
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Genet., 26 July 2024
Sec. Applied Genetic Epidemiology
Volume 15 - 2024 | https://doi.org/10.3389/fgene.2024.1432055
 Nanyan Xiang1†
Nanyan Xiang1† Shiqi Su1†
Shiqi Su1† Zeng Wang2†
Zeng Wang2† Yong Yang3
Yong Yang3 Boxi Chen4
Boxi Chen4 Rui Shi2
Rui Shi2 Tao Zheng2
Tao Zheng2 Banghua Liao5
Banghua Liao5 Yifei Lin6,7*
Yifei Lin6,7* Jin Huang6*
Jin Huang6*Background: Benign prostatic hyperplasia (BPH) is a common disease occurring in elderly and middle-aged men, and cardiovascular diseases (CVDs) are one of the major causes of death worldwide. Many observational studies examined have found a strong association between BPH and CVDs, but the causal relationship between them is unclear. The aim of this study was to determine the causal relationship between BPH and CVDs, specifically five diseases: stroke, coronary heart disease (CHD), heart failure, myocardial infarction (MI), and atrial fibrillation (AF).
Methods: In this study, we obtained single nucleotide polymorphisms (SNPs) of patients with BPH from the UK Biobank database and patients with CVDs from the UK Biobank, the HERMES Consortium, and the FinnGen Genome Database, each used as a genetic tool for a Mendelian randomization (MR) study. We used conventional MR analysis to assess potential causal direction between BPH and CVDs, as well as MR-Egger, MR-PRESSO, model-based estimation (MBE) and weighted median methods for sensitivity analysis.
Results: Using a bidirectional two-sample MR study, we found that BPH patients had an increased risk of developing CHD (ConMix OR = 1.152, 95% CI: 1.011–1.235, p = 0.035) and MI (ConMix OR = 1.107.95% CI: 1.022–1.164, p = 0.013), but a decreased risk of stroke (ConMix OR = 0.872, 95% CI: 0.797–0.926, p = 0.002). The reverse study was not statistically significant and further research may be needed.
Conclusion: Our study suggests a potential causal relationship between BPH and CVDs. BPH appears to be a risk factor for CHD and MI, but it may be protective against stroke. There was no evidence of a causal association in the reverse study, and a larger sample size was needed in follow-up to further explore the potential association.
Benign prostate hyperplasia (BPH) is a prevalent chronic disease characterized by the enlargement of the prostate gland, frequently giving rise to lower urinary tract symptom (LUTS) (Dincer et al., 2021). This condition has evolved into a substantial public health concern, affecting over one million individuals globally in 20192). Notably, the incidence of BPH increased by 105.7% from 1990 to 2019. Its prevalence exhibits an age-dependent escalation, peaking at 14.67% among individuals aged over 70, with approximately 80% of men aged over 80 being affected (Zhang et al., 2019; Srinivasan and Wang, 2020; Zhu et al., 2021). Moreover, the economic impact of BPH was also substantial, evidenced by a significant rise in the expenses related to its diagnosis and treatment from 2014 to 2017. During this period, the average annual cost surged to $4 billion in the United States (Vuichoud and Loughlin, 2015; Del Giudice et al., 2022).
Although BPH is not life-threatening, it can present significant challenges marked by certain complications. Particularly within the context of prevalent age-related comorbidities such as cardiovascular diseases (CVDs), hypertension, diabetes, and the metabolic syndrome (Colon and Payne, 2008), certain individuals may experience moderate-to-severe lower urinary tract symptoms (LUTS) (McVary, 2006). Increasing researches have focused on the correlation between the presentation of BPH and the development of CVDs, including heart disease, stroke, and atrial fibrillation (AF) (Hägg et al., 2015; Davies et al., 2018; Sanderson et al., 2022; Wang et al., 2022). A prospective study of Chinese men demonstrated a significant association between BPH and cardiovascular diseases, heart disease, and stroke, particularly in men under 60 years of age (Burgess et al., 2013). Similarly, another study found a higher incidence of AF in individuals with BPH(10). Several observational studies have suggested a correlation between BPH and CVDs. However, due to the absence of randomized controlled trials (RCTs) or long-term cohort studies, the causal relationship between BPH and CVDs remains unclear.
