- 1Department of Emergency Medicine, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, China
- 2Disaster Medical Center, Sichuan University, Chengdu, China
- 3Nursing Key Laboratory of Sichuan Province, Chengdu, China
- 4Department of Dermatology, Mianyang Central Hospital, School of Medicine, University of Electronic Science and Technology of China, Mianyang, China
Background: Diabetic foot ulcers (DFU) are a significant complication of diabetes, with huge implications on patient morbidity and healthcare costs. The objective of this meta-analysis was to evaluate the impacts of stem cells from different sources on wound healing rate in DFU patients.
Methods: We systematically retrieved records via key databases PubMed, Cochrane Library, Web of Science, Embase, China National Knowledge Infrastructure (CNKI) and Wanfang from the inception to October 2024. The Stata 16.0 (Stata Corp, TX) software was used to perform the meta-analysis. Risk of bias in all included studies was evaluated by Cochrane Risk of Bias version 2.
Results: A total of 24 studies involving 1,321 patients were included. There was an increased likelihood of wound healing with peripheral blood-derived stem cells, the most effective cells (odds ratios (OR) = 7.31, 95% CI: 2.90–18.47), followed by adipose-derived stem cells (OR = 5.23, 95% CI: 2.76–9.90), umbilical cord-derived stem cells (OR = 4.94, 95% CI: 0.61–40.03), bone-derived stem cells (OR = 4.36, 95% CI: 2.43–7.85) and other sources stem cells (OR = 3.16, 95% CI: 1.83–5.45). Nevertheless, only umbilical cord-derived stem cells showed statistical significance (p < 0.05). The heterogeneity ranged from non-existent in the adipose and peripheral blood groups (I2 = 0.00%) to moderate in the bone groups (I2 = 26.31%) and other groups (I2 = 30.62%), and substantial in the umbilical cord groups (I2 = 88.37%). Asymmetrical funnel plots pointed to publication bias, but the trim-and-fill method to correct for this brought the effect estimates even lower: based on the pooled OR, corrected OR was 3.40 (95% CI 2.39–4.84). Stem cell therapy was also associated with improvements in several secondary outcomes, suggesting its potential to influence the progression of DFU.
Conclusion: Our study suggested that stem cells from different sources showed potential in promoting wound healing in DFU, although with some variation in effectiveness. Despite some publication bias and moderate heterogeneity, the overall therapeutic effect remained positive. These findings indicated that stem cell therapy might influence the progression of DFU.
Introduction
Diabetic foot ulcers (DFU) refer to a condition in individuals who have been diagnosed with diabetes or have a history of the disease, characterized by infection, ulceration, or degradation of tissue in the foot. This condition frequently occurs alongside complications such as lower extremity neuropathy and/or peripheral arterial disease (Lipsky et al., 2020). Among the severe chronic complications of diabetes, DFU are particularly concerning. These ulcers adversely affect the physical health and significantly diminish the quality of life of affected individuals (Krasilnikova et al., 2022). Additionally, DFU lead to considerable psychological distress and financial strain for both the patients and their families, imposing a significant societal burden. Given its widespread impact, diabetic foot ulceration has become a critical public health issue (Edmonds et al., 2021). Statistics highlight the severity of this condition, showing that between 5% and 24% of patients with diabetic foot ulcers require amputation within 6–18 months after their initial diagnosis. Alarmingly, an amputation occurs approximately every 20 s among this population (Ibrahim, 2017). Moreover, about half of these patients die within 5 years following their amputation (Weledji and Fokam, 2014; Humphries et al., 2016).
Current therapeutic approaches for DFU include a variety of strategies: glycemic control, local wound debridement, changing wound dressings, applying antimicrobial treatments, vascular reconstruction, employing traditional Chinese medicinal compounds, hyperbaric oxygen therapy, and in severe cases, restoring lower limb blood perfusion (Singh et al., 2017; Chen et al., 2023). Despite these diverse interventions, the effectiveness of treatments for severe DFU is often limited, and some methods have inherent drawbacks. For example, extended use of high-dose antibiotics can disrupt normal bacterial balance, leading to secondary fungal infections and the development of drug-resistant bacteria (Liu et al., 2023a). Surgical options are also limited by technical challenges such as recanalization failures, vascular re-occlusions, and impaired microcirculation (Meloni et al., 2020).
Stem cell therapy holds significant promise in enhancing wound healing in DFU, largely due to its potential to regenerate tissue and modulate immune responses. Stem cells, particularly mesenchymal stem cells (MSCs), have been shown to promote wound healing by secreting growth factors and cytokines that accelerate tissue repair and reduce inflammation. For instance, studies have demonstrated that MSCs can improve angiogenesis, increase collagen synthesis, and recruit local and systemic cells involved in wound repair processes. Additionally, the immunomodulatory properties of stem cells help in mitigating the excessive inflammatory responses often seen in diabetic wounds, thereby preventing prolonged inflammation and further tissue damage (Sasaki et al., 2008; Tutuianu et al., 2021; Mimeault et al., 2007). Currently, stem cells used for the treatment of DFU are classified into autologous and allogeneic types, with primary sources including bone marrow, umbilical cord, adipose tissue, and placenta. However, the impact of stem cells from different sources on DFU healing rates has not been fully quantified (Yu et al., 2022).
This study aimed to explore the existing literature on stem cell therapies for DFU, assessing their efficacy in accelerating wound healing based on different cell sources. By providing a clearer understanding of the benefits and limitations of various stem cell therapies, this study seeked to inform clinical decision-making and highlight potential avenues for future research.
Methods
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) checklist for reporting systematic reviews and meta-analysis. The study has been registered on the international prospective register of systematic reviews.
Literature search
We conducted a thorough search to assess the association between cell therapy and ulcer healing. PubMed, Cochrane Library, Web of Science, Embase, China National Knowledge Infrastructure (CNKI) and Wanfang were searched from the inception to October 2024. The following search strategy was used: “stem cell,” or “progenitor cell,” or “mesenchymal stem cells,” or “adipose-derived,” or “bone marrow,” or “peripheral blood,” or “Umbilical cord,” or “mononuclear cell” paired with “diabetic” paired with “foot,” or “ulcer,” or “wound” (Supplementary Table S1).
Study selection
The criteria for the inclusion of studies were pre-established as follows: 1) the study design must be either controlled clinical trials (CCTs); 2) the study population should consist of patients afflicted with diabetic foot ulcers; 3) the intervention group should have received some form of stem cells therapy, including but not limited to bone-derived stem cells, adipose-derived stem cells, or peripheral blood-derived stem cells; 4) the comparison group should have received either standard care or a placebo; 5) the studies must report on the outcome measure of the wound healing rate in patients; 6) the study should have a sample size of 10 or more participants.
Conversely, studies were excluded based on the following criteria: 1) studies that suffered from incomplete data with no possibility of contacting the authors for further information; 2) studies lacking a control group; 3) non-empirical studies such as letters, editorials, conference abstracts, case reports, reviews, and study protocols; 4) animal studies.
