
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 02 April 2025
Sec. Statistical Genetics and Methodology
Volume 16 - 2025 | https://doi.org/10.3389/fgene.2025.1399353
Background: Evidence on the relationship between thyroid function and hepatic fibrosis/cirrhosis are still unclear, with inconsistent conclusions. This Mendelian randomization (MR) study aimed to investigate the potential causal association between thyroid function and hepatic fibrosis/cirrhosis in order to provide new insights for improving prevention and control strategies for this disease.
Methods: Genome-wide association study (GWAS) data on exposures, which included hyperthyroidism, hypothyroidism, and thyroid-stimulating hormone (TSH), were extracted from the MRC Integrative Epidemiology Unit (MRC-IEU) (https://gwas.mrcieu.ac.uk/), and GWAS data for outcomes, including hepatic fibrosis/cirrhosis and chitinase-3-like protein 1 (CHI3L1), were obtained from the FinnGen consortium (https://www.finngen.fi/fi). Inverse variance weighted (IVW), weighted median, and MR-Egger methods were utilized to examine the causal association between thyroid function and the risk of hepatic fibrosis/cirrhosis. Cochran’s Q test was used to assess the heterogeneity of instrumental variables (IVs), while MR-PRESSO and leave-one-out analyses were conducted for sensitivity analysis.
Results: IVW estimates suggested that hypothyroidism had a potential causal association with higher odds of hepatic fibrosis/cirrhosis (OR = 1.247, 95% CI: 1.087–1.431). Leave-one-out results indicated that this potential causal relationship was relatively robust. In addition, we assessed the causal association between hypothyroidism and hepatic fibrosis/cirrhosis before and after removal of outliers with heterogeneity. After removing the outliers, the association was still significant (OR = 1.266, 95% CI: 1.082–1.482, P = 0.0046).
Conclusion: Patients with hypothyroidism may have a higher risk of hepatic fibrosis/cirrhosis, and this finding may provide some references for the early screening and prevention of the disease. However, further studies are needed to explore the specific mechanisms by which hypothyroidism influences hepatic fibrosis/cirrhosis.
Hepatic fibrosis is a significant health burden worldwide, with cirrhosis and hepatoma caused by hepatic fibrosis accounting for many hepatic disease-related deaths each year (Gines et al., 2021; Devarbhavi et al., 2023). In recent years, several observational studies have investigated the association between reduced thyroid function or the reduction in the normal range and hepatic fibrosis (Bano et al., 2020; Rahadini and Rahadina, 2022), which may be related to the regulatory role of thyroid function in lipid metabolism (Mavromati and Jornayvaz, 2021).
It has been found that subclinical hypothyroidism may be associated with hepatic fibrosis/cirrhosis (Bano et al., 2016; Kim et al., 2018); however, the conclusions are inconsistent (D'Ambrosio et al., 2021). In addition, even if thyroid function is within normal ranges, relatively high thyroid stimulating hormone (TSH) levels (Kim et al., 2019; Martinez-Escude et al., 2021a; Martinez-Escude et al., 2021b; Fan et al., 2023; Zhang et al., 2023) and low free thyroxine 4 (FT4) levels (Zhang et al., 2020; Zhang et al., 2022) are risk factors for hepatic fibrosis. However, some studies have found that there is no association between TSH (Liu et al., 2018; Zhang et al., 2020; Du et al., 2021; Guo et al., 2021) and FT4 (Du et al., 2021; Guo et al., 2021) and hepatic fibrosis. To date, few studies have discussed the relationship between hyperthyroidism and hepatic fibrosis/cirrhosis. Bano et al. (2016) found no significant association between hyperthyroidism and non-alcoholic fatty liver disease (NAFLD). In contrast, Labenz et al. (2021) suggested that hyperthyroidism is linked to a lower risk of NAFLD. In addition, serum chitinase-3-like protein 1 (CHI3L1) has recently been found to be a potential marker for the diagnosis of hepatic fibrosis and hepatic cirrhosis, but no study has investigated the relationship between thyroid function and CHI3L1 levels (Tao et al., 2014; Jin et al., 2020; Nishimura et al., 2021). The underlying mechanisms of this association remain unknown but may involve factors such as obesity, insulin resistance, oxidative stress, and mitochondrial dysfunction (Taylor et al., 2013; van Tienhoven-Wind and Dullaart, 2015; Kim et al., 2018). In conclusion, evidence on the association between thyroid function-related indices and hepatic fibrosis/cirrhosis is still limited, and observational studies cannot establish causality. Therefore, the causal association between thyroid function and hepatic fibrosis/cirrhosis needs to be further verified.
Mendelian randomization (MR) is a widely used approach to infer potential causal associations between environmental exposures and diseases (Davey Smith and Hemani, 2014; Sekula et al., 2016), which leverages single-nucleotide polymorphisms (SNPs) as instrumental variants (IVs). MR analysis can avoid reverse causality inferences and capture the long-term effects of exposures on outcomes. This study used MR methods to investigate the potential causal associations between thyroid function and hepatic fibrosis/cirrhosis, providing new insights for developing relevant screening and intervention strategies for the prevention and control of hepatic fibrosis/cirrhosis. The MR approach is widely applied in epigenetics, metabolic disease research, and environmental causal inference and has been thoroughly validated in exploring causal mechanisms. Therefore, this method can provide robust support for causal inference in the current study.