Mendelian randomization (MR) is a method that uses genetic variation as an instrumental variable to determine causal relationships. This approach can effectively overcome bias caused by confounding and reverse causation, making it useful for causal inference studies (Daghlas et al., 2019). Several MR studies have investigated causal effects among BPH and CVDs. For example, depression has been shown to be causally associated with BPH and CVDs, including myocardial infarction (MI), AF, and coronary heart disease (CHD) (Wang et al., 2022). Using the two-sample MR framework, Yong-Bo Wang and Sara Hägg found that obesity and lifestyle factors also had a causal relationship between BPH and CVDs, such as heart failure and ischemic stroke (Hagg et al., 2015). However, no MR study has yet investigated the direct association between BPH and CVDs.
This study utilized a two-sample MR method (Davies et al., 2018) to investigate the causal relationship between BPH and CVDs, including stroke, CHD, heart failure, MI, and AF, with summary statistics from large-scale genome-wide association studies (GWAS), to help facilitate early screening for patients with BPH or CVDs.
In our study, we employed a two-sample MR approach to investigate the causal relationship between BPH and five types of CVDs (stroke, CHD, heart failure, MI, and AF). We obtained BPH summary statistics from a meta-analysis of GWASs from the UK Biobank, while summary data for MI, stroke, and AF was also obtained from the UK Biobank, and heart failure and CHD was obtained from the HERMES Consortium and the FinnGen Genome Database, respectively. Further details on each dataset can be found in the Supplementary Table S1.
To ensure that genetic variants were strongly associated with the exposure under study, we selected genome-wide significant genetic variants associated with BPH and CVDs as instrumental variables for two-sample MR. All these genetic variants were clumped using PLINK (parameters: -clump-p1 5e-8 -- clump-p2 1e-5 --clump-r2 0.01 --clump-kb 500). Notably, no SNP was selected with the threshold of
In this study, we employed various MR methods to examine the causal relationship between BPH and CVDs. To establish a causal effect through MR, three essential conditions must be satisfied: 1) genetic variants must be closely associated with the exposure under study, 2) the genetic variation must be independent of confounding factors, and 3) genetic variants must be associated with the outcome solely through the exposure, with no direct association due to horizontal pleiotropy (Sanderson et al., 2022).
The primary MR analysis was conducted using the inverse variance weighting (IVW) method (Burgess et al., 2013). If there was no horizontal pleiotropy, the results obtained through the IVW method remain unbiased. To address the potential impact of instrumental variable polymorphisms and extreme values, we also utilized the contaminated mixture method (ConMix) (Burgess et al., 2020) and Robust Adjusted Profile Score (RAPS) (Qingyuan et al., 2018), which could identify horizontal pleiotropy and acquire the proper results after removing outliers (Yang and Schooling, 2023). To ensure the validity of our results, we assessed the potential impact of horizontal pleiotropy on the instrumental variables using the MR-Egger test (Bowden et al., 2015). To address the issue of polymorphism, we used the PRESSO method to detect and remove outliers (Sun et al., 2023). Additionally, we used model-based estimation (MBE) and weighted median methods to calibrate our results.
Our analysis was conducted using R software version 4.2.1 and various packages, including "MendelianRandomization” for IVW, weighted median, MBE, and MR-Egger methods, "MR-PRESSO” for MR PRESSO, and "MR.RAPS” for building the RAPS model.
This MR study was based on publicly available GWAS statistics, obviating the need for ethical approvals.
To obtain the independent instrumental variable SNPs, we set the p-value threshold at
First, genetically predicted BPH was found to be positively associated with CHD (ConMix OR = 1.152, 95% CI: 1.011–1.235, p = 0.035; RAPS OR = 1.08, 95% CI: 1.032–1.13, p = 0.001). The results of several other MR methods, including weighted median and IVW, were consistent with a causal effect of BPH on CHD (all OR > 1, p < 0.05) (Table 1; Figure 1). Moreover, the intercept of MR-Egger (p = 0.383) and the GlobalTest.p-value of MR-PRESSO (p = 0.207) were not significant (Supplementary Table S3), which indicated that the horizontal pleiotropy may not exist.
