
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 12 March 2025
Sec. Computational Genomics
Volume 16 - 2025 | https://doi.org/10.3389/fgene.2025.1500061
Background: Preeclampsia (PE), a major obstetric disorder marked by dysfunction in both placental and maternal vascular systems, continues to pose critical challenges in global maternal healthcare. This multisystem pregnancy complication contributes significantly to adverse perinatal outcomes and remains a leading cause of pregnancy-related morbidity worldwide. However, the available treatment options at present remain restricted. Our investigation employs an integrative bioinformatics approach to elucidate critical molecular signatures linked to the interplay between immunological dysregulation and oxidative stress mechanisms in PE pathogenesis.
Methods: In this study, we sourced the dataset from the GEO database with the aim of pinpointing differentially expressed genes (DEGs) between PE samples and control samples. Genes associated with oxidative stress were procured from the Genecards database. Next, we employed a comprehensive approach. This involved integrating WGCNA, GO and KEGG pathway analyses, constructing PPI networks, applying machine learning algorithms, performing gene GSEA, and conducting immune infiltration analysis to identify the key hub genes related to oxidative stress. Diagnostic potential of candidate biomarkers was quantitatively assessed through ROC curve modeling. Additionally, we constructed a miRNA - gene regulatory network for the identified diagnostic genes and predicted potential candidate drugs. In the final step, we validated the significant hub gene using independent external datasets, the hypoxia model of the HTR-8/SVneo cell line, and human placental tissue samples.
Results: At last, leptin (LEP) was identified as a core gene through screening and was found to be upregulated. The results of quantitative real-time polymerase chain reaction (qRT -PCR) and immunohistochemistry validation were consistent with those obtained from the datasets. KEGG analysis revealed that LEP was significantly enriched in “allograft rejection,” “antigen processing,” “ECM receptor interaction” and “graft versus host disease.” GO analysis revealed that LEP was involved in biological processes such as “antigen processing and presentation,” “peptide antigen assembly with MHC protein complex,” “complex of collagen trimers,” “MHC class II protein complex” and “mitochondrial protein containing complex.” Moreover, immune cell analysis indicated that T follicular helper cells, plasmacytoid dendritic cells, neutrophils, and activated dendritic cells were positively correlated with LEP expression, whereas γδT cells, eosinophils, and central memory CD4+ T cells showed a negative correlation. These findings suggest that LEP influences the immune microenvironment of PE through its interaction with arious immune cells. In addition, 28 miRNAs and 15 drugs were predicted to target LEP. Finally, the overexpression of LEP was verified using independent external datasets, the hypoxia model of the HTR-8/SVneo cell line, and human placental tissue.
Conclusion: Through an integrated analytical framework employing WGCNA coupled with three distinct machine learning-driven phenotypic classification models, we discovered a pivotal regulatory gene. This gene has the potential to act as a novel diagnostic biomarker for PE. Moreover, it can be considered as a promising target for drug development related to PE. Notably, it shows a strong correlation with the immune microenvironment, suggesting its crucial role in the complex pathophysiological processes underlying PE.
Preeclampsia (PE) is a pregnancy-specific, multisystemic disorder characterized by clinical manifestations such as new-onset hypertension and proteinuria after 20 weeks of gestation. It stands as a significant contributor to maternal and fetal mortality rates (Mol et al., 2016). The incidence of PE varies based on geographic location, season, nutritional factors, and racial/ethnic backgrounds; however, it impacts approximately 3%–5% of women globally (Mol et al., 2016). Maternal complications associated with PE encompass eclampsia, renal failure, and hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome. The pathophysiological mechanisms of PE encompass endothelial damage, systemic arteriolar spasm, reduced systemic perfusion, and multiorgan dysfunction, which collectively pose a significant threat to the health of both mothers and infants (San Juan-Reyes et al., 2020; Ziganshina et al., 2020).Currently, the only effective treatment is the termination of pregnancy (Tannetta and Sargent, 2013). Unfortunately, the exact etiology and pathogenesis remain unelucidated. However, placental dysfunction, characterized by inadequate trophoblast invasion of spiral arterioles, is thought to play a central role. This dysfunction may be triggered by an imbalance between the production and deactivation of reactive oxygen species (ROS) in the placenta (Mol et al., 2016; Roberts et al., 1989; Rana et al., 2019; Brown et al., 2018).
Oxidative stress plays a significant pathogenic role in PE, with numerous oxidative stress markers showing diagnostic potential (Agrawal et al., 2018). A substantial body of evidence underscores the crucial involvement of immune responses and oxidative stress in the development of PE (Meng et al., 2021; Afrose et al., 2022; Deer et al., 2021a). The integration of immune infiltration, oxidative stress, and bioinformatics approaches provides new insights into the diagnosis and treatment of PE. Furthermore, two external datasets were utilized to validate the identified gene. Ultimately, the gene was validated through quantitative real-time polymerase chain reaction (qRT -PCR) and immunohistochemistry. The findings of this study might offer new perspectives on the role of oxidative stress in placental pathology and could also facilitate the identification of potential biomarkers and therapeutic targets, providing a novel approach to the clinical diagnosis and treatment of PE. The study route is illustrated in Figure 1.
Human PE datasets were extracted from the Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) in July 2023. Three PE datasets were retrieved: GSE10588 (GPL2986), consisting of 17 cases of PE and 26 control cases; GSE74341 (GPL16699), containing 12 PE cases and 10 control cases for validation; and another validation set GSE98224 (GPL6244)) containing 30 PE cases and 18 control samples. Furthermore, 1142 oxidative stress-related genes were extracted from the Genecards database. Pertinent details information is displayed in Table 1.
After normalizing the data, the “limma” R package (version 3.44.3) was used to perform differential expression analysis. The filter criteria were set at |Log2FC|>0.5 and padj<0.05 (Ritchie et al., 2015). The expression heat map of DEGs was generated by employing the “Pheatmap” R package (version 4.1.0) (Ritchie et al., 2015).
Subsequently, differentially expressed genes related to oxidative stress (DEOSGs) were identified by intersecting the DEGs with the 1142 oxidative stress-related genes.
Weighted gene co-expression network analysis (WGCNA) is an algorithm that can screen candidate biomarkers and the co-expressed gene modules with high correlation coefficients (Langfelder and Horvath, 2008). In this study, the “WGCNA” package (version 4.0.3) was used to identify key module and hub genes associated with PE using the GSE10588 dataset. To construct the network, the co-expression relationship was calculated by Pearson’s correlation coefficient. The optimal soft-thresholding factor (β) was chosen to strengthen strong correlations among the DEGs while penalizing the impact of weak correlations. The adjacency matrix was then converted into a Topological Overlap Matrix (TOM). Using the TOM-based dissimilarity measure, genes with similar expression patterns were grouped into distinct modules through hierarchical clustering. A module with a strong correlation to PE was identified based on gene significance and its correlation with clinical subtypes. The genes from this module were subsequently utilized for further analysis.
Eventually, the genes from DEOSGs and key modules were intersected.
Gene Ontology (GO) analyse serves as a comprehensive repository of computable knowledge concerning the functions of genes and gene products (The Gene Ontology Consortium, 2019). Kyoto Encyclopedia of Genes and Genomes (KEGG) analyse is a widely utilized database for pathway enrichment analysis (Kanehisa and Goto, 2000). GO and KEGG analyses were executed using Metascape (http://metascape.org).
In addition, the GeneMANIA (http://genemania.org/search/) online database was utilized to construct a gene interaction network and analyze the functions of the identified genes. The protein-protein interaction networks (PPI) network was built to examine the interactions between protein-coding genes using the GeneMANIA tool.
To identify key biomarkers, we employed several machine learning methods. Least Absolute Shrinkage and Selection Operator (LASSO) logistic regression analysis is a data mining technique that uses an L1 penalty to eliminate less important variables. This approach helps in selecting significant variables for classification models (Zhang et al., 2021). Support Vector Machine-Recursive Feature Elimination (SVM-RFE) analysis is a supervised machine-learning approach that progressively removes less informative features to identify the most crucial genes (Lin et al., 2017). Random Forest (RF) is a suitable methodology that operates without constraints on variable conditions and evaluates the importance of each gene by assessing how well it contributes to the model’s predictive accuracy across multiple decision trees. It is another machine learning method that works well for prediction tasks with continuous variables, offering high accuracy, sensitivity, and specificity (Ellis et al., 2014). These algorithms complement each other by offering different perspectives on feature selection. While LASSO provides penalization to eliminate irrelevant features, Random Forest evaluates feature importance in terms of model accuracy, and SVM-RFE systematically eliminates less relevant features. By using these three methods in parallel, we aimed to increase the robustness of the selected biomarkers and ensure that the identified biomarkers are consistently relevant across different algorithmic approaches. LASSO regression and RF analysis were conducted using the “glmnet” (Zhang et al., 2019) (version 3.3.3) and “randomForest” (Alderden et al., 2018) (version 3.0.2) R packages. The intersection of the key genes identified by the three algorithms was used for further analysis.