Data extraction
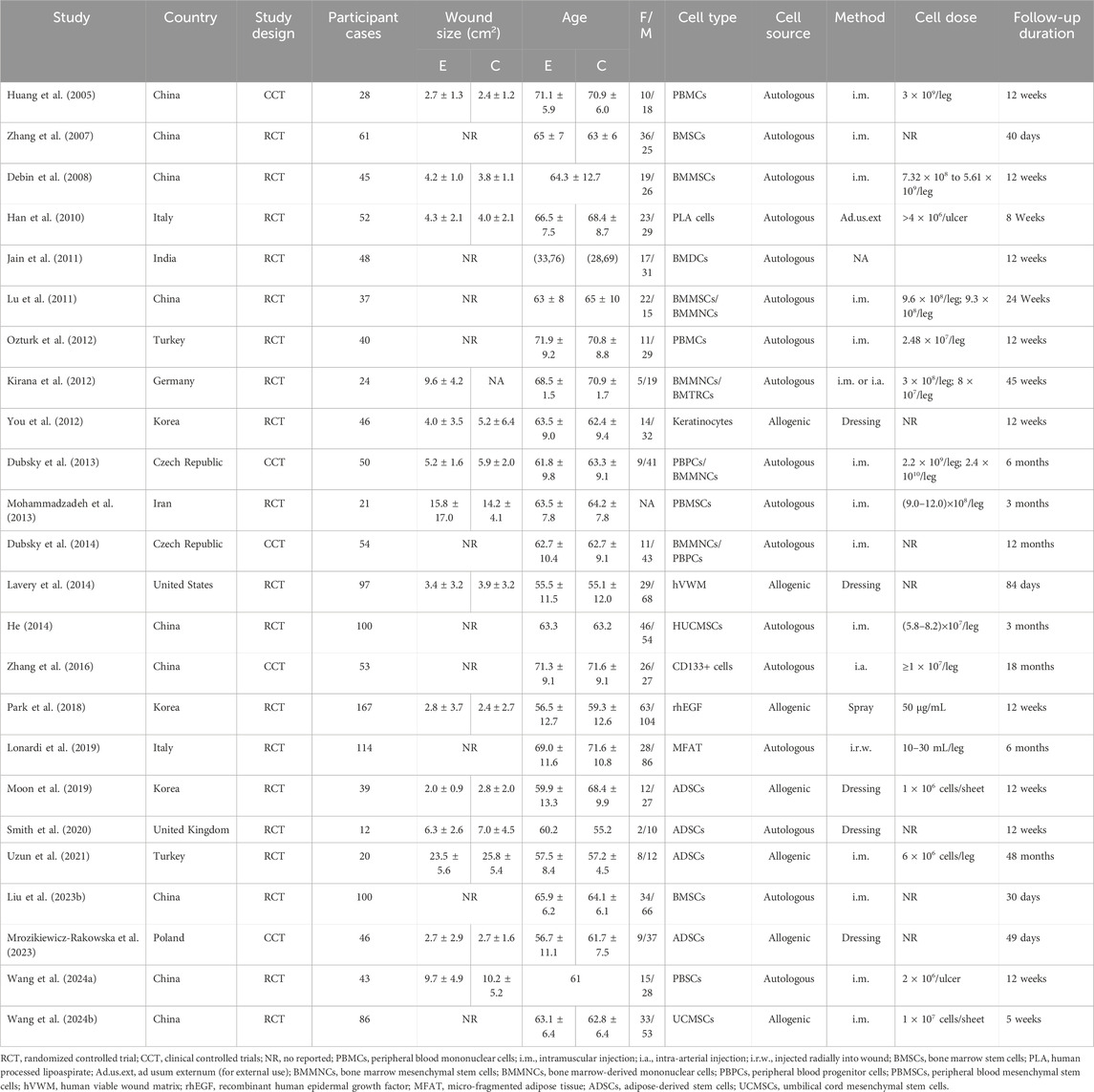
Data extraction was performed by two independent reviewers utilizing standardized forms to ensure accuracy and reliability. Any discrepancies between reviewers were resolved through discussion until a consensus was achieved. The quality of the included studies was appraised based on the pre-specified inclusion criteria. The extracted data encompassed author (s), publication year, country, study design, sample size, demographic data of participants (age, gender), original ulcer size, cell source, donor type, intervention method, cell dose, follow-up time, and reported results.
Quality assessment
The assessment of the risk of bias within the included studies was conducted independently by two reviewers using the Cochrane Collaboration’s risk of bias tool (RoB 2.0). This tool facilitated a systematic evaluation across five domains of bias, namely: bias arising from the randomization process; bias due to deviations from intended interventions; bias due to missing outcome data; bias in measurement of the outcome; and bias in the selection of the reported result. Studies were then categorized based on their risk of bias as low (meeting all or at least four of the low-risk criteria), having some concerns, or high risk, to ensure a rigorous analysis of the evidence.
Statistical analysis
Statistical analyses were performed using the random-effects model, following the DerSimonian and Laird method, to accommodate inherent between-study variability. Pooled odds ratios (OR) and mean differences (MD) with 95% confidence intervals (CI) were calculated to assess the efficacy of stem cell therapies. Heterogeneity among the included studies was quantified using the I2 statistic, where values of 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively. Heterogeneity was also assessed through visual inspection of L’Abbé plots. Moreover, sensitivity analysis was used to test the stability of the results, and publication bias was estimated using a funnel plot, trim-and-fill analysis and Egger’s and Begg’s tests, with a significance threshold of P < 0.05. Subgroup analyses were conducted based on donor source, study design, and follow-up duration to explore potential sources of variability in treatment effects. All statistical analyses were performed using STATA 16.0 software. Significance was set at a p-value of less than 0.05 for all tests.
Results
Literature search
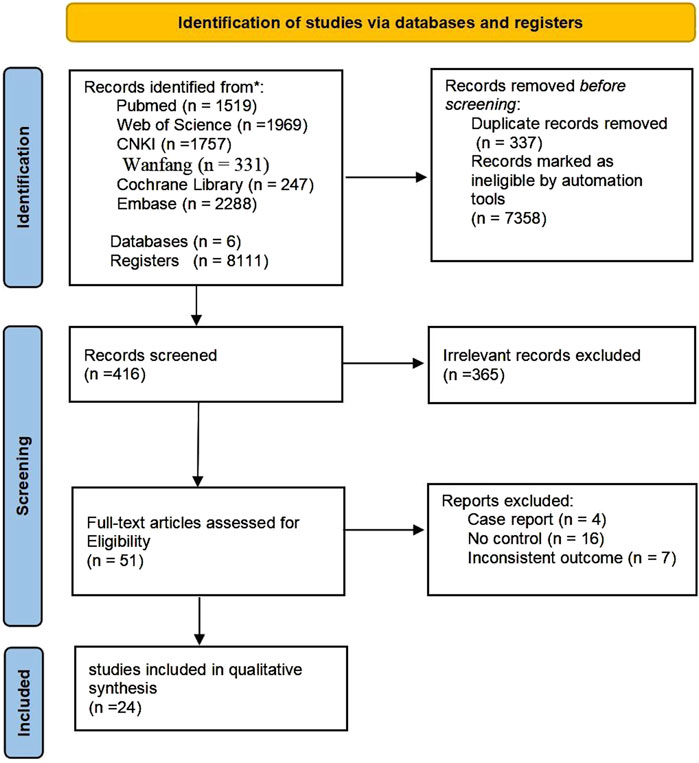
Initially, a comprehensive search across six databases and various registers yielded a total of 8,111 records. Of these, 337 were identified as duplicates and 7358 were automatically disqualified. Following a evaluation of titles and abstracts, 365 articles were excluded based on the inclusion and exclusion criteria. The remaining 51 full-text articles were assessed for eligibility, leading to the exclusion of an additional 98 reports due to various reasons such as non-controlled study designs, inconsistent outcomes, or because they were case reports or reviews. Ultimately, 24 studies met all inclusion criteria and were included in the qualitative synthesis of our meta-analysis (Huang et al., 2005; Zhang et al., 2007; Debin et al., 2008; Han et al., 2010; Jain et al., 2011; Lu et al., 2011; Ozturk et al., 2012; Kirana et al., 2012; You et al., 2012; Dubsky et al., 2013; Mohammadzadeh et al., 2013; Dubský et al., 2014; Lavery et al., 2014; He, 2014; Zhang et al., 2016; Park et al., 2018; Lonardi et al., 2019; Moon et al., 2019; Smith et al., 2020; Uzun et al., 2021; Liu et al., 2023b; Mrozikiewicz-Rakowska et al., 2023; Wang et al., 2024a; Wang et al., 2024b) (Figure 1).
