- 1Department of Laboratory Medicine, Maternal and Child Health Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
- 2Guangxi Key Laboratory of Reproductive Health and Birth Defect Prevention, Maternal and Child Health Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
- 3Department of Clinical Genetics, Maternal and Child Health Hospital of Guangxi Zhuang Autonomous Region, Nanning, China
Objective: This study aimed to evaluate the efficacy of α-thalassemia gene testing as a part of an antenatal intervention program over a 10-year period.
Methods: All patients underwent α-thalassemia gene testing, which included the analysis of three types of deletions and mutations. Rare α-thalassemia gene testing was performed using Sanger sequencing, multiplex ligation-dependent probe amplification, and sequencing techniques. Prenatal diagnosis was performed in high-risk couples using chorionic villus sampling or amniocentesis.
Results: From 2010 to 2019, among the 91,852 patients examined, α-thalassemia mutations were identified in 41.78% of patients. The most frequent α0 gene mutation was--SEA, followed by--THAI. Two rare α0-thalassemia gene mutations at --32.8 and --230, were also observed. A total of 2,235 high-risk couples were identified, of which 562 were affected, including three with the--SEA/--THAI genotype and one with the--SEA/--230 genotype. Additionally, prenatal diagnosis revealed four cases of fetal anemia and/or mild edema, along with two cases of severe fetal edema. Chromosome and gene chip results were normal. Thalassemia gene testing showed an αCSα/αCSα genotype in four patients with anemia and/or mild edema, while two patients with severe fetal edema had one--SEA/αCSα genotype and one--SEA/--GX genotype. Using the cut-off points of 74.6 fL and 24.4 pg as criteria for identifying α0-thalassemia carriers and HbH disease, the detection rate of missed diagnoses in high-risk couples is consistent with national guidelines for standards, potentially saving 10,217,700 ¥.
Conclusion: Routine molecular testing for α-thalassemia in high-risk prenatal populations effectively prevented severe α-thalassemia births. Despite the high cost, the cutoff points proposed by this study suggest that implementing screening using a new parameter has the potential to reduce current expenses.
1 Introduction
α-Thalassemia is a common genetic disorder caused by a defect in the α-globin gene, resulting in hereditary hemolytic anemia. It is the primary cause of fetal hydrops in Southeast Asia, accounting for 33%–62% of cases (He et al., 2017a; Chainarong et al., 2021). Currently, long-term blood transfusion and hematopoietic stem cell transplantation are primary treatment options for severe thalassemia (Songdej et al., 2017; King and Higgs, 2018; Chan et al., 2021). Therefore, it is crucial to implement interventions that target high-risk populations to prevent the birth of children with severe α-thalassemia. Prenatal screening programs are established in countries with a high prevalence of thalassemia (Sorour et al., 2007; Bhukhanvala et al., 2013; Jiang et al., 2021). These programs typically involve initial tests, such as measuring red blood cell indices and quantifying hemoglobin(Hb)A2 levels. Although quantifying HbA2 using techniques such as capillary electrophoresis or high-performance liquid chromatography is effective in detecting most β-thalassemia traits, identifying α-thalassemia traits remains challenging owing to limited screening tests (Han et al., 2019; Mustafa et al., 2020; Jiang et al., 2021; Vachhani et al., 2022). In certain cases, such as individuals with borderline hypochromia, compound triplicated α-globin genes, or β-thalassemia, α0-thalassemia may remain undetected (Sorour et al., 2007; Colaco and Nadkarni, 2021).
Two In China, thalassemia screening strategies have been implemented in high-prevalence regions, including Guangxi, Guangdong, Hainan, Yunnan, Fujian, Hong Kong, and Taiwan (Jiang et al., 2021). Among these regions, Guangxi Province has the highest prevalence, and Hb Barts hydrops fetalis constitutes a significant birth defect within the region. Over the course of a decade, severe cases of α-thalassemia have been successfully eliminated in Guangxi Province since the comprehensive implementation of thalassemia prevention and control strategies in 2010. This study aimed to evaluate the effectiveness of current molecular testing strategies for thalassemia and provide insights into screening and diagnosing high-risk couples expecting a child with Hb Barts hydrops fetalis.
2 Methods
2.1 Subjects and hematological screening strategy
The prenatal thalassemia intervention program was implemented through a three-level network of maternal and child health services in Guangxi with government support (Figure 1). During the period from 2010 to 2019, an extensive cohort of 91,852 cases derived from 20 districts was meticulously scrutinized for this comprehensive study. Notably, among this cohort, a significant proportion of 49,880 cases pertained to the female population, with a median age of 28 years (ranging from 16 to 49 years). The distribution of pregnancy stages within this cohort revealed that 44.20% of the cases were in the early gestational phase, while the remaining 55.80% were in the mid-pregnancy stage. Additionally, the prevalence rates of severe, moderate, and mild anemia were observed to be 0.06%, 6.74%, and 58.47% respectively. All patients underwent routine Hb variant and thalassemia screening, which included hematology and Hb analysis. Molecular testing for α-thalassemia was recommended when the screening results indicated a mean corpuscular volume (MCV) < 82 fL, mean corpuscular hemoglobin (MCH) < 27 pg, totaling 78,302 cases. Even if the screening results were normal, molecular testing was still recommended for genetic counseling if there was a history of fetal hydrops during pregnancy (7,824 cases) or if either spouse was a carrier of α0-thalassemia or Hb H disease (4,726 cases), totaling 13,550 cases. All the patients provided written informed consent.