In this two-sample MR study, the exposure genome-wide association study (GWAS) data, including hyperthyroidism, hypothyroidism, FT4, and TSH, were extracted from the MRC Integrative Epidemiology Unit (MRC-IEU) (https://gwas.mrcieu.ac.uk/), whereas the GWAS data for hepatic fibrosis/cirrhosis and CHI3L1 were obtained from the FinnGen consortium (https://www.finngen.fi/fi). The diagnosis of hyperthyroidism, hypothyroidism, and hepatic fibrosis/cirrhosis was according to the International Classification of Diseases (ICD) 10th Version, with codes of E05, E03, and K74, respectively. We summarized and presented the aggregated information on the data source, GWAS ID, population, and sample size in Table 1.
The GWAS datasets in this study received ethical approval from their respective institutions, with data de-identified, and informed consent was obtained from all participants. Since the database is publicly available, ethical approval was waived by the Institutional Review Board (IRB) of our hospital. In addition, the methods and procedures of this study followed the STROBE-MR checklist to ensure the robustness and reliability of the findings (Skrivankova et al., 2021).
SNPs significantly associated with exposures were selected as potential IVs, with a selection threshold of P < 5.0 × 10−8 (P < 5.0 × 10−6 for IVs associated with FT4 and TSH). SNPs in linkage disequilibrium (LD) or those that were palindromic with intermediate allele frequencies were removed according to the MR principle to ensure that the same allele corresponded to the effects between SNPs and both the exposures and outcomes. The threshold was set to be r2 = 0.001, with a clumping distance of 10,000 kb.
The MR analysis must adhere to three important assumptions in order to minimize bias in the results. (1) Relevance assumption: the selected IVs must exhibit significant associations with the exposure factor (P < 5.0 × 10−8 or P < 5.0 × 10−6). (2) Independence assumption: IVs should be independent of potential confounders. To validate this, we implemented LD clumping procedures for IV selection. Details regarding assumptions (1) and (2) can be found in the section on the selection of single-nucleotide polymorphisms. Horizontal pleiotropy was systematically evaluated using the MR-PRESSO global test (P < 0.05 as the significance threshold) and MR-Egger regression. (3) Exclusion restriction assumption: IVs should influence outcomes solely through exposure. The PhenoScanner V2 database was used to exclude SNPs with documented pleiotropic pathways. Additionally, the intercept term of the MR-Egger regression was examined to assess residual horizontal pleiotropy (A P-value > 0.05 was considered to indicate the absence of horizontal pleiotropic effects).
First, IVs need to be significantly associated with exposures. The strength of association between exposures and IVs was estimated using the following formula: F = r2 * (N-2)/(1-r2), where r2 = 2 * EAF * (1-EAF) *β2/SD2. Here, N is the sample size, EAF is the effect allele frequency, β represents the regression coefficient for the association between exposures and IVs, and SD is the standard deviation. In addition, F < 10 indicates a weak association between IVs and exposures. Second, IVs must be independent of confounders associated with exposures and outcomes. To determine whether this assumption was violated, the MR-Egger regression test was utilized to detect potential horizontal pleiotropy, which refers to confounding effects caused by other diseases (Burgess and Thompson, 2017; Bowden et al., 2019). A significant intercept term in MR-Egger represents the existence of pleiotropy. Third, IVs should affect outcomes solely through exposure, meaning that there should be no horizontal pleiotropy influence of IVs on the outcome.
The potential causal association between thyroid function and hepatic fibrosis/cirrhosis was estimated using the inverse variance weighted (IVW) method, which was evaluated by odds ratios (ORs) with 95% confidence intervals (CIs). The IVW test serves as the primary method to calculate the unbiased estimates of causal effect when horizontal pleiotropy is absent. The weighted median method was also used to assess the causal effect because it can provide a robust and consistent estimate even if nearly 50% of the genetic variants are invalid instruments. The MR-Egger regression was used as its intercept allows for the detection of potential pleiotropy in IVs (P > 0.05 was recognized as no horizontal pleiotropy). In addition, false discovery rate (FDR) correction was utilized to increase the reliability and stability of the results. A P < 0.05 value was considered statistically significant for the potential causal relationships.
The heterogeneity of the significant causal effect was tested using Cochran’s Q test, where IVs with P < 0.05 were considered heterogeneous. Outliers among selected IVs were identified using the MR-Pleiotropy RESidual Sum and Outlier (MR-PRESSO) test (Verbanck et al., 2018). The causal associations between thyroid function and hepatic fibrosis/cirrhosis were assessed after excluding significant outliers. The scatter plots and the leave-one-out test were used for sensitivity analysis. Statistical analyses were performed with R version 4.2.0 (Institute for Statistics and Mathematics, Vienna, Austria). The R package “TwoSampleMR” was used for MR analyses of the causal association between thyroid function and hepatic fibrosis/cirrhosis.