Furthermore, receiver operating characteristic (ROC) curves and the area under the curve (AUC) were employed to assess the diagnostic efficacy. Ultimately, the gene was recognized as a hub gene through both methods combined.
Gene Set Enrichment Analysis (GSEA) is a computational method to analyze gene sets and identify biological functions and signaling pathways (Subramanian et al., 2005). Samples were categorized into high- and low-expression groups based on the gene expression of each diagnostic marker. A GSEA was then conducted to investigate the related biological functions and pathways, with P < 0.05 set as the significance threshold.
To quantify the immune response, we used single-sample GSEA (ssGSEA) to analyze 28 immune-related gene sets. The gene set encompassing 28 immune cell types was derived from a previously published article (Charoentong et al., 2017). Using ssGSEA, a comparison was performed on differential immune cell infiltration patterns and evaluate functional variations in immunoregulatory pathways between cohorts dichotomized by transcript abundance thresholds. The “GSVA” R package was used for this analysis, with P < 0.05 indicating statistical significance.
We utilized the DGIdb (https://www.dgidb.org), a Drug-Gene interaction database (Cotto et al., 2018), to identify potentially druggable targets based on the biomarkers for PE. The gene-drug network was visualized using Cytoscape software (version 3.9.0).
To computationally identify miRNA-mRNA interactions of diagnostic significance, we performed multi-platform target prediction analyses integrating miRanda (http://www.microrna.org/), miRDB (http://mirdb.org/),and TargetScan (http://www.targetscan.org/vert_72/). Consensus predictions derived from tripartite database intersection were subsequently modeled as regulatory networks using Cytoscape (v3.9.0), a graph theory-based visualization platform for biomolecular interaction mapping.
To validate the findings, the expression of key hub genes was verified in external datasets, GSE74341 and GSE98224.
The HTR8/SVneo (CRL-3271; ATCC) cell line was cultured in RPMI-1640 medium (Glibco) supplemented with 5% fetal bovine serum (Glibco) and maintained with antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin). Cells were then incubated at 37°C in 5% CO2 for a duration of 24 h. For experiments involving hypoxia, cells were cultured under serum-free conditions at 37°C in a humidified 3-gas incubator containing 1% O2 and 5% CO2. This experimental condition was selected based on prior research (Koklanaris et al., 2006; Graham et al., 1998), and was aimed at simulating the conditions of PE for a period of 24 h.
Total RNA was extracted utilizing TRIzol reagent (Thermo Fisher Scientific). Complementary DNA (cDNA) was synthesized through reverse transcription using Prime-Script RTase (Takara). Gene expression was normalized to actin and analyzed using the 2−ΔΔCt method. Primer sequences employed for qRT -PCR amplification are mentioned below:
ACTIN
Forward: 5′- TCCGCCCCGCGAGCACAGAG-3′
Reverse: 5′- TCATCATCCATGGTGAGCTGGCGGC -3′,
LEP
Forward: 5′- TCCTCACCAGTATGCCTTCC -3′
Reverse: 5′- TCTGTGGAGTAGCCTGAAGG-3′
The immunohistochemical (IHC) analysis in this study was conducted using placental samples from four PE patients and four healthy controls from Fujian Provincial Maternity and Child Healthcare Hospital to validate our bioinformatics results. The study was reviewed and approved by the Ethics Committee of Fujian Provincial Maternity and Child Healthcare Hospital (2024KY070-02). Fresh placental samples were immediately fixed in 4% paraformaldehyde (PFA, Sigma-Aldrich) at 4°C for 24 h and subsequently embedded in paraffin matrix. Thin sections (4 μm) mounted on charged slides underwent standard deparaffinization procedures followed by antigen unmasking via microwave-assisted retrieval in EDTA buffer (pH 8.0). After hydrogen peroxide-mediated peroxidase inactivation and 10% normal goat serum blocking, sections were probed with leptin-specific rabbit polyclonal antibody (ABclonal, 1:200 dilution) through 4°C overnight incubation. Subsequently, the sections were incubated with the secondary antibody at room temperature for 1.5 h, followed by incubation with 3,3′-diaminobenzidine at room temperature for 20 min. The stained sections were visualized using an optical microscope (Leica), and quantitative analysis of the samples was carried out with the assistance of ImageJ software (version 1.52).
Gene expression data in the placental tissues of individuals with non-PE and PE were retrieved from the GSE10588 dataset in the GEO databases. The integrated dataset consists of 17 PE samples and 26 control samples. A total of 335 DEGs were identified utilizing the Limma method. The heatmap of DEGs is shown in Figure 2.
The gene expression profiles of the PE samples obtained from the GSE10588 dataset were analyzed using the WGCNA technique. This analysis involved constructing a gene co-expression network and identifying co-expression network and identifying co-expression modules using the WGCNA package in R. The dataset comprising 26 normal samples and 17 PE samples, which underwent clustering. Samples exhibiting evident aberrations were excluded based on a predefined threshold, as depicted in Figure 3A. Furthermore, a soft threshold of β = 4 (scale-free R2 = 0.9) was employed to construct a scale-free network, ensuring high connectivity (Figure 3B). Next, four modules were developed based on a gene clustering tree and a dynamic hybrid cutting algorithm (with a minimum of 100 genes per module) (Figure 3C). The module with the highest correlation with PE was brown, r = 0.77, P = 2e-09 (Figure 3D). The significance of the genes in the brown module with the PE genes was cor = 0.67, P = 6.5e-56 (Figure 3E).
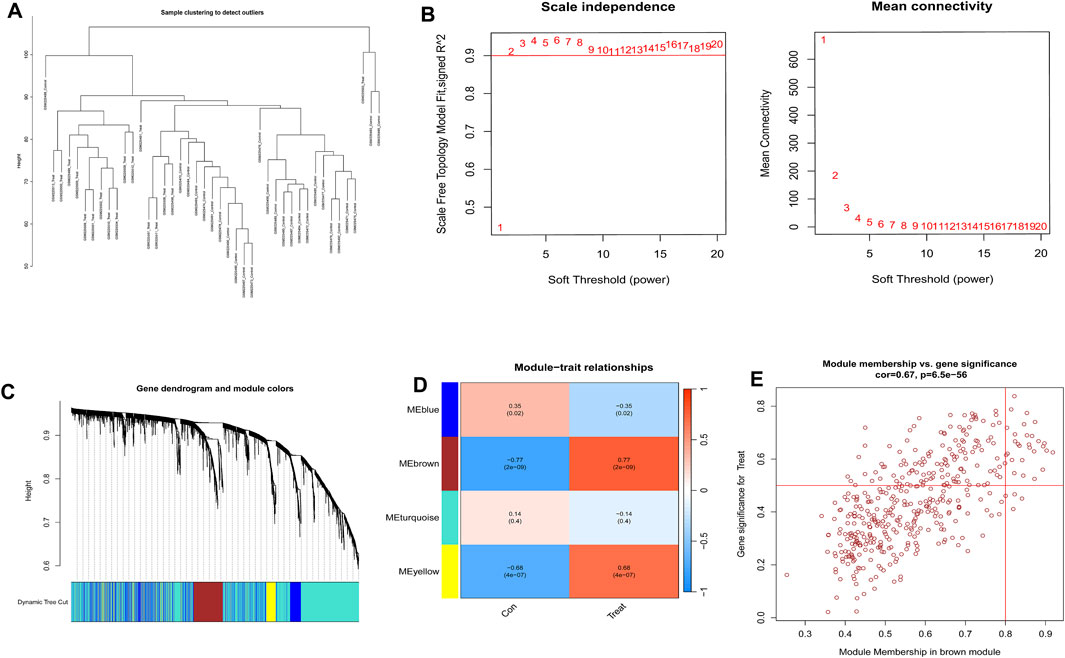
Figure 3. Construction of weighted gene co-expression networks. (A) Sample clustering and detection of outliers. (B) In the process of choosing the soft-threshold power, two aspects were analyzed: the scale-free fit index (displayed on the left) and the mean connectivity (presented on the right). (C) Clustering of co-expression modules was conducted. Each branch in the graph corresponds to a single gene. Genes grouped into the identical module were designated with the same color. (D) Correlations between the modules and normal placenta as well as preeclampsia placenta. (E) The relevance of members in the brown module and preeclampsia.