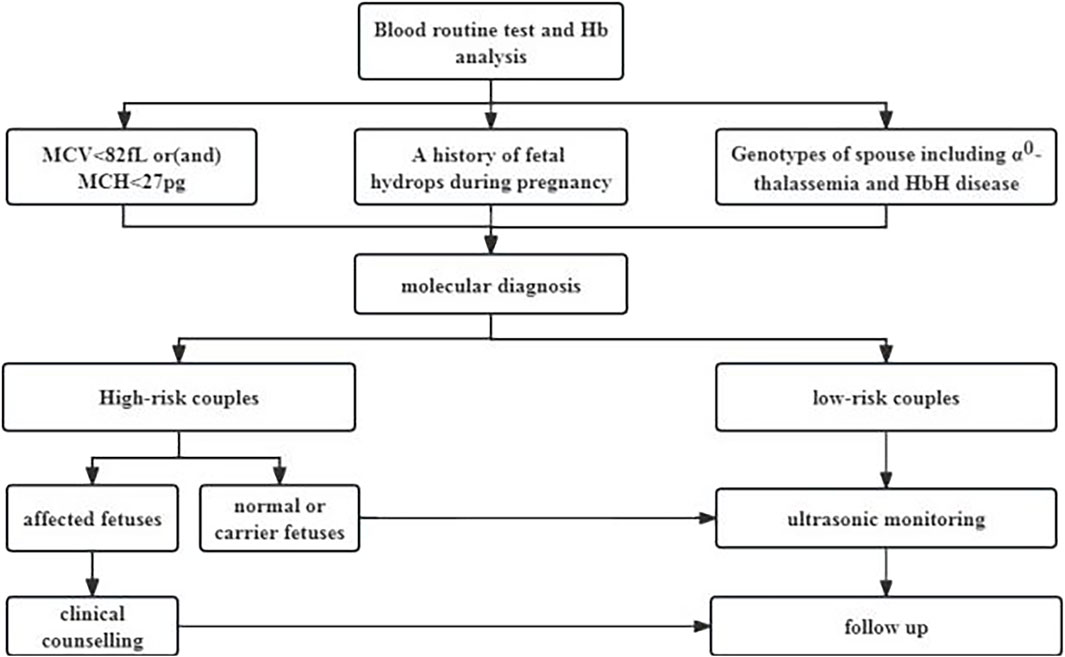
Figure 1. Intervention Strategies, for α-Thalassemia in Individuals of Childbearing Age (Mean corpuscular volume, MCV, Mean corpuscular hemoglobin, MCH; Hemoglobin,Hb).
2.2 Genotype test
Genomic DNAs was extracted from the peripheral blood leukocytes of subjects using a DNA blood extraction kit. Gap-PCR was used to identify common Chinese α-globin gene deletion mutations (-α3.7, -α4.2, and--SEA). Reverse dot-blot hybridization was employed to detect three types of α-thalassemia, αWSα, αCSα, and αQSα, as well as 17 types of β-globin gene mutations. In cases of new mutations, gross deletions in the α-gene cluster were detected using multiplex ligation-dependent probe amplification (MLPA). Additionally, DNA sequencing was performed to identify the unknown and rare α-globin mutations. Couples in which both partners carry α0-gene mutations are deemed to be at a high risk for severe α-thalassemia in the fetus, whereas couples without such mutations are classified as having a low risk.
2.3 Prenatal diagnosis
In couples with α0-thalassemia, pregnancy is considered high risk. Prior to prenatal diagnosis, couples designated as at-risk for pregnancy undergo detailed interviews, particularly regarding the risks of invasive sampling, surgical complications, fetal loss rates, and the option to terminate pregnancy if the fetus is diagnosed with Hb Bart’s hydrops fetalis. Written informed consent was obtained from all pregnant women. Invasive examination techniques include chorionic villus sampling (CVS) in early pregnancy (11–14 weeks) and amniocentesis in mid-pregnancy (18–28 weeks), both of which are performed under ultrasonographic guidance. All pregnant women receiving antenatal care at this hospital underwent consecutive ultrasound examinations at 12–15 weeks, 16–20 weeks, and 25–30 weeks to evaluate the presence of fetal edema (Li et al., 2015). For low-risk pregnant women with fetal edema, prenatal diagnosis is recommended to determine the cause of fetal edema through chromosomal analysis, genetic microarray testing, and thalassemia gene testing.
