
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Genet., 27 March 2025
Sec. RNA
Volume 16 - 2025 | https://doi.org/10.3389/fgene.2025.1556495
Idiopathic Pulmonary Fibrosis (IPF) is a progressive interstitial lung disease characterized by unknown etiology and limited therapeutic options. Recent studies implicate exosomal non-coding RNAs (ncRNAs) as crucial regulators in IPF. These ncRNAs, including long non-coding RNAs (lncRNAs), microRNAs (miRNAs), and circular RNAs (circRNAs), are involved in cellular processes through various mechanisms of selective packaging, intercellular communication, and signaling pathway integration. LncRNAs such as LINC00470 and PVT1 exhibit pro-fibrotic effects, while others like lnc-DC and THRIL show inhibitory roles; some, including UCA1 and MALAT1, demonstrate bidirectional regulation. In miRNAs, pro-fibrotic agents (e.g., miR-486, miR-223) contrast with inhibitory miRNAs (e.g., miR-34a, miR-126), while miR-21 and miR-155 display dual functions. Similarly, circRNAs such as circ_0000479 and circ_0026344 promote fibrosis, whereas circ_0000072 and circ_0000410 act as inhibitors, with certain circRNAs (e.g., circ_002178 and circ_0001246) exhibiting complex regulatory effects. Exosomal ncRNAs modulate key pathways, including TGF-β and Wnt/β-catenin, influencing IPF progression. Despite their potential, challenges remain in exosome isolation, functional characterization of ncRNAs, and clinical translation. Addressing these barriers through innovative research strategies is essential to leverage exosomal ncRNAs in the management and treatment of IPF. This review comprehensively examines the roles of exosomal ncRNAs in IPF, elucidates their mechanisms and pathway interactions, and discusses future perspectives to enhance understanding and therapeutic strategies for this disease.
Idiopathic Pulmonary Fibrosis (IPF) is a disease characterized by progressive interstitial lung disease. Its feature is that the lung tissue gradually becomes fibrotic, leading to respiratory dysfunction and eventually respiratory failure (Adkins and Collard, 2012; Lu and El-Hashash, 2019; Varone et al., 2018). The cause of this disease has not yet been clarified, but it is believed that a combination of genetic factors, environmental exposures, and an aberrant wound healing response contributes to the development of IPF (Figure 1). Its clinical manifestations are often similar to those of many other diseases, making early diagnosis somewhat difficult. According to statistical data, the incidence of IPF has been continuously rising worldwide, especially more pronounced among the elderly population (Gulati and Thannickal, 2019; Martinez et al., 2017; Spagnolo et al., 2015). Although in recent years we have gained a deeper understanding of the pathophysiological mechanisms of IPF, the effective treatment methods for this disease are still limited (Collins and Raghu, 2019; Kropski et al., 2013; Shumar et al., 2021). Currently, the main treatment methods include antifibrotic drugs (such as pirfenidone and nintedanib). However, these drugs can only slow down the progression of the disease to a certain extent and cannot cure or reverse the condition. In addition, lung transplantation is the last resort for IPF patients, but due to the scarcity of donor organs and surgical risks, this measure is not applicable to all patients (Balamugesh and Behera, 2007; George et al., 2019; Mason et al., 2007). Therefore, searching for new biomarkers and therapeutic targets has become one of the hotspots in current IPF research.
In recent years, exosomes, as important mediators for cell-cell interaction and information transmission, have received widespread attention (Nguyen et al., 2016). Exosomes are extracellular vesicles with a diameter of approximately 30–150 nm, capable of delivering various biomolecules in the extracellular environment, including proteins, lipids and RNA, etc., (Paul et al., 2023). The formation of exosomes involves the endogenous membrane system and is released into the extracellular space along with the physiological and pathological changes of cells, reflecting the state of the parent cells (Sharma et al., 2017). Studies have shown that exosomes play a crucial role in multiple biological processes such as tumorigenesis, immune regulation and inflammatory responses (Liu Z. et al., 2021). More importantly, non-coding RNAs (ncRNAs) in exosomes, especially microRNAs (miRNAs) and circular RNAs (circRNAs), have significant functions in regulating intercellular communication, the fibrosis process and inflammatory responses (Li C. et al., 2021).
Non-coding RNAs are a class of RNA molecules that do not encode proteins. Broadly speaking, they include microRNAs (miRNAs), long non-coding RNAs (lncRNAs), circular RNAs (circRNAs) and so on (Li et al., 2019; Zang et al., 2021). These RNA molecules play important roles in gene expression regulation, the cell cycle, development and disease progression (Li et al., 2019; Rodrigo, 2018). In recent years, more and more studies have revealed their crucial roles in pathological conditions such as fibrosis (Amalinei et al., 2010; Leitinger, 2014). For example, miRNAs can regulate post-transcriptional gene expression by binding to target mRNAs, while circRNAs regulate the gene expression network by acting as miRNA sponges (Liu Y. et al., 2018; Panda, 2018). Therefore, non-coding RNAs are not only of great significance in basic biological research but also provide new targets for the diagnosis and treatment of diseases (Garcia-Padilla et al., 2018; Gomes et al., 2013).
Studies on Idiopathic Pulmonary Fibrosis (IPF) have shown that non-coding RNAs abundant in exosomes exhibit significant changes in this disease. For example, the expression of some miRNAs in the exosomes of IPF patients is abnormal, which may be closely related to the occurrence and development of pulmonary fibrosis (Njock et al., 2019). Meanwhile, research on circRNAs also shows that they have a potential role in regulating the activation of fibroblasts and the fibrosis process of lung tissue (Wang et al., 2022; Yao et al., 2018). For example, studies have found that miR-143-5p and miR-342-5p in the exosomes of patients with idiopathic pulmonary fibrosis (IPF) inhibit the fatty acid synthesis of alveolar type II cells and promote fibrosis, thereby exacerbating the condition of IPF. Pirfenidone and nintedanib can improve the condition. Another example is that Gan et al.'s study found that three circRNAs (hsa_circ_0044226, hsa_circ_0004099, hsa_circ_0008898) in patients with IPF increased significantly. Moreover, compared with patients with stable IPF (S-IPF), the level of hsa_circ_0044226 in patients with acute exacerbation of IPF (AE-IPF) was significantly higher. In addition, the upregulation of hsa_circ_0044226 was observed in the blood exosomes of a bleomycin-induced IPF mouse model. The expression levels of hsa_circ_0044226, hsa_circ_0004099, and hsa_circ_0008898 in plasma exosomes introduce a new biomarker paradigm for the diagnosis and progression of IPF. By analyzing non-coding RNAs in exosomes, researchers can reveal their potential biomarkers and therapeutic targets in IPF (Chen et al., 2021; Zhang et al., 2021). Therefore, in-depth research on the functions and mechanisms of non-coding RNAs in exosomes will not only help to understand the pathogenesis of IPF but may also provide an important basis for the development of new diagnostic and treatment options.
All in all, as a serious interstitial lung disease, the established pathological mechanism of idiopathic pulmonary fibrosis still requires further research (Strykowski and Adegunsoye, 2023). With the deepening of research on exosomes and the non-coding RNAs they contain, it may provide new ideas and perspectives for the innovation of diagnosis, prognosis evaluation and treatment strategies in the future (Da et al., 2021; Fattahi et al., 2024; Yang et al., 2016). It is hoped that through the exploration of the relationship between exosome non-coding RNAs and IPF, early diagnosis and more effective intervention strategies for this disease can be finally achieved, contributing to the improvement of the quality of life of IPF patients.
In this comprehensive and in-depth review study, we have extensively covered a vast amount of relevant literature. To ensure the comprehensiveness and reliability of the research, we have carefully selected 197 pieces of literature for systematic analysis from numerous documents. These literature come from a wide range of sources, mainly focusing on internationally authoritative databases such as Web of Science, PubMed, and Embase. They cover multiple disciplinary fields closely related to idiopathic pulmonary fibrosis (IPF) and exosomal non-coding RNAs (ncRNAs), such as basic medicine, clinical medicine, as well as cutting-edge disciplines like cell biology and molecular biology. Through a comprehensive analysis of these literature, we are committed to deeply exploring the key information regarding the pathogenesis, regulatory mechanisms, and potential clinical applications of exosomal ncRNAs in IPF, providing a solid theoretical foundation and strong practical guidance for further research and development in this field. The specific article screening method is as follows: Search Strategy: Taking the PubMed database as the main search platform, Boolean logical operators are comprehensively used for the search. The specific search formula is: (“Idiopathic Pulmonary Fibrosis” [MeSH Terms] OR “Idiopathic Pulmonary Fibrosis” [All Fields]) AND (“Exosomes” [MeSH Terms] OR “Exosomes” [All Fields]) AND (“Non - coding RNAs” [MeSH Terms] OR “Non - coding RNAs” [All Fields] OR “lncRNAs” [All Fields] OR “miRNAs” [All Fields] OR “circRNAs” [All Fields]). Specific Keywords: Use “Idiopathic Pulmonary Fibrosis”, “Exosomes”, “Non - coding RNAs”, “lncRNAs”, “miRNAs”, “circRNAs”, etc., As the core search terms, and further expand related vocabulary according to the search results, such as “IPF pathogenesis”, “exosomal ncRNAs function”, “lncRNAs in IPF”, “miRNAs regulation in IPF”, “circRNAs and IPF fibrosis process”, etc., to ensure the comprehensiveness of the search. Inclusion Criteria: The research content focuses on the relationship between exosomal non-coding RNAs and idiopathic pulmonary fibrosis; the literature type is a research paper or a review article; the language is English; the research has a certain degree of scientificity and reliability, with a reasonable experimental design and substantial data. Exclusion Criteria: Literature irrelevant to the research topic, such as those only involving other lung diseases or studies not related to exosomes; literature of poor quality and unreliable data, such as those with too small a sample size or obvious flaws in the research method; duplicate published literature. Publication Time Range: Considering the rapid progress of research in this field, we mainly searched for literature published in the past 10 years (2014–2024) to obtain the latest research results. However, for some classic and important early literature, such as those with pioneering significance in this field published before 2010, they are also retained to ensure the integrity and systematicness of the review.
Extracellular vesicles (EVs), especially exosomes, are nano-sized structures bound by membranes (with a size range of 30–150 nm) that are released from different cell types. They play a crucial role as mediators in intercellular communication and carry a wide variety of substances like proteins, lipids, and nucleic acids, among which non-coding RNAs (ncRNAs) are included (Sun et al., 2017; Videira and Da, 2020). Exosomal non-coding RNAs have drawn substantial interest as a result of their multifaceted functions in cellular modulation, disease development, as well as their promising value for therapeutic applications (Alidadi et al., 2023; Fadaei et al., 2022).
Exosomal ncRNAs can be transported effectively via biological fluids like blood, urine, and saliva. This enables them to mediate cell-to-cell communication across relatively long distances (Chen et al., 2022; X; Wang et al., 2024; Wang et al., 2023). This transport takes place through multiple mechanisms, such as passive diffusion, active transport, and specific receptor-mediated endocytosis (Bild et al., 2002; Hastings et al., 2004; Nividha et al., 2024). The lipid bilayer of exosomes shields the RNA they carry from being broken down by ribonucleases outside cells, ensuring its stable transfer to recipient cells. When close to target cells, exosomes can enter them via different routes like endocytosis, phagocytosis, or membrane fusion (Kim et al., 2024; 2023; Sabatke et al., 2024). Upon uptake, exosomal ncRNAs are capable of being released into the cytoplasmic compartment, where they commence exerting their regulatory functions. The efficacy of this uptake procedure can be contingent upon numerous factors, such as the specific subtype of recipient cell, the availability of particular receptors on the cell surface, as well as the comprehensive composition of the exosomal membrane (Hushmandi et al., 2024; Willms et al., 2016; Zaborowski et al., 2015). Prior research has emphasized that certain proteins like tetraspanins or integrins present on exosomes can promote their attachment and subsequent uptake by target cells. This notably amplifies the biological influence of the ncRNAs contained within them (Jaquenod et al., 2019).
Exosomal non-coding RNAs, such as microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), are vital in modulating diverse cellular activities. miRNAs, generally short RNA fragments, mainly operate via post-transcriptional regulation. They attach to complementary sites on target mRNAs, triggering mRNA breakdown or impeding translation. These mechanisms greatly impact cell proliferation, apoptosis, and differentiation, rendering miRNAs key elements in maintaining normal cellular function and driving disease development (Nasimi et al., 2024; van Wijk et al., 2022). Conversely, long non-coding RNAs exhibit a wide array of regulatory capabilities. They can serve as structural supports for assembling protein complexes, adjust the conformation of chromatin, or even be converted into building blocks for shorter RNAs. Their regulatory scope is not confined to transcriptional control; rather, they’re empowered to coordinate elaborate signaling cascades. Meanwhile, circular RNAs have also become prominent regulators across diverse biological scenarios. Notably, they possess the special function of acting like “miRNA sponges”. By doing so, they block miRNAs from repressing the translation of their target messenger RNAs. Additionally, CircRNAs can interface with RNA-binding proteins to fine-tune gene expression levels.
Exosomal ncRNAs do not just regulate one particular cellular pathway. Instead, they usually manage a complex web of signaling routes, magnifying their impact on how cells work. This broad regulatory power offers promising opportunities to use exosomal ncRNAs in medical treatments. It's especially relevant for diseases where gene expression goes haywire, like cancer and neurodegenerative disorders.
Mechanisms underlying exosomal ncRNAs: a concise overview of pivotal aspects concerning their modes of functioning (Figure 2).