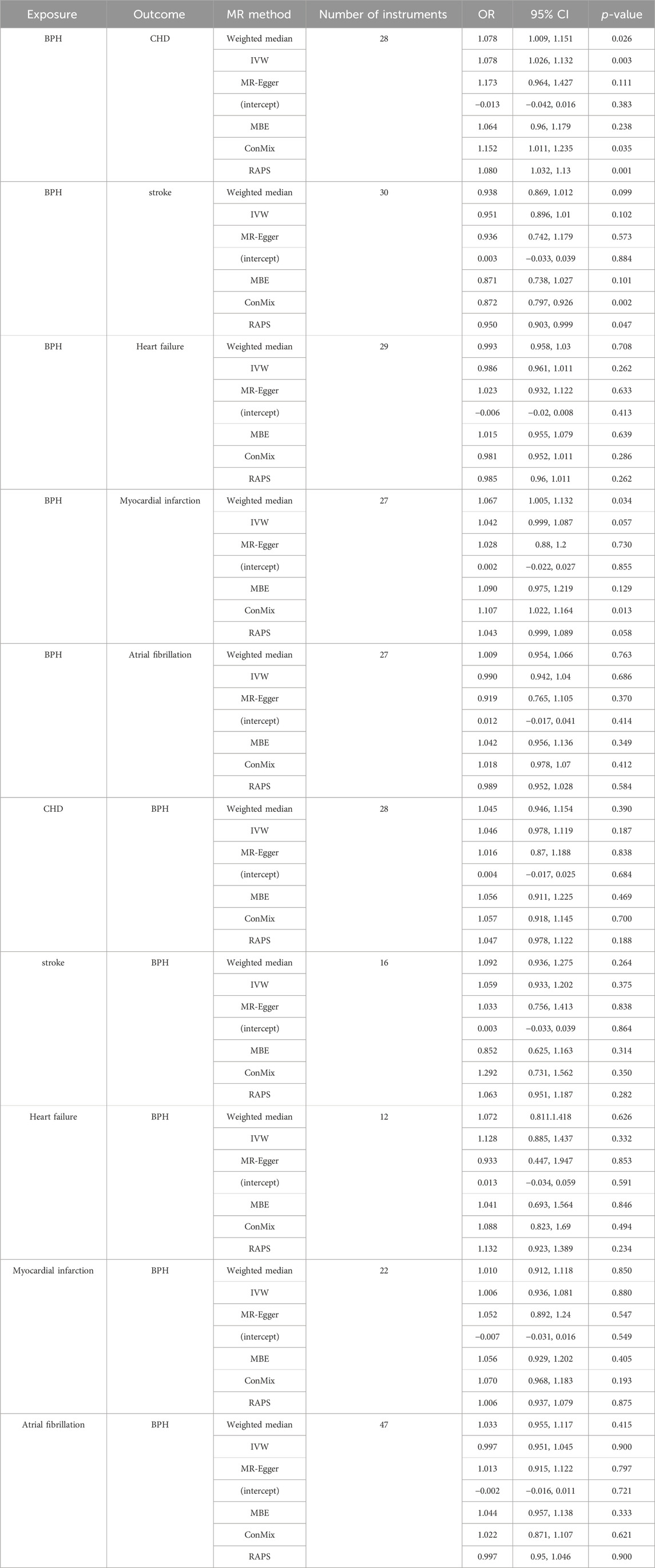
Table 1. Results of the MR analyses testing the causal association between benign BPH and cardiovascular disease (
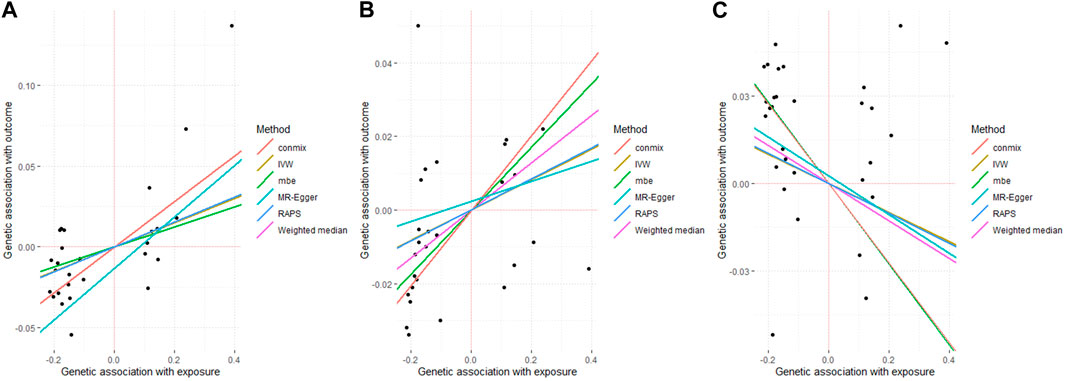
Figure 1. Associated plot of the main MR study investigating the causal effect of benign prostate hyperplasia on cardiovascular diseases. (A) outcome coronary heart disease (B) outcome myocardial infarction (C) outcome stroke.
