
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Genet. , 11 September 2024
Sec. Applied Genetic Epidemiology
Volume 15 - 2024 | https://doi.org/10.3389/fgene.2024.1434975
Background: Previous studies have investigated the association between the haptoglobin rs72294371 polymorphism and coronary artery disease (CAD) risk, but the results are controversial and uncertain. Therefore, this study aimed to systematically review the literature on haptoglobin polymorphism and susceptibility to CAD.
Methods: PubMed, Embase, Web of Science, Cochrane Library, and Wanfang databases were used to identify relevant studies from their inception to April 2024. The pooled odds ratio (OR) with corresponding 95% confidence interval (CI) were used to assess the strength of the association. An OR value greater than one suggested an increased risk; otherwise, it suggested a protective risk.
Results: A total of 15 studies comprising 8,632 individuals (2,988 cases and 5,644 controls) were included. In the current meta-analysis, a significant association between haptoglobin polymorphism and CAD was found under recessive model (OR:0.74, 95% CI:0.60–0.92), dominant model (OR: 0.82, 95% CI: 0.71–0.95), homozygote model (OR: 0.70, 95% CI: 0.53–0.92), and allelic genetic model (OR: 0.80, 95% CI: 0.69–0.94). In the analysis stratified by ethnicity, a statistically significant association was observed in Asians rather than Caucasian population.
Conclusion: This meta-analysis indicates that haptoglobin polymorphism is associated with CAD susceptibility, especially in Asians.
Coronary artery disease (CAD) is the leading cause of death worldwide, accounting for 30% of global mortality (Erdmann et al., 2018). The main cause of CAD is currently believed to be the accumulation of atherosclerotic plaques in blood vessels and subsequent blockage, which leads to cardiac hypoxia and nutrient deprivation (Moras et al., 2024). Despite substantial advancements in diagnosing and predicting CAD outcomes, the exact mechanisms underlying CAD onset remain elusive. Epidemiological studies have shown that the development of CAD is influenced by multiple factors, such as hypertension, smoking, diabetes, and dyslipidaemia (Gray et al., 2024). In addition, recent evidence suggests that environmental factors and genetic polymorphisms play a key role in the development of CAD (Charmet et al., 2018).
Haptoglobin, which is predominantly synthesized in the liver, is a glycoprotein with antioxidant properties (Orchard et al., 2016). It stabilizes free haemoglobin by binding to it, thereby preventing further tissue damage in inflamed regions (Dalan and Liuh Ling, 2018). The gene encoding haptoglobin is located at chromosome 16q22.3 and exists in two allelic forms, HP1 and HP2, which result in three genotypes, HP1-1, HP2-1, and HP2-2 (Dalan and Liuh Ling, 2018). Proteins derived from these genotypes exhibit distinct functional profiles; specifically, the HP2-2 phenotype, compared to the HP1-1 and HP2-1 phenotypes, exhibits compromised antioxidant capacity, potentially enhancing vulnerability to atherosclerosis and increasing the risk of CAD (Cahill et al., 2024; Warren et al., 2024). Although multiple studies have investigated the association between haptoglobin polymorphisms and CAD risk (Levy et al., 2004; Fan et al., 2013; Mewborn et al., 2024; Moras et al., 2024), a clear consensus among these studies has not been reached due to the small sample sizes and the limitations of research heterogeneity. Thus, this meta-analysis aimed to investigate the relationship between haptoglobin polymorphisms and CAD susceptibility by synthesizing findings from published research.
The present meta-analysis adheres to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement (Page et al., 2021). The PRISMA Checklist was described in Supplementary Table S1.
PubMed, Embase, Web of Science, Cochrane Library, and Wanfang databases were used to identify relevant studies from their inception to April 2024. The following keywords were used: haptoglobin, polymorphism, variant, mutation, coronary artery disease, and coronary heart disease. To avoid missing any potential study, the references of the included studies were also manually searched. Languages were limited to English and Chinese. The detailed search strategy was described in Supplementary Table S2.
The eligible studies should match the inclusion criteria: 1) case-control study; 2) evaluate the association between haptoglobin polymorphism and the risk of CAD; 3) report sufficient data to calculate odds ratio (OR) and corresponding 95% confidence interval; 4) control population was consistent with Hardy–Weinberg equilibrium (HWE). The exclusion criteria were as follows: abstracts, case reports, reviews, duplicated studies, or incomplete data.