Characteristics of included articles
Table 1 showed the characteristics of included study. Most studies originated from Asia and Europe, with only one study from North America. Sample sizes varied widely, ranging from 12 to 167 patients, with ages between 56.6 and 64.1 years. Gender distribution differed across studies, with the total number of males slightly exceeding the total number of females. The average wound size ranged from 2.0 cm2 to 23.5 cm2. Stem cell sources included bone marrow, peripheral blood, fat, umbilical cord, and other tissues, with administration methods being either injection or application. The cell doses and follow-up periods varied, with the shortest follow-up being 30 days and the longest reaching 48 months.
Quality assessment
A thorough quality assessment revealed that 20 studies provided detailed descriptions of the randomization methods employed. However, seven studies, despite mentioning randomization, failed to elucidate the specific methods utilized. The risk of bias was categorized as low in twelve articles, with the remaining articles falling into the medium-risk category; notably, no studies were classified as high risk (Figure 2).
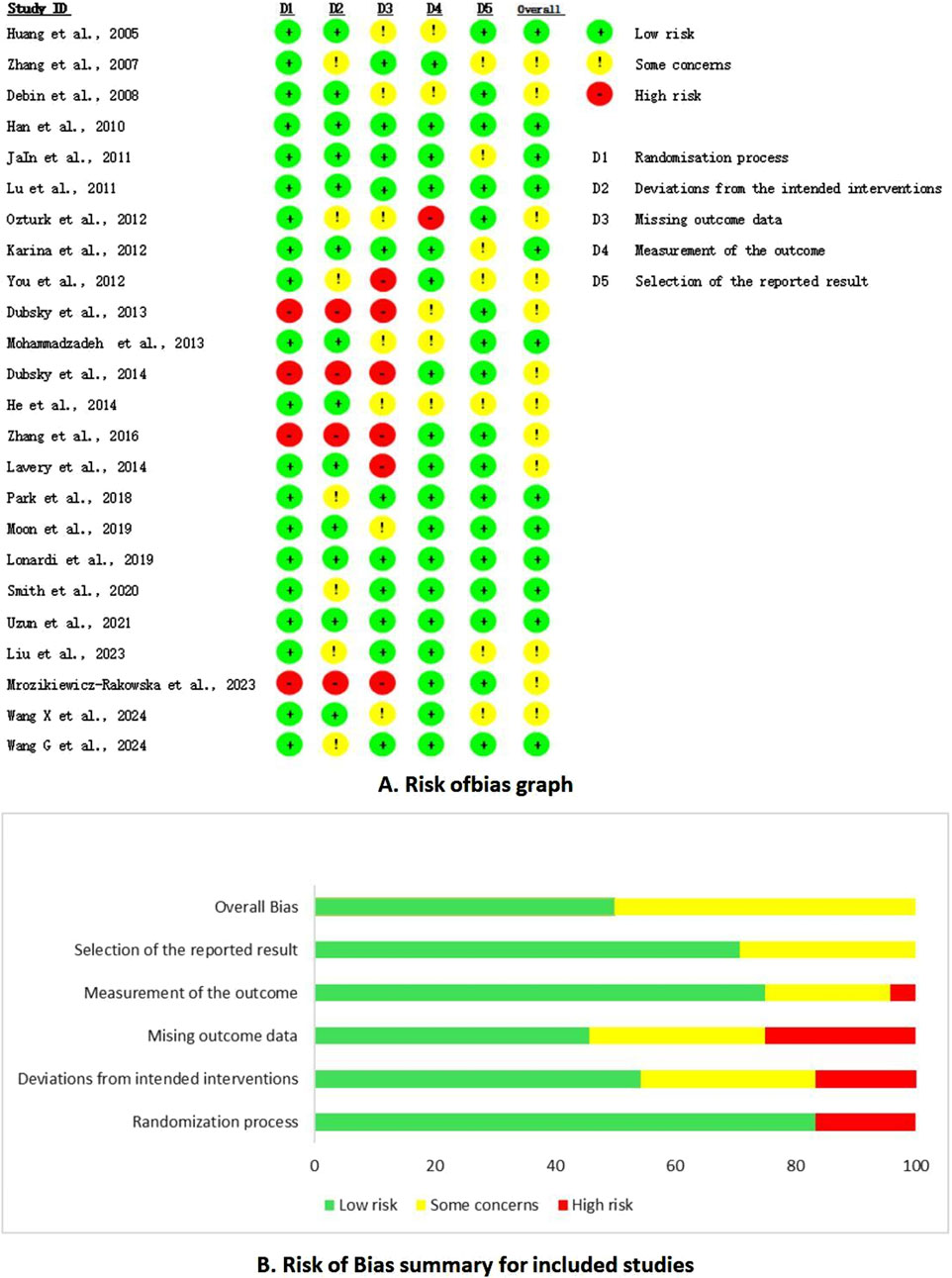
Figure 2. Risk of bias summary. (A) Risk of bias graph. (B) Risk of bias summary for included studies.
Wound healing rate by cell sources
The healing rate of wounds treated with stem cells from different sources showed obvious improvement, with peripheral blood-derived stem cells demonstrating the highest efficacy (OR = 7.31, 95% CI: 2.90–18.47). Adipose-derived stem cells were the second most effective (OR = 5.23, 95% CI: 2.76–9.90), followed by umbilical cord-derived stem cells (OR = 4.94, 95% CI: 0.61–40.03), bone marrow-derived stem cells (OR = 4.36, 95% CI: 2.43–7.85), and stem cells from other sources (OR = 3.16, 95% CI: 1.83–5.45). The heterogeneity ranged from non-existent in the adipose and peripheral blood groups (I2 = 0.00%) to moderate in the bone groups (I2 = 26.31%) and other groups (I2 = 30.62%), and substantial in the umbilical cord groups (I2 = 88.37%). Nevertheless, only umbilical cord-derived stem cells showed statistical significance (p < 0.05) (Figure 3). The L’Abbé plot analysis shows high consistency and low heterogeneity across stem cells from different sources in promoting DFU healing, with most studies aligning closely with the equality line. However, some studies deviated, indicating that certain stem cell sources may exhibit stronger or weaker effects under specific conditions. (Supplementary Figure S1).