The similar positive causality was found for the outcome MI, in 27 SNPs with strict thresholds (ConMix OR = 1.107, 95% CI: 1.022–1.164, p = 0.013; Weighted median OR = 1.067, 95% CI:1.005–1.132, p = 0.034) with the intercept of MR-Egger being not significant (p = 0.855). In addition, the results for RAPS (p = 0.058) and IVW (p = 0.057) (Table 1; Figure 1), MR-PRESSO were marginal significant (p = 0.063) (Supplementary Table S3).
In terms of stroke, the risk of developing stroke was found to have a significant negative causation with genetically predicted BPH (ConMix OR = 0.872, 95% CI: 0.797–0.926, p = 0.002; RAPS OR = 0.950, 95% CI: 0.903–0.999, p = 0.047) whereas the intercept for MR-Egger (p = 0.884) (Table 1; Figure 1) and the GlobalTest.p-value for MR-PRESSO (p = 0.067) were not yet significant (Supplementary Table S3).
For the other two CVD traits including heart failure and AF, there was no evidence to support a causal effect of BPH at a threshold of
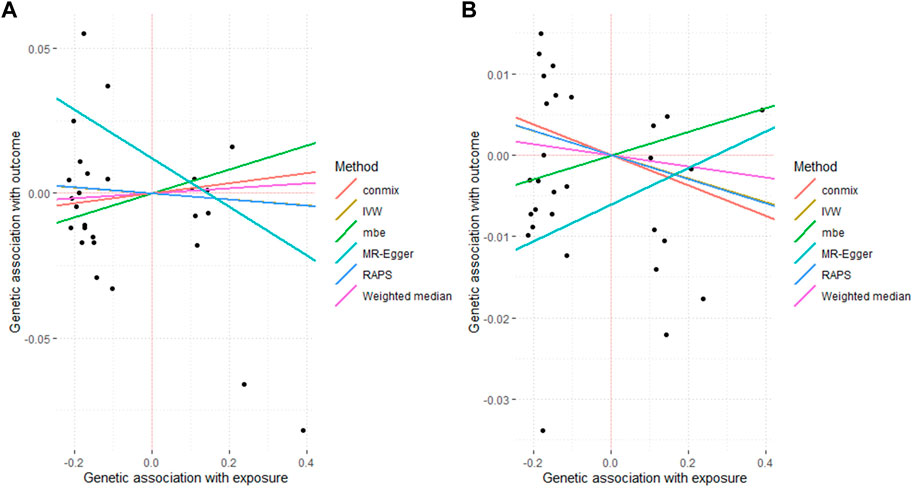
Figure 2. Associated plot of the main MR study investigating the causal effect of benign prostate hyperplasia on cardiovascular diseases. (A) outcome atrial fibrillation (B) outcome heart failure.
Under the major genome-wide significance p-value threshold, a total of 28, 16, 12, 22 and 47 SNPs were obtained for CHD, stroke (
In this study, we utilized large-scale GWAS summary statistics and a two-sample MR approach to explore the causal relationship between BPH and CVDs. Our forward analyses revealed that BPH emerged as a risk factor for CHD and MI, while demonstrating a protective effect against stroke. However, no statistically significant causal relationship was observed between BPH and AF or heart failure. Additionally, our reverse analyses failed to furnish supporting evidence for the influence of CVDs on the occurrence of BPH.