Two investigators completed the data extraction independently, and any disagreement was discussed and resolved with consensus. The following information was extracted from eligible studies: the first author’s name, published year, country of origin, ethnicity, sample size, frequencies of genotypes in cases and controls, and Hardy-Weinberg equilibrium (HWE) in controls. The quality of the selected studies was assessed using the Newcastle–Ottawa Scale (NOS) (Stang, 2010).
Statistical analyses were performed with STATA version 12.0 software (STATA Corp., College Station, TX, United States). Odds ratios (OR) with 95% confidence intervals (CI) were assessed to determine the relationship between the haptoglobin polymorphism and the risk of CAD under dominant (Hp 2-1 + Hp 2-2 vs. Hp 1-1), recessive (Hp 2-2 vs. Hp 1-1 + Hp 2-1), allelic (Hp 2 vs. Hp 1), codominant homozygote (Hp 2-2 vs. Hp 1-1), codominant heterozygote (Hp 2-1 vs. Hp 1-1), and overdominance genetic models (Hp 2-1 vs. Hp 1-1 + Hp 2-2). The HWE of the included studies was assessed by a chi-square test. The heterogeneity among the included studies was evaluated through the chi-squared test and I2 statistic. A fixed effect model (Mantel–Haenszel) was employed when I2 < 50%; otherwise, a random effect model (DerSimonian–Laird) was adopted. Stratified analyses were performed based on ethnicity. A sensitivity test was performed to evaluate the stability of the results. The publication bias was assessed by using Begg’s funnel plot and Egger’s linear regression test. A P-value <0.05 was considered statistically significant.
The study selection process was presented in Figure 1. A total of 161 studies were acquired from PubMed, Embase, Web of Science, Cochrane Library, and Wan Fang databases. Among them, 34 articles were excluded as duplicates, 107 studies were excluded based on titles and abstracts, and 20 articles were excluded after reading the full text. Ultimately, a total of 15 articles (Bilgrami et al., 1980; Surya Prabha et al., 1987; Hong et al., 1997; Levy et al., 2004; Liu et al., 2011; Wobeto et al., 2011; Cahill et al., 2013; Fan et al., 2013; Lee et al., 2013; Pan et al., 2013; Hamdy et al., 2014; Moussa et al., 2014; Cahill et al., 2015; Wang et al., 2018; Mewborn et al., 2024) involving 2,988 cases and 5,644 controls were selected for this meta-analysis. The characteristics of the included studies were listed in Table 1. Studies were conducted between 1979 and 2024. Four studies involved America, 5 studies involved China, 2 studies involved India, and 1 study involved Korea, Brazil, Tunisia, and Egypt. The 4 studies were performed among Caucasians, 8 studies among Asians, 1 among Egyptian, Tunisian, and mixed population. The quality of the selected studies was assessed in Supplementary Table S3.
The pooled association of haptoglobin polymorphism with CAD risk is summarized in Table 2. The combined results showed that a significant association between haptoglobin polymorphism and CAD risk was found under dominant model (OR: 0.82, 95% CI: 0.71–0.95, p = 0.007) (Figure 2), recessive model (OR:0.74, 95% CI:0.60–0.92, p = 0.005) (Figure 3), codominant homozygote model (OR: 0.70, 95% CI: 0.53–0.92, p = 0.010) (Figure 4), allelic genetic model (OR: 0.80, 95% CI: 0.69–0.94, p = 0.005) (Figure 5). However, under the codominant heterozygote (Figure 6) and overdominance genetic model (Figure 7), haptoglobin polymorphism did not significantly associated with CAD risk. In addition, we did the subgroup analysis based on ethnicity (Table 2). Results from the subgroup analysis stratified by ethnicity indicated that the haptoglobin polymorphism was associated with CAD susceptibility in the Asian population than that in the Caucasian population. In the Asian subgroup, there was a significant association between haptoglobin polymorphism and CAD risk under recessive (OR: 0.72, 95% CI: 0.52–0.98, p = 0.036) and dominant model (OR: 0.70,95% CI: 0.53–0.93, p = 0.015), and overdominance genetic model (OR: 1.21,95% CI: 1.03–1.48, p = 0.024). In the Caucasian subgroup, no significant association under all genetic model.