17 overlapping genes were identified by intersecting the critical module genes and DEOSGs utilizing a Venn diagram (Figure 4A). GO and pathway analyses were then performed to explore the biological functions of these overlapping genes using Metascape. The overlapping genes were primarily involved with positive regulation of protein kinase activity, positive regulation of MAPK cascade, organic hydroxy compound metabolic process, response to inorganic substance, transition metal ion transport, and various other biological processes (Figure 4B). Figure 4C revealed the relationships between the GO terms. The PPI network visually depicted the interactions among these 17 hub genes that involved in the development of PE (Figure 4D).
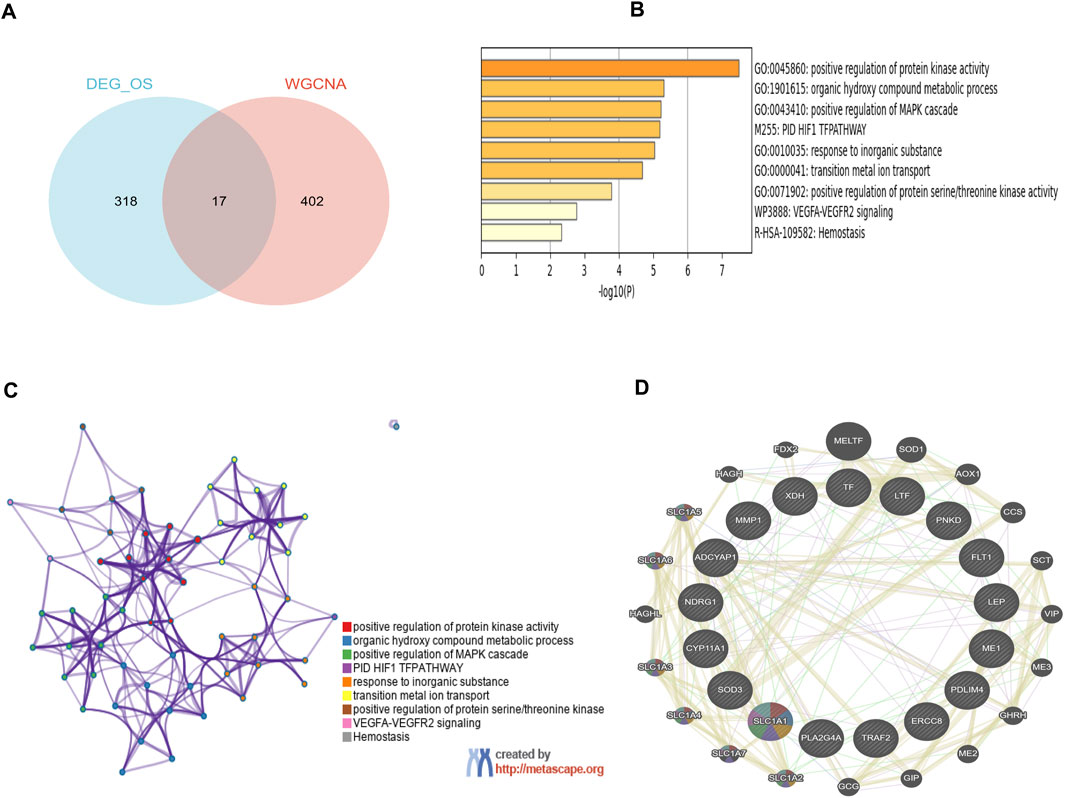
Figure 4. Functional enrichment analysis of key module genes merged with DEOSGs. (A) Venn diagram of key module genes versus DEOSGs. (B) Bar plot of DEOSGs functional enrichment terms. (C) Network relationship plots among all terms. (D) The PPI network of 17 genes constructed by GeneMANIA. The 20 most frequently changed neighboring genes are shown.
In this study, three machine-learning algorithms were utilized to identify feature genes: SVM-RFE (Figure 5A); LASSO regression analysis, which selected five genes based on statistically significant univariable analyses (Figures 5B, C); and the RF algorithm, which ranked genes based on their importance (Figures 5D, E). The Venn diagram displayed the overlap of the three approaches, resulting in the identification of a single gene, namely leptin (LEP)(Figure 5F). Subsequently, a logistic regression model was developed using this candidate gene. The outcomes showcased the superior diagnostic performance of the predictive model, with an AUC of 0.95 in the training set (Figure 5G). These findings suggest that LEP may serve as a promising biomarker for PE.

Figure 5. Screening hub genes by machine learning and GSEA analysis of potential biomarkers. (A) SVM-RFE algorithm. (B, C) LASSO regression algorithm. (D, E) RF algorithm. (F) Venn diagrams for three algorithms. (G) Hub gene in the GSE10588 dataset were analyzed using ROC curves. (H) GO analysis results for LEP. (I) KEGG analysis results for LEP.
Signaling pathways related to the characteristic genes were analyzed using GSEA. KEGG analysis revealed that LEP was significantly enriched in pathways such as “allograft rejection,” “antigen processing,” “asthma,” “autoimmune thyroid disease,” “ECM receptor interaction,” and “graft versus host disease” (Figure 5H). GO analysis identified key biological processes including “antigen processing and presentation,” “peptide antigen assembly with MHC protein complex,” “complex of collagen trimers,” “MHC class II protein complex,” “MHC protein complex,” and “mitochondrial protein-containing complex” (Figure 5I).
To further investigate immune infiltration differences between individuals with PE and healthy controls, we performed ssGSEA. Figure 6A illustrates the distribution of 28 immune cell types in the GSE10588 dataset. Notably, PE samples exhibited significantly higher infiltration levels of several immune cell types, including activated dendritic cells, CD56dim natural killer cells, γδT cell, regulatory T cells, T follicular helper cells, type 1 T helper cells, type 2 T helper cells, and effector memory CD4+ T cells, compared to healthy controls. This suggests that these immune cell types play a central role in PE development (Figure 6B). Furthermore, a positive correlation was observed between LEP expression and T follicular helper cells, plasmacytoid dendritic cells, neutrophils, and activated dendritic cells, while γδT cells, eosinophils, and central memory CD4+ T cells exhibited a negative correlation with LEP (Figure 6C).