The discovery of our research that BPH served as a risk factor for CHD aligned with prior research, which identified an association between CHD and an elevated risk of moderate-to-severe BPH-related LUTS (OR = 1.68; CI: 1.08–2.61; Wald chi-square test = 5.34; p = 0.02) (Wong et al., 2010). Moreover, previous studies have shown that more than half of patients with BPH had erectile dysfunction (Calogero et al., 2019), significantly elevating the risk of CHD ((Dong et al., 2011)). The pathophysiology of erectile dysfunction involved oxidative stress and endothelial dysfunction induced by dyslipidemia, which is also recognized as a risk factor for CHD ((Arvanitis and Lowenstein, 2023)). Consequently, we hypothesized that dyslipidemia played an intermediary role in the causal association between BPH and CHD.
Confirming the link between BPH and MI, our study were consistent with findings from a comprehensive analysis of 702 older men aged 65–80 years (Weisman et al., 2000). This analysis revealed a significant association between BPH and an increased likelihood of experiencing MI. Existing researches consistently characterized BPH as an androgen-dependent condition (Weisman et al., 2000). Additionally, endogenous testosterone, one of the primary androgens, has been found to have a positive causal relationship with MI((Luo et al., 2019)). Therefore, our contention was that androgens played a pivotal role in mediating the impact of BPH on the occurrence of MI.
Interestingly, our discovery that BPH was protective against strokes diverged from a cross-sectional study involving 788 Chinese men, which identified an elevated risk of stroke associated with increased white matter hyperintensity in BPH patients (Yin et al., 2023). This disparity may stem from the inherent limitation of cross-sectional studies and insufficient samples, which often fails to account for causal associations. Additionally, we believe that behavioral changes associated with BPH, such as increased nocturia, may also contribute to this discrepancy. Nocturia, as a common symptom of BPH((Yoong et al., 2005)), is associated with inadequate nocturnal blood pressure reduction (Kato et al., 2024). Reduced blood pressure fluctuation could decrease vascular smooth muscle cell proliferation (Tedla et al., 2017), thereby reducing the risk of arterial stiffness and stroke (Lacolley et al., 2017).
However, our study did not identify a significant causal relationship between BPH and heart failure or AF. This finding differed from a current observational study suggesting that patients with BPH may exhibit a significantly higher risk of concomitant AF ((Hu and Lin, 2018)). The discrepancy observed could be attributed to an inadequate consideration of the causal association between BPH and AF in existing observational studies. Hence, for a more robust validation of our findings, it is crucial to utilize larger sample sizes and explore alternative methodologies, including cohort studies and genetic investigations.
Furthermore, in the reverse analysis, we discovered that these five cardiovascular diseases did not exhibit a significant causal relationship with BPH. Previous research indicated that men with metabolic diseases may undergo changes in the vascular supply and innervation of the prostate gland, ultimately resulting in an elevated cardiovascular risk and major adverse cardiovascular events (MACE) (Gacci et al., 2016). Therefore, we posited that BPH may manifest earlier than CVDs, providing an explanation for our observed results.
Our findings suggested that CVDs were severe complications of BPH, and to some extent supported that CVDs and BPH shared common risk factors in elderly men (Zhang et al., 2023). Thus, timely identification and treatment of BPH can effectively prevent the occurrence of CVDs. Moreover, previous research has demonstrated the notable impact of Pentosan polysulfate (PPS) on the proliferation of smooth muscle cells from vascular tissues and prostate smooth muscle cells, providing an efficacious treatment for both maladies (Elliot et al., 2003). The focus on drugs that can alleviate both BPH and CVDs may provide new avenues for the prevention and treatment of CVDs in patients with BPH((Milani and Djavan, 2005)). Additionally, previous research has found a strong causal relationship between lifestyle habits, particularly sleep levels, and BPH((Jia et al., 2024)). This suggests that lifestyle interventions could serve as a promising strategy for preventing CVDs in patients with BPH. By encouraging patients to adopt healthier lifestyle habits, such as improving sleep quality, the incidence and severity of cardiovascular diseases can potentially be reduced. These findings advocate for a more holistic approach in managing BPH, integrating both medical and lifestyle interventions to enhance patient outcomes and prevent cardiovascular complications.
This research has several strengths. Firstly, we utilized extensive genomic data from the UK Biobank, HERMES Consortium, and the FinnGen Genome Database, making our results more reliable. Secondly, MR analysis was employed to identify the relationship between BPH and CVDs, which could ensure the reliability of results by minimizing residual confounding to the greatest extent possible. Thirdly, we identified causal relationships between BPH and five types of CVDs, providing a new perspective for a deeper understanding of the complex interactions between BPH and CVDs.