Table 2. Summary of meta-analysis of association of haptoglobin polymorphism and coronary artery disease risk.
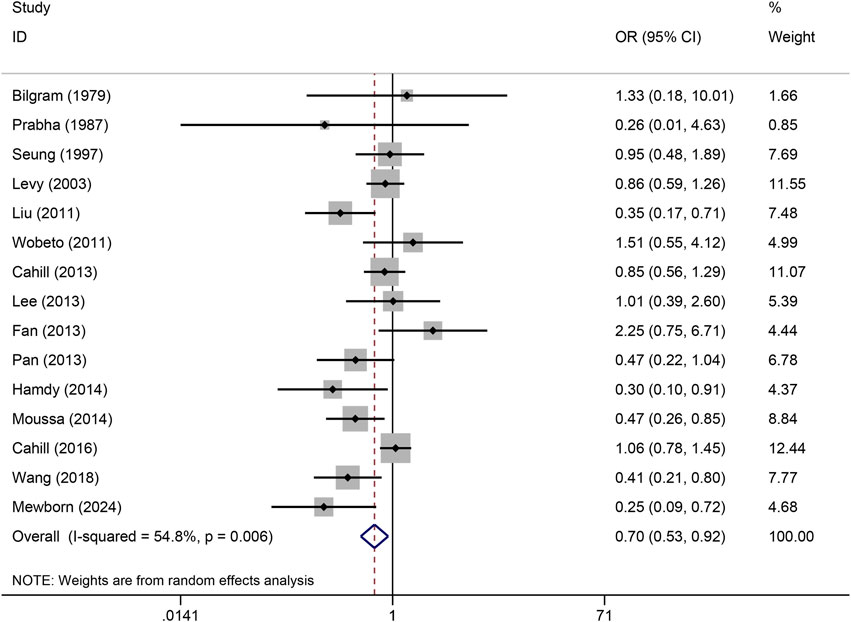
Figure 4. Forest plots of haptoglobin polymorphism and CAD risk under codominant homozygous genetic model.

Figure 6. Forest plots of haptoglobin polymorphism and CAD risk under codominant heterozygote genetic model.
Sensitivity analysis was used to estimate the effect of individual study on the pooled results (Figure 8). Omitting individual studies did not influenced the pooled results, suggesting that our results were reliable. Begg’s and Egger’s tests were used to evaluate the publication bias (Figure 9). The results showed that there was no publication bias under all genetic models (Table 3).
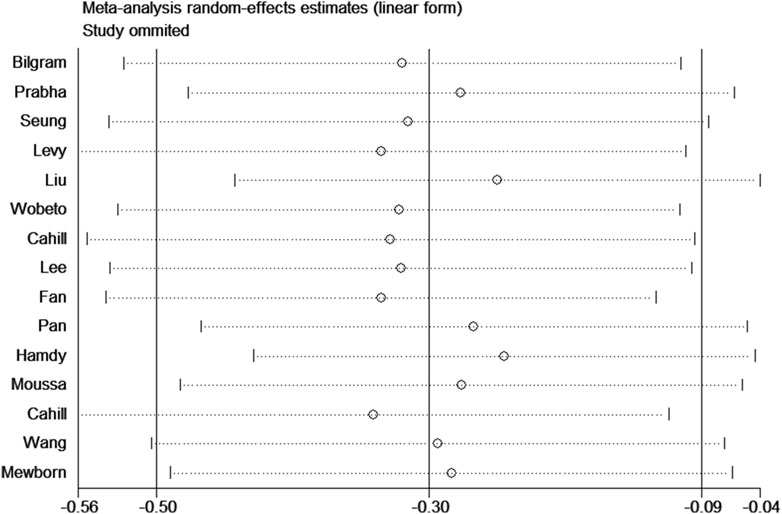
Figure 8. Sensitivity analysis for association between haptoglobin polymorphism and CAD risk under recessive genetic model.