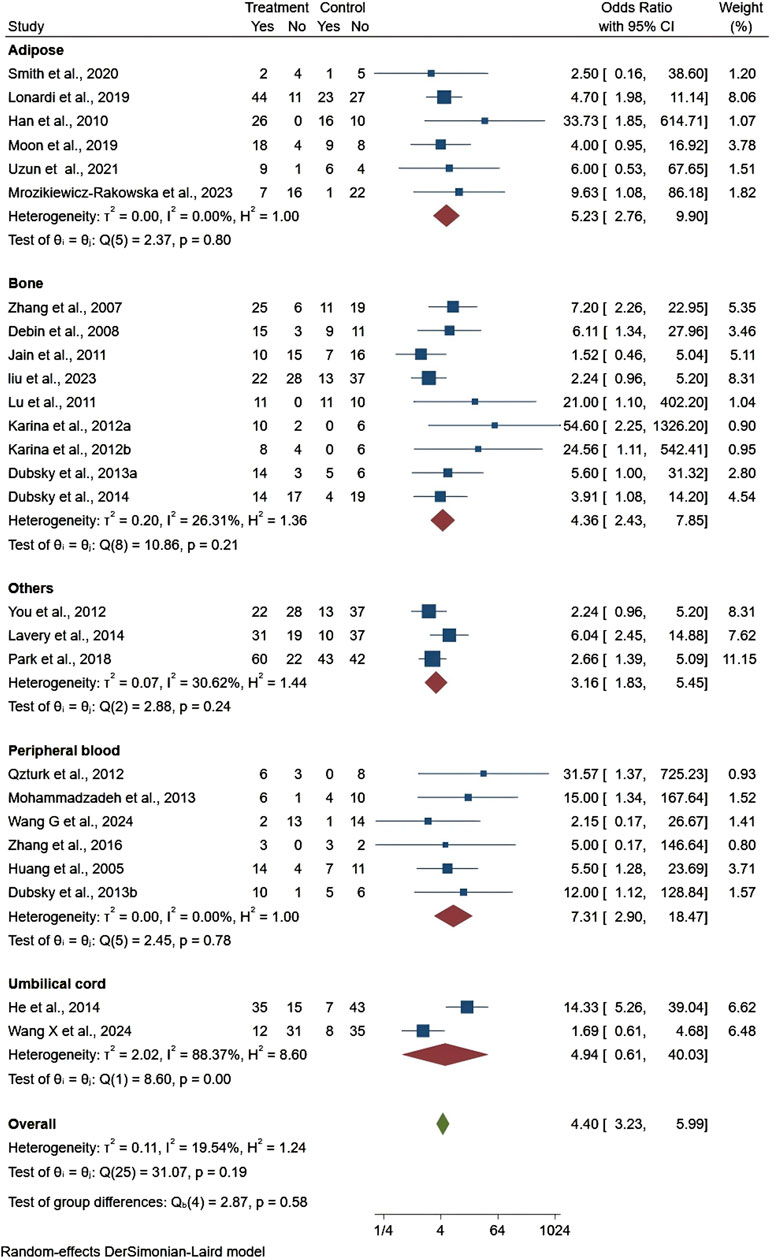
Figure 3. Forest plot showing the effect of stem cells from different sources on ulcer healing rates.
Publication bias
In the funnel plots of studies on different sources of stem cells, only studies on bone derived stem cells showed uneven distribution, indicating potential bias (Supplementary Figure S2). The overall funnel plot shows asymmetry, indicating possible publication bias in the overall analysis (Supplementary Figure S3). Both Begg’s and Egger’s tests suggested the possibility of potential publication bias (P < 0.01). To address this, we performed a trim-and-fill analysis. After adjusting for the imputed studies, the pooled OR decreased from 4.40 (95% CI: 3.23–5.99) to 3.40 (95% CI: 2.39–4.84).
Sensitivity analyses
The included literature demonstrated that excluding each study in turn had no impact on the results (Supplementary Figure S4).
Subgroup analysis
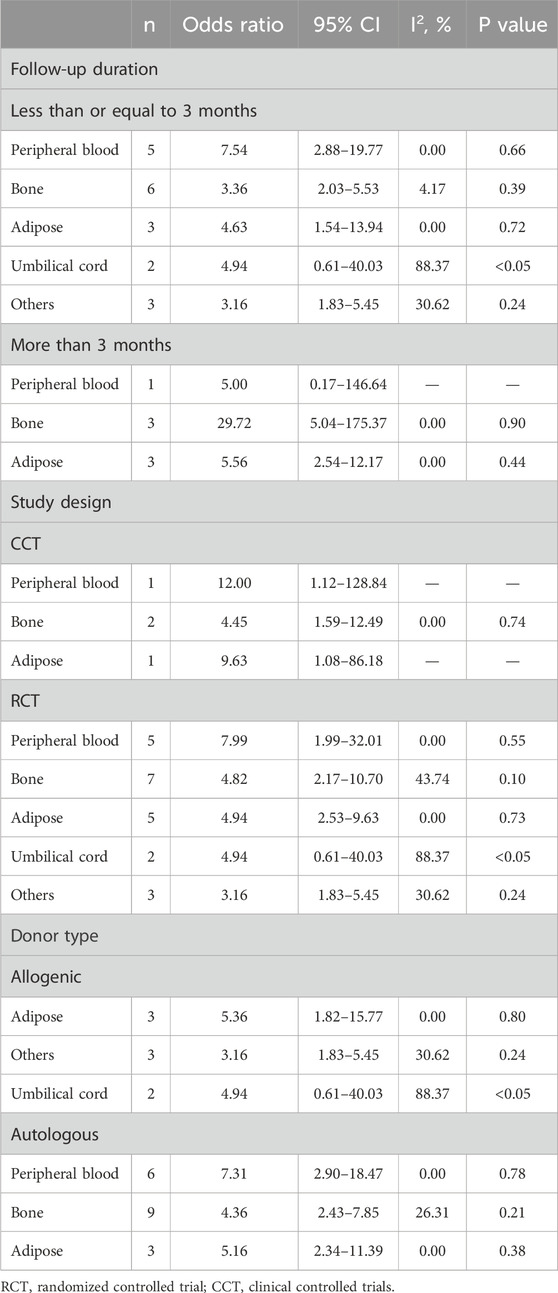
The subgroup analysis investigated the effects of stem cell therapy on wound healing rates in diabetic foot ulcers, stratifying the data by follow-up duration, study design, and donor type (Table 2). In the group with a follow-up time of 3 months or less, peripheral blood stem cells demonstrated the most favorable therapeutic effect (OR = 7.54, 95% CI: 2.88–19.77), though it did not reach statistical significance (P > 0.05), while umbilical cord stem cell therapy had statistically significant efficacy (P < 0.05), despite higher heterogeneity (I2 = 88.37%). In the group with a follow-up duration of more than 3 months, bone marrow-derived stem cells showed the best therapeutic effect (OR = 29.72, 95% CI: 5.04–175.37). Regarding study design, both CCT and RCT studies demonstrated the effectiveness of peripheral blood, bone marrow, and adipose-derived stem cells in treating diabetic foot, with higher heterogeneity observed in the RCT group. Analysis by donor type showed that allogenic stem cells, particularly adipose-derived stem cells (OR = 5.36, 95% CI: 1.82–15.77), also had favorable therapeutic effects for diabetic foot. In autologous stem cell group, peripheral blood-derived stem cells demonstrated the most favorable therapeutic effect compared to bone-derived and adipose-derived stem cells (OR = 7.31, 95% CI: 2.90–18.47).