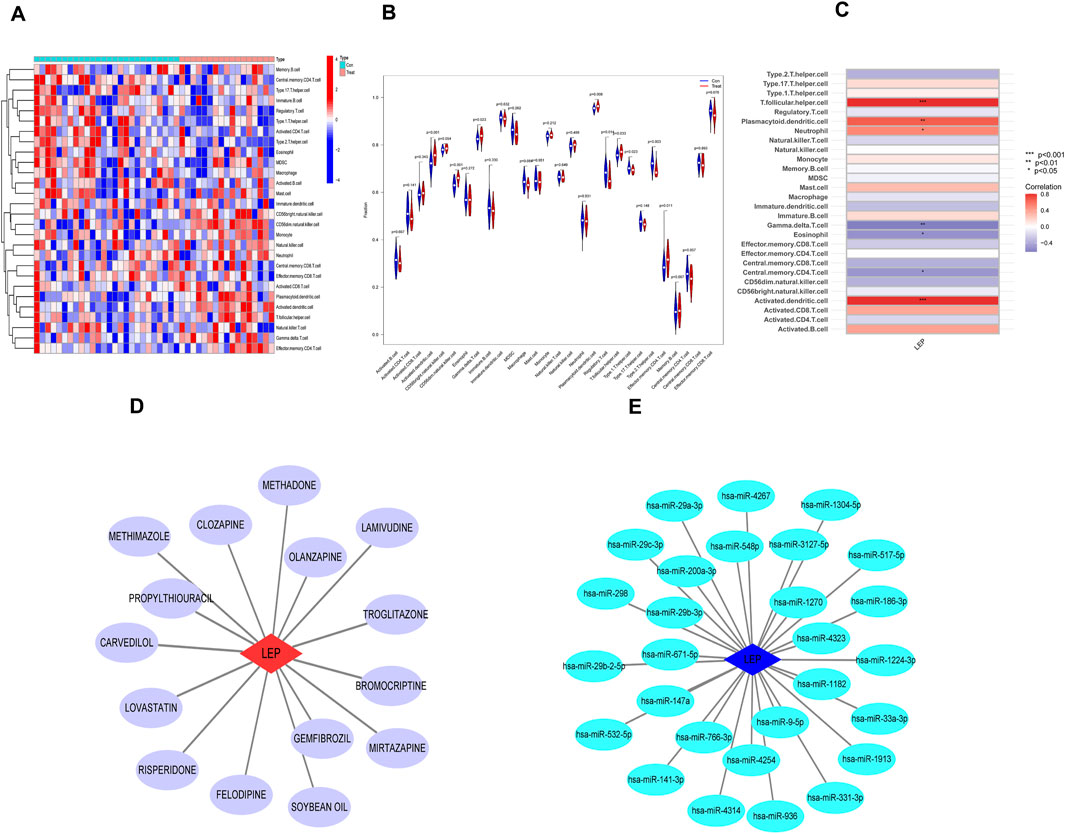
Figure 6. Immune infiltration analysis and potential drug prediction. (A) Heatmap showed the composition of 28 kinds of immune cells in each sample. (B) Comparison regarding the proportion of 28 kinds of immune cells between PE and normal groups visualized by the vioplot. (C) Heatmap of the correlations between the biomarkers and infiltrating immune cells. (D) Hub gene–potential drug network; the red node represents the hub gene, and the violet node represents the potential drug. (E) Interaction between candidate genes and miRNA (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
To identify potential therapeutic drugs for PE, we searched the DGIdb database and identified 15 potential drugs that target LEP. Cytoscape was employed to visualize the drug-gene interaction networks for enhanced clarity and comprehensibility (Figure 6D). However, the underlying mechanism linking these potential drugs to LEP remain unclear. Subsequently, a search of the miRDB, miRanda, and TargetScan databases identified 28 miRNAs that may target LEP. The networks depicting these interactions were visualized using Cytoscape (Figure 6E).
Two independent external datasets (GSE74341 and GSE98224) were utilized to validate the analytical results. The findings revealed that LEP expression was significantly upregulated in placental tissues of PE patients (Figures 7A, B).
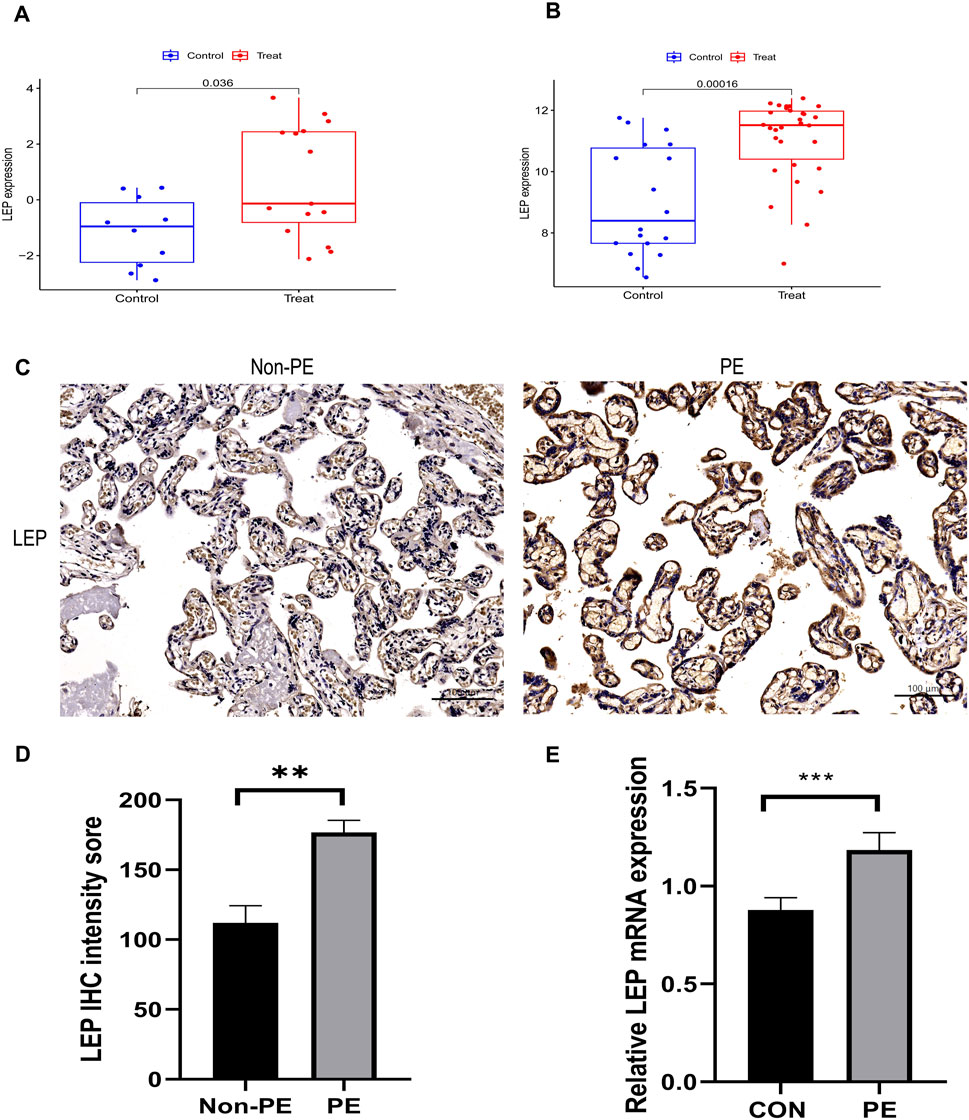
Figure 7. Validation of hub gene. (A) The hub gene in GSE74341 showed significant differences, with p value <0.05. (B) The hub gene in GSE98224 showed significant differences, with p value <0.05. (C) Immunohistochemical staining of placental tissue. (D) Histograms of immunohistochemical staining results. (E) Validation of LEP expression in the HTR-8/SVneo cell line by qRT-PCR analysis of LEP expression under normal oxygen concentration and hypoxia, CON: normal oxygen concentration for 24 h, hypoxia: hypoxia condition for 24 h (**, p < 0.01; ***, p < 0.001). Scale bars = 100 μm.
To validate the bioinformatics results, immunohistochemistry was used to assess the levels of LEP protein in preeclamptic placenta samples (Figure 7C). The results indicated that LEP protein expression was upregulated in preeclamptic placenta samples compared to the control group (Figure 7D).
To further explore the expression of LEP in PE, a hypoxia model was established utilizing the HTR-8/SVneo cell line. Subsequently, LEP expression within this model was evaluated using qRT-PCR. The findings demonstrated a significant upregulation of LEP mRNA expression under hypoxic conditions (Figure 7E).
PE represents a multifaceted clinical syndrome that is exclusive to human pregnancy and plays a major role in causing morbidity and mortality among pregnant women and their neonates (Roberts et al., 1989). Despite its significance, the exact pathogenesis of PE remains incompletely understood, resulting from intricate interactions of various factors. PE can be triggered by a number of conditions such as systemic inflammation, placental dysfunction, hypoxia, immunological dysregulation, and oxidative stress (Jung et al., 2022; George and Granger, 2011).Oxidative stress molecular markers play a critical role in the pathogenesis of PE. During early pregnancy, abnormal invasion of trophoblasts into the maternal uterine spiral arteries leads to oxidative stress, enhancing the production of oxygen-free radicals in the placental environment (Karaşin and Çift, 2020; van Rijn et al., 2008). Certain studies have also revealed that immune response disruptions have a remarkable impact on the development of this obstetrical syndrome (Miller et al., 2022; Santana-Garrido et al., 2022; Lang et al., 2022; Deer et al., 2021b). Furthermore, advances in informatics technologies now allow the identification of diagnostic markers associated with both immune responses and oxidative stress in the context of PE.