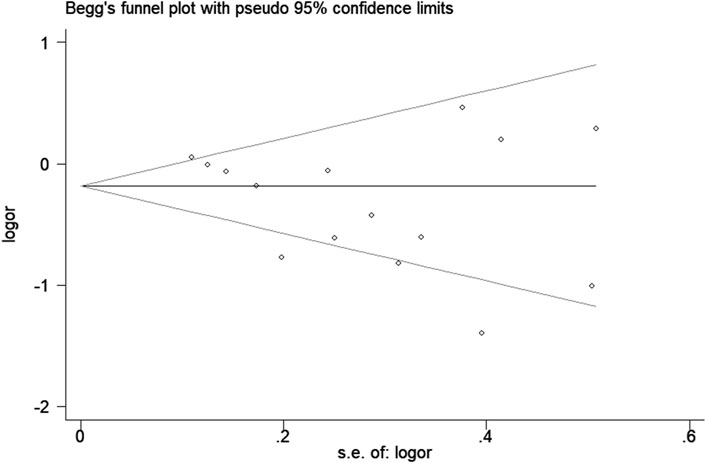
Figure 9. Begg’s funnel plot for association between haptoglobin polymorphism and CAD risk under recessive genetic model.
This study systematically synthesized the published research on the association between haptoglobin polymorphisms and CAD susceptibility. In the pooled analysis of five different genetic models, we found that individuals with the HP2 genotype had a greater risk of CAD than those with the HP1 allele. Subgroup analysis revealed a significant association between haptoglobin polymorphisms and CAD risk in Asian populations, while in Caucasians, haptoglobin polymorphisms were unrelated to CAD.
Current research indicates that haptoglobin primarily exhibits anti-inflammatory effects (Costacou et al., 2015). When inflammation occurs within the body, haptoglobin synthesis increases dramatically to mitigate further inflammation (Sadrzadeh and Bozorgmehr, 2004). Inflammation plays a crucial role in the development of atherosclerosis and in its association with CAD, which poses a significant threat to human health (Boland and Long, 2021). Haptoglobin binds to free haemoglobin (Hb) to form an HP complex, which effectively reduces the consumption of free Hb by carbon monoxide, thereby lowering the risk of cardiovascular complications and CAD. However, compared to individuals with the HP1-1 genotype, individuals with the HP2-2 genotype have a significantly greater risk of developing CAD. Bacquer et al. reported that haptoglobin 1 increases the risk of CAD and its mortality rate (OR 2.09, 95% CI 1.22–3.60) compared to haptoglobin 2 (De Bacquer et al., 2001). Conversely, Mewborn et al. discovered that patients with the HP2-2 genotype have a fourfold greater incidence of CAD than those with the HP1-1 genotype (OR 3.987, 95% CI 1.39–11.43) (Mewborn et al., 2024). In summary, this meta-analysis suggested that haptoglobin polymorphism was associated with an increased risk of CAD in Asian populations.
Individuals with the HP2-2 phenotype exhibit a reduced clearance rate of macrophage-HP-Hb complexes, which can affect iron deposition, oxidative stress, and the accumulation of active macrophages, all of which are consistent with an increased risk of atherosclerotic cardiovascular diseases (Moreno et al., 2008). A greater proportion of participants with lower Hp concentrations exhibited the HP1-1 phenotype than among those with higher concentrations, which is consistent with the higher affinity of haptoglobin 1-1 for binding to HP (Holme et al., 2009). Given the connection between HP and CAD, patients with the HP1-1 phenotype might have a lower risk of CAD due to their lower plasma HP levels (Lee et al., 2013). Due to impaired clearance of the HP-Hb 2-2 complex, there is an increased concentration of Hp–Hb in Hp 2-2 individuals, which allows the complex to bind to other plasma proteins that are not normally bound, such as high-density lipoproteins (HDL) (Goldenstein et al., 2018). Hp can bind directly to the main apolipoprotein ApoA1 of HDL, and thereby tether Hb to which it is complexed to HDL. This process transforms normal anti-atherosclerotic and antioxidant HDL particles into pro-atherosclerotic pro-oxidative dysfunctional HDL particles (Asleh et al., 2019). In addition, the increased attachment of Hb to HDL may lead to endothelial dysfunction through its interference with HDL function. Firstly, Hb binding to HDL leads to oxidative modification of HDL-associated lipids and proteins, resulting in the inactivation of antioxidant enzymes such as glutathione peroxidase and paraoxonase (Navab et al., 2006). Secondly, Hb can oxidise ApoA1, leading to a significant impairment of its ability to promote cholesterol efflux from macrophages (Salvatore et al., 2007). Thus, the prevalence of microvascular endothelial dysfunction is higher in Hp 2-2 individuals because Hp 2-2 is a poorer antioxidant relative to Hp 1-1, especially for glycosylated Hb (Asleh et al., 2019).