The flowchart of the study process is shown in Figure 1. Table 2 shows the selection of IVs and the results of tests for horizontal pleiotropy, strength, and heterogeneity. After quality control, we identified 11 SNPs (related to hyperthyroidism), 62 SNPs (related to hypothyroidism), and 4 SNPs (related to TSH) as IVs for the exposures. The MR-Egger regression test indicated no evidence of horizontal pleiotropy (all P > 0.05). The strength values of the associations between IV exposures were all greater than 20. However, Cochran’s Q test showed potential heterogeneity in the associations between selected IVs and hyperthyroidism (P = 1.09 × 10−4) and hypothyroidism (P = 0.006).
Then, we investigated the potential causal associations between thyroid function and hepatic fibrosis/cirrhosis (Figure 2). After applying the FDR correction, the IVW estimate suggested that hypothyroidism had a positive causal association with hepatic fibrosis/cirrhosis (OR = 1.247, 95% CI: 1.087–1.431), while FT4 was negatively associated with the odds of hepatic fibrosis/cirrhosis (OR = 0.530, 95% CI: 0.282–0.998). In addition, weighted median results similarly suggested a positive causal association between hypothyroidism and hepatic fibrosis/cirrhosis (OR = 1.430, 95% CI: 1.123–1.820). More detailed information on the causal associations between thyroid function and hepatic fibrosis/cirrhosis is provided in Supplementary Table S1.
Through the MR-PRESSO global test, we identified two significant outliers among the IVs associated with hypothyroidism and hepatic fibrosis/cirrhosis and one for the association between FT4 and hepatic fibrosis/cirrhosis. It could also be clearly observed in Figure 3 that the tendency of causal associations between hypothyroidism and FT4 and hepatic fibrosis/cirrhosis evaluated using different MR methods was substantially consistent. Then, we confirmed the reliability of the MR results regarding the potential causal associations between both hypothyroidism and FT4 and hepatic fibrosis/cirrhosis through sensitivity analysis. The leave-one-out test indicated that the potential causal effects of hypothyroidism and FT4 on the risk of hepatic fibrosis/cirrhosis were relatively robust (Figure 4).
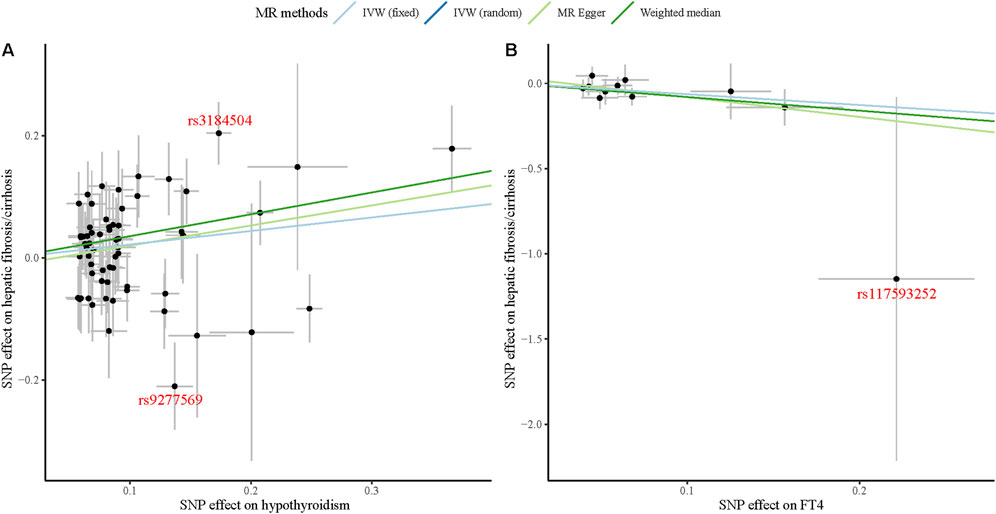
Figure 3. Scatter plots of causal associations between both hypothyroidism (A) and FT4 (B) and hepatic fibrosis/cirrhosis.