WGCNA is a widely used method for bioinformatics analysis. In this study, We WGCNA algorithm to build a gene co-expression network, subsequently leveraging hierarchical clustering for delineating functional modules enriched with tightly interconnected genes. Analysis of the WGCNA results revealed that genes in the brown module exhibited the highest correlation with PE development. After that, a Venn diagram was used to disclose 17 genes that overlapped among the gene sets under study. GO functional analysis illustrated that these genes were involved in processes such as the positive regulation of organic hydroxy compound metabolic process, protein kinase activity, and MAPK cascade positive regulation. Similarly, KEGG analysis demonstrated that these genes were primarily associated with the positive regulation of protein kinase activity, organic hydroxy compound metabolism, positive regulation of MAPK cascade, response to inorganic substance, transition metal ion transport, and several other biological processes. Subsequently, machine-learning techniques, including LASSO, RF, and SVM-RFE, were employed to identify key disease-related genes. Furthermore, ROC curves and AUC values were utilized to assess diagnostic potential of these markers. Ultimately, a potential diagnostic marker for PE, namely, LEP, was identified.
Currently, identifying effective early prediction methods for PE remains a significant challenge. A cohort study conducted in the United States found that the maternal LEP/ceramide (cer) ratio in early pregnancy serves as a superior non-invasive serum biomarker for predicting PE, compared to the sFlt/PlGF ratio (Huang et al., 2021). This suggests that LEP may be more helpful than sFlt/PlGF in predicting PE.LEP is a protein-coding gene released by white adipocytes into the bloodstream, where it plays a crucial role in maintaining energy balance.LEP possesses various endocrine functions and isimplicated in the regulation of immune and inflammatoryresponses, hematopoiesis, angiogenesis, reproduction, anthe promotion of angiogenesis (Pérez-Pérez et al., 2018; Fasshauer and Blüher, 2015; Nieuwenhuis et al., 2020; Childs et al., 2021).LEP acts on variety of immune cells, influencing their development and function, and plays a key role as an immune modulator in the body. Most immune cells express LEP receptors and respond to LEP. In particular, LEP is essential for the development, function, and metabolism of T cells. Research indicates that LEP is critical for the early development of T cells, but is not required for CD8+ T cells (Kim et al., 2010). It has been demonstrated that leptin receptor signaling in T cells enhances cell survival, differentiation, cytokine production, and proliferation differentiatio (Fujita et al., 2002; Saucillo et al., 2014; Lord et al., 1998; Gerriets et al., 2016). Furthermore, LEP promotes B cell homeostasis by inhibiting apoptosis and promoting cell cycle progression (Lam et al., 2010). In vitro studies have demonstrated that LEP can activate B cells, inducing a pro-inflammatory phenotype (Frasca et al., 2020; Agrawal et al., 2011).LEP also binds specifically to LEP receptors on macrophages, promoting their phagocytic activity (Mancuso et al., 2018). Similar to T cells, LEP activation in monocytes induces the expression of an inflammatory phenotype (Jaedicke et al., 2013). LEP has been shown to promote the anti-apoptotic effects of dendritic cells and enhance their maturation (Mattioli et al., 2005; Moraes-Vieira et al., 2014). Studies also indicate that LEP can inhibit neutrophil apoptosis and act as a chemoattractant for neutrophils (Bruno et al., 2005; Ubags et al., 2014; Naylor et al., 2014). In addition, LEP attracts eosinophils and basophils in a manner similar to its effect on neutrophils (Suzukawa et al., 2011; Kato et al., 2011). Furthermore, LEP plays a critical role in the development of natural killer (NK) cells (Tian et al., 2002). Currently, no studies have reported LEP-induced oxidative stress activation in existing PE animal models. However, LEP has been shown to increase ROS levels in vascular smooth muscle cells and promote cell proliferation through the activating of thethe NF-κB pathway (Schroeter et al., 2012; Li et al., 2005). In the placenta of PE patients, an increase in ROS levels can be observed, potentially due to the activation of the NF-κB pathway in trophoblast cells, leading to enhanced ROS production (Wang et al., 2021). Excessively high LEP levels may disrupt glucose and lipid metabolism, leading to the excessive release of lipid peroxides and ROS, which increases the risk of PE (Kutlu et al., 2005). This may help explain our findings. The inflammatory microenvironment in PE patients promotes the infiltration of dendritic cells. However, the increased proportion of conventional dendritic cells and their over-maturation in PE patients may contribute to the increased production of pro-inflammatory cytokines and maternal damage (Zhang et al., 2017; Darmochwal-Kolarz et al., 2003). This finding aligns with our results. Decidual NK (dNK) cells actively mediate two pivotal processes in early gestation: facilitating trophoblast migration and orchestrating structural transformation of spiral arteries, potentially contributing to the development of PE (Kalkunte et al., 2009).This process may be driven by the secretion of vascular endothelial growth factor (VEGF) and placental growth factor placental growth factor (PlGF) by dNK cells, which stimulate spiral artery remodeling (Kalkunte et al., 2009; Gibson et al., 2015). Our results indicate a higher proportion of CD56dim NK cells in preeclamptic women, whereas no significant difference was observed in the proportion of CD56bright NK cells between the PE and control groups. T cells are classified into αβ T cells and γδ T cells based on differences in their T cell receptors. Currently, the role of γδ T cells in the pathogenesis of PE remains unclear.; however, animal studies have shown a significant elevation in γδ T cells in the placenta of PE model mice, with γδ T cell-deficient mice exhibiting improved PE symptoms (Chatterjee et al., 2017). This finding is consistent with our results. During pregnancy, type 1 T helper cell (Th1) immunity exerts pro-inflammatory effects, while type 2 T helper cell (Th2) immunity has anti-inflammatory effects. The balance between Th1 and Th2 immune responses is crucial for a healthy pregnancy. However, in PE patients, the Th1/Th2 ratio in peripheral blood is increased (El-Kabarity and Naguib, 2011). A deficiency of decidual regulatory T cells (Treg cells) is associated with poor spiral artery remodeling and insufficient trophoblast invasion, both of which exacerbate the symptoms of PE (Harmon et al., 2016).
LEP plays a critical role in placental development and function, influencing processes such as invasion, protein synthesis, cell proliferation, and apoptosis in placental cells (Pérez-Pérez et al., 2018; D'Ippolito et al., 2012; Schanton et al., 2018). Multiple studies have underscored LEP’s involvement in the pathophysiology of PE (Pérez-Pérez et al., 2018; Dos Santos et al., 2015; Hauguel-de Mouzon et al., 2006; Gutaj et al., 2020). Notably, PE patients exhibit elevated serum LEP levels compared to individuals without the condition, and elevated levels of this protein have also been reported in the placentas of PE patients. (Song et al., 2016; Kalinderis et al., 2015; Taylor et al., 2015). Multiple investigations have detected both LEP mRNA and protein expression in the placentas of PE patients (Taylor et al., 2015; Mise et al., 1998; Nishizawa et al., 2007), findings consistent with the results of the present study.
On the one hand, the elevated LEP levels may reflect a compensatory response to placental underperfusion, aiming to support neovascularization (Eleuterio et al., 2014; Lepercq et al., 2003). On the other hand, LEP is known to play a role in immune regulation, and disruptions in immune homeostasis may lead to alterations in maternal LEP expression in PE. However, further comprehensive research is needed to fully elucidate the dysregulation of LEP in the context of PE and its broader impact on maternal physiology.
PE is a complex systemic condition, with increasing evidence highlighting the immune system’s pivotal role in its development (Lu and Hu, 2019). Thus, to further investigate this, an analysis of immune infiltration was conducted to compare the immune cell profiles between normal and PE samples. The results revealed a remarkable increase in the infiltration of eight immune cell types in PE samples compared to normal samples. These immune cell types included activated dendritic cells, type 1 T helper cells,CD56dim natural killer cells, γδT cells, macrophages, regulatory T cells, T follicular helper cells, type 2 T helper cells, and effector memory CD4+ T cells.
These findings indicate that LEP may serve as a promising diagnostic and therapeutic marker for PE, potentially exerting a significant role in its pathogenesis. Nevertheless, the study lacks longitudinal data, which could be valuable in understanding the temporal changes in LEP expression and its correlation with disease severity and outcomes; validating the prognostic and therapeutic implications of this model will require a larger sample size, longitudinal data, and comprehensive clinical data from multiple medical center in future research. The potential therapeutic drugs targeting LEP in PE remains to be confirmed, and future research should focus on conducting fundamental studies or clinical trials to validate the feasibility of LEP-targeting drugs in the treatment of PE. Additionally, as this study relies on publicly available data, further experimental investigations are needed to elucidate the biological functions of LEP.