However, there were some limitations that need to be acknowledged. Firstly, only five types of CVDs were selected in the study, and some other types of CVDs, like peripheral artery disease, may be causally related to BPH, and the correlation between them should be confirmed through subsequent studies. Besides, our study population was limited to individuals of European ancestry, and thus caution should be taken when generalizing the results to populations of other ancestries. Finally, in the correlation analysis with BPH, we encountered challenges in excluding female samples from the CVDs group. This may arise from constraints within the GWAS database, potentially introducing statistical bias.
In conclusion, our MR estimates supported an adverse effect of increased BPH on CHD and MI, while concurrently indicating a protective effect of heightened BPH on stroke in men. These established associations contributed to a more profound comprehension of the pathogenesis of both diseases, offering valuable insights for the development of more effective strategies in their prevention and treatment.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
This MR study was based on publicly available GWAS data, obviating the need for ethical approvals. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
NX: Methodology, Software, Validation, Visualization, Writing–original draft. SS: Formal Analysis, Methodology, Validation, Writing–original draft. ZW: Formal Analysis, Methodology, Software, Validation, Writing–review and editing. YY: Data curation, Formal Analysis, Methodology, Software, Writing–review and editing. BC: Formal Analysis, Visualization, Writing–original draft, SR: Validation, Writing–review and editing. TZ: Validation, Writing–review and editing. BL: Validation, Writing–review and editing. YL: Conceptualization, Data curation, Methodology, Resources, Writing–review and editing. JH: Funding acquisition, Project administration, Resources, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Grant No. 32101206), Key Program of Science and Technology Department of Sichuan Province (Grant Nos. 2023YFS0025 and 2023YFS0102); 1⋅3⋅5 project for disciplines of excellence–Clinical Research Fund, West China Hospital, Sichuan University (2023HXFH044).
We would like to thank the United Kingdom biobank, HERMES Consortium, and the FinnGen Genome Database for sharing the GWAS summary data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1432055/full#supplementary-material
Arvanitis, M., and Lowenstein, C. J. (2023). Dyslipidemia. Ann. Intern. Med. 176 (6), Itc81–itc96. doi:10.7326/AITC202306200
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 44 (2), 512–525. doi:10.1093/ije/dyv080
Burgess, S., Butterworth, A., and Thompson, S. G. (2013). Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37 (7), 658–665. doi:10.1002/gepi.21758
Burgess, S., Foley, C. N., Allara, E., Staley, J. R., and Howson, J. M. M. (2020). A robust and efficient method for Mendelian randomization with hundreds of genetic variants. Nat. Commun. 11 (1), 376. doi:10.1038/s41467-019-14156-4
Calogero, A. E., Burgio, G., Condorelli, R. A., Cannarella, R., and La Vignera, S. (2019). Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. aging male official J. Int. Soc. Study Aging Male 22 (1), 12–19. doi:10.1080/13685538.2018.1434772
Colon, I., and Payne, R. E. (2008). Benign prostatic hyperplasia and lower urinary tract symptoms in African Americans and Latinos: treatment in the context of common comorbidities. Am. J. Med. 121 (8 Suppl. 2), S18–S26. doi:10.1016/j.amjmed.2008.05.023