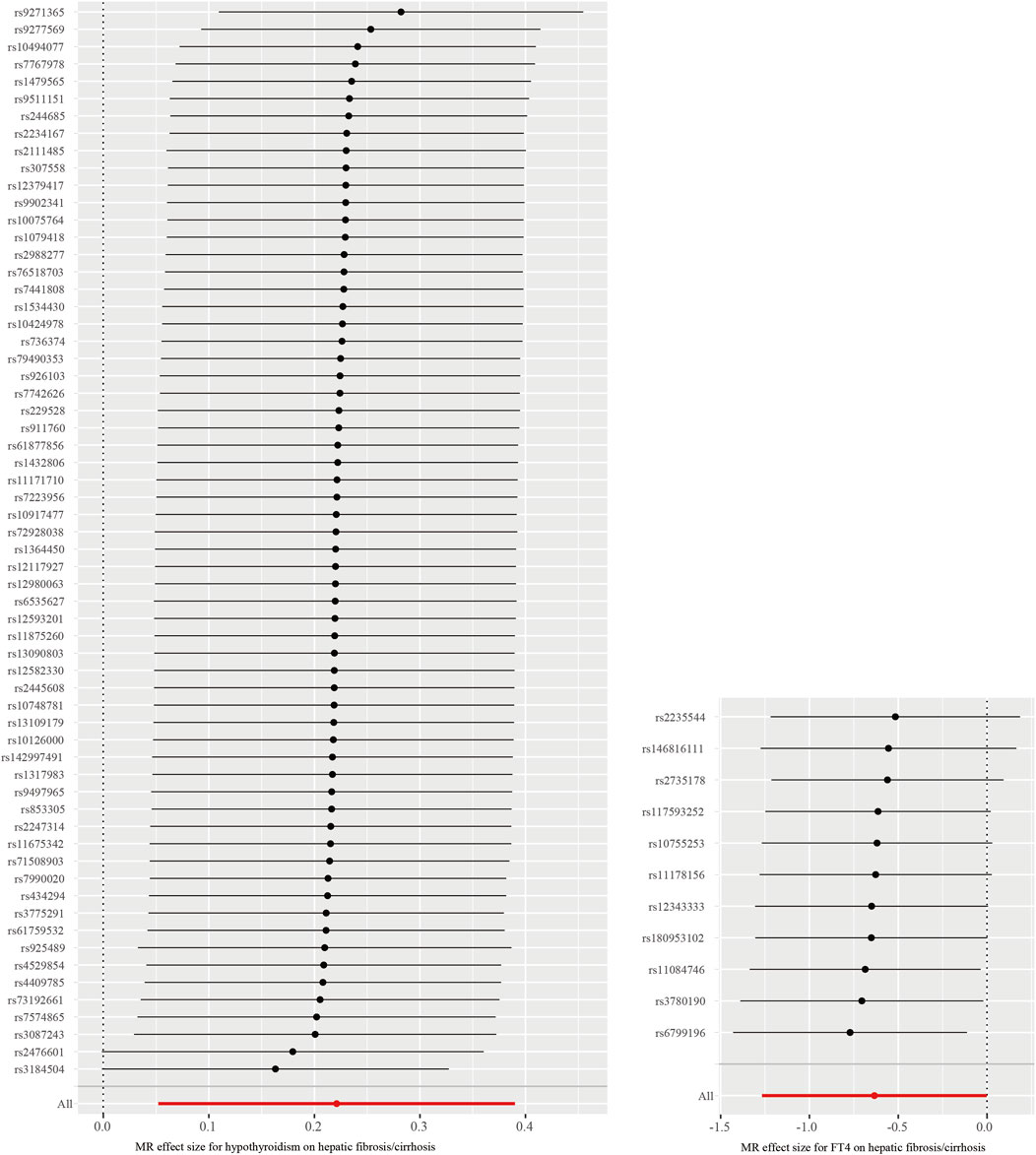
Figure 4. Leave-one-out test of causal associations between both hypothyroidism and FT4 and hepatic fibrosis/cirrhosis.
Moreover, since the heterogeneity test indicated that there was heterogeneity in the associations between selected IVs and hypothyroidism, we assessed the causal relationship between hypothyroidism and hepatic fibrosis/cirrhosis before and after removal of significant outliers (Table 3). According to the IVW results, after the removal of outliers with heterogeneity, the causal association between hypothyroidism and hepatic fibrosis/cirrhosis was still significant (OR = 1.217, 95% CI: 1.055–1.404) (Table 4).
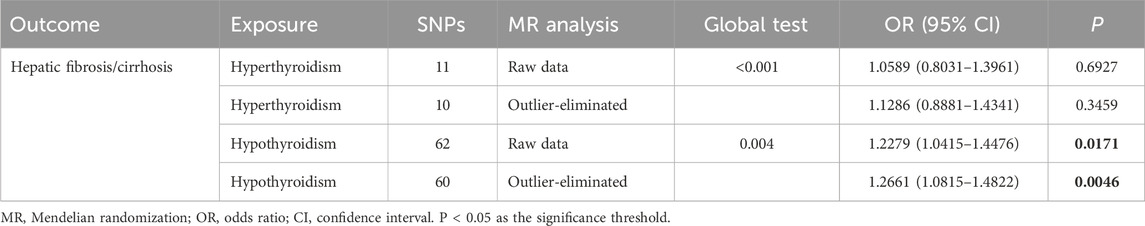
Table 3. Causal relationship between thyroid function and hepatic fibrosis/cirrhosis before and after removal of outliers with heterogeneity.

Table 4. Causal association between thyroid function and hepatic fibrosis/cirrhosis after removal of outliers.
We conducted a two-sample MR analysis to explore the causal associations between different thyroid function-related indices and hepatic fibrosis/cirrhosis. The results showed that hypothyroidism had a positive causal relationship with hepatic fibrosis/cirrhosis, whereas FT4 had a negative association.