In summary, through integrated bioinformatics analyses and experimental verification, we identified LEP as a key biomarker associated with immune infiltration and oxidative stress in PE.
The data presented in the study can be found in the Gene Expression Omnibus (GEO) datasets (http://www.ncbi.nlm.nih.gov/geo/), accession number GSE10588 (GPL2986), GSE74341 (GPL16699) and GSE98224 (GPL6244). Since we utilized existing public data rather than generating new data, there is no need to deposit data in a separate repository. We believe the information provided in the manuscript about the data sources is sufficient, and we are ready to move forward with the publication process.
The studies involving humans were approved by the Ethics Committee of Fujian Provincial Maternity and Child Healthcare Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TY: Data curation, Formal Analysis, Writing–original draft. GW: Data curation, Formal Analysis, Software, Writing–original draft. XX: Supervision, Writing–review and editing. JY: Formal Analysis, Funding acquisition, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Joint Funds for the innovation of Science and Technology, Fujian province (2020Y9134); Fujian Provincial Health Technology Project (2024ZD01005); Fujian Provincial Natural Science Foundation of China (2024Y0035); National Key Clinical Specialty Construction Program of China (Obstetric).
We thank the development team of R and the database. And we also thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that Generative AI was used in the creation of this manuscript. This article uses generative AI to polish the article.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Afrose, D., Chen, H., Ranashinghe, A., Liu, C. C., Henessy, A., Hansbro, P. M., et al. (2022). The diagnostic potential of oxidative stress biomarkers for preeclampsia: systematic review and meta-analysis. Biol. Sex. Differ. 13 (1), 26. doi:10.1186/s13293-022-00436-0
Agrawal, S., Cerdeira, A. S., Redman, C., and Vatish, M. (2018). Meta-analysis and systematic review to assess the role of soluble FMS-like tyrosine kinase-1 and placenta growth factor ratio in prediction of preeclampsia: the SaPPPhirE study. Hypertension 71 (2), 306–316. doi:10.1161/HYPERTENSIONAHA.117.10182
Agrawal, S., Gollapudi, S., Su, H., and Gupta, S. (2011). Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J. Clin. Immunol. 31 (3), 472–478. doi:10.1007/s10875-010-9507-1
Alderden, J., Pepper, G. A., Wilson, A., Whitney, J. D., Richardson, S., Butcher, R., et al. (2018). Predicting pressure injury in critical care patients: a machine-learning model. Am. J. Crit. Care 27 (6), 461–468. doi:10.4037/ajcc2018525
Brown, M. A., Magee, L. A., Kenny, L. C., Karumanchi, S. A., McCarthy, F. P., Saito, S., et al. (2018). The hypertensive disorders of pregnancy: ISSHP classification, diagnosis and management recommendations for international practice. Pregnancy Hypertens. 13, 291–310. doi:10.1016/j.preghy.2018.05.004
Bruno, A., Conus, S., Schmid, I., and Simon, H. U. (2005). Apoptotic pathways are inhibited by leptin receptor activation in neutrophils. J. Immunol. 174 (12), 8090–8096. doi:10.4049/jimmunol.174.12.8090
Charoentong, P., Finotello, F., Angelova, M., Mayer, C., Efremova, M., Rieder, D., et al. (2017). Pan-cancer immunogenomic analyses reveal genotype-immunophenotype relationships and predictors of response to checkpoint blockade. Cell. Rep. 18 (1), 248–262. doi:10.1016/j.celrep.2016.12.019
Chatterjee, P., Chiasson, V. L., Seerangan, G., De Guzman, E., Milad, M., Bounds, K. R., et al. (2017). Depletion of MHC class II invariant chain peptide or γ-δ T-cells ameliorates experimental preeclampsia. Clin. Sci. (Lond) 131 (15), 2047–2058. doi:10.1042/CS20171008
Childs, G. V., Odle, A. K., MacNicol, M. C., and MacNicol, A. M. (2021). The importance of leptin to reproduction. Endocrinology 162 (2), bqaa204. doi:10.1210/endocr/bqaa204
Cotto, K. C., Wagner, A. H., Feng, Y. Y., Kiwala, S., Coffman, A. C., Spies, G., et al. (2018). DGIdb 3.0: a redesign and expansion of the drug-gene interaction database. Nucleic Acids Res. 46 (D1), D1068-D1073–D1073. doi:10.1093/nar/gkx1143
Darmochwal-Kolarz, D., Rolinski, J., Tabarkiewicz, J., Leszczynska-Gorzelak, B., Buczkowski, J., Wojas, K., et al. (2003). Myeloid and lymphoid dendritic cells in normal pregnancy and pre-eclampsia. Clin. Exp. Immunol. 132 (2), 339–344. doi:10.1046/j.1365-2249.2003.02136.x
Deer, E., Reeve, K. E., Amaral, L., Vaka, V. R., Franks, M., Campbell, N., et al. (2021b). CD4+ T cells cause renal and placental mitochondrial oxidative stress as mechanisms of hypertension in response to placental ischemia. Am. J. Physiol. Ren. Physiol. 320 (1), F47–F54. doi:10.1152/ajprenal.00398.2020
Deer, E., Vaka, V. R., McMaster, K. M., Wallace, K., Cornelius, D. C., Amaral, L. M., et al. (2021a). Vascular endothelial mitochondrial oxidative stress in response to preeclampsia: a role for angiotension II type 1 autoantibodies. Am. J. Obstet. Gynecol. MFM 3 (1), 100275. doi:10.1016/j.ajogmf.2020.100275
D'Ippolito, S., Tersigni, C., Scambia, G., and Di Simone, N. (2012). Adipokines, an adipose tissue and placental product with biological functions during pregnancy. Biofactors 38 (1), 14–23. doi:10.1002/biof.201
Dos Santos, E., Duval, F., Vialard, F., and Dieudonné, M. N. (2015). The roles of leptin and adiponectin at the fetal-maternal interface in humans. Horm. Mol. Biol. Clin. Investig. 24 (1), 47–63. doi:10.1515/hmbci-2015-0031
Eleuterio, N. M., Palei, A. C., Rangel Machado, J. S., Tanus-Santos, J. E., Cavalli, R. C., and Sandrim, V. C. (2014). Correlations between circulating levels of adipokines and anti-angiogenic factors in women with BMI <30 and a late-onset preeclampsia. Hypertens. Pregnancy. 33 (1), 72–80. doi:10.3109/10641955.2013.837174
El-Kabarity, R. H., and Naguib, A. H. (2011). Serum levels of IL-18, IL-12 and TH-1/TH-2 ratio in patients with pre-eclampsia. Egypt J. Immunol. 18 (1), 1–8.