The influence of haptoglobin polymorphisms on CAD risk was obviously nonuniform, which might be prompted by, sample size, racial difference, gender and age. Despite our attempt to conduct a comprehensive meta-analysis of haptoglobin gene polymorphisms and CVD risk, it is essential to consider the limitations of the current studies. First, the subgroup analysis based on race suggested that regional and genetic environmental variations might influence the results. The complexity of gene-environment interactions suggests a need for validation of these findings in diverse racial and ethnic populations. Second, our study focused on the association between haptoglobin polymorphisms and CAD susceptibility in Asians and Caucasians, without examining other populations. Finally, the literature search was primarily in English, potentially overlooking studies in other languages.
In summary, this meta-analysis highlights a significant association between haptoglobin polymorphism and CAD risk in Asian populations. Given its limitations, it is recommended that future studies with larger sample sizes and broader subgroups be conducted to further investigate the relationship between haptoglobin polymorphisms and CAD susceptibility, accounting for the potential influence of genetic and environmental factors.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
JW: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Writing–original draft, Writing–review and editing. XZ: Data curation, Formal Analysis, Methodology, Software, Writing–original draft, Writing–review and editing. YS: Data curation, Formal Analysis, Methodology, Software, Writing–review and editing. DC: Data curation, Formal Analysis, Methodology, Software, Writing–review and editing. YR: Data curation, Formal Analysis, Methodology, Software, Writing–review and editing. Jinhua Wang: Conceptualization, Validation, Visualization, Writing–original draft, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1434975/full#supplementary-material
Asleh, R., Levy, A. P., Levy, N. S., Asleh, A., Goldenstein, H., Segol, I., et al. (2019). Haptoglobin phenotype is associated with high-density lipoprotein-bound hemoglobin content and coronary endothelial dysfunction in patients with mild nonobstructive coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 39 (4), 774–786. doi:10.1161/ATVBAHA.118.312232
Bilgrami, G., Tyagi, S. P., and Qasim, A. (1980). Serum haptoglobin in cases of ischemic heart diseases. Jpn. Heart J. 21 (4), 505–510. doi:10.1536/ihj.21.505
Boland, J., and Long, C. (2021). Update on the inflammatory hypothesis of coronary artery disease. Curr. Cardiol. Rep. 23 (2), 6. doi:10.1007/s11886-020-01439-2
Cahill, L. E., Jensen, M. K., Chiuve, S. E., Shalom, H., Pai, J. K., Flint, A. J., et al. (2015). The risk of coronary heart disease associated with glycosylated hemoglobin of 6.5% or greater is pronounced in the haptoglobin 2-2 genotype. J. Am. Coll. Cardiol. 66 (16), 1791–1799. doi:10.1016/j.jacc.2015.07.076
Cahill, L. E., Levy, A. P., Chiuve, S. E., Jensen, M. K., Wang, H., Shara, N. M., et al. (2013). Haptoglobin genotype is a consistent marker of coronary heart disease risk among individuals with elevated glycosylated hemoglobin. J. Am. Coll. Cardiol. 61 (7), 728–737. doi:10.1016/j.jacc.2012.09.063
Cahill, L. E., Warren, R. A., Carew, A. S., Levy, A. P., Sapp, J., Samuel, M., et al. (2024). Haptoglobin phenotype and intensive glycemic control for coronary artery disease risk reduction in people with type 2 diabetes: the ADVANCE study. Diabetes Care 47 (5), 835–843. doi:10.2337/dc23-2165