2.4 Analysis
Statistical analyses were performed using SPSS version 26. Chi-squared test was used to compare the different types of thalassemia, with the significance level set at p < 0.05. Receiver Operating Characteristic (ROC) curve analysis was conducted to determine the optimal cutoff values for MCV and MCH, and the sensitivity (Sen), specificity (Spe), positive predictive values (PPV), and negative predictive values (NPV) of each cutoff were calculated.
3 Results
3.1 Gene mutation spectrum of α-thalassemia
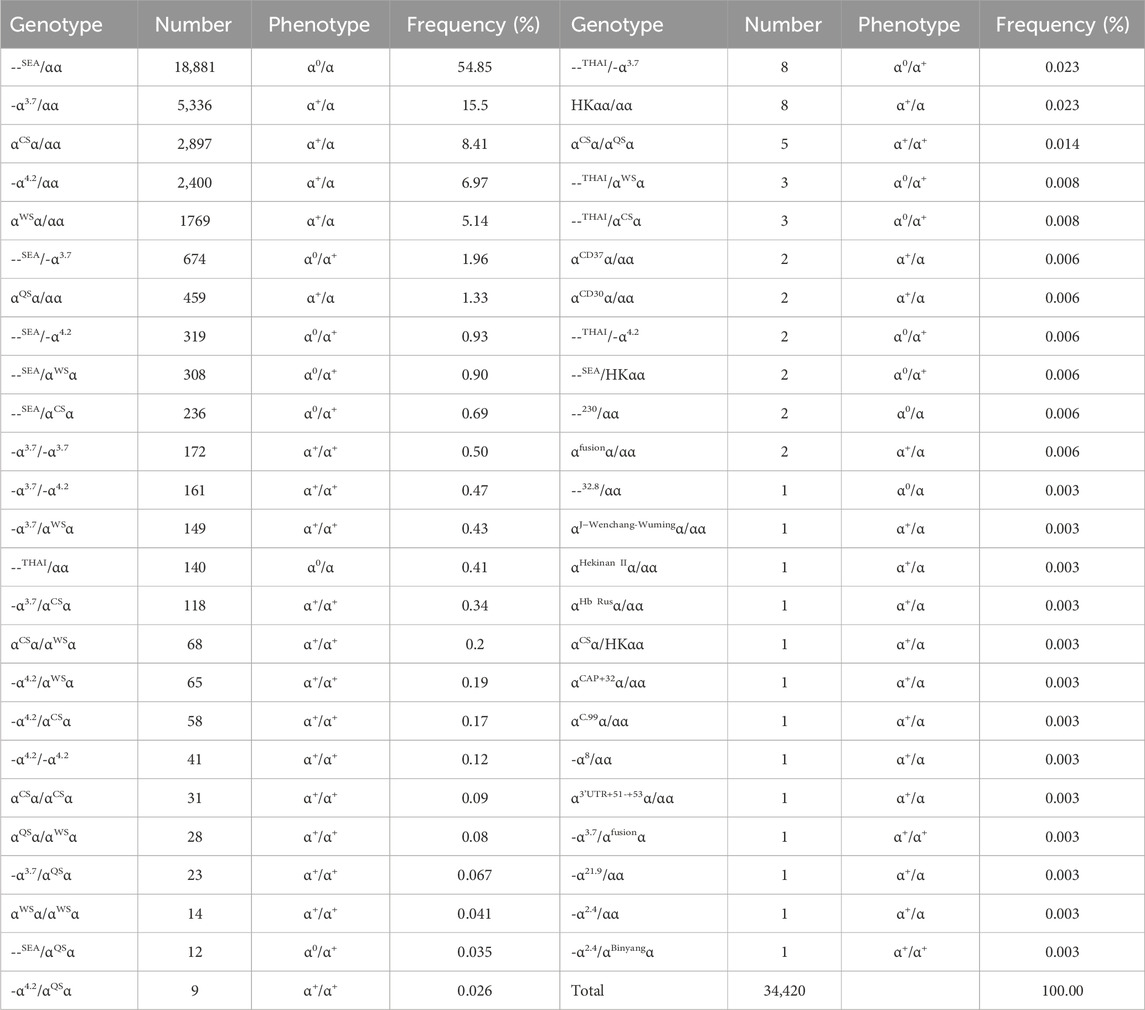
Among the 91,852 subjects, 38,379 (41.78%) had α-thalassemia mutations, including 34,420 (37.47%) with α-thalassemia and 3,959 (4.31%) with α/β-thalassemia. Out of the 34,420 subjects with α-thalassemia, 20,591 (59.82%) had α0-thalassemia and Hb H. Forty-nine genotypes were identified, with the most frequent accounting for 94.27% of all the genotypes, including--SEA/αα, -α3.7/αα, αCSα/αα, -α4.2/αα, αWSα/αα, --SEA/-α3.7, and αQSα/αα. Three rare α0-thalassemia and five rare Hb H disease genotypes were identified: --THAI/αα, --32.8/αα, --230/αα, --THAI/-α3.7, --THAI/αWSα, --THAI/-α4.2, --THAI/αCSα, and--SEA/HKαα (Table 1).