In recent years, the relationship between thyroid function and hepatic fibrosis/cirrhosis has been extensively studied. However, existing evidence from animal experiments or observational studies cannot infer a causal relationship between them. Research in patients from Jiading District Central Hospital, Shanghai, China, showed that low-normal thyroid function increased the risk of advanced fibrosis in patients with metabolic dysfunction-associated fatty liver disease (MAFLD), and the elevated TSH concentrations were linked to advanced hepatic fibrosis (Fan et al., 2023). A cross-sectional study in the Indian population suggested that patients with hepatic cirrhosis showed impaired thyroid function, and that thyroid hormone (TH) levels may help assess the severity and course of cirrhosis (Sikarwar et al., 2022). In a mouse model of non-alcoholic steatohepatitis, TH was observed to reduce lipotoxicity, hepatic inflammation, oxidative stress, and fibrosis (Zhou et al., 2022). An MR study suggested an inverse association between genetically predicted hypothyroidism and hepatocellular carcinoma risk, but there was no evidence observed of a direct causal effect of TSH level and FT4 level on HCC (Lu et al., 2022). On the other hand, Shao et al. (2022) found that FT4 in the normal range was lower in the NAFLD group than in the healthy control group. In the current MR study, we found that patients with hypothyroidism had a higher hepatic fibrosis/cirrhosis risk, while FT4 was negatively associated with the odds of hepatic fibrosis/cirrhosis; however, no significant causal association was observed between TSH and hepatic fibrosis/cirrhosis. Our findings were relatively complementary to the existing literature by filling the gap in inferring causal associations between thyroid function and both hepatic fibrosis and cirrhosis. Furthermore, the observed discrepancies between our findings and prior studies may be attributable to population stratification by ancestry. These preliminary causal associations therefore warrant validation through large-scale multi-ethnic cohorts to elucidate potential ancestry-specific effects.
The mechanisms through which hypothyroidism and FT4 may influence the occurrence of hepatic fibrosis/cirrhosis are still unclear. The liver plays an essential physiological role in TH activation and inactivation, transport, and metabolism, and conversely, TH affects the activities of hepatocytes and hepatic metabolism (Piantanida et al., 2020). Kannt et al. (2021) showed that treatment with hepatic thyroid hormone receptor β (THR-β) in patients with non-alcoholic steatohepatitis (NASH) led to a significant reduction in hepatic steatosis, α-smooth muscle actin content, and the expression of genes involved in fibrogenesis, indicating a decrease in hepatic fibrosis. An upregulation of THR-β was observed in the developing brain under iodine deficiency, indicating an adaptive process coming into play to protect it from the damages that are inflicted due to hypothyroidism (Chattopadhyay et al., 1995). Hence, it is suggested that hypothyroidism may be involved in the development of hepatic fibrosis/cirrhosis by influencing the relative expression of THR-β. In addition, hypothyroidism in hepatic cirrhosis inevitably affects mitochondrial metabolism and functional integrity, as the liver is one of the main target organs for THs (Weitzel et al., 2003). It is known that hypothyroidism in hepatic cirrhosis influences mitochondrial oxidative phosphorylation and disrupts the function of many antioxidant enzymes (Mukherjee et al., 2014). The excessive accumulation of free oxygen radicals in hepatocytes may play a critical role in the development of hepatocellular carcinoma (HCC) in cirrhosis by causing oxygen-free radical-related DNA damage (Sahin et al., 2020). Nevertheless, due to the limitation of GWAS databases, other indices related to thyroid function, such as TH, THR-β, triiodothyronine (T3), and thyroxine (T4), were not available. Therefore, the specific underlying mechanisms behind the causal associations between both hypothyroidism and FT4 and the occurrence of hepatic fibrosis/cirrhosis need to be further explored and elucidated.
As mentioned above, the MR study design is superior to observational studies in clarifying the causal effect of potential risk factors for diseases of interest to a certain extent. By investigating the potential causal association that hypothyroidism increases the odds of hepatic fibrosis/cirrhosis and FT4 decreases the odds of hepatic fibrosis/cirrhosis, our study may provide some references for further exploration to facilitate the recommendation of public health policies and clinical interventions among patients with hepatic diseases and help reduce the incidence and social burden of this disease. Compared with previous research, the current study included a large sample of the European population from the open-access GWAS databases. According to our findings, clinicians should focus on the regular screening and timely treatment of hepatic inflammation or fibrosis in patients with hypothyroidism and monitor serum FT4 levels to maintain them within an appropriate range in order to reduce the further risk of developing hepatic cirrhosis.