Ellis, K., Kerr, J., Godbole, S., Lanckriet, G., Wing, D., and Marshall, S. (2014). A random forest classifier for the prediction of energy expenditure and type of physical activity from wrist and hip accelerometers. Physiol. Meas. 35 (11), 2191–2203. doi:10.1088/0967-3334/35/11/2191
Fasshauer, M., and Blüher, M. (2015). Adipokines in health and disease. Trends Pharmacol. Sci. 36 (7), 461–470. doi:10.1016/j.tips.2015.04.014
Frasca, D., Diaz, A., Romero, M., and Blomberg, B. B. (2020). Leptin induces immunosenescence in human B cells. Cell. Immunol. 348, 103994. doi:10.1016/j.cellimm.2019.103994
Fujita, Y., Murakami, M., Ogawa, Y., Masuzaki, H., Tanaka, M., Ozaki, S., et al. (2002). Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin. Exp. Immunol. 128 (1), 21–26. doi:10.1046/j.1365-2249.2002.01797.x
George, E. M., and Granger, J. P. (2011). Mechanisms and potential therapies for preeclampsia. Curr. Hypertens. Rep. 13 (4), 269–275. doi:10.1007/s11906-011-0204-0
Gerriets, V. A., Danzaki, K., Kishton, R. J., Eisner, W., Nichols, A. G., Saucillo, D. C., et al. (2016). Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur. J. Immunol. 46 (8), 1970–1983. doi:10.1002/eji.201545861
Gibson, D. A., Greaves, E., Critchley, H. O., and Saunders, P. T. (2015). Estrogen-dependent regulation of human uterine natural killer cells promotes vascular remodelling via secretion of CCL2. Hum. Reprod. 30 (6), 1290–1301. doi:10.1093/humrep/dev067
Graham, C. H., Fitzpatrick, T. E., and McCrae, K. R. (1998). Hypoxia stimulates urokinase receptor expression through a heme protein-dependent pathway. Blood 91 (9), 3300–3307. doi:10.1182/blood.v91.9.3300.3300_3300_3307
Gutaj, P., Sibiak, R., Jankowski, M., Awdi, K., Bryl, R., Mozdziak, P., et al. (2020). The role of the adipokines in the most common gestational complications. Int. J. Mol. Sci. 21 (24), 9408. doi:10.3390/ijms21249408
Harmon, A. C., Cornelius, D. C., Amaral, L. M., Faulkner, J. L., Cunningham, M. W., Wallace, K., et al. (2016). The role of inflammation in the pathology of preeclampsia. Clin. Sci. (Lond). 130 (6), 409–419. doi:10.1042/CS20150702
Hauguel-de Mouzon, S., Lepercq, J., and Catalano, P. (2006). The known and unknown of leptin in pregnancy. Am. J. Obstet. Gynecol. 194 (6), 1537–1545. doi:10.1016/j.ajog.2005.06.064
Huang, Q., Hao, S., You, J., Yao, X., Li, Z., Schilling, J., et al. (2021). Early-pregnancy prediction of risk for pre-eclampsia using maternal blood leptin/ceramide ratio: discovery and confirmation. BMJ Open 11 (11), e050963. doi:10.1136/bmjopen-2021-050963
Jaedicke, K. M., Roythorne, A., Padget, K., Todryk, S., Preshaw, P. M., and Taylor, J. J. (2013). Leptin up-regulates TLR2 in human monocytes. J. Leukoc. Biol. 93 (4), 561–571. doi:10.1189/jlb.1211606
Jung, E., Romero, R., Yeo, L., Gomez-Lopez, N., Chaemsaithong, P., Jaovisidha, A., et al. (2022). The etiology of preeclampsia. Am. J. Obstet. Gynecol. 226 (2S), S844–S866. doi:10.1016/j.ajog.2021.11.1356
Kalinderis, M., Papanikolaou, A., Kalinderi, K., Vyzantiadis, T. A., Ioakimidou, A., and Tarlatzis, B. C. (2015). Serum levels of leptin and IP-10 in preeclampsia compared to controls. Arch. Gynecol. Obstet. 292 (2), 343–347. doi:10.1007/s00404-015-3659-4
Kalkunte, S. S., Mselle, T. F., Norris, W. E., Wira, C. R., Sentman, C. L., and Sharma, S. (2009). Vascular endothelial growth factor C facilitates immune tolerance and endovascular activity of human uterine NK cells at the maternal-fetal interface. J. Immunol. 182 (7), 4085–4092. doi:10.4049/jimmunol.0803769
Kanehisa, M., and Goto, S. (2000). KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28 (1), 27–30. doi:10.1093/nar/28.1.27
Karaşin, S. S., and Çift, T. (2020). The role of ischemia-modified albumin as a biomarker in preeclampsia. Rev. Bras. Ginecol. Obstet. 42 (3), 133–139. doi:10.1055/s-0040-1709662
Kato, H., Ueki, S., Kamada, R., Kihara, J., Yamauchi, Y., Suzuki, T., et al. (2011). Leptin has a priming effect on eotaxin-induced human eosinophil chemotaxis. Int. Arch. Allergy Immunol. 155 (4), 335–344. doi:10.1159/000321195
Kim, S. Y., Lim, J. H., Choi, S. W., Kim, M., Kim, S. T., Kim, M. S., et al. (2010). Preferential effects of leptin on CD4 T cells in central and peripheral immune system are critically linked to the expression of leptin receptor. Biochem. Biophys. Res. Commun. 394 (3), 562–568. doi:10.1016/j.bbrc.2010.03.019
Koklanaris, N., Nwachukwu, J. C., Huang, S. J., Guller, S., Karpisheva, K., Garabedian, M., et al. (2006). First-trimester trophoblast cell model gene response to hypoxia. Am. J. Obstet. Gynecol. 194 (3), 687–693. doi:10.1016/j.ajog.2006.01.067
Kutlu, S., Canpolat, S., Aydin, M., Yasar, A., Tuzcu, M., and Baydas, G. (2005). Exogenous leptin increases lipid peroxidation in the mouse brain. Tohoku J. Exp. Med. 206 (3), 233–236. doi:10.1620/tjem.206.233
Lam, Q. L., Wang, S., Ko, O. K., Kincade, P. W., and Lu, L. (2010). Leptin signaling maintains B-cell homeostasis via induction of Bcl-2 and Cyclin D1. Proc. Natl. Acad. Sci. U. S. A. 107 (31), 13812–13817. doi:10.1073/pnas.1004185107
Lang, Q., Wei, J., Tian, M., Wei, S., Yu, X., Zhao, C., et al. (2022). Attenuated effect of zinc gluconate on oxidative stress, inflammation, and angiogenic imbalance in pre-eclampsia rats. Life Sci. 310, 121055. doi:10.1016/j.lfs.2022.121055
Langfelder, P., and Horvath, S. (2008). WGCNA: an R package for weighted correlation network analysis. BMC Bioinforma. 9, 559. doi:10.1186/1471-2105-9-559
Lepercq, J., Guerre-Millo, M., André, J., Caüzac, M., and Hauguel-de Mouzon, S. (2003). Leptin: a potential marker of placental insufficiency. Gynecol. Obstet. Investig. 55 (3), 151–155. doi:10.1159/000071529
Li, L., Mamputu, J. C., Wiernsperger, N., and Renier, G. (2005). Signaling pathways involved in human vascular smooth muscle cell proliferation and matrix metalloproteinase-2 expression induced by leptin: inhibitory effect of metformin. Diabetes 54 (7), 2227–2234. doi:10.2337/diabetes.54.7.2227
Lin, X., Li, C., Zhang, Y., Su, B., Fan, M., and Wei, H. (2017). Selecting feature subsets based on SVM-RFE and the overlapping ratio with applications in bioinformatics. Molecules 23 (1), 52. doi:10.3390/molecules23010052
Lord, G. M., Matarese, G., Howard, J. K., Baker, R. J., Bloom, S. R., and Lechler, R. I. (1998). Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394 (6696), 897–901. doi:10.1038/29795
Lu, H. Q., and Hu, R. (2019). The role of immunity in the pathogenesis and development of pre-eclampsia. Scand. J. Immunol. 90 (5), e12756. doi:10.1111/sji.12756
Mancuso, P., Curtis, J. L., Freeman, C. M., Peters-Golden, M., Weinberg, J. B., and Myers, M. G. (2018). Ablation of the leptin receptor in myeloid cells impairs pulmonary clearance of Streptococcus pneumoniae and alveolar macrophage bactericidal function. Am. J. Physiol. Lung Cell. Mol. Physiol. 315 (1), L78-L86–L86. doi:10.1152/ajplung.00447.2017
Mattioli, B., Straface, E., Quaranta, M. G., Giordani, L., and Viora, M. (2005). Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J. Immunol. 174 (11), 6820–6828. doi:10.4049/jimmunol.174.11.6820
Meng, Y., Li, C., and Liu, C. X. (2021). Immune cell infiltration landscape and immune marker molecular typing in preeclampsia. Bioengineered 12 (1), 540–554. doi:10.1080/21655979.2021.1875707
Miller, D., Motomura, K., Galaz, J., Gershater, M., Lee, E. D., Romero, R., et al. (2022). Cellular immune responses in the pathophysiology of preeclampsia. J. Leukoc. Biol. 111 (1), 237–260. doi:10.1002/JLB.5RU1120-787RR
Mise, H., Sagawa, N., Matsumoto, T., Yura, S., Nanno, H., Itoh, H., et al. (1998). Augmented placental production of leptin in preeclampsia: possible involvement of placental hypoxia. J. Clin. Endocrinol. Metab. 83 (9), 3225–3229. doi:10.1210/jcem.83.9.5117
Mol, B., Roberts, C. T., Thangaratinam, S., Magee, L. A., de Groot, C., and Hofmeyr, G. J. (2016). Pre-eclampsia. Lancet 387 (10022), 999–1011. doi:10.1016/S0140-6736(15)00070-7
Moraes-Vieira, P. M., Larocca, R. A., Bassi, E. J., Peron, J. P. S., Andrade-Oliveira, V., Wasinski, F., et al. (2014). Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur. J. Immunol. 44 (3), 794–806. doi:10.1002/eji.201343592
Naylor, C., Burgess, S., Madan, R., Buonomo, E., Razzaq, K., Ralston, K., et al. (2014). Leptin receptor mutation results in defective neutrophil recruitment to the colon during Entamoeba histolytica infection. mBio 5 (6). doi:10.1128/mBio.02046-14
Nieuwenhuis, D., Pujol-Gualdo, N., Arnoldussen, I., and Kiliaan, A. J. (2020). Adipokines: a gear shift in puberty. Obes. Rev. 21 (6), e13005. doi:10.1111/obr.13005
Nishizawa, H., Pryor-Koishi, K., Kato, T., Kowa, H., Kurahashi, H., and Udagawa, Y. (2007). Microarray analysis of differentially expressed fetal genes in placental tissue derived from early and late onset severe pre-eclampsia. Placenta 28 (5-6), 487–497. doi:10.1016/j.placenta.2006.05.010
Pérez-Pérez, A., Toro, A., Vilariño-García, T., Maymó, J., Guadix, P., Dueñas, J. L., et al. (2018). Leptin action in normal and pathological pregnancies. J. Cell. Mol. Med. 22 (2), 716–727. doi:10.1111/jcmm.13369
Rana, S., Lemoine, E., Granger, J. P., and Karumanchi, S. A. (2019). Preeclampsia: pathophysiology, challenges, and perspectives. Circ. Res. 124 (7), 1094–1112.