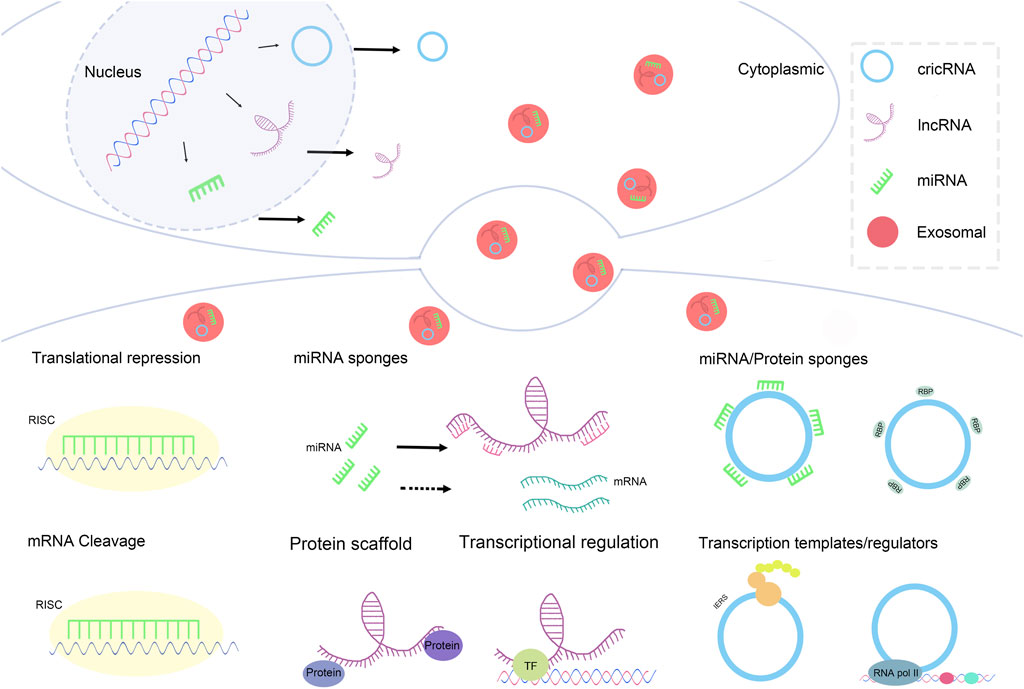
Figure 2. Mechanisms underlying exosomal ncRNAs: a concise overview of pivotal aspects concerning their modes of functioning.
1) Translational Repression: In the cytoplasm, miRNAs can recognize and bind to the 3′untranslated region (3′UTR) of target mRNAs(Han et al., 2018). This binding recruits the RNA-induced silencing complex (RISC). Once the RISC is associated with the miRNA-mRNA complex, it inhibits the binding of ribosomes to the mRNA (Fukaya and Tomari, 2012), thereby suppressing the translation process. This mechanism effectively reduces protein synthesis without leading to mRNA degradation.
2) mRNA Cleavage: In certain instances, after miRNA binds to RISC, it guides RISC to cleave the target mRNA (M. Zhang et al., 2024). This cleavage primarily depends on the complete or near-complete complementary pairing between miRNA and the target mRNA. The cleaved mRNA fragments are subsequently degraded by cellular nucleases, thus completely blocking the synthesis of the protein encoded by that mRNA.
1) Transcriptional Regulation: In the nucleus, lncRNAs can interact with transcription factors (TFs). This interaction can influence gene transcription through various mechanisms (Liu et al., 2018c). For example, lncRNAs can guide transcription factors to specific gene loci to promote the initiation of transcription; alternatively, lncRNAs may alter the activity of transcription factors upon binding, suppressing their association with gene promoters and thereby inhibiting gene transcription (Ferrer and Dimitrova, 2024).
2) miRNA/Protein Sponges: In the cytoplasm, lncRNAs can simultaneously bind to miRNAs and proteins, functioning as miRNA/protein sponges (Dang et al., 2018). By binding miRNAs, they can impact miRNA regulation of target mRNAs; through binding proteins, they can regulate the activity, localization, or stability of those proteins, further influencing various physiological processes within the cell.
3) Protein Scaffold: lncRNAs can also serve as protein scaffolds, facilitating the formation of protein complexes (Ribeiro et al., 2018). They can gather multiple proteins together, allowing these proteins to interact at specific cellular locations and times, thereby forming functional protein complexes that participate in crucial physiological processes such as signal transduction and gene expression regulation.
1) miRNA Sponges: In the cytoplasm, CircRNA can function as miRNA sponges (Hoffmann et al., 2023). CircRNA possesses multiple binding sites for miRNAs, allowing it to sequester large amounts of miRNA, thereby reducing the pool of free miRNA. This decrease diminishes the chances of miRNA binding to target mRNAs, indirectly alleviating the inhibitory effect of miRNA on its target mRNAs and subsequently affecting gene expression.
2) Transcription Templates/Regulators: Within the nucleus, CircRNA can act as a transcription template or regulatory factor involved in the regulation of gene expression (Qi et al., 2021). As a transcription template, CircRNA may participate in the synthesis of other RNA molecules; as a regulatory factor, it can influence the initiation and progression of gene transcription through interactions with other transcription-related factors.
To sum up, exosomal ncRNAs embody a new and active means of cell-to-cell communication. By regulating gene expression, they impact diverse biological processes. Grasping how they’re transported, taken up by cells, and how they work mechanistically will speed up the creation of novel treatment methods. It will also deepen our understanding of disease mechanisms, especially in cancer and other illnesses where ncRNA expression patterns are abnormal (Dill and Naya, 2018; Rodriguez et al., 2018).
In the research on idiopathic pulmonary fibrosis (IPF), microRNAs (miRNAs) in exosomes have been found to be involved in promoting this disease (Elliot et al., 2022). In particular, three miRNAs represented by miR-486, miR-223 and miR-199a have attracted widespread attention in the scientific community. MiR-486 has been shown to be significantly upregulated in the lung tissues and exosomes of IPF patients in multiple studies (Ji et al., 2015). This miRNA can enhance the activation and proliferation of fibroblasts by targeting and inhibiting the expression of certain anti-fibrotic genes. In addition, miR-486 has also been found to promote the deposition of collagen by regulating the synthesis of extracellular matrix components, thus accelerating the fibrosis process of lung tissues (Liu et al., 2019). Its mechanism involves the regulation of cell signaling pathways such as TGF-β and Wnt/β-catenin, driving this complex pathological process. MiR-223 also plays an important role in promoting lung fibrosis (Hou et al., 2023). Studies have shown that the increased expression level of miR-223 is closely related to the severity of IPF (Shan et al., 2015). This miRNA enhances the production of pro-inflammatory cytokines by targeting specific deacetylases and other genes related to the inflammatory response, which in turn triggers the activation and proliferation of fibroblasts. This process not only promotes fibrosis in lung tissues but also leads to the persistence of a chronic inflammatory state, forming a vicious cycle and further aggravating the development of the disease. Furthermore, the role of miR-199a in the fibrosis process has received increasing attention. This miRNA also mediates the progression of IPF through multiple mechanisms. It can not only promote the proliferation of fibroblasts but also enhance the reactivity of lung tissues by inhibiting the expression of certain anti-fibrotic factors, making alveolar epithelial cells unable to be effectively repaired after being damaged, resulting in the formation of fibrosis. Studies also show that miR-199a can further amplify the degree of the fibrosis response by affecting the activity of transforming growth factor-β (TGF-β) and myofibroblasts (Savary et al., 2019). Overall, the important promoting roles played by miR-486, miR-223 and miR-199a in idiopathic pulmonary fibrosis not only provide a new perspective for us to understand the pathogenesis of IPF but also point out the direction for future treatment options. (Table 1)
In the research on idiopathic pulmonary fibrosis (IPF), microRNAs (miRNAs) in exosomes have been found to play inhibitory roles through multiple mechanisms. In particular, five miRNAs, namely, miR-34a, miR-126, miR-146a, miR-143 and miR-137, are especially important in this regard. As a crucial regulator, miR-34a exerts an inhibitory effect on the proliferation and migration of fibroblasts by targeting multiple profibrotic signaling pathways (Zhang et al., 2012). Studies have found that miR-34a can downregulate the expression of transforming growth factor-β (TGF-β), thereby effectively reducing collagen synthesis and alleviating the fibrosis process, demonstrating its potential in protecting lung tissues (Huang et al., 2018). Moreover, miR-126 also shows a significant inhibitory effect in IPF. Studies indicate that this miRNA enhances vascular integrity by regulating endothelial cell functions and inhibiting inflammatory responses, reducing alveolar damage caused by inflammation and the abnormal proliferation of fibroblasts. The upregulation of miR-126 is believed to help promote the repair process of the lungs and prevent the development of fibrosis (Ohta et al., 2020). Meanwhile, miR-146a plays an important role in the pathogenesis of IPF due to its ability to regulate inflammation. It reduces the excessive activation of immune cells by inhibiting the release of pro-inflammatory factors, thereby alleviating the fibrotic response of lung tissues and showing a good protective effect (Hadjicharalambous and Lindsay, 2018). MiR-143 participates in the inhibition of IPF through different mechanisms (Tam et al., 2011). Relevant studies have pointed out that miR-143 can limit the proliferation of fibroblasts and the deposition of collagen by regulating the cell cycle and metabolic pathways. Its ability to inhibit signaling pathways provides new ideas for the treatment of pulmonary fibrosis. At the same time, miR-137 has also been found to have the potential to inhibit idiopathic pulmonary fibrosis (Lee et al., 2010). It further alleviates the degree of fibrosis by interfering with the proliferation signals of fibroblasts and the expression of inflammation-related factors (Table 1).
In conclusion, these five exosomal miRNAs, namely, miR-34a, miR-126, miR-146a, miR-143 and miR-137, play a key role in the inhibitory mechanism of idiopathic pulmonary fibrosis. They not only reduce the proliferation and migration of fibroblasts by inhibiting the expression of profibrotic factors but also promote the repair and regeneration of lung tissues by regulating inflammatory responses, providing new potential targets for the treatment of IPF. Future research is expected to further explore the specific functions of these miRNAs in IPF and their application prospects as biomarkers and targeted therapies, providing a theoretical basis and practical guidance for the improvement of clinical intervention measures.
In the research on idiopathic pulmonary fibrosis (IPF), microRNAs (miRNAs) in exosomes have been found to possess complex dual roles. Among them, four miRNAs, namely, miR-21, miR-155, miR-29 and miR-200, have particularly drawn attention for their promoting and inhibiting effects in this process. MiR-21 has been widely studied and is regarded as a profibrotic factor in IPF. It enhances the proliferation of fibroblasts and the synthesis of collagen by targeting and regulating inhibitory genes (such as PDCD4, TP53, etc.), thus promoting the development of fibrosis in lung tissues (Pandit et al., 2011). Meanwhile, miR-21 also shows the potential to inhibit fibrosis in specific microenvironments, especially in the repair process after acute injury (Barwari et al., 2018). It can promote the remodeling of alveolar epithelial cells by regulating the balance between apoptosis and proliferation. Another important miRNA, miR-155, also demonstrates its duality in the pathology of IPF (Su et al., 2017). As a pro-inflammatory mediator, miR-155 promotes the progression of fibrosis by enhancing the inflammatory response and facilitating the activation of immune cells, and it plays a promoting role in the proliferation and migration of fibroblasts (Chi et al., 2019). However, studies have shown that under certain circumstances, miR-155 can also play a certain protective role by regulating the immune microenvironment and inhibiting chronic inflammation, thereby alleviating the progression of fibrosis. Therefore, miR-155 plays a complex role in the pathogenesis of IPF and is worthy of in-depth study. MiR-29 is another miRNA that exhibits dual roles in regulating the fibrosis process (Ge et al., 2011). In terms of promoting fibrosis, the downregulation of miR-29 is believed to lead to the excessive synthesis of collagen and other extracellular matrix components, thereby exacerbating fibrosis (Kwiecinski et al., 2012). However, in the inhibition mechanism of fibrosis, miR-29 can also alleviate the condition by negatively regulating the expression of profibrotic factors (Jing et al., 2018). Therefore, the expression level and environmental state of miR-29 directly affect its role in IPF, reflecting its potential as an important regulatory factor (Matsushima and Ishiyama, 2016). Finally, the miR-200 family of miRNAs also shows duality in IPF. This miRNA family can promote pathological changes by regulating epithelial-mesenchymal transition (EMT) during the fibrosis process. However, studies have also found that when the expression level of miR-200 increases, they can inhibit fibrosis-related signaling pathways, reduce the activity of fibroblasts, and then alleviate fibrosis (Yang et al., 2012). By regulating the expression of transcription factors and signaling molecules, miR-200 can play diverse roles under different physiological and pathological states (Martinez-Campa et al., 2024). In conclusion, exosomal miRNAs such as miR-21, miR-155, miR-29 and miR-200 play complex dual regulatory roles in idiopathic pulmonary fibrosis, which can both promote the occurrence of inflammation and fibrosis and inhibit these pathological processes under appropriate conditions (Table 1).
In the research on idiopathic pulmonary fibrosis (IPF), recent findings have shown that exosomal long non-coding RNAs (lncRNAs) play a significant promoting role in the development of this disease. In particular, the studies on two types of lncRNAs, namely, LINC00470 and PVT1, have attracted much attention (Huang et al., 2021; Huang W. et al., 2020). The expression of LINC00470 is significantly upregulated in the lung tissues of patients with idiopathic pulmonary fibrosis. Studies have demonstrated that it promotes the fibrosis process by mediating the activities of lung fibrosis-related cell types, such as fibroblasts and alveolar epithelial cells. Specifically, LINC00470 can enhance the activity of the transforming growth factor-β (TGF-β) signaling pathway, facilitating the proliferation and activation of fibroblasts, which in turn leads to the excessive deposition of extracellular matrix components like collagen, and this process directly drives the fibrosis of lung tissues (Huang W. et al., 2020; Nonaka et al., 2008). PVT1 has also been found to be involved in the regulation of fibrosis (Cao et al., 2020; Tian et al., 2022). Studies have shown that PVT1 can compete with miRNAs for binding, regulate the expression of target genes, activate transcription factors and multiple cell signaling pathways, and then influence the apoptosis and proliferation of alveolar epithelial cells (Ji et al., 2021; Li et al., 2018a; Wang et al., 2024). Further research has revealed that the mechanism by which PVT1 promotes pulmonary fibrosis may be closely related to the regulation of the immune response and the remodeling of the inflammatory microenvironment (Table 2).