Secondary outcomes
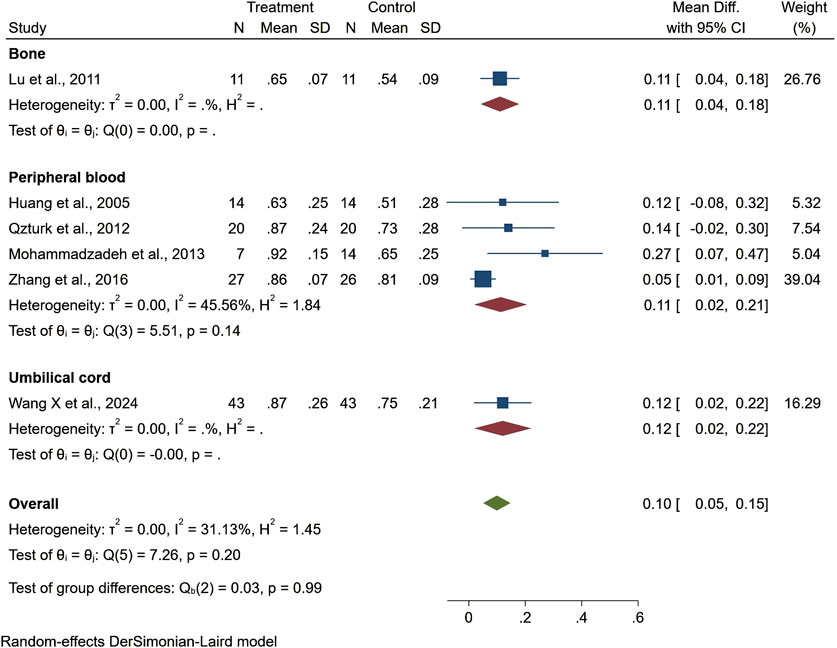
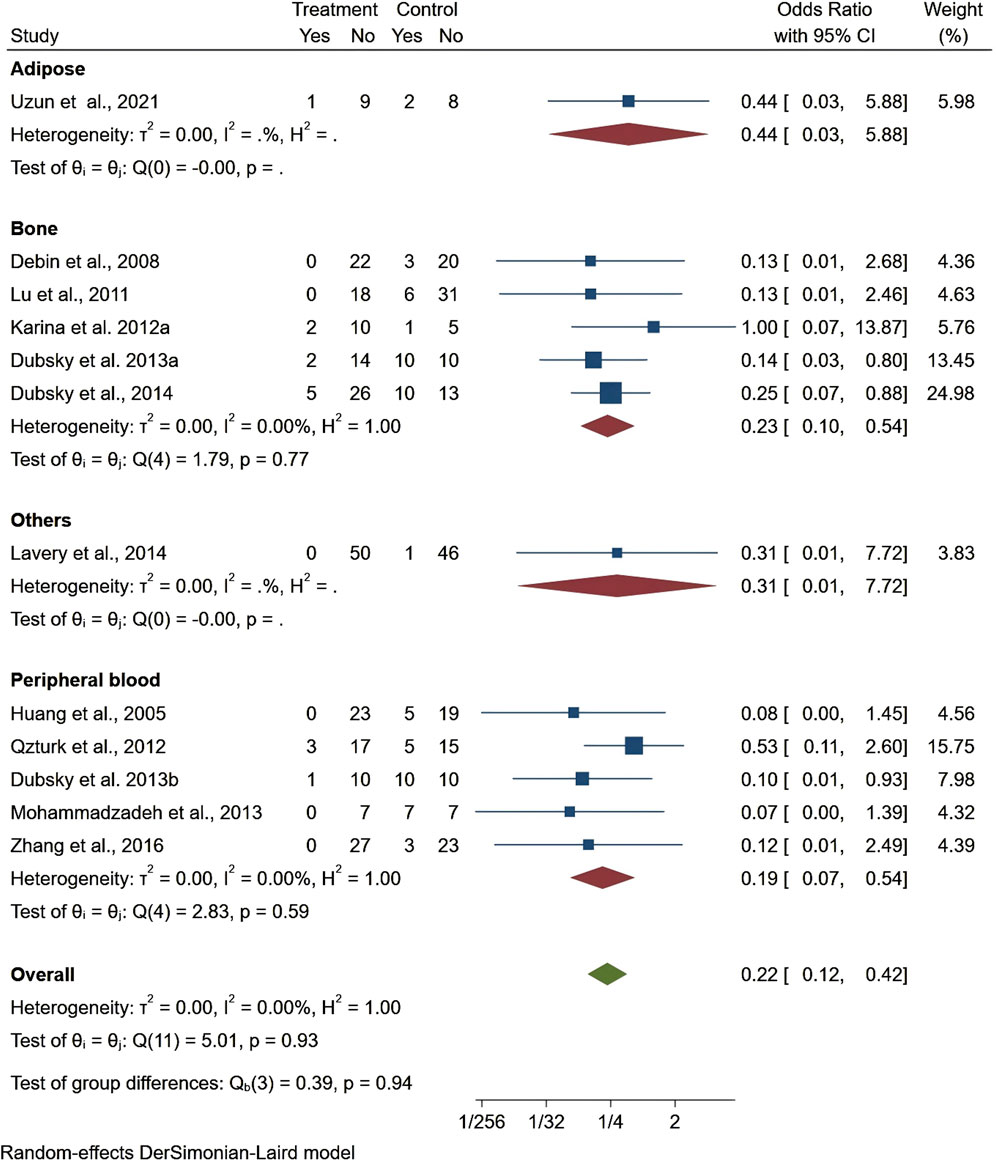
Seven studies reported healing time, highlighting 13.44 days shorter of the trial group than the control one (95% CI −22.76 to −4.13, P < 0.01). Among them, patients treated with stem cells from other sources had significantly shorter ulcer healing times compared to those treated with bone marrow, adipose tissue, or umbilical cord stem cells. However, the results for ulcer healing time showed considerable heterogeneity (I2 = 99.06%), but the overall result was statistically significant (Figure 4). Regarding ankle brachial index (ABI), the results showed a slight improvement (MD = 0.10, 95% CI: 0.05–0.15), but did not reach statistical significance (P > 0.05) (Figure 5). Although the results for amputation rate also did not reach statistical significance (P > 0.05), all stem cell sources demonstrated consistent positive effects (OR = 0.22, 95% CI: 0.12–0.42) (Figure 6).

Figure 4. Forest plot showing the effect of stem cells from different sources on ulcer healing time.
Discussion
This study assessed the efficacy of various stem cell therapies in promoting ulcer healing in patients with DFU. The efficacy varied across different stem cell sources, with peripheral blood-derived cells demonstrating the most potent effect, while adipose-derived, umbilical cord-derived, bone marrow-derived, and other source stem cells also contributed to improved ulcer healing. Heterogeneity across the studies was relatively moderate, suggesting a reasonable consistency among the included studies despite the varied therapeutic outcomes. However, publication bias, as indicated by funnel plot asymmetry and confirmed through the trim-and-fill method, suggested an initial overestimation of treatment effects. Subgroup analysis revealed that treatment outcomes were influenced by follow-up duration, study design, and donor type, although no significant statistical differences were found between the groups except for umbilical cord derived stem cells. Additionally, stem cell therapy demonstrated positive clinical effects in accelerating wound healing, improving ABI, and reducing amputation rates, further highlighting its potential in the management of diabetic foot ulcers.
In recent years, numerous studies have explored the potential efficacy of stem cells in treating diabetic foot ulcers, examining their specific molecular mechanisms, types, and expanded applications, with the goal of advancing the clinical use of stem cell therapy for DFU (Galindo et al., 2011; Xia et al., 2024). Previous studies demonstrated a superior therapeutic impact of cellular interventions, assessing not only therapeutic efficacy but also the critical outcome of amputation rates, although the studies were limited by small sample sizes and a lack of variability in outcome measures (Guo et al., 2017; Dai et al., 2020). More recently, Sun et al., in 2022 analyzed fourteen studies with a total of 683 participants, revealing that cell-based therapy improved several outcomes including wound healing rates, vascular neogenesis in the lower extremities, TcPO2, ABI values, and pain-free walking distances. Additionally, these interventions were linked to reduced amputation rates and resting pain scores. These results are consistent with those of the current study, particularly in terms of vascular neogenesis and improvements in pain management and mobility. However, significant gaps remain in the research on TcPO2, pain-free ambulation, and rest-induced pain, highlighting the need for more comprehensive studies. The analysis also pointed out the lack of subgroup analyses that could differentiate the effects of various cellular therapies, adding a layer of uncertainty to the results (Sun et al., 2022a).