3.2 Gene mutation spectrum of α/β-thalassemia
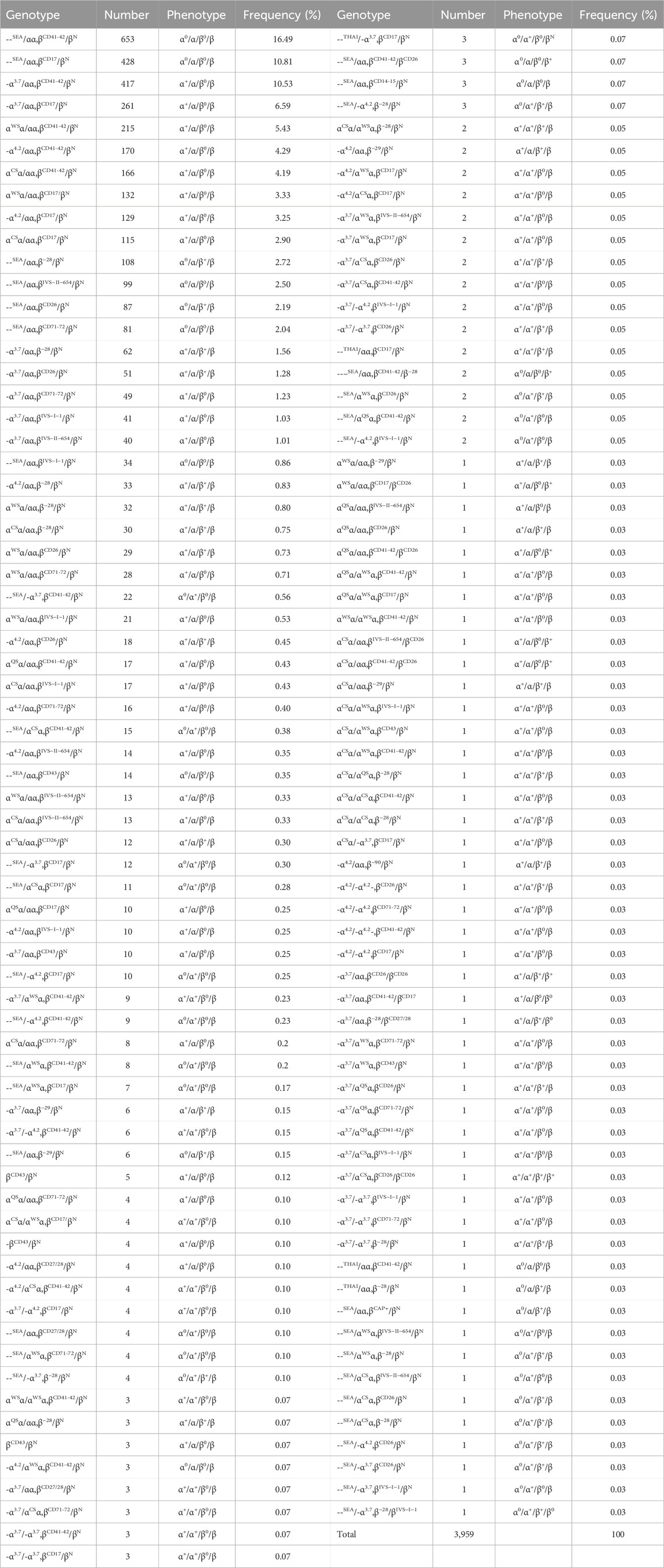
Among the 3,959 cases of α/β-thalassemia, 1,650 (41.68%) were diagnosed with β-thalassemia combined with α0-thalassemia and Hb H disease. A total of 137 genotypes were identified, with the most prevalent accounting for 58.70% of all the genotypes, including βCD41-42/βN combined with --SEA/αα, -α3.7/αα, αWSα/αα, -α4.2/αα, and αCSα/αα. Other common genotypes included βCD17/βN combined with --SEA/αα and -α3.7/αα (Table 2). Additionally, four rare α/β-thalassemia genotypes were identified: ---THAI/-α3.7, βCD17/βN; --THAI/αα, βCD17/βN; --THAI/αα, βCD41-42/βN; and --THAI/αα, β−28/βN. Moreover, two α0 with β0/β+ genotypes were identified, namely, --SEA/αα, βCD41-42/βCD26 (0.07%) and --SEA/αα, βCD41-42/β−28 (0.05%), and one genotype, --SEA/-α3.7, β−28/βIVS−I−1 (0.03%).
