New Zealand: ASMR.
Keywords: Cobalt, mine waste, critical metal, geochemistry, mineralogy, toxicity, mobility
Citation: Ziwa G, Crane R and Hudson-Edwards KA (2025) Geochemistry and mineralogy of Cobalt in mine wastes: examples from Cobalt, Canada and Cornwall, England. Front. Geochem. 3:1543695. doi: 10.3389/fgeoc.2025.1543695
Received: 11 December 2024; Accepted: 16 January 2025;
Published: 07 February 2025.
Edited by:
Liliana Lefticariu, Southern Illinois University Carbondale, United StatesReviewed by:
Aaron Goodman, Colorado School of Mines, United StatesDarrell Nordstrom, National Institute of Standards and Technology (NIST), United States
Copyright © 2025 Ziwa, Crane and Hudson-Edwards. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karen A. Hudson-Edwards, ay5odWRzb24tZWR3YXJkc0BleGV0ZXIuYWMudWs=
 Gabriel Ziwa
Gabriel Ziwa Rich Crane
Rich Crane Karen A. Hudson-Edwards
Karen A. Hudson-Edwards