DFU is a prevalent and severe complication of diabetes, impacting skin, muscle, nerve, and vascular systems, thereby complicating the healing process (Sun et al., 2022b). The pathogenesis is driven by a high-sugar microenvironment that elevates oxidative stress, impairing wound healing (Patel et al., 2019). This environment also facilitates reactions between proteins and glucose, producing harmful byproducts that inhibit cell proliferation and adversely affect vascular and neural functions (Davis et al., 2020). Furthermore, an environment rich in fats and glucose promotes inflammatory responses, reducing neutrophil chemotaxis and macrophage efficacy, and hindering the macrophage phenotype shift from M1 to M2 (Boniakowski et al., 2019; Parisi et al., 2018). These complex mechanisms contribute to the high recurrence and worsening of DFU, placing significant psychological and economic burdens on patients. Addressing these challenges is critical and enhancing the clinical cure rate and accelerating wound healing are priorities in diabetes research. While it has been noted that VEGF and fibroblast growth factor levels are comparable in peripheral and bone marrow-derived cells, levels of interleukin-1b and tumor necrosis factor are higher in peripheral blood, suggesting less efficacy of peripheral blood mononuclear cells compared to bone marrow-derived cells (Iba et al., 2002).
Back to our findings, Peripheral Blood-derived stem cells might be particularly effective due to their high availability and the potential presence of a wide range of progenitor cells, which can contribute to enhanced angiogenesis and tissue regeneration (Rai et al., 2022). Adipose-derived stem cells, while also effective, contain a rich mix of regenerative factors and have been shown to promote wound healing through their anti-inflammatory properties and ability to enhance collagen deposition (Zhang et al., 2020). Bone-derived stem cells are well-noted for their osteogenic and chondrogenic potential but may offer slightly less efficacy in soft tissue regeneration compared to other cell sources (Polymeri et al., 2016). Umbilical cord-derived stem cells, due to their unique regenerative capabilities, including anti-inflammatory, immunomodulatory, and tissue repair properties, show potential in the treatment of diabetic foot ulcers; however, the lack of standardized treatment protocols may result in variability in their efficacy (Yu et al., 2023). These findings underscore the importance of selecting an appropriate stem cell source based on the specific clinical aspects and healing requirements of diabetic foot ulcers. The choice of stem cell type should consider the wound environment, the patient’s overall health status, and the specific healing mechanisms needed (Lopes et al., 2018).
In addition, although our study found that peripheral blood derived stem cells and bone derived stem cells showed the most favorable therapeutic effects at follow-up times of 3 months or less and 3 months or more, respectively, the small overall sample size may be an important influencing factor. For example, a 12-week trial in Turkey used autologous peripheral blood mononuclear cell transplantation to treat DFU, with a cure rate 31 times higher than that of patients receiving standard treatment. However, the total number of patients was only 40 (Ozturk et al., 2012). Another study evaluated the effects of autologous bone marrow mononuclear cells and CD90+ enriched tissue repair cells in treating DFU patients. The treatment effects of the two cell on ulcer healing rates were 54 times and 25 times higher than that of the control group, respectively, but the total sample size was only 24 (Kirana et al., 2012). In contrast, Lonardi et al.’s study included over 100 patients who received adipose-derived stem cell therapy, but the treatment effect in the intervention group was only four times higher than that of the control group (Lonardi et al., 2019). Additionally, we observed that umbilical cord-derived stem cells had a significant effect on wound healing rates. One study using umbilical cord mesenchymal stem cell transplantation in DFU patients found that the observation group had significantly shorter healing times and faster wound closure compared to the control group, with the healing rate approximately 14 times higher (He, 2014). These findings emphasize the need for more and larger sample size studies to confirm the potential of stem cells from various sources in treating DFU.
However, there are some limitations to our meta-analysis. First, the relatively small sample sizes may limit the applicability of our findings to larger, more diverse patient populations. Second, the inherent heterogeneity among the included studies, due to variations in stem cell sources, protocols, wound environments, and patient demographics, could affect the generalizability of the findings. Although we employed a random-effects model to address this variability, the differences in study designs and treatment regimens across studies still pose a challenge in interpreting the pooled results uniformly. Third, the presence of publication bias, as indicated by the asymmetry in funnel plots and confirmed by the trim-and-fill method, suggests that smaller studies with negative results might be underrepresented in the literature, potentially skewing the efficacy estimates. Lastly, the quantitative analysis was limited to published studies; thus, unpublished data and ongoing research could alter the effectiveness and safety profiles presented.
Future research should prioritize understanding the mechanisms behind stem cell-mediated wound healing and how patient-specific factors like genetics and metabolic control influence treatment outcomes. Comparative studies are essential to determine the most effective sources and types of stem cells, while the development of standardized protocols for cell preparation and application will enhance treatment consistency and efficacy. Longitudinal studies should evaluate the long-term effects of therapy, and integration with other treatments could lead to comprehensive care strategies. Additionally, addressing regulatory, ethical, and economic issues will be crucial for facilitating clinical implementation and ensuring the therapy’s cost-effectiveness compared to conventional treatments.
Conclusion
This study shows that stem cell therapy may be a promising method to promote wound healing in patients with diabetes foot ulcers, but its effectiveness varies depending on the source of stem cells. Despite evidence of moderate heterogeneity and publication bias, this analysis demonstrates the overall effectiveness of stem cell therapy in improving clinical outcomes in DFU management.
Author contributions
LeT: Formal Analysis, Project administration, Validation, Writing–original draft. LiT: Data curation, Investigation, Writing–review and editing. BT: Data curation, Writing–review and editing. JZ: Conceptualization, Methodology, Software, Supervision, Visualization, Writing–review and editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1541992/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | L'Abbé plot for trials evaluating the impact of stem cells from different sources on the healing rate in DFU.
SUPPLEMENTARY FIGURE S2 | Funnel plot of stem cells from different sources on the healing rate in DFU.
SUPPLEMENTARY FIGURE S3 | Overall Funnel plot of stem cells on the healing rate in DFU.
SUPPLEMENTARY FIGURE S4 | Sensitivity analysis of stem cells from different sources on the healing rate in DFU.