3.3 The status of α-thalassemia
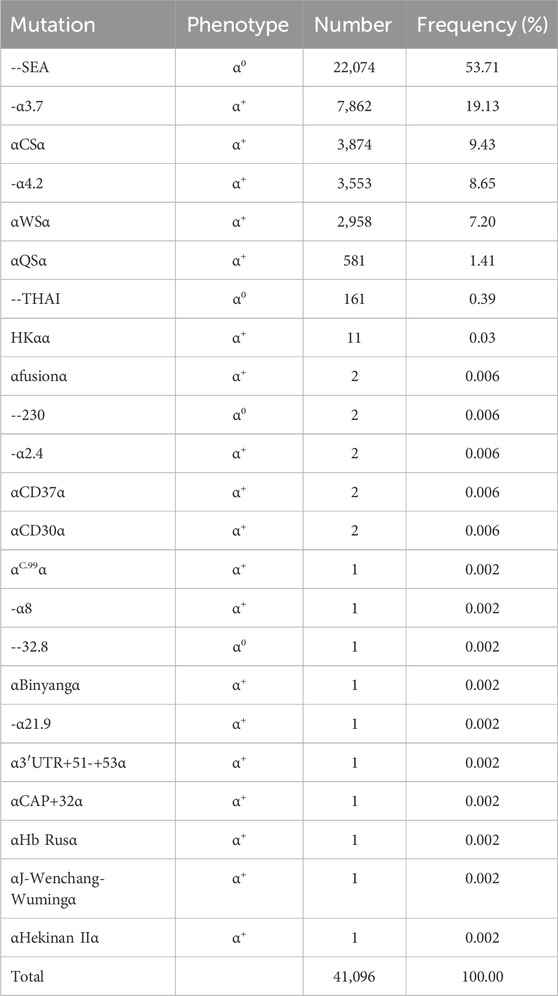
Table 3 illustrates the frequency distribution of mutation types among all α-thalassemia mutant chromosomes based on the identification of twenty-five distinct mutations. Hematology parameters of 10 main types α-thalassemiaand α/β-thalassemia were shown in supplement Table 1. The most predominant mutation observed was--SEA, accounting for 53.71% of all α-mutant chromosomes. The most frequent mutations, in descending order, were -α3.7, αCSα, -α4.2, αWSα, αQSα, and --THAI. Additionally, two rare α0 mutations, namely, --32.8 and --230, were observed. Overall, 22,238 (57.94%) patients had α0-thalassemia mutations.
A total of 2,235 high-risk couples were identified, 562 of which were affected. Among these patients, three had the--SEA/--THAI genotype and one had the--SEA/--230 genotype. All the affected patients opted for pregnancy termination. Additionally, we observed four patients with fetal anemia and/or mild edema, as well as two cases of severe fetal edema during prenatal diagnosis. The chromosomal and genetic microarray results were normal. Thalassemia gene testing revealed an αCSα/αCSα genotype in four patients with anemia and/or mild edema, whereas two patients with severe fetal edema had one--SEA/αCSα genotype and one--SEA/--GX genotype. The status of screening and diagnosis for thalassemia from 2010 to 2019 were shown in supplement Table 2.
3.4 Evaluation of α-thalassemia genetic test
In our study, 13,550 patients had an MCV of >82 fL and an MCH of >27 pg. Among them, 8% (1,084 cases) were carriers of α-thalassemia, including -α3.7/αα (40.96%), αWSα/αα (34.50%), -α4.2/αα (18.73%), αCSα/αα (3.78%), --SEA/αα (0.37%) (one patient had βCD26/βN), αQSα/αα (0.18%), and others (1.48%). ROC curves demonstrated that MCV and MCH could effectively differentiate between α0-thalassemia and α+-thalassemia carriers, with optimal cut-off points of 74.6 fL (The Sen, Spe, PPV, and NPV was 0.963, 0.925, 0.804, and 0.987, respectively.) and 24.4 pg (The Sen, Spe, PPV, and NPV was 0.977, 0.905, 0.767, and 0.992, respectively.), respectively (AUC of 0.965 and 0.976, respectively; Figure 2). Using these cutoff points as criteria for identifying α0-thalassemia carriers and HbH disease, only 69,146 cases required genetic testing for α-thalassemia. This would result in a cost-saving of 10,217,700 ¥ based on the cost of the kit (with an average cost of 450 ¥ for α-thalassemia genetic testing). Three high-risk couples were excluded from this study. A comparison of the adoption of national standards reveals (Cut-off values of 82 fL (the Sen, Spe, PPV, and NPV was 0.999, 0.912, 0.785, and 0.999, respectively.) and 27 pg (The Sen, Spe, PPV, and NPV was 0.999, 0.937, 0.835, and 0.999, respectively.) for MCV and MCH) no differences in the results.