Charmet, R., Duffy, S., Keshavarzi, S., Gyorgy, B., Marre, M., Rossing, P., et al. (2018). Novel risk genes identified in a genome-wide association study for coronary artery disease in patients with type 1 diabetes. Cardiovasc Diabetol. 17 (1), 61. doi:10.1186/s12933-018-0705-0
Costacou, T., Evans, R. W., and Orchard, T. J. (2015). Does the concentration of oxidative and inflammatory biomarkers differ by haptoglobin genotype in type 1 diabetes? Antioxid. Redox Signal 23 (18), 1439–1444. doi:10.1089/ars.2015.6355
Dalan, R., and Liuh Ling, G. (2018). The protean role of haptoglobin and haptoglobin genotypes on vascular complications in diabetes mellitus. Eur. J. Prev. Cardiol. 25 (14), 1502–1519. doi:10.1177/2047487318776829
De Bacquer, D., De Backer, G., Langlois, M., Delanghe, J., Kesteloot, H., and Kornitzer, M. (2001). Haptoglobin polymorphism as a risk factor for coronary heart disease mortality. Atherosclerosis 157 (1), 161–166. doi:10.1016/s0021-9150(00)00690-0
Erdmann, J., Kessler, T., Munoz Venegas, L., and Schunkert, H. (2018). A decade of genome-wide association studies for coronary artery disease: the challenges ahead. Cardiovasc Res. 114 (9), 1241–1257. doi:10.1093/cvr/cvy084
Fan, X., Wang, Y. Y., Xie, L. Y., Zhao, S., Wang, S. Q., Liu, P., et al. (2013). Study on the haptoglobin gene polymorphism in patients with both of diabetes mellitus and coronary heart disease. Chin. J. Cardiovasc. Res. 11 (11), 833–836. doi:10.3969/j.issn.1672-5301.2013.11.001
Goldenstein, H., Levy, N. S., Ward, J., Costacou, T., and Levy, A. P. (2018). Haptoglobin genotype is a determinant of hemoglobin adducts and vitamin e content in HDL. J. Diabetes Res. 2018, 6125420. doi:10.1155/2018/6125420
Gray, M. P., Fatkin, D., Ingles, J., Robertson, E. N., and Figtree, G. A. (2024). Genetic testing in cardiovascular disease. Med. J. Aust. 220 (8), 428–434. doi:10.5694/mja2.52278
Hamdy, G., Hendy, O., Mahmoud, H., El-sebaey, A., Ali, S., and Khalaf, F. (2014). Haptoglobin phenotypes as a risk factor for coronary artery disease in type 2 diabetes mellitus: an Egyptian study. Egypt. J. Med. Hum. Genet. 15, 257–264. doi:10.1016/j.ejmhg.2014.03.003
Holme, I., Aastveit, A. H., Hammar, N., Jungner, I., and Walldius, G. (2009). Haptoglobin and risk of myocardial infarction, stroke, and congestive heart failure in 342,125 men and women in the Apolipoprotein MOrtality RISk study (AMORIS). Ann. Med. 41 (7), 522–532. doi:10.1080/07853890903089453
Hong, S. H., Kang, B. Y., Lim, J. H., Namkoong, Y., Oh, M. Y., Kim, J. Q., et al. (1997). Haptoglobin polymorphism in Korean patients with cardiovascular diseases. Hum. Hered. 47 (5), 283–287. doi:10.1159/000154425
Lee, C. W., Cheng, T. M., Lin, C. P., and Pan, J. P. (2013). Plasma haptoglobin concentrations are elevated in patients with coronary artery disease. PLoS One 8 (10), e76817. doi:10.1371/journal.pone.0076817
Levy, A. P., Larson, M. G., Corey, D., Lotan, R., Vita, J. A., and Benjamin, E. J. (2004). Haptoglobin phenotype and prevalent coronary heart disease in the Framingham offspring cohort. Atherosclerosis 172 (2), 361–365. doi:10.1016/j.atherosclerosis.2003.10.014
Liu, H. B., Shi, Y. P., Guo, X. F., Shang, J., and Xu, G. (2011). Association of haptoglobin 1/2 polymorphism with coronary heart disease in Chinese. Chin. J. Med. Genet. 28 (1), 60–63. doi:10.3760/cma.j.issn.1003-9406.2011.01.014
Mewborn, E. K., Tolley, E. A., Wright, D. B., Doneen, A. L., Harvey, M., and Stanfill, A. G. (2024). Haptoglobin genotype is a risk factor for coronary artery disease in prediabetes: a case-control study. Am. J. Prev. Cardiol. 17, 100625. doi:10.1016/j.ajpc.2023.100625