Both LINC00470 and PVT1 not only provide clues for understanding the underlying pathogenesis but also pave the way for the early diagnosis of IPF and the development of new therapies, highlighting the importance of the close combination of translational medicine and basic research in clinical applications (Benegas et al., 2022; Nakamura and Shimizu, 2023).
In the research related to idiopathic pulmonary fibrosis (IPF), more and more evidence shows that exosomal long non-coding RNAs (lncRNAs) play a crucial role in the inhibitory mechanism of this disease (Kaur et al., 2020; Poulet et al., 2020). In particular, seven lncRNAs, namely, lnc-DC, THRIL, GAS5, LINC00460, FEZF1-AS1, SNHG1 and LncRNA-ATB, have attracted widespread attention. Lnc-DC has been proved in studies to be able to inhibit the occurrence of pulmonary fibrosis by regulating the activation and differentiation of T cells. Specifically, lnc-DC can regulate the expression of anti-inflammatory cytokines and enhance the immune response of T cells, thereby inhibiting the abnormal proliferation of fibroblasts and reducing the formation of fibrosis. The relatively high expression level of THRIL can interact with the expression of tumor necrosis factor-α (TNF-α) and other profibrotic factors, and then inhibit the process of pulmonary fibrosis (Medhat et al., 2022; Sayad et al., 2018). GAS5 exerts its inhibitory effect by inhibiting the transcription of various profibrotic factors and reducing the activation of fibroblasts. Meanwhile, it also affects the regulation of the cell cycle and uses this mechanism to intervene in the fibrosis process of lung tissues (She et al., 2020; Tao et al., 2020). Studies on LINC00460 have shown that its presence in exosomes helps to inhibit the migration and proliferation of fibroblasts. Its mechanism involves regulating multiple key signaling pathways, thus reducing the degree of fibrosis (Yue and Zhang, 2018). Meanwhile, FEZF1-AS1, through its interaction with miRNAs, mediates the inhibition of the inflammatory response and guides the formation of a balance among pro- and anti-fibrotic transcription factors, thereby weakening the progression of idiopathic pulmonary fibrosis (Liu et al., 2024). SNHG1 has also been found to have an inhibitory effect on fibrosis in exosomes. It effectively slows down the development of IPF by downregulating the expression of fibrosis-related genes and interfering with the excessive accumulation of the extracellular matrix (Alvarado-Vasquez et al., 2024; Hewlett et al., 2018). Finally, LncRNA-ATB has been confirmed to have certain potential in the treatment of IPF(Lu et al., 2020; Qi et al., 2017). It plays a positive role in promoting the repair and regeneration of alveolar epithelial cells by regulating the interaction between inflammation and fibrosis, and inhibits the deterioration of fibrosis. In conclusion, these seven exosomal lncRNAs play important roles in the inhibitory mechanism of idiopathic pulmonary fibrosis. Through multiple mechanisms such as interfering with cell signaling pathways, regulating the expression of cytokines and promoting immune functions, they are expected to provide new strategies for the prevention and treatment of IPF (Table 2).
In the research on idiopathic pulmonary fibrosis (IPF), exosomal long non-coding RNAs (lncRNAs) display complex regulatory roles involving both promotion and inhibition. Among them, six lncRNAs, namely, UCA1, MALAT1, HOTAIR, TUG1, H19 and NEAT1, are especially significant. UCA1 can promote the process of pulmonary fibrosis under certain conditions by upregulating the expression of profibrotic factors and activating fibroblast functions, thus accelerating the remodeling of alveolar structures and fibrosis (Li et al., 2021; Yang et al., 2022). However, studies also indicate that UCA1 may have an inhibitory effect in some cellular environments, enabling cells to restore normal functions under stress conditions, reflecting its dual roles in different physiological states (Aalijahan and Ghorbian, 2020). MALAT1 has been widely studied and found to exhibit bidirectional regulatory characteristics at different stages of IPF (Li et al., 2019a). In the early stage of fibrosis development, this lncRNA may promote disease progression and enhance fibroblast proliferation and migration. But after cell injury, MALAT1 may inhibit excessive fibrotic responses by regulating the apoptotic pathway (Huang et al., 2019). HOTAIR also shows a similar duality in IPF. For promotion, it can increase fibroblast activity by activating the Wnt/β-catenin signaling pathway. In terms of inhibition, some studies suggest that HOTAIR may play a catalytic role in lung tissue repair by regulating anti-fibrotic pathways and various cytokines (Hadjicharalambous et al., 2018). TUG1 has a dual function as well. It functions in the profibrotic process by enhancing fibroblast activity (Gu et al., 2019). Meanwhile, under certain conditions, it can inhibit the phosphatidylinositol 3-kinase (PI3K) signaling pathway, thereby reducing the secretion of fibrosis-related cytokines and showing a protective effect. H19 is regarded as playing an important role in understanding the pathology of IPF (Wan et al., 2020). The mechanisms of its simultaneous activation and inhibition are complex and still need further exploration. When cells are in a profibrotic state, H19 can promote fibrosis by increasing collagen synthesis. However, in the repair stage after fibrosis, H19 can inhibit by interfering with the expression of profibrotic factors, helping the regeneration and recovery of alveolar epithelial cells (Tang et al., 2016). Regarding NEAT1, its role in IPF has drawn increasing attention. It may promote the fibrosis and repair processes of cells by regulating the inflammatory response and also show the ability to inhibit the progression of the disease course in some situations, regulating the cell cycle and migration. In summary, these six exosomal lncRNAs are intricately intertwined in the promotion and inhibition mechanisms of idiopathic pulmonary fibrosis. The same lncRNAs exhibit different regulatory effects under different biological backgrounds and cellular states, making them important molecular markers for understanding the pathological process of IPF and providing new ideas for the development of future treatment strategies (Table 2).
In the research on idiopathic pulmonary fibrosis (IPF), circular RNAs (circRNAs) in exosomes are increasingly recognized as playing important roles in promoting the disease. Multiple studies have shown that circular RNAs such as circ_0000479, circ_0026344, circ_0136662, circRNA_002453, circRNA_001569, circRNA_103011, circRNA_004666 and circFBXW7 are all significantly upregulated in IPF, and their abnormal expressions have been confirmed to be closely related to the activation, proliferation of fibroblasts and the fibrosis process (Surendran et al., 2024). Specifically, circ_0000479 promotes the proliferation of fibroblasts and enhances the expression of multiple profibrotic factors by regulating specific signaling pathways, thus directly promoting the fibrosis process (Wang et al., 2020). Similarly, the upregulation of circ_0026344 is associated with the pathological progression of IPF. Studies have found that it promotes the fibrosis of lung tissues by enhancing the synthesis of extracellular matrix components like collagen (Tjin et al., 2017). Moreover, circ_0136662 is found to enhance the activity of fibroblasts and collagen synthesis by influencing the TGF-βsignaling pathway, providing an additional impetus for fibrosis. Its mechanism may involve acting as a sponge for specific miRNAs, thereby relieving the inhibition on profibrotic factors (Kang, 2017). CircRNA_002453 plays an important role in the processes of inflammation and lung fibrosis (Ouyang et al., 2018). It further aggravates the damage to lung tissues and the development of fibrosis by upregulating the expression of pro-inflammatory factors and enhancing the inflammatory response. Another important circRNA, circRNA_001569, has been shown to promote the formation of lung fibrosis by regulating apoptosis and fibroblast functions (Ling et al., 2024). Based on this, studies on circRNA_103011 have shown that its expression is increased in the exosomes of IPF patients and is closely related to the proliferation and migration of fibroblasts (Yang et al., 2018). This circRNA enhances the survival ability of fibroblasts by influencing the cell cycle and metabolic pathways, thus promoting the progression of the disease. Meanwhile, circRNA_004666 is considered to have the potential to regulate fibroblast activity (Fukuda, 2020). It further promotes the formation of fibrosis by targeting and regulating fibrosis-related genes and increasing the activity in signal transduction pathways. CircFBXW7 shows a dual regulatory role in the processes of cell proliferation and apoptosis (Liu T. et al., 2021). By balancing the growth and death mechanisms of cells, it aggravates the inflammatory and fibrotic responses in IPF (Table 3).
In conclusion, exosomal circRNAs such as circ_0000479, circ_0026344, circ_0136662, circRNA_002453, circRNA_001569, circRNA_103011, circRNA_004666 and circFBXW7 are not only significantly upregulated in idiopathic pulmonary fibrosis but also jointly promote the fibrosis process of lung tissues through different mechanisms.
In the research on idiopathic pulmonary fibrosis (IPF), circular RNAs (circRNAs) in exosomes are gradually showing their important roles in inhibiting the fibrosis process (Gan et al., 2024). Multiple studies have demonstrated that eight circRNAs, including circ_0000072, circ_0000410, circ_0018287, circRNA_000839, circ_102001, circRNA_004161, circBIRC6 and circRNA_006227, have the potential to inhibit fibrosis in IPF. Specifically, circ_0000072 has been found to be able to alleviate the progression of fibrosis in lung tissues by regulating fibroblast functions and inhibiting collagen synthesis (Liu G. et al., 2010). Its main mechanism may involve regulating relevant mitochondrial functions and promoting cellular energy metabolism. Moreover, the upregulation of circ_0000410 has been confirmed to effectively inhibit the proliferation and migration of fibroblasts and slow down the occurrence of fibrosis (He et al., 2018). Its effect may be closely related to the inhibition of the expression of multiple profibrotic factors. In this context, studies on circ_0018287 have shown that it inhibits the synthesis of collagen and other extracellular matrix components by acting as a sponge for specific miRNAs, thereby playing a role in inhibiting the progress of IPF (Zhou et al., 2024). Therefore, circ_0018287 not only regulates the fibrosis process at the molecular level but also alleviates lung tissue damage at the cellular level. CircRNA_000839 plays an important regulatory role in the interaction between inflammation and fibrosis (Zhou et al., 2022). It reduces the impact of fibrosis on lung tissues by downregulating the expression of pro-inflammatory factors and inhibiting the activation of fibrotic cells. Another important circRNA, circ_102001, shows its inhibitory effect when regulating fibrosis-related signaling pathways. It weakens the occurrence of fibrosis by reducing the response of fibroblasts to the pathological environment (Gu et al., 2020). Similarly, the research results of circRNA_004161 show that it has a significant upregulation in IPF patients and plays an inhibitory role by influencing the activity and apoptosis process of fibroblasts (Coward et al., 2010). In addition, circBIRC6 is considered to have an inhibitory effect on the occurrence of fibrosis. It alleviates the degree of fibrosis in lung tissues by regulating cell survival pathways (Zhao et al., 2022), while circRNA_006227 shows a significant protective effect in idiopathic pulmonary fibrosis (Prasse, 2015). Its main mechanism may involve slowing down the activation and migration of fibroblasts by regulating the expression of pro-inflammatory and profibrotic signaling pathways. In conclusion, these eight exosomal circRNAs, namely, circ_0000072, circ_0000410, circ_0018287, circRNA_000839, circ_102001, circRNA_004161, circBIRC6 and circRNA_006227, play important roles in the inhibition process of idiopathic pulmonary fibrosis. They work together through different mechanisms to inhibit the proliferation and migration of fibroblasts and the synthesis of extracellular matrix components such as collagen, thereby alleviating the progression of fibrosis in lung tissues. (Table 3)
In the research on idiopathic pulmonary fibrosis (IPF), circular RNAs (circRNAs) in exosomes exhibit complex dual roles, having both the ability to promote fibrosis and the potential to inhibit the pathological process. Specific circRNAs such as circ_002178 and circ_0001246 are mainly considered as factors that promote fibrosis. Circ_002178 promotes the synthesis of extracellular matrix components such as collagen by regulating the proliferation and migration of fibroblasts and enhancing their response to profibrotic signals (Munoz-Felix et al., 2014), thus facilitating the progression of fibrosis. However, some studies also suggest that circ_002178 may play a protective role by regulating the expression of certain inhibitory factors and preventing over - activated fibroblasts (Vasse et al., 2021). On the other hand, circ_0001246 promotes the activation and migration of fibroblasts by interfering with factors related to anti - fibrosis pathways, which in turn leads to continuous damage and fibrosis of lung tissue. However, under certain circumstances, circ_0001246 may also be involved in regulating the apoptotic process of cells and inhibiting continuous damage to the lungs (Nho et al., 2022). In contrast, circHIPK3 and circRNA_0001649 show more significant inhibitory effects in this field (Xiao et al., 2021). CircHIPK3 inhibits miRNAs with profibrotic characteristics through a sponge - like function, limiting the activation of fibroblasts, thereby reducing collagen synthesis and inflammatory responses and helping to protect lung tissue from fibrotic damage. However, the expression of circHIPK3 may also be regulated by certain external factors, and then promote cell proliferation under stress conditions, forming a relatively complex regulatory mechanism (Lu and Zhang, 2020). Similarly, circRNA_0001649 mainly inhibits the excessive deposition of extracellular matrix by down - regulating the expression of profibrotic factors, thereby alleviating the progression of pulmonary fibrosis (Lu et al., 2022). But it has also been found that in a specific microenvironment, it may promote the activation of certain signaling pathways, leading to minimal activation of fibroblasts, thus having a certain bidirectional regulatory role. Finally, circ_ITCH shows complexity with its unique dual role in IPF. It not only plays an inhibitory role in fibrosis by regulating cell proliferation, apoptosis and inflammatory responses, but may also promote the activation of fibroblasts and collagen synthesis when specific upstream signaling pathways are activated (Xu et al., 2020a). Therefore, circ_ITCH presents diverse regulatory properties in the pathogenesis of IPF and may have completely different effects in different microenvironments and pathological states. Overall, circ_002178 and circ_0001246 as factors promoting fibrosis are in contrast to the inhibitory effects of circHIPK3, circRNA_0001649 and circ_ITCH. Their interactions jointly shape the complex pathological characteristics of idiopathic pulmonary fibrosis. The understanding of the dual roles of circRNAs can not only enrich our understanding of the pathology of pulmonary fibrosis but also open up new directions for the development of its treatment plans (Table 3).