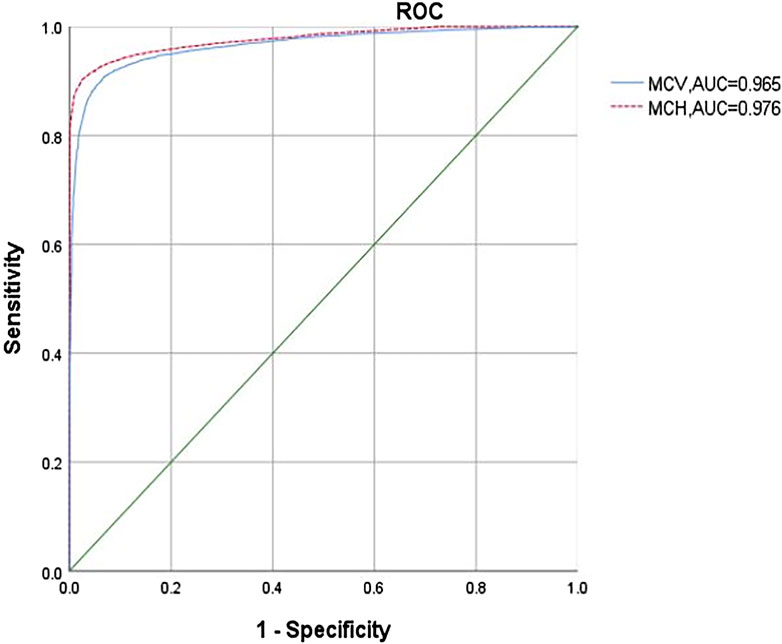
Figure 2. ROC analysis of MCV (Mean corpuscular volume) and MCH (Mean corpuscular hemoglobin) in diagnosing α0-thalassemia.
4 Discussion
In Guangxi, the carrier rate of thalassemia genes is approximately 20%, which is significantly higher than that in other provinces and regions, such as Guangdong (11.6%), Hong Kong (8.4%), Taiwan (5.5%), Sichuan (4.1%), and Jiangxi (2.7%) (Liu et al., 2019; Jiang et al., 2021). As a result, fetal hydrops syndrome (severe α-thalassemia) has consistently been a prominent birth defect in the Guangxi region for many years. The Guangxi government has prioritized the prevention and control of thalassemia by implementing a three-level healthcare network strategy that includes policy interventions by government departments (He et al., 2014). Our center, being one of the three provincial-level prenatal diagnosis centers in Guangxi, plays a crucial role in prenatal diagnosis, birth defect management, and quality control in the region. Our center conducts prenatal diagnosis for thalassemia for over 10,000 people annually, accounting for about one-third of thalassemia prenatal diagnoses in the Guangxi region. With the help of the “Guifuer system,” we perform quality control and follow-up on the screening and diagnostic results entered into the system. No newborn with severe α-thalassemia was identified in the follow-up studies.
In China, thalassemia screening recommends using cut-off values of 82 fL and 27 pg for MCV and MCH, respectively (Jiang et al., 2021). Applying these national criteria for screening for α0-thalassemia can help identify all patients with HbH disease and the majority with α0-thalassemia. In our study, which included both patients with α-thalassemia and α/β-thalassemia, we identified 38,379 patients with α-thalassemia mutations. In this cohort, 57.94% of the patients were classified as having α0-thalassemia and Hb H. Of the 2,235 high-risk couples identified, 562 pregnancies were terminated. These findings highlight the effectiveness of molecular screening procedures in identifying high-risk couples.
However, with increasing population migration and the emergence of new gene variants, relying solely on common α-thalassemia screening may fail to detect certain α0-gene types, resulting in the birth of children with severe α-thalassemia (Henderson et al., 2016; Lai et al., 2017; Nasiri et al., 2020). To accurately identify high-risk couples, we conducted rare gene testing with informed consent from those who tested negative in routine gene testing. In our study, we identified 18 rare gene types, including two α0-thalassemia genes (--32.8 and --230), excluding the--THAI type. Previous research has indicated a relatively common carrier rate of--THAI (0.36%) in Guangxi (He et al., 2017b). Since 2014, molecular testing for this gene has been routinely conducted. In our study, among 562 affected cases, three had the--SEA/--THAI genotype and one had the--SEA/--230. In practical settings, not every individual who tests negative for routine gene testing agrees to undergo rare gene testing because of the high cost. Additionally, we observed two patients with a prenatal diagnosis attributed to severe fetal edema, and the results of α-thalassemia gene testing revealed one--SEA/--GX genotype and one--SEA/αCSα genotype. This indicates a potential association with severe fetal edema.
The screening program aims to identify individuals with α0-thalassemia; however, some patients with α+-thalassemia, particularly those with Hb CS, may be missed. Previous studies have reported that Hb H disease, especially the --/αCSα and αCSα/αCSα genotypes, can result in fetal anemia or edema (He et al., 2016; Sirilert et al., 2019). However, our screening program incorporates Hb analysis, specifically utilizing CE technology, which significantly enhances the detection rate of αCSα/αα and αCSα (Panyasai et al., 2023). In this study, we observed four patients with a prenatal diagnosis attributed to fetal edema or mild anemia, excluding abnormalities in chromosomal and genetic microarray testing results. The α-thalassemia gene testing results showed that four patients had αCSα/αCSα, indicating a potential association with thalassemia. Pregnant women with Hb H often experience severe anemia during pregnancy (Lal and Vichinsky, 2023). Fetails with genotypes αQSα and αCSα typically present with moderate anemia, whereas a few patients may have severe anemia that requires blood transfusion therapy. Therefore, as part of antenatal care, we recommend conducting α+-thalassemia testing to identify Hb H.