The MR method can overcome the limitations of some observational studies such as reverse causality, confounding factors, and various biases. In the current research, the selected IVs for the MR analysis were screened rigorously, with F values of greater than 20, guaranteeing the accuracy of the results. Although the heterogeneity test showed that the selected IVs related to hyperthyroidism and hypothyroidism had potential heterogeneity, we utilized the MR-PRESSO distortion test to screen significant outliers and assessed the causal association between hypothyroidism and hepatic fibrosis/cirrhosis before and after deleting outliers (Verbanck et al., 2018). The leave-one-out method was used for sensitivity analysis. All these tests suggested that the causal associations between both hypothyroidism and FT4 and hepatic fibrosis/cirrhosis were stable and robust. However, there are some limitations. This study included only European populations, so the findings may not be generalizable for extrapolation to individuals of other racial or ethnic backgrounds. Additionally, the GWAS data we used were aggregated rather than individual level, preventing stratification by age, gender, and other factors for further analysis. We also fully acknowledge the critical role of population stratification effects in two-sample MR studies. To address this concern, all GWAS datasets utilized in our analysis incorporated principal component analysis (PCA)-based ancestry adjustment in their original studies, which serves as a methodological safeguard against confounding by population structure. Furthermore, we recognize the importance of expanding population diversity in future research. We propose to validate these associations through large-scale, multi-ancestry analyses to ensure result robustness (Genevieve et al., 2019).
Hypothyroidism may be a potential risk factor for hepatic fibrosis/cirrhosis, while FT4 exhibits a negative causal association with these conditions, indicating that dynamic monitoring and screening of relevant indices may be significant for individuals at high risk of hepatic fibrosis and cirrhosis. Further studies are needed to elucidate the underlying mechanisms of these associations.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
YW: Conceptualization, Formal analysis, Methodology, Writing – original draft, Validation, and Investigation. XZ: Data curation, Investigation, Visualization, and Writing – original draft. QL: Formal analysis, Methodology, Visualization, Writing – review and editing, and Software. QZ: Supervision, Validation, Writing – review and editing. JL: Formal analysis, Supervision, and Writing – review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the Science and Technology Research Project of the Jiangxi Provincial Department of Education of China (GJJ2200184).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2025.1399353/full#supplementary-material
TSH, thyroid stimulating hormone; FT4, free thyroxine 4; NAFLD, non-alcoholic fatty liver disease; CHI3L1, chitinase-3-like protein 1; MR, Mendelian randomization; SNP, single-nucleotide polymorphism; IV, instrumental variant; GWAS, genome-wide association study; MRC-IEU, MRC Integrative Epidemiology Unit; ICD, International Classification of Diseases; IRB, Institutional Review Board; LD, linkage disequilibrium; IVW, inverse variance weighted; OR, odds ratio; CI, confidence interval; FDR, false discovery rate; MR-PRESSO, MR-Pleiotropy RESidual Sum and Outlier; MAFLD, metabolic dysfunction-associated fatty liver disease; TH, thyroid hormone; THR-β, thyroid hormone receptor β; NASH, non-alcoholic steatohepatitis; HCC, hepatocellular carcinoma; T3, triiodothyronine; T4, thyroxine.
Bano, A., Chaker, L., Muka, T., Mattace-Raso, F. U. S., Bally, L., Franco, O. H., et al. (2020). Thyroid function and the risk of fibrosis of the liver, heart, and lung in humans: a systematic review and meta-analysis. Thyroid 30 (6), 806–820. doi:10.1089/thy.2019.0572
Bano, A., Chaker, L., Plompen, E. P., Hofman, A., Dehghan, A., Franco, O. H., et al. (2016). Thyroid function and the risk of nonalcoholic fatty liver disease: the rotterdam study. J. Clin. Endocrinol. Metab. 101 (8), 3204–3211. doi:10.1210/jc.2016-1300
Bowden, J., Del Greco, M. F., Minelli, C., Zhao, Q., Lawlor, D. A., Sheehan, N. A., et al. (2019). Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int. J. Epidemiol. 48 (3), 728–742. doi:10.1093/ije/dyy258
Burgess, S., and Thompson, S. G. (2017). Interpreting findings from Mendelian randomization using the MR-Egger method. Eur. J. Epidemiol. 32 (5), 377–389. doi:10.1007/s10654-017-0255-x