The signal pathways associated with idiopathic pulmonary fibrosis are showed below (Figure 3).
The transforming growth factor-β (TGF-β) signaling pathway plays a central role in the pathogenesis of idiopathic pulmonary fibrosis (IPF). Research indicates that non-coding RNAs in exosomes, especially miR - 21, affect the proliferation and migration of fibroblasts by modulating the TGF-β signaling pathway. miR - 21 can enhance downstream signal transduction by suppressing the expression of the TGF-β inhibitor Smad7, thereby promoting collagen synthesis and deposition. Moreover, the decreased expression of miR - 29 in exosomes also leads to an elevation in TGF-β production, which subsequently triggers alterations in fibrosis-related gene expression, suggesting a close correlation between miR - 29 and the progression of IPF(Guo et al., 2022).
The Wnt/β - catenin signaling pathway also plays a significant role in the process of pulmonary fibrosis. Members of the miR - 135a and miR - 200 family in exosomes have been found to regulate fibrosis-related cell behaviors by inhibiting the activity of β - catenin, a key component of the Wnt signaling pathway. miR - 135a is capable of inhibiting the expression of Wnt target genes, thus impeding the transformation of fibroblasts. The miR - 200 family negatively regulates the epithelial - mesenchymal transition (EMT) process of cells by directly targeting ZEB1, thereby restraining the progression of fibrosis (E. Zhang et al., 2021) (Figure 3).
The PI3K/Akt signaling pathway is involved in multiple biological processes such as cell proliferation, survival, and apoptosis, and its role in idiopathic pulmonary fibrosis is also garnering increasing attention. Studies have demonstrated that miR - 155 in exosomes promotes the proliferation and activation of fibroblasts by activating the PI3K/Akt signaling pathway, consequently aggravating pulmonary fibrosis. Conversely, the decreased expression of miR - 486 leads to the inhibition of the PI3K/Akt pathway, affecting cell proliferation and survival, further highlighting the crucial role of exosomal non - coding RNAs in regulating this pathway (Sun et al., 2019).
The nuclear factor κB (NF - κB) signaling pathway regulates inflammatory responses and cell survival and death, and has a substantial impact on the progression of IPF(Jaffar et al., 2021). The upregulation of miR - 146a in exosomes can inhibit the release of inflammatory factors by interfering with the NF - κB signaling pathway, thereby counteracting the formation of pulmonary fibrosis (Luo et al., 2017). Additionally, research has also discovered that long non - coding RNA - p21 (lincRNA - p21) in exosomes has an inhibitory effect on the NF - κB pathway. By interacting with NF - κB, it influences its transcriptional activity, providing a novel perspective for a better understanding of the connection between inflammation and fibrosis.
The Notch signaling pathway plays an important role in various physiological and pathological processes, particularly in fibroblast activation. miR - nine in exosomes can indirectly reduce the activation and proliferation of fibroblasts by inhibiting the activation of the Notch signaling pathway. Moreover, studies have shown that miR - 126 inhibits the conversion of endothelial cells to fibroblasts by negatively regulating the Notch signaling pathway. The regulation of the Notch signaling pathway will offer new possibilities for the treatment of IPF, emphasizing the significance of exosomal non - coding RNAs in this signaling network (D. Yang et al., 2022; Zhao et al., 2018).
The JAK/STAT signaling pathway is crucial in cytokine signal transduction and is closely related to the progression of pulmonary fibrosis. Research has found that the miR - 17 family in exosomes can inhibit the activation of this signaling pathway by interfering with the phosphorylation of STAT3, thereby reducing the likelihood of fibroblasts transforming into myofibroblasts. In addition, another important constituent in exosomes, miR - 29b, also modifies the activity of the STAT signaling pathway by suppressing the expression of JAK1, further underlining the potential of exosomal non - coding RNAs in regulating the JAK/STAT pathway (Li et al., 2018b).
In summary, exosomal non-coding RNAs play a vital regulatory role in the signal pathways of idiopathic pulmonary fibrosis. Through different signal pathways, these exosomal RNAs can influence the activation, proliferation, and differentiation of fibroblasts, ultimately affecting the occurrence and development of pulmonary fibrosis. Future research is anticipated to further elucidate the specific mechanisms of these informational molecules in IPF and furnish new targets for the development of novel treatment strategies.
Idiopathic Pulmonary Fibrosis (IPF) is a severe lung disease with a complex pathogenesis and a lack of effective treatment options. The research on exosomal non-coding RNAs (ncRNAs) in IPF has brought new hope for solving this difficult problem. After comprehensively analyzing the various roles of exosomal ncRNAs in IPF, we will further explore their profound significance in the research and treatment of this disease, the numerous challenges they face, and the future research directions.
Exosomal ncRNAs play an essential role in revealing the pathogenesis of IPF. Taking long non-coding RNAs (lncRNAs) as an example, Lnc GAS5 among them can inhibit the transcription of various profibrotic factors and reduce the activation of fibroblasts, thus playing an inhibitory role in the fibrosis process. In the pathological environment of IPF, it can precisely intervene in relevant signal transduction and reduce the excessive deposition of extracellular matrix, providing a key clue for us to deeply understand the molecular regulatory links in the pathogenesis of IPF.
In addition, exosomal ncRNAs show great potential in the clinical application of IPF. Given their relative stability in biological fluids, they are expected to become highly valuable non-invasive biomarkers. For example, the significant changes in the expression levels of some miRNAs in the lung tissue and serum exosomes of IPF patients are closely related to the severity of the disease, which provides a strong basis for the early accurate diagnosis and dynamic monitoring of the disease. Meanwhile, based on a deep understanding of their mechanism of action, exosomal ncRNAs have opened up new targets for the development of innovative treatment strategies. For profibrotic ncRNAs, methods such as using antisense oligonucleotides or RNA interference technology to inhibit their function are expected to reduce the degree of pulmonary fibrosis; for ncRNAs with antifibrotic effects, exploring ways to enhance their expression or activity may become an effective treatment approach for IPF. In addition, exosomes themselves, as natural nanocarriers, have excellent biocompatibility and targeting ability and can precisely deliver therapeutic ncRNAs or other drugs to the lesioned parts of the lungs. By modifying their surface molecules, they can specifically recognize and bind to IPF-related cells, achieving efficient treatment while minimizing side effects on normal tissues, bringing unprecedented opportunities for the treatment of IPF.
However, exosomal ncRNAs face many severe challenges in the research process of IPF. At the technical level, the imperfect exosome isolation and identification techniques have become an important bottleneck restricting the progress of research. The commonly used ultracentrifugation method and immunoprecipitation method each have their drawbacks. The ultracentrifugation method is cumbersome, time-consuming, and prone to damage exosomes; the immunoprecipitation method is costly and may have non-specific binding problems. These factors may all affect the purity and quality of exosome samples, thereby interfering with the accurate detection and functional research of ncRNAs in them. In addition, the lack of unified exosome identification standards also greatly reduces the accuracy and reliability of research results, and it is urgent to establish a unified, standardized, and highly sensitive identification system. In the field of functional research, the function of exosomal ncRNAs is complexly affected by many factors, including the diversity of cell sources, the specificity of target cell types, the dynamic changes of tissue microenvironment, and the progression of disease status. The same ncRNA may have completely different functions in different cell environments or disease stages. For example, miR-21 has both a promoting effect on fibrosis and an inhibitory effect in specific situations in IPF. This complexity and variability of functions greatly increase the difficulty of accurately analyzing its mechanism of action in IPF. At the same time, there is a broad and elaborate interaction network between ncRNAs and other biomolecules (such as proteins and DNA), which further complicates functional research and makes our current understanding of their interaction mechanisms still at a relatively preliminary stage. In the clinical translation stage, the lack of ideal preclinical animal models has become a key obstacle to the translation of exosomal ncRNAs treatment strategies from the laboratory to clinical applications. Although the existing animal models can simulate the pathological characteristics of IPF to a certain extent, there are still significant differences from the pathogenesis and pathological manifestations of human IPF, resulting in the difficulty of directly applying the research results obtained in animal models to clinical practice. In addition, the large-scale production, quality control, and standardization of exosomal ncRNAs as therapeutic drugs are also difficult problems that need to be overcome urgently, including how to ensure the stable yield and high quality of exosomes, how to accurately load ncRNAs and maintain their activity, and how to optimize the drug delivery route, reasonably select the dose, and conduct long-term safety evaluations. All these aspects require in-depth and systematic research.
Looking forward to the future, in order to promote the further development of exosomal ncRNAs in IPF research, we need to continue to work hard in several key directions. In terms of technological innovation, there is an urgent need to develop more advanced, efficient, and highly specific exosome isolation methods. For example, combining microfluidic technology with immunomagnetic bead sorting technology to achieve rapid, high-purity isolation of exosomes while minimizing damage and contamination during sample processing. At the same time, efforts should be made to establish a unified, accurate, and comprehensive exosome identification standard. By comprehensively using cutting-edge technologies such as nanoparticle tracking analysis (NTA), proteomics analysis, and single-cell sequencing, exosomes can be accurately characterized from multiple dimensions, significantly improving the accuracy and reliability of exosome identification. In the field of functional mechanism exploration, fully utilize emerging technologies such as single-cell sequencing and spatial transcriptomics to deeply study the spatio-temporal expression patterns and functional heterogeneity of exosomal ncRNAs in IPF, accurately analyze their specific mechanism of action in different cell types, different pathological stages, and different tissue microenvironments, and comprehensively reveal their dynamic interaction networks with other biomolecules. Through large-scale, multicenter clinical studies, widely collect IPF patient samples, deeply analyze the internal relationship between the expression profiles of exosomal ncRNAs and clinical characteristics and treatment responses, rigorously verify their accuracy and clinical application value as biomarkers, and provide a solid theoretical basis for the personalized and precise treatment of IPF. In the process of promoting clinical translation, actively construct animal models that are more in line with the pathogenesis and pathological characteristics of human IPF. For example, using gene editing technology to construct specific IPF animal models or simulating the complex etiology and pathological process of human IPF through a combination of multiple environmental factors and drug induction to provide a more reliable experimental platform for the preclinical research of exosomal ncRNAs treatment strategies. At the same time, vigorously strengthen the research and development of exosomal ncRNAs therapeutic drugs, optimize the production process and quality control standards, deeply explore suitable drug delivery systems and administration routes, and conduct strict and standardized preclinical safety and effectiveness evaluations to lay a solid foundation for their early clinical application. On this basis, actively and orderly carry out clinical trials, comprehensively evaluate the efficacy and safety of exosomal ncRNAs treatment strategies in IPF patients, and effectively promote their translation from laboratory research to clinical practice, bringing new and effective treatment options for IPF patients and ultimately improving their prognosis and quality of life.
YW: Funding acquisition, Writing–original draft. MH: Visualization, Writing–review and editing. HZ: Software, Writing–review and editing. FL: Conceptualization, Funding acquisition, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. Scientific research projects of Nantong Health Commission (MS2024080, QN2024048).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aalijahan, H., and Ghorbian, S. (2020). Clinical application of long non-coding rna-uca1 as a candidate gene in progression of esophageal cancer. Pathol. Oncol. Res. 26 (3), 1441–1446. doi:10.1007/s12253-019-00711-3
Adkins, J. M., and Collard, H. R. (2012). Idiopathic pulmonary fibrosis. Semin. Respir. Crit. Care Med. 33 (5), 433–439. doi:10.1055/s-0032-1325154
Alidadi, M., Hjazi, A., Ahmad, I., Mahmoudi, R., Sarrafha, M., Reza, H. S., et al. (2023). Exosomal non-coding RNAs: emerging therapeutic targets in atherosclerosis. Biochem. Pharmacol. 212, 115572. doi:10.1016/j.bcp.2023.115572
Alvarado-Vasquez, N., Rangel-Escareno, C., de Jesus, R. J., Becerril, C., and Negrete-Garcia, M. C. (2024). The possible role of hypoxia-induced exosomes on the fibroblast metabolism in idiopathic pulmonary fibrosis. Biomed. Pharmacother. 181, 117680. doi:10.1016/j.biopha.2024.117680
Amalinei, C., Caruntu, I. D., Giusca, S. E., and Balan, R. A. (2010). Matrix metalloproteinases involvement in pathologic conditions. Rom. J. Morphol. Embryol. 51 (2), 215–228. [Journal Article; Review].
Ashrafizadeh, M., Ang, H. L., Moghadam, E. R., Mohammadi, S., Zarrin, V., Hushmandi, K., et al. (2020). MicroRNAs and their influence on the ZEB family: mechanistic aspects and therapeutic applications in cancer therapy. Biomolecules 10 (7), 1040. doi:10.3390/biom10071040
Balamugesh, T., and Behera, D. (2007). Idiopathic pulmonary fibrosis. J. Assoc. Physicians India 55, 363–370.