Hematological screening for α-thalassemia can yield false positive and false negative results, leading to fewer missed diagnoses in high-risk couples. In our study, we conducted α-thalassemia gene testing for individuals with a history of fetal edema or cases in which one partner was diagnosed with α0-thalassemia or Hb H disease, even if their MCV was >82 fL and MCH was >27 pg. Among the 13,550 patients, 8% were carriers of α-thalassemia, including 4 cases of--SEA/αα (0.37%). These results are consistent with those reported by Jiang et al. (Jiang et al., 2021), who did not find any patients with --SEA/αα. ROC curves showed that MCV and MCH (cut-off points of 74.6 fL and 24.4 pg) could effectively identify α0-thalassemia carriers and HbH disease. By testing only 69,146 cases of α-thalassemia, cost cost-saving of 10,217,700 ¥ are achieved. The number of missed diagnoses in high-risk couples was consistent with the national screening standards.
The study has a number of limitations. Firstly, the data were collected from a single tertiary hospital, representing only a subset of cases in our region. Therefore, the generalizability of the findings to the broader population may be limited due to the specific sample source. Secondly, although rare α-thalassemia gene testing was implemented, there is a potential risk of underreporting certain genetic variants, which could result in the oversight of severe α-thalassemia cases. Thirdly, a comprehensive cost-benefit analysis was not conducted. However, the proposed cutoff points in this study indicate that the implementation of screening using a new parameter has the potential to reduce current expenses. Further research is needed to validate and optimize this strategy and assess its feasibility.
In summary, our study highlights the success of implementing molecular screening procedures to identify cases of severe α-thalassemia and emphasizes the importance of considering α+-thalassemia testing for HbH disease detection. This information will be valuable in guiding clinical practice and improving the detection and management of α-thalassemia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Medical Ethics Committee of Guangxi Maternal and Child Health Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
DL: Conceptualization, Writing–original draft, Writing–review and editing. LL: Conceptualization, Data curation, Methodology, Software, Writing–original draft, Writing–review and editing. DM: Conceptualization, Data curation, Formal Analysis, Methodology, Writing–review and editing. SH: Conceptualization, Funding acquisition, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Health Department of Guangxi Province (S2023040, S2021071, and ZTJ2020002), Guangxi Medical High-level backbone Talents “139” Plan, Natural Science Foundation of Guangxi (G202003023), and construction project for clinical key specialties in the Guangxi Zhuang Autonomous Region.
Acknowledgments
We thank all of those who participated in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2024.1416047/full#supplementary-material
References
Bhukhanvala, D. S., Sorathiya, S. M., Sawant, P., Colah, R., Ghosh, K., and Gupte, S. C. (2013). Antenatal screening for identification of couples for prenatal diagnosis of severe hemoglobinopathies in surat, South Gujarat. J. Obstet. Gynaecol. India 63, 123–127. doi:10.1007/s13224-012-0271-4
Chainarong, N., Muangpaisarn, W., and Suwanrath, C. (2021). Etiology and outcome of non-immune hydrops fetalis in relation to gestational age at diagnosis and intrauterine treatment. J. Perinatol. 41, 2544–2548. doi:10.1038/s41372-021-01202-7
Chan, W. Y. K., Lee, P. P. W., Lee, V., Chan, G. C. F., Leung, W., Ha, S. Y., et al. (2021). Outcomes of allogeneic transplantation for hemoglobin Bart's hydrops fetalis syndrome in Hong Kong. Pediatr. Transpl. 25, e14037. doi:10.1111/petr.14037
Colaco, S., and Nadkarni, A. (2021). Borderline HbA2 levels: dilemma in diagnosis of beta-thalassemia carriers. Mutat. Res. Rev. Mutat. Res. 788, 108387. doi:10.1016/j.mrrev.2021.108387
Han, W. P., Huang, L., Li, Y. Y., Han, Y. Y., Li, D., An, B. Q., et al. (2019). Reference intervals for HbA2 and HbF and cut-off value of HbA2 for β-thalassemia carrier screening in a Guizhou population of reproductive age. Clin. Biochem. 65, 24–28. doi:10.1016/j.clinbiochem.2018.11.007
He, S., Qin, Q., Yi, S., Wei, Y., Lin, L., Chen, S., et al. (2017b). Prevalence and genetic analysis of α- and β-thalassemia in Baise region, a multi-ethnic region in southern China. Gene 619, 71–75. doi:10.1016/j.gene.2016.02.014