Chattopadhyay, N., Kher, R., Virmani, J., and Godbole, M. M. (1995). Differential expression of alpha- and beta-thyroid hormone receptor genes in the developing rat brain under hypothyroidism. Biol. Neonate 67 (1), 64–71. doi:10.1159/000244145
D'Ambrosio, R., Campi, I., Maggioni, M., Perbellini, R., Giammona, E., Stucchi, R., et al. (2021). The relationship between liver histology and thyroid function tests in patients with non-alcoholic fatty liver disease (NAFLD). PLoS One 16 (4), e0249614. doi:10.1371/journal.pone.0249614
Davey Smith, G., and Hemani, G. (2014). Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23 (R1), R89–R98. doi:10.1093/hmg/ddu328
Devarbhavi, H., Asrani, S. K., Arab, J. P., Nartey, Y. A., Pose, E., and Kamath, P. S. (2023). Global burden of liver disease: 2023 update. J. Hepatol. 79 (2), 516–537. doi:10.1016/j.jhep.2023.03.017
Du, J., Chai, S., Zhao, X., Sun, J., Zhang, X., and Huo, L. (2021). Association between thyroid hormone levels and advanced liver fibrosis in patients with type 2 diabetes mellitus and non-alcoholic fatty liver disease. Diabetes Metab. Syndr. Obes. 14, 2399–2406. doi:10.2147/DMSO.S313503
Fan, H., Li, L., Liu, Z., Zhang, P., Wu, S., Han, X., et al. (2023). Low thyroid function is associated with an increased risk of advanced fibrosis in patients with metabolic dysfunction-associated fatty liver disease. BMC Gastroenterol. 23 (1), 3. doi:10.1186/s12876-022-02612-3
Genevieve, L. W., Mariaelisa, G., Katherine, K. N., Ran, T., Jeffrey, H., Gignoux, C. R., et al. (2019). Genetic analyses of diverse populations improves discovery for complex traits. Nature 570 (7762), 514–518. doi:10.1038/s41586-019-1310-4
Gines, P., Krag, A., Abraldes, J. G., Sola, E., Fabrellas, N., and Kamath, P. S. (2021). Liver cirrhosis. Lancet 398 (10308), 1359–1376. doi:10.1016/S0140-6736(21)01374-X
Guo, W., Qin, P., Li, X. N., Wu, J., Lu, J., Zhu, W. F., et al. (2021). Free triiodothyronine is associated with hepatic steatosis and liver stiffness in euthyroid Chinese adults with non-alcoholic fatty liver disease. Front. Endocrinol. (Lausanne) 12, 711956. doi:10.3389/fendo.2021.711956
Jin, X., Fu, B., Wu, Z. J., Zheng, X. Q., Hu, J. H., Jin, L. F., et al. (2020). Serum chitinase-3-like protein 1 is a biomarker of liver fibrosis in patients with chronic hepatitis B in China. Hepatobiliary Pancreat. Dis. Int. 19 (4), 384–389. doi:10.1016/j.hbpd.2020.05.009
Kannt, A., Wohlfart, P., Madsen, A. N., Veidal, S. S., Feigh, M., and Schmoll, D. (2021). Activation of thyroid hormone receptor-beta improved disease activity and metabolism independent of body weight in a mouse model of non-alcoholic steatohepatitis and fibrosis. Br. J. Pharmacol. 178 (12), 2412–2423. doi:10.1111/bph.15427
Kim, D., Kim, W., Joo, S. K., Bae, J. M., Kim, J. H., and Ahmed, A. (2018). Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin. Gastroenterol. Hepatol. 16 (1), 123–131. doi:10.1016/j.cgh.2017.08.014
Kim, D., Yoo, E. R., Li, A. A., Fernandes, C. T., Tighe, S. P., Cholankeril, G., et al. (2019). Low-normal thyroid function is associated with advanced fibrosis among adults in the United States. Clin. Gastroenterol. Hepatol. 17 (11), 2379–2381. doi:10.1016/j.cgh.2018.11.024
Labenz, C., Kostev, K., Armandi, A., Galle, P. R., and Schattenberg, J. M. (2021). Impact of thyroid disorders on the incidence of non-alcoholic fatty liver disease in Germany. United Eur. Gastroenterol. J. 9 (7), 829–836. doi:10.1002/ueg2.12124
Liu, Y., Wang, W., Yu, X., and Qi, X. (2018). Thyroid function and risk of non-alcoholic fatty liver disease in euthyroid subjects. Ann. Hepatol. 17 (5), 779–788. doi:10.5604/01.3001.0012.3136
Lu, L., Wan, B., Li, L., and Sun, M. (2022). Hypothyroidism has a protective causal association with hepatocellular carcinoma: a two-sample Mendelian randomization study. Front. Endocrinol. (Lausanne) 13, 987401. doi:10.3389/fendo.2022.987401
Martinez-Escude, A., Pera, G., Costa-Garrido, A., Rodriguez, L., Arteaga, I., Exposito-Martinez, C., et al. (2021a). TSH levels as an independent risk factor for NAFLD and liver fibrosis in the general population. J. Clin. Med. 10 (13), 2907. doi:10.3390/jcm10132907
Martinez-Escude, A., Pera, G., Rodriguez, L., Arteaga, I., Exposito-Martinez, C., Toran-Monserrat, P., et al. (2021b). Risk of liver fibrosis according to TSH levels in euthyroid subjects. J. Clin. Med. 10 (7), 1350. doi:10.3390/jcm10071350
Mavromati, M., and Jornayvaz, F. R. (2021). Hypothyroidism-associated dyslipidemia: potential molecular mechanisms leading to NAFLD. Int. J. Mol. Sci. 22 (23), 12797. doi:10.3390/ijms222312797