Barwari, T., Eminaga, S., Mayr, U., Lu, R., Armstrong, P. C., Chan, M. V., et al. (2018). Inhibition of profibrotic microRNA-21 affects platelets and their releasate. JCI Insight 3 (21), e123335. doi:10.1172/jci.insight.123335
Batchu, S. N., Hughson, A., Gerloff, J., Fowell, D. J., and Korshunov, V. A. (2013). Role of axl in early kidney inflammation and progression of salt-dependent hypertension. Hypertension 62 (2), 302–309. doi:10.1161/HYPERTENSIONAHA.113.01382
Bendoraite, A., Knouf, E. C., Garg, K. S., Parkin, R. K., Kroh, E. M., O'Briant, K. C., et al. (2010). Regulation of miR-200 family microRNAs and ZEB transcription factors in ovarian cancer: evidence supporting a mesothelial-to-epithelial transition. Gynecol. Oncol. 116 (1), 117–125. doi:10.1016/j.ygyno.2009.08.009
Benegas, U. M., Ramirez, R. J., and Sanchez, G. M. (2022). Idiopathic pulmonary fibrosis. Radiol. Engl. Ed. 64 (Suppl. 3), 227–239. doi:10.1016/j.rxeng.2022.10.009
Bild, A. H., Turkson, J., and Jove, R. (2002). Cytoplasmic transport of Stat3 by receptor-mediated endocytosis. EMBO. J. 21 (13), 3255–3263. doi:10.1093/emboj/cdf351
Buratin, A., Borin, C., Tretti, P. C., Dal Molin, A., Orsi, S., Binatti, A., et al. (2023). Circfbxw7 in patients with t-cell all: depletion sustains myc and notch activation and leukemia cell viability. Exp. Hematol. Oncol. 12 (1), 12. [Letter]. doi:10.1186/s40164-023-00374-6
Cao, L., Qin, P., Zhang, J., Qiao, H., Shi, P., and Huo, H. (2020). Lncrna pvt1 suppresses the progression of renal fibrosis via inactivation of tgf-beta signaling pathway. Drug Des. Devel Ther. 14, 3547–3557. doi:10.2147/DDDT.S245244
Chandran, R. R., Xie, Y., Gallardo-Vara, E., Adams, T., Garcia-Milian, R., Kabir, I., et al. (2021). Distinct roles of KLF4 in mesenchymal cell subtypes during lung fibrogenesis. Nat. Commun. 12 (1), 7179. doi:10.1038/s41467-021-27499-8
Chen, J., Yu, X., and Zhang, X. (2022). Advances on biological functions of exosomal non-coding RNAs in osteoarthritis. Cell. biochem. Funct. 40 (1), 49–59. doi:10.1002/cbf.3679
Chen, P., Wan, D., Zheng, D., Zheng, Q., Wu, F., and Zhi, Q. (2016). Long non-coding RNA UCA1 promotes the tumorigenesis in pancreatic cancer. Biomed. Pharmacother. 83, 1220–1226. doi:10.1016/j.biopha.2016.08.041
Chen, Q., Li, Y., Liu, Y., Xu, W., and Zhu, X. (2021). Exosomal non-coding rnas-mediated crosstalk in the tumor microenvironment. Front. Cell. Dev. Biol. 9, 646864. doi:10.3389/fcell.2021.646864
Chen, X., Ding, Z., Li, T., Jiang, W., Zhang, J., and Deng, X. (2020). MicroR-26b targets high mobility group, AT-hook 2 to ameliorate myocardial infarction-induced fibrosis by suppression of cardiac fibroblasts activation. Curr. Neurovasc. Res. 17 (2), 204–213. doi:10.2174/1567202617666200506101258
Cheng, H., Chen, L., Fang, Z., Wan, Q., Du, Z., Ma, N., et al. (2022). The effect of miR-138 on the proliferation and apoptosis of breast cancer cells through the NF-κB/VEGF signaling pathway. Cell. Mol. Biol. (Noisy-le-grand). 68 (2), 132–137. doi:10.14715/cmb/2022.68.2.19
Chi, L., Xiao, Y., Zhu, L., Zhang, M., Xu, B., Xia, H., et al. (2019). microRNA-155 attenuates profibrotic effects of transforming growth factor-beta on human lung fibroblasts. J. Biol. Regul. Homeost. Agents. 33 (5), 1415–1424. doi:10.23812/19-41A
Collins, B. F., and Raghu, G. (2019). Antifibrotic therapy for fibrotic lung disease beyond idiopathic pulmonary fibrosis. Eur. Respir. Rev. 28 (153), 190022. doi:10.1183/16000617.0022-2019
Coward, W. R., Saini, G., and Jenkins, G. (2010). The pathogenesis of idiopathic pulmonary fibrosis. Ther. Adv. Respir. Dis. 4 (6), 367–388. doi:10.1177/1753465810379801
Da, M., Jiang, H., Xie, Y., Jin, W., and Han, S. (2021). The biological roles of exosomal long non-coding RNAs in cancers. OncoTargets Ther. 14, 271–287. doi:10.2147/OTT.S281175
Dang, Y., Wei, X., Xue, L., Wen, F., Gu, J., and Zheng, H. (2018). Long non-coding RNA in glioma: target miRNA and signaling pathways. Clin. Lab. 64 (6), 887–894. doi:10.7754/Clin.Lab.2018.180107
Davis-Dusenbery, B. N., Chan, M. C., Reno, K. E., Weisman, A. S., Layne, M. D., Lagna, G., et al. (2011). down-regulation of Kruppel-like factor-4 (KLF4) by microRNA-143/145 is critical for modulation of vascular smooth muscle cell phenotype by transforming growth factor-beta and bone morphogenetic protein 4. J. Biol. Chem. 286 (32), 28097–28110. doi:10.1074/jbc.M111.236950
Deng, Z., He, Y., Yang, X., Shi, H., Shi, A., Lu, L., et al. (2017). MicroRNA-29: a crucial player in fibrotic disease. Mol. Diagn. Ther. 21 (3), 285–294. doi:10.1007/s40291-016-0253-9
Dill, T. L., and Naya, F. J. (2018). A hearty dose of noncoding RNAs: the imprinted DLK1-DIO3 locus in cardiac development and disease. J. Cardiovasc. Dev. Dis. 5 (3), 37. doi:10.3390/jcdd5030037
Elliot, S., Catanuto, P., Pereira-Simon, S., Xia, X., Shahzeidi, S., Roberts, E., et al. (2022). Urine-derived exosomes from individuals with IPF carry pro-fibrotic cargo. Elife 11, e79543. doi:10.7554/eLife.79543
Fadaei, S., Zarepour, F., Parvaresh, M., Motamedzadeh, A., Tamehri, Z. S., Sheida, A., et al. (2022). Epigenetic regulation in myocardial infarction: non-coding RNAs and exosomal non-coding RNAs. Front. Cardiovasc. Med. 9, 1014961. doi:10.3389/fcvm.2022.1014961
Fattahi, M., Alamdari-Palangi, V., Rahimi, J. K., Ehtiati, S., Ojaghi, S., Rahimi-Jaberi, A., et al. (2024). Exosomal long non-coding RNAs in glioblastoma. Clin. Chim. Acta. 553, 117705. doi:10.1016/j.cca.2023.117705
Ferrer, J., and Dimitrova, N. (2024). Transcription regulation by long non-coding RNAs: mechanisms and disease relevance. Nat. Rev. Mol. Cell Biol. 25 (5), 396–415. doi:10.1038/s41580-023-00694-9
Fukaya, T., and Tomari, Y. (2012). MicroRNAs mediate gene silencing via multiple different pathways in drosophila. Mol. Cell. 48 (6), 825–836. doi:10.1016/j.molcel.2012.09.024
Fukuda, K. (2020). Corneal fibroblasts: function and markers. Exp. Eye Res. 200, 108229. doi:10.1016/j.exer.2020.108229
Gan, W., Song, W., Gao, Y., Zheng, X., Wang, F., Zhang, Z., et al. (2024). Exosomal circRNAs in the plasma serve as novel biomarkers for IPF diagnosis and progression prediction. J. Transl. Med. 22 (1), 264. doi:10.1186/s12967-024-05034-9
Garcia-Padilla, C., Aranega, A., and Franco, D. (2018). The role of long non-coding RNAs in cardiac development and disease. AIMS Genet. 5 (2), 124–140. doi:10.3934/genet.2018.2.124
Ge, Y., Chen, G., Sun, L., and Liu, F. (2011). Microrna-29 and fibrosis diseases. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 36 (9), 908–912. doi:10.3969/j.issn.1672-7347.2011.09.017
Geng, H., and Tan, X. D. (2016). Functional diversity of long non-coding rnas in immune regulation. Genes Dis. 3 (1), 72–81. doi:10.1016/j.gendis.2016.01.004
George, P. M., Patterson, C. M., Reed, A. K., and Thillai, M. (2019). Lung transplantation for idiopathic pulmonary fibrosis. Lancet Resp. Med. 7 (3), 271–282. doi:10.1016/S2213-2600(18)30502-2
Gomes, A. Q., Nolasco, S., and Soares, H. (2013). Non-coding RNAs: multi-tasking molecules in the cell. Int. J. Mol. Sci. 14 (8), 16010–16039. doi:10.3390/ijms140816010
Gu, W., Yuan, Y., Wang, L., Yang, H., Li, S., Tang, Z., et al. (2019). Long non-coding RNA TUG1 promotes airway remodelling by suppressing the miR-145-5p/DUSP6 axis in cigarette smoke-induced COPD. J. Cell. Mol. Med. 23 (11), 7200–7209. doi:10.1111/jcmm.14389
Gu, X., Jiang, Y. N., Wang, W. J., Zhang, J., Shang, D. S., Sun, C. B., et al. (2020). Comprehensive circrna expression profile and construction of circrna-related cerna network in cardiac fibrosis. Biomed. Pharmacother. 125, 109944. doi:10.1016/j.biopha.2020.109944
Gulati, S., and Thannickal, V. J. (2019). The aging lung and idiopathic pulmonary fibrosis. Am. J. Med. Sci. 357 (5), 384–389. [Journal Article; Research Support, N.I.H., Extramural; Research Support, U.S. Gov't, Non-P.H.S.; Review]. doi:10.1016/j.amjms.2019.02.008
Guo, X., Sunil, C., Adeyanju, O., Parker, A., Huang, S., Ikebe, M., et al. (2022). PD-L1 mediates lung fibroblast to myofibroblast transition through Smad3 and β-catenin signaling pathways. Sci. Rep. 12 (1), 3053. doi:10.1038/s41598-022-07044-3
Hadjicharalambous, M. R., and Lindsay, M. A. (2020). Idiopathic pulmonary fibrosis: pathogenesis and the emerging role of long non-coding rnas. Int. J. Mol. Sci. 21 (2), 524. doi:10.3390/ijms21020524
Hadjicharalambous, M. R., Roux, B. T., Feghali-Bostwick, C. A., Murray, L. A., Clarke, D. L., and Lindsay, M. A. (2018). Long non-coding RNAs are central regulators of the IL-1β-induced inflammatory response in normal and idiopathic pulmonary lung fibroblasts. Front. Immunol. 9, 2906. doi:10.3389/fimmu.2018.02906
Han, S., Kim, D., Shivakumar, M., Lee, Y. J., Garg, T., Miller, J. E., et al. (2018). The effects of alternative splicing on mirna binding sites in bladder cancer. [Journal Article; Research Support, N.I.H., Extramural; Research Support, U.S. Gov't, P.H.S.]. PLoS One. 13 (1), e190708. doi:10.1371/journal.pone.0190708
Hao, B., and Zhang, J. (2019). Mirna-21 inhibition suppresses the human epithelial ovarian cancer by targeting pten signal pathway. Saudi J. Biol. Sci. 26 (8), 2026–2029. doi:10.1016/j.sjbs.2019.08.008
Hastings, R. H., Folkesson, H. G., and Matthay, M. A. (2004). Mechanisms of alveolar protein clearance in the intact lung. Am. J. Physiol.-Lung Cell. Mol. Physiol. 286 (4), L679–L689. doi:10.1152/ajplung.00205.2003
He, Y., Tsou, P. S., Khanna, D., and Sawalha, A. H. (2018). Methyl-cpg-binding protein 2 mediates antifibrotic effects in scleroderma fibroblasts. Ann. Rheum. Dis. 77 (8), 1208–1218. doi:10.1136/annrheumdis-2018-213022
Hewlett, J. C., Kropski, J. A., and Blackwell, T. S. (2018). Idiopathic pulmonary fibrosis: epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol. 71-72, 112–127. doi:10.1016/j.matbio.2018.03.021
Hoffmann, M., Schwartz, L., Ciora, O. A., Trummer, N., Willruth, L. L., Jankowski, J., et al. (2023). Circrna-sponging: a pipeline for extensive analysis of circrna expression and their role in mirna sponging. bioRxiv, 524495. doi:10.1101/2023.01.19.524495
Hou, L., Zhu, Z., Jiang, F., Zhao, J., Jia, Q., Jiang, Q., et al. (2023). Human umbilical cord mesenchymal stem cell-derived extracellular vesicles alleviated silica induced lung inflammation and fibrosis in mice via circpwwp2a/mir-223-3p/nlrp3 axis. Ecotoxicol. Environ. Saf. 251, 114537. doi:10.1016/j.ecoenv.2023.114537
Huang, G., Du, M. Y., Zhu, H., Zhang, N., Lu, Z. W., Qian, L. X., et al. (2018). Mirna-34a reversed tgf-beta-induced epithelial-mesenchymal transition via suppression of smad4 in npc cells. Biomed. Pharmacother. 106, 217–224. doi:10.1016/j.biopha.2018.06.115
Huang, S., Li, C., Huang, J., Luo, P., Mo, D., and Wang, H. (2020a). Lncrna fezf1-as1 promotes non-small lung cancer cell migration and invasion through the up-regulation of notch1 by serving as a sponge of mir-34a. BMC Pulm. Med. 20 (1), 110. doi:10.1186/s12890-020-1154-6
Huang, S., Zhang, L., Song, J., Wang, Z., Huang, X., Guo, Z., et al. (2019). Long noncoding RNA MALAT1 mediates cardiac fibrosis in experimental postinfarct myocardium mice model. J. Cell. Physiol. 234 (3), 2997–3006. doi:10.1002/jcp.27117