He, S., Wang, L., Pan, P., Wei, H., Meng, D., Du, J., et al. (2017a). Etiology and perinatal outcome of nonimmune hydrops fetalis in southern China. AJP Rep. 7, e111–e115. doi:10.1055/s-0037-1603890
He, S., Zhang, Q., Li, D., Chen, S., Tang, Y., Chen, Q., et al. (2014). Prevention and control of Hb Bart's disease in Guangxi Zhuang autonomous region, China. Eur. J. Obstet. Gynecol. Reprod. Biol. 178, 138–141. doi:10.1016/j.ejogrb.2014.03.034
He, Y., Zhao, Y., Lou, J. W., Liu, Y. H., and Li, D. Z. (2016). Fetal anemia and hydrops fetalis associated with homozygous Hb constant spring (HBA2: c.427T > C). Hemoglobin 40, 97–101. doi:10.3109/03630269.2015.1126721
Henderson, S. J., Timbs, A. T., McCarthy, J., Gallienne, A. E., Proven, M., Rugless, M. J., et al. (2016). Ten years of routine α- and β-globin gene sequencing in UK hemoglobinopathy referrals reveals 60 novel mutations. Hemoglobin 40, 75–84. doi:10.3109/03630269.2015.1113990
Jiang, F., Zuo, L., Li, J., Chen, G., Tang, X., Zhou, J., et al. (2021). Evaluation of intervention strategy of thalassemia for couples of childbearing ages in Centre of Southern China. J. Clin. Lab. Anal. 35, e23990. doi:10.1002/jcla.23990
King, A. J., and Higgs, D. R. (2018). Potential new approaches to the management of the Hb Bart's hydrops fetalis syndrome: the most severe form of α-thalassemia. Hematol. Am. Soc. Hematol. Educ. Program 2018, 353–360. doi:10.1182/asheducation-2018.1.353
Lai, K., Huang, G., Su, L., and He, Y. (2017). The prevalence of thalassemia in mainland China: evidence from epidemiological surveys. Sci. Rep. 7, 920. doi:10.1038/s41598-017-00967-2
Lal, A., and Vichinsky, E. (2023). The clinical phenotypes of alpha thalassemia. Hematol. Oncol. Clin. North Am. 37, 327–339. doi:10.1016/j.hoc.2022.12.004
Li, X., Zhou, Q., Huang, H., Tian, X., and Peng, Q. (2015). Z-score reference ranges for normal fetal heart sizes throughout pregnancy derived from fetal echocardiography. Prenat. Diagn 35, 117–124. doi:10.1002/pd.4498
Liu, J., Huang, X., Li, Y., Li, P., Zhang, X., and Zhang, S. (2019). Prevalence and molecular characterization of thalassemia in the Guangxi Zhuang Autonomous Region of southern China. Hemoglobin 43, 78–82. doi:10.1080/03630269.2019.1577732
Mustafa, I., Firdous, N., Shebl, F. M., Shi, Z., Saeed, M., Zahir, Z., et al. (2020). Genetic epidemiology of beta-thalassemia in the Maldives: 23 years of a beta-thalassemia screening program. Gene 741, 144544. doi:10.1016/j.gene.2020.144544
Nasiri, A., Rahimi, Z., and Vaisi-Raygani, A. (2020). Hemoglobinopathies in Iran: an updated review. Int. J. Hematol. Oncol. Stem Cell Res. 14, 140–150. doi:10.18502/ijhoscr.v14i2.2679
Panyasai, S., Satthakarn, S., and Phasit, A. (2023). Effective screening of hemoglobin Constant Spring and hemoglobin Paksé with several forms of α- and β-thalassemia in an area with a high prevalence and heterogeneity of thalassemia using capillary electrophoresis. Heliyon 9, e19116. doi:10.1016/j.heliyon.2023.e19116
Sirilert, S., Charoenkwan, P., Sirichotiyakul, S., Tongprasert, F., Srisupundit, K., Luewan, S., et al. (2019). Prenatal diagnosis and management of homozygous hemoglobin constant spring disease. J. Perinatol. 39, 927–933. doi:10.1038/s41372-019-0397-7
Songdej, D., Babbs, C., and Higgs, D. R.BHFS International Consortium (2017). An international registry of survivors with Hb Bart's hydrops fetalis syndrome. Blood 129, 1251–1259. doi:10.1182/blood-2016-08-697110
Sorour, Y., Heppinstall, S., Porter, N., Wilson, G. A., Goodeve, A. C., Rees, D., et al. (2007). Is routine molecular screening for common alpha-thalassaemia deletions necessary as part of an antenatal screening programme? J. Med. Screen 14, 60–61. doi:10.1258/096914107781261981
Keywords: α-thalassemia, antenatal screening, hematological parameters, prenatal diagnosis, cost-saving
Citation: Li D, Liang L, Meng D and He S (2024) Routine antenatal molecular testing for α-thalassemia at a tertiary referral hospital in China: ten years of experience. Front. Genet. 15:1416047. doi: 10.3389/fgene.2024.1416047
Received: 11 April 2024; Accepted: 20 May 2024;
Published: 04 June 2024.
Edited by:
Enrique Medina-Acosta, State University of Northern Rio de Janeiro, BrazilReviewed by:
Paloma Ropero, Hospital Clínico San Carlos, SpainWanjun Zhou, Southern Medical University, China
Thais Louvain De Souza, Faculdade de Medicina de Campos, Brazil
Copyright © 2024 Li, Liang, Meng and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongming Li, ankyMDA3MDcxOEAxMjYuY29t; Sheng He, Z3hjc3F4eWpzX2hlc0AxNjMuY29t
†These authors have contributed equally to this work
 Dongming Li
Dongming Li Lifang Liang2†
Lifang Liang2†