Ritchie, M. E., Phipson, B., Wu, D., Hu, Y., Law, C. W., Shi, W., et al. (2015). Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43 (7), e47. doi:10.1093/nar/gkv007
Roberts, J. M., Taylor, R. N., Musci, T. J., Rodgers, G. M., Hubel, C. A., and McLaughlin, M. K. (1989). Preeclampsia: an endothelial cell disorder. Am. J. Obstet. Gynecol. 161 (5), 1200–1204. doi:10.1016/0002-9378(89)90665-0
San Juan-Reyes, S., Gómez-Oliván, L. M., Islas-Flores, H., and Dublán-García, O. (2020). Oxidative stress in pregnancy complicated by preeclampsia. Arch. Biochem. Biophys. 681, 108255. doi:10.1016/j.abb.2020.108255
Santana-Garrido, Á., Reyes-Goya, C., Espinosa-Martín, P., Sobrevia, L., Beltrán, L. M., Vázquez, C. M., et al. (2022). Oxidative and inflammatory imbalance in placenta and kidney of sFlt1-induced early-onset preeclampsia rat model. Antioxidants (Basel). 11 (8), 1608. doi:10.3390/antiox11081608
Saucillo, D. C., Gerriets, V. A., Sheng, J., Rathmell, J. C., and Maciver, N. J. (2014). Leptin metabolically licenses T cells for activation to link nutrition and immunity. J. Immunol. 192 (1), 136–144. doi:10.4049/jimmunol.1301158
Schanton, M., Maymó, J. L., Pérez-Pérez, A., Sánchez-Margalet, V., and Varone, C. L. (2018). Involvement of leptin in the molecular physiology of the placenta. Reproduction 155 (1), R1-R12–R12. doi:10.1530/REP-17-0512
Schroeter, M. R., Stein, S., Heida, N. M., Leifheit-Nestler, M., Cheng, I. F., Gogiraju, R., et al. (2012). Leptin promotes the mobilization of vascular progenitor cells and neovascularization by NOX2-mediated activation of MMP9. Cardiovasc Res. 93 (1), 170–180. doi:10.1093/cvr/cvr275
Song, Y., Gao, J., Qu, Y., Wang, S., Wang, X., and Liu, J. (2016). Serum levels of leptin, adiponectin and resistin in relation to clinical characteristics in normal pregnancy and preeclampsia. Clin. Chim. Acta. 458, 133–137. doi:10.1016/j.cca.2016.04.036
Subramanian, A., Tamayo, P., Mootha, V. K., Mukherjee, S., Ebert, B. L., Gillette, M. A., et al. (2005). Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A. 102 (43), 15545–15550. doi:10.1073/pnas.0506580102
Suzukawa, M., Nagase, H., Ogahara, I., Han, K., Tashimo, H., Shibui, A., et al. (2011). Leptin enhances survival and induces migration, degranulation, and cytokine synthesis of human basophils. J. Immunol. 186 (9), 5254–5260. doi:10.4049/jimmunol.1004054
Tannetta, D., and Sargent, I. (2013). Placental disease and the maternal syndrome of preeclampsia: missing links. Curr. Hypertens. Rep. 15 (6), 590–599. doi:10.1007/s11906-013-0395-7
Taylor, B. D., Ness, R. B., Olsen, J., Hougaard, D. M., Skogstrand, K., Roberts, J. M., et al. (2015). Serum leptin measured in early pregnancy is higher in women with preeclampsia compared with normotensive pregnant women. Hypertension 65 (3), 594–599. doi:10.1161/HYPERTENSIONAHA.114.03979
The Gene Ontology Consortium (2019). The gene Ontology resource: 20 years and still GOing strong. Nucleic Acids Res. 47 (D1), D330–D338. doi:10.1093/nar/gky1055
Tian, Z., Sun, R., Wei, H., and Gao, B. (2002). Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem. Biophys. Res. Commun. 298 (3), 297–302. doi:10.1016/s0006-291x(02)02462-2
Ubags, N. D., Vernooy, J. H., Burg, E., Hayes, C., Bement, J., Dilli, E., et al. (2014). The role of leptin in the development of pulmonary neutrophilia in infection and acute lung injury. Crit. Care Med. 42 (2), e143–e151. doi:10.1097/CCM.0000000000000048
van Rijn, B. B., Franx, A., Sikkema, J. M., van Rijn, H. J., Bruinse, H. W., and Voorbij, H. A. (2008). Ischemia modified albumin in normal pregnancy and preeclampsia. Hypertens. Pregnancy 27 (2), 159–167. doi:10.1080/10641950701885147
Wang, Z., Zhao, G., Zibrila, A. I., Li, Y., Liu, J., and Feng, W. (2021). Acetylcholine ameliorated hypoxia-induced oxidative stress and apoptosis in trophoblast cells via p38 MAPK/NF-κB pathway. Mol. Hum. Reprod. 27 (8), gaab045. doi:10.1093/molehr/gaab045
Zhang, J., Yu, R., Guo, X., Zou, Y., Chen, S., Zhou, K., et al. (2021). Identification of TYR, TYRP1, DCT and LARP7 as related biomarkers and immune infiltration characteristics of vitiligo via comprehensive strategies. Bioengineered 12 (1), 2214–2227. doi:10.1080/21655979.2021.1933743
Zhang, M., Zhu, K., Pu, H., Wang, Z., Zhao, H., Zhang, J., et al. (2019). An immune-related signature predicts survival in patients with lung adenocarcinoma. Front. Oncol. 9, 1314. doi:10.3389/fonc.2019.01314
Zhang, W., Zhou, Y., and Ding, Y. (2017). Lnc-DC mediates the over-maturation of decidual dendritic cells and induces the increase in Th1 cells in preeclampsia. Am. J. Reprod. Immunol. 77 (6). doi:10.1111/aji.12647
Keywords: leptin, biomarker, preeclampsia, immune infiltration, machine learning, WGCNA
Citation: Yu T, Wang G, Xu X and Yan J (2025) Identification and validation of key biomarkers associated with immune and oxidative stress for preeclampsia by WGCNA and machine learning. Front. Genet. 16:1500061. doi: 10.3389/fgene.2025.1500061
Received: 10 October 2024; Accepted: 19 February 2025;
Published: 12 March 2025.
Edited by:
Lei Chen, Shanghai Maritime University, ChinaReviewed by:
Showkat A. Dar, National Institutes of Health (NIH), United StatesCopyright © 2025 Yu, Wang, Xu and Yan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xia Xu, eHV4aWEwNjIzQGZqbXUuZWR1LmNu; Jianying Yan, eWFuankyMDE5QGZqbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.