References
Boniakowski, A. M., denDekker, A. D., Davis, F. M., Joshi, A., Kimball, A. S., Schaller, M., et al. (2019). SIRT3 regulates macrophage-mediated inflammation in diabetic wound repair. J. Invest Dermatol 139 (12), 2528–2537.e2. doi:10.1016/j.jid.2019.05.017
Chen, Y., Wang, Y., and Zhang, T. (2023). Efficacy of Chinese and western medical techniques in treating diabetic foot ulcers with necrotizing fasciitis of the lower leg. Int. J. Low. Extrem Wounds 23 (1), 70–79. doi:10.1177/15347346221150865
Dai, J., Jiang, C., Chen, H., and Chai, Y. (2020). Treatment of diabetic foot with autologous stem cells: a meta-analysis of randomized studies. Stem Cells Int. 2020, 6748530. doi:10.1155/2020/6748530
Davis, F. M., Tsoi, L. C., Wasikowski, R., denDekker, A., Joshi, A., Wilke, C., et al. (2020). Epigenetic regulation of the PGE2 pathway modulates macrophage phenotype in normal and pathologic wound repair. JCI Insight 5 (17), e138443. doi:10.1172/jci.insight.138443
Debin, L., Youzhao, J., Ziwen, L., Xiaoyan, L., Zhonghui, Z., and Bing, C. (2008). Autologous transplantation of bone marrow mesenchymal stem cells on diabetic patients with lower limb ischemia. J. Med. Coll. PLA 23 (2), 106–115. doi:10.1016/s1000-1948(08)60031-3
Dubsky, M., Jirkovska, A., Bem, R., Fejfarova, V., Pagacova, L., Sixta, B., et al. (2013). Both autologous bone marrow mononuclear cell and peripheral blood progenitor cell therapies similarly improve ischaemia in patients with diabetic foot in comparison with control treatment. Diabetes Metab. Res. Rev. 29 (5), 369–376. doi:10.1002/dmrr.2399
Dubský, M., Jirkovská, A., Bem, R., Fejfarová, V., Pagacová, L., Nemcová, A., et al. (2014). Comparison of the effect of stem cell therapy and percutaneous transluminal angioplasty on diabetic foot disease in patients with critical limb ischemia. Cytotherapy 16 (12), 1733–1738. doi:10.1016/j.jcyt.2014.08.010
Edmonds, M., Manu, C., and Vas, P. (2021). The current burden of diabetic foot disease. J. Clin. Orthop. Trauma 17, 88–93. doi:10.1016/j.jcot.2021.01.017
Galindo, L. T., Filippo, T. R., Semedo, P., Ariza, C. B., Moreira, C. M., Camara, N. O. S., et al. (2011). Mesenchymal stem cell therapy modulates the inflammatory response in experimental traumatic brain injury. Neurol. Res. Int. 2011, 564089. doi:10.1155/2011/564089
Guo, J., Dardik, A., Fang, K., Huang, R., and Gu, Y. (2017). Meta-analysis on the treatment of diabetic foot ulcers with autologous stem cells. Stem Cell Res. Ther. 8 (1), 228. doi:10.1186/s13287-017-0683-2
Han, S. K., Kim, H. R., and Kim, W. K. (2010). The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound Repair Regen. 18 (4), 342–348. doi:10.1111/j.1524-475X.2010.00593.x
He, Y. Q. (2014). Clinical research on the umbilical cord mesenchymal stem cells therapy in the treatment of type 2 diabetes skin ulcer. Chin. J. Coal Ind. Med. 17 (4), 532–534. doi:10.11723/mtgyyx.1007-9564.201404005
Huang, P., Li, S., Han, M., Xiao, Z., Yang, R., and Han, Z. C. (2005). Autologous transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells improves critical limb ischemia in diabetes. Diabetes Care 28 (9), 2155–2160. doi:10.2337/diacare.28.9.2155
Humphries, M. D., Brunson, A., Li, C. S., Melnikow, J., and Romano, P. S. (2016). Amputation trends for patients with lower extremity ulcers due to diabetes and peripheral artery disease using statewide data. J. Vasc. Surg. 64 (6), 1747–1755.e3. doi:10.1016/j.jvs.2016.06.096
Iba, O., Matsubara, H., Nozawa, Y., Fujiyama, S., Amano, K., Mori, Y., et al. (2002). Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation 106 (15), 2019–2025. doi:10.1161/01.cir.0000031332.45480.79
Ibrahim, A. (2017). IDF clinical practice recommendation on the diabetic foot: a guide for healthcare professionals. Diabetes Res. Clin. Pract. 127, 285–287. doi:10.1016/j.diabres.2017.04.013
Jain, P., Perakath, B., Jesudason, M. R., and Nayak, S. (2011). The effect of autologous bone marrow-derived cells on healing chronic lower extremity wounds: results of a randomized controlled study. Ostomy Wound Manage 57 (7), 38–44.
Kirana, S., Stratmann, B., Prante, C., Prohaska, W., Koerperich, H., Lammers, D., et al. (2012). Autologous stem cell therapy in the treatment of limb ischaemia induced chronic tissue ulcers of diabetic foot patients. Int. J. Clin. Pract. 66 (4), 384–393. doi:10.1111/j.1742-1241.2011.02886.x
Krasilnikova, O. A., Baranovskii, D. S., Lyundup, A. V., Shegay, P. V., Kaprin, A. D., and Klabukov, I. D. (2022). Stem and somatic cell monotherapy for the treatment of diabetic foot ulcers: review of clinical studies and mechanisms of action. Stem Cell Rev. Rep. 18 (6), 1974–1985. doi:10.1007/s12015-022-10379-z
Lavery, L. A., Fulmer, J., Shebetka, K. A., Regulski, M., Vayser, D., Fried, D., et al. (2014). The efficacy and safety of Grafix(®) for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int. Wound J. 11 (5), 554–560. doi:10.1111/iwj.12329
Lipsky, B. A., Senneville, É., Abbas, Z. G., Aragón-Sánchez, J., Diggle, M., Embil, J. M., et al. (2020). Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 36 (Suppl. 1), e3280. doi:10.1002/dmrr.3280
Liu, Y., He, L., Cheng, L., Li, X., Gao, M., Li, Q., et al. (2023a). Enhancing bone grafting outcomes: A. Comprehensive review of antibacterial artificial composite bone scaffolds. Med. Sci. Monit. 29, e939972. doi:10.12659/MSM.939972
Liu, P. Y., Ma, H. W., and Zhang, Y. Q. (2023b). Effect of autologous bone marrow stem cell transplantation on diabetic foot. Hebei Med. J. 45 (4), 579–581. doi:10.3969/j.issn.1002-7386.2023.04.025
Lonardi, R., Leone, N., Gennai, S., Trevisi Borsari, G., Covic, T., and Silingardi, R. (2019). Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: a randomized controlled single-center clinical trial (MiFrAADiF). Stem Cell Res. Ther. 10 (1), 223. doi:10.1186/s13287-019-1328-4
Lopes, L., Setia, O., Aurshina, A., Liu, S., Hu, H., Isaji, T., et al. (2018). Stem cell therapy for diabetic foot ulcers: a review of preclinical and clinical research. Stem Cell Res. Ther. 9 (1), 188. doi:10.1186/s13287-018-0938-6
Lu, D., Chen, B., Liang, Z., Deng, W., Jiang, Y., Li, S., et al. (2011). Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res. Clin. Pract. 92 (1), 26–36. doi:10.1016/j.diabres.2010.12.010
Meloni, M., Izzo, V., Da Ros, V., Morosetti, D., Stefanini, M., Brocco, E., et al. (2020). Characteristics and outcome for persons with diabetic foot ulcer and No-option critical limb ischemia. J. Clin. Med. 9 (11), 3745. doi:10.3390/jcm9113745
Mimeault, M., Hauke, R., and Batra, S. K. (2007). Stem cells: a revolution in therapeutics-recent advances in stem cell biology and their therapeutic applications in regenerative medicine and cancer therapies. Clin. Pharmacol. Ther. 82 (3), 252–264. doi:10.1038/sj.clpt.6100301