Daghlas, I., Dashti, H. S., Lane, J., Aragam, K. G., Rutter, M. K., Saxena, R., et al. (2019). Sleep duration and myocardial infarction. J. Am. Coll. Cardiol. 74 (10), 1304–1314. doi:10.1016/j.jacc.2019.07.022
Davies, N. M., Holmes, M. V., and Davey Smith, G. (2018). Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ Clin. Res. ed. 362, k601. doi:10.1136/bmj.k601
Del Giudice, F., Oh, J. K., Basran, S., Nicaise, E., Song, P. H., Kim, W., et al. (2022). Recent trends in the diagnostic and surgical management of benign prostatic hyperplasia in the U.S. From 2004 to 2017: annual changes in the selection of treatment options and medical costs. Appl. Sci. 12 (17), 8697. doi:10.3390/app12178697
Dincer, E., Ipek, O. M., Sarikaya Kayipmaz, S., and Hanci, B. (2021). Giant prostatic hyperplasia: case presentation of the second largest prostate adenoma. Afr. J. Urology 27 (1), 27. doi:10.1186/s12301-021-00131-3
Dong, J. Y., Zhang, Y. H., and Qin, L. Q. (2011). Erectile dysfunction and risk of cardiovascular disease: meta-analysis of prospective cohort studies. J. Am. Coll. Cardiol. 58 (13), 1378–1385. doi:10.1016/j.jacc.2011.06.024
Elliot, S. J., Zorn, B. H., McLeod, D. G., Moul, J. W., Nyberg, L., Striker, L. J., et al. (2003). Pentosan polysulfate decreases prostate smooth muscle proliferation and extracellular matrix turnover. Prostate cancer prostatic Dis. 6 (2), 138–142. doi:10.1038/sj.pcan.4500632
Gacci, M., Corona, G., Sebastianelli, A., Serni, S., De Nunzio, C., Maggi, M., et al. (2016). Male lower urinary tract symptoms and cardiovascular events: a systematic review and meta-analysis. Eur. Urol. 70 (5), 788–796. doi:10.1016/j.eururo.2016.07.007
Hagg, S., Fall, T., Ploner, A., Magi, R., Fischer, K., Draisma, H. H., et al. (2015). Adiposity as a cause of cardiovascular disease: a Mendelian randomization study. Int. J. Epidemiol. 44 (2), 578–586. doi:10.1093/ije/dyv094
Hu, W. S., and Lin, C. L. (2018). Increased risk of atrial fibrillation in patients with benign prostatic hyperplasia: a population-based cohort study. Clin. Cardiol. 41 (10), 1374–1378. doi:10.1002/clc.23063
Jia, F., Wei, Z., Kong, X., Mao, Y., and Yang, Y. (2024). Causal associations between lifestyle habits and risk of benign prostatic hyperplasia: a two-sample mendelian randomization study. journals gerontology Ser. A, Biol. Sci. Med. Sci. 79 (1), glad187. doi:10.1093/gerona/glad187
Kato, Y., Akaihata, H., Takezawa, K., Maekawa, S., Matsuoka, K., Fukuhara, S., et al. (2024). Association between nocturnal polyuria and 24-h blood pressure fluctuations in males with lower urinary tract symptoms: a multicenter prospective study. Int. J. urology official J. Jpn. Urological Assoc. 31 (3), 259–264. doi:10.1111/iju.15354
Lacolley, P., Regnault, V., Segers, P., and Laurent, S. (2017). Vascular smooth muscle cells and arterial stiffening: relevance in development, aging, and disease. Physiol. Rev. 97 (4), 1555–1617. doi:10.1152/physrev.00003.2017
Luo, S., Au Yeung, S. L., Zhao, J. V., Burgess, S., and Schooling, C. M. (2019). Association of genetically predicted testosterone with thromboembolism, heart failure, and myocardial infarction: mendelian randomisation study in UK Biobank. BMJ Clin. Res. ed. 364, l476. doi:10.1136/bmj.l476
McVary, K. T. (2006). BPH: epidemiology and comorbidities. Am. J. Manag. care 12 (5 Suppl. l), S122–S128.
Milani, S., and Djavan, B. (2005). Lower urinary tract symptoms suggestive of benign prostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int. 95 (Suppl. 4), 29–36. doi:10.1111/j.1464-410X.2005.05485.x
Qingyuan, Z., Jingshu, W., Jack, B., and Dylan, S.S. (2018). Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann. Statist. 48 (3), 1742–1769.