Mukherjee, S., Samanta, L., Roy, A., Bhanja, S., and Chainy, G. B. (2014). Supplementation of T3 recovers hypothyroid rat liver cells from oxidatively damaged inner mitochondrial membrane leading to apoptosis. Biomed. Res. Int. 2014, 590897. doi:10.1155/2014/590897
Nishimura, N., De Battista, D., McGivern, D. R., Engle, R. E., Tice, A., Fares-Gusmao, R., et al. (2021). Chitinase 3-like 1 is a profibrogenic factor overexpressed in the aging liver and in patients with liver cirrhosis. Proc. Natl. Acad. Sci. U. S. A. 118 (17), e2019633118. doi:10.1073/pnas.2019633118
Piantanida, E., Ippolito, S., Gallo, D., Masiello, E., Premoli, P., Cusini, C., et al. (2020). The interplay between thyroid and liver: implications for clinical practice. J. Endocrinol. Investig. 43 (7), 885–899. doi:10.1007/s40618-020-01208-6
Rahadini, A. A. D., and Rahadina, A. (2022). Association between hypothyroidism and liver fibrosis risk: a systematic review and meta-analysis. Clin. Exp. Hepatol. 8 (3), 188–194. doi:10.5114/ceh.2022.118594
Sahin, T., Oral, A., Turker, F., and Kocak, E. (2020). Can hypothyroidism be a protective factor for hepatocellular carcinoma in cirrhosis? Med. Baltim. 99 (11), e19492. doi:10.1097/MD.0000000000019492
Sekula, P., Del Greco, M. F., Pattaro, C., and Kottgen, A. (2016). Mendelian randomization as an approach to assess causality using observational data. J. Am. Soc. Nephrol. 27 (11), 3253–3265. doi:10.1681/ASN.2016010098
Shao, C., Cheng, Q., Zhang, S., Xiang, X., and Xu, Y. (2022). Serum level of free thyroxine is an independent risk factor for non-alcoholic fatty liver disease in euthyroid people. Ann. Palliat. Med. 11 (2), 655–662. doi:10.21037/apm-21-3890
Sikarwar, S. S., Usmani, M. H., and Kapur, K. S. (2022). A clinical study of cirrhosis with special reference to thyroid function. J. Assoc. Physicians India 70 (4), 11–12.
Skrivankova, V. W., Richmond, R. C., Woolf, B. A. R., Yarmolinsky, J., Davies, N. M., Swanson, S. A., et al. (2021). Strengthening the reporting of observational studies in Epidemiology using mendelian randomization: the STROBE-MR statement. JAMA 326 (16), 1614–1621. doi:10.1001/jama.2021.18236
Tao, H., Yang, J. J., Shi, K. H., Huang, C., Zhang, L., Lv, X. W., et al. (2014). The significance of YKL-40 protein in liver fibrosis. Inflamm. Res. 63 (4), 249–254. doi:10.1007/s00011-013-0698-9
Taylor, P. N., Razvi, S., Pearce, S. H., and Dayan, C. M. (2013). Clinical review: a review of the clinical consequences of variation in thyroid function within the reference range. J. Clin. Endocrinol. Metab. 98 (9), 3562–3571. doi:10.1210/jc.2013-1315
van Tienhoven-Wind, L. J., and Dullaart, R. P. (2015). Low-normal thyroid function and the pathogenesis of common cardio-metabolic disorders. Eur. J. Clin. Investig. 45 (5), 494–503. doi:10.1111/eci.12423
Verbanck, M., Chen, C. Y., Neale, B., and Do, R. (2018). Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50 (5), 693–698. doi:10.1038/s41588-018-0099-7
Weitzel, J. M., Iwen, K. A., and Seitz, H. J. (2003). Regulation of mitochondrial biogenesis by thyroid hormone. Exp. Physiol. 88 (1), 121–128. doi:10.1113/eph8802506
Zhang, X., Chen, Y., Ye, H., Luo, Z., Li, J., Chen, Z., et al. (2023). Correlation between thyroid function, sensitivity to thyroid hormones and metabolic dysfunction-associated fatty liver disease in euthyroid subjects with newly diagnosed type 2 diabetes. Endocrine 80 (2), 366–379. doi:10.1007/s12020-022-03279-2
Zhang, X., Zhang, J., Dai, Y., and Qin, J. (2020). Serum thyroid hormones levels are significantly associated with nonalcoholic fatty liver disease in euthyroid Chinese population. Clin. Lab. 66 (10). doi:10.7754/Clin.Lab.2020.200219
Zhang, Y., Li, J., and Liu, H. (2022). Correlation between the thyroid hormone levels and nonalcoholic fatty liver disease in type 2 diabetic patients with normal thyroid function. BMC Endocr. Disord. 22 (1), 144. doi:10.1186/s12902-022-01050-2
Keywords: causal association, hepatic fibrosis/cirrhosis, hyperthyroidism, hypothyroidism, Mendelian randomization study, single-nucleotide polymorphisms, thyroid function
Citation: Wang Y, Zhang X, Li Q, Zhang Q and Liu J (2025) Thyroid function and hepatic fibrosis/cirrhosis: a two-sample Mendelian randomization study. Front. Genet. 16:1399353. doi: 10.3389/fgene.2025.1399353
Received: 26 March 2024; Accepted: 11 March 2025;
Published: 02 April 2025.
Edited by:
Akira Sugawara, Tohoku University, JapanReviewed by:
Georgia Damoraki, National and Kapodistrian University of Athens, GreeceCopyright © 2025 Wang, Zhang, Li, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Liu, MTI5MTkyODE3OEBxcS5jb20=; Qing Zhang, aGhoMzM1N0BzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.