Huang, T., Wang, Y. J., Huang, M. T., Guo, Y., Yang, L. C., Liu, X. J., et al. (2021). Linc00470 accelerates the proliferation and metastasis of melanoma through promoting apex1 expression. Cell Death Dis. 12 (5), 410. doi:10.1038/s41419-021-03612-z
Huang, W., Liu, J., Yan, J., Huang, Z., Zhang, X., Mao, Y., et al. (2020b). Lncrna linc00470 promotes proliferation through association with nf45/nf90 complex in hepatocellular carcinoma. Hum. Cell. 33 (1), 131–139. doi:10.1007/s13577-019-00288-8
Hushmandi, K., Saadat, S. H., Raei, M., Aref, A. R., Reiter, R. J., Nabavi, N., et al. (2024). The science of exosomes: understanding their formation, capture, and role in cellular communication. Pathol. Res. Pract. 259, 155388. doi:10.1016/j.prp.2024.155388
Jaffar, J., Glaspole, I., Symons, K., and Westall, G. (2021). Inhibition of NF-κB by ACT001 reduces fibroblast activity in idiopathic pulmonary fibrosis. Biomed. Pharmacother. 138, 111471. doi:10.1016/j.biopha.2021.111471
Jaquenod, D. G. C., Santalla, M., and Das, S. (2019). Exosomal non-coding RNAs (Exo-ncRNAs) in cardiovascular health. J. Mol. Cell. Cardiol. 137, 143–151. doi:10.1016/j.yjmcc.2019.09.016
Ji, X., Li, Z., Wang, W., and Chen, J. (2021). Downregulation of long non-coding rna pvt1 enhances fracture healing via regulating microrna-497-5p/hmga2 axis. Bioengineered 12 (1), 8125–8134. doi:10.1080/21655979.2021.1987099
Ji, X., Wu, B., Fan, J., Han, R., Luo, C., Wang, T., et al. (2015). The anti-fibrotic effects and mechanisms of MicroRNA-486-5p in pulmonary fibrosis. Sci. Rep. 5, 14131. doi:10.1038/srep14131
Jiang, J., Gao, G., Pan, Q., Liu, J., Tian, Y., and Zhang, X. (2022). Circular rna circhipk3 is downregulated in diabetic cardiomyopathy and overexpression of circhipk3 suppresses pten to protect cardiomyocytes from high glucose-induced cell apoptosis. Bioengineered 13 (3), 6272–6279. doi:10.1080/21655979.2022.2031395
Jiao, D., Zhang, H., Jiang, Z., Huang, W., Liu, Z., Wang, Z., et al. (2018). MicroRNA-34a targets sirtuin 1 and leads to diabetes-induced testicular apoptotic cell death. J. Mol. Med. 96 (9), 939–949. doi:10.1007/s00109-018-1667-0
Jing, X., Yang, J., Jiang, L., Chen, J., and Wang, H. (2018). Microrna-29b regulates the mitochondria-dependent apoptotic pathway by targeting bax in doxorubicin cardiotoxicity. Cell Physiol. Biochem. 48 (2), 692–704. doi:10.1159/000491896
Kang, H. (2017). Role of MicroRNAs in TGF-β signaling pathway-mediated pulmonary fibrosis. Int. J. Mol. Sci. 18 (12), 2527. doi:10.3390/ijms18122527
Kaur, G., Singh, K., Maremanda, K. P., Li, D., Chand, H. S., and Rahman, I. (2020). Differential plasma exosomal long non-coding rnas expression profiles and their emerging role in e-cigarette users, cigarette, waterpipe, and dual smokers. [Comparative Study; Journal Article; Research Support, N.I.H., Extramural; Research Support, U.S. Gov't, P.H.S.]. PLoS One 15 (12), e243065. doi:10.1371/journal.pone.0243065
Khalil, W., Xia, H., Bodempudi, V., Kahm, J., Hergert, P., Smith, K., et al. (2015). Pathologic regulation of collagen i by an aberrant protein phosphatase 2a/histone deacetylase c4/microrna-29 signal axis in idiopathic pulmonary fibrosis fibroblasts. Am. J. Respir. Cell. Mol. Biol. 53 (3), 391–399. doi:10.1165/rcmb.2014-0150OC
Kim, G., Zhu, R., Zhang, Y., Jeon, H., Shirinichi, F., and Wang, Y. (2024). Fluorescent chiral quantum dots to unveil origin-dependent exosome uptake and cargo release. ACS Appl. Bio Mater. 7 (5), 3358–3374. doi:10.1021/acsabm.4c00296
Kim, G., Zhu, R., Zhang, Y., Jeon, H., and Wang, Y. (2023). Fluorescent chiral quantum dots to unveil origin-dependent exosome uptake and cargo release. bioRxiv, 572689. doi:10.1101/2023.12.20.572689
Kropski, J. A., Lawson, W. E., Young, L. R., and Blackwell, T. S. (2013). Genetic studies provide clues on the pathogenesis of idiopathic pulmonary fibrosis. Dis. Model. Mech. 6 (1), 9–17. doi:10.1242/dmm.010736
Kuse, N., Kamio, K., Azuma, A., Matsuda, K., Inomata, M., Usuki, J., et al. (2020). Exosome-derived microrna-22 ameliorates pulmonary fibrosis by regulating fibroblast-to-myofibroblast differentiation in vitro and in vivo. J. Nippon. Med. Sch. 87 (3), 118–128. doi:10.1272/jnms.JNMS.2020_87-302
Kwiecinski, M., Elfimova, N., Noetel, A., Tox, U., Steffen, H. M., Hacker, U., et al. (2012). Expression of platelet-derived growth factor-C and insulin-like growth factor I in hepatic stellate cells is inhibited by miR-29. Lab. Invest. 92 (7), 978–987. doi:10.1038/labinvest.2012.70
Lee, J. S., Collard, H. R., Raghu, G., Sweet, M. P., Hays, S. R., Campos, G. M., et al. (2010). Does chronic microaspiration cause idiopathic pulmonary fibrosis? [Journal article; research support, N.I.H., extramural; review]. Am. J. Med. 123 (4), 304–311. doi:10.1016/j.amjmed.2009.07.033
Leitinger, B. (2014). Discoidin domain receptor functions in physiological and pathological conditions. Int. Rev. Cell Mol. Biol. 310, 39–87. doi:10.1016/B978-0-12-800180-6.00002-5
Li, C., Ni, Y. Q., Xu, H., Xiang, Q. Y., Zhao, Y., Zhan, J. K., et al. (2021a). Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct. Target. Ther. 6 (1), 383. doi:10.1038/s41392-021-00779-x
Li, D., Zhang, C., Li, J., Che, J., Yang, X., Xian, Y., et al. (2019a). Long non-coding RNA MALAT1 promotes cardiac remodeling in hypertensive rats by inhibiting the transcription of MyoD. Aging (Albany NY) 11 (20), 8792–8809. doi:10.18632/aging.102265
Li, H., Chen, S., Liu, J., Guo, X., Xiang, X., Dong, T., et al. (2018a). Long non-coding RNA PVT1-5 promotes cell proliferation by regulating miR-126/SLC7A5 axis in lung cancer. Biochem. Biophys. Res. Commun. 495 (3), 2350–2355. doi:10.1016/j.bbrc.2017.12.114
Li, H., Wang, Z., Zhang, J., Wang, Y., Yu, C., Zhang, J., et al. (2018b). Feifukang ameliorates pulmonary fibrosis by inhibiting jak-stat signaling pathway. BMC Complement. Altern. Med. 18 (1), 234. doi:10.1186/s12906-018-2297-3
Li, M., Duan, L., Li, Y., and Liu, B. (2019b). Long noncoding RNA/circular noncoding RNA-miRNA-mRNA axes in cardiovascular diseases. Life Sci. 233, 116440. doi:10.1016/j.lfs.2019.04.066
Li, Y., Sun, W., Pan, H., Yuan, J., Xu, Q., Xu, T., et al. (2021b). Lncrna-pvt1 activates lung fibroblasts via mir-497-5p and is facilitated by foxm1. Ecotoxicol. Environ. Saf. 213, 112030. doi:10.1016/j.ecoenv.2021.112030
Li, Y., Xu, J. Z., Gu, C. X., Liu, G. L., and Tian, K. (2019c). Carvacrol suppresses inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes. J. Cell. Biochem. 120 (5), 8169–8176. doi:10.1002/jcb.28098
Lian, Y., Yan, C., Xu, H., Yang, J., Yu, Y., Zhou, J., et al. (2018). A novel lncrna, linc00460, affects cell proliferation and apoptosis by regulating klf2 and cul4a expression in colorectal cancer. Mol. Ther. Nucleic Acids 12, 684–697. doi:10.1016/j.omtn.2018.06.012
Ling, S., Kwak, D., Takuwa, Y., Ge, C., Franceschi, R., and Kim, K. K. (2024). Discoidin domain receptor 2 signaling through PIK3C2α in fibroblasts promotes lung fibrosis. J. Pathol. 262 (4), 505–516. doi:10.1002/path.6253
Liu, G., Cooley, M. A., Jarnicki, A. G., Borghuis, T., Nair, P. M., Tjin, G., et al. (2019). Fibulin-1c regulates transforming growth factor-β activation in pulmonary tissue fibrosis. JCI Insight 5 (16), e124529. doi:10.1172/jci.insight.124529
Liu, G., Friggeri, A., Yang, Y., Milosevic, J., Ding, Q., Thannickal, V. J., et al. (2010a). Mir-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J. Exp. Med. 207 (8), 1589–1597. doi:10.1084/jem.20100035
Liu, M., Song, L., Lai, Y., Gao, F., and Man, J. (2024). LncRNA FEZF1-AS1 promotes pulmonary fibrosis via up-regulating EZH2 and targeting miR-200c-3p to regulate the ZEB1 pathway. Sci. Rep. 14 (1), 26044. doi:10.1038/s41598-024-74570-7
Liu, T., Hooda, J., Atkinson, J. M., Whiteside, T. L., Oesterreich, S., and Lee, A. V. (2021a). Exosomes in breast cancer - mechanisms of action and clinical potential. Mol. Cancer Res. 19 (6), 935–945. doi:10.1158/1541-7786.MCR-20-0952
Liu, X., Li, F., Sun, S. Q., Thangavel, M., Kaminsky, J., Balazs, L., et al. (2010b). Fibroblast-specific expression of AC6 enhances beta-adrenergic and prostacyclin signaling and blunts bleomycin-induced pulmonary fibrosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 298 (6), L819–L829. doi:10.1152/ajplung.00429.2009
Liu, Y., Li, Y., Xu, Q., Yao, W., Wu, Q., Yuan, J., et al. (2018b). Long non-coding RNA-ATB promotes EMT during silica-induced pulmonary fibrosis by competitively binding miR-200c. Biochim. Biophys. Acta-Mol. Basis Dis. 1864 (2), 420–431. doi:10.1016/j.bbadis.2017.11.003
Liu, Y., Tang, G., and Li, J. (2022). Long non-coding rna neat1 participates in ventilator-induced lung injury by regulating mir-20b expression. Mol. Med. Rep. 25 (2), 66. doi:10.3892/mmr.2022.12582
Liu, Y. C., Hong, H. C., Yang, C. D., Lee, W. H., Huang, H. T., and Huang, H. D. (2018a). Ouroboros resembling competitive endogenous loop (orcel) in circular rnas revealed through transcriptome sequencing dataset analysis. BMC Genomics 19 (Suppl. 2), 171. doi:10.1186/s12864-018-4456-9
Liu, Z., Dai, J., and Shen, H. (2018c). Systematic analysis reveals long noncoding RNAs regulating neighboring transcription factors in human cancers. Biochim. Biophys. Acta-Mol. Basis Dis. 1864 (9 Pt B), 2785–2792. doi:10.1016/j.bbadis.2018.05.006
Liu, Z., Ma, T., Duan, J., Liu, X., and Liu, L. (2021b). Microrna-223-induced inhibition of the fbxw7 gene affects the proliferation and apoptosis of colorectal cancer cells via the notch and akt/mtor pathways. Mol. Med. Rep. 23 (2), 154. doi:10.3892/mmr.2020.11793
Lu, G., and Zhang, Y. (2020). Long non-coding rna atb promotes human non-small cell lung cancer proliferation and metastasis by suppressing mir-141-3p. PLoS One 15 (2), e0229118. doi:10.1371/journal.pone.0229118
Lu, H., Han, X., Ren, J., Ren, K., Li, Z., and Sun, Z. (2020). Circular rna hipk3 induces cell proliferation and inhibits apoptosis in non-small cell lung cancer through sponging mir-149. Cancer Biol. Ther. 21 (2), 113–121. doi:10.1080/15384047.2019.1669995
Lu, Q., and El-Hashash, A. (2019). Cell-based therapy for idiopathic pulmonary fibrosis. Stem Cell Investig. 6, 22. doi:10.21037/sci.2019.06.09
Lu, Y., Zhao, J., Tian, Y., Shao, D., Zhang, Z., Li, S., et al. (2022). Dichotomous roles of men1 in macrophages and fibroblasts in bleomycin-induced pulmonary fibrosis. Int. J. Mol. Sci. 23 (10), 5385. doi:10.3390/ijms23105385
Luo, Q., Ren, Z., Zhu, L., Shao, Y., Xie, Y., Feng, Y., et al. (2017). Involvement of microRNA-146a in the inflammatory response of S tatus epilepticus rats. CNS Neurol. Disord.-Drug Targets 16 (6), 686–693. doi:10.2174/1871527316666170505123956
Martinez, F. J., Collard, H. R., Pardo, A., Raghu, G., Richeldi, L., Selman, M., et al. (2017). Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 3, 17074. doi:10.1038/nrdp.2017.74
Martinez-Campa, C., Alvarez-Garcia, V., Alonso-Gonzalez, C., Gonzalez, A., and Cos, S. (2024). Melatonin and its role in the epithelial-to-mesenchymal transition (EMT) in cancer. Cancers 16 (5), 956. doi:10.3390/cancers16050956
Mason, D. P., Brizzio, M. E., Alster, J. M., McNeill, A. M., Murthy, S. C., Budev, M. M., et al. (2007). Lung transplantation for idiopathic pulmonary fibrosis. Ann. Thorac. Surg. 84 (4), 1121–1128. doi:10.1016/j.athoracsur.2007.04.096