Sanderson, E., Glymour, M. M., Holmes, M. V., Kang, H., Morrison, J., Munafò, M. R., et al. (2022). Mendelian randomization. Nat. Rev. Methods Prim. 2 (1), 6. doi:10.1038/s43586-021-00092-5
Srinivasan, A., and Wang, R. (2020). An update on minimally invasive surgery for benign prostatic hyperplasia: techniques, risks, and efficacy. world J. men's health 38 (4), 402–411. doi:10.5534/wjmh.190076
Sun, Y., Ding, Z., Guo, Y., Yuan, J., Zhu, C., Pan, Y., et al. (2023). Causal inference of central nervous system-regulated hormones in COVID-19: a bidirectional two-sample mendelian randomization study. J. Clin. Med. 12 (4), 1681. doi:10.3390/jcm12041681
Tedla, Y. G., Yano, Y., Carnethon, M., and Greenland, P. (2017). Association between long-term blood pressure variability and 10-year progression in arterial stiffness: the multiethnic study of atherosclerosis. Hypertens. (Dallas, Tex 1979) 69 (1), 118–127. doi:10.1161/HYPERTENSIONAHA.116.08427
Vuichoud, C., and Loughlin, K. R. (2015). Benign prostatic hyperplasia: epidemiology, economics and evaluation. Can. J. urology 22 (Suppl. 1), 1–6.
Wang, Y. B., Yang, L., Deng, Y. Q., Yan, S. Y., Luo, L. S., Chen, P., et al. (2022). Causal relationship between obesity, lifestyle factors and risk of benign prostatic hyperplasia: a univariable and multivariable Mendelian randomization study. J. Transl. Med. 20 (1), 495. doi:10.1186/s12967-022-03722-y
Weisman, K. M., Larijani, G. E., Goldstein, M. R., and Goldberg, M. E. (2000). Relationship between benign prostatic hyperplasia and history of coronary artery disease in elderly men. Pharmacotherapy 20 (4), 383–386. doi:10.1592/phco.20.5.383.35053
Wong, S. Y., Woo, J., Leung, J. C., and Leung, P. C. (2010). Depressive symptoms and lifestyle factors as risk factors of lower urinary tract symptoms in Southern Chinese men: a prospective study. aging male official J. Int. Soc. Study Aging Male 13 (2), 113–119. doi:10.3109/13685530903440432
Yang, G., and Schooling, C. M. (2023). Investigating sex-specific associations of lipid traits with type 2 diabetes, glycemic traits and sex hormones using Mendelian randomization. Cardiovasc. Diabetol. 22 (1), 3. doi:10.1186/s12933-022-01714-2
Yin, F., He, Q. D., Chen, J., Gui, T. J., Cai, R. J., Wang, Y., et al. (2023). Benign prostatic hyperplasia associated with white matter hyperintensities in men. Clin. neurology Neurosurg. 229, 107738. doi:10.1016/j.clineuro.2023.107738
Yoong, H. F., Sundaram, M. B., and Aida, Z. (2005). Prevalence of nocturnal polyuria in patients with benign prostatic hyperplasia. Med. J. Malays. 60 (3), 294–296.
Zhang, J., Latour, C. D., Olawore, O., Pate, V., Friedlander, D. F., Stürmer, T., et al. (2023). Cardiovascular outcomes of α-blockers vs 5-α reductase inhibitors for benign prostatic hyperplasia. JAMA Netw. open 6 (11), e2343299. doi:10.1001/jamanetworkopen.2023.43299
Zhang, W., Zhang, X., Li, H., Wu, F., Wang, H., Zhao, M., et al. (2019). Prevalence of lower urinary tract symptoms suggestive of benign prostatic hyperplasia (LUTS/BPH) in China: results from the China Health and Retirement Longitudinal Study. BMJ open 9 (6), e022792. doi:10.1136/bmjopen-2018-022792
Keywords: benign prostatic hyperplasia, cardiovascular diseases, mendelian randomization, genetic epidemiology, causal relationship
Citation: Xiang N, Su S, Wang Z, Yang Y, Chen B, Shi R, Zheng T, Liao B, Lin Y and Huang J (2024) Exploring the causal relationship: bidirectional mendelian randomization study on benign prostatic hyperplasia and cardiovascular diseases. Front. Genet. 15:1432055. doi: 10.3389/fgene.2024.1432055
Received: 13 May 2024; Accepted: 05 July 2024;
Published: 26 July 2024.
Edited by:
Xiaolong Wang, Temple University, United StatesReviewed by:
Peng Wang, Coriell Institute For Medical Research, United StatesCopyright © 2024 Xiang, Su, Wang, Yang, Chen, Shi, Zheng, Liao, Lin and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Huang, bWljaGFlbF9odWFuZ2ppbkAxNjMuY29t; Yifei Lin, eWxpbkBoc3BoLmhhcnZhcmQuZWR1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.