Mohammadzadeh, L., Samedanifard, S. H., Keshavarzi, A., Alimoghaddam, K., Larijani, B., Ghavamzadeh, A., et al. (2013). Therapeutic outcomes of transplanting autologous granulocyte colony-stimulating factor-mobilised peripheral mononuclear cells in diabetic patients with critical limb ischaemia. Exp. Clin. Endocrinol. Diabetes 121 (1), 48–53. doi:10.1055/s-0032-1311646
Moon, K. C., Suh, H. S., Kim, K. B., Han, S. K., Young, K. W., Lee, J. W., et al. (2019). Potential of allogeneic adipose-derived stem cell-hydrogel complex for treating diabetic foot ulcers. Diabetes 68 (4), 837–846. doi:10.2337/db18-0699
Mrozikiewicz-Rakowska, B., Szabłowska-Gadomska, I., Cysewski, D., Rudziński, S., Płoski, R., Gasperowicz, P., et al. (2023). Allogenic adipose-derived stem cells in diabetic foot ulcer treatment: clinical effectiveness, safety, survival in the wound site, and proteomic impact. Int. J. Mol. Sci. 24 (2), 1472. doi:10.3390/ijms24021472
Ozturk, A., Kucukardali, Y., Tangi, F., Erikci, A., Uzun, G., Bashekim, C., et al. (2012). Therapeutical potential of autologous peripheral blood mononuclear cell transplantation in patients with type 2 diabetic critical limb ischemia. J. Diabetes Complicat. 26 (1), 29–33. doi:10.1016/j.jdiacomp.2011.11.007
Parisi, L., Gini, E., Baci, D., Tremolati, M., Fanuli, M., Bassani, B., et al. (2018). Macrophage polarization in chronic inflammatory diseases: killers or builders? J. Immunol. Res. 2018, 8917804. doi:10.1155/2018/8917804
Park, K. H., Han, S. H., Hong, J. P., Han, S. K., Lee, D. H., Kim, B. S., et al. (2018). Topical epidermal growth factor spray for the treatment of chronic diabetic foot ulcers: a phase III multicenter, double-blind, randomized, placebo-controlled trial. Diabetes Res. Clin. Pract. 142, 335–344. doi:10.1016/j.diabres.2018.06.002
Patel, S., Srivastava, S., Singh, M. R., and Singh, D. (2019). Mechanistic insight into diabetic wounds: pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 112, 108615. doi:10.1016/j.biopha.2019.108615
Polymeri, A., Giannobile, W. V., and Kaigler, D. (2016). Bone marrow stromal stem cells in tissue engineering and regenerative medicine. Horm. Metab. Res. 48 (11), 700–713. doi:10.1055/s-0042-118458
Rai, V., Moellmer, R., and Agrawal, D. K. (2022). Stem cells and angiogenesis: implications and limitations in enhancing chronic diabetic foot ulcer healing. Cells 11 (15), 2287. doi:10.3390/cells11152287
Sasaki, M., Abe, R., Fujita, Y., Ando, S., Inokuma, D., and Shimizu, H. (2008). Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 180 (4), 2581–2587. doi:10.4049/jimmunol.180.4.2581
Singh, G. D., Brinza, E. K., Hildebrand, J., Waldo, S. W., Foley, T. R., Laird, J. R., et al. (2017). Midterm outcomes after infrapopliteal interventions in patients with critical limb ischemia based on the TASC II classification of below-the-knee arteries. J. Endovasc. Ther. 24 (3), 321–330. doi:10.1177/1526602817704643
Smith, O. J., Leigh, R., Kanapathy, M., Macneal, P., Jell, G., Hachach-Haram, N., et al. (2020). Fat grafting and platelet-rich plasma for the treatment of diabetic foot ulcers: a feasibility-randomised controlled trial. Int. Wound J. 17 (6), 1578–1594. doi:10.1111/iwj.13433
Sun, Y., Zhao, J., Zhang, L., Li, Z., and Lei, S. (2022a). Effectiveness and safety of stem cell therapy for diabetic foot: a meta-analysis update. Stem Cell Res. Ther. 13 (1), 416. doi:10.1186/s13287-022-03110-9
Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., et al. (2022b). IDF Diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. doi:10.1016/j.diabres.2021.109119
Tutuianu, R., Rosca, A. M., Iacomi, D. M., Simionescu, M., and Titorencu, I. (2021). Human mesenchymal stromal cell-derived exosomes promote in vitro wound healing by modulating the biological properties of skin keratinocytes and fibroblasts and stimulating angiogenesis. Int. J. Mol. Sci. 22 (12), 6239. doi:10.3390/ijms22126239
Uzun, E., Güney, A., Gönen, Z. B., Özkul, Y., Kafadar, İ. H., Günay, M., et al. (2021). Intralesional allogeneic adipose-derived stem cells application in chronic diabetic foot ulcer: phase I/2 safety study. Foot Ankle Surg. 27 (6), 636–642. doi:10.1016/j.fas.2020.08.002
Wang, X. F., Gao, S. W., and Zhang, Z. (2024a). The curative effect of umbilical cord mesenchymal stem cells transplantation combined with vacuum sealing drainage for diabetic foot ulcer. Anhui Med. Pharm. J. 28 (4), 777–781. doi:10.3969/j.issn.1009-6469.2024.04.030
Wang, G. Y., Li, Q. Y., and Jia, Y. P. (2024b). Therapeutic effect of peripheral blood stem cells combined with exosomes on diabetic foot ulcer wounds. Chin. J. Aesthet. Med. 33 (8), 33–37.
Weledji, E. P., and Fokam, P. (2014). Treatment of the diabetic foot - to amputate or not? BMC Surg. 14, 83. doi:10.1186/1471-2482-14-83
Xia, Y., Wu, P., Chen, H., and Chen, Z. (2024). Advances in stem cell therapy for diabetic foot. Front. Genet. 15, 1427205. doi:10.3389/fgene.2024.1427205
You, H. J., Han, S. K., Lee, J. W., and Chang, H. (2012). Treatment of diabetic foot ulcers using cultured allogeneic keratinocytes--a pilot study. Wound Repair Regen. 20 (4), 491–499. doi:10.1111/j.1524-475X.2012.00809.x
Yu, Q., Qiao, G. H., Wang, M., Yu, L., Sun, Y., Shi, H., et al. (2022). Stem cell-based therapy for diabetic foot ulcers. Front. Cell Dev. Biol. 10, 812262. doi:10.3389/fcell.2022.812262
Yu, X., Liu, P., Li, Z., and Zhang, Z. (2023). Function and mechanism of mesenchymal stem cells in the healing of diabetic foot wounds. Front. Endocrinol. (Lausanne) 14, 1099310. doi:10.3389/fendo.2023.1099310
Zhang, Z., Liu, W. S., and Zhao, X. L. (2007). Autologous bone marrow stem cell transplantation for treatment of diabetes foot ulcer. Prac. Clin. Med. 8 (8), 58–60.
Zhang, X., Lian, W., Lou, W., Han, S., Lu, C., Zuo, K., et al. (2016). Transcatheter arterial infusion of autologous CD133(+) cells for diabetic peripheral artery disease. Stem Cells Int. 2016, 6925357. doi:10.1155/2016/6925357
Keywords: stem cells, different sources, diabetic foot ulcer, wound healing rate, meta-analysis
Citation: Tong L, Tang L, Tang B and Zhang J (2025) Impacts of stem cells from different sources on wound healing rate in diabetic foot ulcers: a systematic review and meta-analysis. Front. Genet. 15:1541992. doi: 10.3389/fgene.2024.1541992
Received: 09 December 2024; Accepted: 31 December 2024;
Published: 28 January 2025.
Edited by:
Francesco De Francesco, Other, ItalyReviewed by:
Armel Hervé Nwabo Kamdje, University of Garoua, CameroonPratima Kumari, Georgia State University, United States
Copyright © 2025 Tong, Tang, Tang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianna Zhang, empud2VzdGNoaW5hNTIwQG91dGxvb2suY29t
 Le Tong1,2,3
Le Tong1,2,3 Bangli Tang
Bangli Tang Jianna Zhang
Jianna Zhang