Matsushima, S., and Ishiyama, J. (2016). Microrna-29c regulates apoptosis sensitivity via modulation of the cell-surface death receptor, fas, in lung fibroblasts. Am. J. Physiol.-Lung Cell. Mol. Physiol. 311 (6), L1050-L1061–L1061. doi:10.1152/ajplung.00252.2016
McMillan, D. H., Woeller, C. F., Thatcher, T. H., Spinelli, S. L., Maggirwar, S. B., Sime, P. J., et al. (2013). Attenuation of inflammatory mediator production by the NF-κB member RelB is mediated by microRNA-146a in lung fibroblasts. Am. J. Physiol.-Lung Cell. Mol. Physiol. 304 (11), L774–L781. doi:10.1152/ajplung.00352.2012
Medhat, E., Ayeldeen, G., Hosni, A. H., Shaker, O., Gheita, T., and Salama, A. S. (2022). Hotair and thril long non coding rnas and their target genes in rheumatoid arthritis patients. Rep. Biochem. Mol. Biol. 10 (4), 614–621. doi:10.52547/rbmb.10.4.697
Munoz-Felix, J. M., Perretta-Tejedor, N., Eleno, N., Lopez-Novoa, J. M., and Martinez-Salgado, C. (2014). ALK1 heterozygosity increases extracellular matrix protein expression, proliferation and migration in fibroblasts. Biochim. Biophys. Acta 1843 (6), 1111–1122. doi:10.1016/j.bbamcr.2014.02.017
Nakamura, Y., and Shimizu, Y. (2023). Cellular and molecular control of lipid metabolism in idiopathic pulmonary fibrosis: clinical application of the lysophosphatidic acid pathway. Cells 12 (4), 548. doi:10.3390/cells12040548
Nasimi, S. A., Fanoodi, A., Maharati, A., Akhlaghipour, I., Bina, A. R., Saburi, E., et al. (2024). Role of micrornas in tumor progression by regulation of kinesin motor proteins. Int. J. Biol. Macromol. 270 (Pt 1), 132347. doi:10.1016/j.ijbiomac.2024.132347
Nguyen, H. P., Simpson, R. J., Salamonsen, L. A., and Greening, D. W. (2016). Extracellular vesicles in the intrauterine environment: challenges and potential functions. Biol. Reprod. 95 (5), 109. doi:10.1095/biolreprod.116.143503
Nho, R. S., Ballinger, M. N., Rojas, M. M., Ghadiali, S. N., and Horowitz, J. C. (2022). Biomechanical force and cellular stiffness in lung fibrosis. Am. J. Pathol. 192 (5), 750–761. doi:10.1016/j.ajpath.2022.02.001
Nividha, , Maiti, A., Parihar, K., Chakraborty, R., Agarwala, P., Sasmal, D. K., et al. (2024). Enzyme-regulated non-thermal fluctuations enhance ligand diffusion and receptor-mediated endocytosis. bioRxiv, 627033. doi:10.1101/2024.12.05.627033
Njock, M. S., Guiot, J., Henket, M. A., Nivelles, O., Thiry, M., Dequiedt, F., et al. (2019). Sputum exosomes: promising biomarkers for idiopathic pulmonary fibrosis. Thorax 74 (3), 309–312. doi:10.1136/thoraxjnl-2018-211897
Nonaka, M., Pawankar, R., Fukumoto, A., and Yagi, T. (2008). Heterogeneous response of nasal and lung fibroblasts to transforming growth factor-beta 1. Clin. Exp. Allergy. 38 (5), 812–821. doi:10.1111/j.1365-2222.2008.02959.x
Ohta, M., Kihara, T., Toriuchi, K., Aoki, H., Iwaki, S., Kakita, H., et al. (2020). IL-6 promotes cell adhesion in human endothelial cells via microRNA-126-3p suppression. Exp. Cell Res. 393 (2), 112094. doi:10.1016/j.yexcr.2020.112094
Ouyang, Q., Huang, Q., Jiang, Z., Zhao, J., Shi, G. P., and Yang, M. (2018). Using plasma circRNA_002453 as a novel biomarker in the diagnosis of lupus nephritis. Mol. Immunol. 101, 531–538. doi:10.1016/j.molimm.2018.07.029
Panda, A. C. (2018). Circular RNAs act as miRNA sponges. Adv. Exp. Med. Biol. 1087, 67–79. doi:10.1007/978-981-13-1426-1_6
Pandit, K. V., Milosevic, J., and Kaminski, N. (2011). MicroRNAs in idiopathic pulmonary fibrosis. Transl. Res. 157 (4), 191–199. doi:10.1016/j.trsl.2011.01.012
Papa, A., Wan, L., Bonora, M., Salmena, L., Song, M. S., Hobbs, R. M., et al. (2014). Cancer-associated PTEN mutants act in a dominant-negative manner to suppress PTEN protein function. Cell. 157 (3), 595–610. doi:10.1016/j.cell.2014.03.027
Paul, N., Sultana, Z., Fisher, J. J., Maiti, K., and Smith, R. (2023). Extracellular vesicles-crucial players in human pregnancy. Placenta 140, 30–38. doi:10.1016/j.placenta.2023.07.006
Penke, L. R., Speth, J. M., Huang, S. K., Fortier, S. M., Baas, J., and Peters-Golden, M. (2022). KLF4 is a therapeutically tractable brake on fibroblast activation that promotes resolution of pulmonary fibrosis. JCI Insight 7 (16), e160688. doi:10.1172/jci.insight.160688
Poulet, C., Njock, M. S., Moermans, C., Louis, E., Louis, R., Malaise, M., et al. (2020). Exosomal long non-coding rnas in lung diseases. Int. J. Mol. Sci. 21 (10), 3580. doi:10.3390/ijms21103580
Prasse, A. (2015). Idiopathic pulmonary fibrosis. Pneumologie 69 (10), 608–614. 615. doi:10.1055/s-0034-1393036
Putta, S., Lanting, L., Sun, G., Lawson, G., Kato, M., and Natarajan, R. (2012). Inhibiting microrna-192 ameliorates renal fibrosis in diabetic nephropathy. J. Am. Soc. Nephrol. 23 (3), 458–469. doi:10.1681/ASN.2011050485
Qi, J. J., Liu, Y. X., and Lin, L. (2017). High expression of long non-coding RNA ATB is associated with poor prognosis in patients with renal cell carcinoma. Eur. Rev. Med. Pharmacol. Sci. 21 (12), 2835–2839.
Qi, Y., Han, W., Chen, D., Zhao, J., Bai, L., Huang, F., et al. (2021). Engineering circular RNA regulators to specifically promote circular RNA production. Theranostics 11 (15), 7322–7336. doi:10.7150/thno.56990
Que, W., Liu, H., and Yang, Q. (2022). CircPRKCH modulates extracellular matrix formation and metabolism by regulating the miR-145/HGF axis in osteoarthritis. Arthritis Res. Ther. 24 (1), 216. doi:10.1186/s13075-022-02893-9
Ribeiro, D. M., Zanzoni, A., Cipriano, A., Delli, P. R., Spinelli, L., Ballarino, M., et al. (2018). Protein complex scaffolding predicted as a prevalent function of long non-coding RNAs. Nucleic. acids. Res. 46 (2), 917–928. doi:10.1093/nar/gkx1169
Rodrigo, G. (2018). Post-transcriptional bursting in genes regulated by small rna molecules. Phys. Rev. E. 97 (3-1), 32401. doi:10.1103/PhysRevE.97.032401
Rodriguez, B. R., Ortega, G. A., Hidalgo, M. A., Zentella, D. A., Villarreal-Garza, C., Avila-Moreno, F., et al. (2018). Long non-coding RNAs: implications in targeted diagnoses, prognosis, and improved therapeutic strategies in human non- and triple-negative breast cancer. Clin. Epigenetics. 10, 88. doi:10.1186/s13148-018-0514-z
Sabatke, B., Rossi, I. V., Sana, A., Bonato, L. B., and Ramirez, M. I. (2024). Extracellular vesicles biogenesis and uptake concepts: a comprehensive guide to studying host-pathogen communication. Mol. Microbiol. 122 (5), 613–629. doi:10.1111/mmi.15168
Savary, G., Dewaeles, E., Diazzi, S., Buscot, M., Nottet, N., Fassy, J., et al. (2019). The long noncoding RNA DNM3OS is a reservoir of FibromiRs with major functions in lung fibroblast response to TGF-β and pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 200 (2), 184–198. doi:10.1164/rccm.201807-1237OC
Sayad, A., Hajifathali, A., Omrani, M. D., Arsang-Jang, S., Hamidieh, A. A., and Taheri, M. (2018). Expression of tnf- and hnrnpl-related immunoregulatory long non-coding rna (thril) in acute myeloid leukemia: is there any correlation?Iran. J. Allergy Asthma Immunol. 17 (3), 274–280.
Schober, A., Nazari-Jahantigh, M., Wei, Y., Bidzhekov, K., Gremse, F., Grommes, J., et al. (2014). MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1. Nat. Med. 20 (4), 368–376. doi:10.1038/nm.3487
Shan, Z., Qin, S., Li, W., Wu, W., Yang, J., Chu, M., et al. (2015). An endocrine genetic signal between blood cells and vascular smooth muscle cells: role of MicroRNA-223 in smooth muscle function and atherogenesis. J. Am. Coll. Cardiol. 65 (23), 2526–2537. doi:10.1016/j.jacc.2015.03.570
Sharma, S., Zuniga, F., Rice, G. E., Perrin, L. C., Hooper, J. D., and Salomon, C. (2017). Tumor-derived exosomes in ovarian cancer - liquid biopsies for early detection and real-time monitoring of cancer progression. Oncotarget 8 (61), 104687–104703. doi:10.18632/oncotarget.22191
She, Q., Shi, P., Xu, S. S., Xuan, H. Y., Tao, H., Shi, K. H., et al. (2020). Dnmt1 methylation of lncrna gas5 leads to cardiac fibroblast pyroptosis via affecting nlrp3 axis. Inflammation 43 (3), 1065–1076. doi:10.1007/s10753-020-01191-3
Shen, F., Liu, P., Xu, Z., Li, N., Yi, Z., Tie, X., et al. (2019). Circrna_001569 promotes cell proliferation through absorbing mir-145 in gastric cancer. J. Biochem. 165 (1), 27–36. doi:10.1093/jb/mvy079
Shumar, J. N., Chandel, A., and King, C. S. (2021). Antifibrotic therapies and progressive fibrosing interstitial lung disease (pf-ild): building on inbuild. J. Clin. Med. 10 (11), 2285. doi:10.3390/jcm10112285
Spagnolo, P., Sverzellati, N., Rossi, G., Cavazza, A., Tzouvelekis, A., Crestani, B., et al. (2015). Idiopathic pulmonary fibrosis: an update. Ann. Med. 47 (1), 15–27. doi:10.3109/07853890.2014.982165
Strykowski, R., and Adegunsoye, A. (2023). Idiopathic pulmonary fibrosis and progressive pulmonary fibrosis. Immunol. Allerg. Clin. North Am. 43 (2), 209–228. doi:10.1016/j.iac.2023.01.010
Su, L. C., Huang, A. F., Jia, H., Liu, Y., and Xu, W. D. (2017). Role of microRNA-155 in rheumatoid arthritis. Int. J. Rheum. Dis. 20 (11), 1631–1637. doi:10.1111/1756-185X.13202
Sun, H. J., Zhu, X. X., Cai, W. W., and Qiu, L. Y. (2017). Functional roles of exosomes in cardiovascular disorders: a systematic review. Eur. Rev. Med. Pharmacol. Sci. 21 (22), 5197–5206. doi:10.26355/eurrev_201711_13840
Sun, J., Jin, T., Niu, Z., Guo, J., Guo, Y., Yang, R., et al. (2022). Lncrna dach1 protects against pulmonary fibrosis by binding to srsf1 to suppress ctnnb1 accumulation. Acta Pharm. Sin. B 12 (9), 3602–3617. doi:10.1016/j.apsb.2022.04.006
Sun, X. H., Wang, X., Zhang, Y., and Hui, J. (2019). Exosomes of bone-marrow stromal cells inhibit cardiomyocyte apoptosis under ischemic and hypoxic conditions via miR-486-5p targeting the PTEN/PI3K/AKT signaling pathway. Thromb. Res. 177, 23–32. doi:10.1016/j.thromres.2019.02.002
Surendran, A., Huang, C., and Liu, L. (2024). Circular RNAs and their roles in idiopathic pulmonary fibrosis. Respir. Res. 25 (1), 77. doi:10.1186/s12931-024-02716-2
Tam, T. S., Bastian, I., Zhou, X. F., Vander, H. M., Michael, M. Z., Gibbins, I. L., et al. (2011). Microrna-143 expression in dorsal root ganglion neurons. Cell. Tissue. Res. 346 (2), 163–173. doi:10.1007/s00441-011-1263-x
Tang, Y., He, R., An, J., Deng, P., Huang, L., and Yang, W. (2016). The effect of h19-mir-29b interaction on bleomycin-induced mouse model of idiopathic pulmonary fibrosis. Biochem. Biophys. Res. Commun. 479 (3), 417–423. doi:10.1016/j.bbrc.2016.09.028
Tao, H., Shi, P., Zhao, X. D., Xuan, H. Y., and Ding, X. S. (2020). MeCP2 inactivation of LncRNA GAS5 triggers cardiac fibroblasts activation in cardiac fibrosis. Cell. Signal. 74, 109705. doi:10.1016/j.cellsig.2020.109705
Tian, C., Hu, S., Yu, J., Li, W., Li, P., and Huang, H. (2022). Creb1 transcription-activated lncrna pvt1 promotes cardiac fibrosis via mir-145/hcn1 axis. Int. J. Cardiol. 353, 88–95. doi:10.1016/j.ijcard.2022.01.024
Tjin, G., White, E. S., Faiz, A., Sicard, D., Tschumperlin, D. J., Mahar, A., et al. (2017). Lysyl oxidases regulate fibrillar collagen remodelling in idiopathic pulmonary fibrosis. Dis. Model. Mech. 10 (11), 1301–1312. doi:10.1242/dmm.030114
Tu, B., Song, K., Zhou, Y., Sun, H., Liu, Z. Y., Lin, L. C., et al. (2023). METTL3 boosts mitochondrial fission and induces cardiac fibrosis by enhancing LncRNA GAS5 methylation. Pharmacol. Res. 194, 106840. doi:10.1016/j.phrs.2023.106840