Moras, E., Zaid, S., Gandhi, K., Barman, N., Birnbaum, Y., Virani, S. S., et al. (2024). Pharmacotherapy for coronary artery disease and acute coronary syndrome in the aging population. Curr. Atheroscler. Rep. 26, 231–248. doi:10.1007/s11883-024-01203-9
Moreno, P. R., Purushothaman, K. R., Purushothaman, M., Muntner, P., Levy, N. S., Fuster, V., et al. (2008). Haptoglobin genotype is a major determinant of the amount of iron in the human atherosclerotic plaque. J. Am. Coll. Cardiol. 52 (13), 1049–1051. doi:10.1016/j.jacc.2008.06.029
Moussa, A., Rejeb, J., Omezzine, A., Rebhi, L., Boumaiza, I., Kacem, S., et al. (2014). Association between haptoglobin 2-2 genotype and coronary artery disease and its severity in a tunisian population. Biochem. Genet. 52 (5-6), 269–282. doi:10.1007/s10528-014-9646-9
Navab, M., Anantharamaiah, G. M., Reddy, S. T., Van Lenten, B. J., Ansell, B. J., and Fogelman, A. M. (2006). Mechanisms of disease: proatherogenic HDL--an evolving field. Nat. Clin. Pract. Endocrinol. Metab. 2 (9), 504–511. doi:10.1038/ncpendmet0245
Orchard, T. J., Backlund, J. C., Costacou, T., Cleary, P., Lopes-Virella, M., Levy, A. P., et al. (2016). Haptoglobin 2-2 genotype and the risk of coronary artery disease in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study (DCCT/EDIC). J. Diabetes Complicat. 30 (8), 1577–1584. doi:10.1016/j.jdiacomp.2016.07.014
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The prisma 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pan, W. W., Wang, Y. Y., Xie, L. Y., Wang, S. Q., Zhao, S., Fan, X., et al. (2013). Association between haptoglobin polymorphism and coronary artery stenosis in diabetics. China Mod. Dr. 51 (1), 30–32.
Sadrzadeh, S. M., and Bozorgmehr, J. (2004). Haptoglobin phenotypes in health and disorders. Am. J. Clin. Pathol. 121 (Suppl. l), S97–S104. doi:10.1309/8GLX5798Y5XHQ0VW
Salvatore, A., Cigliano, L., Bucci, E. M., Corpillo, D., Velasco, S., Carlucci, A., et al. (2007). Haptoglobin binding to apolipoprotein A-I prevents damage from hydroxyl radicals on its stimulatory activity of the enzyme lecithin-cholesterol acyl-transferase. Biochemistry 46 (39), 11158–11168. doi:10.1021/bi7006349
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25 (9), 603–605. doi:10.1007/s10654-010-9491-z
Surya Prabha, P., Padma, T., and Ramaswamy, M. (1987). Haptoglobin patterns in essential hypertension and associated conditions--increased risk for Hp 2-2. Hum. Hered. 37 (6), 345–348. doi:10.1159/000153732
Wang, Y. Y., Li, W., Sui, K. X., Zhang, Q. Y., and Ma, X. Y. (2018). Association between haptoglobin genotype and coronary artery stenosis in patient with premature coronary artery disease. Biomed. Res. 29 (8), 1631–1636. doi:10.4066/biomedicalresearch.29-17-1597
Warren, R. A., Bancks, M. P., Carew, A. S., Levy, A. P., Sapp, J., Bahnson, J., et al. (2024). Intensive lifestyle intervention in type 2 diabetes and risk of incident coronary artery disease for the common haptoglobin phenotypes: the Look ahead study. Cardiovasc Diabetol. 23 (1), 82. doi:10.1186/s12933-024-02164-8
Keywords: coronary artery disease, haptoglobin, polymorphism, meta-analysis, susceptibility
Citation: Wang J, Zhou X, Su Y, Chai D, Ruan Y and Wang J (2024) Association between haptoglobin polymorphism and coronary artery disease: a meta-analysis. Front. Genet. 15:1434975. doi: 10.3389/fgene.2024.1434975
Received: 20 May 2024; Accepted: 28 August 2024;
Published: 11 September 2024.
Edited by:
Phillip E. Melton, University of Tasmania, AustraliaReviewed by:
Emina Colak, University of Belgrade, SerbiaCopyright © 2024 Wang, Zhou, Su, Chai, Ruan and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinhua Wang, d2oxNDg2QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.