van Wijk, N., Zohar, K., and Linial, M. (2022). Challenging cellular homeostasis: spatial and temporal regulation of mirnas. Int. J. Mol. Sci. 23 (24), 16152. doi:10.3390/ijms232416152
Varone, F., Sgalla, G., Iovene, B., Bruni, T., and Richeldi, L. (2018). Nintedanib for the treatment of idiopathic pulmonary fibrosis. Expert Opin. Pharmacother. 19 (2), 167–175. doi:10.1080/14656566.2018.1425681
Vasse, G. F., Van Os, L., De Jager, M., Jonker, M. R., Borghuis, T., Van Den Toorn, L. T., et al. (2021). Adipose stromal cell-secretome counteracts profibrotic signals from ipf lung matrices. Front. Pharmacol. 12, 669037. doi:10.3389/fphar.2021.669037
Vickers, K. C., Landstreet, S. R., Levin, M. G., Shoucri, B. M., Toth, C. L., Taylor, R. C., et al. (2014). MicroRNA-223 coordinates cholesterol homeostasis. Proc. Natl. Acad. Sci. U. S. A. 111 (40), 14518–14523. doi:10.1073/pnas.1215767111
Videira, R. F., and Da, C. M. P. (2020). Non-coding RNAs in cardiac intercellular communication. Front. Physiol. 11, 738. doi:10.3389/fphys.2020.00738
Wan, X., Tian, X., Du, J., Lu, Y., and Xiao, Y. (2020). Long non-coding RNA H19 deficiency ameliorates bleomycin-induced pulmonary inflammation and fibrosis. Respir. Res. 21 (1), 290. doi:10.1186/s12931-020-01534-6
Wang, B., Ding, X., Ding, C., Tesch, G., Zheng, J., Tian, P., et al. (2020). WNT1-inducible-signaling pathway protein 1 regulates the development of kidney fibrosis through the TGF-β1 pathway. Faseb. J. 34 (11), 14507–14520. doi:10.1096/fj.202000953R
Wang, J., Zhang, Y., Lian, W., and Gan, M. (2024). Long non-coding rna pvt1 facilitates radiation-induced vascular endothelial cell injury through sponging microrna-9-5p. Radiat. Res. 202 (4), 670–676. doi:10.1667/RADE-24-00089.1
Wang, L. M., Zhang, L. L., Wang, L. W., Zhu, L., and Ma, X. X. (2019). Influence of miR-199a on rats with non-small cell lung cancer via regulating the HIF-1α/VEGF signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 23 (23), 10363–10369. doi:10.26355/eurrev_201912_19675
Wang, S., Luo, W., Huang, J., Chen, M., Ding, J., Cheng, Y., et al. (2022). The combined effects of circular rna methylation promote pulmonary fibrosis. Am. J. Respir. Cell. Mol. Biol. 66 (5), 510–523. doi:10.1165/rcmb.2021-0379OC
Wang, X., Yang, M., Zhu, J., Zhou, Y., and Li, G. (2024). Role of exosomal non-coding rnas in ovarian cancer (review). Int. J. Mol. Med. 54 (4), 87. doi:10.3892/ijmm.2024.5411
Wang, Z., Feng, C., Song, K., Qi, Z., Huang, W., and Wang, Y. (2020). Lncrna-h19/mir-29a axis affected the viability and apoptosis of keloid fibroblasts through acting upon col1a1 signaling. J. Cell. Biochem. 121 (11), 4364–4376. doi:10.1002/jcb.29649
Wang, Z., Tan, W., Li, B., Zou, J., Li, Y., Xiao, Y., et al. (2023). Exosomal non-coding RNAs in angiogenesis: functions, mechanisms and potential clinical applications. Heliyon 9 (8), e18626. doi:10.1016/j.heliyon.2023.e18626
Wei, Y., Yan, X., Yan, L., Hu, F., Ma, W., Wang, Y., et al. (2017). Inhibition of microRNA‑155 ameliorates cardiac fibrosis in the process of angiotensin II‑induced cardiac remodeling. Mol. Med. Rep. 16 (5), 7287–7296. doi:10.3892/mmr.2017.7584
Weng, X. Q., and Wang, W. (2022). Mir-137 modulates human gastric cancer cell proliferation, apoptosis, and migration by targeting ezh2. Crit. Rev. Eukaryot. Gene. Expr. 32 (4), 31–40. doi:10.1615/CritRevEukaryotGeneExpr.2022041013
Willms, E., Johansson, H. J., Mager, I., Lee, Y., Blomberg, K. E., Sadik, M., et al. (2016). Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 6, 22519. doi:10.1038/srep22519
Xiao, L., Ma, X. X., Luo, J., Chung, H. K., Kwon, M. S., Yu, T. X., et al. (2021). Circular rna circhipk3 promotes homeostasis of the intestinal epithelium by reducing microrna 29b function. Gastroenterology 161 (4), 1303–1317.e3. doi:10.1053/j.gastro.2021.05.060
Xu, B., Jin, X., Yang, T., Zhang, Y., Liu, S., Wu, L., et al. (2020a). Upregulated lncrna thril/tnf-alpha signals promote cell growth and predict poor clinical outcomes of osteosarcoma. OncoTargets Ther. 13, 119–129. doi:10.2147/OTT.S235798
Xu, F., Xu, F., Xie, S., Zuo, W., Wen, G., Zhao, T., et al. (2020b). MicroRNA-448 overexpression inhibits fibroblast proliferation and collagen synthesis and promotes cell apoptosis via targeting ABCC3 through the JNK signaling pathway. J. Cell. Physiol. 235 (2), 1374–1385. doi:10.1002/jcp.29056
Yang, D., Xu, P., Su, H., Zhong, W., Xu, J., Su, Z., et al. (2022). The histone methyltransferase DOT1L is a new epigenetic regulator of pulmonary fibrosis. Cell Death Dis. 13 (1), 60. doi:10.1038/s41419-021-04365-5
Yang, G., Tian, Y., Li, C., Xia, J., Qi, Y., Yao, W., et al. (2022). LncRNA UCA1 regulates silicosis-related lung epithelial cell-to-mesenchymal transition through competitive adsorption of miR-204-5p. Toxicol. Appl. Pharmacol. 441, 115977. doi:10.1016/j.taap.2022.115977
Yang, H., Fu, H., Xu, W., and Zhang, X. (2016). Exosomal non-coding RNAs: a promising cancer biomarker. Clin. Chem. Lab. Med. 54 (12), 1871–1879. doi:10.1515/cclm-2016-0029
Yang, J., Zhang, Y., Huang, L., Lan, Y. J., and Zhang, Q. (2019). The inhibition effect of mir-29c on lung fibroblasts transdifferentiation induced by sio(2). Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 37 (5), 321–326. doi:10.3760/cma.j.issn.1001-9391.2019.05.001
Yang, L., Herrera, J., Gilbertsen, A., Xia, H., Smith, K., Benyumov, A., et al. (2018). Il-8 mediates idiopathic pulmonary fibrosis mesenchymal progenitor cell fibrogenicity. Am. J. Physiol.-Lung Cell. Mol. Physiol. 314 (1), L127-L136–L136. doi:10.1152/ajplung.00200.2017
Yang, S., Banerjee, S., de Freitas, A., Sanders, Y. Y., Ding, Q., Matalon, S., et al. (2012). Participation of miR-200 in pulmonary fibrosis. Am. J. Pathol. 180 (2), 484–493. doi:10.1016/j.ajpath.2011.10.005
Yang, S., Cui, H., Xie, N., Icyuz, M., Banerjee, S., Antony, V. B., et al. (2013). miR-145 regulates myofibroblast differentiation and lung fibrosis. Faseb. J. 27 (6), 2382–2391. doi:10.1096/fj.12-219493
Yao, J., Dai, Q., Liu, Z., Zhou, L., and Xu, J. (2018). Circular RNAs in organ fibrosis. Adv. Exp. Med. Biol. 1087, 259–273. doi:10.1007/978-981-13-1426-1_21
Yue, Q. Y., and Zhang, Y. (2018). Effects of linc00460 on cell migration and invasion through regulating epithelial-mesenchymal transition (emt) in non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 22 (4), 1003–1010. doi:10.26355/eurrev_201802_14382
Zaborowski, M. P., Balaj, L., Breakefield, X. O., and Lai, C. P. (2015). Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience 65 (8), 783–797. doi:10.1093/biosci/biv084
Zang, H., Zhang, Q., and Li, X. (2021). Non-coding rna networks in pulmonary hypertension. Front. Genet. 12, 703860. doi:10.3389/fgene.2021.703860
Zerr, P., Palumbo-Zerr, K., Huang, J., Tomcik, M., Sumova, B., Distler, O., et al. (2016). Sirt1 regulates canonical TGF-β signalling to control fibroblast activation and tissue fibrosis. Ann. Rheum. Dis. 75 (1), 226–233. doi:10.1136/annrheumdis-2014-205740
Zhang, C., Yao, Z., Zhu, M., Ma, X., Shi, T., Li, H., et al. (2012). Inhibitory effects of microrna-34a on cell migration and invasion of invasive urothelial bladder carcinoma by targeting notch1. J. Huazhong Univ. Sci. Technol. Med. Sci. 32 (3), 375–382. doi:10.1007/s11596-012-0065-z
Zhang, E., Geng, X., Shan, S., Li, P., Li, S., Li, W., et al. (2021). Exosomes derived from bone marrow mesenchymal stem cells reverse epithelial-mesenchymal transition potentially via attenuating Wnt/β-catenin signaling to alleviate silica-induced pulmonary fibrosis. Toxicol. Mech. Methods. 31 (9), 655–666. doi:10.1080/15376516.2021.1950250
Zhang, F., Geng, L., Zhang, J., Han, S., Guo, M., Xu, Y., et al. (2024). Mir-486-5p diagnosed atrial fibrillation, predicted the risk of left atrial fibrosis, and regulated angiotensin ii-induced cardiac fibrosis via modulating pi3k/akt signaling through targeting foxo1. Mol. Cell. Biochem. 480, 1077–1087. doi:10.1007/s11010-024-05027-8
Zhang, H., Song, M., Guo, J., Ma, J., Qiu, M., and Yang, Z. (2021). The function of non-coding RNAs in idiopathic pulmonary fibrosis. Open Med. 16 (1), 481–490. doi:10.1515/med-2021-0231
Zhang, J., Zhang, P., Wang, L., Piao, H. L., and Ma, L. (2014). Long non-coding RNA HOTAIR in carcinogenesis and metastasis. Acta Biochim. Biophys. Sin. 46 (1), 1–5. doi:10.1093/abbs/gmt117
Zhang, L., Yang, Z., Huang, W., and Wu, J. (2019). H19 potentiates let-7 family expression through reducing PTBP1 binding to their precursors in cholestasis. Cell Death Dis. 10 (3), 168. doi:10.1038/s41419-019-1423-6
Zhang, M., and Zhang, C. (2024). Preferential cleavage of upstream targets in concatenated mirna/sirna target sites support a 5'-3' scanning model for risc target recognition. Biochem. Biophys. Res. Commun. 703, 149662. doi:10.1016/j.bbrc.2024.149662
Zhang, Z., Wang, X., Cao, S., Han, X., Wang, Z., Zhao, X., et al. (2018). The long noncoding rna tug1 promotes laryngeal cancer proliferation and migration. Cell Physiol. Biochem. 49 (6), 2511–2520. doi:10.1159/000493876
Zhao, P., Ma, G., and Ma, L. (2023). Circ_0000479 promotes proliferation, invasion, migration and inflammation and inhibits apoptosis of rheumatoid arthritis fibroblast-like synoviocytes via miR-766/FKBP5 axis. J. Orthop. Surg. Res. 18 (1), 220. doi:10.1186/s13018-023-03700-0
Zhao, S., Xiao, X., Sun, S., Li, D., Wang, W., Fu, Y., et al. (2018). MicroRNA-30d/JAG1 axis modulates pulmonary fibrosis through Notch signaling pathway. Pathol. Res. Pract. 214 (9), 1315–1323. doi:10.1016/j.prp.2018.02.014
Zhao, X., Sun, W., Guo, B., and Cui, L. (2022). Circular RNA BIRC6 depletion promotes osteogenic differentiation of periodontal ligament stem cells via the miR-543/PTEN/PI3K/AKT/mTOR signaling pathway in the inflammatory microenvironment. Stem Cell Res. Ther. 13 (1), 417. doi:10.1186/s13287-022-03093-7
Zhou, J., Chen, Y., He, M., Li, X., and Wang, R. (2022). Role of circular rnas in pulmonary fibrosis. Int. J. Mol. Sci. 23 (18), 10493. doi:10.3390/ijms231810493
Zhou, Z., Xie, Y., Wei, Q., Zhang, X., and Xu, Z. (2024). Revisiting the role of MicroRNAs in the pathogenesis of idiopathic pulmonary fibrosis. Front. Cell. Dev. Biol. 12, 1470875. doi:10.3389/fcell.2024.1470875
Zhu, G., Chai, J., Ma, L., Duan, H., and Zhang, H. (2013). Downregulated microRNA-32 expression induced by high glucose inhibits cell cycle progression via PTEN upregulation and Akt inactivation in bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 433 (4), 526–531. doi:10.1016/j.bbrc.2013.03.018
Keywords: idiopathic pulmonary fibrosis, exosomes, non-coding RNAs, function, review
Citation: Wei Y, Hong M, Zhu H and Li F (2025) Recent progress in exosomal non-coding RNAs research related to idiopathic pulmonary fibrosis. Front. Genet. 16:1556495. doi: 10.3389/fgene.2025.1556495
Received: 08 January 2025; Accepted: 10 March 2025;
Published: 27 March 2025.
Edited by:
Guilherme Targino Valente, São Paulo State University, BrazilReviewed by:
Rinku Sharma, Brigham and Women’s Hospital, United StatesCopyright © 2025 Wei, Hong, Zhu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Li, bGlmZW5nMUBudHNkbHJteXkud2Vjb20ud29yaw==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.